Wild Blackberry Fruit (Rubus fruticosus L.) as Potential Functional Ingredient in Food: Ultrasound-Assisted Extraction Optimization, Ripening Period Evaluation, Application in Muffin, and Consumer Acceptance
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
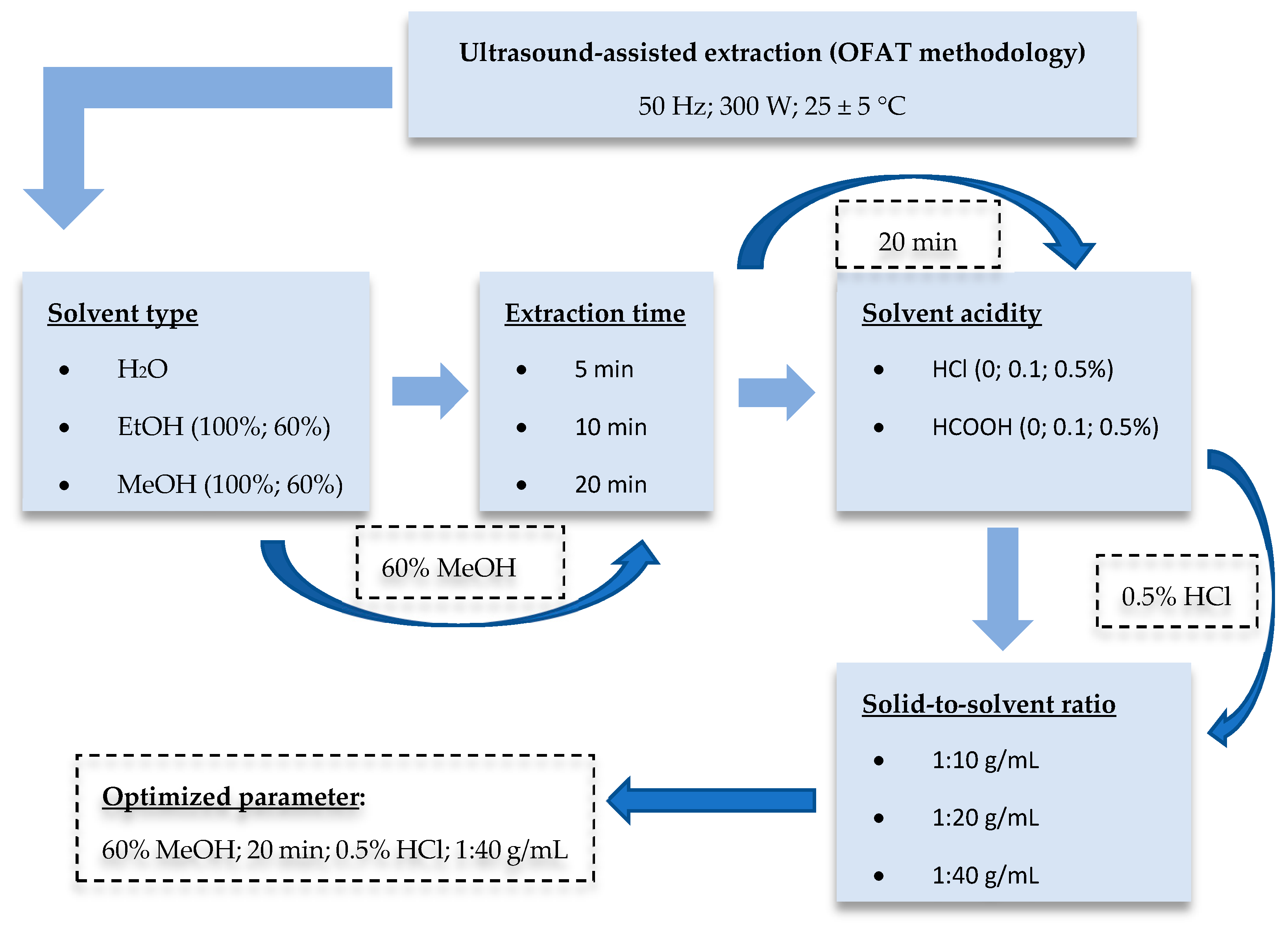
2.3. Optimization Ultrasound-Assisted Extraction (UAE) Procedure
2.4. Muffin Preparation
2.5. Extraction of Muffin Samples
2.6. Phytochemical Analysis
2.7. Consumer Acceptance
2.8. Statistical Analysis
3. Results and Discussion
3.1. Optimization of Ultrasound-Assisted Extraction (UAE)
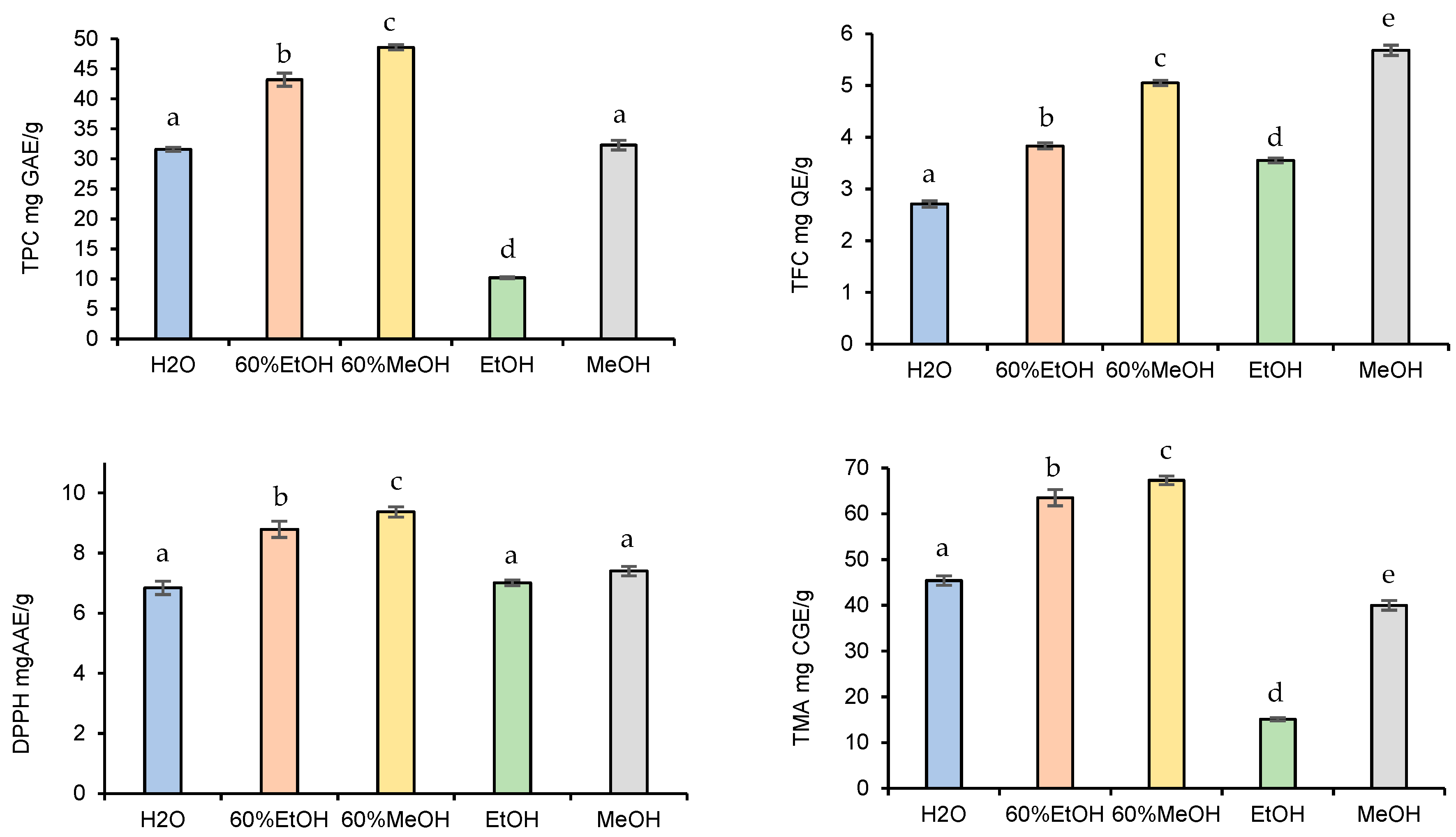
3.1.1. Effect of Solvent Type
3.1.2. Effect of Sonication Time and Acidification
3.1.3. Effect of Solid-to-Solvent Ratio
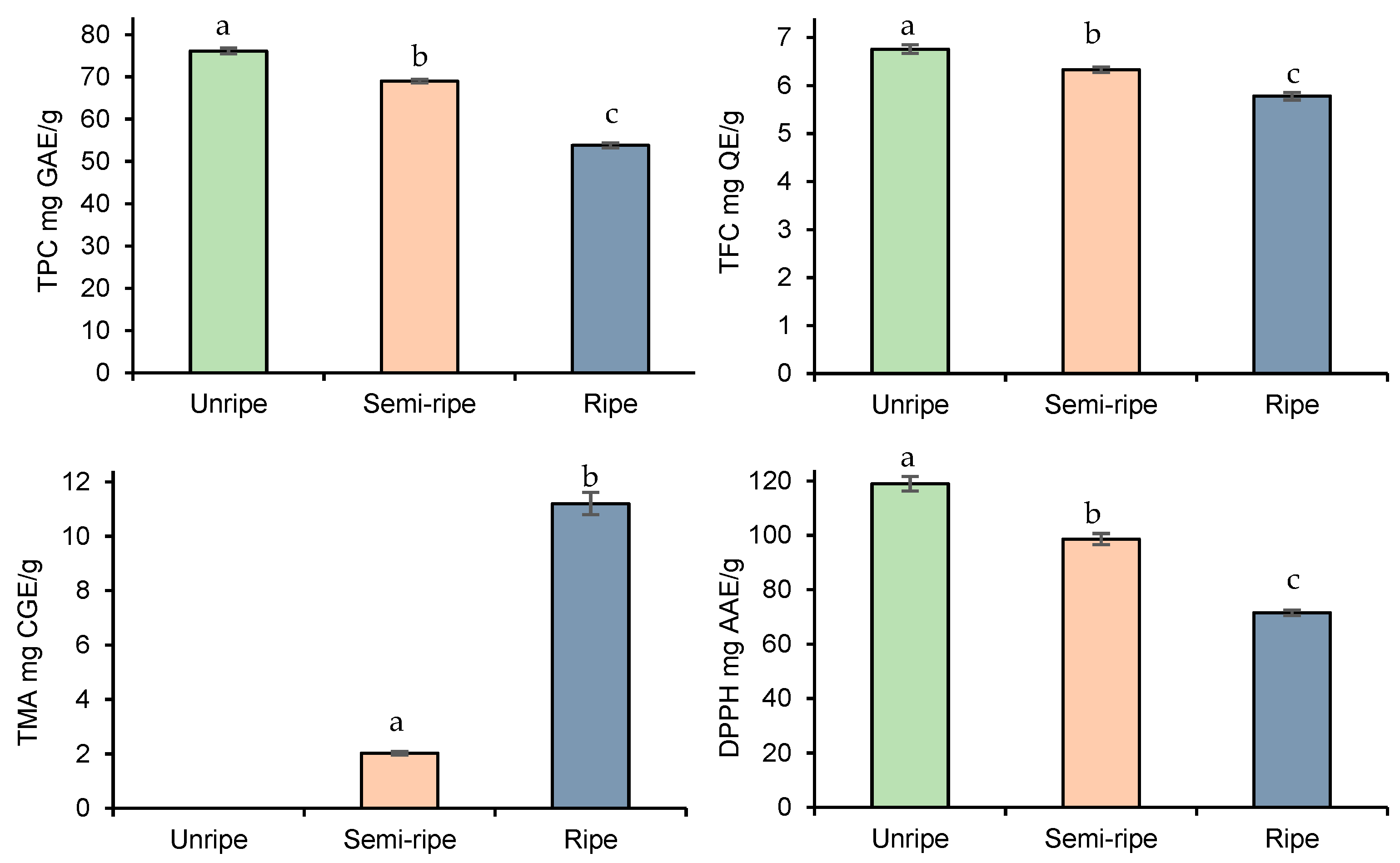
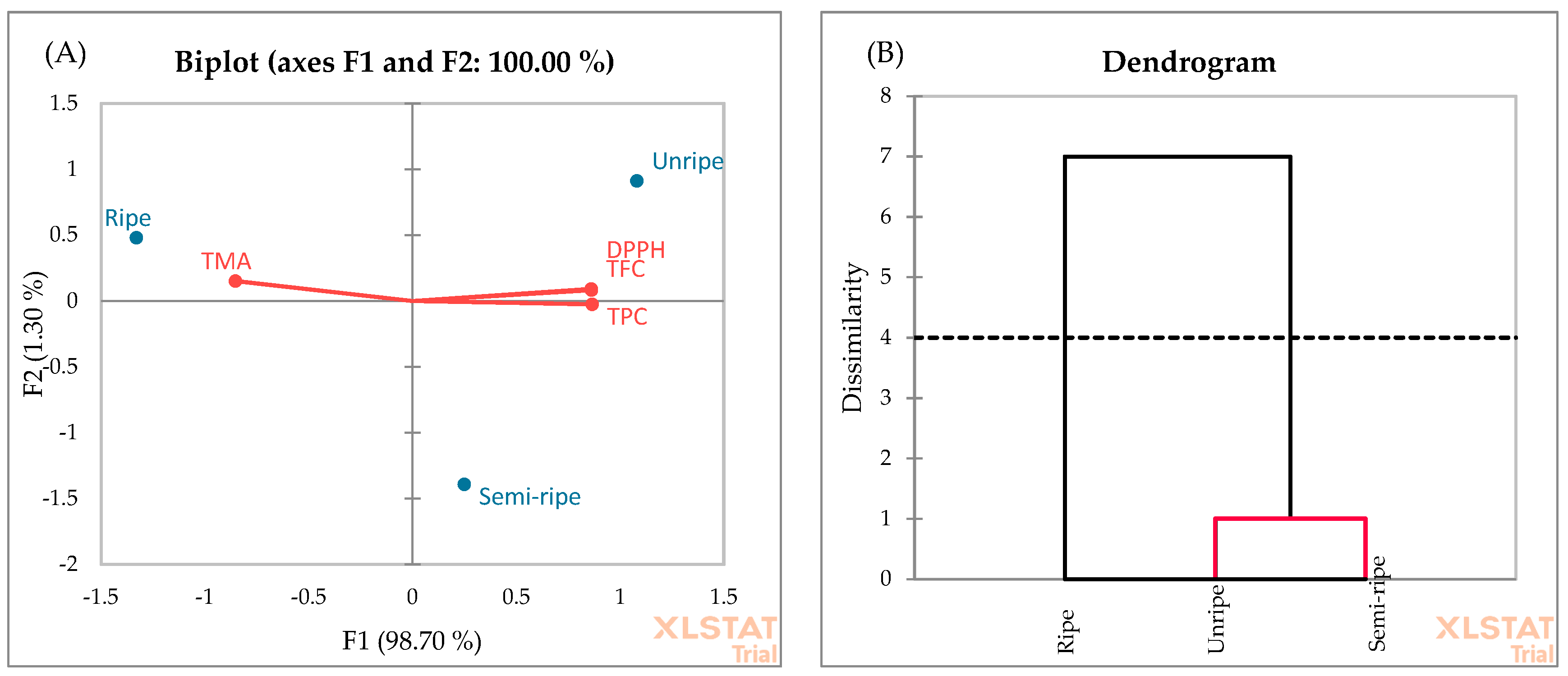
3.2. Ripening Period Evaluation
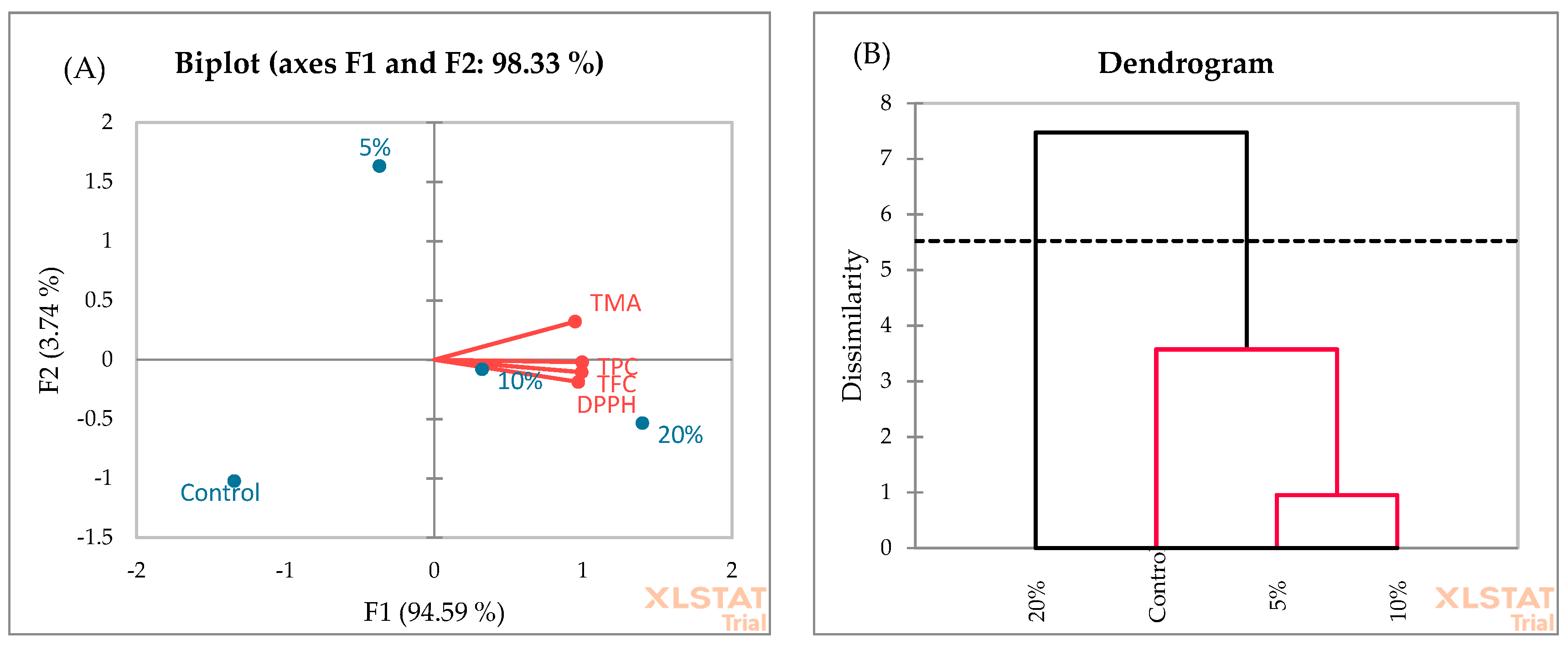
3.3. Fortified Muffin Characterization
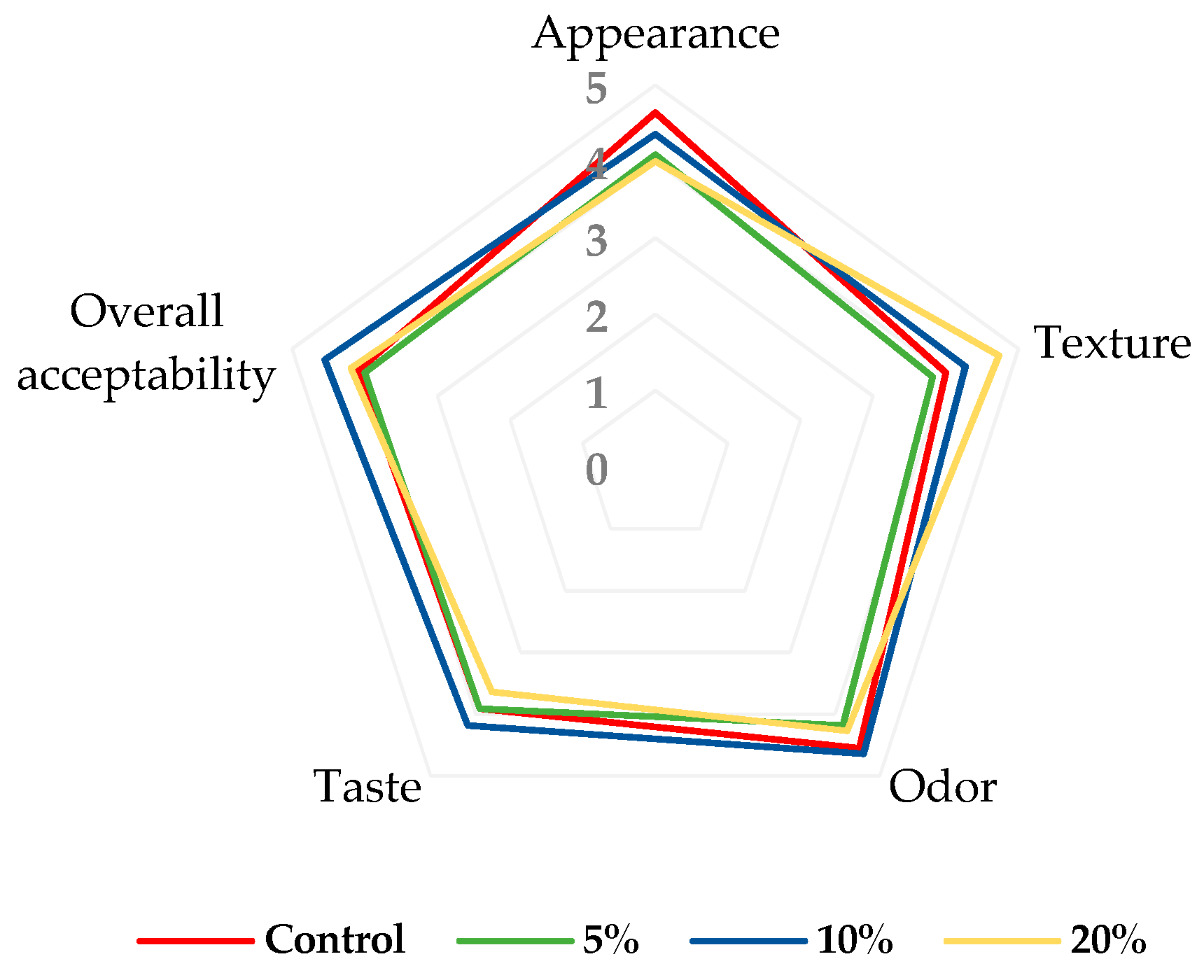
3.4. Acceptance Evaluation
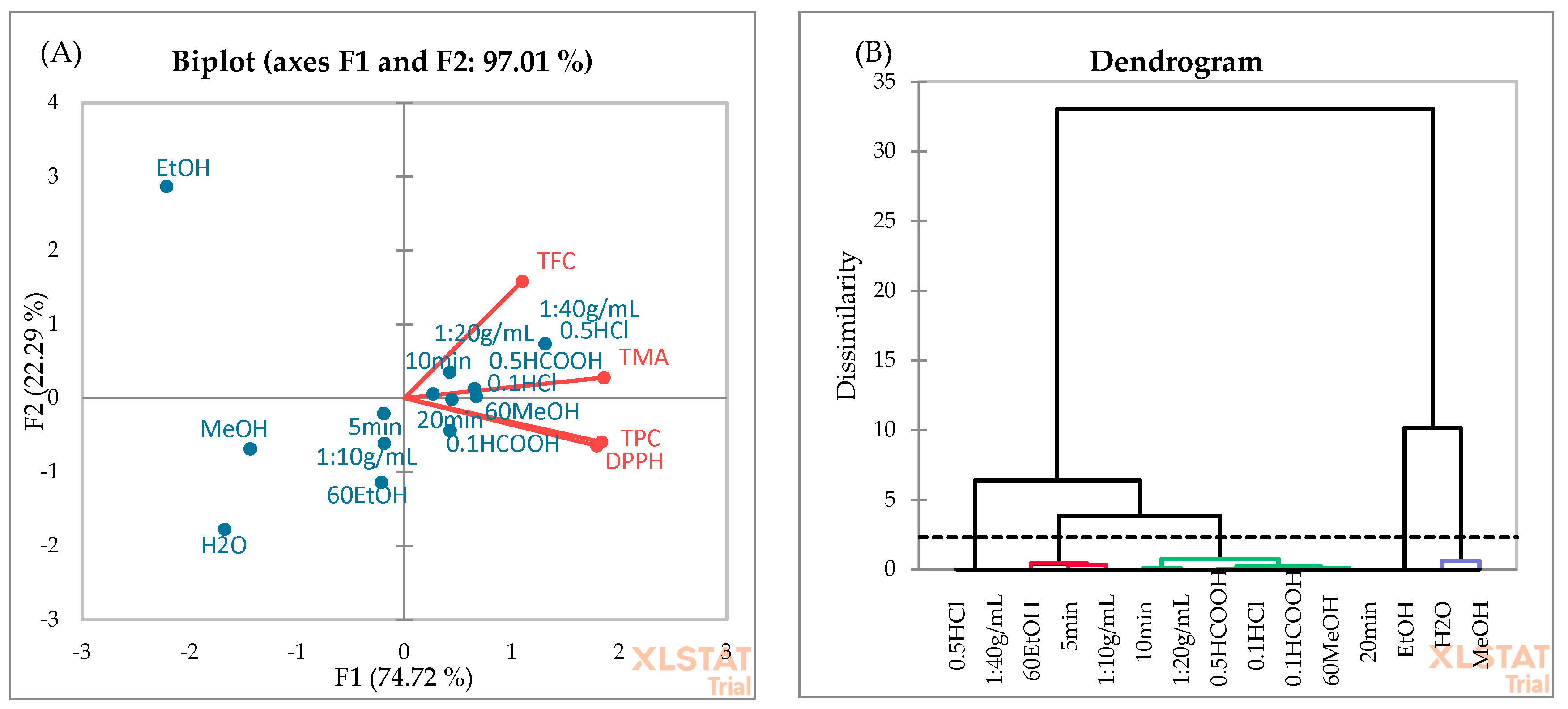
3.5. Principal Component Analysis (PCA) and Agglomerative Hierarchical Clustering (AHC)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Achaglinkame, M.A.; Aderibigbe, R.O.; Hensel, O.; Sturm, B.; Korese, J.K. Nutritional characteristic of four underutilized edible wild fruits of dietary interest in Ghana. Foods 2019, 8, 104. [Google Scholar] [CrossRef]
- Liu, Z.; Dong, B.; Liu, C.; Zong, Y.; Shao, Y.; Liu, B.; Yue, H. Variation of anthocyanin content in fruits of wild and cultivated Lycium ruthenicum. Ind. Crops Prod. 2020, 146, 112208. [Google Scholar] [CrossRef]
- Paun, N.; Botoran, O.R.; Niculescu, V.C. Total phenolic, anthocyanins HPLC-DAD-MS determination and antioxidant capacity in black grape skins and blackberries: A comparative study. Appl. Sci. 2022, 12, 936. [Google Scholar] [CrossRef]
- Ercisli, S.; Sagbas, H.I. Wild edible fruits: A rich source of biodiversity. ANADOLU Ege Tarımsal Araştırma Enstitüsü Derg. 2017, 27, 116–122. [Google Scholar]
- Hidalgo, G.I.; Almajano, M.P. Red fruits: Extraction of antioxidants, phenolic content, and radical scavenging determination: A review. Antioxidants 2017, 6, 7. [Google Scholar] [CrossRef]
- Verma, R.; Gangrade, T.; Punasiya, R.; Ghulaxe, C. Rubus fruticosus (blackberry) use as an herbal medicine. Pharmacogn. Rev. 2014, 8, 101–104. [Google Scholar] [CrossRef]
- Kaume, L.; Howard, L.R.; Devareddy, L. The blackberry fruit: A review on its composition and chemistry metabolism and bioavailability, and health benefits. J. Agric. Food Chem. 2012, 60, 5716–5727. [Google Scholar] [CrossRef] [PubMed]
- Kafkas, E.; Koşar, M.; Türemiş, N.; Başer, K.H.C. Analysis of sugars, organic acids and vitamin C contents of blackberry genotypes from Turkey. Food Chem. 2006, 7, 732–736. [Google Scholar] [CrossRef]
- Duttaroy, A.K. Regulation of functional foods in European Union: Assessment of health claim by the European food safety authority. In Nutraceutical and Functional Food Regulations in the United States and around the World, 3rd ed.; Bagchi, D., Ed.; Elsevier: Amsterdam, The Netherland, 2019; pp. 267–276. [Google Scholar] [CrossRef]
- Banwo, K.; Olojede, A.O.; Adesulu-Dahunsi, A.T.; Verma, D.K.; Thakur, M.; Tripathy, S.; Singh, S.; Patel, A.R.; Gupta, A.K.; Aguilar, C.N.; et al. Functional importance of bioactive compounds of foods with potential health benefits: A review on recent trends. Food Biosci. 2021, 43, 101320. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef]
- Li, W.; Chen, H.; Xu, B.; Wang, Y.; Zhang, C.; Cao, Y.; Xing, X. Research progress on classification sources and functions of dietary polyphenols for prevention and treatment of chronic diseases. J. Future Foods 2023, 3, 289–305. [Google Scholar] [CrossRef]
- Pucel, N.; Sarkar, D.; Labbe, R.G.; Khanongnuch, C.; Shetty, K. Improving Health Targeted Food Quality of Blackberry: Pear Fruit Synergy Using Lactic Acid Bacterial Fermentation. Front. Sustain. Food Syst. 2021, 5, 703672. [Google Scholar] [CrossRef]
- Mildner-Szkudlarz, S.; Bajerska, J.; Górnaś, P.; Seglina, D.; Pilarska, A.; Jesionowski, T. Physical and bioactive properties of muffins enriched with raspberry and cranberry pomace powder: A promising application of fruit by-products rich in biocompounds. Plant Foods Hum. Nutr. 2016, 71, 165–173. [Google Scholar] [CrossRef]
- Hosseininejad, S.; Larrea, V.; Moraga, G.; Hernando, I. Evaluation of the Bioactive Compounds, and Physicochemical and Sensory Properties of Gluten-Free Muffins Enriched with Persimmon ‘Rojo Brillante’ Flour. Foods 2022, 11, 3357. [Google Scholar] [CrossRef]
- dos Santos, S.S.; Paraíso, C.M.; Romanini, E.B.; Correa, V.G.; Peralta, R.M.; da Costa, S.C.; de Oliveira Santos Junior, O.; Visentainer, J.V.; Reis, M.H.M.; Madrona, G.S. Bioavailability of blackberry pomace microcapsules by using different techniques: An approach for yogurt application. Innov. Food Sci. Emerg. Technol. 2022, 81, 103111. [Google Scholar] [CrossRef]
- Pereira, A.P.A.; Clerici, M.T.P.S.; Schmiele, M.; Pastore, G.M. Blackberries (Rubus sp.) and whole grain wheat flour in cookies: Evaluation of phenolic compounds and technological properties. J. Food Sci. Technol. 2019, 56, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Yusoff, I.M.; Taher, Z.M.; Rahmat, Z.; Chua, L.S. A review of ultrasound-assisted extraction for plant bioactive compounds: Phenolics, flavonoids, thymols, saponins and proteins. Food Res. Int. 2022, 157, 111268. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Király, G. Alien Rubus species in Hungary: Distribution, habitats and threats. Dendrobiology 2018, 80, 1–11. [Google Scholar] [CrossRef]
- Sochor, M.; Trávníček, B.; Király, G. Ploidy level variation in the genus Rubus in the Pannonian Basin and the northern Balkans, and evolutionary implications. Plant Syst. Evol. 2019, 305, 611–626. [Google Scholar] [CrossRef]
- Tukassar, A.; Shukat, R.; Iahtisham-UlHaq; Butt, M.S.; Nayik, G.A.; Ramniwas, S.; Al Obaid, S.; Ali Alharbi, S.; Ansari, M.J.; Konstantinos Karabagias, I.; et al. Compositional profiling and sensory analysis of cauliflower by-products-enriched muffins. Food Sci. Nutr. 2023, 11, 6020–6031. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdicphosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Cuesta-Riaño, C.S.; Castro-Guascaa, M.P.; Tarazona-Díaz, M.P. Anthocyanin Extract from Blackberry Used as an Indicator of Hydrogen Potential. Int. J. Fruit Sci. 2022, 22, 224–234. [Google Scholar] [CrossRef]
- Işik, E.; Şahin, S.; Demir, C. Determination of total phenolic content of raspberry and blackberry cultivations immobilized horseradish peroxidase bioreactor. J. Food Compos. Anal. 2011, 24, 944–949. [Google Scholar] [CrossRef]
- Assefa, A.D.; Ko, E.Z.; Moon, S.H.; Keum, Y.S. Antioxidant and antiplatelet activities of flavonoid-rich fractions of three citrus fruits from Korea. 3 Biotech 2016, 6, 109. [Google Scholar] [CrossRef]
- Rodríguez, R.; Alvarez-Sabatel, S.; Ríos, Y.; Rioja, P.; Talens, C. Effect of microwave technology and upcycled orange fibre on the quality of gluten-free muffins. LWT 2022, 158, 113148. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Álvarez, J.A.F.; Barbero, G.; Ayuso, J. Extraction of antioxidants from blackberry (Rubus ulmifolius L.): Comparison between ultrasound- and microwave-assisted extraction techniques. Agronomy 2019, 9, 745. [Google Scholar] [CrossRef]
- Celli, G.B.; Ghanem, A.; Brooks, M.S.-L. Optimization of ultrasound-assisted extraction of anthocyanins from haskap berries (Lonicera caerulea L.) using response surface methodology. Ultrason. Sonochem. 2015, 27, 449–455. [Google Scholar] [CrossRef]
- Ivanovic, J.; Tadic, V.; Dimitrijevic, S.; Stamenic, M.; Petrovic, S.; Zizovic, I. Antioxidant properties of the anthocyanin-containing ultrasonic extract from blackberry cultivar “Čačanska Bestrna”. Ind. Crops Prod. 2014, 53, 274–281. [Google Scholar] [CrossRef]
- Yılmaz, F.M.; Karaaslan, M.; Vardin, H. Optimization of extraction parameters on the isolation of phenolic compounds from sour cherry (Prunus cerasus L.) pomace. J. Food Sci. Technol. 2015, 52, 2851–2859. [Google Scholar] [CrossRef]
- Iglesias-Carres, L.; Mas-Capdevila, A.; Sancho-Pardo, L.; Bravo, F.I.; Mulero, M.; Muguerza, B.; Arola-Arnal, A. Optimized Extraction by Response Surface Methodology Used for the Characterization and Quantification of Phenolic Compounds in Whole Red Grapes (Vitis vinifera). Nutrients 2018, 10, 1931. [Google Scholar] [CrossRef]
- Stanoeva, J.P.; Balshikevska, E.; Stefova, M.; Tusevski, O.; Simic, S.G. Comparison of the effect of acids in solvent mixtures for extraction of phenolic compounds from Aronia melanocarpa. Nat. Prod. Commun. 2020, 15, 1934578X20934675. [Google Scholar]
- Weremfo, A.; Adulley, F.; Dabie, K.; Abassah-Oppong, S.; Peprah-Yamoah, E. Optimization of ultrasound-assisted extraction of phenolic antioxidants from turkey berry (Salonum torvum Sw) fruits using response surface methodology. J. Appl. Res. Med. Aromat. Plants 2022, 30, 100387. [Google Scholar]
- Li, J.; Shi, C.; Shen, D.; Han, T.; Wu, W.; Lyu, L.; Li, W. Composition and Antioxidant Activity of Anthocyanins and Non-Anthocyanin Flavonoids in Blackberry from Different Growth Stages. Foods 2022, 11, 2902. [Google Scholar] [CrossRef]
- Belwal, T.; Pandey, A.; Bhatt, I.D.; Rawal, R.S.; Luo, Z. Trends of polyphenolics and anthocyanins accumulation along ripening stages of wild edible fruits of Indian Himalayan region. Sci. Rep. 2019, 9, 5894. [Google Scholar] [CrossRef]
- Zielinski, A.A.F.; Goltz, C.; Yamato, M.A.C.; Ávila, S.; Hirooka, E.Z.; Wosiacki, G.; Nogueira, A.; Demiate, I.M. Blackberry (Rubus spp.): Influence of ripening and processing on levels of phenolic compounds and antioxidant activity of the ‘Brazos’ and ‘Tupy’ varieties grown in Brazil. Ciência Rural 2015, 45, 744–749. [Google Scholar] [CrossRef]
- Samaniego, I.; Brito, B.; Viera, W.; Cabrera, A.; Llerena, W.; Kannangara, T.; Vilcacundo, R.; Angós, I.; Carrillo, W. Influence of the Maturity Stage on the Phytochemical Composition and the Antioxidant Activity of Four Andean Blackberry Cultivars (Rubus glaucus Benth) from Ecuador. Plants 2020, 9, 1027. [Google Scholar] [CrossRef]
- Jazić, M.; Kukrić, Z.; Vulić, J.; Četojević-Simin, D. Polyphenolic composition, antioxidant and antiproliferative effects of wild and cultivated blackberries (Rubus fruticosus L.) pomace. Int. J. Food Sci. Technol. 2018, 54, 194–201. [Google Scholar] [CrossRef]
- Travičić, V.; Šovljanski, O.; Tomić, A.; Perović, M.; Milošević, M.; Ćetković, N.; Antov, M. Augmenting Functional and Sensorial Quality Attributes of Kefir through Fortification with Encapsulated Blackberry Juice. Foods 2023, 12, 4163. [Google Scholar] [CrossRef] [PubMed]
- Lončarević, I.; Pajin, B.; Fišteš, A.; Šaponjac, V.T.; Petrović, J.; Jovanović, P.; Vulić, J.; Zarić, D. Enrichment of white chocolate with blackberry juice encapsulate: Impact on physical properties, sensory characteristics and polyphenol content. LWT 2018, 924, 458–464. [Google Scholar] [CrossRef]
- Górnaś, P.; Juhņeviča-Radenkova, K.; Radenkovs, V.; Mišina, I.; Pugajeva, I.; Soliven, A.; Segliņa, D. The impact of different baking condition on the stability of the extractable polyphenols in muffins enriched by strawberry, sour cherry, raspberry or black currant pomace. LWT Food Sci. Technol. 2016, 65, 946–953. [Google Scholar] [CrossRef]
- Ifie, I.; Marshall, L.J. Food processing and its impact on phenolic constituents in food. Cogent Food Agric. 2018, 4, 1. [Google Scholar] [CrossRef]

| TPC (mg GAE/g) | TFC (mg QE/g) | TMA (mg CGE/g) | Antioxidant Activity | ||
|---|---|---|---|---|---|
| DPPH (mg AAE/g) | IC50 (µg/mL) | ||||
| Extraction time (min) | |||||
| 5 | 40.5 ± 0.70 a | 4.46 ± 0.02 a | 9.06 ± 0.14 a | 58.2 ± 0.81 a | 67.2 ± 0.65 a |
| 10 | 45.5 ± 0.74 b | 4.82 ± 0.07 b | 9.79 ± 0.28 b | 60.5 ± 1.18 a | 63.3 ± 0.51 b |
| 20 | 48.6 ± 0.42 c | 5.05 ± 0.05 c | 9.37 ± 0.17 c | 67.3 ± 0.94 b | 56.1 ± 0.91 c |
| Acidification (v/v%) | |||||
| 0 | 48.6 ± 0.42 c | 5.05 ± 0.05 c | 9.37 ± 0.17 c | 67.3 ± 0.94 b | 56.1 ± 0.91 c |
| HCOOH | |||||
| 0.1 | 49.8 ± 0.49 d | 4.72 ± 0.09 d | 9.36 ± 0.14 c | 68.5 ± 1.45 b | 55.4 ± 0.49 c |
| 0.5 | 51.2 ± 0.80 d | 5.31 ± 0.08 e | 9.47 ± 0.21 c | 69.9 ± 1.1 b | 54.1 ± 0.53 c |
| HCl | |||||
| 0.1 | 50.6 ± 0.45 cd | 5.15 ± 0.12 ce | 9.77 ± 0.20 c | 69.6± 0.73 b | 54.7 ± 0.62 c |
| 0.5 | 53.8 ± 0.59 e | 5.78 ± 0.08 f | 11.2 ± 0.41 d | 71.5 ± 0.96 bc | 52.3 ± 0.59 d |
| Solid-to-Solvent Ratio (g/mL) | TPC (mg GAE/g) | TFC (mg QE/g) | TMA (mg CGE/g) | Antioxidant Activity | |
|---|---|---|---|---|---|
| DPPH (mg AAE/g) | IC50 (µg/mL) | ||||
| 1:10 | 44.2 ± 0.84 a | 4.00 ± 0.08 a | 9.53 ± 0.12 a | 52.4 ± 0.60 a | 73.2 ± 1.12 a |
| 1:20 | 48.7 ± 0.58 b | 5.10 ± 0.09 b | 10.0 ± 0.20 a | 58.0 ± 1.20 a | 69.2 ± 0.77 a |
| 1:40 | 53.8 ± 0.59 c | 5.78 ± 0.08 c | 11.2 ± 0.41 b | 71.5 ± 0.96 b | 52.3 ± 0.63 b |
| Sample | TPC (mg GAE/g) | TFC (mg QE/g) | TMA (mg CGE/g) | Antioxidant Activity | |
|---|---|---|---|---|---|
| DPPH (mg AAE/g) | IC50 (mg/mL) | ||||
| Control | 0.20 ± 0.006 a | 0.09 ± 0.001 a | - | 1.36 ± 0.006 a | 3.15 ± 0.029 a |
| 5% | 1.18 ± 0.027 b | 0.20 ± 0.005 b | 0.15 ± 0.004 a | 1.42 ± 0.013 b | 2.90 ± 0.025 b |
| 10% | 1.64 ± 0.033 c | 0.30 ± 0.004 c | 0.17 ± 0.005 b | 1.61 ± 0.017 c | 2.39 ± 0.013 c |
| 20% | 3.15 ± 0.022 d | 0.52 ± 0.006 d | 0.23 ± 0.007 c | 1.70 ± 0.009 d | 1.65 ± 0.007 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sik, B.; Ajtony, Z.; Lakatos, E.; Székelyhidi, R. Wild Blackberry Fruit (Rubus fruticosus L.) as Potential Functional Ingredient in Food: Ultrasound-Assisted Extraction Optimization, Ripening Period Evaluation, Application in Muffin, and Consumer Acceptance. Foods 2024, 13, 666. https://doi.org/10.3390/foods13050666
Sik B, Ajtony Z, Lakatos E, Székelyhidi R. Wild Blackberry Fruit (Rubus fruticosus L.) as Potential Functional Ingredient in Food: Ultrasound-Assisted Extraction Optimization, Ripening Period Evaluation, Application in Muffin, and Consumer Acceptance. Foods. 2024; 13(5):666. https://doi.org/10.3390/foods13050666
Chicago/Turabian StyleSik, Beatrix, Zsolt Ajtony, Erika Lakatos, and Rita Székelyhidi. 2024. "Wild Blackberry Fruit (Rubus fruticosus L.) as Potential Functional Ingredient in Food: Ultrasound-Assisted Extraction Optimization, Ripening Period Evaluation, Application in Muffin, and Consumer Acceptance" Foods 13, no. 5: 666. https://doi.org/10.3390/foods13050666
APA StyleSik, B., Ajtony, Z., Lakatos, E., & Székelyhidi, R. (2024). Wild Blackberry Fruit (Rubus fruticosus L.) as Potential Functional Ingredient in Food: Ultrasound-Assisted Extraction Optimization, Ripening Period Evaluation, Application in Muffin, and Consumer Acceptance. Foods, 13(5), 666. https://doi.org/10.3390/foods13050666





