The Potential of Pediococcus acidilactici Cell-Free Supernatant as a Preservative in Food Packaging Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Culture
2.2. Cell-Free Supernatant (CFS) Production
2.3. Viable Cell Count
2.4. Antimicrobial Activity Testing with Agar Well Diffusion
2.5. CFS-Pa Characterization
2.5.1. Total Protein Quantification Using Bicinchoninic Acid (BCA) Assay
2.5.2. Lactic Acid Profile Measurement
2.5.3. pH Adjustment/Neutralization
2.5.4. Pepsin Digestion Assay
2.5.5. Catalase Activity Assay
2.5.6. SDS-PAGE Analysis
2.5.7. Minimal Inhibitory Concentration (MIC) Test
2.5.8. Time–Kill Assay
2.5.9. Antioxidant Activity Assay
2.6. Production and Antimicrobial Activity of CFS-Pa-Loaded Bacterial Cellulose
2.6.1. Bacterial Cellulose Production by Komagataeibacter intermedius
2.6.2. CFS-Pa-Loaded Bacterial Cellulose Preparation and Antimicrobial Activity Testing
2.7. Data Analysis
3. Results and Discussion
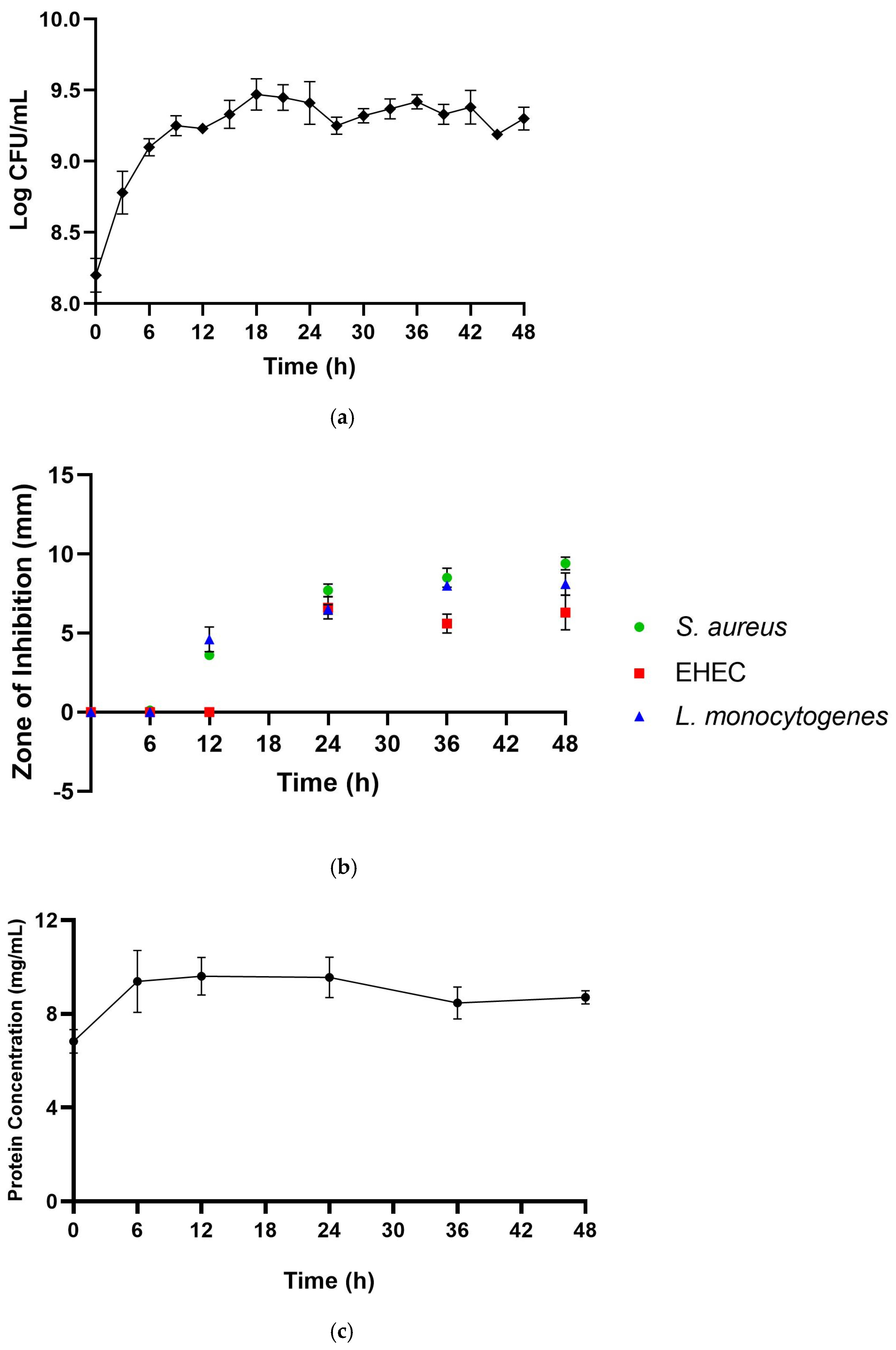
3.1. The Optimal Incubation Period for the Production of CFS-Pa with the Highest Antimicrobial Activity
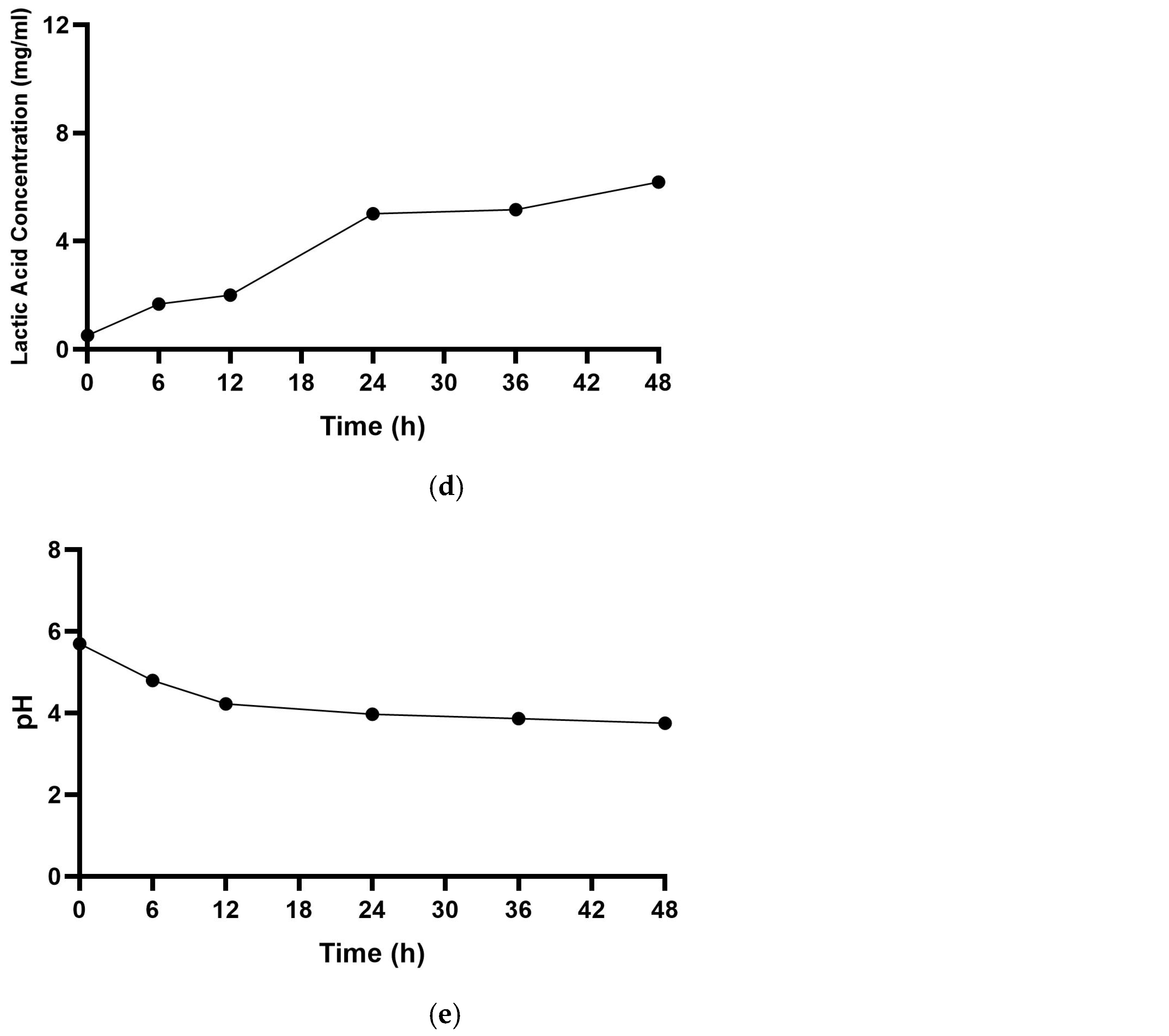
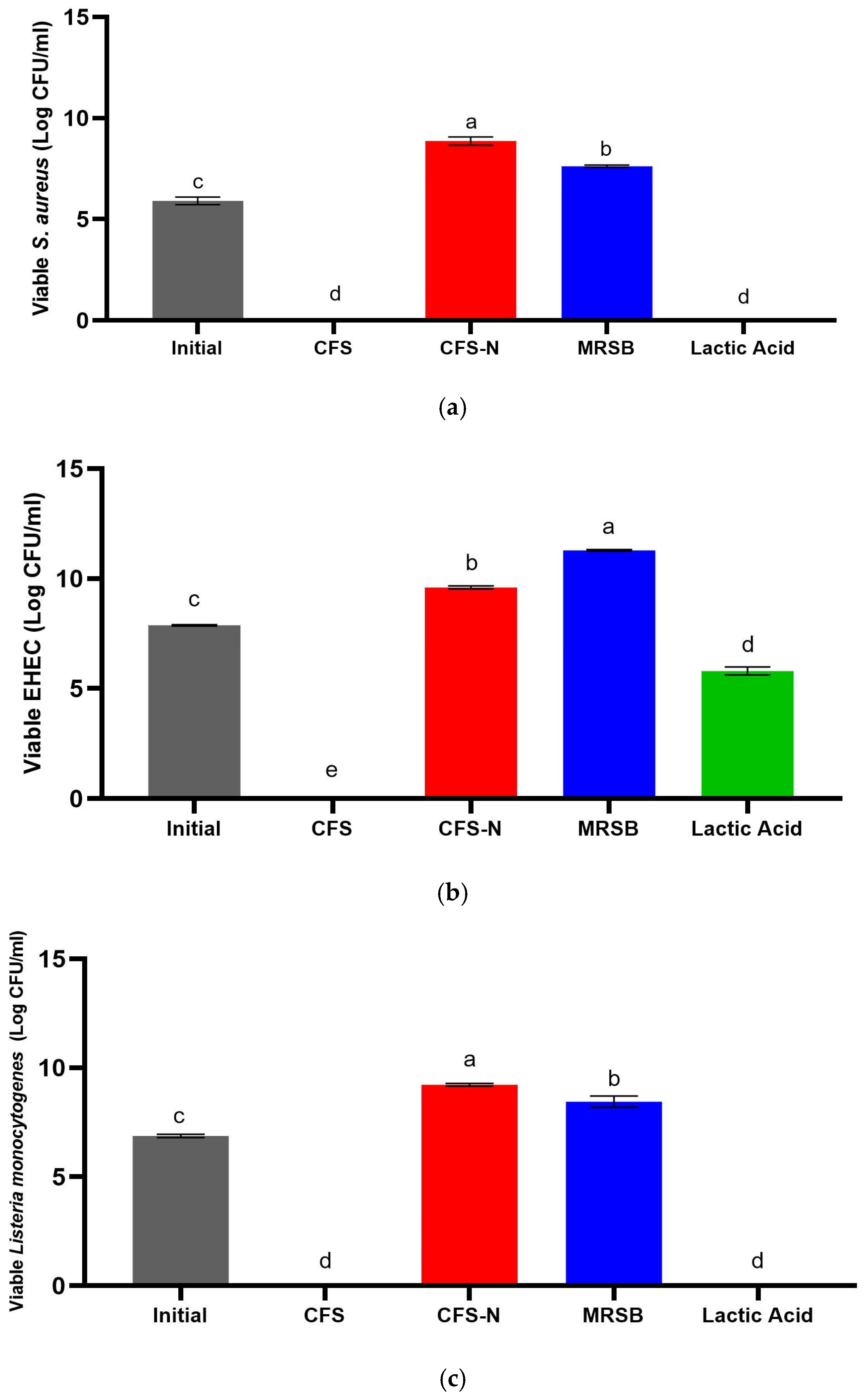
3.2. Primary Metabolites Responsible for the Antimicrobial Activity of CFS-Pa
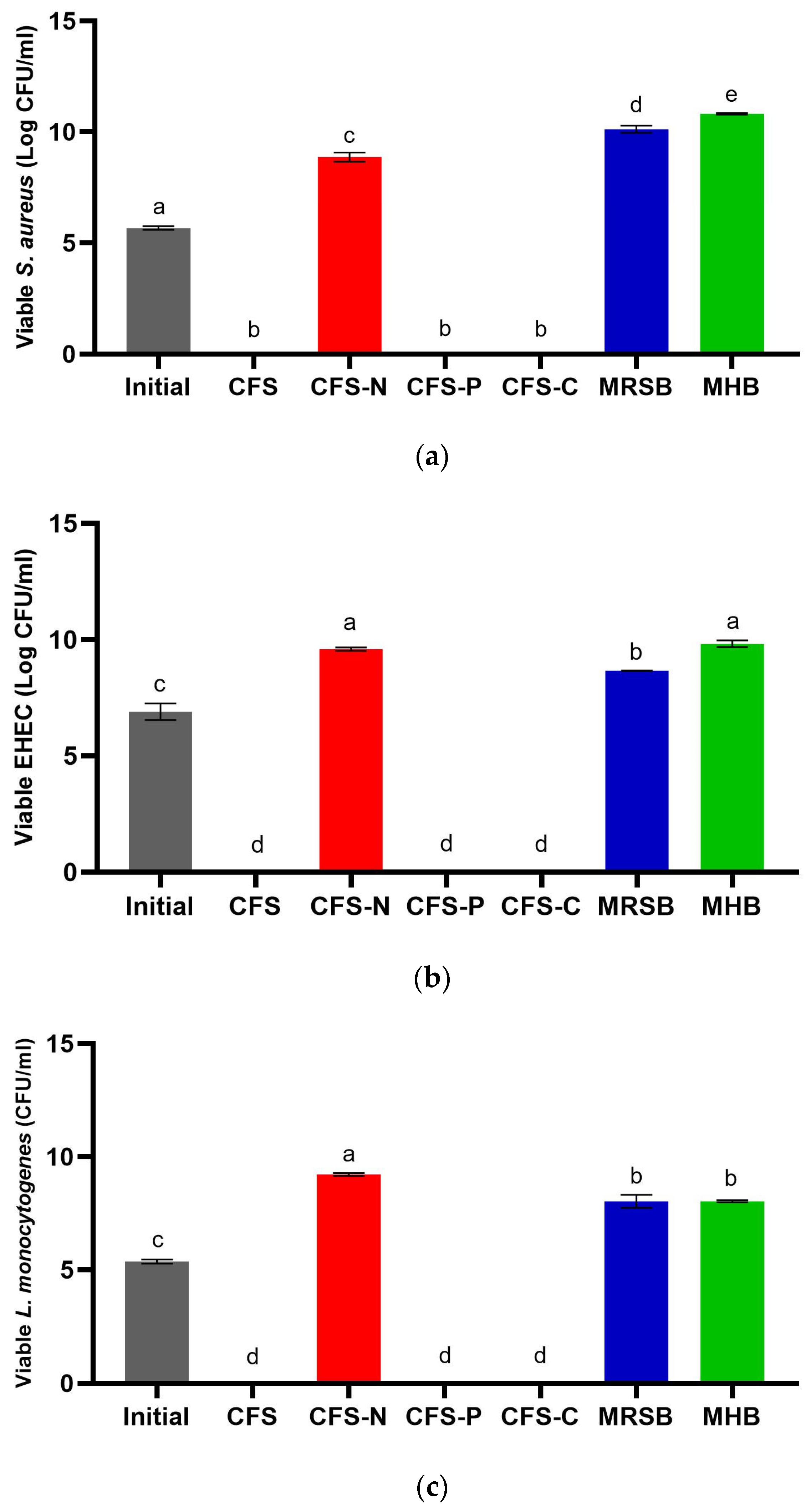
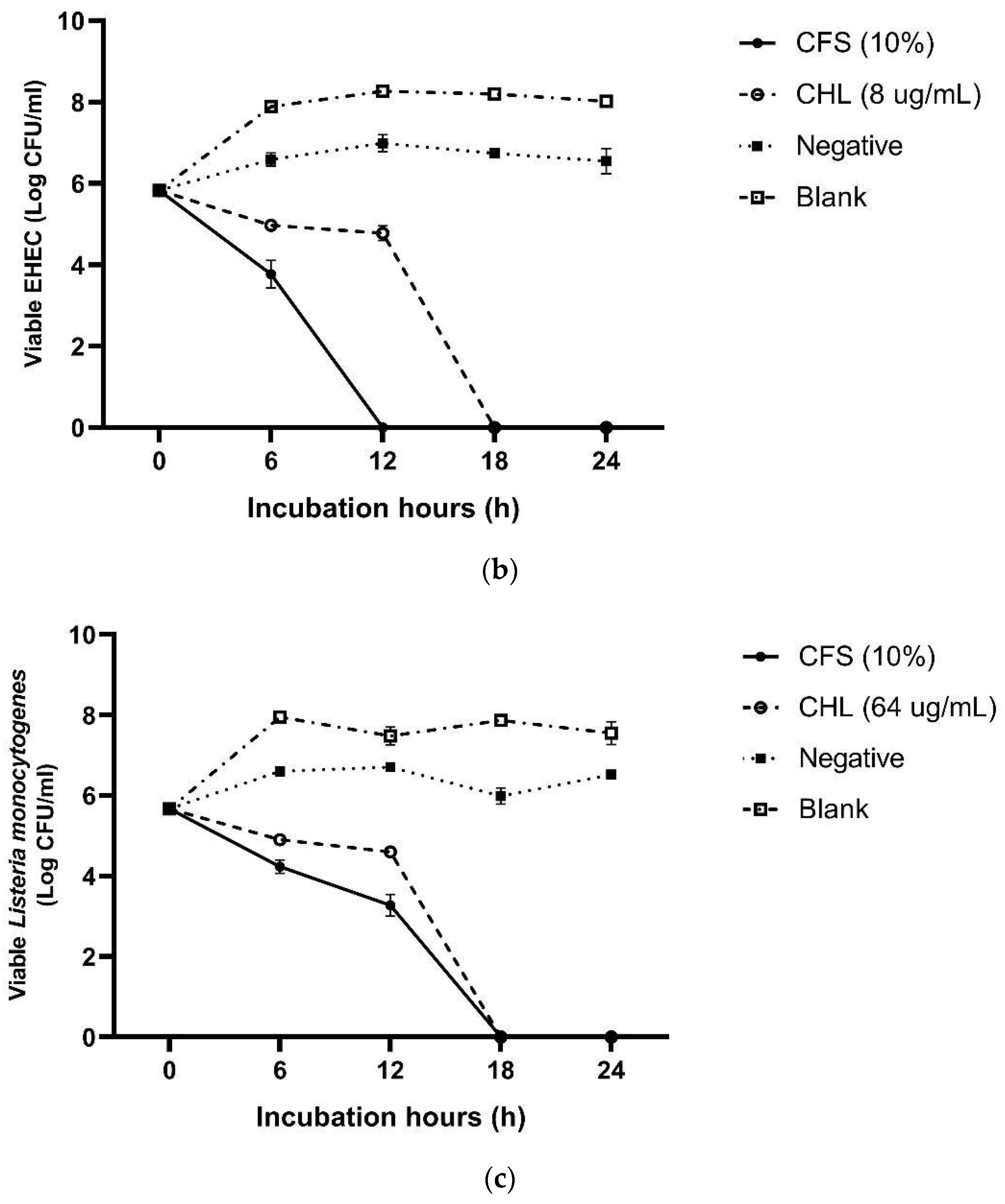
3.3. Determination of Minimum Inhibitory Concentration (MIC) and Time–Kill Assay
3.4. Antioxidant Activity of CFS-Pa
3.5. Potential Application of CFS-Pa-Loaded BC against Foodborne Pathogens
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amit, S.K.; Uddin, M.M.; Rahman, R.; Islam, S.M.R.; Khan, M.S. A Review on Mechanisms and Commercial Aspects of Food Preservation and Processing. Agric. Food Secur. 2017, 6, 51. [Google Scholar] [CrossRef]
- Witkowski, M.; Grajeta, H.; Gomułka, K. Hypersensitivity Reactions to Food Additives—Preservatives, Antioxidants, Flavor Enhancers. Int. J. Environ. Res. Public. Health 2022, 19, 11493. [Google Scholar] [CrossRef] [PubMed]
- Cen, T.; Zhang, X.; Xie, S.; Li, D. Preservatives Accelerate the Horizontal Transfer of Plasmid-Mediated Antimicrobial Resistance Genes via Differential Mechanisms. Environ. Int. 2020, 138, 105544. [Google Scholar] [CrossRef]
- Bouarab Chibane, L.; Degraeve, P.; Ferhout, H.; Bouajila, J.; Oulahal, N. Plant Antimicrobial Polyphenols as Potential Natural Food Preservatives. J. Sci. Food Agric. 2019, 99, 1457–1474. [Google Scholar] [CrossRef] [PubMed]
- Falleh, H.; Ben Jemaa, M.; Saada, M.; Ksouri, R. Essential Oils: A Promising Eco-Friendly Food Preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef]
- Stoyanova, L.G.; Ustyugova, E.A.; Netrusov, A.I. Antibacterial Metabolites of Lactic Acid Bacteria: Their Diversity and Properties. Appl. Biochem. Microbiol. 2012, 48, 229–243. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Ayivi, R.D.; Zimmerman, T.; Siddiqui, S.A.; Altemimi, A.B.; Fidan, H.; Esatbeyoglu, T.; Bakhshayesh, R.V. Lactic Acid Bacteria as Antimicrobial Agents: Food Safety and Microbial Food Spoilage Prevention. Foods 2021, 10, 3131. [Google Scholar] [CrossRef]
- Fugaban, J.I.I.; Vazquez Bucheli, J.E.; Park, Y.J.; Suh, D.H.; Jung, E.S.; Franco, B.D.G.d.M.; Ivanova, I.V.; Holzapfel, W.H.; Todorov, S.D. Antimicrobial Properties of Pediococcus acidilactici and Pediococcus pentosaceus Isolated from Silage. J. Appl. Microbiol. 2022, 132, 311–330. [Google Scholar] [CrossRef] [PubMed]
- Thorakkattu, P.; Khanashyam, A.C.; Shah, K.; Babu, K.S.; Mundanat, A.S.; Deliephan, A.; Deokar, G.S.; Santivarangkna, C.; Nirmal, N.P. Postbiotics: Current Trends in Food and Pharmaceutical Industry. Foods 2022, 11, 3094. [Google Scholar] [CrossRef]
- Hamad, G.M.; Abdelmotilib, N.M.; Darwish, A.M.G.; Zeitoun, A.M. Commercial Probiotic Cell-Free Supernatants for Inhibition of Clostridium Perfringens Poultry Meat Infection in Egypt. Anaerobe 2020, 62, 102181. [Google Scholar] [CrossRef]
- Arrioja-Bretón, D.; Mani-López, E.; Palou, E.; López-Malo, A. Antimicrobial Activity and Storage Stability of Cell-Free Supernatants from Lactic Acid Bacteria and Their Applications with Fresh Beef. Food Control 2020, 115, 107286. [Google Scholar] [CrossRef]
- Kim, H.; Kang, S.-S. Antifungal Activities against Candida Albicans, of Cell-Free Supernatants Obtained from Probiotic Pediococcus acidilactici HW01. Arch. Oral Biol. 2019, 99, 113–119. [Google Scholar] [CrossRef]
- Liu, W.-X.; Wang, J.-J.; Xiao, X.-K.; Chen, C.-R.; Lu, X.; Zhang, X.-Y.; Lin, L.-B.; Wang, F. Antimicrobial Effects and Metabolomics Analysis of Cell-Free Supernatant Produced by Pediococcus acidilactici LWX 401 Isolated from Yunnan Traditional Pickles. LWT 2024, 191, 115626. [Google Scholar] [CrossRef]
- Khorshidian, N.; Khanniri, E.; Mohammadi, M.; Mortazavian, A.M.; Yousefi, M. Antibacterial Activity of Pediocin and Pediocin-Producing Bacteria Against Listeria Monocytogenes in Meat Products. Front. Microbiol. 2021, 12, 709959. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, E.; Calzada, J.; Arqués, J.L.; Rodríguez, J.M.; Nuñez, M.; Medina, M. Antimicrobial Activity of Pediocin-Producing Lactococcus Lactis on Listeria Monocytogenes, Staphylococcus aureus and Escherichia coli O157:H7 in Cheese. Int. Dairy J. 2005, 15, 51–57. [Google Scholar] [CrossRef]
- Raccach, M. Pediococcus. In Encyclopedia of Food Microbiology, 2nd ed.; Academic Press: Oxford, UK, 2014; pp. 1–5. [Google Scholar]
- Mani-López, E.; Arrioja-Bretón, D.; López-Malo, A. The Impacts of Antimicrobial and Antifungal Activity of Cell-free Supernatants from Lactic Acid Bacteria in Vitro and Foods. Compr. Rev. Food Sci. Food Saf. 2022, 21, 604–641. [Google Scholar] [CrossRef]
- Dike, K.S.; Sanni, A.I. Influence of Starter Culture of Lactic Acid Bacteria on the Shelf Life of Agidi, an Indigenous Fermented Cereal Product. Afr. J. Biotechnol. 2010, 9, 7922–7927. [Google Scholar] [CrossRef]
- Macáková, K.; Afonso, R.; Saso, L.; Mladěnka, P. The Influence of Alkaloids on Oxidative Stress and Related Signaling Pathways. Free Radic. Biol. Med. 2019, 134, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Šušković, J.; Kos, B.; Beganović, J.; Leboš Pavunc, A.; Habjanič, K.; Matošić, S. Antimicrobial Activity—The Most Important Property of Probiotic and Starter Lactic Acid Bacteria. Food Technol. Biotechnol. 2010, 48, 296–307. [Google Scholar]
- Guerra, N.P.; Bernárdez, P.F.; Castro, L.P. Fed-Batch Pediocin Production on Whey Using Different Feeding Media. Enzym. Microb. Technol. 2007, 41, 397–406. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M. Bacterial Cellulose as a Biodegradable Food Packaging Material: A Review. Food Hydrocoll. 2021, 113, 106530. [Google Scholar] [CrossRef]
- Tirta, G.D.; Martin, L.; Bani, M.D.; Kho, K.; Pramanda, I.T.; Pui, L.P.; How, Y.H.; Lim, C.S.Y.; Devanthi, P.V.P. Spray Drying Encapsulation of Pediococcus acidilactici at Different Inlet Air Temperatures and Wall Material Ratios. Foods 2022, 12, 165. [Google Scholar] [CrossRef]
- Ahn, H.; Kim, J.; Kim, W.J. Isolation and Characterization of Bacteriocin-Producing Pediococcus acidilactici HW01 from Malt and Its Potential to Control Beer Spoilage Lactic Acid Bacteria. Food Control 2017, 80, 59–66. [Google Scholar] [CrossRef]
- Prabhurajeshwar, C.; Chandrakanth, K. Evaluation of Antimicrobial Properties and Their Substances against Pathogenic Bacteria In-Vitro by Probiotic Lactobacilli Strains Isolated from Commercial Yoghurt. Clin. Nutr. Exp. 2019, 23, 97–115. [Google Scholar] [CrossRef]
- De Marco, S.; Sichetti, M.; Muradyan, D.; Piccioni, M.; Traina, G.; Pagiotti, R.; Pietrella, D. Probiotic Cell-Free Supernatants Exhibited Anti-Inflammatory and Antioxidant Activity on Human Gut Epithelial Cells and Macrophages Stimulated with LPS. Evid. Based Complement. Alternat. Med. 2018, 2018, 1756308. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Ramana, K.V.; Sabapathy, S.N.; Bawa, A.S. Physico-Mechanical Properties of Chemically Treated Bacterial (Acetobacter xylinum) Cellulose Membrane. World J. Microbiol. Biotechnol. 2005, 21, 1323–1327. [Google Scholar] [CrossRef]
- Dos Santos, G.R.; Soeiro, V.S.; Talarico, C.F.; Ataide, J.A.; Lopes, A.M.; Mazzola, P.G.; Oliveira, T.J.; Oliveira Junior, J.M.; Grotto, D.; Jozala, A.F. Bacterial Cellulose Membranes as Carriers for Nisin: Incorporation, Antimicrobial Activity, Cytotoxicity and Morphology. Polymers 2022, 14, 3497. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.; Fan, L.; Yan, J.; Jiang, Y.; Doucette, C.; Fillmore, S.; Walker, B. Influence of Culture Media, pH and Temperature on Growth and Bacteriocin Production of Bacteriocinogenic Lactic Acid Bacteria. AMB Express 2018, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Papagianni, M.; Anastasiadou, S. Pediocins: The Bacteriocins of Pediococci. Sources, Production, Properties and Applications. Microb. Cell Factories 2009, 8, 3. [Google Scholar] [CrossRef]
- Li, Z.; Song, Q.; Wang, M.; Ren, J.; Liu, S.; Zhao, S. Comparative Genomics Analysis of Pediococcus acidilactici Species. J. Microbiol. 2021, 59, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Porto, M.C.W.; Kuniyoshi, T.M.; Azevedo, P.O.S.; Vitolo, M.; Oliveira, R.P.S. Pediococcus spp.: An Important Genus of Lactic Acid Bacteria and Pediocin Producers. Biotechnol. Adv. 2017, 35, 361–374. [Google Scholar] [CrossRef]
- Mani-López, E.; García, H.S.; López-Malo, A. Organic Acids as Antimicrobials to Control Salmonella in Meat and Poultry Products. Food Res. Int. 2012, 45, 713–721. [Google Scholar] [CrossRef]
- Kovanda, L.; Zhang, W.; Wei, X.; Luo, J.; Wu, X.; Atwill, E.R.; Vaessen, S.; Li, X.; Liu, Y. In Vitro Antimicrobial Activities of Organic Acids and Their Derivatives on Several Species of Gram-Negative and Gram-Positive Bacteria. Molecules 2019, 24, 3770. [Google Scholar] [CrossRef]
- Moon, G.-S.; Kim, M.-H.; Kim, W.-J. Synergistic Effects of Bacteriocin-Producing Pediococcus Acidilactici K10 and Organic Acids on Inhibiting Escherichia coli O157:H7 and Applications in Ground Beef. J. Microbiol. Biotechnol. 2002, 12, 936–942. [Google Scholar]
- Erttmann, S.F.; Gekara, N.O. Hydrogen Peroxide Release by Bacteria Suppresses Inflammasome-Dependent Innate Immunity. Nat. Commun. 2019, 10, 3493. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, W.J.; Kang, S.-S. Inhibitory Effect of Bacteriocin-Producing Lactobacillus brevis DF01 and Pediococcus acidilactici K10 Isolated from Kimchi on Enteropathogenic Bacterial Adhesion. Food Biosci. 2019, 30, 100425. [Google Scholar] [CrossRef]
- Castellano, P.; Peña, N.; Ibarreche, M.P.; Carduza, F.; Soteras, T.; Vignolo, G. Antilisterial Efficacy of Lactobacillus Bacteriocins and Organic Acids on Frankfurters. Impact on Sensory Characteristics. J. Food Sci. Technol. 2018, 55, 689–697. [Google Scholar] [CrossRef]
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The Minimum Inhibitory Concentration of Antibiotics: Methods, Interpretation, Clinical Relevance. Pathogens 2021, 10, 165. [Google Scholar] [CrossRef]
- Pankey, G.A.; Sabath, L.D. Clinical Relevance of Bacteriostatic versus Bactericidal Mechanisms of Action in the Treatment of Gram-Positive Bacterial Infections. Clin. Infect. Dis. 2004, 38, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Park, H.-O.; Yoo, W. Anti-Melanogenic and Antioxidant Effects of Cell-Free Supernatant from Lactobacillus gasseri BNR17. Microorganisms 2022, 10, 788. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Franco, R.; Navarro, G.; Martínez-Pinilla, E. Antioxidants versus Food Antioxidant Additives and Food Preservatives. Antioxidants 2019, 8, 542. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Ke, W.C.; Bai, J.; Li, F.H.; Xu, D.M.; Ding, Z.T.; Guo, X.S. The Effect of Pediococcus Acidilactici J17 with High-Antioxidant Activity on Antioxidant, A-tocopherol, B-carotene, Fatty Acids, and Fermentation Profiles of Alfalfa Silage Ensiled at Two Different Dry Matter Contents. Anim. Feed Sci. Technol. 2020, 268, 114614. [Google Scholar] [CrossRef]
- İncili, G.K.; Karatepe, P.; Akgöl, M.; Kaya, B.; Kanmaz, H.; Hayaloğlu, A.A. Characterization of Pediococcus acidilactici Postbiotic and Impact of Postbiotic-Fortified Chitosan Coating on the Microbial and Chemical Quality of Chicken Breast Fillets. Int. J. Biol. Macromol. 2021, 184, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Aziz, T.; Sun, H.; Ullah, A.; Ali, A.; Cheng, L.; Ullah, R.; Khan, F.U. Advances and Applications of Cellulose Bio-Composites in Biodegradable Materials. J. Polym. Environ. 2023, 31, 2273–2284. [Google Scholar] [CrossRef]
- Kamaruddin, I.; Dirpan, A.; Bastian, F. The Novel Trend of Bacterial Cellulose as Biodegradable and Oxygen Scavenging Films for Food Packaging Application: An Integrative Review. IOP Conf. Ser. Earth Environ. Sci. 2021, 807, 022066. [Google Scholar] [CrossRef]
- Devanthi, P.V.P.; Pratama, F.; Kho, K.; Taherzadeh, M.J.; Aslanzadeh, S. The Effect of Dekkera Bruxellensis Concentration and Inoculation Time on Biochemical Changes and Cellulose Biosynthesis by Komagataeibacter Intermedius. J. Fungi 2022, 8, 1206. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, H.M.C.; Barud, H.; Farinas, C.S.; Vasconcellos, V.M.; Claro, A.M. Bacterial Cellulose as a Raw Material for Food and Food Packaging Applications. Front. Sustain. Food Syst. 2019, 3, 7. [Google Scholar] [CrossRef]
- Deng, Z.; Jung, J.; Zhao, Y. Development, Characterization, and Validation of Chitosan Adsorbed Cellulose Nanofiber (CNF) Films as Water Resistant and Antibacterial Food Contact Packaging. LWT Food Sci. Technol. 2017, 83, 132–140. [Google Scholar] [CrossRef]
- Abral, H.; Pratama, A.B.; Handayani, D.; Mahardika, M.; Aminah, I.; Sandrawati, N.; Sugiarti, E.; Muslimin, A.N.; Sapuan, S.M.; Ilyas, R.A. Antimicrobial Edible Film Prepared from Bacterial Cellulose Nanofibers/Starch/Chitosan for a Food Packaging Alternative. Int. J. Polym. Sci. 2021, 2021, 6641284. [Google Scholar] [CrossRef]
- Masmoudi, F.; Bessadok, A.; Dammak, M.; Jaziri, M.; Ammar, E. Biodegradable Packaging Materials Conception Based on Starch and Polylactic Acid (PLA) Reinforced with Cellulose. Environ. Sci. Pollut. Res. 2016, 23, 20904–20914. [Google Scholar] [CrossRef] [PubMed]
- Efthymiou, M.-N.; Tsouko, E.; Papagiannopoulos, A.; Athanasoulia, I.-G.; Georgiadou, M.; Pispas, S.; Briassoulis, D.; Tsironi, T.; Koutinas, A. Development of Biodegradable Films Using Sunflower Protein Isolates and Bacterial Nanocellulose as Innovative Food Packaging Materials for Fresh Fruit Preservation. Sci. Rep. 2022, 12, 6935. [Google Scholar] [CrossRef] [PubMed]
- Padrão, J.; Gonçalves, S.; Silva, J.P.; Sencadas, V.; Lanceros-Méndez, S.; Pinheiro, A.C.; Vicente, A.A.; Rodrigues, L.R.; Dourado, F. Bacterial Cellulose-Lactoferrin as an Antimicrobial Edible Packaging. Food Hydrocoll. 2016, 58, 126–140. [Google Scholar] [CrossRef]
- Mapelli, C.; Musatti, A.; Barbiroli, A.; Saini, S.; Bras, J.; Cavicchioli, D.; Rollini, M. Cellulose Nanofiber (CNF)–sakacin-A Active Material: Production, Characterization and Application in Storage Trials of Smoked Salmon. J. Sci. Food Agric. 2019, 99, 4731–4738. [Google Scholar] [CrossRef]
- Maresca, D.; Mauriello, G. Development of Antimicrobial Cellulose Nanofiber-Based Films Activated with Nisin for Food Packaging Applications. Foods 2022, 11, 3051. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Gidley, M.J.; Dykes, G.A. Potential of a Nisin-Containing Bacterial Cellulose Film to Inhibit Listeria Monocytogenes on Processed Meats. Food Microbiol. 2008, 25, 471–478. [Google Scholar] [CrossRef]


| CFS-Pa Concentration after Dilution | MRS Broth | MHB | ||||||
|---|---|---|---|---|---|---|---|---|
| 50% | 40% | 30% | 20% | 10% | 5% | |||
| Staphylococcus aureus | − | − | − | − | − | + | + | + |
| EHEC | − | − | − | − | − | + | + | + |
| Listeria monocytogenes | − | − | − | − | − | + | + | + |
| pH of CFS-Pa stock | 3.75 ± 0.02 | 3.88 ± 0.08 | 4.09 ± 0.01 | 4.37 ± 0.02 | 4.44 ± 0.07 | 5.16 ± 0.01 | 5.73 | 7.30 |
| Pathogens | Zone of Inhibition (mm) |
|---|---|
| S. aureus | 16.37 ± 1.14 |
| L. monocytogenes | 12.10 ± 0.78 |
| EHEC | 3.17 ± 0.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kho, K.; Kadar, A.D.; Bani, M.D.; Pramanda, I.T.; Martin, L.; Chrisdianto, M.; Pratama, F.; Devanthi, P.V.P. The Potential of Pediococcus acidilactici Cell-Free Supernatant as a Preservative in Food Packaging Materials. Foods 2024, 13, 644. https://doi.org/10.3390/foods13050644
Kho K, Kadar AD, Bani MD, Pramanda IT, Martin L, Chrisdianto M, Pratama F, Devanthi PVP. The Potential of Pediococcus acidilactici Cell-Free Supernatant as a Preservative in Food Packaging Materials. Foods. 2024; 13(5):644. https://doi.org/10.3390/foods13050644
Chicago/Turabian StyleKho, Katherine, Adinda Darwanti Kadar, Mario Donald Bani, Ihsan Tria Pramanda, Leon Martin, Matthew Chrisdianto, Ferren Pratama, and Putu Virgina Partha Devanthi. 2024. "The Potential of Pediococcus acidilactici Cell-Free Supernatant as a Preservative in Food Packaging Materials" Foods 13, no. 5: 644. https://doi.org/10.3390/foods13050644
APA StyleKho, K., Kadar, A. D., Bani, M. D., Pramanda, I. T., Martin, L., Chrisdianto, M., Pratama, F., & Devanthi, P. V. P. (2024). The Potential of Pediococcus acidilactici Cell-Free Supernatant as a Preservative in Food Packaging Materials. Foods, 13(5), 644. https://doi.org/10.3390/foods13050644






