The Effect of Opuntia ficus Mucilage Pectin and Citrus aurantium Extract Added to a Food Matrix on the Gut Microbiota of Lean Humans and Humans with Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Food Matrix
2.2. Gastrointestinal Conditions and the Obtention of Microbiota
2.3. Administration of the Food Matrix to the Digestive Tract Simulator (ARIS)
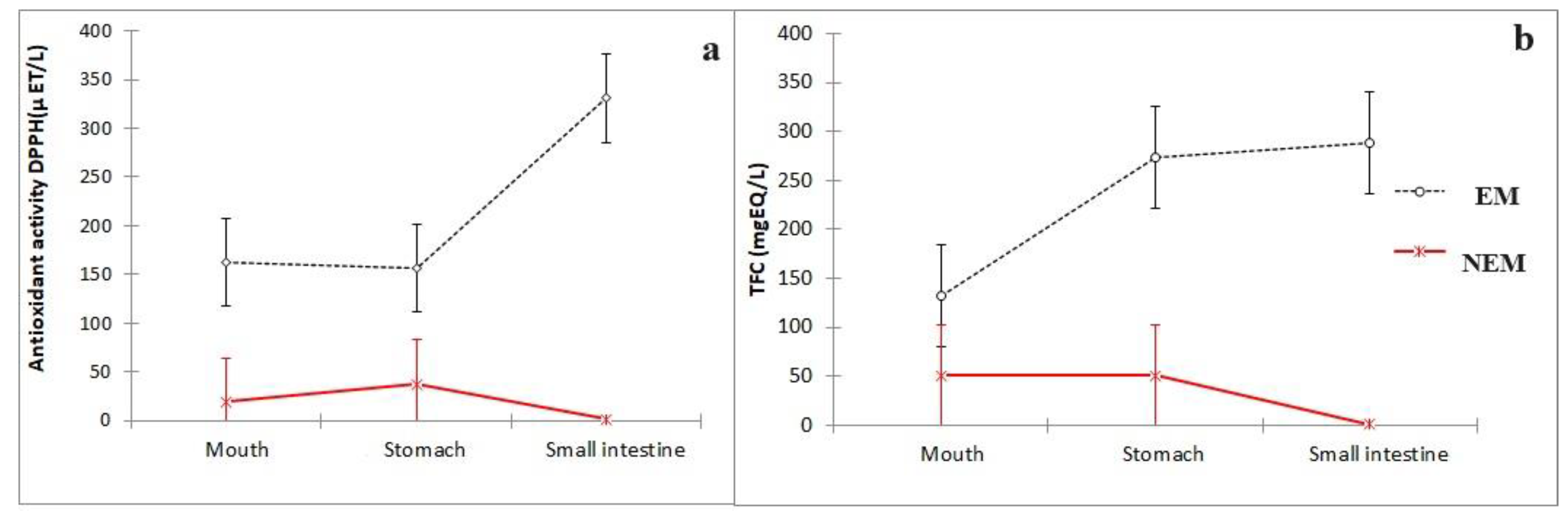
2.4. Determination of Antioxidant Activity and Total Flavonoid Content in the Mouth, Stomach, and Small Intestine
2.5. Microbiological Analysis during Large Intestine Simulation
2.6. Analysis of Short-Chain Fatty Acids during the Large Intestine Simulation
2.7. Taxonomic Analysis of Intestinal Microbiota via Bacterial DNA Extraction, 16S RNA Amplification, Library Construction, and Massive Sequencing
2.8. Statistical Analysis
3. Results and Discussion
3.1. Changes in Antioxidant Activity and Total Flavonoid Content during the First Part of the Digestion Process
3.2. Abundance and Identity of Initial Microbiota: Comparison between Microbiota from People Who Are Lean and People with Obesity
3.3. Taxonomic Analysis
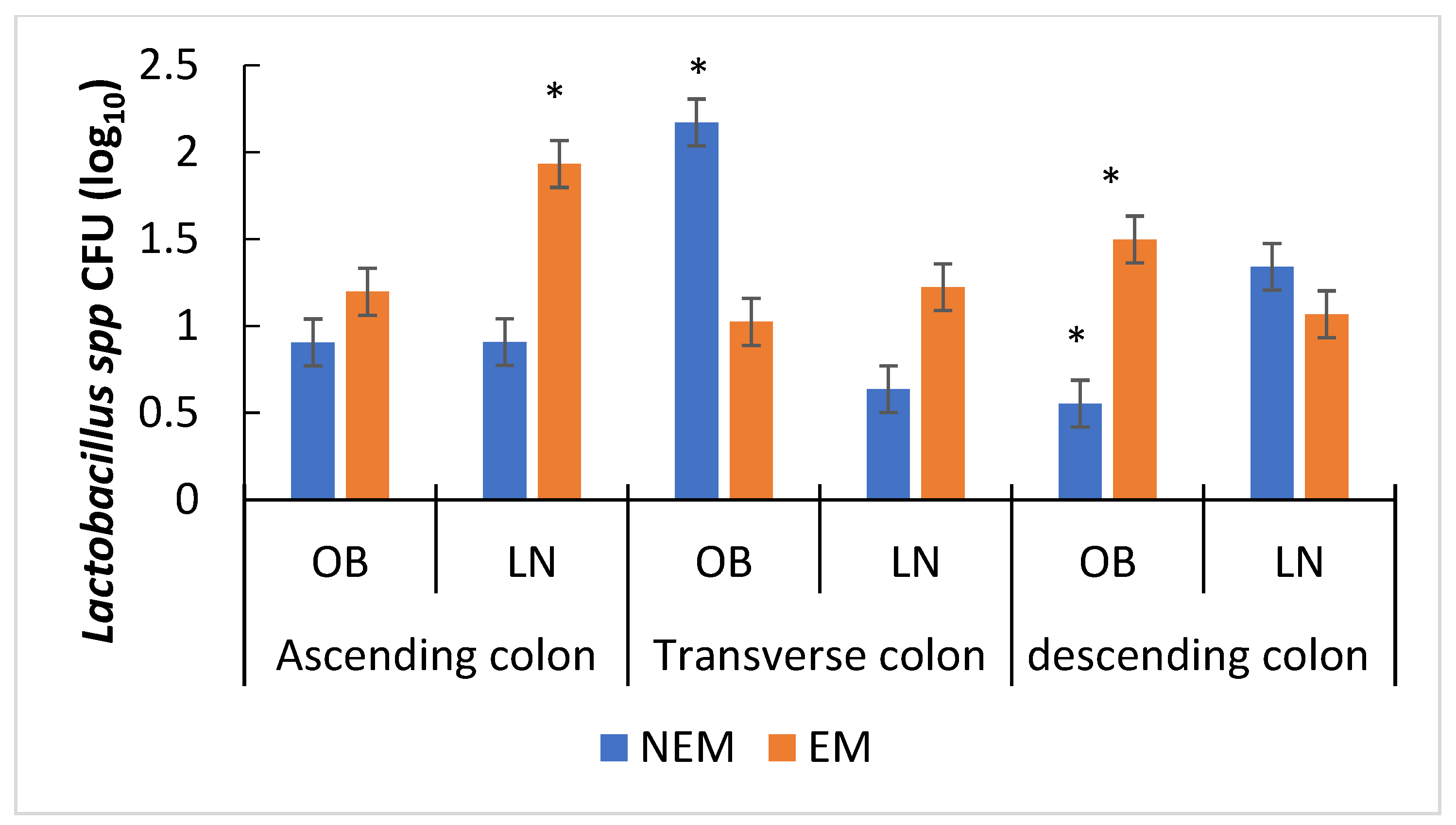
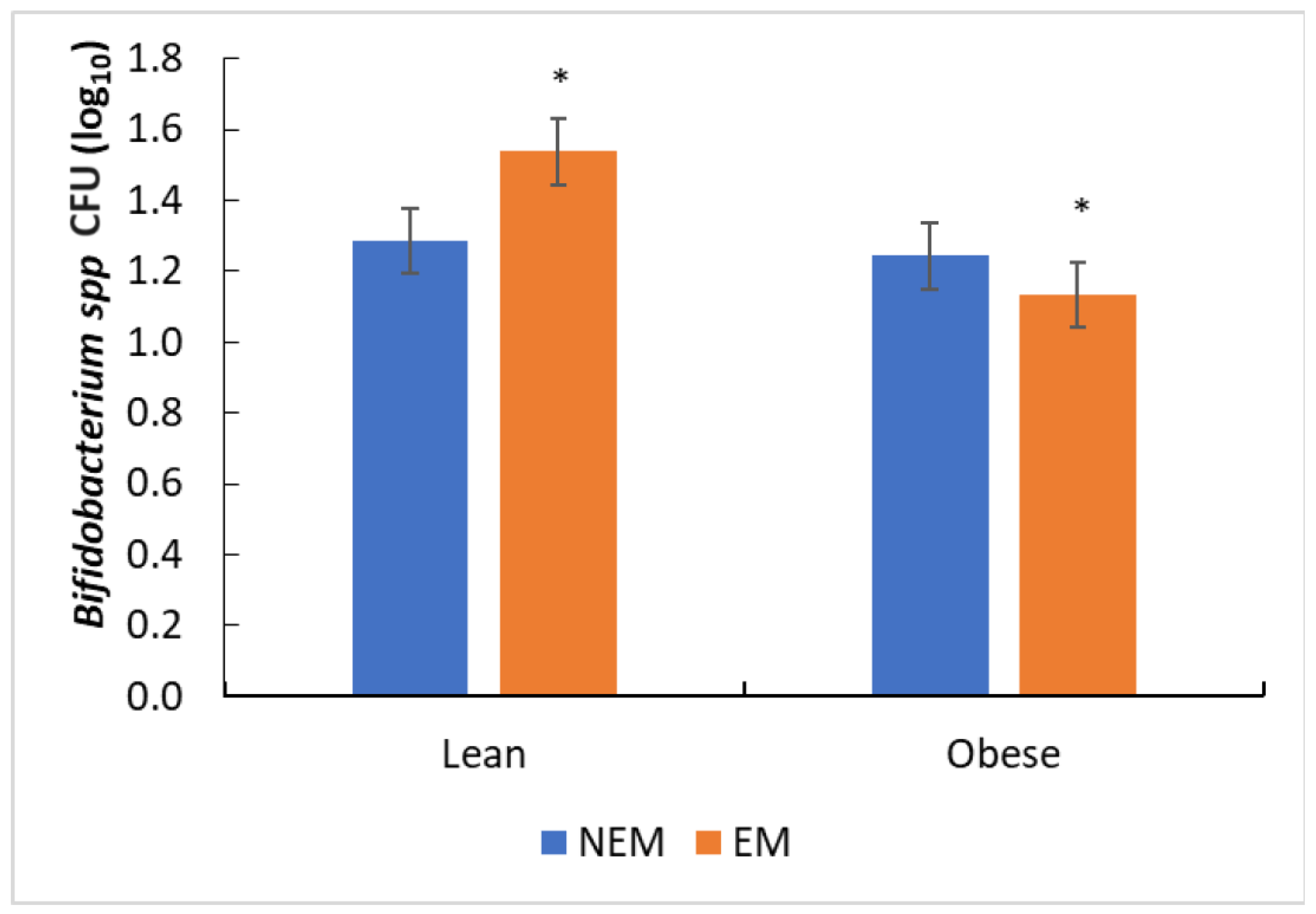
3.4. Effect of the Food Matrix on Bacterial Growth
3.4.1. Effect of the Food Matrix on Probiotic Bacteria Growth
3.4.2. Effect of the Food Matrix on the Growth of Potentially Pathogenic Microbiota
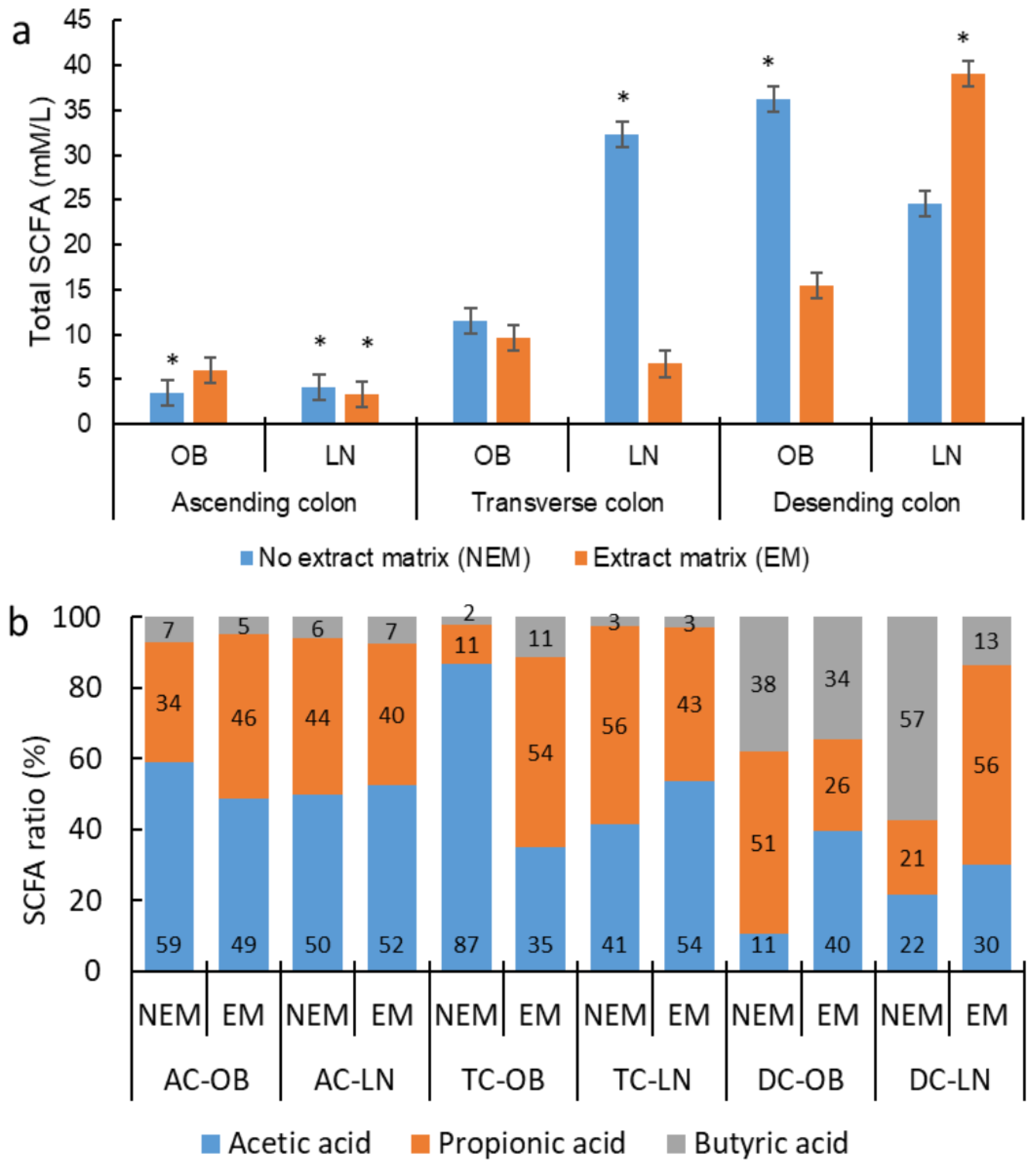
3.5. Short-Chain Fatty Acid Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Million, M.; Angelakis, E.; Maraninchi, M.; Henry, M.; Giorgi, R.; Valero, R.; Vialettes, B.; Raoult, D. Correlation between Body Mass Index and Gut Concentrations of Lactobacillus Correlation between Body Mass Index and Gut Concentrations of Lactobacillus Reuteri, Bifidobacterium Animalis, Methanobrevibacter Smithii and Escherichia Coli. Int. J. Obes. 2013, 37, 1460–1466. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Shao, H.; Fang, Z.; Zhao, Y.; Cao, C.Y.; Li, Q. Mechanism and Effects of Polyphenol Derivatives for Modifying Collagen. ACS Biomater. Sci. Eng. 2019, 5, 4272–4284. [Google Scholar] [CrossRef] [PubMed]
- Boulangé, C.L.; Neves, A.L.; Chilloux, J.; Nicholson, J.K.; Dumas, M.E. Impact of the Gut Microbiota on Inflammation, Obesity, and Metabolic Disease. Genome Med. 2016, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Barberán, F.A.; Espín, J.C. Effect of Food Structure and Processing on (Poly)Phenol–Gut Microbiota Interactions and the Effects on Human Health. Annu. Rev. Food Sci. Technol. 2019, 10, 221–238. [Google Scholar] [CrossRef]
- Walters, W.A.; Xu, Z.; Knight, R. Meta-Analysis of Human Gut Microbes Associated with Obesity and IBD. FEBS Lett. 2014, 588, 4223–4233. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, T.F.S.; Grze, M.; Gouveia, C. Faecal Levels of Bifidobacterium and Clostridium Coccoides but Not Plasma Lipopolysaccharide Are Inversely Related to Insulin and HOMA Index in Women. Clin. Nutr. 2013, 32, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Khodaiyan, F.; Kazemi, M.; Najari, Z. Optimization and Characterization of Pectin Extracted from Sour Orange Peel by Ultrasound Assisted Method. Int. J. Biol. Macromol. 2018, 125, 621–629. [Google Scholar] [CrossRef]
- Xian, Y.; Fan, R.; Shao, J.; Mulcahy Toney, A.; Chung, S.; Ramer-Tait, A.E. Polyphenolic Fractions Isolated from Red Raspberry Whole Fruit, Pulp, and Seed Differentially Alter the Gut Microbiota of Mice with Diet-Induced Obesity. J. Funct. Foods 2020, 76, 104288. [Google Scholar] [CrossRef]
- Bayar, N.; Kriaa, M.; Kammoun, R. Extraction and Characterization of Three Polysaccharides Extracted from Opuntia Ficus Indica Cladodes. Int. J. Biol. Macromol. 2016, 92, 441–450. [Google Scholar] [CrossRef]
- Pérez-Martínez, J.D.; Sánchez-Becerril, M.; Ornelas-Paz, J.D.J.; González-Chávez, M.M.; Ibarra-Junquera, V.; Escalante-Minakata, P. The Effect of Extraction Conditions on the Chemical Characteristics of Pectin from Opuntia Ficus Indica Cladode Flour. J. Polym. Environ. 2013, 21, 1040–1051. [Google Scholar] [CrossRef]
- Cruz-Rubio, J.M.; Mueller, M.; Viernstein, H.; Loeppert, R.; Praznik, W. Prebiotic Potential and Chemical Characterization of the Poly and Oligosaccharides Present in the Mucilage of Opuntia Ficus-Indica and Opuntia Joconostle. Food Chem. 2021, 362, 130167. [Google Scholar] [CrossRef] [PubMed]
- El-Mostafa, K.; Kharrassi, Y.E.; Badreddine, A.; Andreoletti, P.; Vamecq, J.; Saïd, M.H.; Kebbaj, E.; Latruffe, N.; Lizard, G.; Nasser, B.; et al. Nopal Cactus (Opuntia Ficus-Indica) as a Source of Bioactive Compounds for Nutrition, Health and Disease. Molecules 2014, 8, 14879–14901. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Arauza, J.C.; Ornelas-Paz, J.D.J.; Pimentel-gonzález, D.J.; Mendoza, S.R.; Elena, R.; Guerra, S.; María, L.; Paz, T. Prebiotic Effect of Mucilage and Pectic-Derived Oligosaccharides from Nopal (Opuntia Ficus-Indica). Food Sci. Biotechnol. 2012, 21, 997–1003. [Google Scholar] [CrossRef]
- Nuñez-López, M.A.; Paredes-López, O.; Reynoso-Camacho, R. Functional and Hypoglycemic Properties of Nopal Cladodes (O. Ficus-Indica) at Different Maturity Stages Using in Vitro and in Vivo Tests. J. Agric. Food Chem. 2013, 61, 10981–10986. [Google Scholar] [CrossRef]
- Sánchez-Tapia, M.; Aguilar-López, M.; Pérez-Cruz, C.; Pichardo-Ontiveros, E.; Wang, M.; Donovan, S.M.; Tovar, A.R.; Torres, N. Nopal (Opuntia Ficus Indica) Protects from Metabolic Endotoxemia by Modifying Gut Microbiota in Obese Rats Fed High Fat/Sucrose Diet Mónica. Sci. Rep. 2017, 7, 4716. [Google Scholar] [CrossRef]
- Alves-Santos, A.M.; Sandra, C.; Sugizaki, A.; Lima, G.C. Prebiotic Effect of Dietary Polyphenols: A Systematic Review. J. Funct. Foods 2020, 74, 104169. [Google Scholar] [CrossRef]
- Duque, A.L.R.F.; Monteiro, M.; Adorno, M.A.T.; Sakamoto, I.K.; Sivieri, K. An Exploratory Study on the Influence of Orange Juice on Gut Microbiota Using a Dynamic Colonic Model. Food Res. Int. 2016, 84, 160–169. [Google Scholar] [CrossRef]
- Estrada-Sierra, N.A.; Padilla-Camberos, E.; Cervantes-Martinez, J.; Bravo, S. Physicochemical Interactions between Flavonoids/Polysacarides Present in Industrialized Citrus Products (Citrus Limetta Riso, Citus Aurantium). Impact on the Hypoglycemic and Hypocolesterolemic Effect in an Animal Model. In Proceedings of the 32nd EFFoST International Conferences, Nantes, France, 6–8 November 2018. [Google Scholar]
- Lirin- Mary, M.K.; Varghese, S.E. Role of Bitter Orange in Reducing Fat. Eur. J. Pharm. Med. Res. 2017, 4, 242–250. [Google Scholar]
- Maksoud, S.; Abdel-Massih, R.M.; Rajha, H.N.; Louka, N.; Chemat, F.; Barba, F.J.; Debs, E. Citrus Aurantium L. Active Constituents, Biological Effects and Extraction Methods. an Updated Review. Molecules 2021, 26, 5832. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Sierra, N.A.; Rincon-Enriquez, G.; Urías-Silvas, J.E.; Bravo, S.D.; Villanueva-Rodríguez, S.J. Impact of Ripening, Harvest Season, and the Nature of Solvents on Antioxidant Capacity, Flavonoid, and p-Synephrine Concentrations in Citrus Aurantium Extracts from Residue. Future Foods 2022, 6, 100153. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, P.; Yuan, B.; Ji, W.; Cheng, Z.; Qiu, X. Real-Space Identification of Intermolecular Bonding with Atomic Force Microscopy. Science 2013, 342, 611–614. [Google Scholar] [CrossRef]
- Makarewicz, M.; Drożdż, I.; Tarko, T.; Duda-Chodak, A. The Interactions between Polyphenols and Microorganisms, Especially Gut Microbiota. Antioxidants 2021, 10, 188. [Google Scholar] [CrossRef]
- Leena, M.M.; Silva, G.M.; Vinitha, K.; Moses, J.A.; Anandharamakrishnan, C. Synergistic Potential of Nutraceuticals: Mechanisms and Prospects for Futuristic Medicine. Food Funct. 2020, 11, 9317–9337. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Romero, J.d.J.; Arce-Reynoso, A.; Parra-Torres, C.G.; Zamora-Gasga, V.M.; Mendivil, E.J.; Sáyago-Ayerdi, S.G. In Vitro Gastrointestinal Digestion Affects the Bioaccessibility of Bioactive Compounds in Hibiscus Sabdariffa Beverages. Molecules 2023, 28, 1824. [Google Scholar] [CrossRef] [PubMed]
- Alminger, M.; Aura, A.M.; Bohn, T.; Dufour, C.; El, S.N.; Gomes, A.; Karakaya, S.; Martínez-Cuesta, M.C.; Mcdougall, G.J.; Requena, T.; et al. In Vitro Models for Studying Secondary Plant Metabolite Digestion and Bioaccessibility. Compr. Rev. Food Sci. Food Saf. 2014, 13, 413–436. [Google Scholar] [CrossRef]
- Garcia-Gamboa, R.; Gradilla-Hernandez, M.S.; Ostirz-Basurto, R.I.; Garcia-Reyes, R.A.; González-Avila, M. Assessment of Intermediate- and Long- Chains Agave Fructan Fermentation on the Growth of Intestinal Bacteria Cultured in a Gastrointestinal Tract Simulator Evaluación. Rev. Mex. Ing. Quim. 2020, 12, 505–511. [Google Scholar] [CrossRef]
- Gonzalez-Avila, M.; Vazquez-Castro, P.; Vazquez-Garcia, F.; Martinez-Mireles, J.R.; Chavez-Bautista, S.J.; Ruiz-Alvarez, B.E.; Pliego-Sandoval, J.E. Automated System for Simulating the Human Digestive Tract. World Intelectuall Property Organization. WIPO Patent WO 2017/065595 A2, 20 April 2017. [Google Scholar]
- Nakajima, V.M.; Macedo, G.A.; Macedo, J.A. Citrus Bioactive Phenolics: Role in the Obesity Treatment. LWT—Food Sci. Technol. 2014, 59, 1205–1212. [Google Scholar] [CrossRef]
- Tounsi, M.S.; Wannes, W.A.; Ouerghemmi, I.; Jegham, S.; Ben Njima, Y.; Hamdaoui, G.; Zemni, H.; Marzouk, B. Juice Components and Antioxidant Capacity of Four Tunisian Citrus Varieties. J. Sci. Food Agric. 2011, 91, 142–151. [Google Scholar] [CrossRef]
- Pietro-Femia, A.; Luceri, C.; Dolara, P.; Giannini, A.; Biggeri, A.; Salvadori, M.; Clune, Y.; Collins, K.J.; Paglierani, M.; Caderni, G.; et al. Antitumorigenic Activity of the Prebiotic Inulin Enriched with Oligofructose in Combination with the Probiotics Lactobacillus Rhamnosus and Bifidobacterium Lactis on Azoxymethane-Induced Colon Carcinogenesis in Rats. Carcinogenesis 2002, 23, 1953–1960. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Liu, S.; Xie, J.; Chen, Y.; Zheng, Y.; Zhang, X.; Zhao, E.; Wang, Z.; Xu, H.; Yu, Q. The Recovery, Catabolism and Potential Bioactivity of Polyphenols from Carrot Subjected to in Vitro Simulated Digestion and Colonic Fermentation. Food Res. Int. 2021, 143, 110263. [Google Scholar] [CrossRef] [PubMed]
- Attri, S.; Goel, G. Influence of Polyphenol Rich Seabuckthorn Berries Juice on Release of Polyphenols and Colonic Microbiota on Exposure to Simulated Human Digestion Model. Food Res. Int. 2018, 111, 314–323. [Google Scholar] [CrossRef]
- Balakrishnan, G.; Schneider, R.G. Quinoa Flavonoids and Their Bioaccessibility during in Vitro Gastrointestinal Digestion. J. Cereal Sci. 2020, 95, 103070. [Google Scholar] [CrossRef]
- Tarko, T.; Duda-Chodak, A. Influence of Food Matrix on the Bioaccessibility of Fruit Polyphenolic Compounds. J. Agric. Food Chem. 2020, 68, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Crovesy, L.; Masterson, D.; Rosado, E.L. Profile of the Gut Microbiota of Adults with Obesity: A Systematic Review. Eur. J. Clin. Nutr. 2020, 74, 1251–1262. [Google Scholar] [CrossRef]
- Drissi, F.; Raoult, D.; Merhej, V. Metabolic Role of Lactobacilli in Weight Modification in Humans and Animals. Microb. Pathog. 2017, 106, 182–194. [Google Scholar] [CrossRef]
- Benahmed, A.G.; Gasmi, A.; Doşa, A.; Chirumbolo, S.; Mujawdiya, P.K.; Aaseth, J.; Dadar, M.; Bjørklund, G. Association between the Gut and Oral Microbiome with Obesity. Anaerobe 2020, 70, 102248. [Google Scholar] [CrossRef]
- Selma, M.V.; Romo-vaquero, M.; García-villalba, R.; González-sarrías, A.; Tomás-barberán, F.A.; Espín, J.C. The Human Gut Microbial Ecology Associated with Overweight and Obesity Determines Ellagic Acid Metabolism. Food Funct. 2016, 74, 1769–1774. [Google Scholar] [CrossRef]
- Jinatham, V.; Kullawong, N.; Kespechara, K.; Gentekaki, E.; Popluechai, S. Comparison of Gut Microbiota between Lean and Obese Adult Thai Individuals. Microbiol. Biotechnol. Lett. 2018, 46, 277–287. [Google Scholar] [CrossRef]
- Andoh, A.; Nishida, A.; Takahashi, K.; Inatomi, O.; Imaeda, H.; Bamba, S.; Kito, K.; Sugimoto, M.; Kobayashi, T. Comparison of the Gut Microbial Community between Obese and Lean Peoples Using 16S Gene Sequencing in a Japanese Population. J. Clin. Biochem. Nutr. 2016, 59, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Kasai, C.; Sugimoto, K.; Moritani, I.; Tanaka, J.; Oya, Y.; Inoue, H.; Tameda, M.; Shiraki, K.; Ito, M.; Takei, Y.; et al. Comparison of the Gut Microbiota Composition between Obese and Non-Obese Individuals in a Japanese Population, as Analyzed by Terminal Restriction Fragment Length Polymorphism and next-Generation Sequencing. BMC Gastroenterol. 2015, 15, 100. [Google Scholar] [CrossRef]
- Lee, S.; Keirsey, K.I.; Kirkland, R.; Grunewald, Z.I.; Fischer, J.G.; de La Serre, C.B. Blueberry Supplementation Influences the Gut Microbiota, Inflammation, and Insulin Resistance in High-Fat-Diet-Fed Rats. J. Nutr. 2018, 148, 209–219. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Wen, Z.; Liu, W.; Meng, L.; Huang, H. Oscillospira—A Candidate for the next-Generation Probiotics. Gut Microbes 2021, 13, 1987783. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Wang, H.Z.; Lv, X.M.; Yi, Y.; Zheng, D.; Gou, M.; Nie, Y.; Hu, B.; Nobu, M.K.; Narihiro, T.; Tang, Y.Q. Using DNA-Based Stable Isotope Probing to Reveal Novel Propionate- and Acetate-Oxidizing Bacteria in Propionate-Fed Mesophilic Anaerobic Chemostats. Sci. Rep. 2019, 9, 17396. [Google Scholar] [CrossRef] [PubMed]
- Rau, M.; Rehman, A.; Dittrich, M.; Groen, A.K.; Hermanns, H.M.; Seyfried, F.; Beyersdorf, N.; Dandekar, T.; Rosenstiel, P.; Geier, A. Fecal SCFAs and SCFA-Producing Bacteria in Gut Microbiome of Human NAFLD as a Putative Link to Systemic T-Cell Activation and Advanced Disease. United Eur. Gastroenterol. J. 2018, 6, 1496–1507. [Google Scholar] [CrossRef]
- Buzzanca, D.; Alessandria, V.; Botta, C.; Seif Zadeh, N.; Ferrocino, I.; Houf, K.; Cocolin, L.; Rantsiou, K. Transcriptome Analysis of Arcobacter Butzleri Infection in a Mucus-Producing Human Intestinal In Vitro Model. Microbiol. Spectr. 2023, 11, e02071-22. [Google Scholar] [CrossRef]
- Riva, A.; Kolimár, D.; Spittler, A.; Wisgrill, L.; Herbold, C.W.; Abrankó, L.; Berry, D. Conversion of Rutin, a Prevalent Dietary Flavonol, by the Human Gut Microbiota. Front. Microbiol. 2020, 11, 585428. [Google Scholar] [CrossRef]
- Sasaki, K.; Sasaki, D.; Sasaki, K.; Nishidono, Y.; Yamamori, A.; Tanaka, K.; Kondo, A. Growth Stimulation of Bifidobacterium from Human Colon Using Daikenchuto in an in Vitro Model of Human Intestinal Microbiota. Sci. Rep. 2021, 11, 4580. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, H.; Wang, X.; Yu, X.; Hu, C.; Zhang, X. The Family Coriobacteriaceae Is a Potential Contributor to the Beneficial Effects of Roux-En-Y Gastric Bypass on Type 2 Diabetes. Surg. Obes. Relat. Dis. 2018, 14, 584–593. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, J.; Xiong, B.; Zhang, C.; Kang, B.; Gao, Y.; Li, Z.; Ge, W.; Cheng, S.; Hao, Y.; et al. Microbiota from Alginate Oligosaccharide-Dosed Mice Successfully Mitigated Small Intestinal Mucositis. Microbiome 2020, 8, 112. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Yan, Y.; Kim, E.B.; Lee, B.; Marco, M.L. Short Communication: Effect of Milk and Milk Containing Lactobacillus Casei on the Intestinal Microbiota of Mice. J. Dairy. Sci. 2014, 97, 2049–2055. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Kong, Q.; Li, X.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. A High-Fat Diet Increases Gut Microbiota Biodiversity and Energy Expenditure Due to Nutrient Difference. Nutrients 2020, 12, 3197. [Google Scholar] [CrossRef] [PubMed]
- Nagano, T.; Yano, H. Effect of Dietary Cellulose Nanofiber and Exercise on Obesity and Gut Microbiota in Mice Fed a High-Fat-Diet. Biosci. Biotechnol. Biochem. 2020, 84, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Burton, K.J.; Krüger, R.; Scherz, V.; Münger, L.H.; Picone, G.; Vionnet, N.; Bertelli, C.; Greub, G.; Capozzi, F.; Vergères, G. Trimethylamine-N-Oxide Postprandial Response in Plasma and Urine Is Lower after Fermented Compared to Non-Fermented Dairy Consumption in Healthy Adults. Nutrients 2020, 12, 234. [Google Scholar] [CrossRef] [PubMed]
- Selle, A.; Brosseau, C.; Dijk, W.; Duval, A.; Bouchaud, G.; Rousseaux, A.; Bruneau, A.; Cherbuy, C.; Mariadassou, M.; Cariou, V.; et al. Prebiotic Supplementation During Gestation Induces a Tolerogenic Environment and a Protective Microbiota in Offspring Mitigating Food Allergy. Front. Immunol. 2022, 12, 745535. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, B.; Bhattacharya, A.; Adak, A.; Das, S.; Khan, M.R. A Human and Animal Based Study Reveals That a Traditionally Fermented Rice Beverage Alters Gut Microbiota and Fecal Metabolites for Better Gut Health. Fermentation 2023, 9, 126. [Google Scholar] [CrossRef]
- Chung, C.S.; Chang, P.F.; Liao, C.H.; Lee, T.H.; Chen, Y.; Lee, Y.C.; Wu, M.S.; Wang, H.P.; Ni, Y.H. Differences of Microbiota in Small Bowel and Faeces between Irritable Bowel Syndrome Patients and Healthy Subjects. Scand. J. Gastroenterol. 2016, 51, 410–419. [Google Scholar] [CrossRef]
- Gil-Sánchez, I.; Cueva, C.; Sanz-Buenhombre, M.; Guadarrama, A.; Moreno-Arribas, M.V.; Bartolomé, B. Dynamic Gastrointestinal Digestion of Grape Pomace Extracts: Bioaccessible Phenolic Metabolites and Impact on Human Gut Microbiota. J. Food Compos. Anal. 2018, 68, 41–52. [Google Scholar] [CrossRef]
- Multari, S.; Carafa, I.; Barp, L.; Caruso, M.; Licciardello, C.; Larcher, R.; Tuohy, K.; Martens, S. Effects of Lactobacillus spp. on the Phytochemical Composition of Juices from Two Varieties of Citrus Sinensis L. Osbeck: ‘Tarocco’ and ‘Washington Navel. Lwt 2020, 125, 109205. [Google Scholar] [CrossRef]
- Degrain, A.; Manhivi, V.; Remize, F.; Garcia, C.; Sivakumar, D. Effect of Lactic Acid Fermentation on Color, Phenolic Compounds and Antioxidant Activity in African Nightshade. Microorganisms 2020, 8, 1324. [Google Scholar] [CrossRef] [PubMed]
- Bussolo de Souza, C.; Roeselers, G.; Troost, F.; Jonkers, D.; Koenen, M.E.; Venema, K. Prebiotic Effects of Cassava Bagasse in TNO’s in Vitro Model of the Colon in Lean versus Obese Microbiota. J. Funct. Foods 2014, 11, 210–220. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Alcántara, C.; Žugčić, T.; Abdelkebir, R.; Carmen, M.; García-pérez, J.V.; Režek, A.; Gavahian, M.; Barba, F.J.; Lorenzo, J.M. Impact of Ultrasound-Assisted Extraction and Solvent Composition on Bioactive Compounds and in Vitro Biological Activities of Thyme and Rosemary. Food Res. Int. 2020, 134, 109242. [Google Scholar] [CrossRef] [PubMed]
- Parkar, S.G.; Trower, T.M.; Stevenson, D.E. Fecal Microbial Metabolism of Polyphenols and Its Effects on Human Gut Microbiota. Anaerobe 2013, 23, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Patán, F.; Barroso, E.; Van De Wiele, T.; Jiménez-Girón, A.; Martín-Alvarez, P.J.; Moreno-Arribas, M.V.; Martínez-Cuesta, M.C.; Peláez, C.; Requena, T.; Bartolomé, B. Comparative in Vitro Fermentations of Cranberry and Grape Seed Polyphenols with Colonic Microbiota. Food Chem. 2015, 183, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Edwards, A.; Fernández-Romero, A.; Carmona, M.; Thuissard-Vasallo, I.; Schmeda-Hirschmann, G.; Larrosa, M. Effects of Gastrointestinal Digested Polyphenolic Enriched Extracts of Chilean Currants (Ribes magellanicum and Ribes punctatum) on in Vitro Fecal Microbiota. Food Res. Int. 2020, 129, 108848. [Google Scholar] [CrossRef] [PubMed]
- Elshahed, M.S.; Miron, A.; Aprotosoaie, A.C.; Farag, M.A. Pectin in Diet: Interactions with the Human Microbiome, Role in Gut Homeostasis, and Nutrient-Drug Interactions. Carbohydr. Polym. 2021, 255, 117388. [Google Scholar] [CrossRef] [PubMed]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef]
- Yutin, N.; Galperin, M.Y. A Genomic Update on Clostridial Phylogeny: Gram-Negative Spore Formers and Other Misplaced Clostridia. Environ. Microbiol. 2013, 15, 2631–2641. [Google Scholar] [CrossRef]
- Zofou, D.; Shu, G.L.; Foba-Tendo, J.; Tabouguia, M.O.; Assob, J.C.N. In Vitro and in Vivo Anti-Salmonella Evaluation of Pectin Extracts and Hydrolysates from “Cas Mango” (Spondias Dulcis). Evid.-Based Complement. Altern. Med. 2019, 2019, 3578402. [Google Scholar] [CrossRef]
- Mullineaux-Sanders, C.; Sanchez-Garrido, J.; Hopkins, E.G.D.; Shenoy, A.R.; Barry, R.; Frankel, G. Citrobacter rodentium–Host–Microbiota Interactions: Immunity, Bioenergetics and Metabolism. Nat. Rev. Microbiol. 2019, 17, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Michel-Barba, M.G.; Espinosa-Andrews, H.; García-Reyes, R.A.; Desjardins, Y.; González-Ávila, M. Effect of Blueberry Extract, Carriers, and Combinations on the Growth Rate of Probiotic and Pathogenic Bacteria. Int. J. Food Sci. Nutr. 2019, 70, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Agus, S.; Achmadi, S.S.; Mubarik, N.R. Antibacterial Activity of Naringenin-Rich Fraction of Pigeon Pea Leaves toward Salmonella Thypi. Asian Pac. J. Trop. Biomed. 2017, 7, 725–728. [Google Scholar] [CrossRef]
- Cueva, C.; Jiménez-Girón, A.; Muñoz-González, I.; Esteban-Fernández, A.; Gil-Sánchez, I.; Dueñas, M.; Martín-Álvarez, P.J.; Pozo-Bayón, M.A.; Bartolomé, B.; Moreno-Arribas, M.V. Application of a New Dynamic Gastrointestinal Simulator (SIMGI) to Study the Impact of Red Wine in Colonic Metabolism. Food Res. Int. 2015, 72, 149–159. [Google Scholar] [CrossRef]
- Sadeghi Ekbatan, S.; Sleno, L.; Sabally, K.; Khairallah, J.; Azadi, B.; Rodes, L.; Prakash, S.; Donnelly, D.J.; Kubow, S. Biotransformation of Polyphenols in a Dynamic Multistage Gastrointestinal Model. Food Chem. 2016, 204, 453–462. [Google Scholar] [CrossRef]
- Tuohy, K.M.; Conterno, L.; Gasperotti, M.; Viola, R. Up-Regulating the Human Intestinal Microbiome Using Whole Plant Foods, Polyphenols, and/or Fiber. J. Agric. Food Chem. 2012, 60, 8776–8782. [Google Scholar] [CrossRef]
- Cuevas-Tena, M.; Alegria, A.; Lagarda, M.J.; Venema, K. Impact of Plant Sterols Enrichment Dose on Gut Microbiota from Lean and Obese Subjects Using TIM-2 in Vitro Fermentation Model. J. Funct. Foods 2019, 54, 164–174. [Google Scholar] [CrossRef]
- Ferreira-Lazarte, A.; Moreno, F.J.; Cueva, C.; Gil-Sánchez, I.; Villamiel, M. Behaviour of Citrus Pectin during Its Gastrointestinal Digestion and Fermentation in a Dynamic Simulator (Simgi®). Carbohydr. Polym. 2019, 207, 382–390. [Google Scholar] [CrossRef]
- Aguirre, M.; De Souza, C.B.; Venema, K. The Gut Microbiota from Lean and Obese Subjects Contribute Differently to the Fermentation of Arabinogalactan and Inulin. PLoS ONE 2016, 11, e0159236. [Google Scholar] [CrossRef]
- Liu, Y.; Duan, X.; Duan, S.; Li, C.; Hu, B.; Liu, A.; Wu, Y.; Wu, H.; Chen, H.; Wu, W.; et al. Effects of: In Vitro Digestion and Fecal Fermentation on the Stability and Metabolic Behavior of Polysaccharides from Craterellus Cornucopioides. Food Funct. 2020, 11, 6899–6910. [Google Scholar] [CrossRef]







| Bacterial Family | Obese Microbiota | Lean Microbiota | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| *AC | **TC | ***DC | AC | TC | DC | |||||||
| *EM | *NEM | EM | NEM | EM | NEM | EM | NEM | EM | NEM | EM | NEM | |
| Lachnospiraceae | X | X | X | X | X | X | X | X | X | X | X | X |
| Enterobacteriaceae | X | X | X | X | X | X | ||||||
| Mitochondria | X | X | X | X | X | X | ||||||
| Morganellaceae | X | X | X | X | X | X | ||||||
| Rhizobiaceae | X | X | X | X | X | X | ||||||
| Sphingomonadaceae | X | X | X | X | X | X | ||||||
| Microbacteriaceae | X | X | X | X | X | |||||||
| Xanthobacteraceae | X | X | X | X | X | |||||||
| Beijerinckiaceae | X | X | X | X | ||||||||
| Comamonadaceae | X | X | X | X | ||||||||
| Xanthomonadaceae | X | X | X | |||||||||
| Alcaligenaceae | X | X | ||||||||||
| Arcobacteraceae | X | X | ||||||||||
| Fusobacteriaceae | X | X | ||||||||||
| Kaistiaceae | X | X | ||||||||||
| Sutterellaceae | X | X | ||||||||||
| Tannerellaceae | X | X | ||||||||||
| Synergistaceae | X | |||||||||||
| Erysipelatoclostridiaceae | X | X | X | X | X | X | ||||||
| Erysipelotrichaceae | X | X | X | X | X | X | ||||||
| Bifidobacteriaceae | X | X | X | X | ||||||||
| Coriobacteriaceae | X | X | ||||||||||
| Leuconostocaceae | X | X | ||||||||||
| Acidaminococcaceae | X | X | X | X | X | X | X | X | X | X | X | |
| Enterococcaceae | X | X | X | X | X | X | X | X | X | X | X | |
| Lactobacillaceae | X | X | X | X | X | X | X | X | X | X | X | |
| Eubacteriaceae | X | X | X | X | X | X | X | X | X | X | ||
| Chloroplast | X | X | X | X | X | X | X | X | X | |||
| Veillonellaceae | X | X | X | X | X | X | X | X | ||||
| Anaerovoracaceae | X | X | X | X | X | X | ||||||
| Oscillospiraceae | X | X | X | X | X | X | ||||||
| Atopobiaceae | X | X | X | X | X | |||||||
| Desulfovibrionaceae | X | X | X | X | X | |||||||
| Propionibacteriaceae | X | X | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estrada-Sierra, N.A.; Gonzalez-Avila, M.; Urias-Silvas, J.-E.; Rincon-Enriquez, G.; Garcia-Parra, M.D.; Villanueva-Rodriguez, S.J. The Effect of Opuntia ficus Mucilage Pectin and Citrus aurantium Extract Added to a Food Matrix on the Gut Microbiota of Lean Humans and Humans with Obesity. Foods 2024, 13, 587. https://doi.org/10.3390/foods13040587
Estrada-Sierra NA, Gonzalez-Avila M, Urias-Silvas J-E, Rincon-Enriquez G, Garcia-Parra MD, Villanueva-Rodriguez SJ. The Effect of Opuntia ficus Mucilage Pectin and Citrus aurantium Extract Added to a Food Matrix on the Gut Microbiota of Lean Humans and Humans with Obesity. Foods. 2024; 13(4):587. https://doi.org/10.3390/foods13040587
Chicago/Turabian StyleEstrada-Sierra, Nancy Abril, Marisela Gonzalez-Avila, Judith-Esmeralda Urias-Silvas, Gabriel Rincon-Enriquez, Maria Dolores Garcia-Parra, and Socorro Josefina Villanueva-Rodriguez. 2024. "The Effect of Opuntia ficus Mucilage Pectin and Citrus aurantium Extract Added to a Food Matrix on the Gut Microbiota of Lean Humans and Humans with Obesity" Foods 13, no. 4: 587. https://doi.org/10.3390/foods13040587
APA StyleEstrada-Sierra, N. A., Gonzalez-Avila, M., Urias-Silvas, J.-E., Rincon-Enriquez, G., Garcia-Parra, M. D., & Villanueva-Rodriguez, S. J. (2024). The Effect of Opuntia ficus Mucilage Pectin and Citrus aurantium Extract Added to a Food Matrix on the Gut Microbiota of Lean Humans and Humans with Obesity. Foods, 13(4), 587. https://doi.org/10.3390/foods13040587







