Modeling and Optimization of Herb-Fortified Fresh Kombucha Cheese: An Artificial Neural Network Approach for Enhancing Quality Characteristics
Abstract
1. Introduction
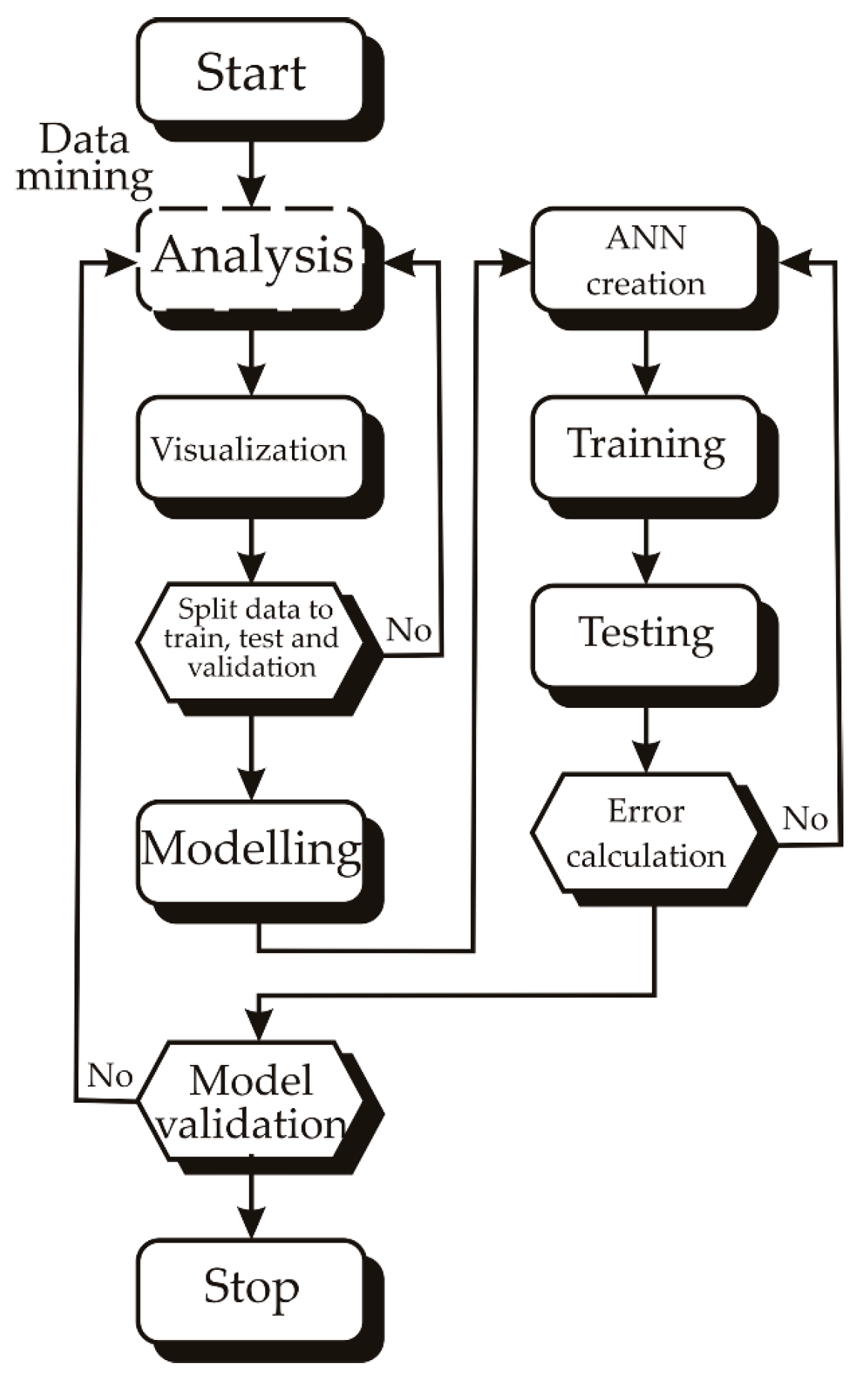
2. Statistical Analysis
2.1. ANN Modeling
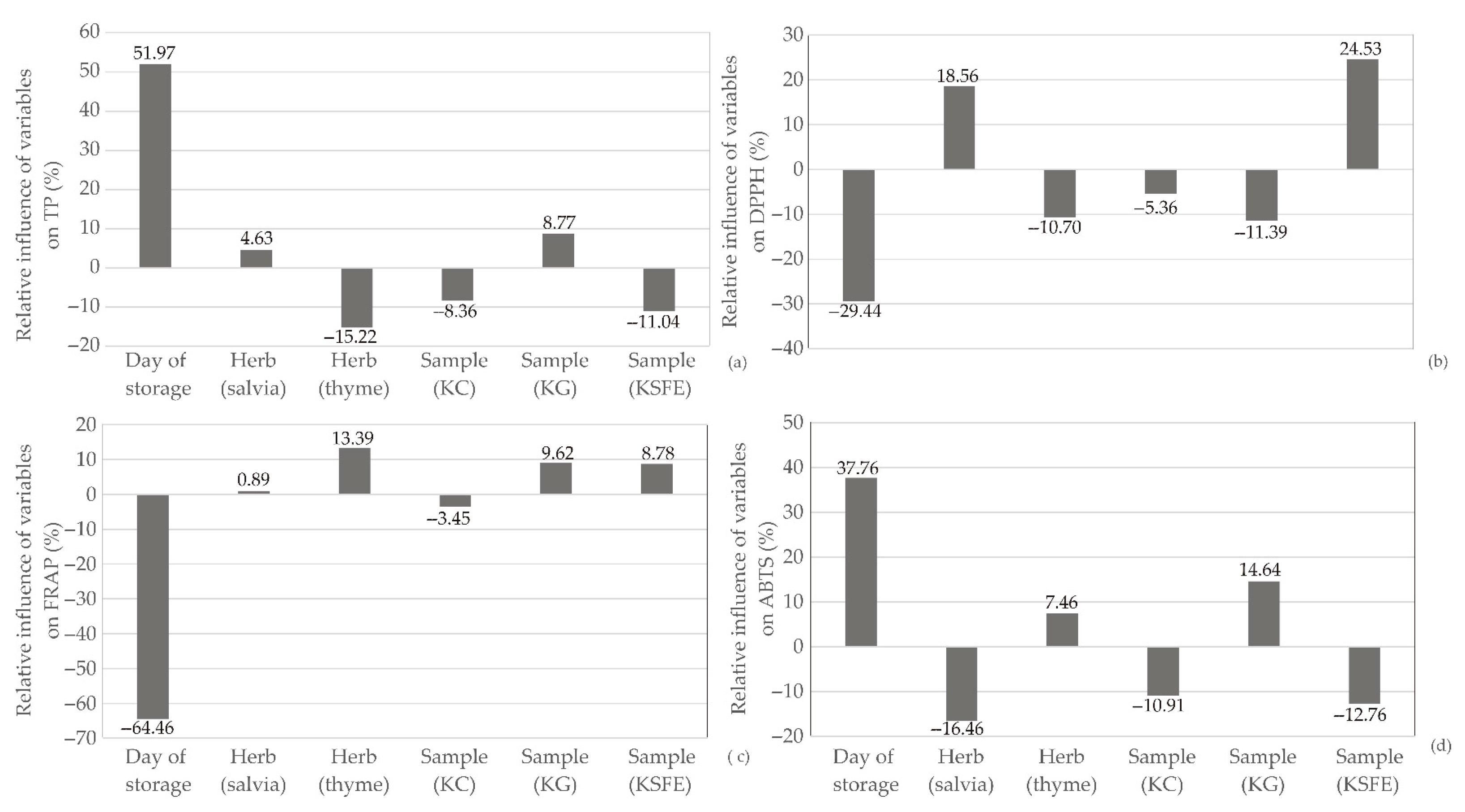
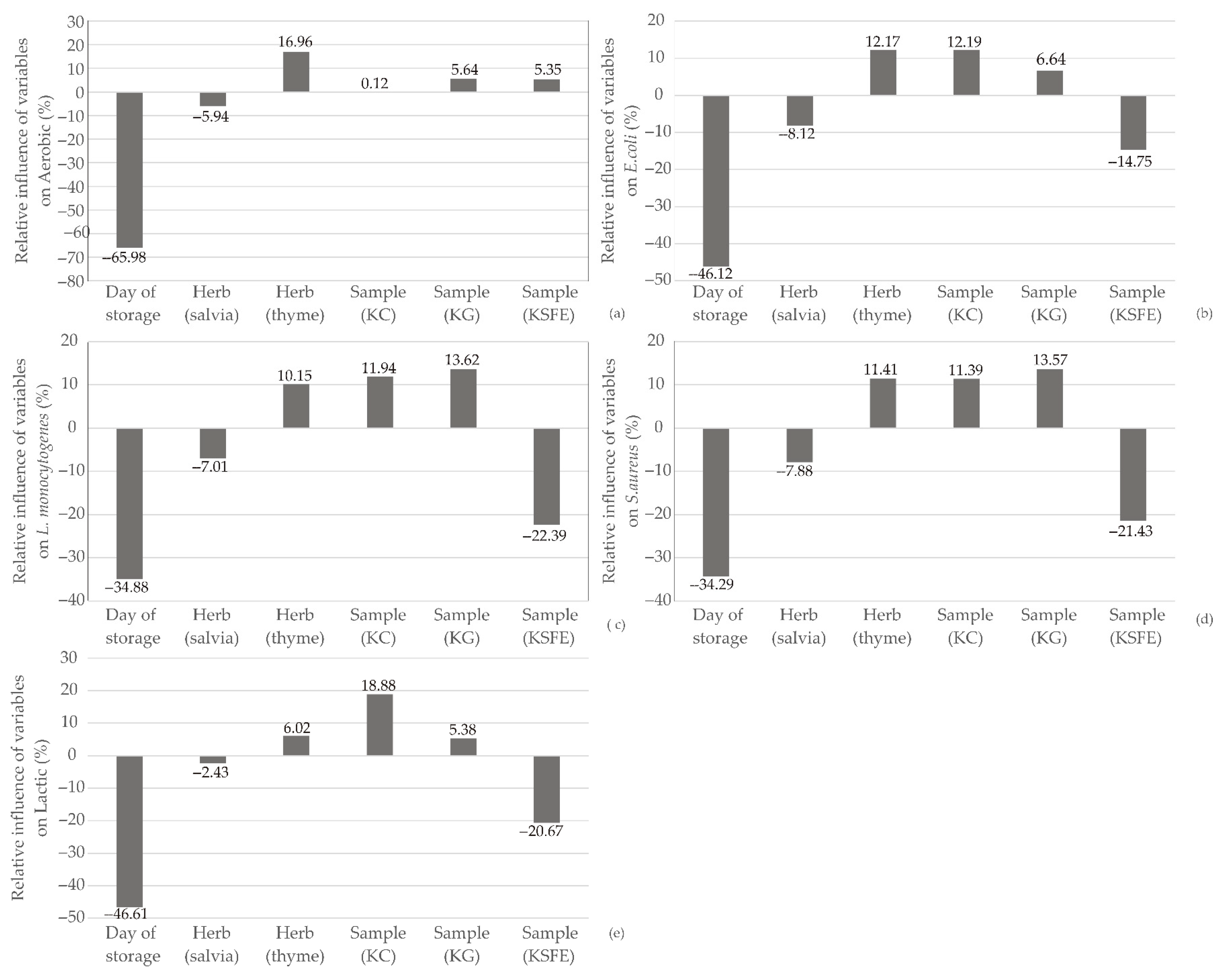
2.2. Global Sensitivity Analysis
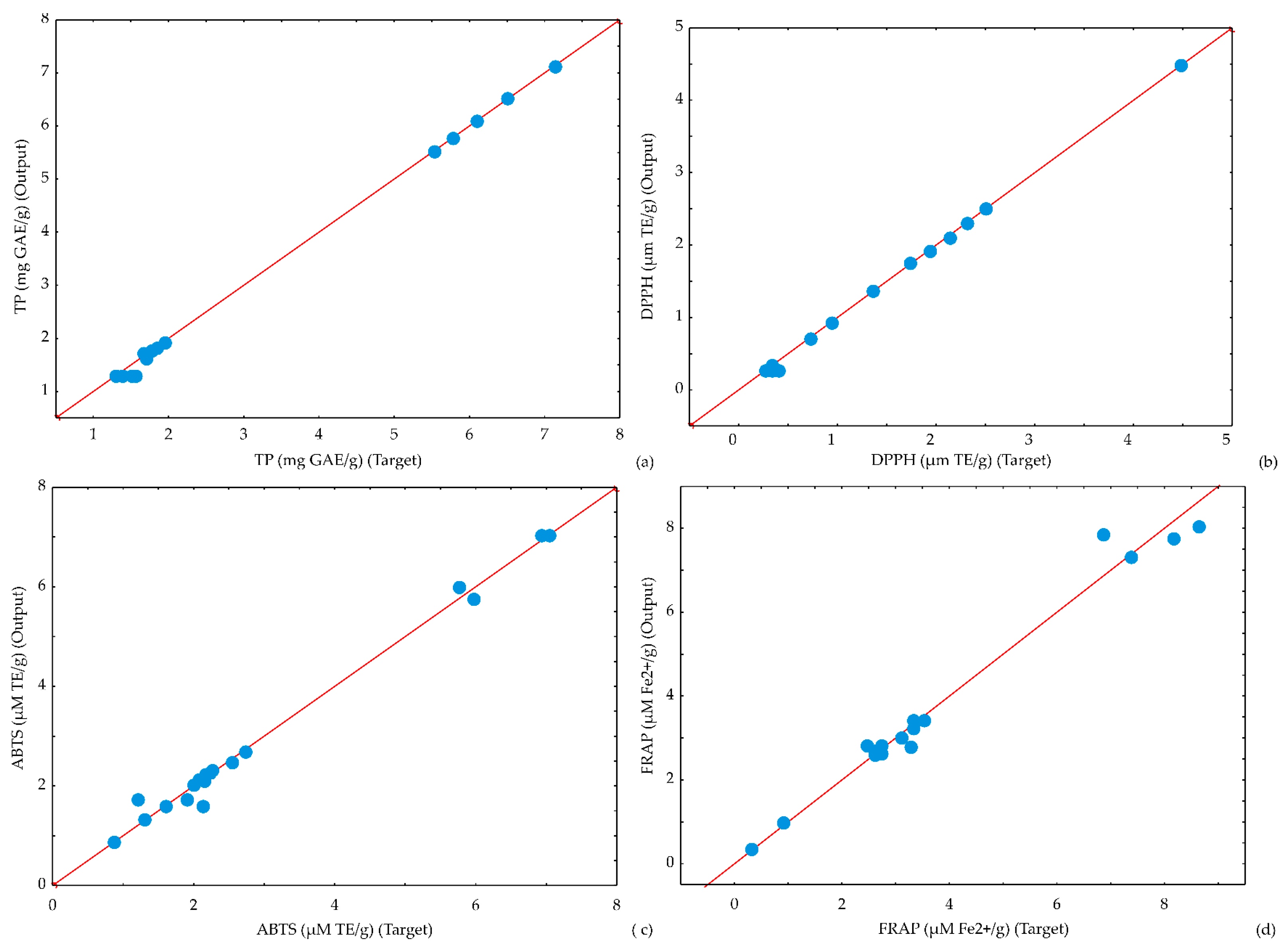
2.3. The Accuracy of the Model
3. Results and Discussion
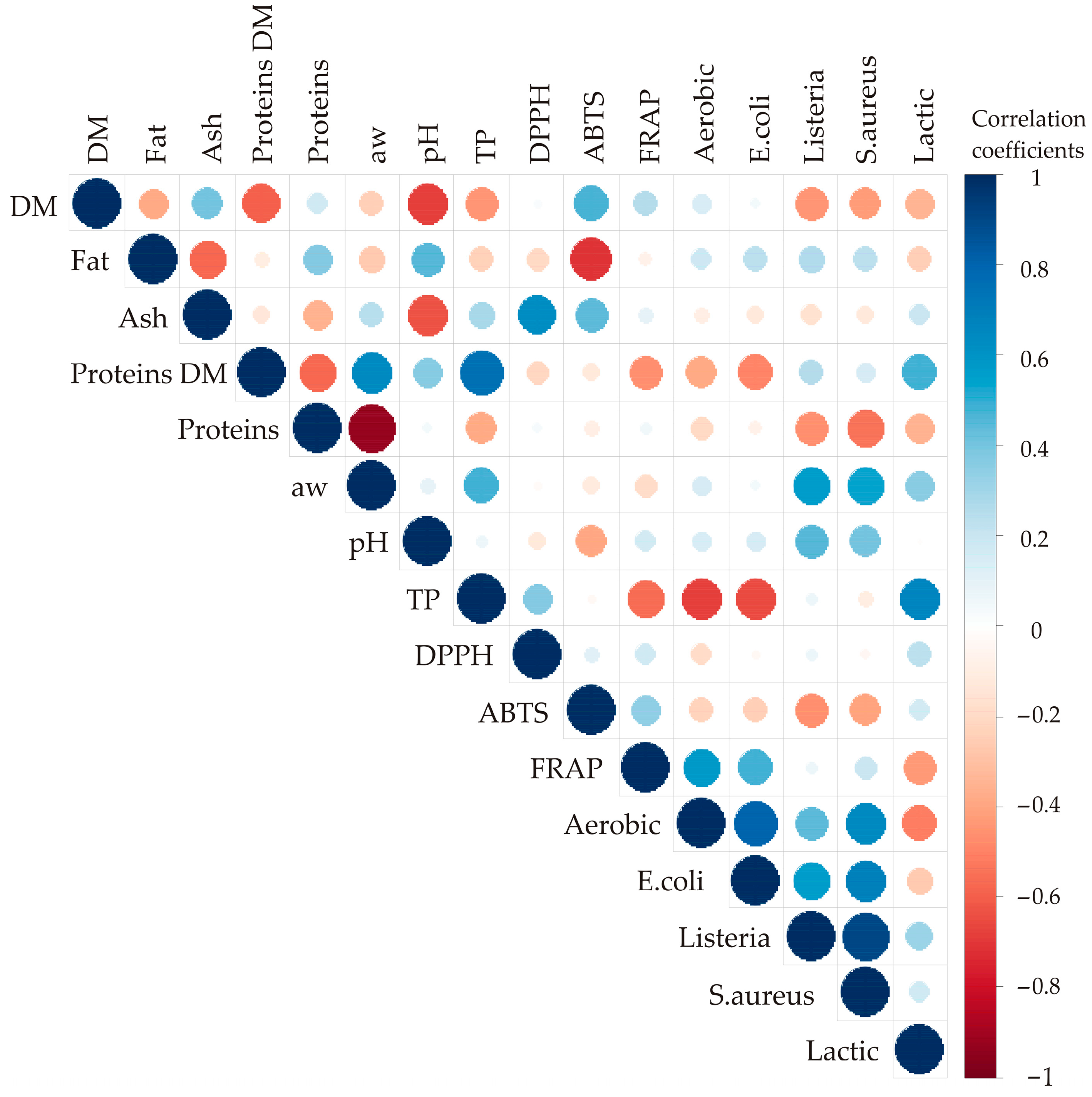
3.1. Correlation Analysis
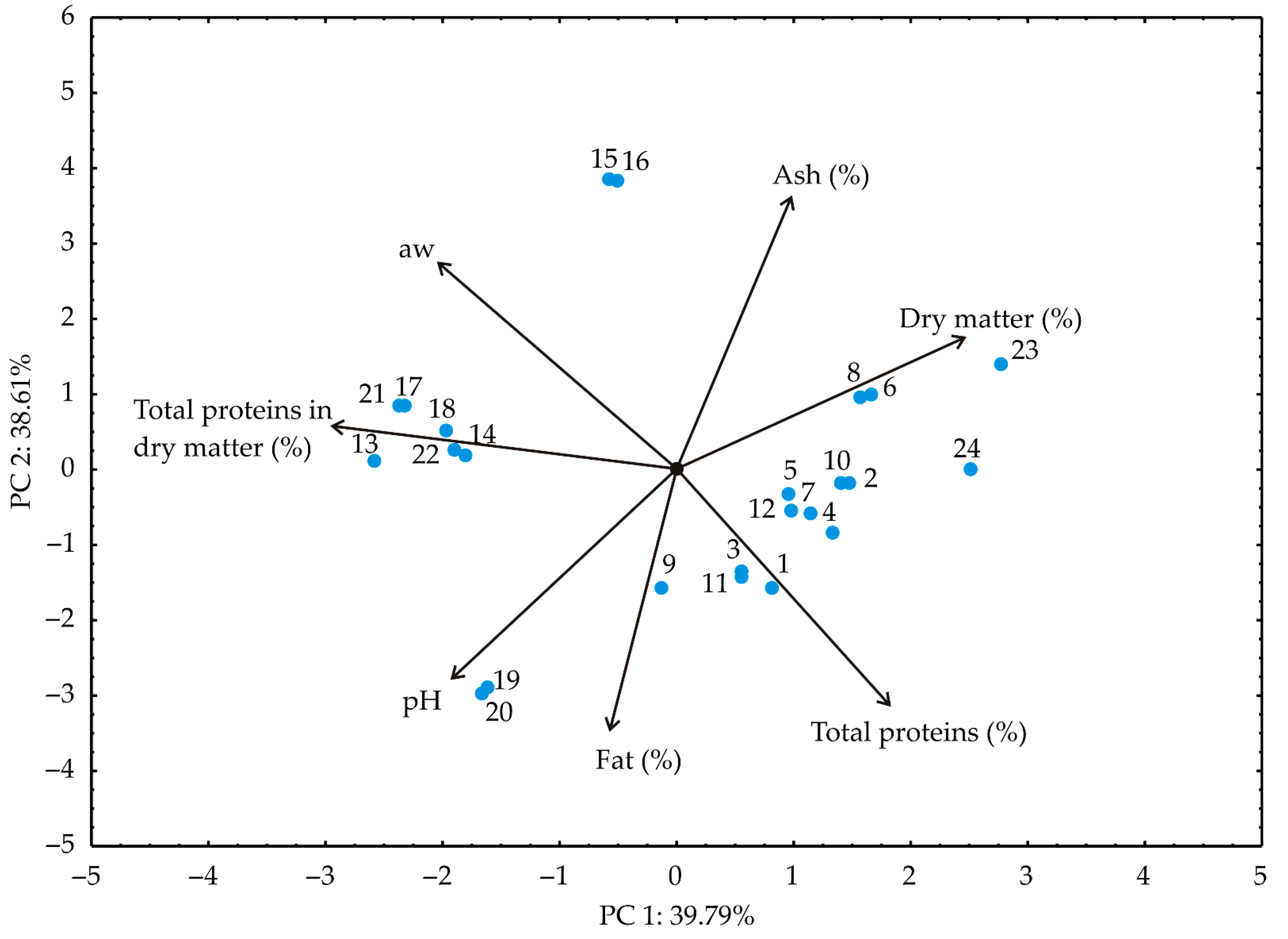
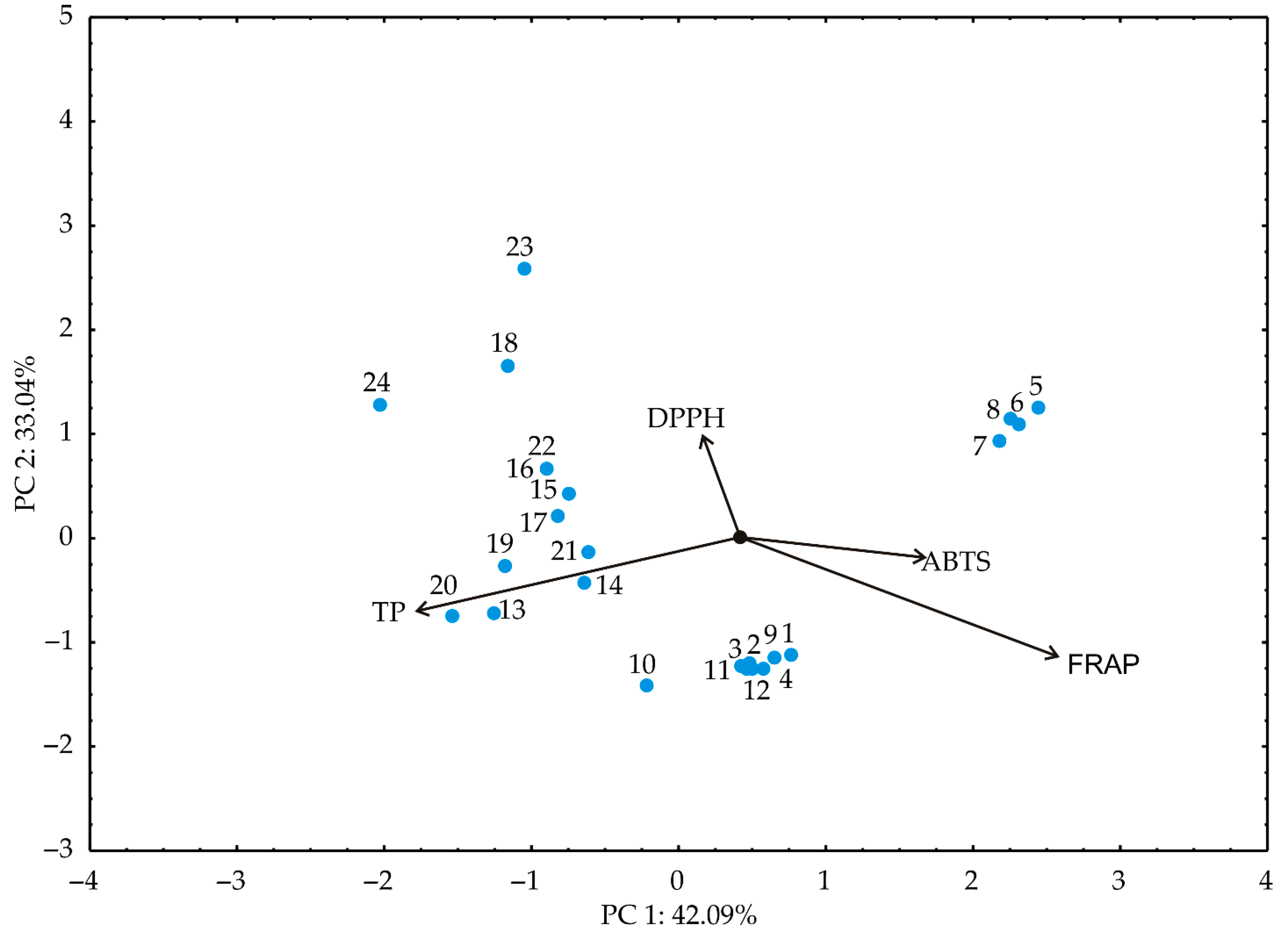
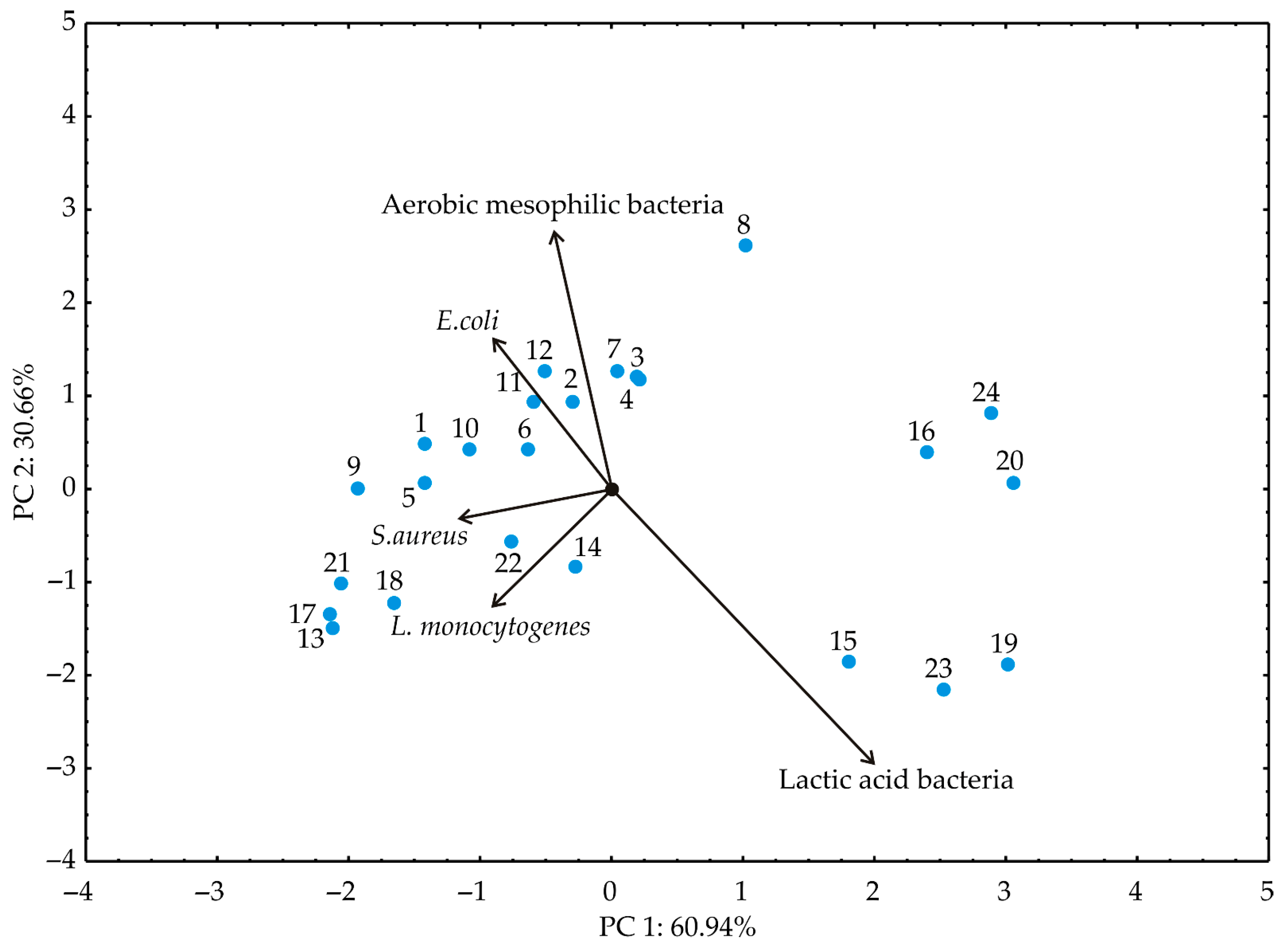
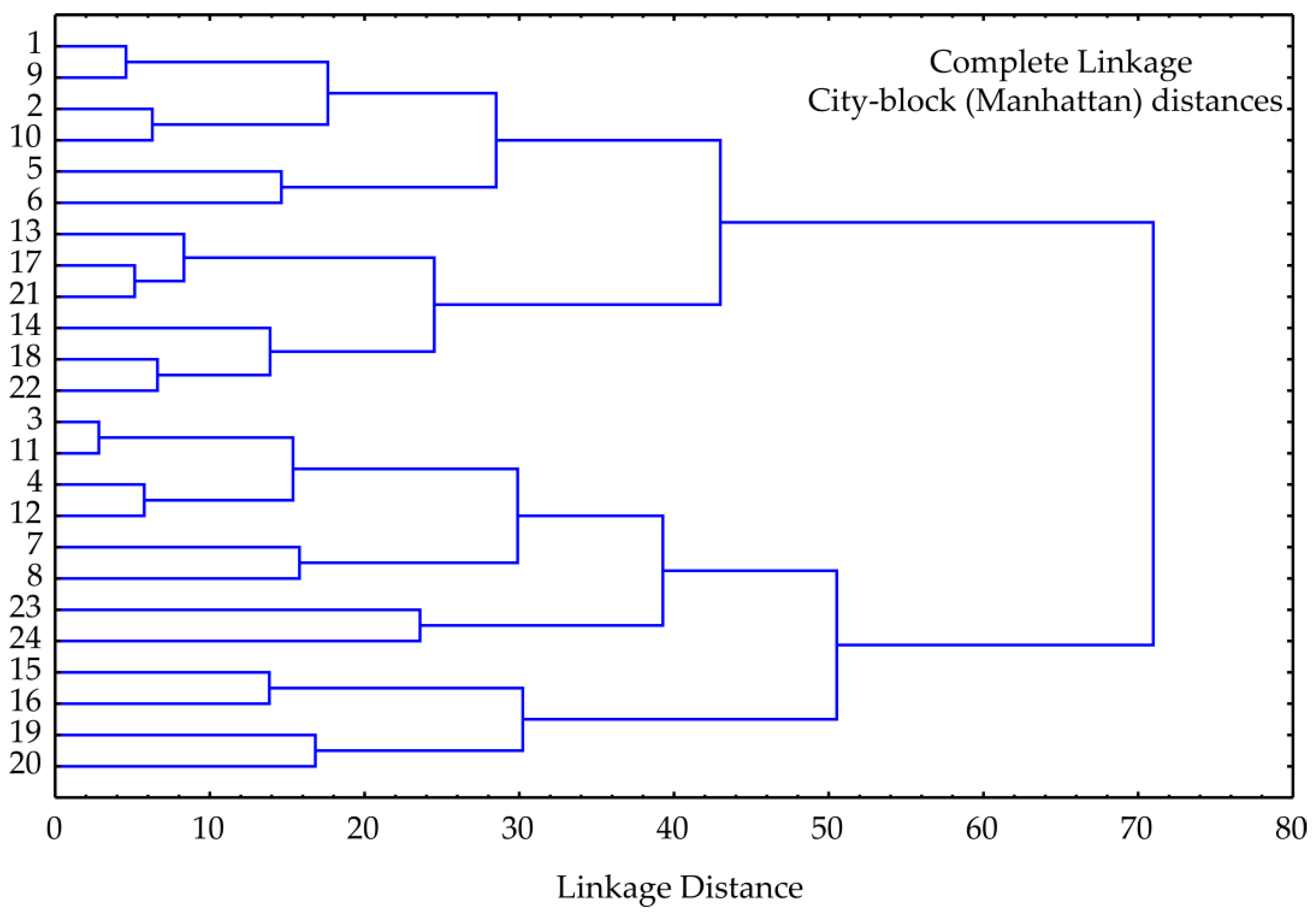
3.2. PCA and Cluster Analysis
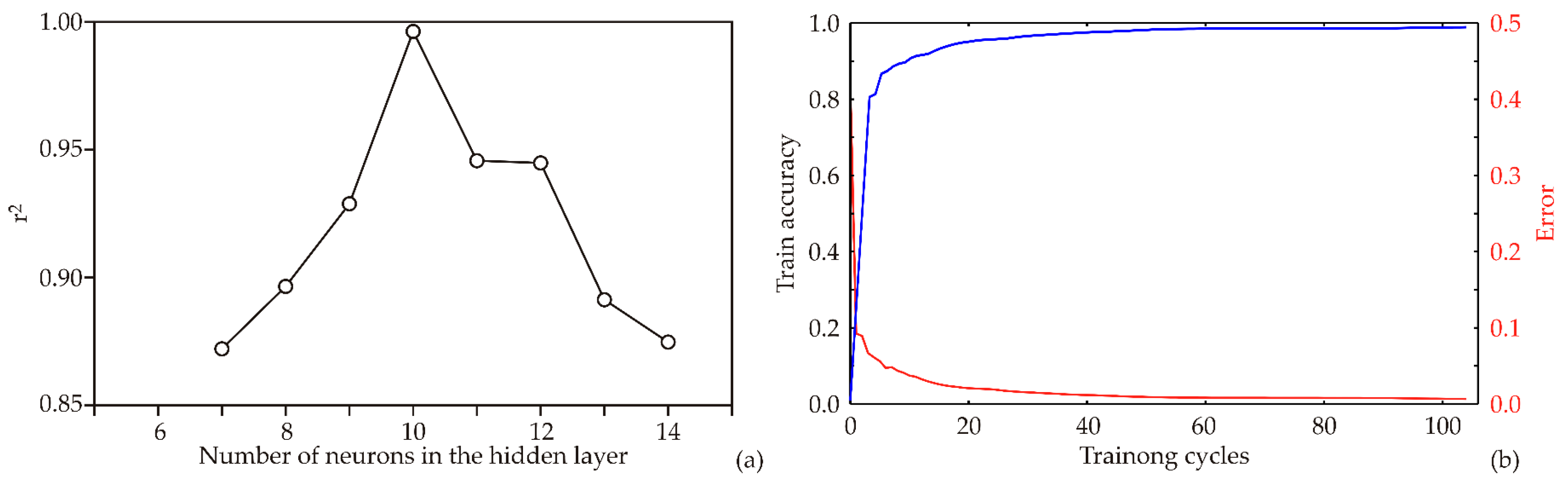
3.3. Artificial Neural Network Modeling
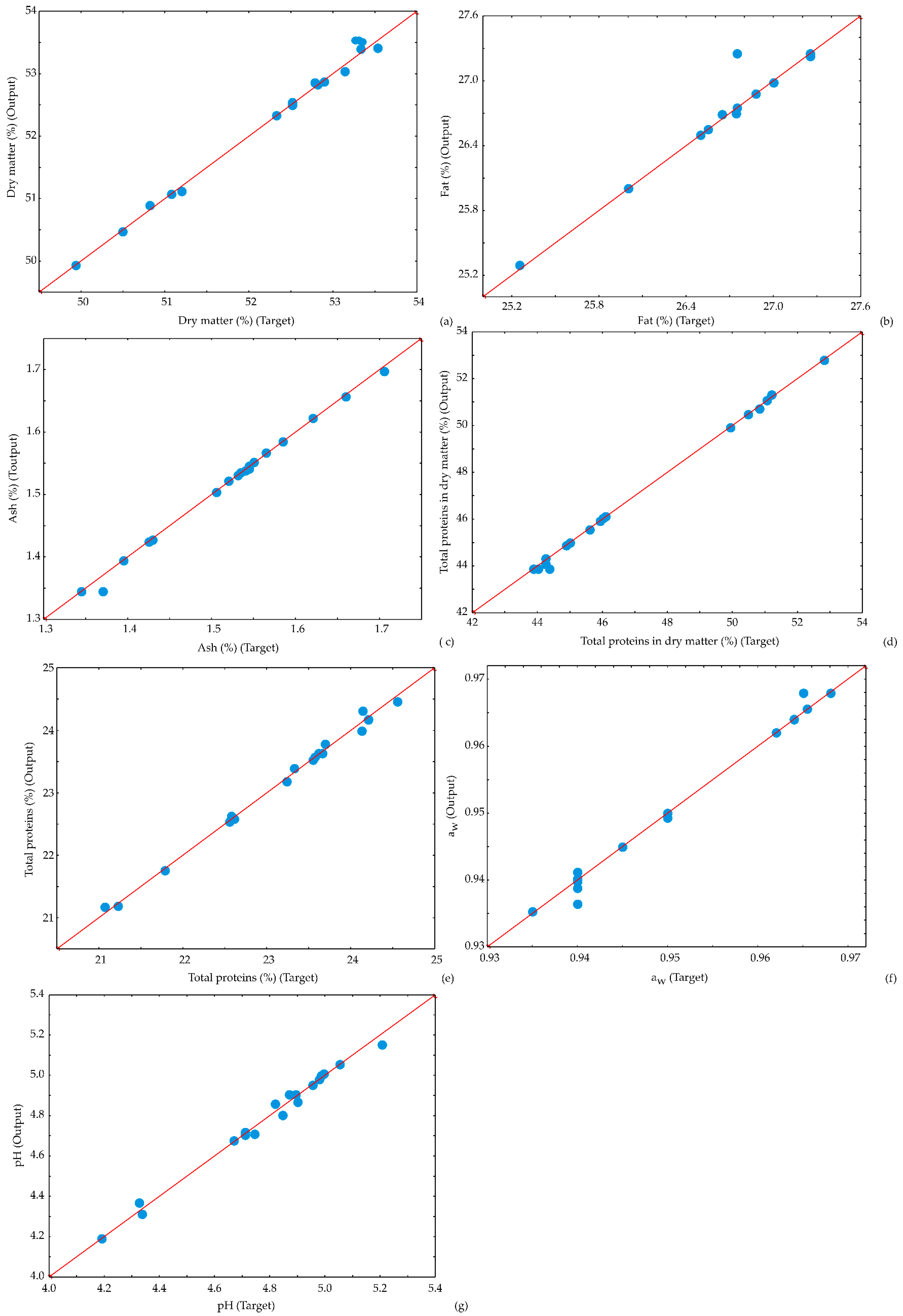
3.4. The Accuracy of the Model
3.5. Global Sensitivity Analysis—Yoon’s Interpretation Method
3.6. Multi-Objective Optimization of the Outputs of the ANN
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stangierski, J.; Weiss, D.; Kaczmarek, A. Multiple regression models and Artificial Neural Network (ANN) as prediction tools of changes in overall quality during the storage of spreadable processed Gouda cheese. Eur. Food Res. Technol. 2019, 245, 2539–2547. [Google Scholar] [CrossRef]
- Guiné, R. The use of artificial neural networks (ANN) in food process engineering. Int. J. Food Eng. 2019, 5, 15–21. [Google Scholar] [CrossRef]
- Vásquez, N.; Magán, C.; Oblitas, J.; Chuquizuta, T.; Avila-George, H.; Castro, W. Comparison between artificial neural network and partial least squares regression models for hardness modeling during the ripening process of Swiss-type cheese using spectral profiles. J. Food Eng. 2018, 219, 8–15. [Google Scholar] [CrossRef]
- Goyal, S.; Kumar Goyal, G. Shelflife Prediction of Processed Cheese Using Artificial Intelligence ANN Technique. Hrvat. Časopis Prehrambenu Tehnol. Biotehnol. Nutr. 2012, 7, 184–187. [Google Scholar]
- Sánchez-González, J.A.; Oblitas-Cruz, J.F. Application of Weibull analysis and artificial neural networks to predict the useful life of the vacuum packed soft cheese. Rev. Fac. De Ing. 2017, 82, 53–59. [Google Scholar] [CrossRef]
- Ghasemi-Varnamkhasti, M.; Mohammad-Razdari, A.; Yoosefian, S.H.; Izadi, Z.; Siadat, M. Aging discrimination of French cheese types based on the optimization of an electronic nose using multivariate computational approaches combined with response surface method (RSM). LWT 2019, 111, 85–98. [Google Scholar] [CrossRef]
- Jakopović, K.; Barukčić Jurina, I.; Marušić Radovčić, N.; Božanić, R.; Jurinjak Tušek, A. Reduced Sodium in White Brined Cheese Production: Artificial Neural Network Modeling for the Prediction of Specific Properties of Brine and Cheese during Storage. Fermentation 2023, 9, 783. [Google Scholar] [CrossRef]
- Horiuchi, J.I.; Shimada, T.; Funahashi, H.; Tada, K.; Kobayashi, M.; Kanno, T. Artificial neural network model with a culture database for prediction of acidification step in cheese production. J. Food Eng. 2004, 63, 459–465. [Google Scholar] [CrossRef]
- Cevoli, C.; Cerretani, L.; Gori, A.; Caboni, M.F.; Toschi, T.G.; Fabbri, A. Classification of Pecorino cheeses using electronic nose combined with artificial neural network and comparison with GC–MS analysis of volatile compounds. Food Chem. 2011, 129, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Soto-Barajas, M.C.; González-Martín, M.I.; Salvador-Esteban, J.; Hernández-Hierro, J.M.; Moreno-Rodilla, V.; Vivar-Quintana, A.M.; Revilla, I.; Ortega, I.L.; Morón-Sancho, R.; Curto-Diego, B. Prediction of the type of milk and degree of ripening in cheeses by means of artificial neural networks with data concerning fatty acids and near infrared spectroscopy. Talanta 2013, 116, 50–55. [Google Scholar] [CrossRef]
- Ebrahimpour, M.; Yu, W.; Young, B. Artificial neural network modelling for cream cheese fermentation pH prediction at lab and industrial scales. Food Bioprod. Process. 2021, 126, 81–89. [Google Scholar] [CrossRef]
- Santos, L.S.; Cardozo, R.M.; Nunes, N.M.; Inácio, A.B.; Pires, A.C.D.S.; Pinto, M.S. Easy classification of traditional Minas cheeses using artificial neural networks and discriminant analysis. Int. J. Dairy Technol. 2017, 70, 492–498. [Google Scholar] [CrossRef]
- Hayaloglu, A.A. Cheese with Herbs, Spices and Condiments. In McNamara, Encyclopedia of Dairy Sciences, 3rd ed.; McSweeney, P.L.H., McNamara, J.P., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 137–145. [Google Scholar] [CrossRef]
- Hamasadek, Z.O.; Hadi, C.S.; Wally, N.M.; Balaky, H.H. Antimicrobial Activity of some Herbs against Pathogenic Microorganisms in Raw Soft Cheese (Kurdish Cheese). J. Univ. Anbar Pure Sci. 2023, 17, 1–10. [Google Scholar] [CrossRef]
- Shinde, S.P.; Kankhare, D.H.; Andhare, B.C.; Kamble, D.K. Standardization of Level of Herbs in Quarg Cheese. Asian J. Dairy Food Res. 2023. [Google Scholar] [CrossRef]
- Setyawardani, T.; Sumarmono, J.; Dwiyanti, H.; Arkan, N.D. Yield, Composition, Texture, and Sensory Characteristics of Cottage Cheese Produced with the Incorporation of Different Herb Extracts. Anim. Prod. 2023, 47, 109–117. [Google Scholar] [CrossRef]
- Ghanimah, M.; Hanafy, N.; Hassanein, A.; Hashim, M. Utilisation of water extract of green chilli pepper in the manufacture of low-fat fresh cheese. Int. J. Dairy Technol. 2018, 71, 693–698. [Google Scholar] [CrossRef]
- Racette, C.M.; Drake, M.A. Consumer perception of natural hot-pepper cheeses. J. Dairy Sci. 2022, 105, 2166–2179. [Google Scholar] [CrossRef]
- El-Sayed, S.M.; Ibrahim, O.A.; Kholif, A.M. Characterization of novel Ras cheese supplemented with Jalapeno red pepper. J. Food Process. Preserv. 2020, 44, 14535. [Google Scholar] [CrossRef]
- Wahba, N.M.; Ahmed, A.S.; Ebraheim, Z.Z. Antimicrobial effects of pepper, parsley, and dill and their roles in the microbiological quality enhancement of traditional Egyptian Kareish cheese. Foodborne Pathog. Dis. 2010, 17, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Tuminello, L.; Carpino, S.; Campo, P.; Licitra, G. The wooden vat acts as an important natural inoculation system of the milk, in the cheese making process of the Piacentinu Ennese cheese. Sci. E Tec. Latt.-Casearia 2010, 61, 251–263. [Google Scholar]
- Kirby, C.J.; Brooker, B.E.; Law, B.A. Accelerated ripening of cheese using liposome-encapsulated enzyme. Int. J. Food Sci. Technol. 1987, 22, 355–375. [Google Scholar] [CrossRef]
- Ahmed, L.I.; Ibrahim, N.; Abdel-Salam, A.B.; Fahim, K.M. Potential application of ginger, clove and thyme essential oils to improve soft cheese microbial safety and sensory characteristics. Food Biosci. 2021, 42, 101177. [Google Scholar] [CrossRef]
- Hassanien, M.F.R.; Mahgoub, S.A.; El-Zahar, K.M. Soft cheese supplemented with black cumin oil: Impact on food borne pathogens and quality during storage. Saudi J. Biol. Sci. 2014, 21, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Shahdadi, F. Effect of edible coating of Basil seed mucilage with different levels of black Caraway extract on quality and shelf life of lactic cheese. J. Food Sci. Technol. 2022, 18, 315–324. [Google Scholar]
- Vitalini, S.; Nalbone, L.; Bernardi, C.; Iriti, M.; Costa, R.; Cicero, N.; Giarratana, F.; Vallone, L. Ginger and parsley essential oils: Chemical composition, antimicrobial activity, and evaluation of their application in cheese preservation. Nat. Prod. Res. 2023, 37, 2742–2747. [Google Scholar] [CrossRef] [PubMed]
- Raeisi, M.; Tajik, H.; Razavi, R.S.; Maham, M.; Moradi, M.; Hajimohammadi, B.; Naghili, H.; Hashemi, M.; Mehdizadeh, T. Essential oil of tarragon (Artemisia dracunculus) antibacterial activity on Staphylococcus aureus and Escherichia coli in culture media and Iranian white cheese. Iran. J. Microbiol. 2012, 4, 30. [Google Scholar]
- Schuh, J.; Batisteli, P.; Gargetti, A.; Zapparoli, A.; Balsan, T.I.; Gilioli, A.; Zanetti, V.C.; Foralosso, F.B.; Vargas Junior, A.; Fronza, N.; et al. Basil, marjoram, nutmeg and oregano essential oils as natural preservatives of Quark-type cheese. Food Sci. Technol. 2022, 42, 31322. [Google Scholar] [CrossRef]
- Belewu, M.A.; Belewu, K.Y.; Adebisi, M.B.; Esan, O.T.; Fashola, Y.A.; Belewu, N.O. Physicochemical evaluation of garlic oil on the nutritive and shelf-life of cheese. Int. J. Nat. 2013, 4, 699–701. [Google Scholar]
- Alimardanova, M.; Tlevlessova, D.; Bakiyeva, V.; Akpanov, Z. Revealing the features of the formation of the properties of processed cheese with wild onions. East.-Eur. J. Enterp. Technol. 2021, 4, 112. [Google Scholar] [CrossRef]
- Solhi, P.; Azadmard-Damirchi, S.; Hesari, J.; Hamishehkar, H. Production of the processed cheese containing tomato powder and evaluation of its rheological, chemical and sensory characteristics. J. Food Sci. Technol. 2020, 57, 2198–2205. [Google Scholar] [CrossRef]
- Vukić, V.; Iličić, M.; Vukić, D.; Kocić-Tanackov, S.; Pavlić, B.; Bjekić, M.; Kanurić, K.; Degenek, J.; Zeković, Z. The application of kombucha inoculum as an innovative starter culture in fresh cheese production. LWT 2021, 151, 112142. [Google Scholar] [CrossRef]
- Vukić, V.; Vukić, D.; Pavlić, B.; Iličić, M.; Kocić-Tanackov, S.; Kanurić, K.; Bjekić, M.; Zeković, Z. Antimicrobial potential of kombucha fresh cheese with the addition of sage (Salvia officinalis L.) and its preparations. Food Funct. 2023, 14, 3348–3356. [Google Scholar] [CrossRef] [PubMed]
- Degenek, J.; Kanurić, K.; Iličić, M.; Vukić, D.; Mrkonjić, Ž.; Pavlić, B.; Zeković, Z.; Vukić, V. Fortification of fresh kombucha cheese with wild thyme (Thymus serpyllum L.) herbal dust and its influence on antioxidant activity. Food Biosci. 2023, 56, 103161. [Google Scholar] [CrossRef]
- Correia, P.; Vitor, A.; Tenreiro, M.; Correia, A.C.; Pinto, A.; Correia, P.; Madanelo, J.; Vacas, M.; Guiné, R. Influence of different processing parameters in physical and sensorial properties of Serra de Estrela cheese. J. Hyg. Eng. Des. 2014, 8, 135–140. [Google Scholar]
- Bezie, A.; Regasa, H. The role of starter culture and enzymes/rennet for fermented dairy products manufacture-A Review. Nutr. Food Sci. Int. J. 2019, 9, 21–27. [Google Scholar]
- Troch, T.; Lefébure, É.; Baeten, V.; Colinet, F.; Gengler, N.; Sindic, M. Cow milk coagulation: Process description, variation factors and evaluation methodologies. A review. Biotechnol. Agron. Société Environ. 2017, 21, 276–287. [Google Scholar] [CrossRef]
- Panthi, R.R.; Kelly, A.L.; O’Callaghan, D.J.; Sheehan, J.J. Measurement of syneretic properties of rennet-induced curds and impact of factors such as concentration of milk: A review. Trends Food Sci. Technol. 2019, 91, 530–540. [Google Scholar] [CrossRef]
- Guinee, T.P.; O’callaghan, D.J. Control and prediction of quality characteristics in the manufacture and ripening of cheese. Technol. Cheesemak. 2010, 8904, 260. [Google Scholar]
- Guinee, T.P. Salting and the role of salt in cheese. Int. J. Dairy Technol. 2004, 57, 99–109. [Google Scholar] [CrossRef]
- Meng, Y.; Sun, J.; Zhang, G.; Yu, T.; Piao, H. Unlock the power of bovine milk-derived exosomes for degenerative diseases associated with aging. J. Funct. Foods. 2023, 109, 105788. [Google Scholar] [CrossRef]
- Fusco, V.; Chieffi, D.; Fanelli, F.; Logrieco, A.F.; Cho, G.S.; Kabisch, J.; Böhnlein, C.; Franz, C.M. Microbial quality and safety of milk and milk products in the 21st century. Compr. Rev. Food Sci. 2020, 19, 2013–2049. [Google Scholar] [CrossRef] [PubMed]
- Benedet, A.; Manuelian, C.L.; Penasa, M.; Cassandro, M.; Righi, F.; Sternieri, M.; Galimberti, P.; Zambrini, A.V.; De Marchi, M. Factors associated with herd bulk milk composition and technological traits in the Italian dairy industry. J. Dairy Sci. 2018, 101, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wen, J.; Wang, J. Effect of environmental temperature and humidity on milk production and milk composition of Guanzhong dairy goats. Vet. Anim. Sci. 2020, 9, 100121. [Google Scholar] [CrossRef] [PubMed]
- D’amico, D.J. Microbiological quality and safety issues in cheesemaking. Cheese Microbes 2014, 2, 251–309. [Google Scholar]
- Curto, B.; Moreno, V.; García-Esteban, J.A.; Blanco, F.J.; González, I.; Vivar, A.; Revilla, I. Accurate prediction of sensory attributes of cheese using near-infrared spectroscopy based on artificial neural network. Sensors 2020, 20, 3566. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Fan, C.; Wang, Y.; Xue, W. Limited memory BFGS algorithm for the matrix approximation problem in Frobenius norm. Comput. Appl. Math. 2020, 39, 43. [Google Scholar] [CrossRef]
- Mohammed, N.A.; Ismail, Z.Z. Prediction of pollutants removal from cheese industry wastewater in constructed wetland by artificial neural network. Int. J. Sci. Environ. Technol. 2021, 19, 9775–9790. [Google Scholar] [CrossRef]
- Rajković, D.; Marjanović Jeromela, A.; Pezo, L.; Lončar, B.; Zanetti, F.; Monti, A.; Kondić Špika, A. Yield and quality prediction of winter rapeseed—Artificial neural network and random forest models. Agronomy 2021, 12, 58. [Google Scholar] [CrossRef]
- Rezazadeh Eidgahee, D.; Rafiean, A.H.; Haddad, A. A novel formulation for the compressive strength of IBP-based geopolymer stabilized clayey soils using ANN and GMDH-NN approaches. Iran. J. Sci. Technol.—Trans. Civ. Eng. 2020, 44, 219–229. [Google Scholar] [CrossRef]
- Yoon, G.H.; Donoso, A.; Carlos Bellido, J.; Ruiz, D. Highly efficient general method for sensitivity analysis of eigenvectors with repeated eigenvalues without passing through adjacent eigenvectors. Int. J. Numer. Methods Eng. 2020, 121, 4473–4492. [Google Scholar] [CrossRef]
- Makowski, D.; Ben-Shachar, M.S.; Patil, I.; Lüdecke, D. Methods and algorithms for correlation analysis in R. J. Open Source Softw. 2020, 5, 2306. [Google Scholar] [CrossRef]
- Middendorf, D.; Bindrich, U.; Siemer, C.; Töpfl, S.; Heinz, V. Affecting casein micelles by pulsed electrical field (PEF) for inclusion of lipophilic organic compounds. Appl. Sci. 2021, 11, 4611. [Google Scholar] [CrossRef]
- Ćurčić, L.; Lončar, B.; Pezo, L.; Stojić, N.; Prokić, D.; Filipović, V.; Pucarević, M. Chemometric approach to pesticide residue analysis in surface water. Water 2022, 14, 4089. [Google Scholar] [CrossRef]
- Ferreira, S.M.; Matos, L.C.; Santos, L. Harnessing the potential of chestnut shell extract to enhance fresh cheese: A sustainable approach for nutritional enrichment and shelf-life extension. J. Food Meas. Charact. 2023, 18, 1559–1573. [Google Scholar] [CrossRef]
- Dalaka, E.; Politis, I.; Theodorou, G. Antioxidant Activity of Sweet Whey Derived from Bovine, Ovine and Caprine Milk Obtained from Various Small-Scale Cheese Plants in Greece before and after in vitro Simulated Gastrointestinal Digestion. Antioxidants 2023, 12, 1676. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, U.; Cummins, E.; Valero, A.; Walsh, D.; Dalmasso, M.; Jordan, K.; Duffy, G. Farm to Fork Quantitative Risk Assessment of Listeria monocytogenes Contamination in Raw and Pasteurized Milk Cheese in Ireland. Risk Anal. 2015, 35, 1140–1153. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.; Mikiciuk, G.; Kisiel, A.; Mikiciuk, M.; Paliwoda, D.; Sas-Paszt, L.; Cembrowska-Lech, D.; Krzemińska, A.; Kozioł, A.; Brysiewicz, A. Machine Learning Approaches for Forecasting the Best Microbial Strains to Alleviate Drought Impact in Agriculture. Agriculture 2023, 13, 1622. [Google Scholar] [CrossRef]
- Mudoor Sooresh, M.; Willing, B.P.; Bourrie, B.C. Opportunities and Challenges of Understanding Community Assembly in Spontaneous Food Fermentation. Foods 2023, 12, 673. [Google Scholar] [CrossRef] [PubMed]
- Rabunal, J.R.; Dorado, J. (Eds.) Artificial Neural Networks in Real-Life Applications; IGI Global: Hershey, PA, USA, 2006. [Google Scholar]
- Abiodun, O.I.; Jantan, A.; Omolara, A.E.; Dada, K.V.; Mohamed, N.A.; Arshad, H. State-of-the-art in artificial neural network applications: A survey. Heliyon 2018, 4, 11. [Google Scholar] [CrossRef]
- Demir, H.; Demir, H.; Lončar, B.; Pezo, L.; Brandić, I.; Voća, N.; Yilmaz, F. Optimization of Caper Drying using Response Surface Methodology and Artificial Neural Networks for Energy Efficiency Characteristics. Energies 2023, 16, 1687. [Google Scholar] [CrossRef]
- Dayhoff, J.E.; DeLeo, J.M. Artificial neural networks: Opening the black box. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2001, 91, 1615–1635. [Google Scholar] [CrossRef]
- Abdolrasol, M.G.; Hussain, S.S.; Ustun, T.S.; Sarker, M.R.; Hannan, M.A.; Mohamed, R.; Ali, J.A.; Mekhilef, S.; Milad, A. Artificial neural networks based optimization techniques: A review. Electronics 2021, 10, 2689. [Google Scholar] [CrossRef]
- Sousa, J.C.; Bernardo, H. Benchmarking of load forecasting methods using residential smart meter data. Appl. Sci. 2022, 12, 9844. [Google Scholar] [CrossRef]



| No | Herb | Sample | Day of Storage |
|---|---|---|---|
| 1 | thyme | KC | 0 |
| 2 | thyme | KC | 10 |
| 3 | thyme | KC | 20 |
| 4 | thyme | KC | 30 |
| 5 | thyme | KG | 0 |
| 6 | thyme | KG | 10 |
| 7 | thyme | KG | 20 |
| 8 | thyme | KG | 30 |
| 9 | thyme | KSFE | 0 |
| 10 | thyme | KSFE | 10 |
| 11 | thyme | KSFE | 20 |
| 12 | thyme | KSFE | 30 |
| 13 | salvia | KC | 0 |
| 14 | salvia | KC | 10 |
| 15 | salvia | KC | 20 |
| 16 | salvia | KC | 30 |
| 17 | salvia | KG | 0 |
| 18 | salvia | KG | 10 |
| 19 | salvia | KG | 20 |
| 20 | salvia | KG | 30 |
| 21 | salvia | KSFE | 0 |
| 22 | salvia | KSFE | 10 |
| 23 | salvia | KSFE | 20 |
| 24 | salvia | KSFE | 30 |
| Performance | Error | Training Algorithm | Error Function | Activation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Net. Name | Train. | Test | Valid. | Train. | Test | Valid. | Hidden | Output | ||
| MLP 6-10-16 | 0.993 | 0.992 | 0.992 | 0.112 | 0.110 | 0.099 | BFGS 895 | SOS | Tanh | Logistic |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Day of storage | 5.946 | −74.377 | −5.191 | −0.123 | 35.947 | 19.789 | 69.472 | 18.844 | 11.077 | −9.741 |
| Herb (salvia) | −22.188 | 19.571 | 2.858 | −0.875 | −23.021 | −1.460 | −20.183 | −24.839 | 24.732 | −0.049 |
| Herb (thyme) | 23.436 | −8.728 | −1.138 | 0.791 | 23.777 | −8.585 | 5.219 | 17.235 | −22.868 | 3.855 |
| Sample (KC) | −0.108 | −7.028 | 3.362 | 22.847 | −16.967 | 4.949 | 11.619 | 30.853 | −28.909 | −0.042 |
| Sample (KG) | 1.287 | −3.620 | −2.503 | −21.715 | 8.757 | 4.618 | −37.262 | −22.961 | 22.947 | −0.295 |
| Sample (KSFE) | 0.132 | 21.571 | 0.872 | −1.102 | 9.015 | −19.489 | 10.619 | −15.595 | 7.802 | 4.176 |
| Bias | 1.441 | 10.877 | 1.697 | −0.082 | 0.821 | −10.059 | −15.175 | −7.666 | 1.981 | 3.787 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Bias | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| DM | 58.183 | 8.481 | 32.653 | −42.411 | −16.508 | 26.829 | 35.997 | 29.942 | 54.881 | −4.307 | 0.671 |
| Fat | −7.297 | −31.416 | 70.373 | −66.801 | 44.395 | −34.262 | −97.084 | 50.442 | −64.959 | −79.962 | −1.562 |
| Ash | −5.044 | −7.549 | −9.331 | 14.156 | −10.769 | −49.339 | 20.769 | −12.630 | 13.787 | −41.212 | 1.185 |
| Proteins DM | −35.584 | −2.777 | 39.702 | −54.536 | 3.466 | 47.129 | −5.042 | 33.668 | −52.901 | 10.158 | −2.158 |
| Proteins | −20.417 | −3.559 | 7.086 | −9.984 | 25.723 | 11.569 | −13.381 | 5.889 | −10.779 | 3.842 | −0.324 |
| aw | 35.435 | 16.330 | −28.452 | 38.750 | −51.027 | −33.065 | 45.172 | −18.905 | 36.649 | −6.693 | 4.102 |
| pH | −1.433 | 14.026 | 11.830 | −17.768 | 25.875 | 18.149 | −44.432 | 33.513 | −16.784 | 6.469 | −0.361 |
| TP | −73.083 | −36.334 | −15.210 | −21.810 | 19.576 | 21.696 | 8.571 | −25.220 | −22.743 | 4.998 | 0.670 |
| DPPH | −18.678 | 34.277 | −50.497 | 33.291 | 3.410 | −50.724 | 5.340 | 17.083 | 34.110 | −28.422 | −1.852 |
| ABTS | 17.065 | 0.087 | 17.405 | −23.008 | 1.717 | 18.474 | 3.723 | 14.220 | 13.383 | 3.014 | −2.949 |
| FRAP | 3.087 | 6.057 | −1.503 | 2.053 | 3.001 | −1.930 | −4.765 | 5.701 | 3.037 | −0.763 | −0.582 |
| Aerobic | 65.168 | 59.848 | 7.543 | −10.703 | 2.055 | 3.993 | −31.010 | 23.972 | −10.132 | −2.797 | 0.059 |
| E. coli | 16.318 | 2.112 | 4.819 | −6.670 | −10.296 | 3.710 | −7.232 | 3.367 | −6.008 | −0.189 | 0.417 |
| L. monocytogenes | 3.493 | −3.286 | 4.604 | −6.459 | −3.850 | 4.743 | −11.311 | 2.547 | −10.371 | 0.625 | 1.358 |
| S. aureus | 5.659 | −4.874 | 6.536 | −9.038 | −5.145 | 4.751 | −15.700 | 3.467 | −14.378 | −0.910 | 0.171 |
| Lactic | 7.317 | −1.893 | 1.916 | −2.428 | −8.363 | 0.305 | −4.583 | 0.212 | −4.536 | −1.266 | 0.608 |
| χ2 | RMSE | MBE | MPE | SSE | AARD | r2 | |
|---|---|---|---|---|---|---|---|
| DM | 0.080 | 0.094 | −0.028 | 0.128 | 0.146 | 1.205 | 0.994 |
| Fat | 0.128 | 0.119 | −0.028 | 0.146 | 0.242 | 0.707 | 0.936 |
| Ash | 0.000 | 0.006 | 0.002 | 0.202 | 0.001 | 0.054 | 0.996 |
| Proteins DM | 0.170 | 0.137 | 0.035 | 0.166 | 0.317 | 1.365 | 0.998 |
| Proteins | 0.045 | 0.071 | −0.003 | 0.232 | 0.089 | 0.914 | 0.995 |
| aw | 0.000 | 0.001 | 0.000 | 0.072 | 0.000 | 0.012 | 0.990 |
| pH | 0.006 | 0.027 | 0.001 | 0.418 | 0.013 | 0.361 | 0.990 |
| TP | 0.083 | 0.096 | 0.042 | 3.361 | 0.134 | 0.987 | 0.999 |
| DPPH | 0.023 | 0.051 | 0.025 | 7.456 | 0.036 | 0.552 | 0.999 |
| ABTS | 0.350 | 0.197 | −0.005 | 5.905 | 0.699 | 2.390 | 0.990 |
| FRAP | 0.953 | 0.325 | −0.005 | 6.407 | 1.905 | 3.790 | 0.980 |
| Aerobic | 0.856 | 0.534 | −0.236 | 3.468 | 5.508 | 5.868 | 0.698 |
| E. coli | 0.482 | 0.401 | −0.169 | 12.928 | 3.172 | 4.612 | 0.867 |
| L. momocytogens | 0.777 | 0.509 | −0.022 | 8.927 | 6.208 | 7.013 | 0.610 |
| S. aureus | 0.438 | 0.382 | −0.085 | 6.110 | 3.328 | 4.477 | 0.703 |
| Lactic | 6.838 | 1.510 | 0.681 | 13.734 | 43.561 | 17.815 | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lončar, B.; Pezo, L.; Iličić, M.; Kanurić, K.; Vukić, D.; Degenek, J.; Vukić, V. Modeling and Optimization of Herb-Fortified Fresh Kombucha Cheese: An Artificial Neural Network Approach for Enhancing Quality Characteristics. Foods 2024, 13, 548. https://doi.org/10.3390/foods13040548
Lončar B, Pezo L, Iličić M, Kanurić K, Vukić D, Degenek J, Vukić V. Modeling and Optimization of Herb-Fortified Fresh Kombucha Cheese: An Artificial Neural Network Approach for Enhancing Quality Characteristics. Foods. 2024; 13(4):548. https://doi.org/10.3390/foods13040548
Chicago/Turabian StyleLončar, Biljana, Lato Pezo, Mirela Iličić, Katarina Kanurić, Dajana Vukić, Jovana Degenek, and Vladimir Vukić. 2024. "Modeling and Optimization of Herb-Fortified Fresh Kombucha Cheese: An Artificial Neural Network Approach for Enhancing Quality Characteristics" Foods 13, no. 4: 548. https://doi.org/10.3390/foods13040548
APA StyleLončar, B., Pezo, L., Iličić, M., Kanurić, K., Vukić, D., Degenek, J., & Vukić, V. (2024). Modeling and Optimization of Herb-Fortified Fresh Kombucha Cheese: An Artificial Neural Network Approach for Enhancing Quality Characteristics. Foods, 13(4), 548. https://doi.org/10.3390/foods13040548









