Effect of Different Edible Trichosanthes Germplasm on Its Seed Oil to Enhance Antioxidant and Anti-Aging Activity in Caenorhabditis elegans
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
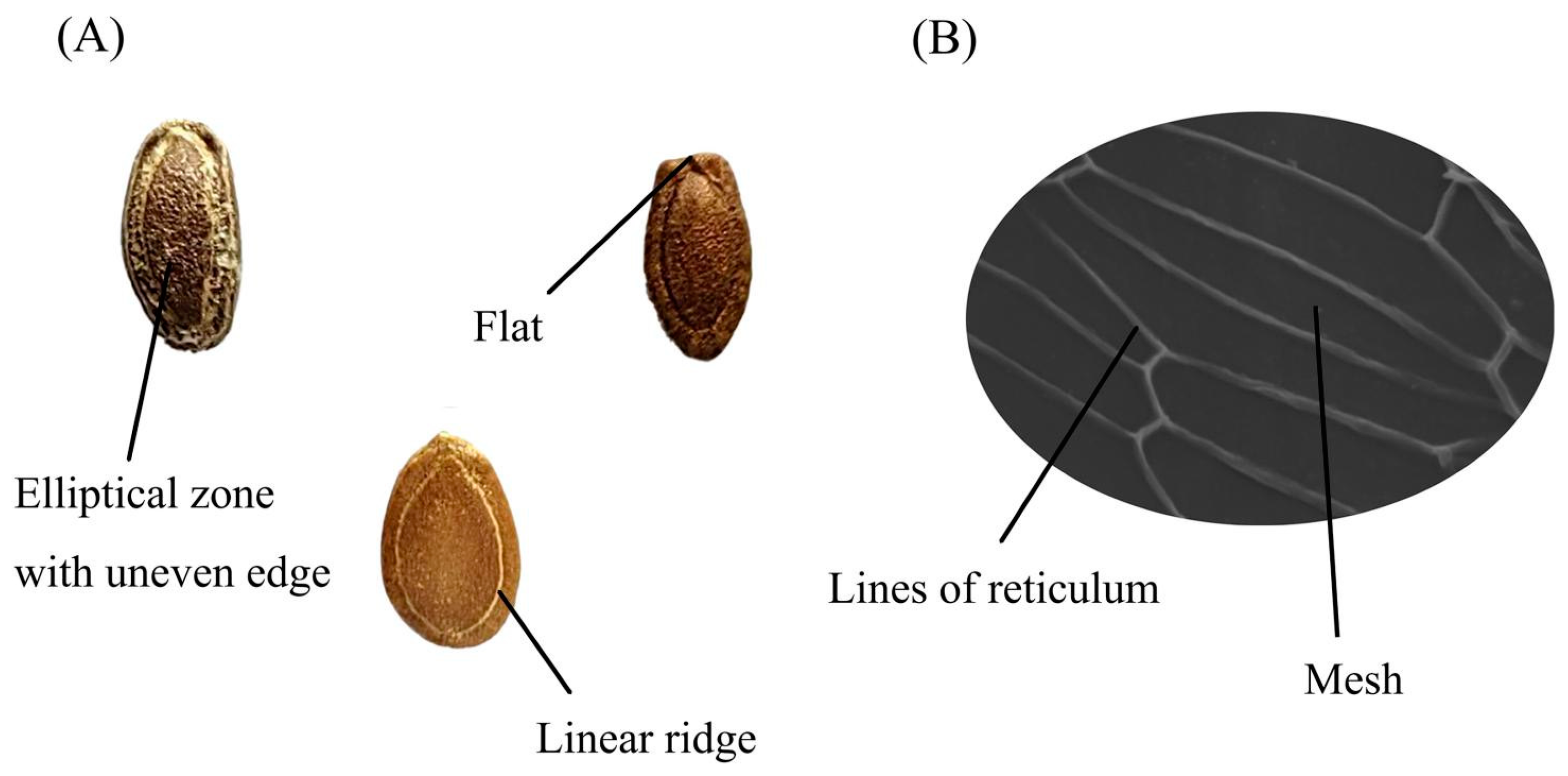
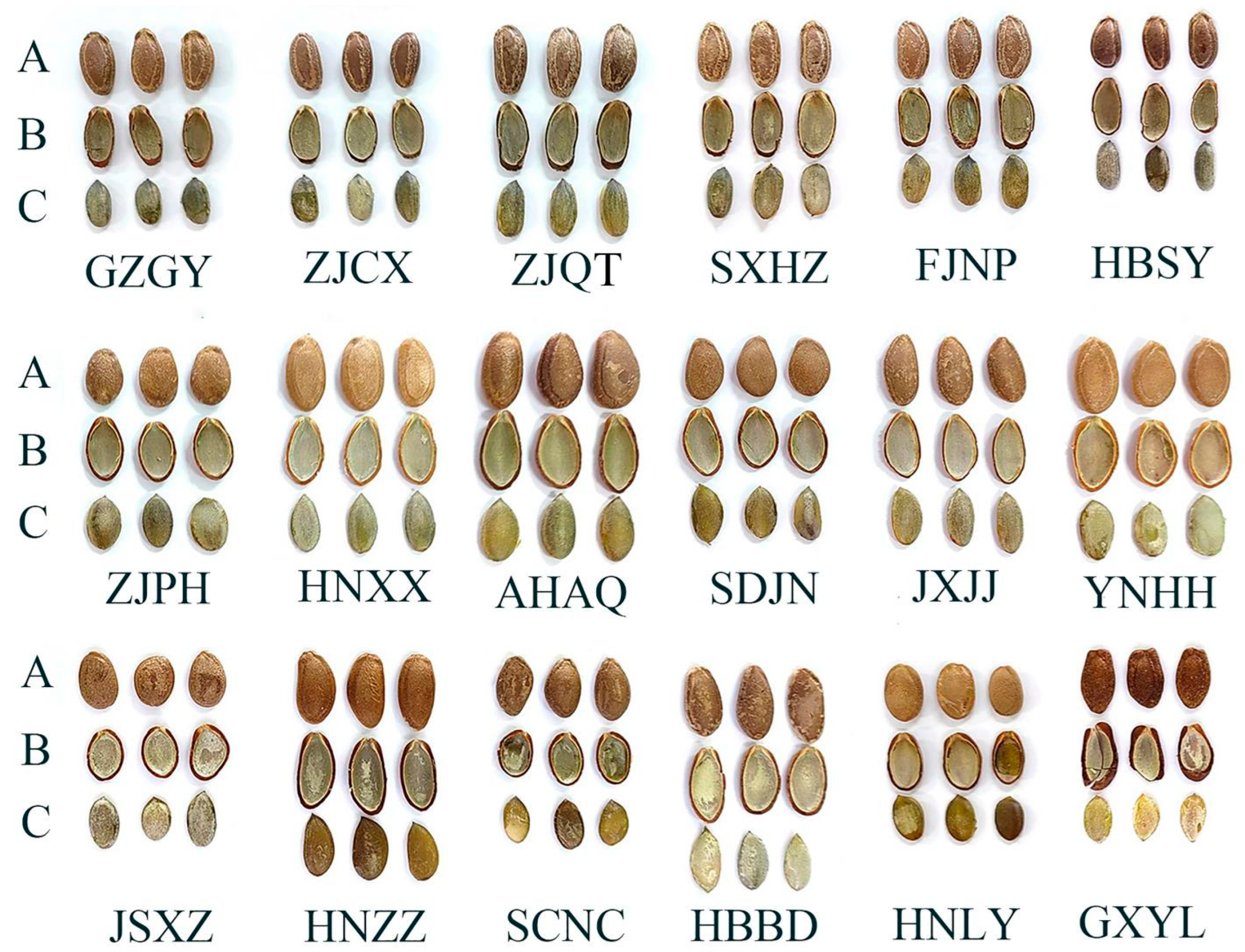
2.2. Seed Morphological Characteristics Analysis
2.3. Seed Microscopic Characteristics Analysis
2.4. DNA Extraction and PCR Amplification
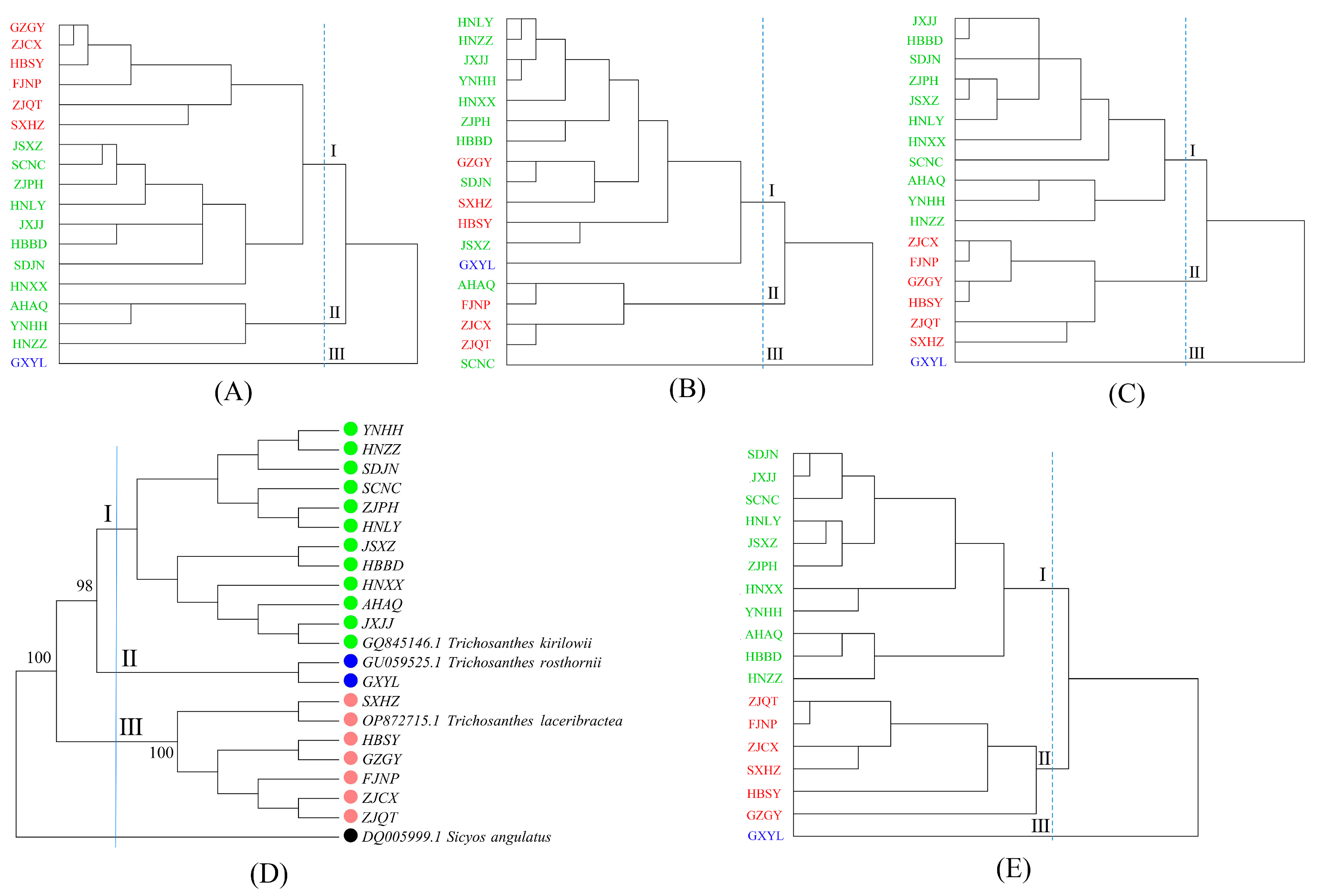
2.5. Sequence Alignment and Phylogenetic Analysis
2.6. Preparation of Seed Oils
2.7. Fatty Acid Composition Analysis
2.8. C. elegans Assays
2.8.1. Antioxidant Activity Assay
2.8.2. Lifespan Assay
2.8.3. Lipofuscin Assay
2.8.4. Healthspan Assay
2.9. Data Analysis
3. Results
3.1. Species Classification of Edible Trichosanthes Germplasm
3.2. Yield and Fatty Acid Composition of Seed Oil
3.3. Antioxidant Activity of Seed Oil
3.4. Anti-Aging Activity of Seed Oil
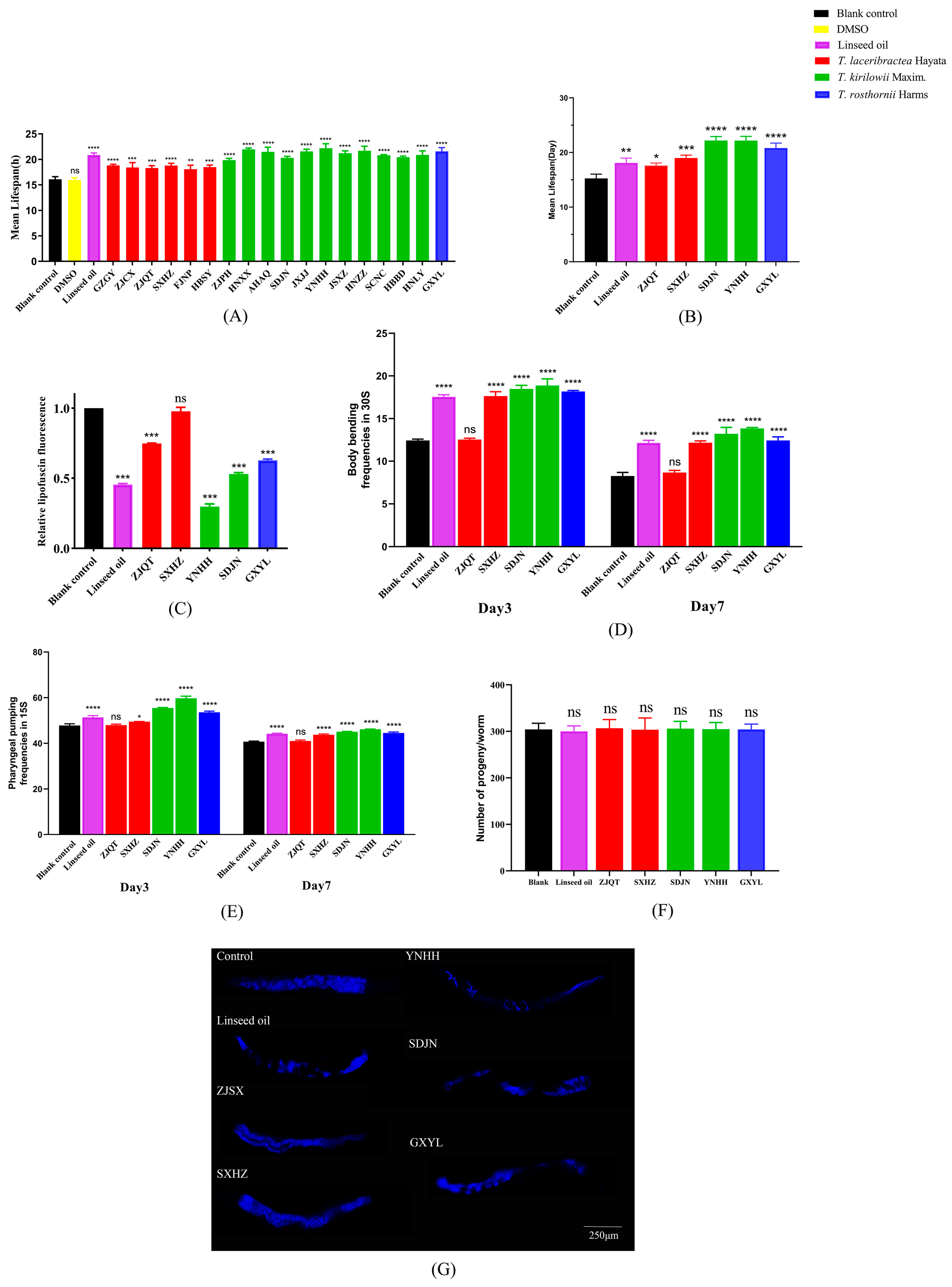
3.4.1. Effect on Lifespan of C. elegans
3.4.2. Effect on Lipofuscin Accumulation of C. elegans
3.4.3. Effect on the Healthspan of C. elegans
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, L.; Yue, C.; Cheng, J.; Lou, Z. Taxonomy of Medicinal Plants in Genus Trichosanthes L.; Shanghai Science and Technology Press: Shanghai, China, 2009. [Google Scholar]
- Committee for the Pharmacopoeia of China. Pharmacopoeia of the People’s Republic of China, Part 1; China Medical Science and Technology Press: Beijing, China, 2020. [Google Scholar]
- Zhang, H.Q.; Liu, P.; Duan, J.A.; Dong, L.; Shang, E.X.; Qian, D.W.; Zhu, Z.; Li, H.; Li, W.W. Comparative analysis of carbohydrates, nucleosides and amino acids in different parts of Trichosanthes kirilowii Maxim. by (ultra) high-performance liquid chromatography coupled with tandem mass spectrometry and evaporative light scattering detector methods. Molecules 2019, 24, 1440. [Google Scholar] [CrossRef]
- Yu, X.; Tang, L.; Wu, H.; Zhang, X.; Luo, H.; Guo, R.; Xu, M.; Yang, X.; Fan, J.; Wang, Z.; et al. Trichosanthis Fructus: Botany, traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2018, 224, 177–194. [Google Scholar] [CrossRef]
- Shen, G.; Xu, L.; Huang, W.; Chen, H.; Zheng, Q. Construction of DNA fingerprint library of cantaloupe cultivars (Lines) based on SSR markers. Mol. Plant Breed. 2023, 21, 3349–3355. [Google Scholar] [CrossRef]
- Jin, X.L.; Sang, G.F.; Du, X. Fatty acid composition and heavy mental accumulation characteristics of Fructus Trichosanthis seed. Fresen. Environ. Bull. 2019, 28, 1978–1980. [Google Scholar]
- Dalda-Sekerci, A. Comprehensive assessment of genetic diversity in chrysanthemum germplasm using morphological, biochemical and retrotransposon-based molecular markers. Genet. Resour. Crop Evol. 2023, 70, 2321–2336. [Google Scholar] [CrossRef]
- Dalawai, D.; Murthy, H.N. Pollen and seed morphology of selected species of Andrographis (Acanthaceae) from India. Grana 2021, 60, 459–476. [Google Scholar] [CrossRef]
- Sevindik, E.; Okan, K.; Sevindik, M.; Ercisli, S. Genetic diversity and phylogenetic analyses of Juglans regia L. (Juglandaceae) populations using RAPD, ISSR markers and nrDNA ITS regions. Erwerbs-Obstbau 2023, 65, 311–320. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Wei, S.; Liu, X.; He, J.; Zhang, W.; Du, J. Trichosanthes kirilowii Maxim seed kernel oil: The optimization of ultrasound-assisted extraction and evaluation of its potential as a novel biodiesel feedstock. Sustain. Chem. Pharm. 2023, 31, 100903. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, X.; Fan, C.; Wu, W.; Zhang, W.; Wang, Y. Health benefits, food applications, and sustainability of microalgae-derived N-3 PUFA. Foods 2022, 11, 1883. [Google Scholar] [CrossRef] [PubMed]
- Panda, C.; Varadharaj, S.; Voruganti, V.S. PUFA, genotypes and risk for cardiovascular disease. Prostaglandins Leukot. Essent. Fat. Acids 2022, 176, 102377. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Gou, X.; Xu, G.; Tang, X.; Zhou, J.; He, X.; Li, C. Study on free radical scavenging activity of Trichosanthes kirilowii seed oil. Food Sci. 2008, 29, 77–79. [Google Scholar]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Wu, S.; Jiang, L.; Lei, L.; Fu, C.; Huang, J.; Hu, Y.; Dong, Y.; Chen, J.; Zeng, Q. Crosstalk between G-quadruplex and ROS. Cell Death Dis. 2023, 14, 37. [Google Scholar] [CrossRef]
- Chen, C.; Fu, W.; Chang, Q.; Zheng, B.; Zhang, Y.; Zeng, H. Moisture distribution model describes the effect of water content on the structural properties of lotus seed resistant starch. Food Chem. 2019, 286, 449–458. [Google Scholar] [CrossRef]
- Liu, C.; Lin, Q.; He, J. Methods and terminology of study on seed morphology from China. Acta Bot. Sin. 2004, 24, 178–188. [Google Scholar]
- Doyle, J.; Doyle, J. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- de Boer, H.J.; Schaefer, H.; Thulin, M.; Renner, S.S. Evolution and loss of long-fringed petals: A case study using a dated phylogeny of the snake gourds, Trichosanthes (Cucurbitaceae). BMC Evol. Biol. 2012, 12, 108. [Google Scholar] [CrossRef]
- Desai, S.N.; Jadhav, A.J.; Holkar, C.R.; Pawar, B.G.; Pinjari, D.V. Extraction and microencapsulation of Buchanania lanzan Spreng seed oil. Chem. Pap. 2022, 76, 3521–3530. [Google Scholar] [CrossRef]
- GB5009.168-2016; National Food Safety Standard-Determination of Fatty Acids in Foods. National Standard of the People’s Republic of China. Standards Press of China: Beijing, China, 2016.
- Gu, J.; Li, Q.; Liu, J.; Ye, Z.; Feng, T.; Wang, G.; Wang, W.; Zhang, Y. Ultrasonic-assisted extraction of polysaccharides from Auricularia auricula and effects of its acid hydrolysate on the biological function of Caenorhabditis elegans. Int. J. Biol. Macromol. 2021, 167, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, W.; Zhu, C.; Gao, X.; Chu, W. Evaluation of antioxidative and neuroprotective activities of total flavonoids from sea buckthorn (Hippophae rhamnoides L.). Front. Nutr. 2022, 9, 861097. [Google Scholar] [CrossRef] [PubMed]
- Büchter, C.; Koch, K.; Freyer, M.; Baier, S.; Saier, C.; Honnen, S.; Wätjen, W. The mycotoxin beauvericin impairs development, fertility and life span in the nematode Caenorhabditis elegans accompanied by increased germ cell apoptosis and lipofuscin accumulation. Toxicol. Lett. 2020, 334, 102–109. [Google Scholar] [CrossRef]
- Sun, M.; Chen, X.; Cao, J.; Cui, X.; Wang, H. Polygonum multiflorum Thunb extract extended the lifespan and healthspan of Caenorhabditis elegans via DAF-16/SIR-2.1/SKN-1. Food Funct. 2021, 12, 8774–8786. [Google Scholar] [CrossRef]
- Sohal, R.S.; Brunk, U.T. Lipofuscin as an indicator of oxidative stress and aging. Adv. Exp. Med. Biol. 1989, 266, 17–26. [Google Scholar] [CrossRef]
- Cao, L.; Huang, Y.; Xie, J.; Peng, S.; Xu, R.; Zhu, X. Genetic diversity of Trichosanthis Fructus for seeds cultivating. Chin. Tradit. Herb. Drugs 2017, 48, 4316–4322. [Google Scholar]
- Chen, W.; Liu, K.; Cai, X.; Cong, Y. Micromorphology features of seed surface of fourteen species in Impatiens (Balsaminaceae) in relation to their taxonomic significance. Acta Bot. Yunnanica 2017, 29, 625–631. [Google Scholar]
- Huang, L.; Yue, C.; Zheng, J.; Cheng, J. SEM examination on seed coat of Trichosanthes and its taxonomic significance. Bull. Bot. Res. 1999, 19, 298–301. [Google Scholar]
- Zhou, T.; Huang, L.; Cui, G.; Jiang, W.K.; Wang, M. Phylogenetic analysis of Trichosanthes based on the nrDNA ITS region. Bull. Bot. Res. 2010, 30, 568–575. [Google Scholar]
- Hao, D.; Xiao, P. Pharmaceutical resource discovery from traditional medicinal plants: Pharmacophylogeny and pharmacophylogenomics. Chin. Herb. Med. 2020, 12, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Tomé-Rodríguez, S.; Barba-Palomeque, F.; Ledesma-Escobar, C.A.; Miho, H.; Díez, C.M.; Priego-Capote, F. Influence of genetic and interannual factors on the fatty acids profile of virgin olive oil. Food Chem. 2023, 422, 136175. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Chang, K.; Lin, B.; Lin, W.; Su, C.; Tsai, J.; Liao, Y.; Hung, L.; Chang, W.; Chen, B. Oleic acid-induced NOX4 is dependent on ANGPTL4 expression to promote human colorectal cancer metastasis. Theranostics 2020, 10, 7083–7099. [Google Scholar] [CrossRef]
- Xi, X.; Zhu, Y.; Tong, Y.; Yang, X.; Tang, N.; Ma, S. Assessment of the genetic diversity of different Job’s Tears (Coix lacryma-jobi L.) accessions and the active composition and anticancer effect of its seed oil. PLoS ONE 2016, 11, e0153269. [Google Scholar] [CrossRef]
- Guerra-Vazquez, C.M.; Martinez-Avila, M.; Guajardo-Flores, D.; Antunes-Ricardo, M. Punicic acid and its role in the prevention of neurological disorders: A review. Foods 2022, 11, 252. [Google Scholar] [CrossRef]
- Dhavamani, S.; Rao, Y.P.C.; Lokesh, B.R. Total antioxidant activity of selected vegetable oils and their influence on total antioxidant values in vivo: A photochemiluminescence based analysis. Food Chem. 2014, 164, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Kennedy, B.K. Does longer lifespan mean longer healthspan? Trends Cell Biol. 2016, 26, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Zhu, L.; Yen, K.; Tissenbaum, H.A. Uncoupling lifespan and healthspan in Caenorhabditis elegans longevity mutants. Proc. Natl. Acad. Sci. USA 2015, 112, E277–E286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lv, T.; Li, M.; Xue, T.; Liu, H.; Zhang, W.; Ding, X.; Zhuang, Z. Anti-aging effect of polysaccharide from Bletilla striate on nematode Caenorhabditis elegans. Pharmacogn. Mag. 2015, 11, 449–454. [Google Scholar] [CrossRef]
- Lin, H.; Gao, Y.; Zhang, C.; Ma, B.; Wu, M.; Cui, X.; Wang, H. Autophagy regulation influences β-Amyloid toxicity in transgenic Caenorhabditis elegans. Front. Aging Neurosci. 2022, 14, 885145. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, P.M.; Mc Intyre, S.A.; Thomas, D.C. Beyond the extra respiration of phagocytosis: NADPH oxidase 2 in adaptive immunity and inflammation. Front. Immunol. 2021, 12, 733918. [Google Scholar] [CrossRef]
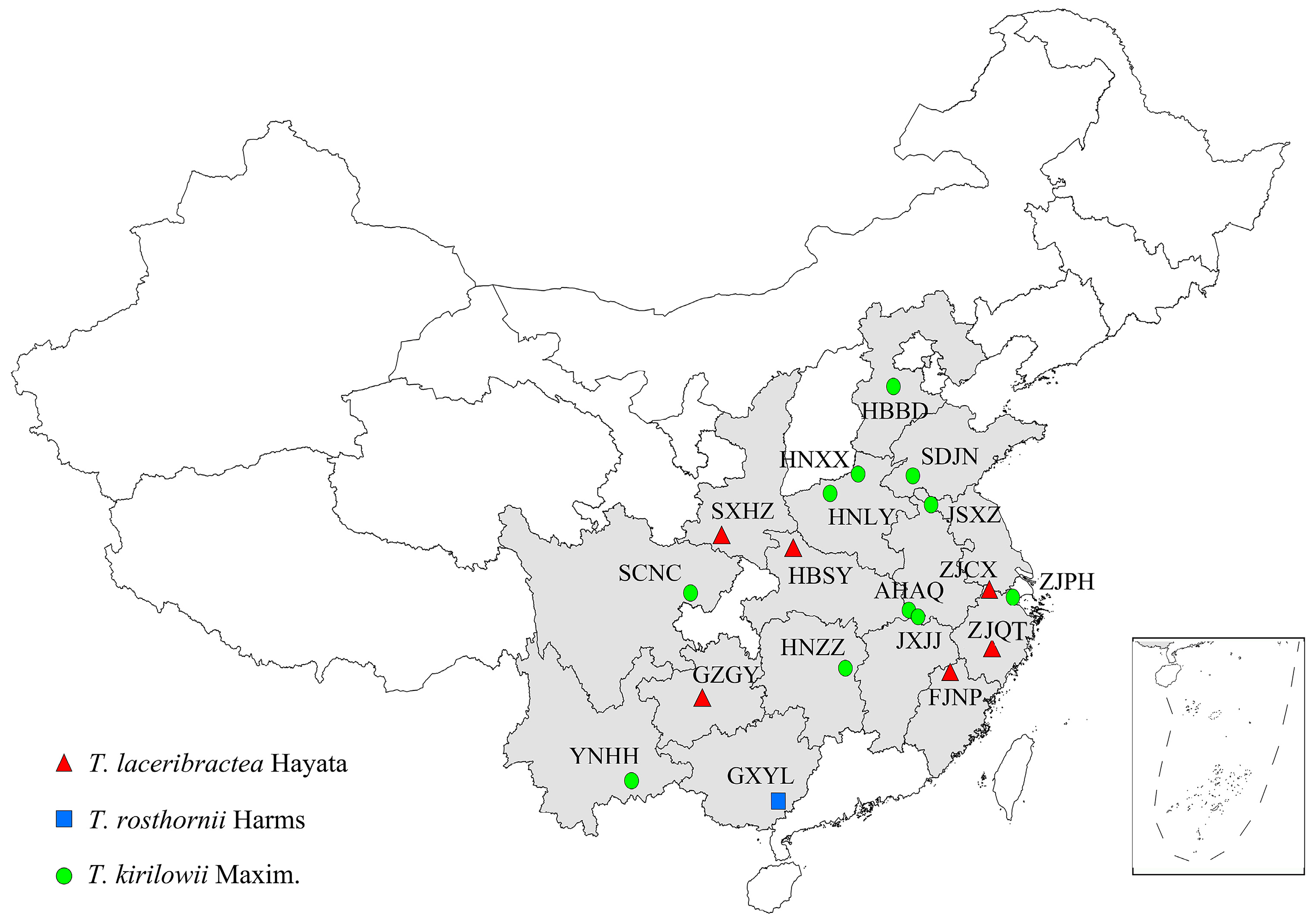
| Code | Geographic Localities | Longitude (E) Latitude (N) | Species | GenBank Accession Number |
|---|---|---|---|---|
| GZGY | Guiyang, Guizhou Province | 106°37′22.73″ (E) 26°40′43.14″ (N) | T. laceribractea Hayata | OR043672 |
| ZJCX | Changxing, Zhejiang Province | 119°54′36.40″ (E) 31°01′35.87″ (N) | T. laceribractea Hayata | OR043673 |
| ZJQT | Qingtian, Zhejiang Province | 120°17′22.38″(E) 28°08′23.53″(N) | T. laceribractea Hayata | OR043674 |
| SXHZ | Hanzhong, Shanxi Province | 107°01′54.98″ (E) 33°04′4.22″ (N) | T. laceribractea Hayata | OR043675 |
| FJNP | Nanping, Fujian Province | 118°07′13.54″ (E) 27°19′54.30″ (N) | T. laceribractea Hayata | OR043676 |
| HBSY | Shiyan, Hubei Province | 110°48′46.26″ (E) 32°35′30.30″ (N) | T. laceribractea Hayata | OR043677 |
| GXYL | Yulin, Guangxi Province | 110°03′4.50″ (E) 22°34′46.45″ (N) | T. rosthornii Harms | OR043666 |
| ZJPH | Pinghu, Zhejiang Province | 121°0′57.82″ (E) 30°40′33.06″ (N) | T. kirilowii Maxim. | OR043979 |
| HNXX | Xinxiang, Henan Province | 113°54′21.53″ (E) 35°22′18.48″ (N) | T. kirilowii Maxim. | OR043980 |
| AHAQ | Anqing, Anhui Province | 116°7′44.94″ (E) 30°9′12.96″ (N) | T. kirilowii Maxim. | OR043981 |
| SDJN | Jining, Shandong Province | 116°35′47.36″ (E) 35°24′29.52″ (N) | T. kirilowii Maxim. | OR043983 |
| JXJJ | Jiujiang, Jiangxi Province | 116°0′5.18″ (E) 29°42′19.80″ (N) | T. kirilowii Maxim. | OR043984 |
| YNHH | Honghe, Yunnan Province | 103°22′32.16″ (E) 23°21′51.19″ (N) | T. kirilowii Maxim. | OR043985 |
| JSXZ | Xuzhou, Jiangsu Province | 117°11′7.94″ (E) 34°17′17.63″ (N) | T. kirilowii Maxim. | OR043986 |
| HNZZ | Zhuzhou, Hunan Province | 113°10′27.19″ (E) 27°51′22.86″ (N) | T. kirilowii Maxim. | OR043987 |
| SCNC | Nanchong, Sichuan Province | 106°07′8.00″ (E) 30°46′53.50″ (N) | T. kirilowii Maxim. | OR043988 |
| HBBD | Baoding, Hebei Province | 115°27′31.50″ (E) 38°52′39.25″ (N) | T. kirilowii Maxim. | OR043989 |
| HNLY | Luoyang, Henan Province | 112°30′38.81″ (E) 34°42′15.52″ (N) | T. kirilowii Maxim. | OR043982 |
| Code | Qualitative Characteristics | Quantitative Characteristics | Microscopic Characteristics | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Color | Elliptical Zone with Uneven Edge | Obvious Linear Ridge | Umbilicus-End Is Flat | Length (mm) | Width (mm) | Thickness (mm) | 100-Seed Weight (g) | Mesh Area (μm2) | Width of the Lines of the Reticulum (μm) | |
| GZGY | Chocolate brown | Yes | Yes | No | 14.11 ± 0.82 H–J | 7.27 ± 0.53 G | 4.68 ± 0.47 BC | 19.91 ± 0.42 KL | 1570.51 ± 39.24 E | 1.361 ± 0.054 H |
| ZJCX | Chocolate brown | Yes | Yes | No | 13.54 ± 0.97 IJ | 7.42 ± 0.82 G | 4.63 ± 0.44 C | 22.42 ± 0.33 HI | 2792.78 ± 51.86 B | 0.903 ± 0.007 I |
| ZJQT | Chocolate brown | Yes | Yes | No | 15.27 ± 1.85 FG | 8.45 ± 0.20 EF | 5.57 ± 0.48 A | 32.93 ± 0.39 B | 2950.39 ± 44.28 A | 0.803 ± 0.014 IJ |
| SXHZ | Chocolate brown | Yes | Yes | No | 16.00 ± 0.79 EF | 8.57 ± 0.51 EF | 5.40 ± 1.03 AB | 27.64 ± 0.51 C–E | 1601.48 ± 35.5 E | 0.841 ± 0.065 IJ |
| FJNP | Chocolate brown | Yes | Yes | No | 14.67 ± 0.68 G–I | 8.52 ± 0.66 EF | 4.65 ± 0.58 BC | 26.90 ± 0.43 DE | 2291.83 ± 118.30 C | 0.631 ± 0.048 K |
| HBSY | Chocolate brown | Yes | Yes | No | 14.26 ± 1.47 H-J | 8.20 ± 1.47 FG | 4.69 ± 1.01 BC | 20.03 ± 0.57 J-L | 1882.51 ± 85.11 D | 2.593 ± 0.098 C |
| GXYL | Dark brown | No | Yes | Yes | 14.72 ± 1.30 F–H | 9.90 ± 1.04 B–D | 3.82 ± 0.50 DE | 21.18 ± 0.46 I–K | 517.89 ± 22.04 I | 3.074 ± 0.037 B |
| AHAQ | Dark brown | No | Yes | No | 18.38 ± 1.55 AB | 10.64 ± 0.65 B | 4.72 ± 0.66 BC | 28.06 ± 0.76 CD | 2161.85 ± 113.66 C | 0.729 ± 0.053 JK |
| HNZZ | Dark brown | No | No | No | 18.21 ± 0.99 AB | 9.92 ± 0.66 B–D | 4.41 ± 0.23 CD | 37.83 ± 0.45 A | 1020.60 ± 43.68 FG | 1.642 ± 0.052 EF |
| ZJPH | Brown | No | No | No | 14.71 ± 0.85 G–I | 10.18 ± 1.00 BC | 4.47 ± 0.38 CD | 20.62 ± 0.33 I–L | 1140.17 ± 88.12 F | 2.035 ± 0.079 D |
| SDJN | Brown | No | No | No | 16.44 ± 0.76 D–F | 9.05 ± 0.57 D–F | 4.35 ± 0.74 CD | 21.38 ± 0.49 I–K | 1488.44 ± 14.78 E | 1.516 ± 0.041 FG |
| JXJJ | Brown | No | No | No | 17.67 ± 0.79 B–D | 9.26 ± 0.48 C–E | 4.17 ± 0.58 C–E | 23.79 ± 0.37 GH | 881.65 ± 24.51 G | 1.656 ± 0.033 E |
| JSXZ | Brown | No | No | No | 13.90 ± 0.93 IJ | 9.04 ± 0.70 D–F | 4.15 ± 0.67 C–E | 21.93 ± 1.79 H–J | 1875.18 ± 54.52 D | 2.024 ± 0.022 D |
| SCNC | Brown | No | No | No | 14.06 ± 1.15 H–J | 9.33 ± 0.97 C–E | 4.60 ± 0.45 C | 24.52 ± 1.54 FG | 1804.22 ± 14.90 D | 4.240 ± 0.086 A |
| HBBD | Brown | No | No | No | 17.83 ± 0.57 A–C | 9.67 ± 0.97 CD | 3.73 ± 0.74 DE | 19.14 ± 0.65 GH | 1458.33 ± 52.88 E | 2.152 ± 0.091 D |
| YNHH | Light brown | No | Yes | No | 19.09 ± 1.39 A | 11.73 ± 0.93 A | 4.37 ± 0.61 CD | 28.87 ± 0.43 C | 910.82 ± 9.61 G | 1.655 ± 0.025 EF |
| HNLY | Light brown | No | No | No | 13.29 ± 0.93 J | 8.40 ± 0.27 EF | 4.45 ± 0.43 CD | 26.04 ± 0.28 EF | 1014.93 ± 45.09 FG | 1.626 ± 0.048 E–G |
| HNXX | Light brown | No | Yes | No | 16.72 ± 1.50 C–E | 9.59 ± 0.68 CD | 3.58 ± 0.75 E | 18.83 ± 0.32 L | 691.58 ± 45.29 H | 1.494 ± 0.027 GH |
| Code | Oil Yield (%) | UFA (%) | PA (%) | SA (%) | OA (%) | LA (%) | TA (%) |
|---|---|---|---|---|---|---|---|
| YNHH | 48.88 ± 0.34 DE | 92.75 ± 0.16 A | 4.17 ± 0.09 DEF | 2.60 ± 0.06 I | 6.98 ± 0.19 K | 39.46 ± 0.23 AB | 45.53 ± 0.59 A |
| JSXZ | 54.82 ± 0.27 AB | 92.73 ± 0.29 A | 3.76 ± 0.15 FGH | 2.87 ± 0.11 GH | 16.48 ± 0.64 J | 37.94 ± 0.99 BCD | 37.68 ± 1.96 B |
| JXJJ | 47.53 ± 0.88 FG | 92.59 ± 0.47 A | 4.01 ± 0.48 EFG | 2.79 ± 0.03 HI | 22.39 ± 1.16 G | 34.67 ± 0.27 FGH | 34.79 ± 1.38 BCD |
| HNLY | 48.37 ± 0.07 D–F | 92.58 ± 0.05 A | 3.78 ± 0.04 FGH | 3.07 ± 0.01 FG | 24.70 ± 0.21 F | 34.87 ± 0.04 FG | 32.50 ± 0.21 DEF |
| HNXX | 45.12 ± 0.36 H | 92.55 ± 0.06 AB | 4.22 ± 0.03 DEF | 2.73 ± 0.02 HI | 6.22 ± 0.06 K | 35.88 ± 0.09 EF | 49.19 ± 0.28 A |
| AHAQ | 47.88 ± 0.68 E–G | 92.19 ± 0.05 ABC | 3.20 ± 0.04 H | 4.02 ± 0.04 BC | 32.43 ± 0.27 B | 33.30 ± 0.17 GHI | 25.88 ± 0.41 GH |
| ZJPH | 48.31 ± 0.65 D–G | 91.94 ± 0.05 A–D | 4.11 ± 0.03 D–G | 3.42 ± 0.02 DE | 20.42 ± 0.19 H | 37.04 ± 0.02 CDE | 33.63 ± 0.14 B–E |
| SCNC | 47.42 ± 0.38 FG | 91.67 ± 0.22 BCD | 4.48 ± 0.11 DE | 3.22 ± 0.11 DEF | 17.29 ± 0.56 IJ | 36.32 ± 0.22 DEF | 37.22 ± 0.44 BC |
| HBBD | 44.24 ± 0.50 H | 91.62 ± 0.04 CD | 3.84 ± 0.01 FG | 3.99 ± 0.03 BC | 28.70 ± 0.25 CD | 33.66 ± 0.07 GHI | 28.63 ± 0.37 FGH |
| SDJN | 55.61 ± 0.66 A | 91.60 ± 0.11 CD | 4.45 ± 0.06 DE | 3.45 ± 0.04 D | 19.07 ± 0.11 HI | 38.55 ± 0.12 BC | 33.31 ± 0.13 CDE |
| GXYL | 42.28 ± 0.48 I | 91.50 ± 0.05 CD | 4.70 ± 0.03 D | 3.17 ± 0.02 EF | 16.48 ± 0.08 J | 37.78 ± 0.18 BCD | 37.01 ± 0.32 BC |
| HNZZ | 49.33 ± 0.36 D | 91.20 ± 0.05 D | 3.52 ± 0.02 GH | 4.71 ± 0.03 A | 42.58 ± 0.31 A | 29.82 ± 0.17 J | 18.32 ± 0.21 I |
| ZJCX | 48.89 ± 0.54 DE | 89.65 ± 0.26 E | 6.90 ± 0.18 BC | 3.09 ± 0.06 FG | 29.13 ± 0.36 C | 32.92 ± 0.44 IH | 26.72 ± 1.16 GH |
| ZJQT | 44.92 ± 0.44 H | 89.65 ± 0.10 E | 6.57 ± 0.05 C | 3.44 ± 0.05 DE | 26.30 ± 0.09 EF | 33.08 ± 0.01 IH | 29.58 ± 0.26 E–H |
| GZGY | 44.29 ± 0.49 H | 89.24 ± 0.08 E | 6.58 ± 0.05 C | 3.80 ± 0.03 C | 22.64 ± 0.17 G | 32.62 ± 0.24 IH | 33.66 ± 0.50 B–E |
| SXHZ | 52.39 ± 0.45 C | 89.16 ± 0.97 E | 7.25 ± 0.64 B | 3.24 ± 0.29 DEF | 26.25 ± 2.15 EF | 36.07 ± 2.21 EF | 25.54 ± 5.23 H |
| FJNP | 54.23 ± 0.36 B | 87.97 ± 0.76 F | 7.46 ± 0.49 B | 4.21 ± 0.24 B | 27.07 ± 1.55 DE | 33.13 ± 0.90 IH | 26.95 ± 3.22 GH |
| HBSY | 47.08 ± 0.41 G | 87.38 ± 0.43 F | 8.20 ± 0.25 A | 4.03 ± 0.16 BC | 15.81 ± 0.63 J | 40.40 ± 0.87 A | 29.99 ± 1.67 EFG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Li, S.; Zhu, Y.; Zhu, R.; Du, X.; Cui, X.; Wang, H.; Cheng, Z. Effect of Different Edible Trichosanthes Germplasm on Its Seed Oil to Enhance Antioxidant and Anti-Aging Activity in Caenorhabditis elegans. Foods 2024, 13, 503. https://doi.org/10.3390/foods13030503
Wang W, Li S, Zhu Y, Zhu R, Du X, Cui X, Wang H, Cheng Z. Effect of Different Edible Trichosanthes Germplasm on Its Seed Oil to Enhance Antioxidant and Anti-Aging Activity in Caenorhabditis elegans. Foods. 2024; 13(3):503. https://doi.org/10.3390/foods13030503
Chicago/Turabian StyleWang, Wenqian, Shan Li, Yunguo Zhu, Ruilin Zhu, Xiling Du, Xianghuan Cui, Hongbing Wang, and Zhou Cheng. 2024. "Effect of Different Edible Trichosanthes Germplasm on Its Seed Oil to Enhance Antioxidant and Anti-Aging Activity in Caenorhabditis elegans" Foods 13, no. 3: 503. https://doi.org/10.3390/foods13030503
APA StyleWang, W., Li, S., Zhu, Y., Zhu, R., Du, X., Cui, X., Wang, H., & Cheng, Z. (2024). Effect of Different Edible Trichosanthes Germplasm on Its Seed Oil to Enhance Antioxidant and Anti-Aging Activity in Caenorhabditis elegans. Foods, 13(3), 503. https://doi.org/10.3390/foods13030503






