Abstract
Notwithstanding the increased interest in wild edible plants, little is known on how some domestic thermal processes can affect their content. The aim of this study was to investigate the amounts of minerals, B1 and B2 vitamins, tocols, and carotenoids in raw, boiled, and steamed wild edible plants, namely, Sonchus asper (L.) Hill s.l., Sonchus oleraceus L., Cichorium intybus L., and Beta vulgaris L. var cicla. All vegetables were confirmed as high sources of lutein (from 6 to 9 mg/100 g) and β-carotene (from 2 to 5 mg/100 g). Quite high amounts of violaxanthin and neoxanthin were found. Alfa-tocopherol and γ-tocopherol were the main tocols, with same contents in raw and processed vegetables (about 2.5 mg/100 g). The most abundant macro element and trace element were, respectively, potassium and iron. B1 and B2 vitamins were found in low amounts in almost all plants, with the exception of thiamine in Beta vulgaris (about 1.6 mg/100 g). Boiling led to a significant loss of minerals (up to 60%) and B-group vitamins (up to 100%), while, among carotenoids, it only affected violaxanthin levels (up to 90%). Steamed vegetables showed only a slight reduction, about 20%, in β-carotene and lutein, with a marked decrease in violaxanthin and neoxanthin. One hundred grams of all fresh and cooked plants can be claimed as a source of vitamin A and E.
Keywords:
wild edible plants; vitamin activity; tocols; carotenoids; B-group vitamins; minerals; boiling; steaming 1. Introduction
The human body cannot produce minerals and vitamins; therefore, they need to be supplied by food. Wild edible plants (WEPs) have been recognised to have health effects against several chronic disorders [1]. WEPs have high amounts of fibre, proteins, and several minerals and provide high contents of bioactive compounds, such as polyphenols, vitamins, tocols, and carotenoids, which play an important therapeutic and nutritional role [1,2,3,4].
In this regard, some minerals are the main components of bones, while others participate to several life processes. Among B-group vitamins, thiamine (vitamin B1) acts as a co-factor to assist in the activity of different enzymes in wholegrains, cereals, nuts, seeds, liver, and pulses. Riboflavin (vitamin B2) is involved in flavoprotein enzymes and, apart by leafy green vegetables, good amounts are provided by milk products and whole cereals [1,5].
Carotenoids are pigments divided into carotenes and xanthophylls. Carotenes are precursors of retinol, better known as vitamin A. Among xanthophylls, lutein and zeaxanthin are antioxidants. The available evidence suggests that lutein is associated with the reduction of the development of chronic diseases and, even if with controversial results, and has a beneficial impact on the visual function [6,7]. In higher plants, essential photoprotective functions have been assigned to lutein in the heat dissipation of excess light energy [8]. Different carotenoids, such as α-carotene, β-carotene, β-cryptoxanthin, lutein, and epoxycarotenoids, in their free or esterified forms, are provided in the diet through yellow/orange fruits and vegetables, such as green leafy ones [1,5,9,10,11].
Tocols are structurally similar compounds. The most common ones are tocopherols, namely, α-tocopherol (α-T), β-tocopherol (β-T), γ-tocopherol (γ-T), and δ-tocopherol (δ-T), and tocotrienols, namely, α-tocotrienol (α-T3), β-tocotrienol (β-T3), γ-tocotrienol (γ-T3), and δ-tocotrienol (δ-T3). According to recent evidence, only α-tocopherol seems to exhibit vitamin E activity and it is still unclear whether other tocol molecules can prevent vitamin E avitaminosis in humans as α-T does [12]. Studies have shown that a dietary intake of tocols helps to prevent the occurrence of neoplasms and heart diseases [12,13]. Foods containing the highest contents of tocols are vegetables, in particular seeds and derived oils [13].
Almost all green leafy vegetables are consumed after different domestic processes, such as boiling, steaming, frying, and microwaving. This leads to the degradation of heat-sensitive compounds and/or the leaching of water-soluble nutrients in the cooking water, with the consequent loss of bioactive constituents, such as carotenoids, polyphenols, and certain vitamins and minerals. The extent of loss depends on the type of vegetable, genotype, structure, compound organization in the food matrix, and cooking methods [14,15,16,17,18]. The degree of these changes may be influenced by temperature and time conditions, oxygen occurrence, water, light, pH, and the presence of other antioxidants [18,19,20]. Some plant enzymes (such as lipoxygenase) also accelerate compound degradation [21]. When subjected to heat treatments, carotenoids and tocols may degrade and thus undergo a disruption of their molecular structure. In particular, the cis-isomerization of carotenoids is one of the most frequent reactions [22]. Published papers on fruits and vegetables show the impact of different cooking processes on bioactive compounds, with controversial results. While, in some cases, a decrease in nutrients is found [14,18,23], in others, an increase is reported. This finding is due to the liberation of bound compounds, resulting in a higher bioavailability and extractability [14,15,16,17]. Some research studies attribute these increases to the loss of moisture and the leaching of soluble solids in the cooking water, which concentrate the sample per unit weight, when data are expressed on a dry weight basis [23,24].
In recent years, the attention on WEPs has quickly grown. However, there is still little information on wild plants of popular consumption, especially in terms of nutritional changes caused by domestic cooking. Therefore, the aim of the present work was to investigate the amounts of minerals, vitamin B1 and B2, carotenoids, and tocols of raw and in-house processed wild green leafy vegetables.
The basal leaves of four WEPs, Sonchus oleraceus L., Sonchus asper (L.) Hill s.l., Beta vulgaris L. var. cicla, and Cichorium intybus L., were investigated as raw and after boiling and steaming treatments. Spinacia oleracea L. was used as a control green leafy vegetable. The contribution to the vitamin activity of the investigated compounds and the percentage of the recommended daily allowance (RDA) were also taken into consideration.
The findings of this study not only could be of interest for a nutritional evaluation, but also for generating awareness among consumers regarding the extent to which certain common domestic thermal treatments could induce changes in some water-soluble and fat-soluble bioactives.
2. Materials and Methods
2.1. Plant Material
The basal leaves of four WEPs were investigated: Sonchus oleraceus L., Sonchus asper (L.) Hill s.l., Beta vulgaris L. var. cicla (chard), and Cichorium intybus L. (chicory). The investigated WEPs were chosen on the basis of their wide diffusion and mode of consumption in the south of Italy, according to [25]. WEPs were collected, during spring, in 2021, in Italy, in particular in the regions of Molise (Ripalimosani-CB) and Campania (Circello-BN). Spinacia oleracea L. (spinach) was bought in local markets. For each sample, a minimum of 1 kg was gathered. Damaged parts and foreign bodies were removed, and some leaves were immediately stored at −20 °C in order to be analysed as raw.
2.2. Samples Preparation and Cooking Treatments
On fresh leaves of each vegetable two cooking treatments, traditional boiling and steaming, were carried out. The conditions used for cooking were those described in Fratianni et al. [23]. Three repetitions were carried out on each sample, for each type of cooking treatment. One hundred grams of fresh leaves were boiled in 1 L of boiling water (vegetable to water ratio 1:10) for 10 min, according to the common conditions used in the literature [26] and their consumption [23]. Steaming was carried out by placing, on a rack, 100 g of leaves over boiling water, in a closed water bath for 10 min. Aliquots of raw samples, boiled, and steamed samples were freeze-dried (Genesis 25SES freeze-dryer, VirTis Co., Gardiner, NY, USA). The freeze-dried samples were milled using a water-cooled mill (IKA®-Werke GmbH & Co. KG, Staufen, Germany), to avoid the overheating of the mass, and stored, under vacuum, at −20 °C until analysis. The moisture of raw, thermally processed, and freeze-dried leaves was determined by measuring weight loss after heating the samples at 130 °C [27].
2.3. Chemicals
Solvents, at the highest purity, and other analytical grade reagents were acquired from Sigma (Sigma Aldrich, St. Louis, MO, USA). Alfa-carotene, 9-cis-β-carotene, 13-cis-β-carotene, violaxanthin, and neoxanthin standards were purchased from CaroteNature (Lupsingen, Switzerland); lutein, zeaxanthin, and β-cryptoxanthin were acquired from Extrasynthese (Z.I. Lyon-Nord, Genay, France). All-trans-β-carotene, thiamine, and riboflavin were purchased from Sigma Chemicals; α, β, γ, and δ-tocopherol standards were acquired from Merck (Darmstadt, Germany); α, β, γ, and δ-tocotrienol standards were obtained as in Panfili et al. [28].
2.4. Determination of Minerals
Macro and trace elements were determined in each sample, according to the procedure of Kawashima and Valente Soares [29] with some modifications. Two grams of each sample were weighed into polytetrafluoroethylene digestion vessels, and then 5 mL HNO3 (65%) and 1 mL H2O were added. The vessels were digested in a microwave digestion system (MARS 6, CEM, Matthews, NC, USA), using the following digestion program: (1) ramp to reach 120 °C and holding for 10 min; (2) ramp to reach 180 °C and holding for 10 min; and (3) ramp to reach 210 °C and holding for 10 min. Before each digestion, the vessels were decontaminated with 50% HNO3 + 20% HF + 20% HCl in the microwave digestion system, rinsed with bi-distillate water, and air-dried. At the end of the procedure, after appropriate dilutions with bi-distilled water, the samples were analysed using Inductively Coupled Plasma Optical Emission Spectroscopy (iCAP 6200 DUO, Thermo Scientific, Waltham, MA, USA), and Ca, Fe, K, Mg, P, Na, Cu, Zn, and Se content was determined. A multi-element certified stock solution (Periodic table mix 3 for ICP; Merck, Darmstadt, Germany) was used to prepare the calibration standards for the measurement using ICP-OES. Spinach leaf standard reference material (NIST-1570A, Sigma Aldrich, St. Louis, MO, USA) was analysed to verify the analytical quality of the data (Table S1).
2.5. Thiamine and Riboflavin Analysis
A total of 0.4 g of sample was weighted in 100 mL volumetric flasks, and 2 mL of 0.1 N HCl was added, followed by heating in a water bath at 100 °C for 30 min as in [5]. The extraction was carried out according to Hasselmann et al. [30]. Each sample was analysed in triplicate. Thiamine and riboflavin were identified and quantified by means of known solutions of their corresponding standards. The analytical quality of the data was verified through the analysis of a certified reference vegetable sample (BCR®-485, Sigma Aldrich, St. Louis, MO, USA) and a reference wholemeal flour sample (BCR®-121, Sigma Aldrich, St. Louis, MO, USA) (Table S1).
2.6. Determination of Tocols
Tocols were extracted on 0.1 g of a freeze-dried sample, using the method of alkaline hydrolysis of the food matrix [28], which, from the literature, has been proven as the most effective extraction method of some bioactives [12]. The residues were suspended in a n-hexane:isopropyl alcohol solution (99:1 v/v). A HPLC Dionex system (Dionex, Sunnyvale, CA, USA), equipped with an Ultimate 3000 Pump, was used. The chromatographic separation of compounds was performed using a 5 µm Luna column, with a silica stationary phase (250 mm × 4.6 mm i.d.) (Phenomenex, Torrance, CA, USA). A n-hexane:ethyl acetate:acetic acid solution (97.3:1.8:0.9 v/v/v) was used as the mobile phase, at a flow rate of 1.6 mL/min. Tocols were detected using a RF 2000 spectrofluorimeter (Dionex, Sunnyvale, CA, USA), set at an excitation and emission wavelength of 290 nm and 330 nm, respectively, and identified and quantified through known standard solutions. Data were processed using a Dionex Chromeleon Version 6.6 chromatography system (Dionex, Sunnyvale, CA, USA). Vitamin E activity was expressed as tocopherol equivalent (TE), calculated as in Sheppard et al. [31]. The performances of the analytical method are reported in [28]. The analysis of α-tocopherol from a certified material (fortified breakfast cereal, NIST-SRM 3233, Sigma Aldrich, St. Louis, MO, USA) is shown in Table S1.
2.7. Determination of Carotenoids
Carotenoids were extracted by using the saponification method of Fratianni et al. [23], under the conditions already reported for tocols. Compounds were analysed through a normal (for xanthophylls) and a reverse phase HPLC (for carotenes). A HPLC Dionex (Sunnyvale, CA, USA) analytical system, consisting of a 50 µL injector loop (Rheodyne, Idex Health & Science, Northbrook, IL, USA) and an Ultimate 3000 pump system, was used. For the reverse phase, the mobile phase was methanol:methylterbutylether:water, at a flow rate of 1 mL/min., under a gradient profile as in Mouly et al. [32], by using a 5 µm C30 YMC (Hampsted, NC, USA) stainless steel column (250 × 4.6 mm i.d.). Samples were suspended in methanol/methylterbutylether 50:50 (v/v). Under normal phase conditions, the mobile phase was n-hexane:isopropyl alcohol, in a multilinear gradient elution from 10% (A) to 20% (B) of isopropyl alcohol in n-hexane, as reported elsewhere [23]. A 5 µm Luna column, with a silica stationary phase (250 mm × 4.6 mm i.d.), was used (Phenomenex, Torrance, CA, USA). Data were processed using a Dionex Chromeleon Version 6.6 chromatography system (Sunnyvale, CA, USA). Carotenoids were spectrophotometrically detected at 450 nm. Spectral characteristics and the comparison of retention times with those of available standards were used to identify carotenoids. Known standard solutions were used for carotenoid quantification. Vitamin A activity was expressed as retinol equivalent (RE), calculated as in EFSA [33], considering carotene amounts. The performances of the analytical method, together with recovery tests and the analysis of a reference certified vegetable sample (BCR®-485), are reported in [10,34].
2.8. Statistical Analysis
For raw leaves and for each cooking treatment, three samples were investigated (biological replicates). Each sample was analysed in triplicate (technical replicates). Results are reported as the average of three determinations on three samples. Statistical data are expressed as mean ± standard deviation. An ANOVA test was applied to the data, using a Statistical Software Package (IBM SPSS Statistic 26 for Windows) (SPSS Inc., Chicago, IL, USA). Statistical significance was attributed to p values < 0.05 and was obtained by using a least significant difference (LSD) test.
3. Results and Discussion
3.1. Levels of Minerals
All vegetables were a good source of nutritional minerals (Table 1). Amounts of mineral constituents in vegetable leaves could be variable, depending on the species, environmental conditions, location, and parameters of the applied thermal processing [3,18,29]. Despite the variability, potassium was considerably higher than sodium, as is common in plant foods. The highest levels of K were found in Sonchus and spinach leaves (on average, 460 mg/100 g), so 100 g of these vegetables were able to cover 15% of the RDA, and could be declared as a source of this element [35]. The investigated Sonchus plants and spinach were a good source of Na (on average, about 130 mg/100 g), while poor levels were found for chicory (1 mg/100 g). With the exception of the lower values of S. oleraceus, the mean Ca content was about 70 mg/100 g. P (about 30–70 mg/100 g) and Mg contents (ranges 15–65 mg/100 g) were all in quite good amounts. The levels of trace elements did not exceed 5 mg/100 g, but it is worth noting that 100 g of all plants were able to fulfil 100% of the RDA for Se [35]. The average values were in the range of those found in the literature, even if with slight differences [1,3,4,29]. In particular, in Sonchus oleraceus, Na ranged from 44 to 270 mg/100 g, K from 320 to 790 mg/100 g, and Ca from 130 to 230 mg/100 g. For trace elements, Fe ranged from 0.6 to 1.2 mg/100 g and Zn from 0.5 to 0.9 mg/100 g [3]. Similar values were highlighted for Sonchus asper for Na (85–211 mg/100 g), K (440–630 mg/100 g), Ca (80–175 mg/100 g), Fe (2–4 mg/100 g), and Zn (0.8–1 mg/100 g) [1]. In the analysed chicory sample, lower Na levels were found compared to the published data (20–270 mg/100 g), while the contents of K and Ca fell within the lower limit of the reported ranges (50–4000 mg/100 g and 45–2000 mg/100 g, respectively). Similar amounts of Fe (0–2.0 mg/100 g) and Zn (0.1–0.5 mg/100 g) were found [1,3,4,29]. Comparing the analysed Beta vulgaris sample to a similar genus, such as Beta maritima L., the latter showed higher levels of Na and K (45–288 mg/100 g and 597–2356 mg/100 g, respectively) and lower Ca contents (19–25 mg/100 g). Fe and Zn were in the reported ranges (1.4–4.0 mg/100 g and 0.6–1.3 mg/100 g, respectively) [4]. Finally, for spinach, the values from the literature were similar to the investigated ones, about 95, 500, and 65 mg/100 g for Na, K, and Ca, respectively, 1.0 mg/100 for Fe, and 0.3 mg/100 g for Zn [29].

Table 1.
Levels of minerals in raw, boiled, and steamed green leafy vegetables (mg/100 g f.w.).
Boiling affected mineral contents (20–60% decreases), with the highest extents for K and Mg (40–60%). Ca losses ranged from 15% in chicory to 50% in S. asper; those of P, Fe, Zn, and Se ranged from 30 to 40%. The overall results did not change if expressed on dry weight. In the literature, a different behaviour has been observed for minerals and trace elements during thermal treatments, due to the different forms in which they are present in the plant tissues [29,36]. Notwithstanding losses as to raw samples, 15% of the RDA for Se and K was still covered, respectively, by 100 g and 150 g of the vegetables. The decrease in nutrients during boiling is caused by leaching or diffusion [18]. With the exception of K in spinach and chicory (about 40% losses), steaming did not significantly influence the mineral content (p < 0.05).
3.2. Content of Carotenoids
The main identified carotenoids were violaxanthin, neoxanthin, lutein, zeaxanthin, β-cryptoxanthin, α-carotene, 13-cis-β-carotene, β-carotene, and 9-cis-β-carotene. Table 2 reports the amounts of the individual and total carotenoids (TC), expressed as mg/100 g of fresh weight (f.w.), in fresh, boiled, and steamed samples. The amounts of carotenoids found refer to total compounds (free, esterified, and linked to the food matrix), due to the method of alkaline hydrolysis of the food matrix and solvent extraction used.

Table 2.
Carotenoid content in raw, boiled, and steamed green leafy vegetables (mg/100 g f.w.).
In all analysed plants, both fresh and cooked ones, the two carotenoids at the highest amounts were lutein and β-carotene. In particular, in raw samples, lutein ranged from about 6 to 9 mg/100 g f.w (on average, 50% of TC), and β-carotene from about 2 to 5 mg/100 g f.w. (on average, 18% of TC in all samples, with the exception of chicory where it was about 33% of TC). There is no defined dietary reference intake for lutein; however, a consumption of 6 to 14 mg of lutein per day has been proposed to lower the risk of macular degeneration and cataract [37]. Therefore, a daily consumption of two portions (160 g) [38] of the investigated green vegetables could fulfil an intake ≥ 10 mg/day. Quite high amounts of violaxanthin and neoxanthin were also found, ranging from 0.7 mg/100 g in S. oleraceus to 1.6 mg/100 g in B. vulgaris, with a combined average contribution of 16% of TC. The overall data are of the same order of magnitude as those found in different green leafy vegetables, even though they were obtained without the use of the alkaline hydrolysis method. Such data are reported for spinach, regarding epoxycarotenoids [39], lutein (from 0.2 to 8.7 mg/100 g), and β-carotene (from 0.02 to 4.6 mg/100 g) [9,40,41], chard (about 2 mg/100 g of lutein and β-carotene) [40], and chicory, for lutein, β-carotene (in the ranges of 1.8–8.4 mg/100 g and 1.0–4.9 mg/100 g, respectively), and expoxycarotenoids [40,42]. Similar results are also reported in other studies, where the total compound content was analysed in Sonchus [5], spinach, chard [10], and chicory [43].
The epoxycarotenoids violaxanthin and neoxanthin were the main molecules that, after domestic treatment, underwent the highest significant reduction. In particular, after boiling, violaxanthin losses significantly ranged from about 20% (S. asper) to 90% (B. vulgaris), while significant decreases in neoxanthin were observed only in B. vulgaris (60%). Greater losses were observed after steaming: in particular, violaxanthin was the most susceptible carotenoid, with decreases ranging from about 40% (Sp. oleracea) to 100% (S. asper and C. intybus). Losses of neoxanthin were observed to a lesser extent (up to 60% in B. vulgaris). Similar results were obtained when data were expressed on dry weight, considering solid losses, as in Fratianni et al. [23]. The high susceptibility of epoxycarotenoids to thermal treatment has been proven in vegetable foods [44].
Considering the other investigated carotenoids, boiling did not cause significant decreases (p < 0.05). On the contrary, a small increase was observed, in some plants, for zeaxanthin and α-carotene. In our case, the complete extraction on the investigated compounds due to the alkaline hydrolysis of the food matrix excluded the increment of their extractability due to heat treatment. As already demonstrated by the authors in a recent paper [23], the leaching of soluble solids after domestic cooking could be responsible for their apparent gain in cooked vegetables. Therefore, considering solid losses, compounds were not statistically affected by boiling, if data were expressed on dry weight [23]. Steaming caused a slight significant decrease in β-carotene, in almost all vegetables (on average, about 20%), and lutein, only in S. asper (about 20%) and Sp. oleracea (about 10%). Data are in accordance with some studies in which losses were lower during boiling than under other domestic treatments, like steaming [14,23,26]. This behaviour could be ascribed to the activities of some oxidative enzymes that may affect compounds early in the process, where thermal conditions were not sufficient for their inactivation [26,45]. No increases in cis isomers were found under any of the applied domestic treatments [22,41]. The loss of epoxycarotenoids caused a corresponding slight significant decrease in total carotenoids, after steaming, in all vegetables (Table 2). From the literature, the effect of different food treatments on carotenoids is difficult to evaluate, due to the various process parameters that could affect their levels, such as temperature, heating time, oxygen, light, pH, and the type of food matrix [14,15,16,17,19], but, also, due to the different analytical procedures carried out for their determination [23].
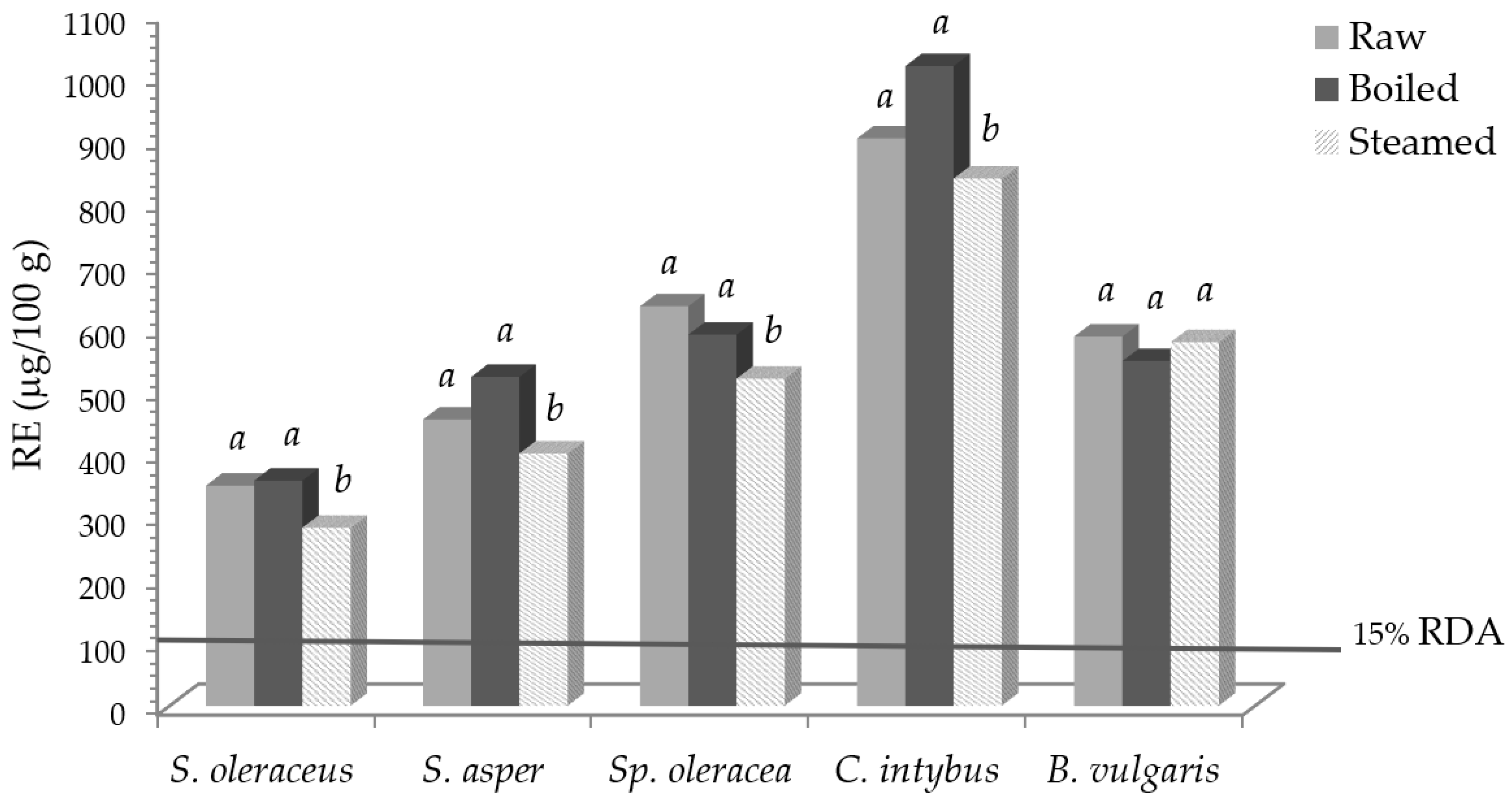
Vitamin A activity (RE) and the required percentage of the recommended daily allowance (RDA), before and after domestic cooking, are reported in Figure 1.
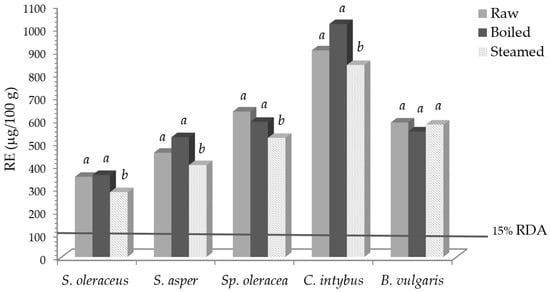
Figure 1.
Vitamin A activity (RE) in leafy vegetables before and after domestic cooking and the expressed percentage of the RDA. Different letters in the same vegetable indicate a statistically significant difference among treatments at p < 0.05.
Taking into account values for vitamin A, expressed as retinol equivalent (RE) [33], and its recommended daily allowance (RDA) of 800 μg/day of RE [35], 100 g of fresh S. oleraceus, S. asper, Sp. Oleracea, C. intybus, and B. vulgaris contributed to 44, 57, 79, 122, and 73% of the RDA, respectively. Notwithstanding the reported slight carotenoid losses, an intake of 100 g of some fresh, boiled, and steamed vegetables was able to cover 15% of the RDA, so, according to [35], they can be declared as “sources of vitamin A”.
3.3. Contents of Tocols and B-Group Vitamins
The contents of single and total tocols, thiamine, and riboflavin, expressed as mg/100 g f.w, are given in Table 3.

Table 3.
Tocol and B-group vitamin content in raw, boiled, and steamed green leafy vegetables (mg/100 g f.w.).
Riboflavin was found at low amounts in all plants. S. oleraceus, S. asper, spinach, and chicory had good levels of thiamine, while chard had the highest amounts, able to cover 100% of the RDA [35]. Few data are reported on B-complex vitamins in WEPs; however, what was found is quite in accordance with the published literature on some green leafy vegetables [1,5]. Almost a total reduction was observed for both vitamins after boiling, with the exception of chard (40% for B1 and 75% for B2). The main reasons for the reduction in thiamine and riboflavin are ascribed to their water solubility and degradation during thermal processing [18].
In all investigated samples only tocopherols were found, as also reported, for similar vegetables, in different papers [1,5,23,43,46]. In particular, α-tocopherol was the main detected compound (on average, 2.2 mg/100 g), representing 79% of total tocopherols in C. intybus, 87% in S. oleraceus and S. asper, and 90% in Sp. oleracea and B. vulgaris. Beta-tocopherol (β-T) was detected at low amounts, exclusively in B. vulgaris, while γ-tocopherol (γ-T) was found in all vegetables, with contents, in raw samples, ranging from 0.2 mg/100 g (B. vulgaris) to 0.9 mg/100 g (C. intybus). Total tocol content was similar in all investigated plants, with an average of 2.5 mg/100 g. Boiling and steaming did not significantly affect single and total tocol amounts. These findings did not change if data were expressed on dry weight, considering weight losses, as reported in Fratianni et al. [23]. A different behaviour has been observed in the literature for tocols, after thermal treatments, depending on the same factors already cited for carotenoids [12,14,23,46].
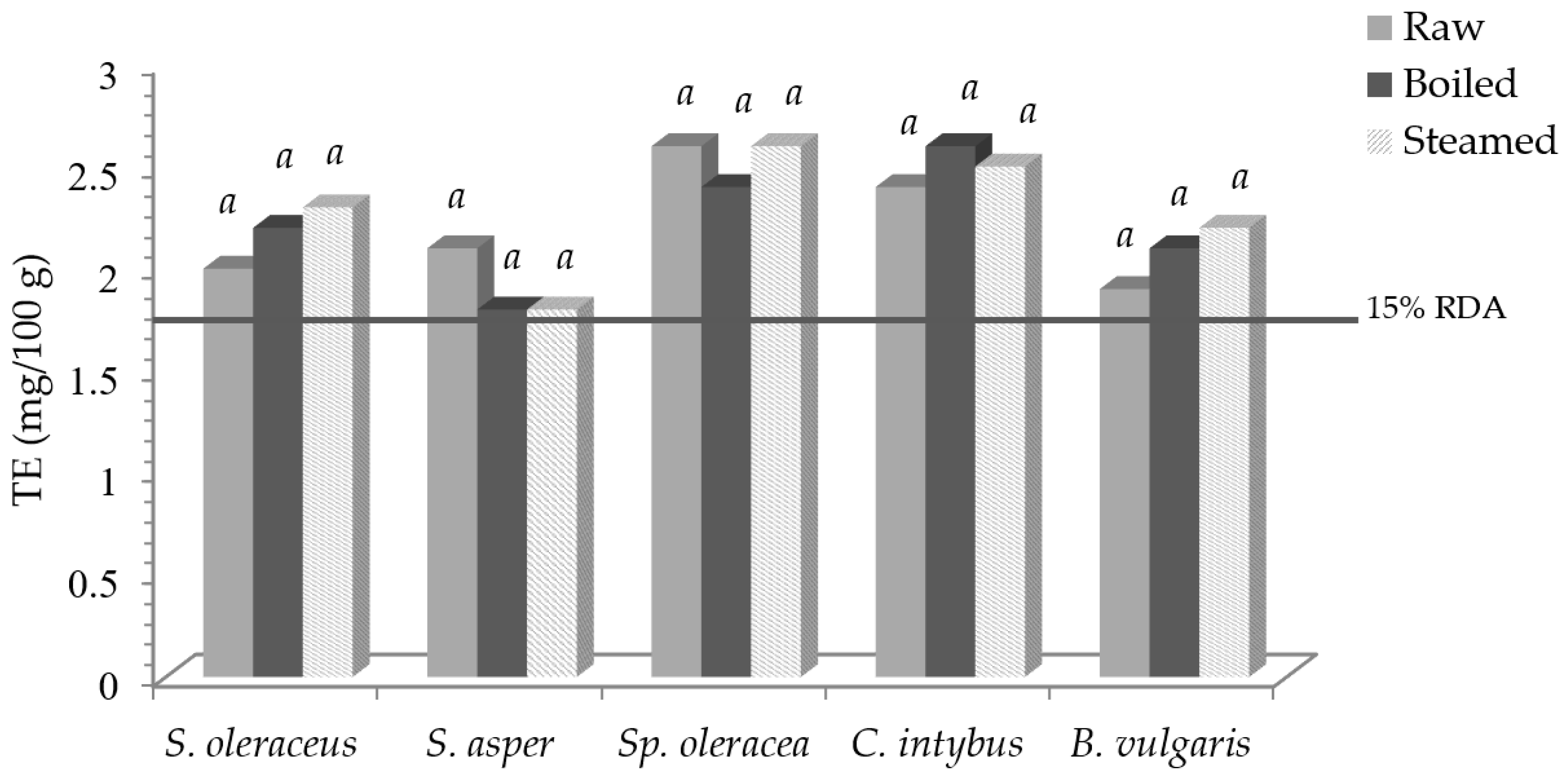
Figure 2 reports vitamin E activity, expressed as tocopherol equivalent (TE), and the expected percentage of the RDA.
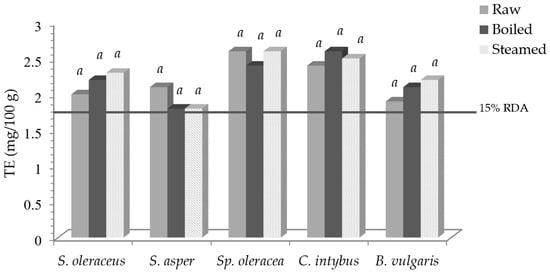
Figure 2.
Vitamin E activity (TE) in leafy vegetables before and after domestic cooking and the expected percentage of the RDA. Same letters in the same vegetable indicate a not statistically significant difference among treatments at p ≥ 0.05.
Taking into account the RDA for vitamin E, which is 12 mg/day of TE [35], 100 g of fresh S. oleraceus, S. asper, Sp. oleracea, C. intybus, and B. vulgaris contributed to 17, 18, 22, 26, and 16% of the RDA, respectively, with not significant variations as to the cooked products. Therefore, an intake of 100 g of fresh, boiled, and steamed samples was able to cover 15% of the RDA, so, according to [35], all vegetables can be claimed to be “sources of vitamin E”.
4. Conclusions
The results from this work confirm that the investigated wild vegetables represent a good source of minerals and tocols, mostly in the form of α-tocopherol, and a relevant source of carotenoids, being important contributors of lutein and β-carotene. The amounts of the less studied carotene isomers (α-carotene, 9-cis, and 13-cis-β-carotene) and of the epoxycarotenoids violaxanthin and neoxanthin are also given. Since some of the investigated WEPs can be declared as a source of vitamins and minerals, they can contribute to the realization of different functional foods. Boiling affects the content of vitamin B1 and B2 and the amount of minerals. Further research should be carried out to find the proper conditions, such as the vegetable/water ratio, to reduce their leaching. The contents of tocols and carotenoids are not significantly affected by boiling, while a slight carotenoid decrease is observed after steaming. Even giving less contribution to total carotenoids, the epoxycarotenoids show a major susceptibility to thermal treatments. This evidence could suggest their use as process and product indicators, to ascertain the impacts of thermal treatments on food and find the proper process parameters to minimize the extent of losses. Considering the overall findings, they could help to establish appropriate additional databases of bioactive compound content in wild plants, either raw or cooked, mainly where the existing knowledge is absent or weak. This can be the case of most published data on liposoluble compounds, like tocols and carotenoids, that could be intimately associated with the vegetable matrix. Without using an extraction procedure able to recover them from the food matrix, data could not refer to their total amounts, giving a wrong estimation of their real content.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods13030472/s1, Table S1: Bias (%) for Ca, P, K, Na, Cu, Zn, α-tocopherol, B1 and B2 vitamins as to values of certified reference materials.
Author Contributions
Conceptualization, A.F.; Methodology, D.A., P.A. and G.P.; Validation, A.F. and G.P.; Formal analysis, G.I., C.V. and F.M.; Investigation, A.F., D.A., G.I., C.V., F.M. and P.A.; Data curation, A.F., D.A., G.I., C.V., F.M. and P.A.; Writing—original draft, G.I. and C.V.; Writing—review & editing, A.F., D.A. and G.P.; Supervision, A.F. and G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tardío, J.; Sánchez-Mata, M.C.; Morales, R.; Molina, M.; García-Herrera, P.; Morales, P.; Díez-Marqués, C.M.; Fernández-Ruiz, V.; Càmara, M.; Pardo-de-Santayana, M.; et al. Ethnobotanical and food composition monographs of selected Mediterranean wild edible plants. In Mediterranean Wild Edible Plants. Ethnobotany and Food Composition Tables; Tardío, J., Sánchez-Mata, M.C., Eds.; Springer: New York, NY, USA, 2016; pp. 273–470. [Google Scholar] [CrossRef]
- Southon, S.; Faulks, R. Health benefits of increased fruit and vegetable consumption. In Fruit and Vegetable Processing: Improving Quality, 9th ed.; Jongen, W., Ed.; Woodhead Publishing Ltd.: Cambridge, UK, 2002; p. 521. [Google Scholar]
- Herrera, P.G.; Sánchez-Mata, M.C.; Cámara, M.; Fernández-Ruiz, V.; Díez-Marqués, C.M.; Molina, M.J.; Tardío, J. Nutrient composition of six wild edible Mediterranean Asteraceae plants of dietary interest. J. Food Comp. Anal. 2014, 34, 163–170. [Google Scholar] [CrossRef]
- Clemente-Villalba, J.; Burló, F.; Hernández, F.; Carbonell-Barrachina, Á.A. Valorization of wild edible plants as food ingredients and their economic value. Foods 2023, 12, 1012. [Google Scholar] [CrossRef] [PubMed]
- Panfili, G.; Niro, S.; Bufano, A.; D’Agostino, A.; Fratianni, A.; Paura, B.; Cinquanta, L. Bioactive compounds in wild Asteraceae edible plants consumed in the Mediterranean diet. Plant Foods Hum. Nutr. 2020, 75, 540–546. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Scientific Opinion on the substantiation of health claims related to lutein and maintenance of normal vision (ID 1603, 1604, further assessment) pursuant to Article 13(1) of Regulation (EC) No 1924/20061. EFSA J. 2012, 10, 2716. [Google Scholar]
- Mitra, S.; Rauf, A.; Tareq, A.M.; Jahan, S.; Emran, T.B.; Shahriar, T.G.; Dhama, K.; Alhumaydhi, F.A.; Aljohani, A.S.M.; Rebezov, M.; et al. Potential health benefits of carotenoid lutein: An updated review. Food Chem. Toxicol. 2021, 154, 112328. [Google Scholar] [CrossRef] [PubMed]
- Jahns, P.; Holzwarth, A.R. The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim. Biophys. Acta 2012, 1817, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Prasad, K.N.; Kong, K.W.; Jiang, Y.; Ismail, A. Carotenoids and their isomers: Color pigments in fruits and vegetables. Molecules 2011, 16, 1710–1738. [Google Scholar] [CrossRef] [PubMed]
- Fratianni, A.; Mignogna, R.; Niro, S.; Panfili, G. Determination of lutein from fruit and vegetables through an alkaline hydrolysis extraction method and HPLC analysis. J. Food Sci. 2015, 80, 2686–2691. [Google Scholar] [CrossRef]
- Saini, R.K.; Nile, S.H.; Park, S. Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Res. Int. 2015, 76, 735–750. [Google Scholar] [CrossRef]
- Górnaś, P.; Baškirovs, G.; Siger, A. Free and esterified tocopherols, tocotrienols and other extractable and non-extractable tocochromanol-related molecules: Compendium of knowledge, future perspectives and recommendations for chromatographic techniques, tools, and approaches used for tocochromanol determination. Molecules 2022, 27, 6560. [Google Scholar] [CrossRef]
- Shahidi, F.; de Camargo, A.C. Tocopherols and tocotrienols in common and emerging dietary sources: Occurrence, applications, and health benefits. Int. J. Mol. Sci. 2016, 17, 1745. [Google Scholar] [CrossRef]
- Ruiz-Rodriguez, A.; Marín, F.R.; Ocaña, A.; Soler-Rivas, C. Effect of domestic processing on bioactive compounds. Phytochem. Rev. 2008, 7, 345–384. [Google Scholar] [CrossRef]
- Bureau, S.; Mouhoubi, S.; Touloumet, L.; Garcia, C.; Moreau, F.; Bédouet, V.; Renard, C.M.G.C. Are folates, carotenoids and vitamin C affected by cooking? Four domestic procedures are compared on a large diversity of frozen vegetables. LWT Food Sci. Technol. 2015, 64, 735–741. [Google Scholar] [CrossRef]
- Prasanna, K.D.; Gunathilake, P.; Somathilaka Ranaweera, K.K.D.; Vasantha Rupasinghe, H.P. Effect of different cooking methods on polyphenols, carotenoids and antioxidant activities of selected edible leaves. Antioxidants 2018, 7, 117. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, Y.; Lai, S.; Cao, H.; Guan, Y.; Cheang, W.S.; Liu, B.; Zhao, K.; Miao, S.; Riviere, C.; et al. Effects of domestic cooking process on the chemical and biological properties of dietary phytochemicals. Trends Food Sci. Technol. 2019, 85, 55–66. [Google Scholar] [CrossRef]
- Korus, A. Changes in the content of minerals, B-group vitamins and tocopherols in processed kale leaves. J. Food Comp. Anal. 2020, 89, 103464. [Google Scholar] [CrossRef]
- Penicaud, C.; Achir, N.; Dhuique-Mayer, C.; Dornier, M.; Bohuon, P. Degradation of β-carotene during fruit and vegetable processing or storage: Reaction mechanisms and kinetic aspects: A review. Fruits 2011, 66, 417–440. [Google Scholar] [CrossRef]
- Ngamwonglumlert, L.; Devahastin, S.; Chiewchan, N.; Raghavan, V. Plant carotenoids evolution during cultivation, postharvest storage, and food processing: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1561–1604. [Google Scholar] [CrossRef]
- Laus, M.N.; Tozzi, D.; Soccio, M.; Fratianni, A.; Panfili, G.; Pastore, D. Dissection of antioxidant activity of durum wheat (Triticum durum Desf.) grains as evaluated by the new LOX/RNO method. J. Cereal Sci. 2012, 56, 214–222. [Google Scholar] [CrossRef]
- Schieber, A.; Carle, R. Occurrence of carotenoid cis-isomers in food: Technological, analytical, and nutritional implications. Trends Food Sci. Technol. 2005, 16, 416–422. [Google Scholar] [CrossRef]
- Fratianni, A.; D’Agostino, A.; Niro, S.; Bufano, A.; Paura, B.; Panfili, G. Loss or gain of lipophilic bioactive compounds in vegetables after domestic cooking? Effect of steaming and boiling. Foods 2021, 10, 960. [Google Scholar] [CrossRef] [PubMed]
- Bergström, L. Nutrient losses and gains in the preparation of food: NLG project. Food Chem. 1996, 57, 77–78. [Google Scholar] [CrossRef]
- Paura, B.; Di Marzio, P.; Salerno, G.; Brugiapaglia, E.; Bufano, A. Design a database of Italian vascular alimurgic flora (AlimurgITA): Preliminary results. Plants 2021, 10, 743. [Google Scholar] [CrossRef] [PubMed]
- Miglio, C.; Chiavaro, E.; Visconti, A.; Fogliano, V.; Pellegrini, N. Effects of different cooking methods on nutritional and physicochemical characteristics of selected vegetables. J. Agric. Food Chem. 2008, 56, 139–147. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- Panfili, G.; Fratianni, A.; Irano, M. Normal phase high-performance liquid chromatography method for the determination of tocopherols and tocotrienols in cereals. J. Agric. Food Chem. 2003, 51, 3940–3944. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, L.M.; Valente Soares, L.M. Mineral profile of raw and cooked leafy vegetables consumed in Southern Brazil. J. Food Comp. Anal. 2003, 16, 605–611. [Google Scholar] [CrossRef]
- Hasselmann, C.; Franck, D.; Grimm, P.; Diop, P.A.; Soules, C. High-performance liquid chromatographic analysis of thiamin and riboflavin in dietetic foods. J. Micronutr. Anal. 1989, 5, 269–279. [Google Scholar]
- Sheppard, A.J.; Pennington, J.A.T.; Weihrauch, J.L. Analysis and distribution of vitamin E in vegetable oil and foods. In Vitamin E in Health and Disease; Packer, L., Fuchs, J., Eds.; Marcel—Dekker: New York, NY, USA, 1993; pp. 9–31. [Google Scholar]
- Mouly, P.P.; Gaydou, E.M.; Corsetti, J. Determination of the geographical origin of Valencia orange juice using carotenoid liquid chromatographic profiles. J. Chromatogr. A 1999, 844, 149–159. [Google Scholar] [CrossRef]
- EFSA. Scientific opinion on dietary reference values for vitamin A. EFSA J. 2015, 13, 4028. [Google Scholar] [CrossRef]
- Panfili, G.; Fratianni, A.; Irano, M. Improved normal-phase high-performance liquid chromatography procedure for the determination of carotenoids in cereals. J. Agric. Food Chem. 2004, 52, 6373–6377. [Google Scholar] [CrossRef]
- Regulation, E.U. No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers. Annex XIII. Off. J. Eur. Union 2011, 50, 18–63. [Google Scholar]
- García-Herrera, P.; Morales, P.; Cámara, M.; Fernández-Ruiz, V.; Tardío, J.; Sánchez-Mata, M.C. Nutritional and phytochemical composition of Mediterranean wild vegetables after culinary treatment. Foods 2020, 9, 1761. [Google Scholar] [CrossRef]
- Alves-Rodrigues, A.; Shao, A. The science behind lutein. Toxicol. Lett. 2004, 150, 57–83. [Google Scholar] [CrossRef]
- SINU-Società Italiana di Nutrizione Umana. LARN—Livelli di Assunzione di Riferimento di Nutrienti ed Energia per la Popolazione Italiana, 4th ed.; SINU-INRAN, SICS: Milano, Italy, 2014. [Google Scholar]
- Biehler, E.; Alkerwi, A.; Hoffmann, L.; Krause, E.; Guillaume, M.; Lair, M.L.; Bohn, T. Contribution of violaxanthin, neoxanthin, phytoene and phytofluene to total carotenoid intake: Assessment in Luxembourg. J. Food Compost. Anal. 2011, 25, 56–65. [Google Scholar] [CrossRef]
- Reif, C.; Arrigoni, E.; Schärer, H.; Nyström, L.; Hurrell, R.F. Carotenoid database of commonly eaten Swiss vegetables and their estimated contribution to carotenoid intake. J. Food Compost. Anal. 2013, 29, 64–72. [Google Scholar] [CrossRef]
- Dias, M.G.; Borge, G.I.A.; Kljak, K.; Mandić, A.I.; Mapelli-Brahm, P.; Olmedilla-Alonso, B.; Pintea, A.M.; Ravasco, F.; Šaponjac, V.T.; Sereikaitė, J.; et al. European database of carotenoid levels in foods. Factors affecting carotenoid content. Foods 2021, 10, 912. [Google Scholar] [CrossRef]
- Žnidarčič, D.; Ban, D.; Šircelj, H. Carotenoid and chlorophyll composition of commonly consumed leafy vegetables in Mediterranean countries. Food Chem. 2011, 129, 1164–1168. [Google Scholar] [CrossRef]
- Delfine, S.; Fratianni, A.; D’Agostino, A.; Panfili, G. Effects of drought stress on physiological responses and bioactive compounds in chicory (Cichorium intybus L.): Opportunity for a sustainable agriculture. Foods 2022, 11, 3725. [Google Scholar] [CrossRef] [PubMed]
- Melèndez-Martínez, A.J.; Britton, G.; Vicario, I.M.; Heredia, F.J. The complex carotenoid pattern of orange juices from concentrate. Food Chem. 2008, 109, 546–553. [Google Scholar] [CrossRef]
- Severini, C.; Giuliani, R.; De Filippis, A.; Derossi, A.; De Pilli, T. Influence of different blanching methods on colour, ascorbic acid and phenolics content of broccoli. J. Food Sci. Technol. 2016, 53, 501–510. [Google Scholar] [CrossRef]
- Knecht, K.; Sandfuchs, K.; Kulling, S.E.; Bunzel, D. Tocopherol and tocotrienol analysis in raw and cooked vegetables: A validated method with emphasis on sample preparation. Food Chem. 2010, 169, 20–27. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).