Migration of Nutrient Substances and Characteristic Changes of Chicken White Soup Emulsion from Chicken Skeleton during Cooking
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of White Chicken Soup
2.3. Migration of Nutrient Substances
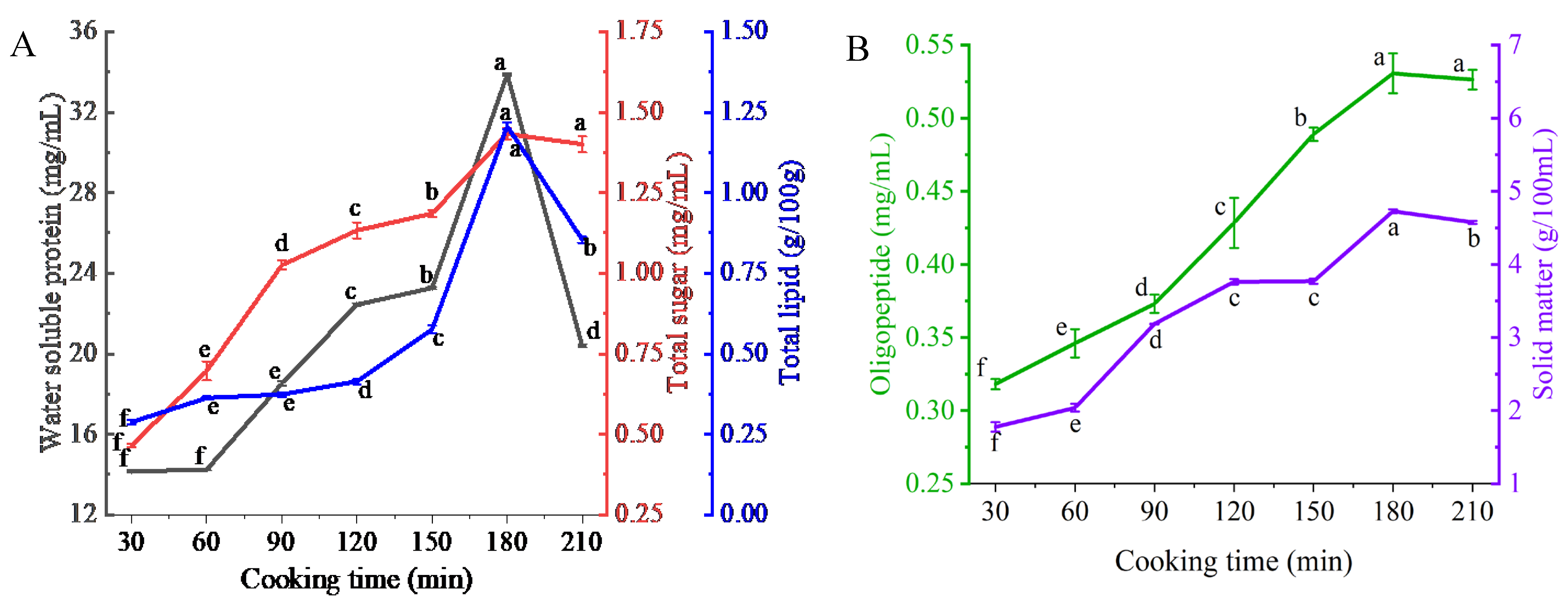
2.3.1. Water-Soluble Proteins
2.3.2. Total Sugars
2.3.3. Solids
2.3.4. Oligopeptides
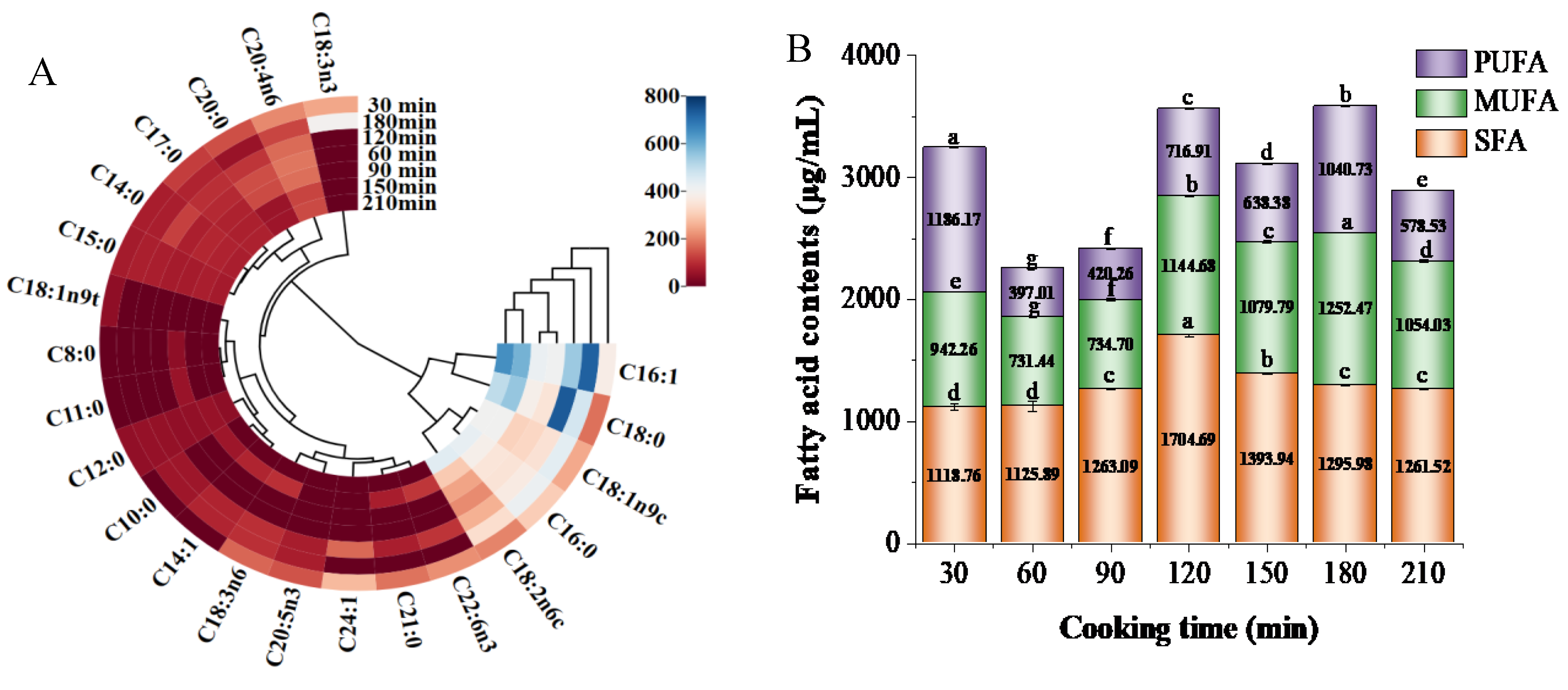
2.3.5. Total Lipids and Composition of the Fatty Acids (FA)
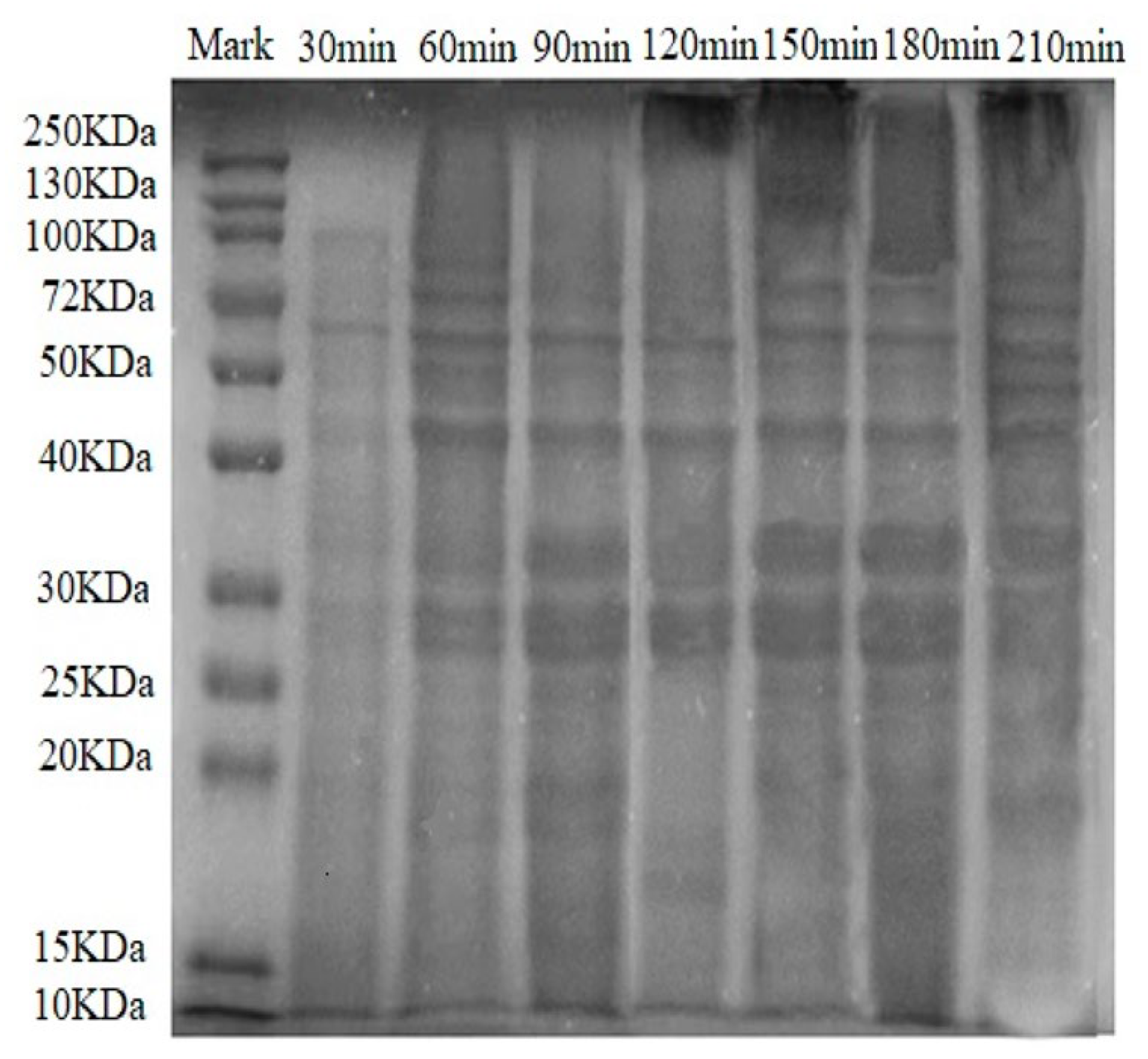
2.3.6. Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.4. Characteristic of White Chicken Soup
2.4.1. Color Analysis
2.4.2. Zeta Potential Analysis
2.4.3. Particle Size
2.4.4. Rheological Measurements
2.4.5. Interfacial Tension and Measurement of the Contact Angle
2.4.6. Physical Stability
2.4.7. Storage Stability
2.5. Sensory Evaluation
2.6. Statistical Analysis
3. Results and Discussion
3.1. Migration of Nutrient Substances
3.2. Composition of Fatty Acids
3.3. SDS-PAGE Analysis
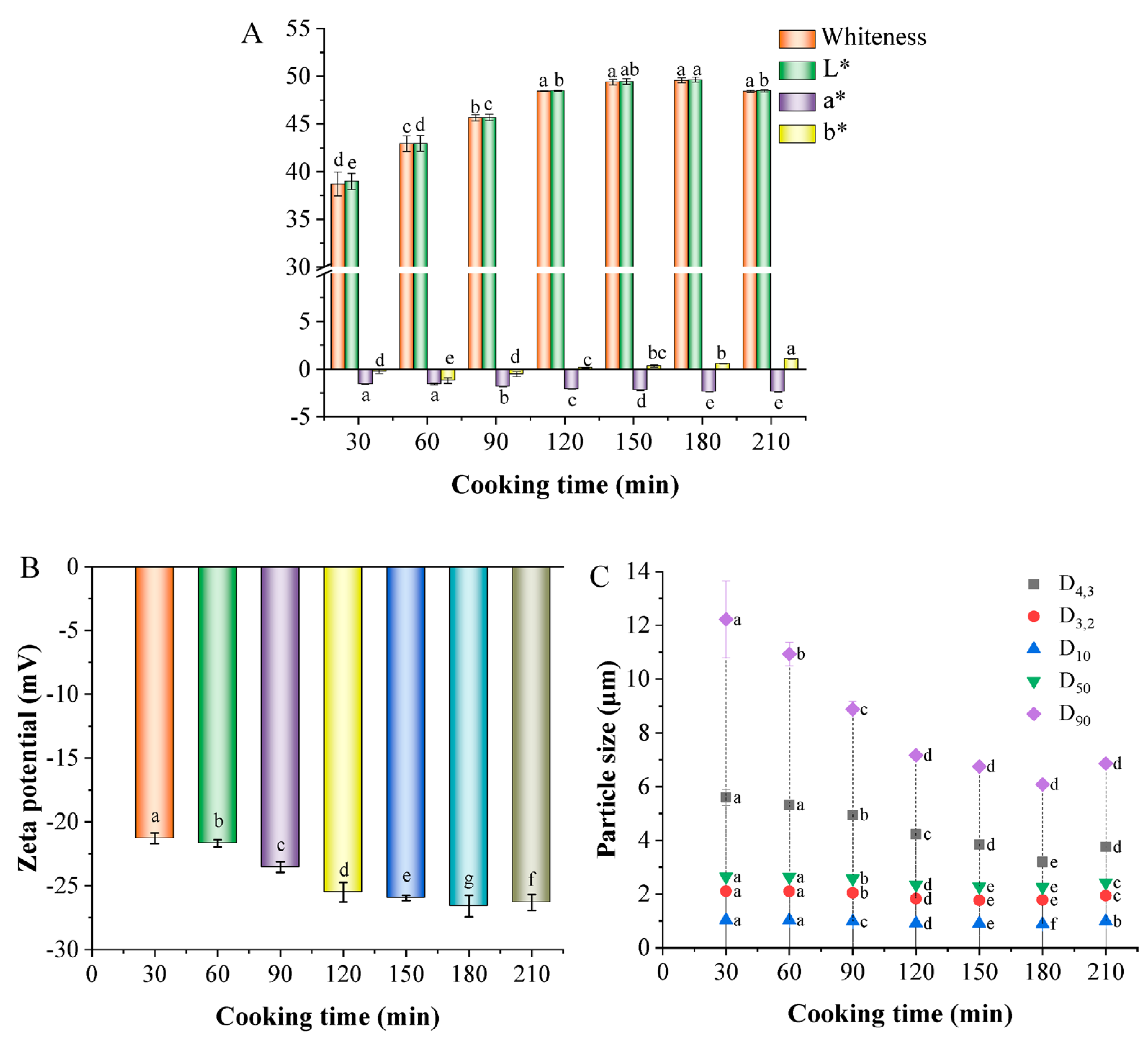
3.4. Characteristics of White Chicken Soup
3.4.1. Color
3.4.2. Zeta Potential
3.4.3. Particle Size
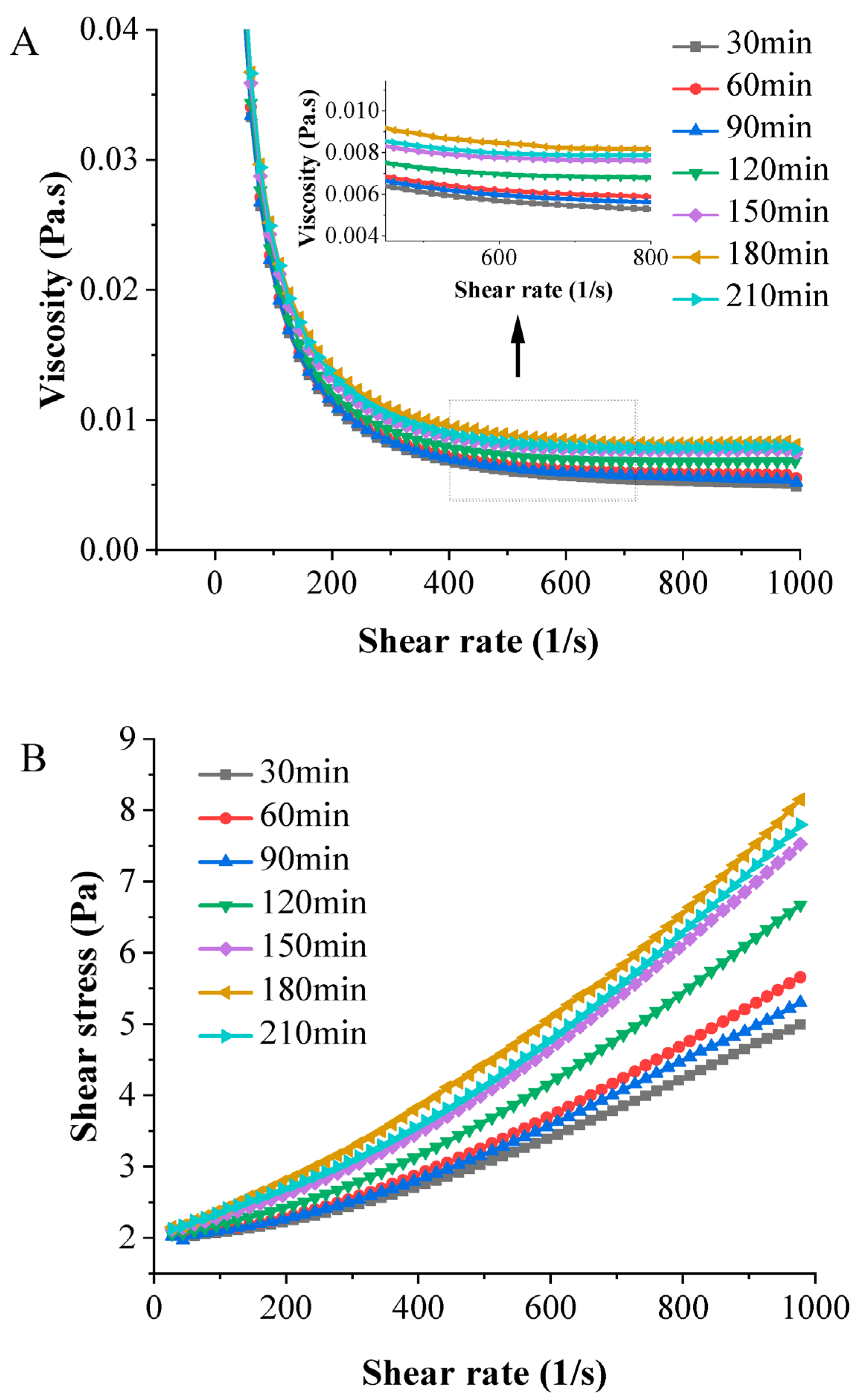
3.4.4. Rheology
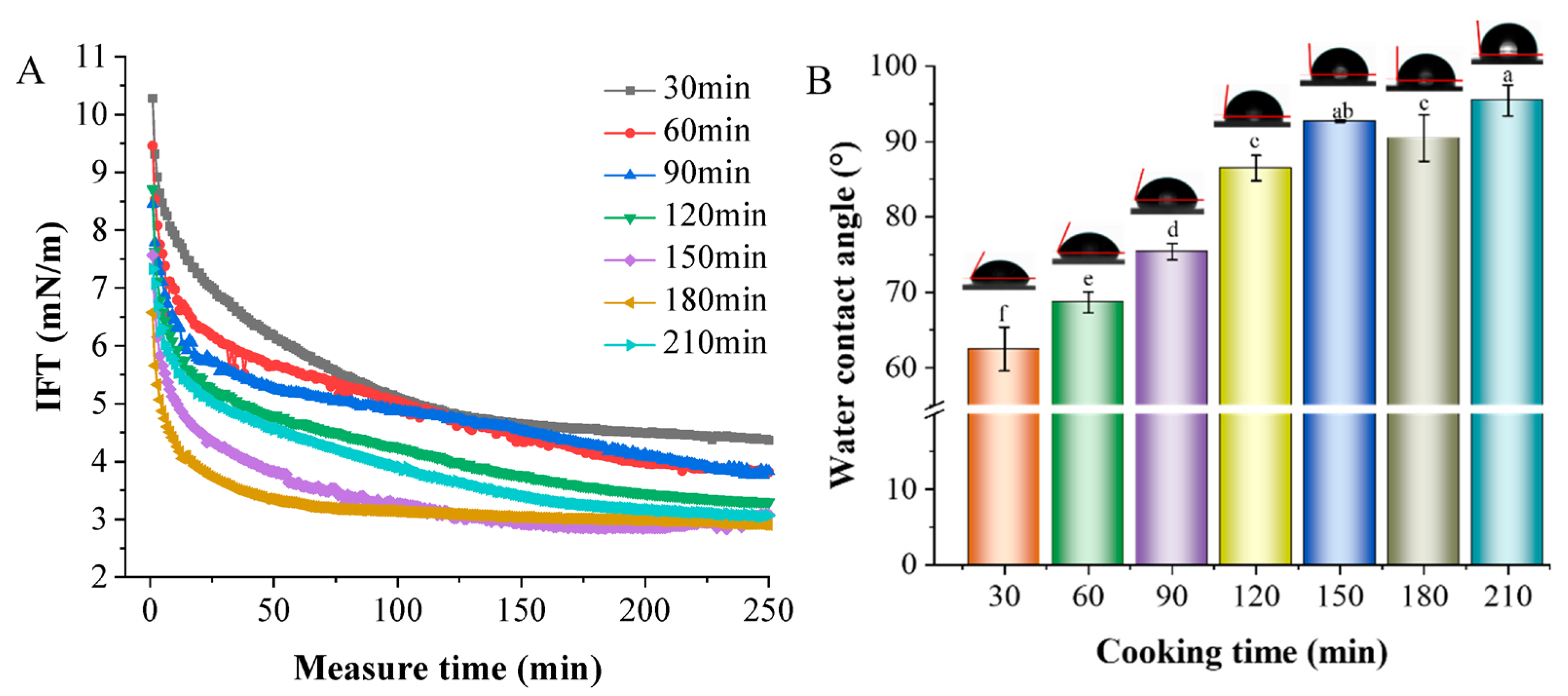
3.4.5. IFT and Contact Angle Analysis
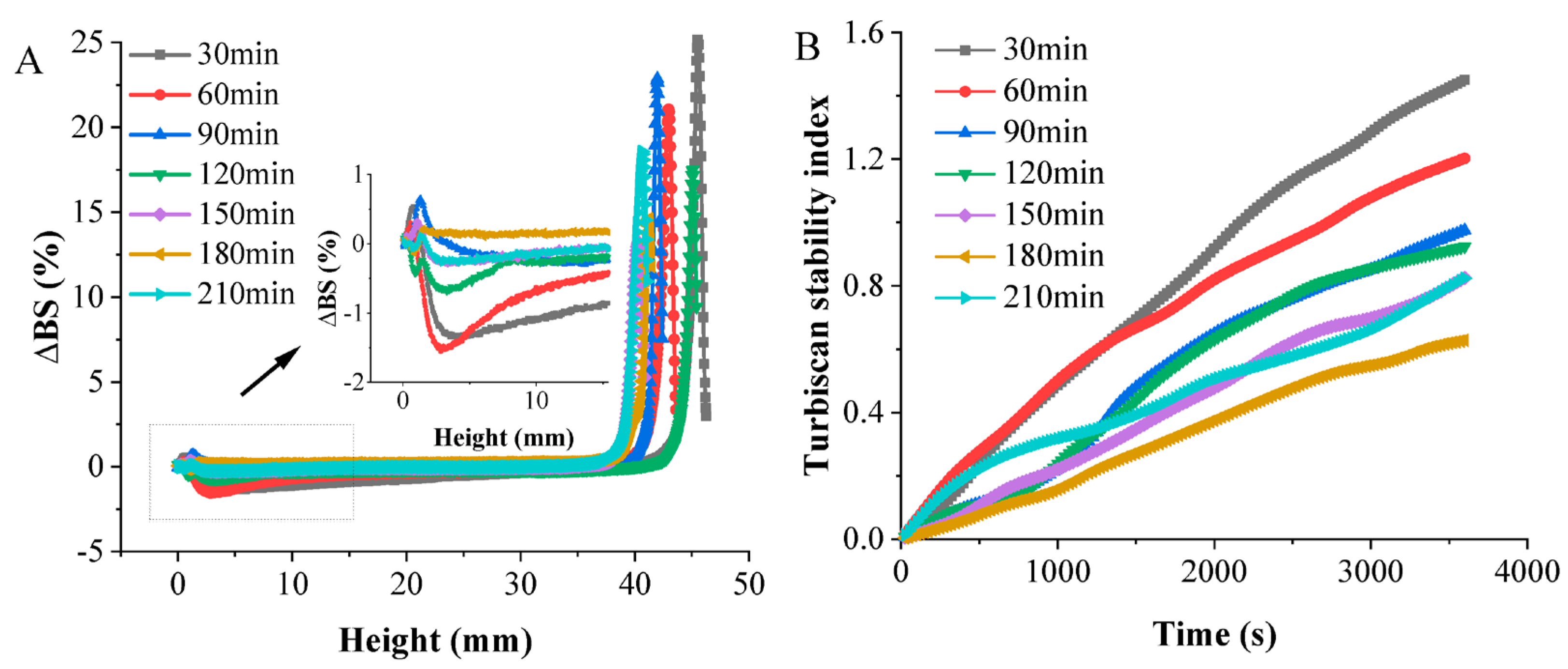
3.4.6. Physical Stability
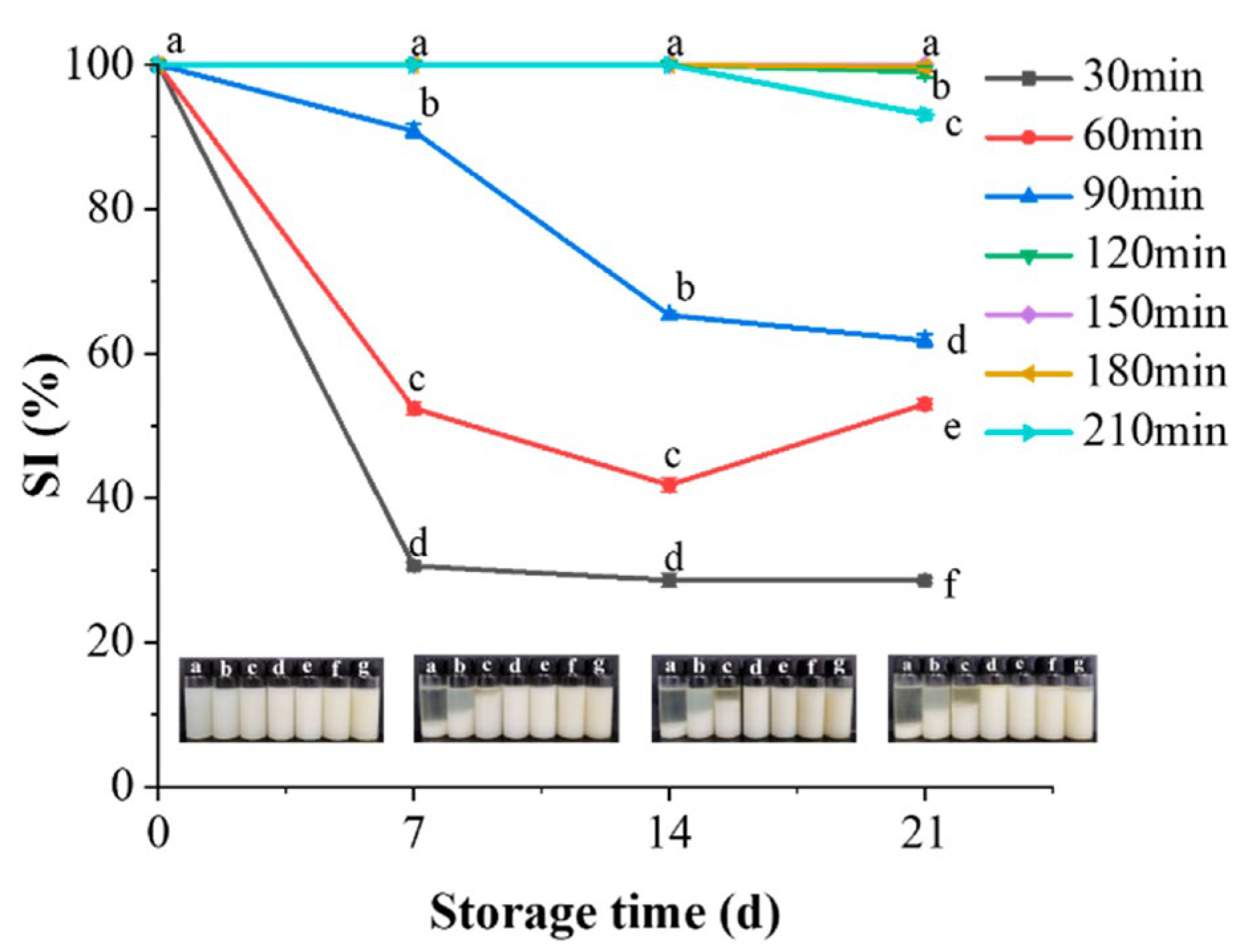
3.4.7. Storage Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, L.; Zhou, C.C.; Zhang, J.C.; Huang, Y.; Zhao, J.; Sun, B.G.; Zhang, Y.Y. Characteristics of umami peptides identified from porcine bone soup and molecular docking to the taste receptor T1R1/T1R3. Food Chem. 2022, 387, 132870. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Zhou, J.W.; Gao, D.; Xu, E.B.; Guo, M.M.; Liu, D.H. Desorption of nutrients and flavor compounds formation during the cooking of bone soup. Food Control. 2022, 132, 108408. [Google Scholar] [CrossRef]
- Zhu, W.H.; He, W.; Wang, W.X.; Bu, Y.; Li, X.P.; Li, J.R.; Zhang, Y.Y. Effects of thermoultrasonic treatment on characteristics of micro-nano particles and flavor in Greenland halibut bone soup. Ultrason. Sonochem. 2021, 79, 105785. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhu, L.H.; Li, H.H.; Tang, H.G.; Yang, H.J.; Zhao, K.; Kong, F.B.; Yin, T.; Yao, Q.; Chen, L.H. Effects of micro-/nano-scaled chicken bones on heat- induced gel properties of low-salt pork batter: Physicochemical characteristics, water distribution, texture, and microstructure. Food Chem. 2021, 373, 131574. [Google Scholar] [CrossRef]
- Akramzadeh, N.; Ramezani, Z.; Ferdousi, R.; Akbari-Adergani, B.; Mohammadi, A.; Karimian-khosroshahi, N.; Famenin, B.K.; Pilevar, Z.; Hosseini, H. Effect of chicken raw materials on physicochemical and microbiological properties of mechanically deboned chicken meat. Vet. Res. Forum. 2020, 11, 153–158. [Google Scholar] [CrossRef]
- Bu, Y.; Han, M.L.; He, W.; Zhu, W.H.; Zhang, Y.Y.; Li, X.P.; Li, J.R.; Zhang, Y. Comprehensive evaluation of the Greenland halibut bone soup quality traits under different thermoultrasonic power. J. Sci. Food Agric. 2022, 2, 603–612. [Google Scholar] [CrossRef]
- Gao, G.Z.; Wang, H.Q.; Zhou, J.W.; Rao, P.F.; Ke, L.J.; Lin, J.J.; Pan, B.S.; Zhang, Y.; Wang, Q. Isolation and characterization of bioactive proteoglycan–lipid nanoparticles from freshwater clam (Corbicula fluminea Muller) soup. J. Agric. Food Chem. 2021, 69, 1610–1618. [Google Scholar] [CrossRef]
- Ke, L.J.; Wang, H.Q.; Gao, G.Z.; Rao, P.F.; He, L.; Zhou, J.W. Direct interaction of food derived colloidal micro/nanoparticles with oral macrophages. NPJ Sci. Food. 2017, 1, 3. [Google Scholar] [CrossRef]
- Pérez-Palacios, T.; Eusebio, J.; Palma, S.F.; Carvalho, M.J.; Mir-Bel, J.; Antequera, T. Taste compounds and consumer acceptance of chicken soups as affected by cooking conditions. Int. J. Food Prop. 2017, 20, S154–S165. [Google Scholar] [CrossRef]
- Chen, X.; Yu, Y.G.; Zhang, Y.S.; Zeng, P.; Zhang, X.Y. Effects of degree of wet superfine grinding on physicochemical properties and component contents of skeleton pulp of Lateolabrax japonicus. J. Food Sci. Technol. 2020, 38, 94–102. [Google Scholar] [CrossRef]
- Qian, X.L.; Fan, X.Y.; Su, H.; Zhang, J.; Tao, N.P.; Zhong, J.; Wang, X.C.; Han, B.S. Migration of lipid and other components and formation of micro/nano-sized colloidal structure in Tuna (Thunnus obesus) head soup. LWT Food Sci. Technol. 2019, 111, 69–76. [Google Scholar] [CrossRef]
- Fan, X.Y.; Li, X.P.; Tao, N.P.; Zhang, J.; Wang, M.F.; Qian, X.L.; Su, H.; Zhong, J. Effect of salt addition time on the nutritional profile of Thunnus obesus head soup and the formation of micro/nano-Sized particle structure. Molecules 2019, 24, 4447. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Tao, N.P.; Su, H.; Zhang, J.; Zhong, J. Migration of nutrients and formation of micro/nano-sized particles in Atlantic salmon (Salmo salar) and bighead carp (Aristichthys nobilis) head soups. Food Biosci. 2020, 36, 100646. [Google Scholar] [CrossRef]
- Laly, S.J.; Sankar, T.V.; Panda, S.K. Effect of pressure cooking alone and in combination with other treatments on shrimp allergic protein, tropomyosin. J. Food Sci. Technol. 2021, 59, 1193–1201. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Dai, L.; Sun, Q.J.; McClements, D.J.; Xu, X.F. Effect of molecular weight on the interfacial and emulsifying characteristics of rice glutelin hydrolysates. Food Hydrocoll. 2022, 128, 107560. [Google Scholar] [CrossRef]
- Xi, Y.K.; Luo, Z.G.; Lu, X.X.; Peng, X.C. Modulation of cyclodextrin particle amphiphilic properties to stabilize pickering emulsion. J. Agric. Food Chem. 2018, 66, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Kim, H. Effect of mucilage extracted from Corchorus olitorius leaves on bovine serum albumin (BSA)-stabilized oil-in-water emulsions. Polymers 2023, 15, 113. [Google Scholar] [CrossRef]
- Wang, M.M.; Huang, B.H.; Fan, C.H.; Zhao, K.L.; Hu, H.; Xu, X.Y.; Pan, S.Y.; Liu, F.X. Characterization and functional properties of mango peel pectin extracted by ultrasound assisted citric acid. Int. J. Biol. Macromol. 2016, 91, 794–803. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, Z.; Zang, M.; Zhang, K.; Li, D.; Li, X. Flavor profile analysis of instant and traditional Lanzhou beef bouillons using HS-SPME-GC/MS, electronic nose and electronic tongue. Bioengineering 2022, 9, 582. [Google Scholar] [CrossRef]
- Ma, C.W.; Tian, X.J.; Li, Y.S.; Guo, J.J.; Wang, X.H.; Chen, S.H.; Bai, L.; Wang, Y.; Zhang, Y.F.; Wang, W.H. Using high-temperature cooking for different times for bone soup: Physicochemical properties, protein oxidation and nutritional value. J. Food Compost. Anal. 2023, 122, 105467. [Google Scholar] [CrossRef]
- Ni, D.D.; Li, H.J.; He, Z.F.; Zhang, D.; Deng, D.C. Changes in nutritional quality during processing of giant salamander soup. Food Sci. 2017, 38, 119–124. [Google Scholar] [CrossRef]
- Li, Y.L.; Fan, D.M.; Zhao, Y.L.; Wang, M.F. Effects of quercetin and cinnamaldehyde on the nutrient release from beef into soup during stewing process. LWT Food Sci. Technol. 2020, 131, 109712. [Google Scholar] [CrossRef]
- Ni, Y.Q.; Wu, N.; Wang, X.C. Correlation analysis between the formation of volatile compounds and lipids in hepatopancreas of female Chinese mitten crab during cooking. Food Sci. 2017, 38, 127–132. [Google Scholar] [CrossRef]
- Lekjing, S.; Venkatachalam, K.; Wangbenmad, C. Biochemical evaluation of novel seabass (Lates calcarifer) fish essence soup prepared by prolonged boiling process. Arab. J. Chem. 2021, 14, 103365. [Google Scholar] [CrossRef]
- Guan, H.N.; Yang, C.; Tian, Y.L.; Feng, C.M.; Gai, S.M.; Liu, D.Y.; Diao, X.Q. Changes in stability and volatile flavor compounds of self-emulsifying chicken soup formed during the stewing process. LWT Food Sci. Technol. 2023, 175, 114520. [Google Scholar] [CrossRef]
- Fu, J.N.; Li, S.B.; Xu, M.Z.; Zhang, D.Q.; Chen, L. Changes in physicochemical properties and formation process of colloidal nanoparticles (CNPs) during the lamb soup stewing. Food Chem. 2023, 442, 138290. [Google Scholar] [CrossRef]
- Moussa, E.R.W.H.; Shereen, A.N.; Manal, A.; Mehanni, A.E.; Rasha, A.E. Nutritional value and fatty acid composition of household cooking on fish fatty acids profile using atherogenicity and thrombogenicity indices. J. Food Chem. Nutr. 2014, 2, 27–41. [Google Scholar]
- Tufan, B.; Koese, S. Variations in lipid and fatty acid contents in different body parts of Black Sea whiting, Merlangius merlangus euxinus (Nordmann, 1840). Int. J. Food Sci. Technol. 2014, 49, 373–384. [Google Scholar] [CrossRef]
- Sequeira-Munoz, A.; Chevalier, D.; LeBail, A.; Ramaswamy, H.S.; Simpson, B.K. Physicochemical changes induced in carp (Cyprinus carpio) fillets by high pressure processing at low temperature. Innov. Food Sci. Emerg. Technol. 2006, 7, 13–18. [Google Scholar] [CrossRef]
- Qi, J.; Yan, H.M.; Xu, Y.; Peng, Y.L.; Jia, C.K.; Ye, M.; Fan, Z.H.; Xiong, G.Y.; Mei, L.; Xu, X.L. Effect of short-term frozen storage of raw meat on aroma retention of chicken broth: A perspective on physicochemical properties of broth. LWT Food Sci. Technol. 2022, 162, 113480. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Tong, Y.; Manyande, A.; Du, H.Y. Effective discrimination of flavours and tastes of Chinese traditional fish soups made from different regions of the silver carp using an electronic nose and electronic tongue. Czech J. Food Sci. 2020, 38, 84–93. [Google Scholar] [CrossRef]
- Guo, X.; Wang, Y.Q.; Lu, S.L.; Wang, J.Y.; Fu, H.H.; Gu, B.Y.; Lyu, B.; Wang, Q.L. Changes in proteolysis, protein oxidation, flavor, color and texture of dry-cured mutton ham during storage. LWT Food Sci. Technol. 2021, 149, 111860. [Google Scholar] [CrossRef]
- Riazi, F.; Zeynali, F.; Hoseini, E.; Behmadi, H.; Savadkoohi, S. Oxidation phenomena and color properties of grape pomace on nitrite-reduced meat emulsion systems. Meat Sci. 2016, 121, 350–358. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, X.X.; Hussain, M.; Li, X.D.; Liu, L.; Liu, Y.B.; Ma, S.Y.; Kouame, K.J.E.; Li, C.M.; Leng, Y.B.; et al. Influence of milk fat globule membrane and milk protein concentrate treated by ultrasound on the structural and emulsifying stability of mimicking human fat emulsions. Ultrason. Sonochem. 2022, 82, 105881. [Google Scholar] [CrossRef]
- Kashaninejad, M.; Razavi, S.M.A. Influence of thermosonication treatment on the average size of fat globules, emulsion stability, rheological properties and color of camel milk cream. LWT Food Sci. Technol. 2020, 132, 109852. [Google Scholar] [CrossRef]
- Zou, J.; Xu, M.J.; Zou, Y.F.; Yang, B. Chemical compositions and sensory characteristics of pork rib and Silkie chicken soups prepared by various cooking techniques. Food Chem. 2021, 345, 128755. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Liang, L.; Huang, Y.; Zhang, Y.Y.; Sun, B.G.; Li, L. Effect of ginger on chemical composition, physical and sensory characteristics of chicken soup. Foods 2021, 10, 1456. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E. Hydrocolloids as emulsifiers and emulsion stabilisers. Food Hydrocoll. 2009, 23, 1473–1482. [Google Scholar] [CrossRef]
- Albano, K.M.; Cavallieri, A.L.F.; Nicoletti, V.R. Electrostatic interaction between proteins and polysaccharides: Physicochemical aspects and applications in emulsion stabilization. Food Rev. Int. 2018, 35, 54–89. [Google Scholar] [CrossRef]
- He, W.; Zhu, W.H.; Bu, Y.; Wang, W.X.; Li, X.P.; Li, J.R.; Zhang, Y.Y. Formation of colloidal micro-nano particles and flavor characteristics of Greenland halibut bone soup. J. Food Sci. 2022, 87, 216–230. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Xu, Y.; Xie, X.F.; Zhang, W.W.; Wang, H.H.; Xu, X.L.; Xiong, G.Y. Gelatin enhances the flavor of chicken broth: A perspective on the ability of emulsions to bind volatile compounds. Food Chem. 2020, 333, 127463. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Yoo, W.; Yoo, B. Effect of thickener type on the rheological properties of hot thickened soups suitable for elderly people with swallowing difficulty. Prev. Nutr. Food Sci. 2014, 19, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.N.; Qu, D.N.; Zhao, G.L.; Yang, L.N.; Zhu, L.J.; Song, H.; Liu, H. Characterization of the structure and properties of the isolating interfacial layer of oil–water emulsions stabilized by soy hull polysaccharide: Effect of pH changes. Food Chem. 2022, 370, 131029. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, H.T.; Xia, X.F.; Sun, F.D.; Kong, B.H. Effect of ultrasound-assisted immersion thawing on emulsifying and gelling properties of chicken myofibrillar protein. LWT Food Sci. Technol. 2021, 142, 111016. [Google Scholar] [CrossRef]
- Wongsagonsup, R.; Pujchakarn, T.; Jitrakbumrung, S.; Chaiwat, W.; Fuongfuchat, A.; Varavinit, S.; Dangtip, S.; Suphantharika, M. Effect of cross-linking on physicochemical properties of tapioca starch and its application in soup product. Carbohydr. Polym. 2014, 101, 656–665. [Google Scholar] [CrossRef]
- Li, D.; Zhao, Y.; Wang, X.; Tang, H.L.; Wu, N.; Wu, F.; Yu, D.Y.; Elfalleh, W. Effects of (+)-catechin on a rice bran protein oil-in-water emulsion: Droplet size, zeta-potential, emulsifying properties, and rheological behavior. Food Hydrocoll. 2020, 98, 105306. [Google Scholar] [CrossRef]
- Dai, L.; Sun, C.X.; Wei, Y.; Mao, L.; Gao, Y.X. Characterization of Pickering emulsion gels stabilized by zein/gum arabic complex colloidal nanoparticles. Food Hydrocoll. 2018, 74, 239–248. [Google Scholar] [CrossRef]
- Jiang, F.; Zhu, Y.L.; Hu, W.X.; Li, M.Q.; Liu, Y.J.; Feng, J.J.; Lv, X.; Yu, X.Z.; Du, S.K. Characterization of quinoa starch nanoparticles as a stabilizer for oil in water Pickering emulsion. Food Chem. 2023, 427, 136697. [Google Scholar] [CrossRef]
- Zhao, G.L.; Wang, S.N.; Li, Y.Y.; Yang, L.N.; Song, H. Properties and microstructure of pickering emulsion synergistically stabilized by silica particles and soy hull polysaccharides. Food Hydrocoll. 2023, 134, 108084. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, Z.C.; Deng, Y.Y.; Dong, H.; Zhang, Y.; Tang, X.J.; Li, P.; Liu, G.; Zhang, M.W. Comparison of the effects of different food-grade emulsifiers on the properties and stability of a casein-maltodextrin-soybean oil compound emulsion. Molecules 2020, 25, 458. [Google Scholar] [CrossRef]
- Su, J.H.; Flanagan, J.; Hemar, Y.; Singh, H. Synergistic effects of polyglycerol ester of polyricinoleic acid and sodium caseinate on the stabilisation of water–oil–water emulsions. Food Hydrocoll. 2006, 20, 261–268. [Google Scholar] [CrossRef]
- Igartúa, D.E.; Cabezas, D.M.; Palazolo, G.G. Effects of pH, protein: Polysaccharide ratio, and NaCl-added concentration on whey protein isolate and soluble soybean polysaccharides electrostatic-complexes formation. Food Chem. Adv. 2022, 1, 100123. [Google Scholar] [CrossRef]
- Ziółkowska, D.; Lamkiewicz, J.; Shyichuk, A. Structure and flocculation of ion associates of carrageenan and poly (ciallyldimethylammonium chloride) depending on the component ratio. Molecules 2022, 27, 8075. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Han, M.L.; Bu, Y.; Zhu, W.H.; Li, J.R.; Li, X.P. Flavor mechanism of micro-nanoparticles and correlation analysis between flavor substances in thermoultrasonic treated fishbone soup. Ultrason. Sonochem. 2023, 93, 106299. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, H.; Tian, Y.; Feng, C.; Leng, S.; Zhao, S.; Liu, D.; Diao, X. Migration of Nutrient Substances and Characteristic Changes of Chicken White Soup Emulsion from Chicken Skeleton during Cooking. Foods 2024, 13, 410. https://doi.org/10.3390/foods13030410
Guan H, Tian Y, Feng C, Leng S, Zhao S, Liu D, Diao X. Migration of Nutrient Substances and Characteristic Changes of Chicken White Soup Emulsion from Chicken Skeleton during Cooking. Foods. 2024; 13(3):410. https://doi.org/10.3390/foods13030410
Chicago/Turabian StyleGuan, Haining, Yanli Tian, Chunmei Feng, Siqi Leng, Shifa Zhao, Dengyong Liu, and Xiaoqin Diao. 2024. "Migration of Nutrient Substances and Characteristic Changes of Chicken White Soup Emulsion from Chicken Skeleton during Cooking" Foods 13, no. 3: 410. https://doi.org/10.3390/foods13030410
APA StyleGuan, H., Tian, Y., Feng, C., Leng, S., Zhao, S., Liu, D., & Diao, X. (2024). Migration of Nutrient Substances and Characteristic Changes of Chicken White Soup Emulsion from Chicken Skeleton during Cooking. Foods, 13(3), 410. https://doi.org/10.3390/foods13030410



