The Impact of Various Types of Cooking on the Fate of Hg and Se in Predatory Fish Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Chemicals and Reagents
2.3. Sampling
2.4. Analytical Procedures
2.4.1. Sample Preparation
2.4.2. Culinary Treatments
- (i)
- Boiling: 300 g of ultra-pure water was placed in a 500 mL beaker and brought to boiling. Then, the sample was immersed into the boiling water during 15 min. The internal temperature of the fish fillets was recorded at the beginning and every 5 min.
- (ii)
- Frying: 300 mL of sunflower oil was placed into a 500 mL beaker and brought to a temperature of 130 °C. Then, the sample was immersed during 5 min; the internal temperature of the fish fillets was recorded at the start, halftime, and at the end of the cooking procedure.
- (iii)
- Grilling: Each sample was cooked without any addition of sunflower oil or other ingredient for 15 min in a conventional (Teflon) frying pan. They were first cooked for 5 min each side, and then 2.5 min each side, for a total of 15 min. This allowed a homogenous cooking throughout the sample and prevented it from burning. The internal temperature of the fish fillets was measured each time the sample was turned.
- (iv)
- Steaming: The steam cooker was filled with ultra-pure water up to its minimum level. The apparatus was heated until steam was produced (≅3 min), and then the sample was introduced into the adequate plastic recipients and cooked for 15 min; the temperature was checked every 5 min during the sample steaming. The sample was hold on a metal-free (plastic) holder to avoid any contamination with trace metals, including Hg.
2.4.3. Determination of HgT and SeT by ICP-MS
2.4.4. Internal Quality Control
2.5. Statistical Data Treatment
3. Results and Discussion
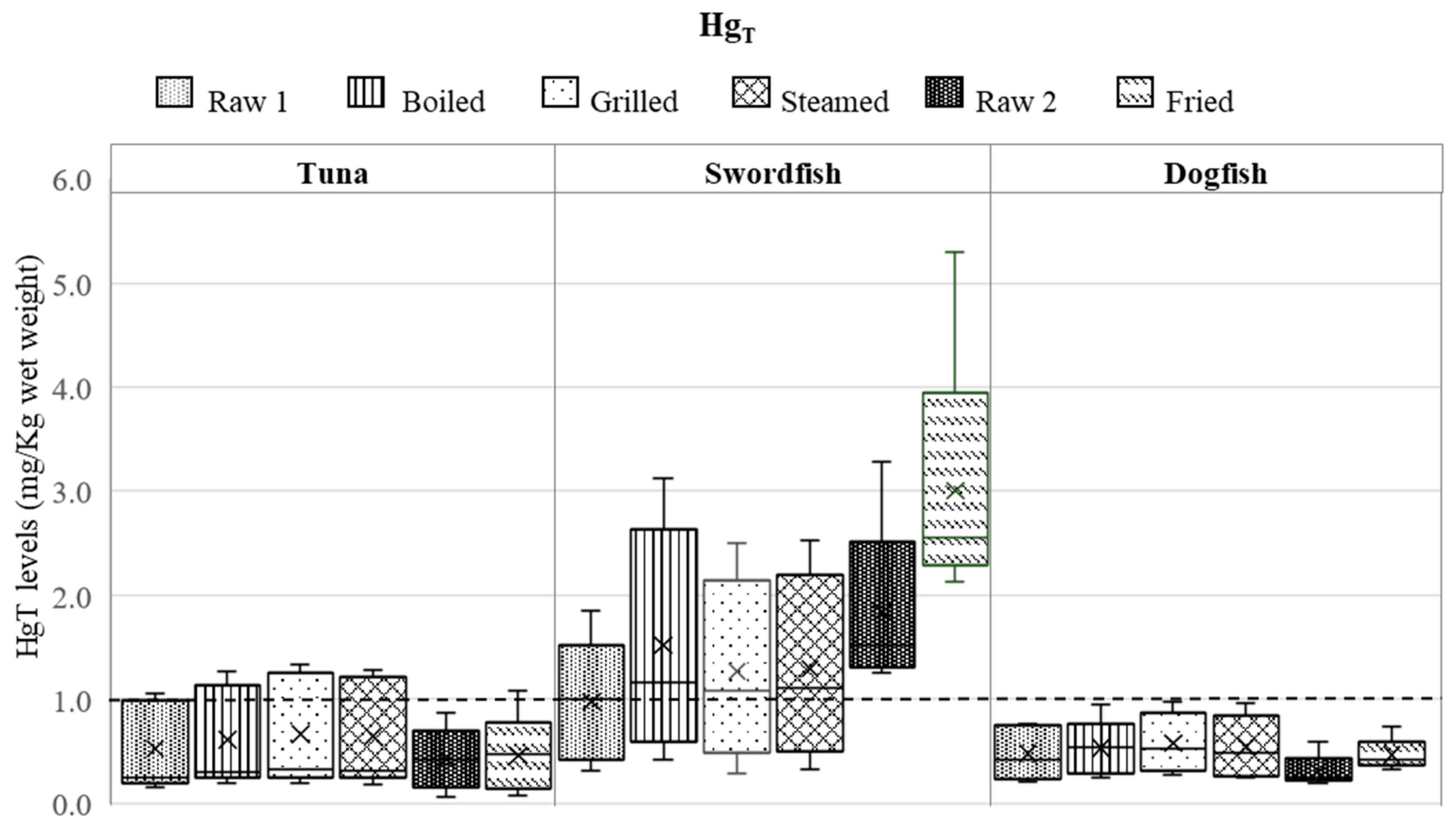
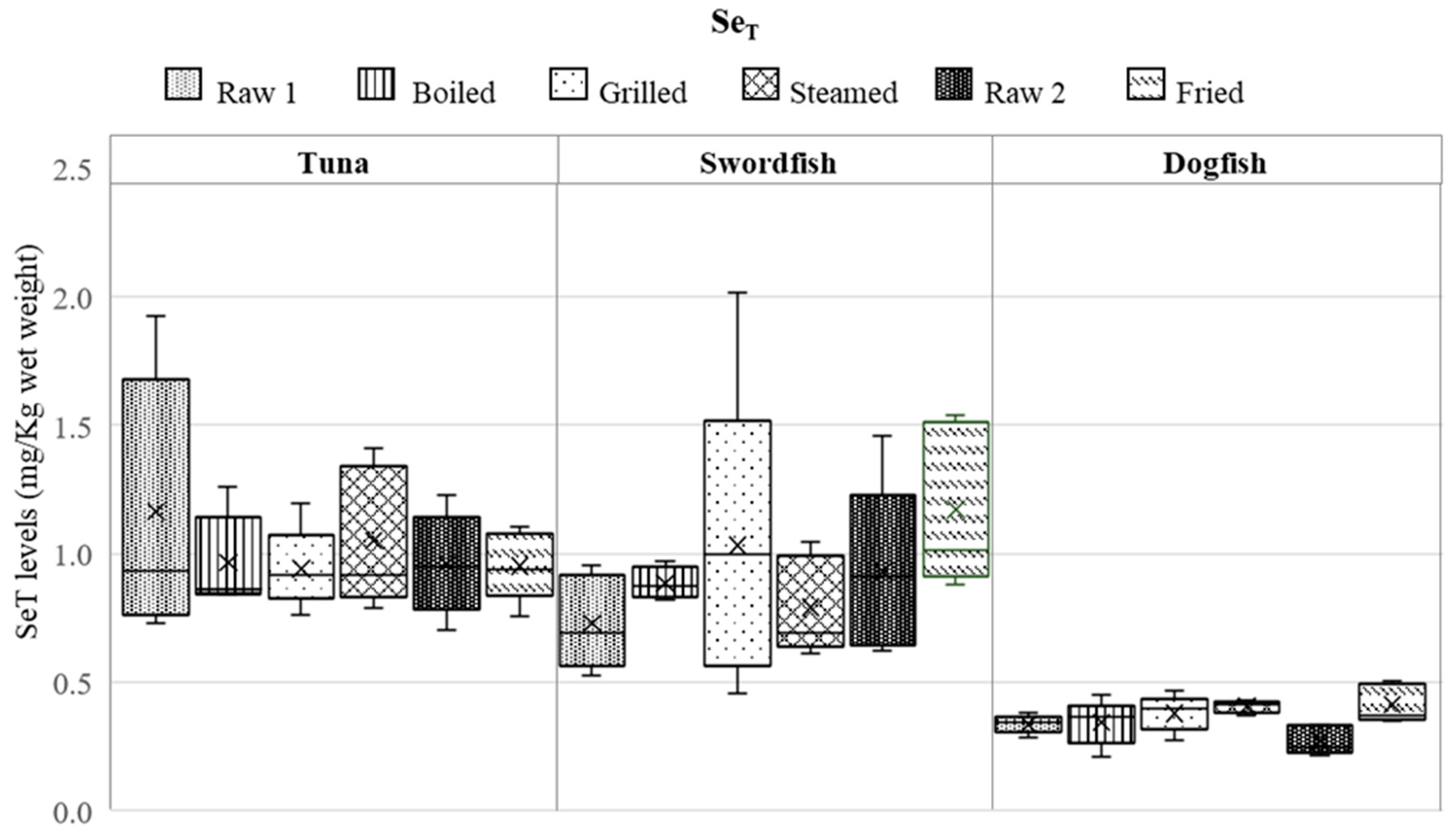
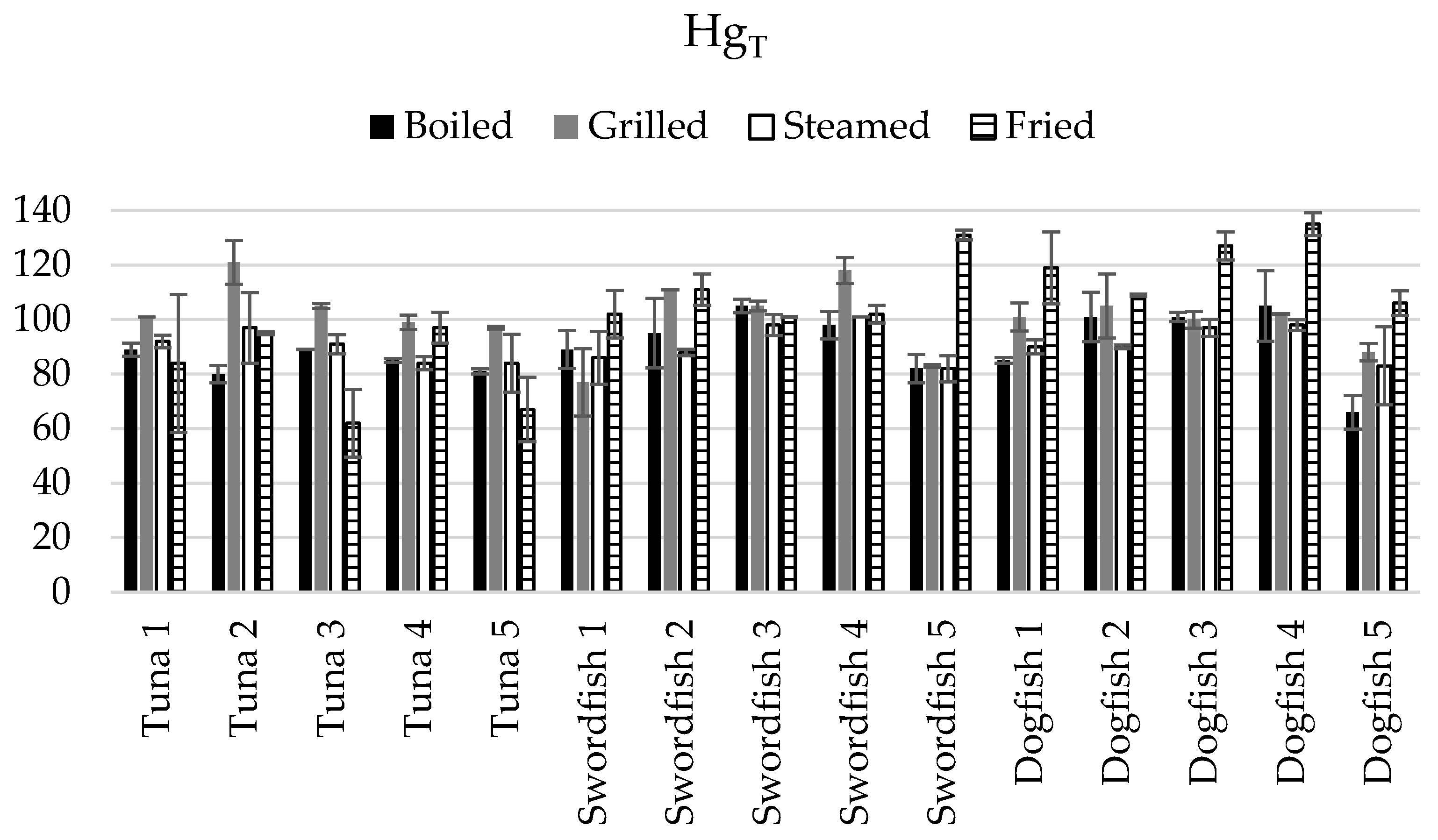
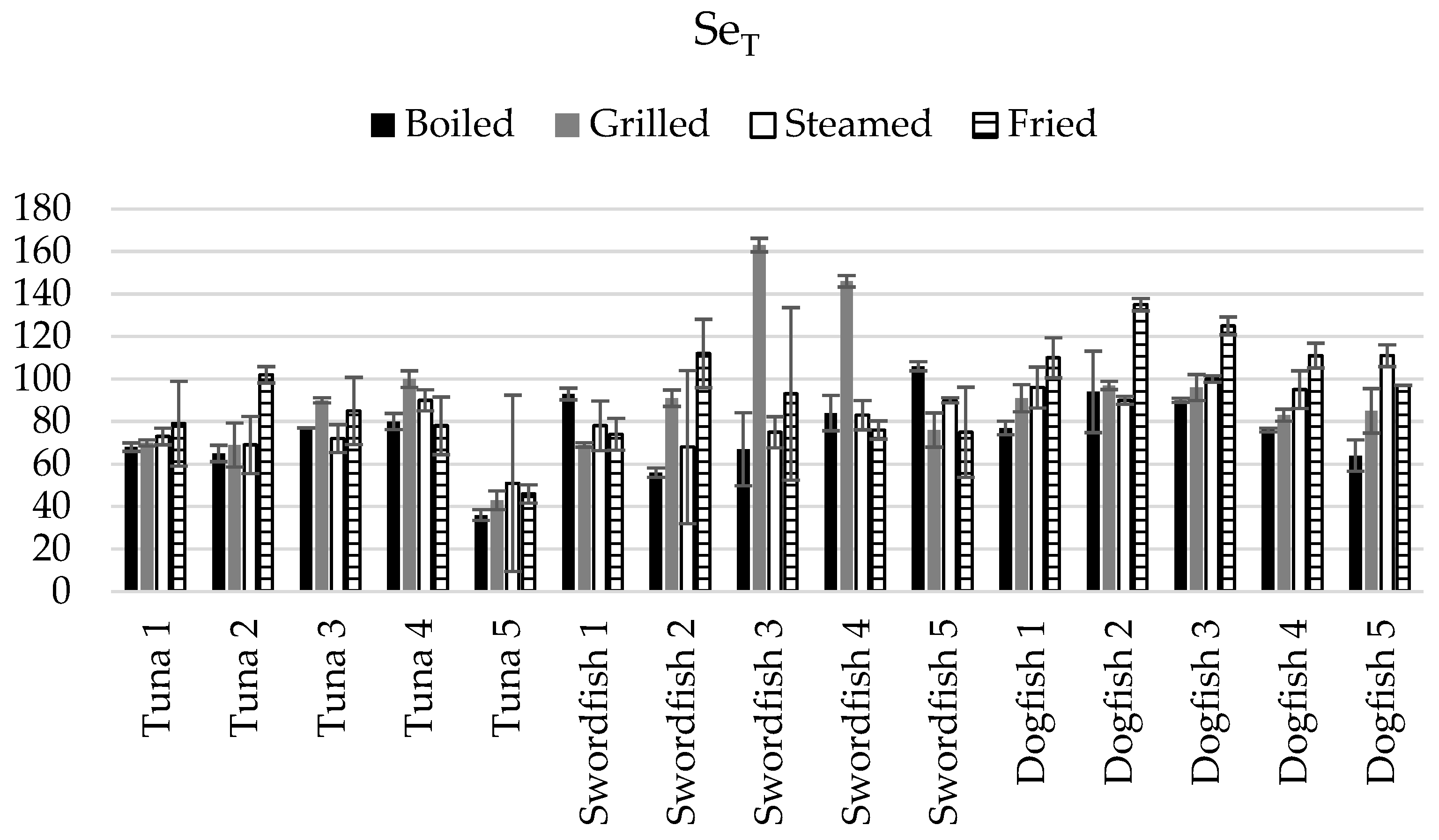
3.1. Influence of Various Cooking Modes on HgT and SeT Levels
3.2. Assessment of Se/Hg Molar Ratios in Raw and Cooked Fish
3.3. Assessment of the Net Gain or Loss in Terms of HgT and SeT in the Cooked Fish Samples
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. La Situation Mondiale des Pêches et de L’aquaculture; FAO: Rome, Italy, 2020. [Google Scholar]
- Liao, W.; Wang, G.; Zhao, W.; Zhang, M.; Wu, Y.; Liu, X.; Li, K. Change in mercury speciation in seafood after cooking and gastrointestinal digestion. J. Hazard. Mater. 2019, 375, 130–137. [Google Scholar] [CrossRef]
- Costa, F.; Mieiro, C.; Pereira, M.; Coelho, J. Mercury bioaccessibility in fish and seafood: Effect of method, cooking and trophic level on consumption risk assessment. Mar. Pollut. Bull. 2022, 179, 113736. [Google Scholar] [CrossRef] [PubMed]
- Mateu, M.B.; Llovet, M.; Bonancia, B.M.; Roig, J.L.D.; Linares-Vidal, V. Effects of cooking process on the concentrations of mercury, selenium and GPx activity in Tuna (Thunnus thynnus). Toxicol. Lett. 2015, 2, S64. [Google Scholar] [CrossRef]
- Ouédraogo, O.; Amyot, M. Effects of various cooking methods and food components on bioaccessibility of mercury from fish. Environ. Res. 2011, 111, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Cabanero, A.I.; Madrid, Y.; Cámara, C. Selenium and mercury bioaccessibility in fish samples. Anal. Chim. Acta 2004, 526, 51–61. [Google Scholar] [CrossRef]
- Bosch, A.C.; O’Neill, B.; Sigge, G.O.; Kerwath, S.E.; Hoffman, L.C. Heavy metals in marine fish meat and consumer health: A review. J. Sci. Food Agric. 2015, 96, 32–48. [Google Scholar] [CrossRef]
- Jitaru, P.; Adams, F. Toxicity, sources and biogeochemical cycle of mercury. J. Phys. IV 2004, 121, 185–193. [Google Scholar] [CrossRef]
- Kumari, S.; Amit; Jamwal, R.; Mishra, N.; Singh, D.K. Recent developments in environmental mercury bioremediation and its toxicity: A review. Environ. Nanotechnol. Monit. Manag. 2020, 13, 100283. [Google Scholar] [CrossRef]
- Deng, L.; Li, Y.; Yan, X.; Xiao, J.; Yang, R. Ultrasensitive and highly selective detection of bioaccumulation of methyl-mercury in fish samples via Ag⁰/Hg⁰ amalgamation. Anal Chem. 2015, 87, 2452–2458. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 364, 5–24. [Google Scholar]
- Wood, C.; Farrell, A.; Brauner, C. Fish Physiology: Homeostasis and Toxicology of Essential Metals; Part A; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Khan, M.A.; Wang, F. Mercury-selenium compounds and their toxicological significance: Toward a molecular understanding of the mercury-selenium antagonism. Environ. Toxicol. Chem. 2009, 28, 1567–1577. [Google Scholar] [CrossRef] [PubMed]
- Castriotta, L.; Rosolen, V.; Biggeri, A.; Ronfani, L.; Catelan, D.; Mariuz, M.; Bin, M.; Brumatti, L.V.; Horvat, M.; Barbone, F. The role of mercury, selenium and the Se-Hg antagonism on cognitive neurodevelopment: A 40-month follow-up of the Italian mother-child PHIME cohort. Int. J. Hyg. Environ. Health 2020, 230, 113604. [Google Scholar] [CrossRef] [PubMed]
- Gajdosechova, Z.; Mester, Z.; Feldmann, J.; Krupp, E.M. The role of selenium in mercury toxicity—Current analytical techniques and future. Trends Anal. Chem. 2018, 104, 95–109. [Google Scholar] [CrossRef]
- Reilly, C. Introduction. In Selenium in Food and Health; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Manceau, A.; Gaillot, A.C.; Glatzel, P.; Cherel, Y.; Bustamante, P. In Vivo Formation of HgSe Nanoparticles and Hg—Tetraselenolate Complex from Methylmercury in Seabirds—Implications for the Hg-Se Antagonism. Environ. Sci. Technol. 2021, 55, 1515–1526. [Google Scholar] [CrossRef]
- Tinggi, U.; Perkins, A.V. Selenium Status: Its Interactions with Dietary Mercury Exposure and Implications in Human Health. Nutrients 2022, 14, 5308. [Google Scholar] [CrossRef] [PubMed]
- Ralston, N.V.; Ralston, C.R.; Blackwell, J.L.; Raymond, L.J. Dietary and tissue selenium in relation to methylmercury toxicity. NeuroToxicology 2008, 29, 802–811. [Google Scholar] [CrossRef]
- Vicente-Zurdo, D.; Gómez-Gómez, B.; Pérez-Corona, M.T.; Madrid, Y. Impact of fish growing conditions and cooking methods on selenium species in swordfish and salmon fillets. J. Food Compos. Anal. 2019, 83, 103275. [Google Scholar] [CrossRef]
- Costa, F.D.N.; Korn, M.G.A.; Brito, G.B.; Ferlin, S.; Fostier, A.H. Preliminary results of mercury levels in raw and cooked seafood and their public health impact. Food Chem. 2016, 192, 837–841. [Google Scholar] [CrossRef]
- Mieiro, C.; Coelho, J.; Dolbeth, M.; Pacheco, M.; Duarte, A.; Pardal, M.; Pereira, M. Fish and mercury: Influence of fish fillet culinary practices on human risk. Food Control 2016, 60, 575–581. [Google Scholar] [CrossRef]
- FAO/INFOODS. Global Food Composition Database for Fish and Shellfish Version 1.0-uFiSh1.0; FAO/INFOODS Global Food Composition: Rome, Italy, 2016. [Google Scholar]
- Chevallier, E.; Chekri, R.; Zinck, J.; Guérin, T.; Noel, L. Simultaneous determination of 31 elements in foodstufs by ICP-MS after closed-vessel microwave digestion: Method validation based on the accuracy profile. J. Food Compost. Anal. 2015, 41, 35–41. [Google Scholar] [CrossRef]
- Harrington, C.F.; Merson, S.A.; Silva, T.M.D. Method to reduce the memory effect of mercury in the analysis of fish tissue using inductively coupled plasma mass spectrometry. Anal. Chim. Acta 2004, 505, 247–254. [Google Scholar] [CrossRef]
- Li, Y.-F.; Chen, C.; Li, B.; Wang, Q.; Wang, J.; Gao, Y.; Zhao, Y.; Chai, Z. Simultaneous speciation of selenium and mercury in human urine samples from long-term mercury-exposed populations with supplementation of selenium-enriched yeast by HPLC-ICP-MS. J. Anal. At. Spectrom. 2007, 22, 925–930. [Google Scholar] [CrossRef]
- FDA. Mercury in Food and Dietary Supplements. 2023. Available online: https://www.fda.gov/food/environmental-contaminants-food/mercury-food-and-dietary-supplements (accessed on 27 March 2023).
- ANSES. Ciqual French Food Composition Table. 2023. Available online: https://ciqual.anses.fr/ (accessed on 14 March 2023).
- EFSA NDA Panel. Scientific opinion on the tolerable upper intake level for selenium. EFSA J. 2022, 21, e07704. [Google Scholar]
- Burger, J.; Gochfeld, M. Selenium and mercury molar ratios in saltwater fish from New Jersey: Individual and species variability complicate use in human health fish consumption advisories. Environ. Res. 2012, 114, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Braaten, H.F.V.; de Wit, H.A.; Harman, C.; Hageström, U.; Larssen, T. Effects of sample preservation and storage on mercury speciation in natural stream water. Int. J. Environ. Anal. Chem. 2014, 94, 381–384. [Google Scholar] [CrossRef]
- Salim, R. Effect of storage on the distribution of trace elements (lead,’ cadmium, copper, zinc and mercury) in natural water. J. Environ. Sci. Health Part A Environ. Sci. Eng. 1987, 22, 59–69. [Google Scholar] [CrossRef]
- Selin, N.E. Global Biogeochemical Cycling of Mercury: A Review. Annu. Rev. Environ. Resour. 2009, 34, 43–63. [Google Scholar] [CrossRef]
- Burger, J.; Gochfeld, M. Mercury and selenium levels in 19 species of saltwater fish from New Jersey as a function of species, size, and season. Sci. Total. Environ. 2011, 409, 1418–1429. [Google Scholar] [CrossRef]
- Schartup, A.T.; Thackray, C.P.; Qureshi, A.; Dassuncao, C.; Gillespie, K.; Hanke, A.; Sunderland, E.M. Climate change and overfishing increase neurotoxicant in marine predators. Nature 2018, 572, 648–650. [Google Scholar] [CrossRef]
- Milea, Ș.-A.; Lazăr, N.-N.; Simionov, I.-A.; Petrea, Ș.-M.; Călmuc, M.; Călmuc, V.; Georgescu, P.-L.; Iticescu, C. Effects of cooking methods and co-ingested foods on mercury bioaccessibility in pontic shad (Alosa immaculata). Curr. Res. Food Sci. 2023, 7, 100599. [Google Scholar] [CrossRef]
- Perugini, M.; Zezza, D.; Tulini, S.M.R.; Abete, M.C.; Monaco, G.; Conte, A.; Olivieri, V.; Amorena, M. Effect of cooking on total mercury content in Norway lobster and European hake and public health impact. Mar. Pollut. Bull. 2015, 109, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Lobo, F.; Lavison-Bompard, G.; Guérin, T.; Parinet, J. Effect of home cooking processes on chlordecone content in beef and investigation of its by-products and metabolites by HPLC-HRMS/MS. Environ. Int. 2020, 144, 106077. [Google Scholar] [CrossRef] [PubMed]
| Nebuliser | Quartz concentric (Micromist) |
| Spray chamber | Scott-type double-pass water cooled (2 °C) |
| RF power | 1500 W |
| Reflected power | <10 W |
| Plasma gas flow | 15.0 L min−1 |
| Nebulizer gas flow | ~1.0 L min−1 (optimised daily) |
| Auxiliary gas flow | ~1.0 L min−1 (optimised daily) |
| Isotopes monitored and detection mode | 202Hg—conventional (no gaz mode) 82Se—use of He as colisison gas |
| Culinary Treatment | n(Se)/n(Hg) ± SD 1 (n = 5) | ||
|---|---|---|---|
| Tuna | Swordfish | Dogfish | |
| Raw 1 | 3.0 ± 1.3 | 1.1 ± 0.7 | 0.9 ± 0.6 |
| Boiling | 2.4 ± 1.4 | 0.9 ± 0.8 | 0.8 ± 0.5 |
| Grilling | 2.3 ± 1.6 | 1.0 ± 0.5 | 0.8 ± 0.5 |
| Steaming | 2.5 ± 1.6 | 0.9 ± 0.7 | 1.0 ± 0.6 |
| Raw 2 | 4.7 ± 5.3 | 0.6 ± 0.2 | 1.0 ± 0.3 |
| Frying | 4.3 ± 4.2 | 0.4 ± 0.1 | 0.9 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, M.; Douis, L.; Silva, J.A.L.d.; Castanheira, I.; Leufroy, A.; Jitaru, P. The Impact of Various Types of Cooking on the Fate of Hg and Se in Predatory Fish Species. Foods 2024, 13, 374. https://doi.org/10.3390/foods13030374
Ribeiro M, Douis L, Silva JALd, Castanheira I, Leufroy A, Jitaru P. The Impact of Various Types of Cooking on the Fate of Hg and Se in Predatory Fish Species. Foods. 2024; 13(3):374. https://doi.org/10.3390/foods13030374
Chicago/Turabian StyleRibeiro, Mariana, Laurène Douis, José Armando Luísa da Silva, Isabel Castanheira, Axelle Leufroy, and Petru Jitaru. 2024. "The Impact of Various Types of Cooking on the Fate of Hg and Se in Predatory Fish Species" Foods 13, no. 3: 374. https://doi.org/10.3390/foods13030374
APA StyleRibeiro, M., Douis, L., Silva, J. A. L. d., Castanheira, I., Leufroy, A., & Jitaru, P. (2024). The Impact of Various Types of Cooking on the Fate of Hg and Se in Predatory Fish Species. Foods, 13(3), 374. https://doi.org/10.3390/foods13030374








