Investigation of Effects of Cushioning Packaging on the Physiological and Quality Changes in Chinese Olive Fruits During Cold Chain Transportation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chinese Olive Fruits and Treatment
2.2. Evaluation of the Decay Rate and Weight Loss
2.3. Assessment of Chlorophyll and Total Flavonoid Content
2.4. Measurement of MDA Content and Cell Membrane Permeability
2.5. Measurement of AsA and GSH Contents
2.6. Determination of CAT, APX, PPO, LOX, and POD Activities
2.7. Analysis of Metabolites
2.8. Statistical Analysis
3. Results
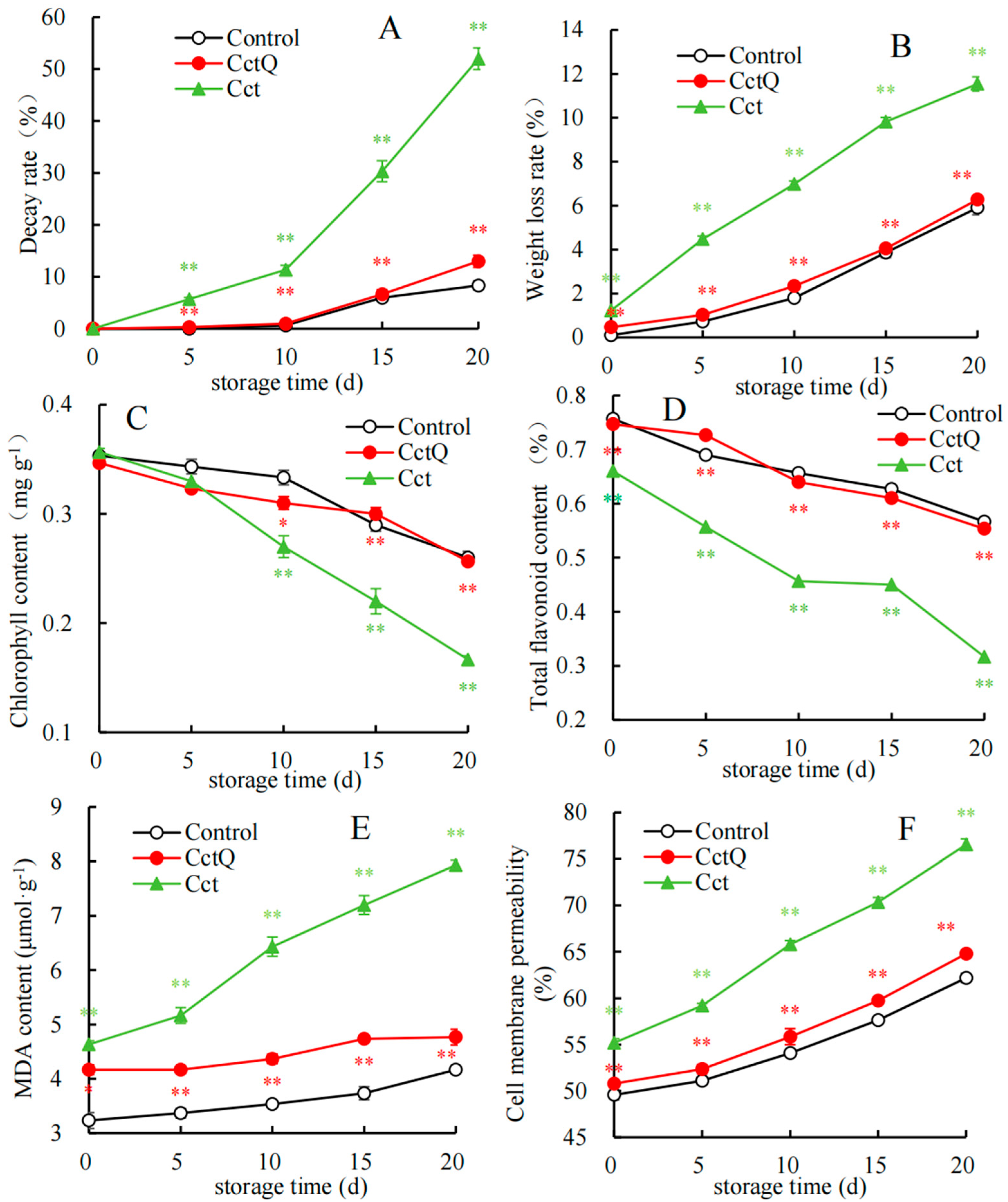
3.1. Changes in Storage Quality of Fruit
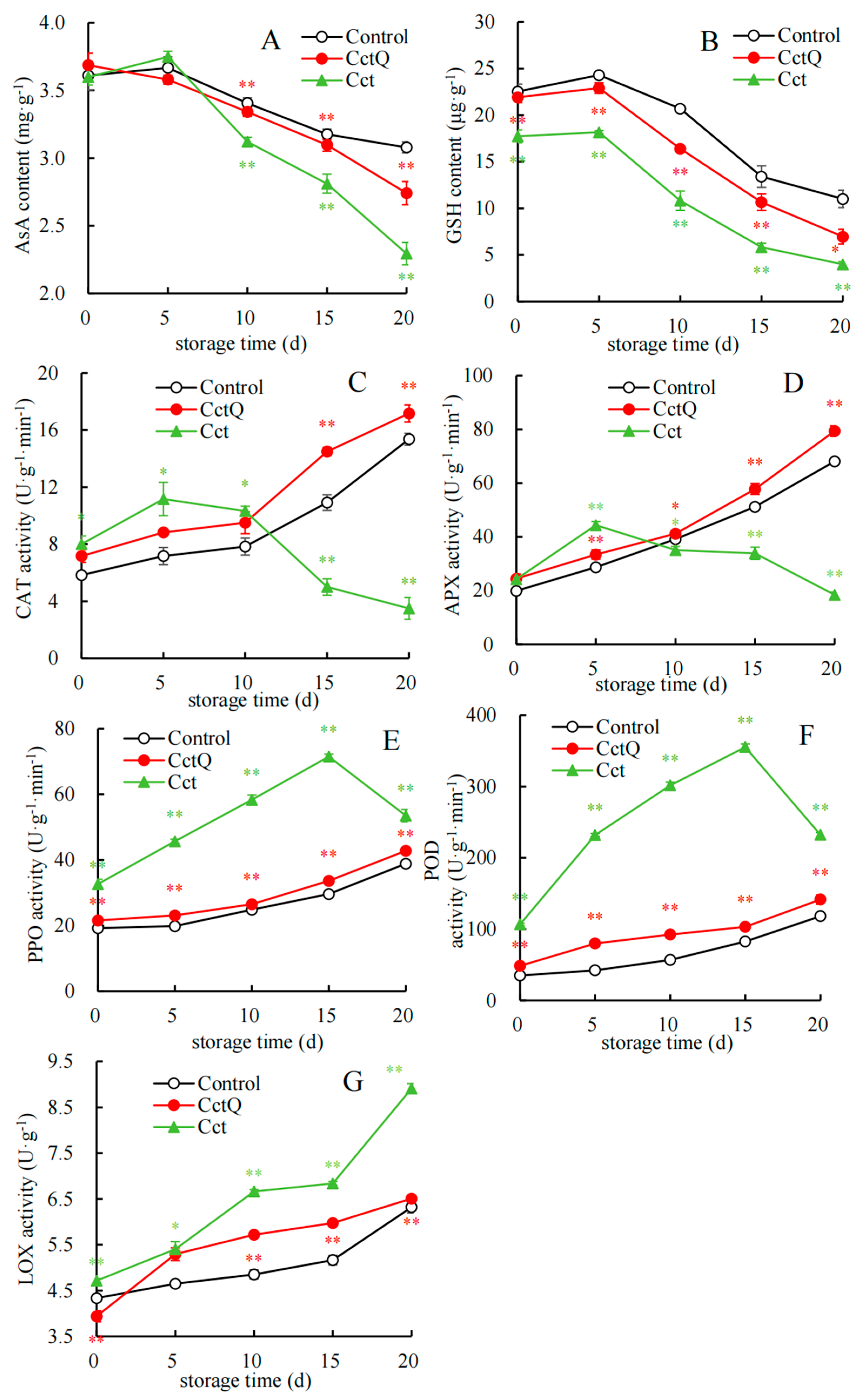
3.2. Investigation of Antioxidative Enzyme Activities and Non-Enzymatic Antioxidant Contents in Chinese Olives
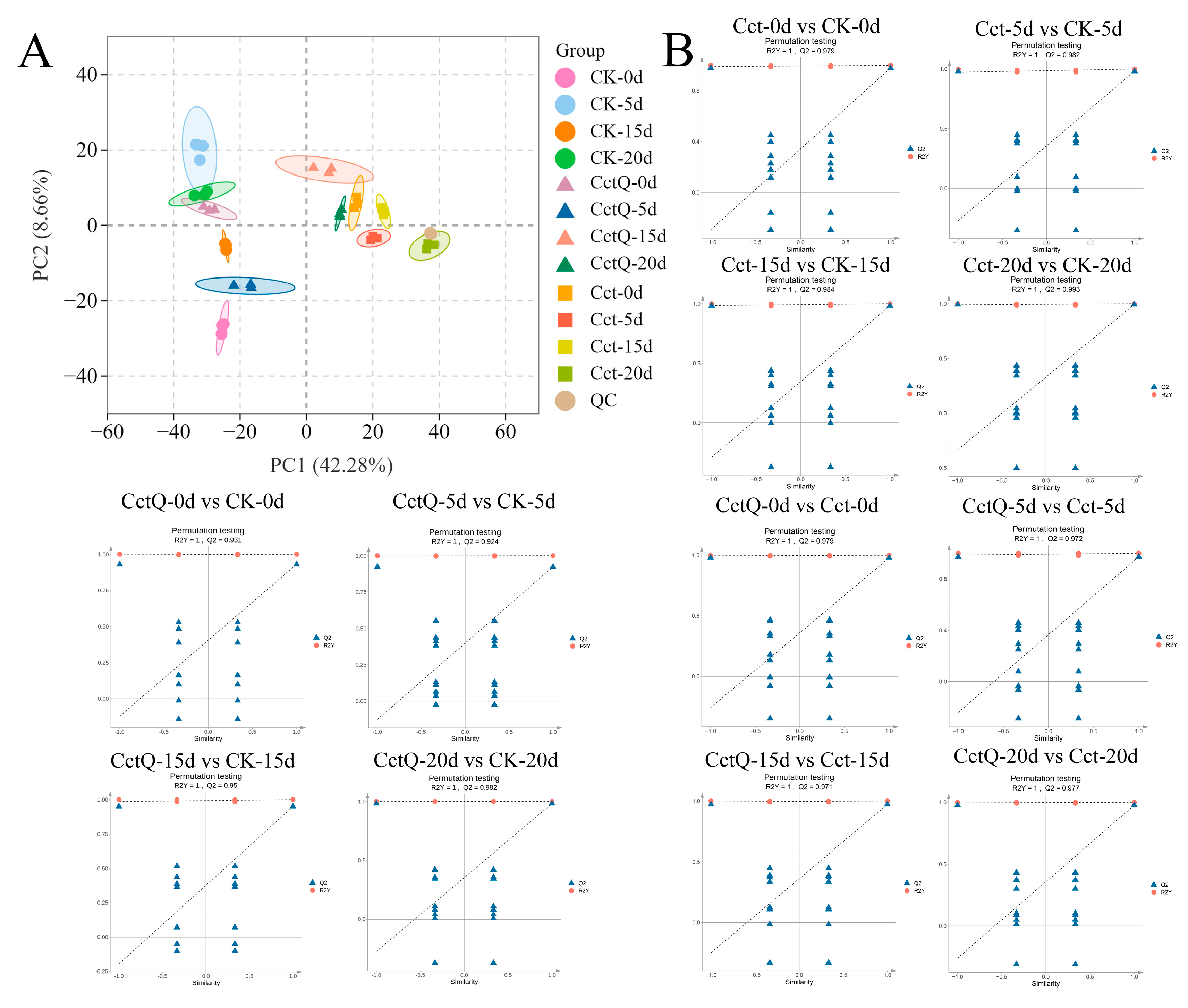
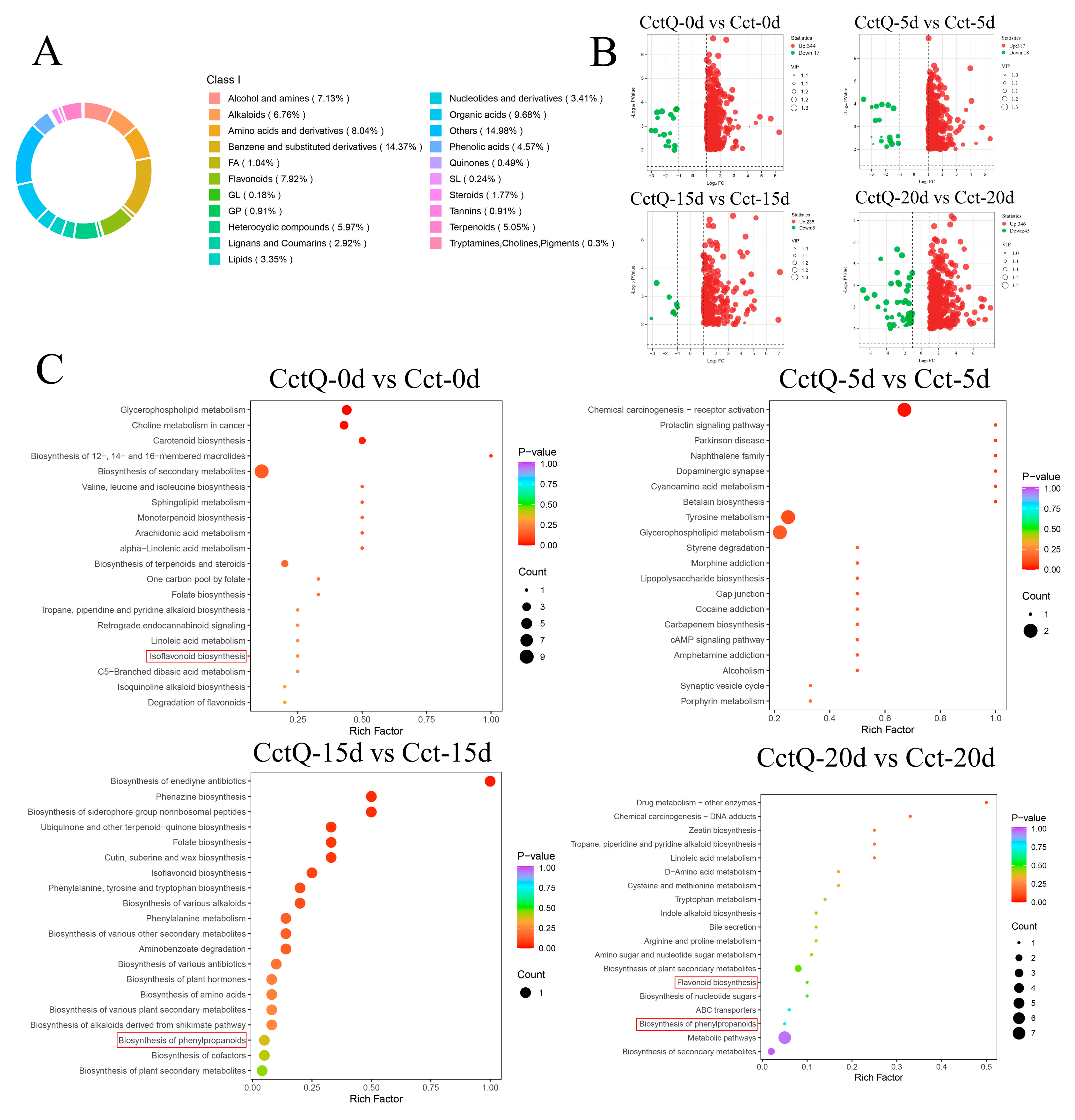
3.3. Metabolic Profiles of Chinese Olive Fruits
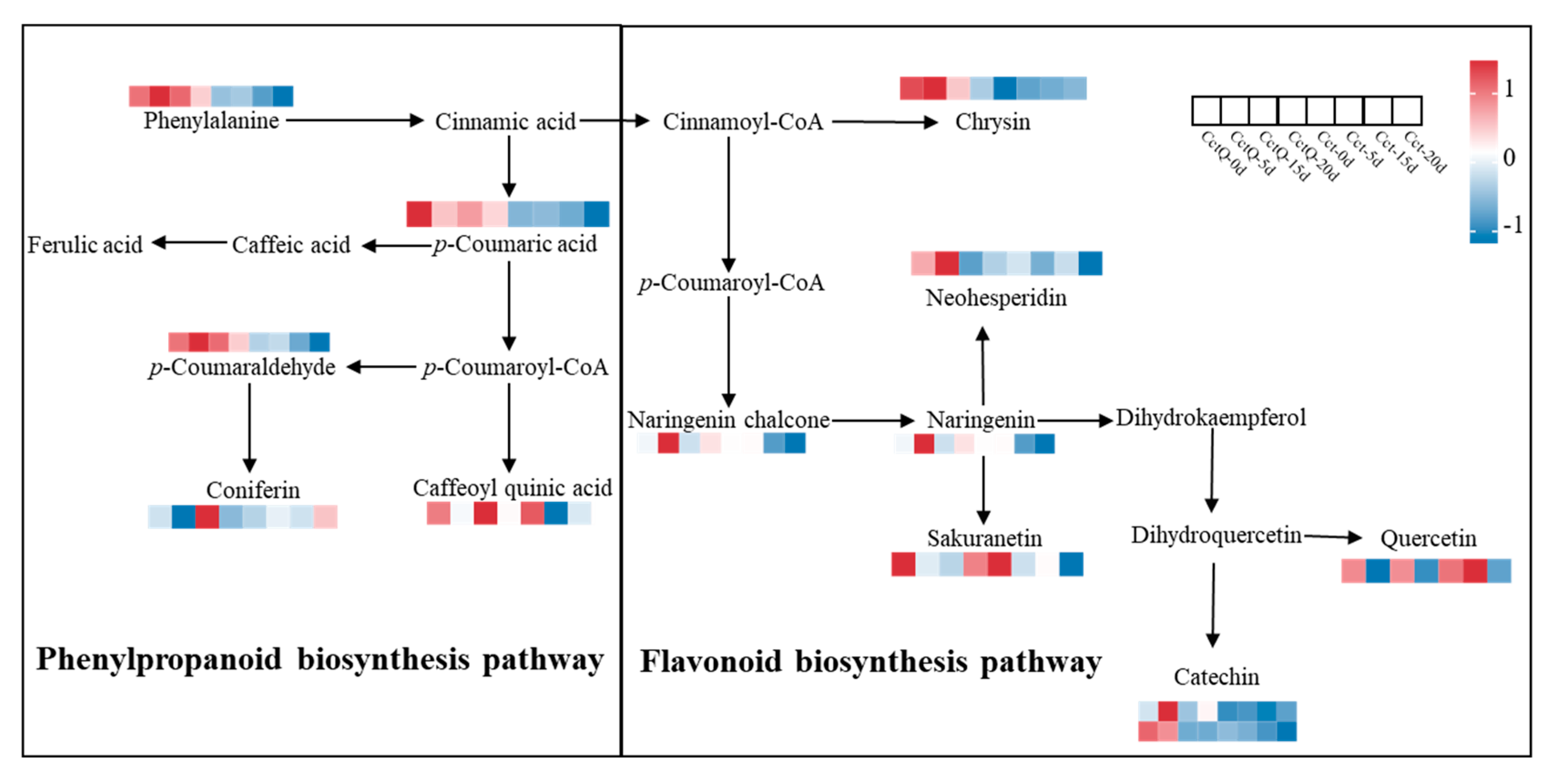
3.4. Analysis of Key Metabolites of Pathways Related to the Antioxidant Capacity
4. Discussion
4.1. Cushioning Packaging Influences the Post-Harvest Quality Parameters of Chinese Olives During the Shelf Life
4.2. The Impact of Cushioning Packaging on the Variations in Antioxidant Enzyme Activity and Antioxidant Substancs Contents in Chinese Olives Throughout the Shelf Life
4.3. Non-Targeted Metabolomic Analysis of Buffer Packaging Effects on the Changes in the Antioxidant Metabolite Contents of Chinese Olives During Shelf Life
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuang, X.Y.; Chen, Y.Z.; Lin, H.T.; Lin, H.; Chen, G.; Lin, Y.F.; Chen, Y.H.; Wang, H.; Fan, Z.Q. Comprehensive analyses of membrane lipids and phenolics metabolisms reveal the developments of chilling injury and browning in Chinese olives during cold storage. Food Chem. 2023, 416, 135754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.H.; Zhang, M.; Law, C.L.; Ma, Y.M. Effect of vibration and broken cold chain on the evolution of cell wall polysaccharides during fruit cucumber (Cucumis sativus L.) shriveling under simulated transportation. Food Packag. Shelf Life 2023, 38, 101126. [Google Scholar] [CrossRef]
- Yin, M.S.; Huo, L.F.; Li, N.; Zhu, H.L.; Zhu, Z.Q.; Hu, J.Y. Packaging performance evaluation and freshness intelligent prediction modeling in grape transportation. Food Control 2024, 165, 110684. [Google Scholar] [CrossRef]
- Chaiwong, S.; Saengrayap, R.; Rattanakaran, J.; Chaithanarueang, A.; Arwatchananukul, S.; Aunsri, N.; Tontiwattanakul, K.; Jitkokkruad, K.; Kitazawa, H.; Trongsatitkul, T. Natural rubber latex cushioning packaging to reduce vibration damage in guava during simulated transportation. Postharvest Biol. Technol. 2023, 199, 112273. [Google Scholar] [CrossRef]
- Zhou, R.; Su, S.Q.; Li, Y.F. Effects of Cushioning Materials on the Firmness of Huanghua Pears (Pyrus pyrifolia Nakai cv. Huanghua) during Distribution and Storage. Packag. Technol. Sci. 2008, 21, 1–11. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Jin, P.; Guo, X.F.; Li, Y.; Fan, C.; Wang, J.; Zheng, Y.H. Enhancement of storage quality and antioxidant capacity of harvested sweet cherry fruit by immersion with β-aminobutyric acid. Postharvest Biol. Technol. 2016, 118, 71–78. [Google Scholar] [CrossRef]
- Yang, Z.C.; Lin, M.H.; Yang, X.Z.; Zhu, C.Q.; Wu, D.; Chen, K.S. Mechanisms of the response of apple fruit to postharvest compression damage analyzed by integrated transcriptome and metabolome. Food Chem. X 2023, 20, 100972. [Google Scholar] [CrossRef]
- Ma, L.L.; Zheng, Y.Y.; Sang, Z.Z.; Ge, Y.H.; Bai, C.M.; Fu, A.Z.; Wang, Q.; Watkins, C.B.; Zuo, J.H. Multi-omics analysis reveals the mechanism of calcium-reduced quality deterioration in mechanically injured green pepper fruit. Postharvest Biol. Technol. 2023, 204, 112437. [Google Scholar] [CrossRef]
- Zhang, H.L.; Shan, T.T.; Chen, Y.; Lin, M.S.; Chen, Y.Z.; Lin, L.J.; Chen, Y.H.; Wang, H.; Fan, Z.Q.; Lin, H.T.; et al. Salicylic acid treatment delayed the browning development in the pericarp of fresh longan by regulating the metabolisms of ROS and membrane lipid. Sci. Hortic. 2023, 318, 112073. [Google Scholar] [CrossRef]
- Kaur, N.; Shahwar, D.; Hassan, F.E.; Ahmed, Z.F.R. Antioxidant and antibacterial activities of date palm fruit (Phoenix dactylifera L.) in response to postharvest application with natural elicitors. Acta Hortic. 2023, 1364, 187–194. [Google Scholar] [CrossRef]
- Wang, L.; Shi, K.L.; Song, Q.Y.; Wang, Y.Y.; Wu, T.Y.; Wang, X.Y.; Liu, Z.K.; Jin, P.; Zheng, Y.H.; Chen, D. Glycine betaine enhances chilling tolerance in peach fruit by modulating PpbHLH130-mediated antioxidant metabolism. Postharvest Biol. Technol. 2024, 218, 113166. [Google Scholar] [CrossRef]
- Wang, C.L.; Du, J.M.; Hou, D.H.; Guo, X.H.; Liu, Q.T.; Liu, L.W.; Fang, Y.; Kou, L.P. Quality retention and delay postharvest senescence of figs (Ficus carica L.) using 1-methylcyclopropene and modified atmosphere packaging during cold storage. Food Biosci. 2023, 53, 102748. [Google Scholar] [CrossRef]
- Kaur, N.; Somasundram, C.; Razali, Z.; Ahmed, Z.F.R. Sustainable Aloe vera/Chitosan-based Edible Coatings Reduce Postharvest Loss of Stored Fresh Figs (Ficus carica L.). Front. Sustain. Food Syst. 2024, 8, 1459600. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.S.; Malik, A.U.; Anjum, M.A.; Nawaz, A.; Shah, H.M.S. Modified atmosphere packaging delays enzymatic browning and maintains quality of harvested litchi fruit during low temperature storage. Sci. Hortic. 2019, 254, 14–20. [Google Scholar] [CrossRef]
- Ali, M.; Khalid, N.; Akhtar, A.; Aslam, S.; Ahmed, Z.F.; Zhang, X. Pulsed Light for Fresh Quality Preservation of Fruits and Vegetables. In Sustainable Postharvest Technologies for Fruits and Vegetables; CRC Press: Boca Raton, FL, USA, 2025; pp. 111–118. [Google Scholar] [CrossRef]
- Fan, Z.Q.; Lin, B.; Lin, H.T.; Lin, M.S.; Chen, J.Y.; Lin, Y.F. γ-Aminobutyric acid treatment reduces chilling injury and improves quality maintenance of cold-stored Chinese olive fruit. Food Chem. X. 2022, 13, 100208. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.J.; Lin, H.T.; Zheng, S.J.; Chen, Y.Z.; Zhang, H.L.; Lin, M.S.; Fan, Z.Q.; Wang, H.; Chen, Y.H.; Lin, Y.F. Metabolisms of ROS and membrane lipid participate in Pestalotiopsis microspora-induced disease occurrence of harvested Chinese olives. Postharvest Biol. Technol. 2024, 210, 112720. [Google Scholar] [CrossRef]
- Lin, H.T.; Xi, Y.F.; Chen, S.J. A review of enzymatic browning in fruit during storage. J. Fuzhou Univ. (Nat. Sci. Ed.) 2002, 30, 696–703. [Google Scholar]
- Chen, Y.M.; Lin, B.; Lin, Y.F.; Sang, Y.Y.; Lin, M.S.; Fan, Z.Q.; Chen, Y.H.; Wang, H.; Lin, H.T. Involvements of membrane lipid and phenolic metabolism in reducing browning and chilling injury of cold-stored Chinese olive by γ-aminobutyric acid treatment. Postharvest Biol. Technol. 2024, 209, 112664. [Google Scholar] [CrossRef]
- Yu, Z.Q.; Tang, D.T.; Zhang, Z.K.; Jiang, Y.M.; Yang, J.L.; Pan, Y.G. Tert-Butylhydroquinone retards longan fruit deterioration by regulating membrane lipid and energy metabolisms. Food Chem. 2024, 457, 140041. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Q.; Huang, X.; Yang, H.; Zhang, S.S.; Lyu, L.F.; Li, W.L.; Wu, W.L. Analysis of flavonoid-related metabolites in different tissues and fruit developmental stages of blackberry based on metabolome analysis. Food Res. Int. 2023, 163, 112313. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Reymick, O.O.; Liu, Z.; Zhou, Y.; Wang, X.; Feng, Z.; Tao, N.G. Citral enhances disease resistance in postharvest citrus fruit through inducing jasmonic acid pathway and accumulating phenylpropanoid compounds. Postharvest Biol. Technol. 2024, 207, 112633. [Google Scholar] [CrossRef]
- Fan, Z.Q.; Lin, B.; Lin, Y.F.; Chen, Y.Z.; Chen, G.; Chen, J.Y.; Wang, H.; Chen, Y.H.; Lin, H.T. Alleviation of chilling injury in cold-stored Chinese olive (Canarium album Lour.) fruit by γ-aminobutyric acid treatment in relation to ROS metabolism. Sci. Hortic. 2024, 327, 112851. [Google Scholar] [CrossRef]
- Kafkaletou, M.; Fasseas, C.; Tsantili, E. Increased firmness and modified cell wall composition by ethylene were reversed by the ethylene inhibitor 1-methylcyclopropene (1-MCP) in the non-climacteric olives harvested at dark green stage—Possible implementation of ethylene for olive quality. J. Plant Physiol. 2019, 238, 63–71. [Google Scholar] [CrossRef]
- Wu, Y.J.; Lin, H.T.; Lin, Y.F.; Shi, J.; Xue, S.; Hung, Y.C.; Chen, Y.H.; Wang, H. Effects of biocontrol bacteria Bacillus amyloliquefaciens LY-1 culture broth on quality attributes and storability of harvested litchi fruit. Postharvest Biol. Technol. 2017, 132, 81–87. [Google Scholar] [CrossRef]
- Liang, F.Y.; Liu, C.S.; Geng, J.W.; Chen, N.C.; Lai, W.D.; Mo, H.T.; Liu, K.D. Chitosan–fucoidan encapsulating cinnamaldehyde composite coating films: Preparation, pH-responsive release, antibacterial activity and preservation for litchi. Carbohyd Polym. 2024, 333, 121968. [Google Scholar] [CrossRef]
- Zhang, X.L.; Zhang, Z.Q.; Li, J.; Wu, L.J.; Guo, J.Y.; Ouyang, L.Q.; Xia, Y.Y.; Huang, X.M.; Pang, X.Q. Correlation of leaf senescence and gene expression/activities of chlorophyll degradation enzymes in harvested Chinese flowering cabbage (Brassica rapa var. parachinensis). J. Plant Physiol. 2011, 168, 2081–2087. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.T.; Tang, H.L.; Lei, D.Y.; Zhao, B.; Zhou, X.; Yao, W.T.; Fan, J.M.; Lin, Y.X.; Chen, Q.; Wang, Y.; et al. Exogenous melatonin maintains postharvest quality in kiwiberry fruit by regulating sugar metabolism during cold storage. LWT—Food Sci. Technol. 2023, 174, 114385. [Google Scholar] [CrossRef]
- Si, J.; Ye, B.B.; Liu, Z.L.; Xiao, X.M.; Yang, Y.Y.; Fan, Z.Q.; Shan, W.; Kuang, J.F.; Lu, W.J.; Su, X.G.; et al. Transcriptional repression of MaRBOHs by MaHsf26 is associated with heat shock-alleviated chilling injury in banana fruit. Postharvest Biol. Technol. 2022, 193, 112056. [Google Scholar] [CrossRef]
- Song, W.W.; Fu, X.X.; Cao, D.T.; Liang, X.G.; Xiao, S.L.; Yuan, M.X.; Huang, Y.J.; Zhou, Q.H.; Wei, H.Y.; Wang, J.W.; et al. The delaying effect of Clausena lansium extract on pear ring rot is related to its antifungal activity and induced disease resistance. Postharvest Biol. Technol. 2024, 212, 112847. [Google Scholar] [CrossRef]
- Hou, Y.Y.; Deng, R.; Shataer, D.; Hong, J.Y.; Wang, L.; Jin, P.; Zhao, Y.T. L-Glutamate treatment alleviates chilling injury of prune (Prunus domestica L.) fruit by regulating ROS homeostasis, GABA shunt, and energy metabolism. Food Biosci. 2024, 461, 140899. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.U.; Singh, Z.; Shah, H.M.S.; Woodward, A.; Yamoah, E.A. Methyl jasmonate advances fruit ripening, colour development, and improves antioxidant quality of ‘Yoho’ and ‘Jiro’ persimmon. Food Chem. 2024, 459, 140360. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Sajjad, M.; Xue, S.D.; Zhou, M.J.; Li, G.H.; Xu, Y.C.; Liu, L.; Zhu, J.T.; Meng, Q.T.; Jin, Q.M.; Du, H.; et al. Decoding comparative taste and nutrition regulation in Chinese cabbage via integrated metabolome and transcriptome analysis. Food Res. Int. 2024, 195, 114943. [Google Scholar] [CrossRef]
- Rahman, F.U.; Zhu, Q.N.; Zhang, K.Y.; Kang, X.M.; Wang, X.T.; Chen, W.X.; Li, X.P.; Zhu, X.Y. Transcriptome and metabolome analyses provide insights into the fruit softening disorder of papaya fruit under postharvest heat stress. Food Chem. 2024, 460, 140771. [Google Scholar] [CrossRef] [PubMed]
- Lufu, R.; Ambaw, A.; Opara, U.L. The Influence of Internal Packaging (Liners) on Moisture Dynamics and Physical and Physiological Quality of Pomegranate Fruit during Cold Storage. Foods 2021, 10, 1388. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.Z.; Li, N.; Lin, H.T.; Lin, M.S.; Chen, Y.H.; Wang, H.; Ritenour, M.A.; Lin, Y.F. Effects of chitosan treatment on the storability and quality properties of longan fruit during storage. Food Chem. 2020, 306, 125627. [Google Scholar] [CrossRef] [PubMed]
- Li, B.R.; Li, M.Q.; Liu, J.; Sun, W.W.; Min, D.D.; Li, F.J.; Li, X.A. Methyl salicylate pretreatment maintains quality and antioxidant capacity of fresh-cut pitaya fruit by modulating phenylpropanoid metabolism and antioxidant system. Sci. Hortic. 2023, 309, 111705. [Google Scholar] [CrossRef]
- Bai, L.J.; Wu, Y.T.; Lin, H.T.; Su, W.B.; Fan, Z.Q. MaERF9 and MaERF113 transcription factors involve in chilling injury development by regulating membrane lipid metabolism of postharvest banana fruit. Postharvest Biol. Technol. 2025, 219, 113230. [Google Scholar] [CrossRef]
- Ding, X.C.; Liu, S.; Duan, X.W.; Pan, X.J.; Dong, B.Y. MAPK cascade and ROS metabolism are involved in GABA-induced disease resistance in red pitaya fruit. Postharvest Biol. Technol. 2023, 200, 112324. [Google Scholar] [CrossRef]
- Lin, Y.F.; Lin, H.T.; Zhang, S.; Chen, Y.H.; Chen, M.Y.; Lin, Y.X. The role of active oxygen metabolism in hydrogen peroxide-induced pericarp browning of harvested longan fruit. Postharvest Biol. Technol. 2014, 96, 42–48. [Google Scholar] [CrossRef]
- Feng, Y.Y.; Liu, T.F.; Dong, T.T.; Wang, Q.G. Endogenous compound chenodeoxycholic acid in potatoes can delay enzymatic browning by inducing antioxidant capacity. Postharvest Biol. Technol. 2024, 210, 112781. [Google Scholar] [CrossRef]
- Chen, Y.H.; Sun, J.Z.; Lin, H.T.; Lin, M.S.; Lin, Y.F.; Wang, H.; Hung, Y.C. Salicylic acid treatment suppresses Phomopsis longanae Chi-induced disease development of postharvest longan fruit by modulating membrane lipid metabolism. Postharvest Biol. Technol. 2020, 164, 111168. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Lin, H.T.; Zhang, H.L.; Chen, Y.; Lin, M.S.; Zheng, Y.; Fan, Z.Q.; Wang, H.; Chen, Y.H.; Lin, Y.F. Dicyclohexylcarbodiimide and disodium succinate regulate the browning development in fresh longan pericarp by modulating the antioxidant system and the metabolisms of membrane lipids and phenolics. Postharvest Biol. Technol. 2023, 203, 112388. [Google Scholar] [CrossRef]
- Jiang, Y.Q.; Wang, X.D.; Li, X.J.; Wang, Z.Y.; Wang, H.F.; Li, W.H.; Liu, T.; Li, X.H.; Jiang, Y.B.; Tang, Y. Combination of 1-methylcyclopropene and phytic acid inhibits surface browning and maintains texture and aroma of fresh-cut peaches. Postharvest Biol. Technol. 2023, 200, 112328. [Google Scholar] [CrossRef]
- Xie, G.F.; Liu, N.; Zhang, Y.; Tan, S.M.; Xu, Y.Q.; Luo, Z.S. Postharvest MeJA maintains the shelf quality of kiwifruit after cold storage by regulating antioxidant capacity and activating the disease resistance. Postharvest Biol. Technol. 2024, 211, 112827. [Google Scholar] [CrossRef]
- Wang, L.J.; Lu, W.X.; Ran, L.Y.; Dou, L.W.; Yao, S.; Hu, J.; Fan, D.; Li, C.F.; Luo, K.M. R2R3-MYB transcription factor MYB6 promotes anthocyanin and proanthocyanidin biosynthesis but inhibits secondary cell wall formation in Populus tomentosa. Plant J. 2019, 99, 733–751. [Google Scholar] [CrossRef]
- Choo, T.M. Breeding barley for resistance to Fusarium head blight and mycotoxin accumulation. Plant Breed. Rev. 2010, 26, 123–169. [Google Scholar]
- Liu, Y.; Ji, D.; Turgeon, R.; Chen, J.; Lin, T.; Huang, J.; Luo, J.; Zhu, Y.; Zhang, C.; Lv, Z. Physiological and Proteomic Responses of Mulberry Trees (Morus alba. L.) to Combined Salt and Drought Stress. Int. J. Mol. Sci. 2019, 20, 2486. [Google Scholar] [CrossRef]
- Ramzan, T.; Shahbaz, M.; Maqsood, M.F.; Zulfiqar, U.; Saman, R.U.; Lili, N.; Irshad, M.; Maqsood, S.; Haider, A.; Shahzad, B.; et al. Phenylalanine supply alleviates the drought stress in mustard (Brassica campestris) by modulating plant growth, photosynthesis, and antioxidant defense system. Plant Physiol. Bioch. 2023, 201, 107828. [Google Scholar] [CrossRef]
- Rao, M.J.; Duan, M.Z.; Shad, M.A.; Aslam, M.Z.; Wang, J.B.; Wang, L.Q. Widely targeted LC-MS/MS approach provides insights into variations in bioactive flavonoid compounds and their antioxidant activities in green, red, and purple sugarcane. LWT—Food Sci. Technol. 2024, 209, 116792. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.; Fu, F.; Li, J.; Liu, J.; Du, K.; Zhu, B.; Guo, Z.; Pan, T.; She, W. Investigation of Effects of Cushioning Packaging on the Physiological and Quality Changes in Chinese Olive Fruits During Cold Chain Transportation. Foods 2024, 13, 4133. https://doi.org/10.3390/foods13244133
Lin H, Fu F, Li J, Liu J, Du K, Zhu B, Guo Z, Pan T, She W. Investigation of Effects of Cushioning Packaging on the Physiological and Quality Changes in Chinese Olive Fruits During Cold Chain Transportation. Foods. 2024; 13(24):4133. https://doi.org/10.3390/foods13244133
Chicago/Turabian StyleLin, Han, Fanghao Fu, Jinghai Li, Jiahui Liu, Kaiyang Du, Bingxia Zhu, Zhixiong Guo, Tengfei Pan, and Wenqin She. 2024. "Investigation of Effects of Cushioning Packaging on the Physiological and Quality Changes in Chinese Olive Fruits During Cold Chain Transportation" Foods 13, no. 24: 4133. https://doi.org/10.3390/foods13244133
APA StyleLin, H., Fu, F., Li, J., Liu, J., Du, K., Zhu, B., Guo, Z., Pan, T., & She, W. (2024). Investigation of Effects of Cushioning Packaging on the Physiological and Quality Changes in Chinese Olive Fruits During Cold Chain Transportation. Foods, 13(24), 4133. https://doi.org/10.3390/foods13244133




