Effect of Genetic Polymorphism of Bovine β-Casein Variants (A1 and A2) on Yoghurt Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Milk Collection
2.2. Yoghurt Production
2.3. Physicochemical Parameters
2.3.1. Total Solids, pH, Protein Content and Acidity
2.3.2. Water-Holding Capacity (WHC) and Spontaneous Syneresis
2.3.3. Color and Texture
2.4. Microbiological Analyses
2.5. Sensory Characteristics
2.6. Statistical Analyses
3. Results and Discussion
3.1. Genetics of Cows Included in This Study
3.2. Total Solids and Protein Content
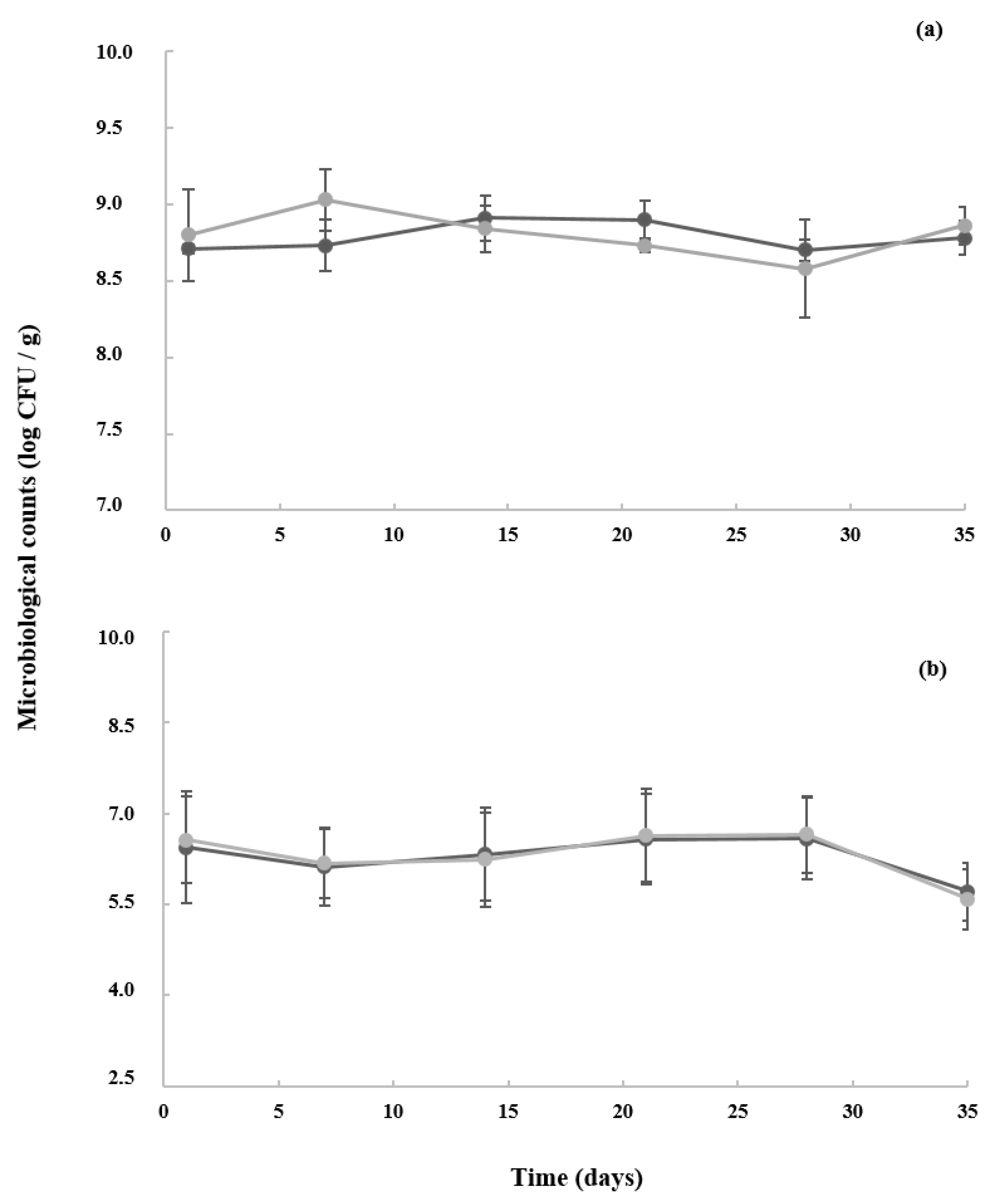
3.3. Microbiological Counts of Starters
3.4. pH and Acidity
3.5. Water-Holding Capacity and Spontaneous Syneresis
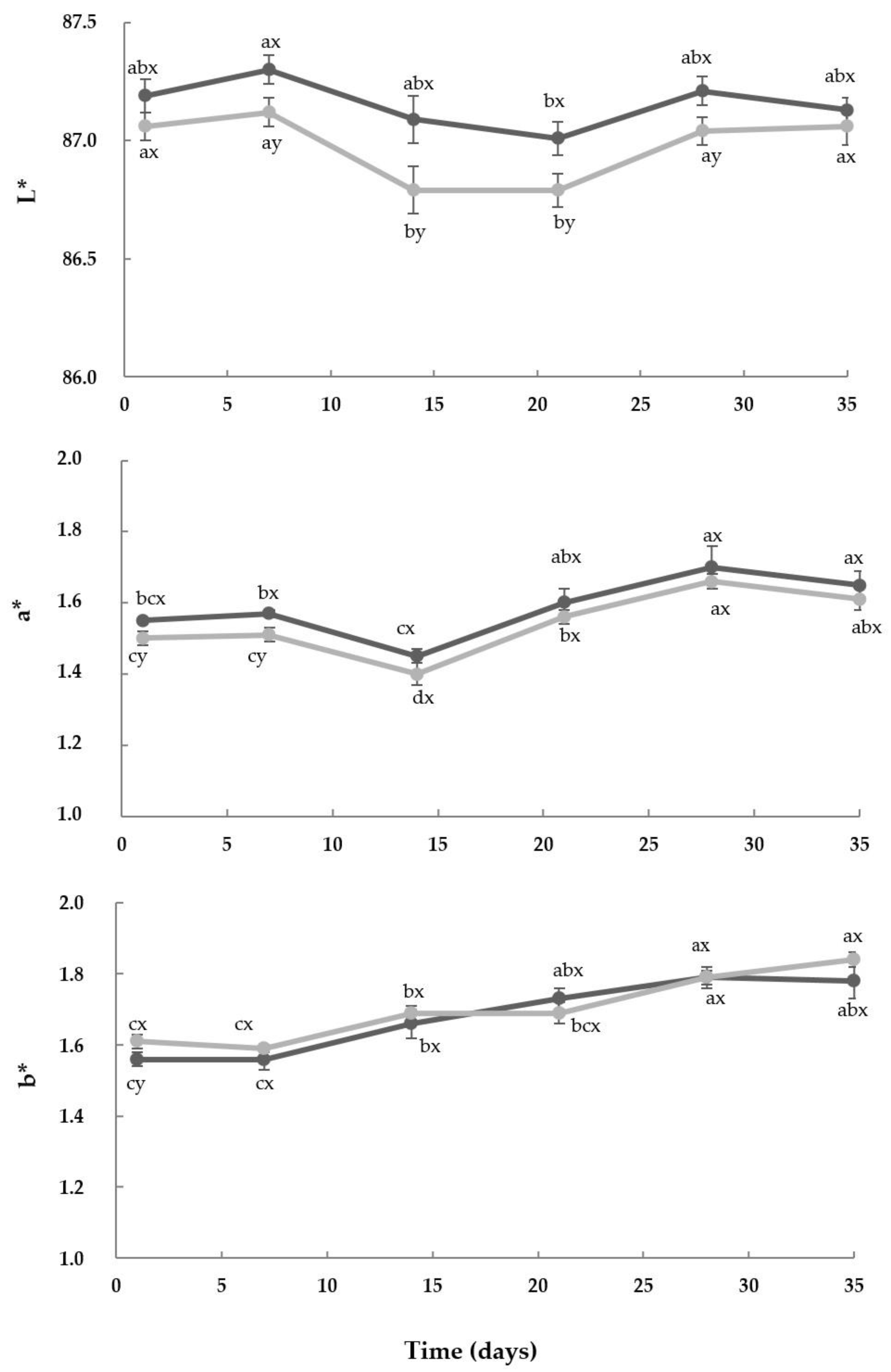
3.6. Color and Texture
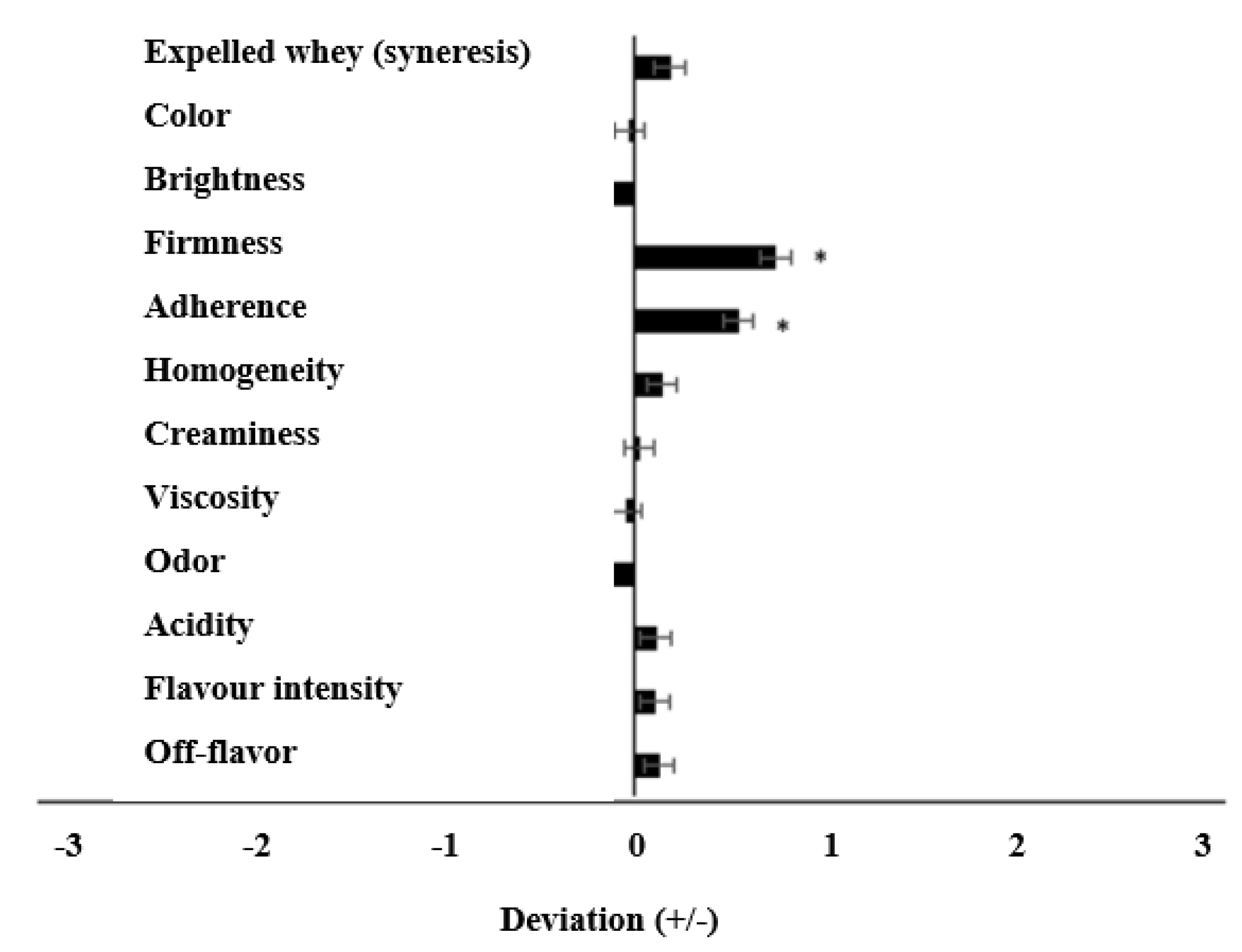
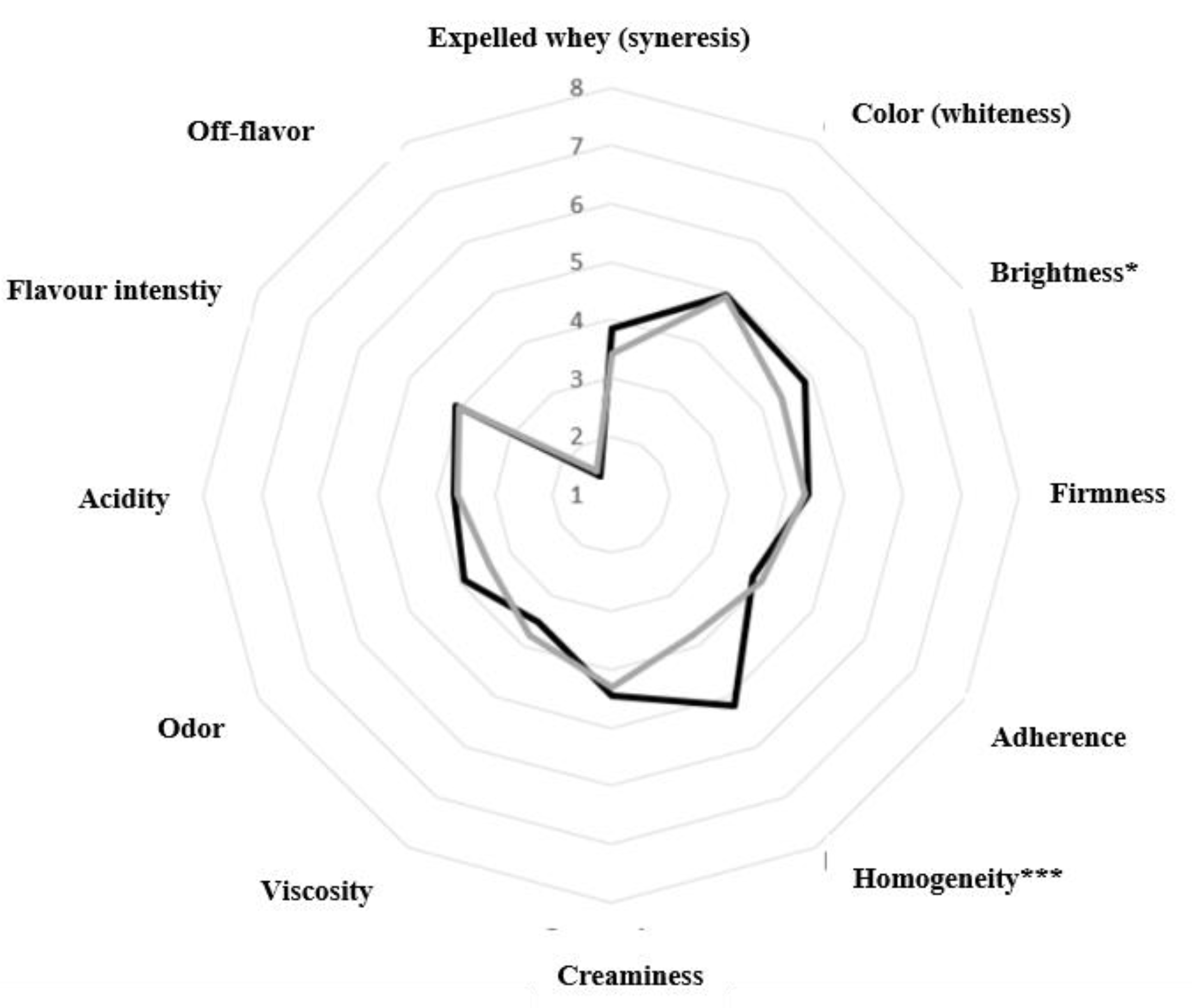
3.7. Sensory Evaluation of Yoghurts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, S.; Shah, T.; Sabara, P.; Bhatia, D.; Panchal, K.; Italiya, J.; Koringa, P.; Rank, D.N. Understanding Functional Implication of β-Casein Gene Variants in Four Cattle Breeds Characterized Using AmpliSeq Approach. 3 Biotech 2020, 10, 414. [Google Scholar] [CrossRef]
- Farrell, H.M.; Jimenez-Flores, R.; Bleck, G.T.; Brown, E.M.; Butler, J.E.; Creamer, L.K.; Hicks, C.L.; Hollar, C.M.; Ng-Kwai-Hang, K.F.; Swaisgood, H.E. Nomenclature of the Proteins of Cows’ Milk—Sixth Revision. J. Dairy Sci. 2004, 87, 1641–1674. [Google Scholar] [CrossRef] [PubMed]
- Summer, A.; Di Frangia, F.; Ajmone Marsan, P.; De Noni, I.; Malacarne, M. Occurrence, Biological Properties and Potential Effects on Human Health of β-Casomorphin 7: Current Knowledge and Concerns. Crit. Rev. Food Sci. Nutr. 2020, 60, 3705–3723. [Google Scholar] [CrossRef]
- Daniloski, D.; McCarthy, N.A.; Vasiljevic, T. Impact of Heating on the Properties of A1/A1, A1/A2, and A2/A2 β-Casein Milk Phenotypes. Food Hydrocoll. 2022, 128, 107064. [Google Scholar] [CrossRef]
- Lambers, T.T.; Broeren, S.; Heck, J.; Bragt, M.; Huppertz, T. Processing Affects Beta-Casomorphin Peptide Formation during Simulated Gastrointestinal Digestion in Both A1 and A2 Milk. Int. Dairy J. 2021, 121, 105099. [Google Scholar] [CrossRef]
- Nguyen, H.T.H.; Schwendel, H.; Harland, D.; Day, L. Differences in the Yoghurt Gel Microstructure and Physicochemical Properties of Bovine Milk Containing A1A1 and A2A2 β-Casein Phenotypes. Food Res. Int. 2018, 112, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Soumitra Banerjee A2 Milk: The Unknown Story About a Milk Protein. Act Sci Nutr. Heal. 2018, 2, 28–31.
- Asledottir, T.; Le, T.T.; Petrat-Melin, B.; Devold, T.G.; Larsen, L.B.; Vegarud, G.E. Identification of Bioactive Peptides and Quantification of β-Casomorphin-7 from Bovine β-Casein A1, A2 and I after Ex Vivo Gastrointestinal Digestion. Int. Dairy J. 2017, 71, 98–106. [Google Scholar] [CrossRef]
- De Noni, I.; FitzGerald, R.J.; Korhonen, H.J.T.; Le Roux, Y.; Livesey, C.T.; Thorsdottir, I.; Tomé, D.; Witkamp, R. Review of the Potential Health Impact of β-Casomorphins and Related Peptides. EFSA J. 2009, 7, 231r. [Google Scholar] [CrossRef]
- Walstra, P.; Wouters, J.T.M.; Geurts, T.J. Dairy Science and Technology; CRC Press: Boca Raton, FL, USA, 2005; ISBN 9780429116148. [Google Scholar]
- Nagaoka, S. Yogurt Production. In Methods in Molecular Biology; Springer Protocols; Humana Press: New York, NY, USA, 2019; Volume 1887, pp. 45–54. ISBN 978-1-4939-8906-5. [Google Scholar]
- Ketto, I.A.; Knutsen, T.M.; Øyaas, J.; Heringstad, B.; Ådnøy, T.; Devold, T.G.; Skeie, S.B. Effects of Milk Protein Polymorphism and Composition, Casein Micelle Size and Salt Distribution on the Milk Coagulation Properties in Norwegian Red Cattle. Int. Dairy J. 2017, 70, 55–64. [Google Scholar] [CrossRef]
- Laiho, S.; Williams, R.P.W.; Poelman, A.; Appelqvist, I.; Logan, A. Effect of Whey Protein Phase Volume on the Tribology, Rheology and Sensory Properties of Fat-Free Stirred Yoghurts. Food Hydrocoll. 2017, 67, 166–177. [Google Scholar] [CrossRef]
- Daniloski, D.; Vasiljevic, T.; Freitas, D.; Comunian, T.A.; Brodkorb, A.; McCarthy, N.A. Physicochemical and simulated gastric digestion properties of A1/A1, A1/A2 and A2/A2 yoghurts. Food Hydrocoll. 2024, 157, 110430. [Google Scholar] [CrossRef]
- Juan, B.; Trujillo, A.J. Acid and Rennet Coagulation Properties of A2 Milk. Foods 2022, 11, 3648. [Google Scholar] [CrossRef]
- ISO-IDF ISO 6731:2010|IDF 21:2010; Milk, Cream and Evaporated Milk Determination of Total Solids Content (Reference Method). ISO: Geneva, Switzerland; IDF: Brussel, Belgium, 2010.
- ISO-IDF ISO 8968-1|IDF 20-1; Milk and Milk Products. Determination of Nitrogen Content. ISO: Geneva, Switzerland; IDF: Brussel, Belgium, 2014.
- Akalın, A.S.; Unal, G.; Dinkci, N.; Hayaloglu, A.A. Microstructural, Textural, and Sensory Characteristics of Probiotic Yogurts Fortified with Sodium Calcium Caseinate or Whey Protein Concentrate. J. Dairy Sci. 2012, 95, 3617–3628. [Google Scholar] [CrossRef]
- Salvador, A.; Fiszman, S.M. Textural and Sensory Characteristics of Whole and Skimmed Flavored Set-Type Yogurt during Long Storage. J. Dairy Sci. 2004, 87, 4033–4041. [Google Scholar] [CrossRef] [PubMed]
- Gaurav, S.; Raja, B. (Eds.) Digital Color Imaging Handbook; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781315220086. [Google Scholar]
- Comin, A.; Cassandro, M.; Chessa, S.; Ojala, M.; Dal Zotto, R.; De Marchi, M.; Carnier, P.; Gallo, L.; Pagnacco, G.; Bittante, G. Effects of Composite β- and κ-Casein Genotypes on Milk Coagulation, Quality, and Yield Traits in Italian Holstein Cows. J. Dairy Sci. 2008, 91, 4022–4027. [Google Scholar] [CrossRef] [PubMed]
- Gai, N.; Uniacke-Lowe, T.; O’regan, J.; Faulkner, H.; Kelly, A.L. Effect of Protein Genotypes on Physicochemical Properties and Protein Functionality of Bovine Milk: A Review. Foods 2021, 10, 2409. [Google Scholar] [CrossRef] [PubMed]
- Hallén, E.; Allmere, T.; Lundén, A.; Andrén, A. Effect of Genetic Polymorphism of Milk Proteins on Rheology of Acid-Induced Milk Gels. Int. Dairy J. 2009, 19, 399–404. [Google Scholar] [CrossRef]
- Glantz, M.; Devold, T.G.; Vegarud, G.E.; Lindmark Månsson, H.; Stålhammar, H.; Paulsson, M. Importance of Casein Micelle Size and Milk Composition for Milk Gelation. J. Dairy Sci. 2010, 93, 1444–1451. [Google Scholar] [CrossRef] [PubMed]
- Chandan, R.C. An Overview of Yogurt Production and Composition. In Yogurt in Health and Disease Prevention; Press, A., Ed.; Elsevier: Amsterdam, The Netherland, 2017; pp. 31–47. [Google Scholar]
- Beal, C.; Skokanova, J.; Latrille, E.; Martin, N.; Corrieu, G. Combined Effects of Culture Conditions and Storage Time on Acidification and Viscosity of Stirred Yogurt. J. Dairy Sci. 1999, 82, 673–681. [Google Scholar] [CrossRef]
- Kneifel, W.; Jaros, D.; Erhard, F. Microflora and Acidification Properties of Yogurt and Yogurt-Related Products Fermented with Commercially Available Starter Cultures. Int. J. Food Microbiol. 1993, 18, 179–189. [Google Scholar] [CrossRef]
- Codex STAN 243-2003; Codex Standard for Fermented Milks. No. CODEX Stan. Alimentarius Commission: Rome, Italy, 2003; pp. 1–5.
- Guan, C.; Chen, X.; Zhao, R.; Yuan, Y.; Huang, X.; Su, J.; Ding, X.; Chen, X.; Huang, Y.; Gu, R. A Weak Post-acidification Lactobacillus Helveticus UV Mutant with Improved Textural Properties. Food Sci. Nutr. 2021, 9, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Sodini, I.; Lucas, A.; Oliveira, M.N.; Remeuf, F.; Corrieu, G. Effect of Milk Base and Starter Culture on Acidification, Texture, and Probiotic Cell Counts in Fermented Milk Processing. J. Dairy Sci. 2002, 85, 2479–2488. [Google Scholar] [CrossRef] [PubMed]
- Tamime, A.Y.; Robinson, R.K. Tamime and Robinson’s Yoghurt: Science and Technology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2007; ISBN 9781845692131. [Google Scholar]
- Wang, Y.; Feng, K.; Jin, J.; Safian Murad, M.; Mu, G.; Wu, X. Comparison on Properties between Normal and A2 Bovine Milk Fermented Using Commercial Bacteria Mixed with/without Two Probiotics from Human Milk. Int. J. Biol. Macromol. 2022, 216, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Cais-Sokolinska, D.; Pikul, J. Use of Colour Measurement to Evaluate Yoghurt Quality during Storage. Ital. J. Food Sci. 2006, 18, 63–71. [Google Scholar]
- Francis, F.J. Colorimetry of Foods. In Physical Properties of Foods; Peleg, M., Bagly, E.B., Eds.; The AVI Publishing Company Inc.: Westport, Ireland, 1983; pp. 105–123. [Google Scholar]
- Lee, W.J.; Lucey, J.A. Formation and Physical Properties of Yogurt. Asian-Australasian J. Anim. Sci. 2010, 23, 1127–1136. [Google Scholar] [CrossRef]

| Milk 1 | Lac 3 | DIL 4 | Number of Cows of Each Genetic Variant 2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β-CN | κ-CN | β-LG | ||||||||||||
| A2A2 | A1A2 | A1A1 | AA | BB | AB | BE | AE | EE | AB | BB | AA | |||
| C | 2.1 | 118.8 | 12 | 3 | 4 | 1 | 2 | 4 | 3 | 1 | 8 | 6 | 1 | |
| A2 | 2.4 | 107.7 | 15 | 2 | 3 | 10 | 7 | 3 | 5 | |||||
| Yoghurt | Storage Time (Days) 1 | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 7 | 14 | 21 | 28 | 35 | ||
| pH | C | 4.51 ± 0.02 a | 4.54 ± 0.05 a | 4.48 ± 0.02 ab | 4.50 ± 0.01 ab | 4.46 ± 0.03 b | 4.44 ± 0.04 b |
| A2 | 4.57 ± 0.03 a | 4.55 ± 0.03 ab | 4.48 ± 0.01 ab | 4.45 ± 0.03 b | 4.44 ± 0.03 b | 4.43 ± 0.04 b | |
| Acidity | C | 83.83 ± 1.13 b | 89.65 ± 0.72 a | 91.41 ± 0.90 a | 90.35 ± 0.36 ay | 91.83 ± 0.34 ay | 91.16 ± 0.17 a,y |
| A2 | 84.55 ± 1.07 c | 90.58 ± 0.23 b | 92.58 ± 1.04 a | 94.62 ± 0.31 ax | 93.76 ± 0.82 ax | 94.79 ± 1.10 a,x | |
| Yoghurt | Storage Time (Days) 1 | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 7 | 14 | 21 | 28 | 35 | ||
| WHC | C | 65.47 ± 0.06 ax | 63.79 ± 0.05 ax | 61.83 ± 0.05 ax | 67.06 ± 0.05 ax | 65.01 ± 0.03 ax | 63.74 ± 0.04 ax |
| A2 | 63.31 ± 0.07 ax | 64.68 ± 0.04 ax | 59.46 ± 0.04 ax | 63.54 ± 0.03 ax | 64.61 ± 0.04 ax | 63.05 ± 0.02 ax | |
| Spontaneous syneresis | C | 0.90 ± 0.14 | 1.04 ± 0.27 | 0.83 ± 0.24 | 0.85 ± 0.17 | 0.75 ± 0.14 | 0.62 ± 0.12 |
| A2 | 0.88 ± 0.14 | 0.64 ± 0.12 | 0.86 ± 0.11 | 1.09 ± 0.21 | 0.60 ± 0.09 | 0.75 ± 0.17 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juan Godoy, B.; Codina-Torrella, I.; Trujillo Mesa, A.-J. Effect of Genetic Polymorphism of Bovine β-Casein Variants (A1 and A2) on Yoghurt Characteristics. Foods 2024, 13, 4135. https://doi.org/10.3390/foods13244135
Juan Godoy B, Codina-Torrella I, Trujillo Mesa A-J. Effect of Genetic Polymorphism of Bovine β-Casein Variants (A1 and A2) on Yoghurt Characteristics. Foods. 2024; 13(24):4135. https://doi.org/10.3390/foods13244135
Chicago/Turabian StyleJuan Godoy, Bibiana, Idoia Codina-Torrella, and Antonio-José Trujillo Mesa. 2024. "Effect of Genetic Polymorphism of Bovine β-Casein Variants (A1 and A2) on Yoghurt Characteristics" Foods 13, no. 24: 4135. https://doi.org/10.3390/foods13244135
APA StyleJuan Godoy, B., Codina-Torrella, I., & Trujillo Mesa, A.-J. (2024). Effect of Genetic Polymorphism of Bovine β-Casein Variants (A1 and A2) on Yoghurt Characteristics. Foods, 13(24), 4135. https://doi.org/10.3390/foods13244135









