Impact of a Plant Sterol Food Supplement on Eryptotic and Associated Cardiometabolic Parameters: A Randomized Placebo-Controlled Trial in Statin-Treated Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Sterol-Enriched Food Supplement
2.2. Study Design
2.3. Blood Samples Collection
2.4. Determination of Biochemical Parameters
2.5. Evaluation of Phosphatidylserine Externalization (EPHS) and Forward Scatter (FSC)
2.6. Intracellular Glutathione
2.7. Assessment of the Adhesion to the Endothelium
2.8. Lifestyle Surveys
2.9. Statistical Analysis
3. Results
3.1. Biochemical Parameters
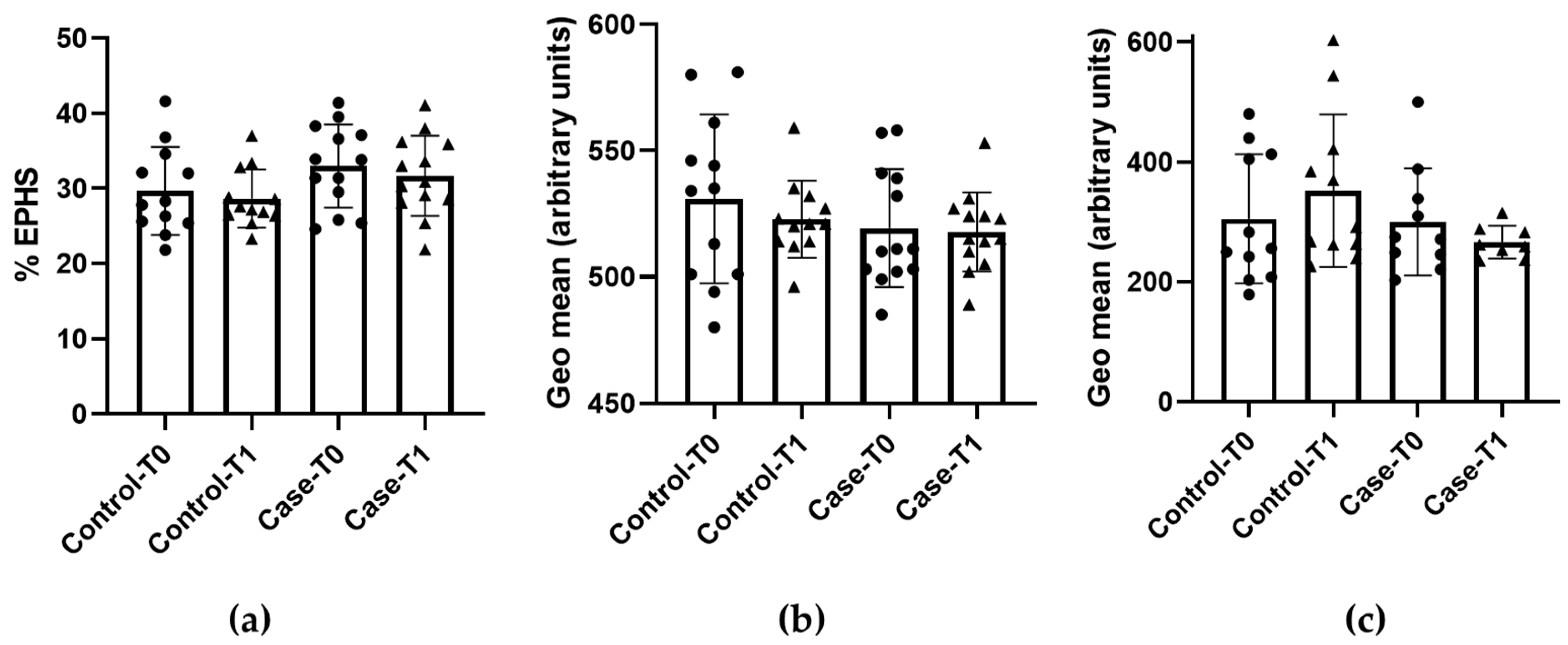
3.2. Eryptosis and Redox Status
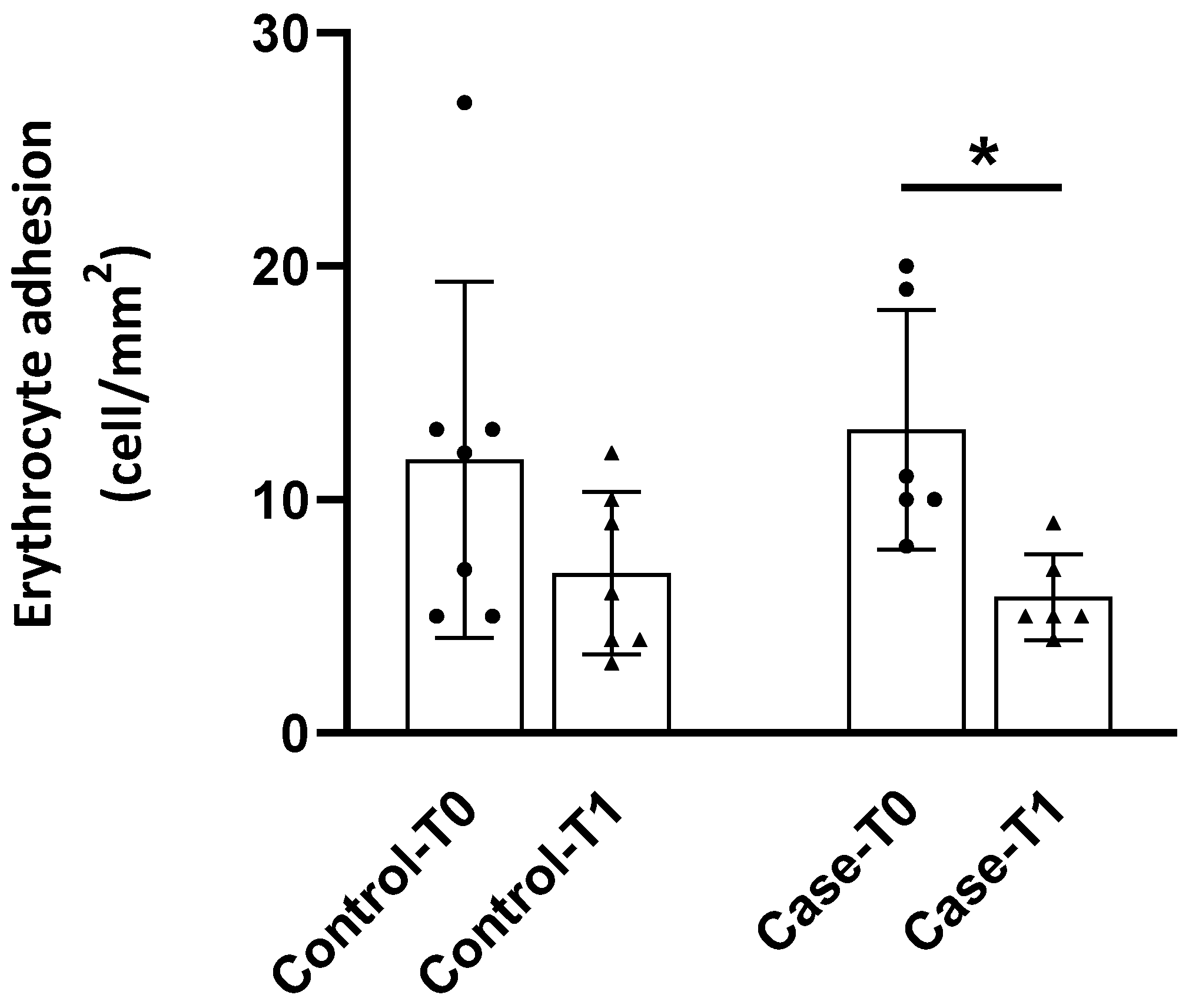
3.3. Adhesion to the Endothelium
3.4. Lifestyle Surveys
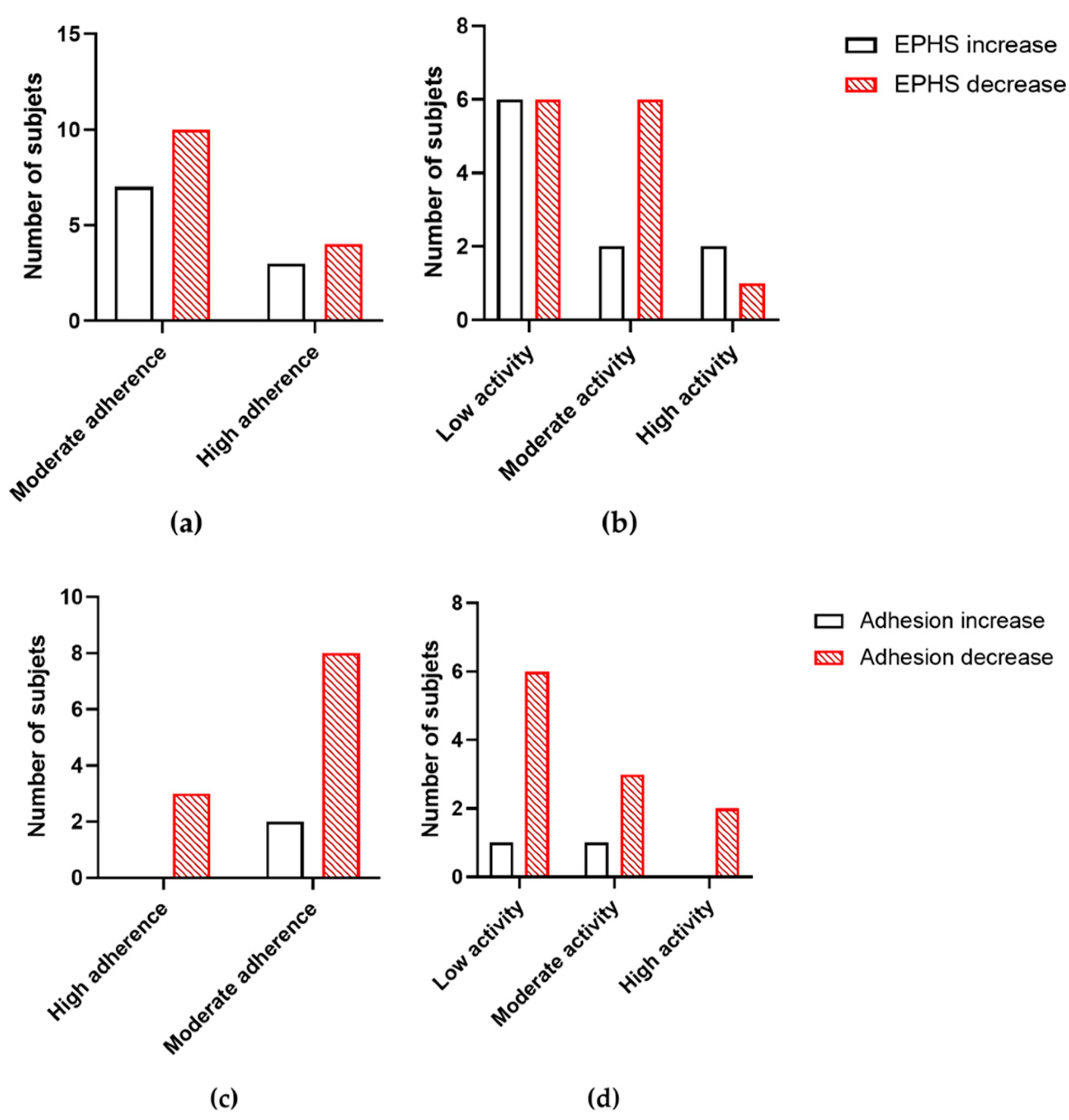
3.5. Correlations Between Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alghareeb, S.A.; Alfhili, M.A.; Fatima, S. Molecular mechanisms and pathophysiological significance of eryptosis. Int. J. Mol. Sci. 2023, 24, 5079. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Abed, M.; Lang, E.; Föller, M. Oxidative stress and suicidal erythrocyte death. Antioxid. Redox Signal. 2014, 21, 138–153. [Google Scholar] [CrossRef]
- Pretorius, E.; du Plooy, J.N.; Bester, J. A comprehensive review on eryptosis. Cell. Physiol. Biochem. 2016, 39, 1977–2000. [Google Scholar] [CrossRef]
- Fang, M.; Xia, F.; Chen, Y.; Shen, Y.; Ma, L.; You, C.; Tao, C.; Hu, X. Role of eryptosis in hemorrhagic stroke. Front. Mol. Neurosci. 2022, 15, 932931. [Google Scholar] [CrossRef] [PubMed]
- Restivo, I.; Attanzio, A.; Tesoriere, L.; Allegra, M. Suicidal erythrocyte death in metabolic syndrome. Antioxidants 2021, 10, 154. [Google Scholar] [CrossRef] [PubMed]
- Cilla, A.; López-García, G.; Collado-Díaz, V.; Blanch-Ruiz, M.A.; Garcia-Llatas, G.; Barberá, R.; Martinez-Cuesta, M.A.; Real, J.T.; Álvarez, Á.; Martínez-Hervás, S. Hypercholesterolemic patients have higher eryptosis and erythrocyte adhesion to human endothelium independently of statin therapy. Int. J. Clin. Pract. 2021, 75, e14771. [Google Scholar] [CrossRef] [PubMed]
- Pinzón-Díaz, C.E.; Calderón-Salinas, J.V.; Rosas-Flores, M.M.; Hernández, G.; López-Betancourt, A.; Quintanar-Escorza, M.A. Eryptosis and oxidative damage in hypertensive and dyslipidemic patients. Mol. Cell. Biochem. 2018, 440, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Rana, R.B.; Jilani, K.; Shahid, M.; Riaz, M.; Ranjha, M.H.; Bibi, I.; Asghar, A.; Irfan, M. Atorvastatin induced erythrocytes membrane blebbing. Dose Response 2019, 17, 1559325819869076. [Google Scholar] [CrossRef] [PubMed]
- Al Mamun Bhuyan, A.; Nüßle, S.; Cao, H.; Zhang, S.; Lang, F. Simvastatin, a novel stimulator of eryptosis, the suicidal erythrocyte death. Cell. Physiol. Biochem. 2017, 43, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Sala, A.; López-García, G.; Attanzio, A.; Tesoriere, L.; Cilla, A.; Barberá, R.; Alegría, A. Effects of plant sterols or β-cryptoxanthin at physiological serum concentrations on suicidal erythrocyte death. J. Agric. Food Chem. 2018, 66, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EU) No 686/2014 of 20 June 2014 amending Regulations (EC) No 983/2009 and (EU) No 384/2010 as regards the conditions of use of certain health claims related to the lowering effect of plant sterols and plant stanols on blood LDL-cholesterol. Off. J. Eur. Union 2014, L182, 27–30.
- Alvarez-Sala, A.; Blanco-Morales, V.; Cilla, A.; Silvestre, R.Á.; Hernández-Álvarez, E.; Granado-Lorencio, F.; Barberá, R.; Garcia-Llatas, G. A positive impact on the serum lipid profile and cytokines after the consumption of a plant sterol-enriched beverage with a milk fat globule membrane: A clinical study. Food Funct. 2018, 9, 5209–5219. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Morales, V.; Silvestre, R.L.Á.; Hernández-Álvarez, E.; Donoso-Navarro, E.; Alegría, A.; Garcia-Llatas, G. Influence of galactooligosaccharides on the positive effect of plant sterol-enriched beverages on cardiovascular risk and sterol colon metabolism. J. Agric. Food Chem. 2022, 70, 532–542. [Google Scholar] [CrossRef]
- Hong, C.G.; Florida, E.; Li, H.; Parel, P.M.; Mehta, N.N.; Sorokin, A.V. Oxidized low-density lipoprotein associates with cardiovascular disease by a vicious cycle of atherosclerosis and inflammation: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2023, 9, 1023651. [Google Scholar] [CrossRef]
- Han, S.; Jiao, J.; Xu, J.; Zimmermann, D.; Actis-Goretta, L.; Guan, L.; Zhao, Y.; Qin, L. Effects of plant stanol or sterol-enriched diets on lipid profiles in patients treated with statins: Systematic review and meta-analysis. Sci. Rep. 2016, 6, 31337. [Google Scholar] [CrossRef] [PubMed]
- Malina, D.M.; Fonseca, F.A.; Barbosa, S.A.; Kasmas, S.H.; Machado, V.A.; França, C.N.; Borges, N.C.; Moreno, R.A.; Izar, M.C. Additive effects of plant sterols supplementation in addition to different lipid-lowering regimens. J. Clin. Lipidol. 2015, 9, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Hervas, S.; Fandos, M.; Real, J.T.; Espinosa, O.; Chaves, F.J.; Saez, G.T.; Salvador, A.; Cerdá, C.; Carmena, R.; Ascaso, J.F. Insulin resistance and oxidative stress in familial combined hyperlipidemia. Atherosclerosis 2008, 199, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Tesoriere, L.; Attanzio, A.; Allegra, M.; Cilla, A.; Gentile, C.; Livrea, M.A. Oxysterol mixture in hypercholesterolemia-relevant proportion causes oxidative stress-dependent eryptosis. Cell. Physiol. Biochem. 2014, 34, 1075–1089. [Google Scholar] [CrossRef]
- Kaliyaperumal, R.; Deng, X.; Meiselman, H.J.; Song, H.; Dalan, R.; Leow, M.K.; Neu, B. Depletion interaction forces contribute to erythrocyte-endothelial adhesion in diabetes. Biochem. Biophys. Res. Commun. 2019, 516, 144–148. [Google Scholar] [CrossRef]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M.; et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Demonty, I.; Ras, R.T.; van der Knaap, H.C.; Duchateau, G.S.; Meijer, L.; Zock, P.L.; Geleijnse, J.M.; Trautwein, E.A. Continuous dose-response relationship of the LDL-cholesterol-lowering effect of phytosterol intake. J. Nutr. 2009, 139, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Naumann, E.; Plat, J.; Kester, A.D.; Mensink, R.P. The baseline serum lipoprotein profile is related to plant stanol induced changes in serum lipoprotein cholesterol and triacylglycerol concentrations. J. Am. Coll. Nutr. 2008, 27, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Kalayci, A.; Gibson, C.M.; Ridker, P.M.; Wright, S.D.; Kingwell, B.A.; Korjian, S.; Chi, G.; Lee, J.J.; Tricoci, P.; Kazmi, S.H.; et al. ApoA-I infusion therapies following acute coronary syndrome: Past, present, and future. Curr. Atheroscler. Rep. 2022, 24, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Primer, K.R.; Psaltis, P.J.; Tan, J.T.M.; Bursill, C.A. The role of high-density lipoproteins in endothelial cell metabolism and diabetes-impaired angiogenesis. Int. J. Mol. Sci. 2020, 21, 3633. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.J.; Woollard, K.J.; Suhartoyo, A.; Stirzaker, R.A.; Shaw, J.; Sviridov, D.; Chin-Dusting, J.P. Neutrophil activation is attenuated by high-density lipoprotein and apolipoprotein A-I in in vitro and in vivo models of inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1333–1341. [Google Scholar] [CrossRef]
| Control T0 (n = 13) | Control T1 (n = 13) | Case T0 (n = 13) | Case T1 (n = 13) | |
|---|---|---|---|---|
| Glucose (mg/dL) | 97.9 ± 10.0 | 93.7 ± 12.6 * | 95.5 ± 9.8 | 93.9 ± 8.4 |
| Insulin (µU/mL) | 9.0 ± 4.5 | 8.0 ± 3.1 | 10.0 ± 5.3 | 9.8 ± 5.4 |
| HOMA index | 2.3 ± 1.3 | 1.9 ± 1.1 | 2.4 ± 1.4 | 2.3 ± 1.4 |
| TC (mg/dL) | 173.1 ± 25.9 | 165.2 ± 27.9 | 172.2 ± 28.2 | 168.1 ± 34.8 |
| HDL-c (mg/dL) | 53.8 ± 13.1 | 55.0 ± 13.2 | 55.5 ± 8.8 | 56.2 ± 10.4 |
| LDL-c (mg/dL) | 101.3 ± 22.7 | 92.0 ± 24.6 * | 98.6 ± 25.7 | 94.9 ± 28.7 |
| TG (mg/dL) | 89.9 ± 24.9 | 91.6 ± 31.9 | 90.8 ± 32.9 | 84.6 ± 25.9 |
| Apo A1 (mg/dL) | 172.1 ± 30.1 | 177.1 ± 31.8 | 163.7 ± 19.4 | 174.1 ± 21.2 * |
| Apo B (mg/dL) | 86.9 ± 15.2 | 84.2 ± 17.2 | 78.6 ± 18.6 | 83.6 ± 18.8 |
| hsCRP | 2.7 ± 2.2 | 2.1 ± 1.1 | 1.5 ± 1.0 | 1.8 ± 0.8 |
| Adherence to the Mediterranean Diet | Low | Moderate | High |
| Total (n = 26) | 0 | 19 (73.1) | 7 (26.9) |
| Level of physical activity | Low | Moderate | High |
| Total (n = 26) | 14 (53.8) | 9 (34.6) | 3 (11.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miedes, D.; Ortega-Luna, R.; Broseta, S.; Martínez-Hervás, S.; Álvarez-Ribelles, Á.; Collado-Díaz, V.; Cilla, A.; Alegría, A. Impact of a Plant Sterol Food Supplement on Eryptotic and Associated Cardiometabolic Parameters: A Randomized Placebo-Controlled Trial in Statin-Treated Patients. Foods 2024, 13, 4108. https://doi.org/10.3390/foods13244108
Miedes D, Ortega-Luna R, Broseta S, Martínez-Hervás S, Álvarez-Ribelles Á, Collado-Díaz V, Cilla A, Alegría A. Impact of a Plant Sterol Food Supplement on Eryptotic and Associated Cardiometabolic Parameters: A Randomized Placebo-Controlled Trial in Statin-Treated Patients. Foods. 2024; 13(24):4108. https://doi.org/10.3390/foods13244108
Chicago/Turabian StyleMiedes, Diego, Raquel Ortega-Luna, Sonia Broseta, Sergio Martínez-Hervás, Ángeles Álvarez-Ribelles, Víctor Collado-Díaz, Antonio Cilla, and Amparo Alegría. 2024. "Impact of a Plant Sterol Food Supplement on Eryptotic and Associated Cardiometabolic Parameters: A Randomized Placebo-Controlled Trial in Statin-Treated Patients" Foods 13, no. 24: 4108. https://doi.org/10.3390/foods13244108
APA StyleMiedes, D., Ortega-Luna, R., Broseta, S., Martínez-Hervás, S., Álvarez-Ribelles, Á., Collado-Díaz, V., Cilla, A., & Alegría, A. (2024). Impact of a Plant Sterol Food Supplement on Eryptotic and Associated Cardiometabolic Parameters: A Randomized Placebo-Controlled Trial in Statin-Treated Patients. Foods, 13(24), 4108. https://doi.org/10.3390/foods13244108








