Bioactive Peptides in Greek Goat Colostrum: Relevance to Human Metabolism
Abstract
1. Introduction
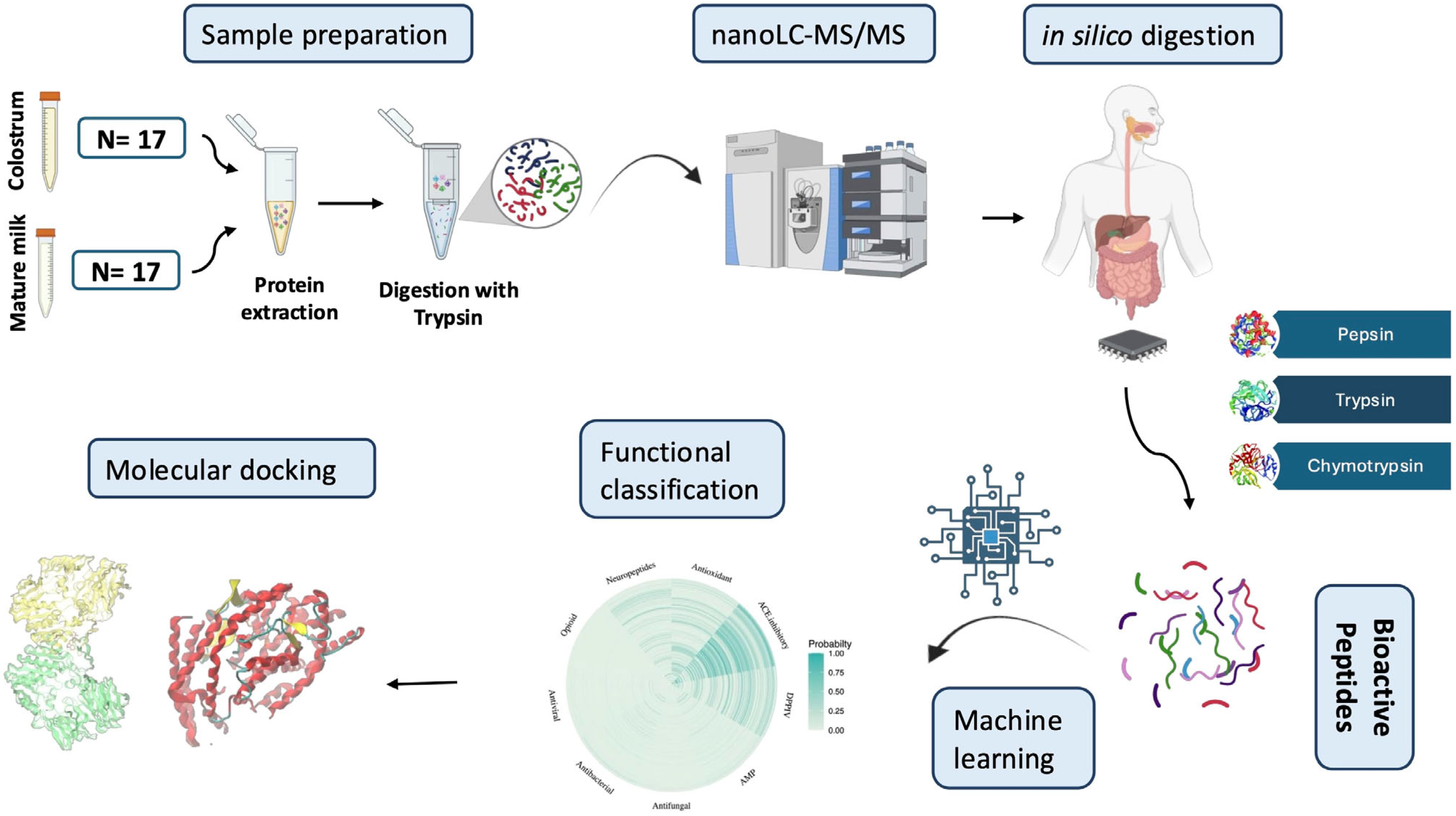
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Proteomic Workflow
2.2.1. Protein Extraction
2.2.2. Peptide Separation and Tandem Mass Spectrometry Analysis
2.3. Data Analysis
2.3.1. Comparative Analysis (Colostrum and Mature Milk)
2.3.2. Protein Digestion and Peptide Functional Annotation
3. Results
3.1. Proteomic Landscape
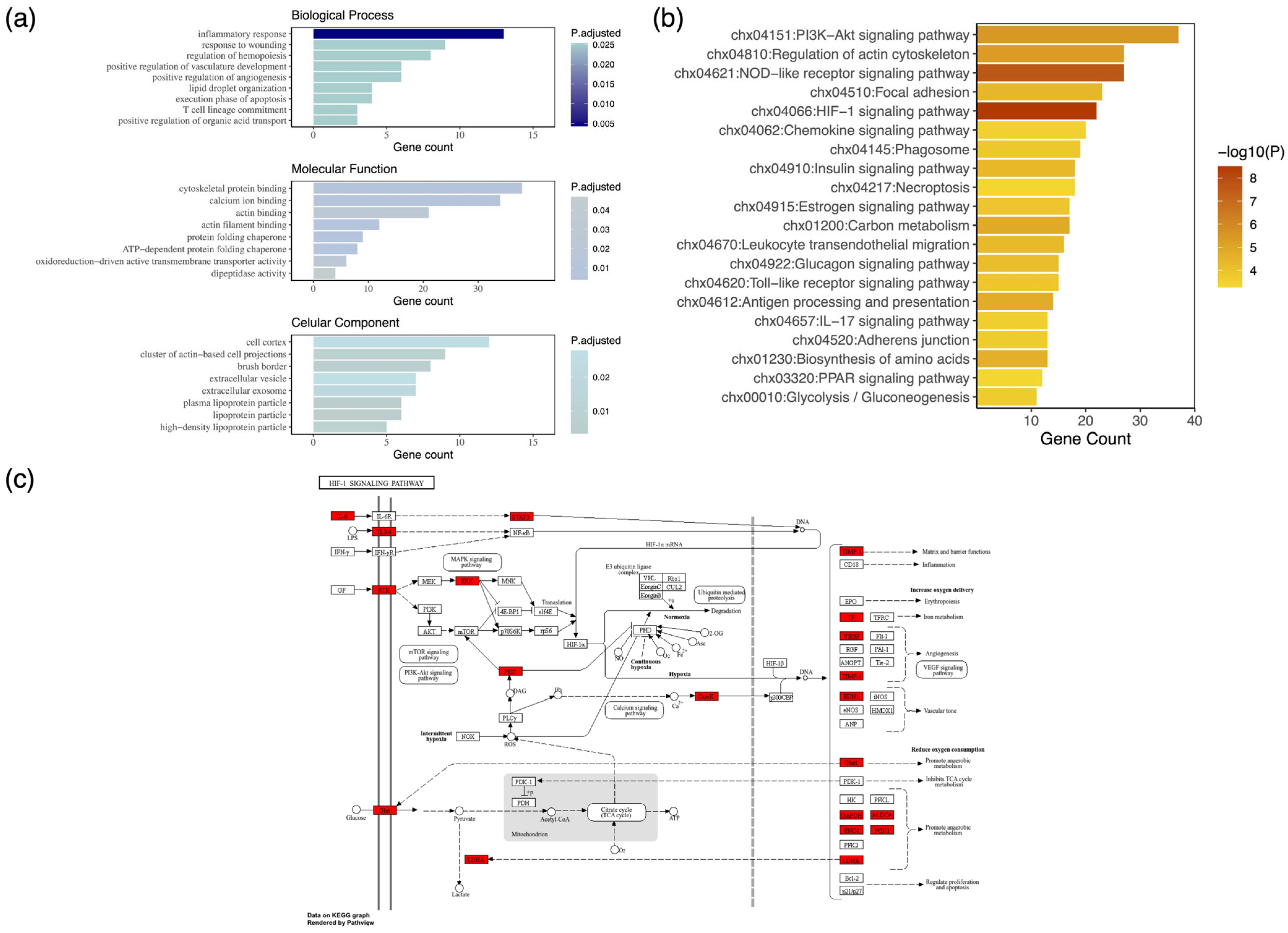
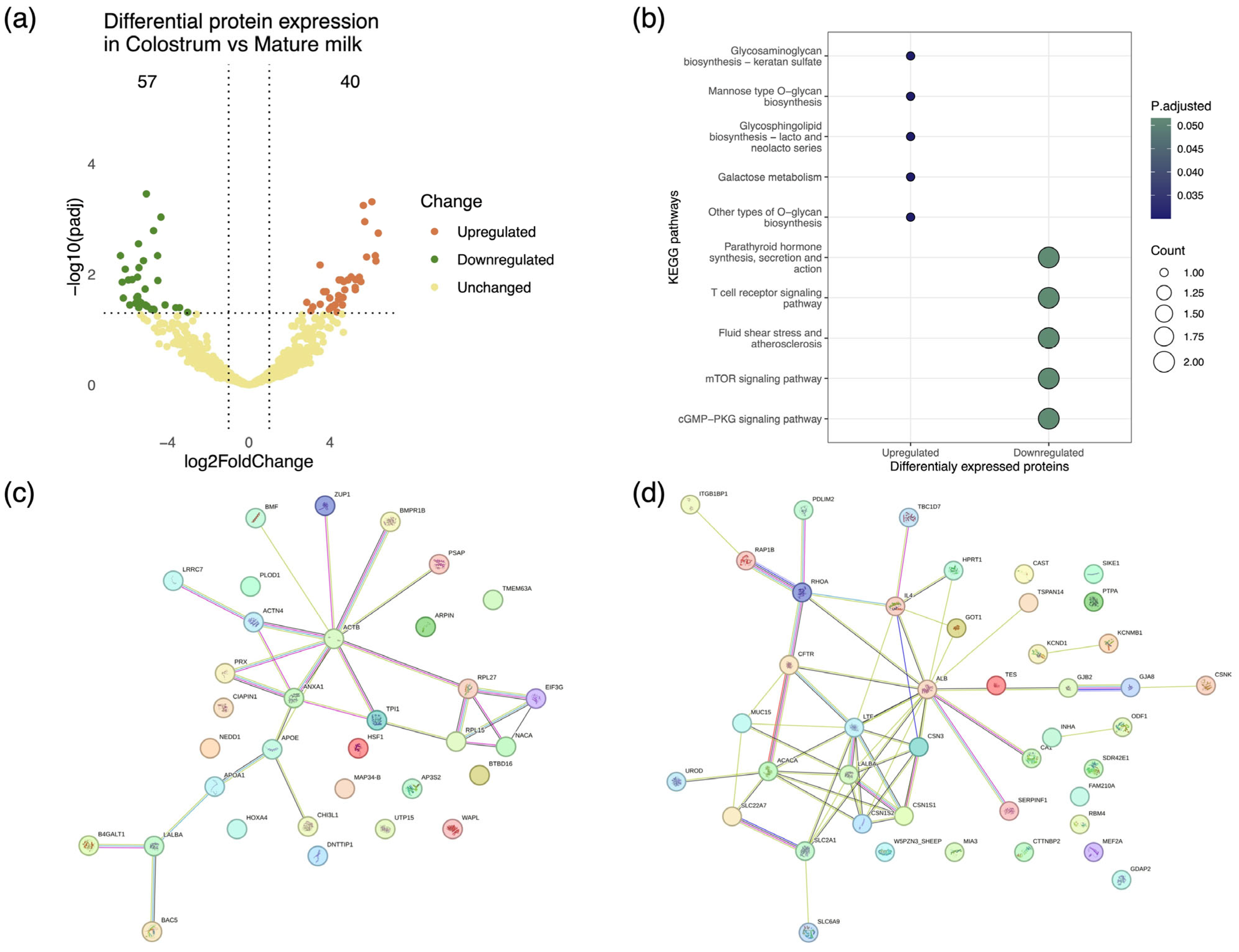
3.2. Comparative Analysis of Colostrum and Mature Milk
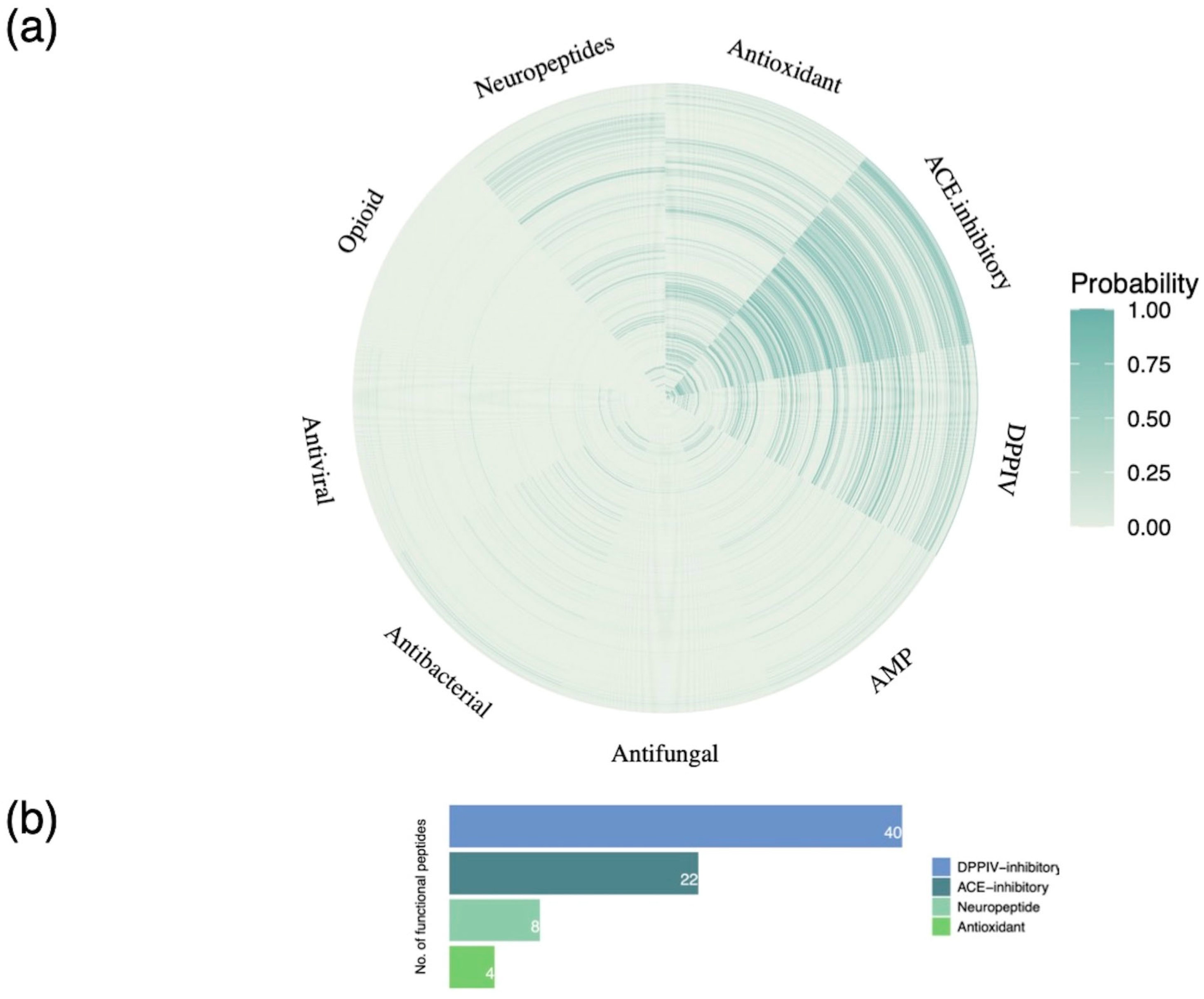
Bioactive Peptides in Colostrum: Functional Classification and Relevance to Human Metabolism
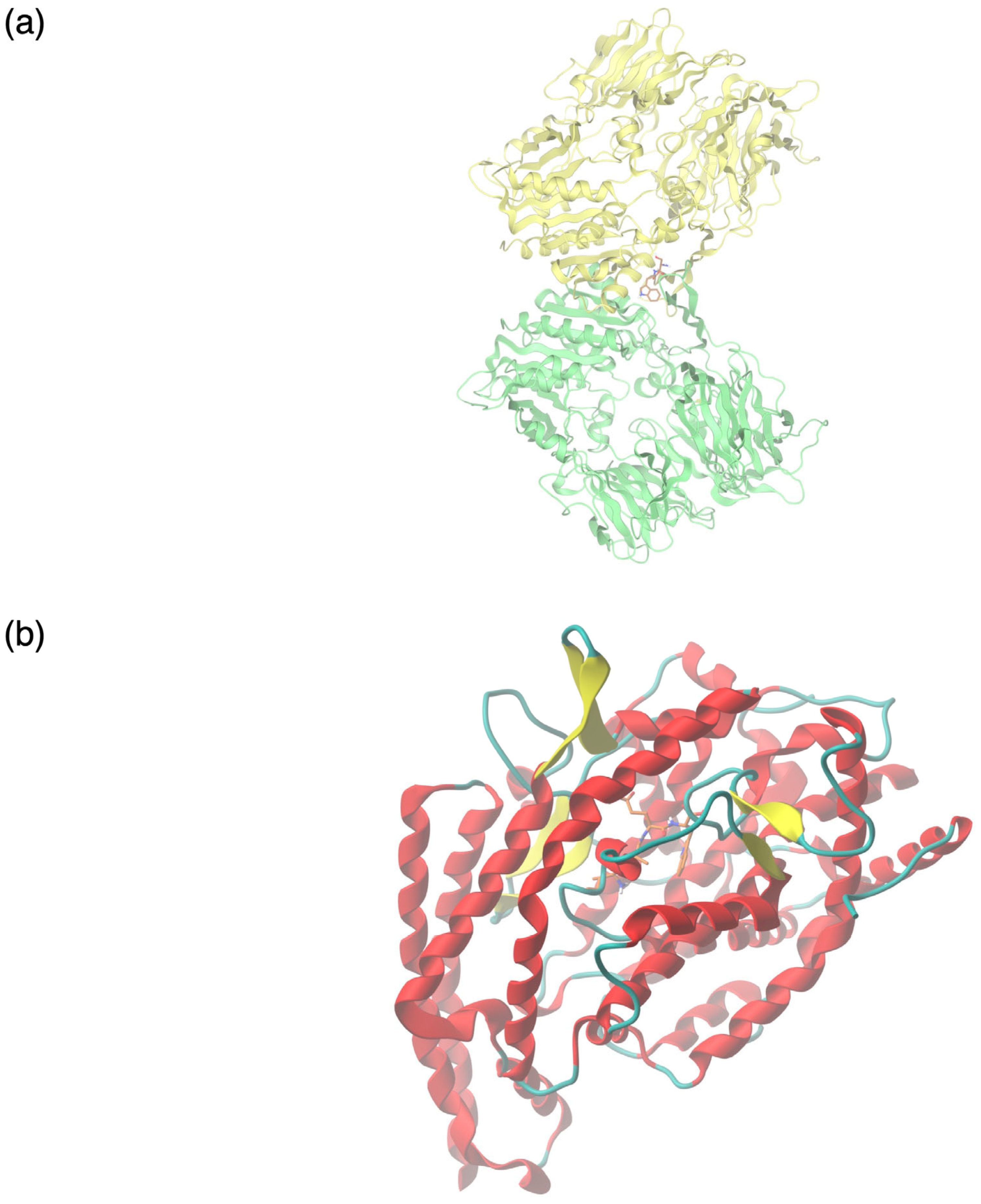
3.3. Bioactive Peptide Screening by Molecular Docking
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdel-Hamid, M.; Yang, P.; Mostafa, I.; Osman, A.; Romeih, E.; Yang, Y.; Huang, Z.; Awad, A.A.; Li, L. Changes in Whey Proteome between Mediterranean and Murrah Buffalo Colostrum and Mature Milk Reflect Their Pharmaceutical and Medicinal Value. Molecules 2022, 27, 1575. [Google Scholar] [CrossRef]
- Hadjipanayiotou, M. Composition of Ewe, Goat and Cow Milk and of Colostrum of Ewes and Goats. Small Rumin. Res. 1995, 18, 255–262. [Google Scholar] [CrossRef]
- Roy, D.; Ye, A.; Moughan, P.J.; Singh, H. Composition, Structure, and Digestive Dynamics of Milk From Different Species—A Review. Front. Nutr. 2020, 7, 577759. [Google Scholar] [CrossRef]
- Mills, S.; Ross, R.P.; Hill, C.; Fitzgerald, G.F.; Stanton, C. Milk Intelligence: Mining Milk for Bioactive Substances Associated with Human Health. Int. Dairy J. 2011, 21, 377–401. [Google Scholar] [CrossRef]
- Nielsen, S.D.; Beverly, R.L.; Qu, Y.; Dallas, D.C. Milk Bioactive Peptide Database: A Comprehensive Database of Milk Protein-Derived Bioactive Peptides and Novel Visualization. Food Chem. 2017, 232, 673–682. [Google Scholar] [CrossRef]
- Sánchez-Macías, D.; Moreno-Indias, I.; Castro, N.; Morales-delaNuez, A.; Argüello, A. From Goat Colostrum to Milk: Physical, Chemical, and Immune Evolution from Partum to 90 Days Postpartum. J. Dairy Sci. 2014, 97, 10–16. [Google Scholar] [CrossRef]
- Agenbag, B.; Swinbourne, A.M.; Petrovski, K.; Van Wettere, W.H.E.J. Lambs Need Colostrum: A Review. Livest. Sci. 2021, 251, 104624. [Google Scholar] [CrossRef]
- Moreno-Indias, I.; Sánchez-Macías, D.; Castro, N.; Morales-delaNuez, A.; Hernández-Castellano, L.E.; Capote, J.; Argüello, A. Chemical Composition and Immune Status of Dairy Goat Colostrum Fractions during the First 10h after Partum. Small Rumin. Res. 2012, 103, 220–224. [Google Scholar] [CrossRef]
- Ruiz, P.; Seseña, S.; Rieiro, I.; Palop, M.L. Effect of Postpartum Time and Season on the Physicochemical Characteristics of Murciano-Granadina Goat Colostrum. Int. J. Dairy Technol. 2015, 68, 88–96. [Google Scholar] [CrossRef]
- Stelwagen, K.; Carpenter, E.; Haigh, B.; Hodgkinson, A.; Wheeler, T.T. Immune Components of Bovine Colostrum and Milk1. J. Anim. Sci. 2009, 87, 3–9. [Google Scholar] [CrossRef]
- Jia, W.; Zhang, R.; Zhu, Z.; Shi, L. LC-Q-Orbitrap HRMS-Based Proteomics Reveals Potential Nutritional Function of Goat Whey Fraction. J. Funct. Foods 2021, 82, 104502. [Google Scholar] [CrossRef]
- Blum, J.W.; Baumrucker, C.R. Colostral and Milk Insulin-like Growth Factors and Related Substances: Mammary Gland and Neonatal (Intestinal and Systemic) Targets. Domest. Anim. Endocrinol. 2002, 23, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, H. Milk-Derived Bioactive Peptides: From Science to Applications. J. Funct. Foods 2009, 1, 177–187. [Google Scholar] [CrossRef]
- Nielsen, S.D.-H.; Liang, N.; Rathish, H.; Kim, B.J.; Lueangsakulthai, J.; Koh, J.; Qu, Y.; Schulz, H.-J.; Dallas, D.C. Bioactive Milk Peptides: An Updated Comprehensive Overview and Database. Crit. Rev. Food Sci. Nutr. 2024, 64, 11510–11529. [Google Scholar] [CrossRef]
- Punia, H.; Tokas, J.; Malik, A.; Sangwan, S.; Baloda, S.; Singh, N.; Singh, S.; Bhuker, A.; Singh, P.; Yashveer, S.; et al. Identification and Detection of Bioactive Peptides in Milk and Dairy Products: Remarks about Agro-Foods. Molecules 2020, 25, 3328. [Google Scholar] [CrossRef]
- Marcone, S.; Belton, O.; Fitzgerald, D.J. Milk-derived Bioactive Peptides and Their Health Promoting Effects: A Potential Role in Atherosclerosis. Br. J. Clin. Pharmacol. 2017, 83, 152–162. [Google Scholar] [CrossRef]
- Sultan, S.; Huma, N.; Butt, M.S.; Aleem, M.; Abbas, M. Therapeutic Potential of Dairy Bioactive Peptides: A Contemporary Perspective. Crit. Rev. Food Sci. Nutr. 2018, 58, 105–115. [Google Scholar] [CrossRef]
- Karami, Z.; Akbari-adergani, B. Bioactive Food Derived Peptides: A Review on Correlation between Structure of Bioactive Peptides and Their Functional Properties. J. Food Sci. Technol. 2019, 56, 535–547. [Google Scholar] [CrossRef]
- Sánchez, A.; Vázquez, A. Bioactive Peptides: A Review. Food Qual. Saf. 2017, 1, 29–46. [Google Scholar] [CrossRef]
- Tonolo, F.; Folda, A.; Cesaro, L.; Scalcon, V.; Marin, O.; Ferro, S.; Bindoli, A.; Rigobello, M.P. Milk-Derived Bioactive Peptides Exhibit Antioxidant Activity through the Keap1-Nrf2 Signaling Pathway. J. Funct. Foods 2020, 64, 103696. [Google Scholar] [CrossRef]
- Gong, H.; Gao, J.; Wang, Y.; Luo, Q.W.; Guo, K.R.; Ren, F.Z.; Mao, X.Y. Identification of Novel Peptides from Goat Milk Casein That Ameliorate High-Glucose-Induced Insulin Resistance in HepG2 Cells. J. Dairy Sci. 2020, 103, 4907–4918. [Google Scholar] [CrossRef] [PubMed]
- Miner-Williams, W.M.; Stevens, B.R.; Moughan, P.J. Are Intact Peptides Absorbed from the Healthy Gut in the Adult Human? Nutr. Res. Rev. 2014, 27, 308–329. [Google Scholar] [CrossRef] [PubMed]
- Tomazou, M.; Oulas, A.; Anagnostopoulos, A.K.; Tsangaris, G.T.; Spyrou, G.M. In Silico Identification of Antimicrobial Peptides in the Proteomes of Goat and Sheep Milk and Feta Cheese. Proteomes 2019, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Campanhon, I.B.; De Aguiar, P.F.; Bezerra, F.F.; Soares, M.R.; Torres, A.G. Human Colostrum in Vitro Protein Digestion: Peptidomics by Liquid Chromatography-Orbitrap-High-Resolution MS and Prospection for Bioactive Peptides via Bioinformatics. Br. J. Nutr. 2024, 131, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Boutrou, R.; Gaudichon, C.; Dupont, D.; Jardin, J.; Airinei, G.; Marsset-Baglieri, A.; Benamouzig, R.; Tomé, D.; Leonil, J. Sequential Release of Milk Protein–Derived Bioactive Peptides in the Jejunum in Healthy Humans. Am. J. Clin. Nutr. 2013, 97, 1314–1323. [Google Scholar] [CrossRef]
- Ashok, N.R.; Aparna, H.S. Empirical and Bioinformatic Characterization of Buffalo (Bubalus bubalis) Colostrum Whey Peptides & Their Angiotensin I-Converting Enzyme Inhibition. Food Chem. 2017, 228, 582–594. [Google Scholar] [CrossRef]
- Sienkiewicz, M.; Szymańska, P.; Fichna, J. Supplementation of Bovine Colostrum in Inflammatory Bowel Disease: Benefits and Contraindications. Adv. Nutr. 2021, 12, 533–545. [Google Scholar] [CrossRef]
- Guberti, M.; Botti, S.; Capuzzo, M.T.; Nardozi, S.; Fusco, A.; Cera, A.; Dugo, L.; Piredda, M.; De Marinis, M.G. Bovine Colostrum Applications in Sick and Healthy People: A Systematic Review. Nutrients 2021, 13, 2194. [Google Scholar] [CrossRef]
- Asbjornsdottir, B.; Sigurdsson, S.; Miranda-Ribera, A.; Fiorentino, M.; Konno, T.; Lan, J.; Gudmundsson, L.S.; Gottfredsson, M.; Lauth, B.; Birgisdottir, B.E.; et al. Evaluating Prophylactic Effect of Bovine Colostrum on Intestinal Barrier Function in Zonulin Transgenic Mice: A Transcriptomic Study. Int. J. Mol. Sci. 2023, 24, 14730. [Google Scholar] [CrossRef]
- Kotsis, Y.; Mikellidi, A.; Aresti, C.; Persia, E.; Sotiropoulos, A.; Panagiotakos, D.B.; Antonopoulou, S.; Nomikos, T. A Low-Dose, 6-Week Bovine Colostrum Supplementation Maintains Performance and Attenuates Inflammatory Indices Following a Loughborough Intermittent Shuttle Test in Soccer Players. Eur. J. Nutr. 2018, 57, 1181–1195. [Google Scholar] [CrossRef]
- Polidori, P.; Rapaccetti, R.; Klimanova, Y.; Zhang, J.-J.; Santini, G.; Vincenzetti, S. Nutritional Parameters in Colostrum of Different Mammalian Species. Beverages 2022, 8, 54. [Google Scholar] [CrossRef]
- Zhou, A.; Liu, G.; Jiang, X. Characteristic of the Components and the Metabolism Mechanism of Goat Colostrum: A Review. Anim. Biotechnol. 2023, 34, 4135–4146. [Google Scholar] [CrossRef] [PubMed]
- Guha, S.; Sharma, H.; Deshwal, G.K.; Rao, P.S. A Comprehensive Review on Bioactive Peptides Derived from Milk and Milk Products of Minor Dairy Species. Food Prod. Process. Nutr. 2021, 3, 2. [Google Scholar] [CrossRef]
- Cunsolo, V.; Fasoli, E.; Saletti, R.; Muccilli, V.; Gallina, S.; Righetti, P.G.; Foti, S. Zeus, Aesculapius, Amalthea and the Proteome of Goat Milk. J. Proteom. 2015, 128, 69–82. [Google Scholar] [CrossRef]
- Anagnostopoulos, A.K.; Katsafadou, A.I.; Pierros, V.; Kontopodis, E.; Fthenakis, G.C.; Arsenos, G.; Karkabounas, S.C.; Tzora, A.; Skoufos, I.; Tsangaris, G.T. Milk of Greek Sheep and Goat Breeds; Characterization by Means of Proteomics. J. Proteom. 2016, 147, 76–84. [Google Scholar] [CrossRef]
- Jia, W.; Zhang, R.; Zhu, Z.; Shi, L. A High-Throughput Comparative Proteomics of Milk Fat Globule Membrane Reveals Breed and Lactation Stages Specific Variation in Protein Abundance and Functional Differences Between Milk of Saanen Dairy Goat and Holstein Bovine. Front. Nutr. 2021, 8, 680683. [Google Scholar] [CrossRef]
- Agradi, S.; González-Cabrera, M.; Argüello, A.; Hernández-Castellano, L.E.; Castro, N.; Menchetti, L.; Brecchia, G.; Vigo, D.; Tuccia, E.; Curone, G. Colostrum Quality in Different Goat Breeds Reared in Northern Italy. Animals 2023, 13, 3146. [Google Scholar] [CrossRef]
- Sarantidi, E.; Ainatzoglou, A.; Papadimitriou, C.; Stamoula, E.; Maghiorou, K.; Miflidi, A.; Trichopoulou, A.; Mountzouris, K.C.; Anagnostopoulos, A.K. Egg White and Yolk Protein Atlas: New Protein Insights of a Global Landmark Food. Foods 2023, 12, 3470. [Google Scholar] [CrossRef]
- Dimou, S.; Georgiou, X.; Sarantidi, E.; Diallinas, G.; Anagnostopoulos, A.K. Profile of Membrane Cargo Trafficking Proteins and Transporters Expressed under N Source Derepressing Conditions in Aspergillus Nidulans. J. Fungi 2021, 7, 560. [Google Scholar] [CrossRef]
- Anagnostopoulos, A.K.; Stravopodis, D.J.; Tsangaris, G.T. Yield of 6,000 Proteins by 1D nLC–MS/MS without Pre-Fractionation. J. Chromatogr. B 2017, 1047, 92–96. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A Universal Enrichment Tool for Interpreting Omics Data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.; Shepherd, L. AnnotationHub: Client to Access AnnotationHub Resources. Available online: https://bioconductor.org/packages/release/bioc/html/AnnotationHub.html (accessed on 10 June 2024).
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2020. Available online: https://www.r-project.org/ (accessed on 10 June 2024).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Buuren, S.V.; Groothuis-Oudshoorn, K. Mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Yu, G. Enrichplot: Visualization of Functional Enrichment Result. 2024. Available online: https://bioconductor.org/packages/release/bioc/html/enrichplot.html (accessed on 11 June 2024).
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING V11: Protein-Protein Association Networks with Increased Coverage, Supporting Functional Discovery in Genome-Wide Experimental Datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM Database of Bioactive Peptides: Current Opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef]
- Mooney, C.; Haslam, N.J.; Pollastri, G.; Shields, D.C. Towards the Improved Discovery and Design of Functional Peptides: Common Features of Diverse Classes Permit Generalized Prediction of Bioactivity. PLoS ONE 2012, 7, e45012. [Google Scholar] [CrossRef]
- Grønning, A.G.; Kacprowski, T.; Scheele, C. MultiPep: A Hierarchical Deep Learning Approach for Multi-Label Classification of Peptide Bioactivities. Biol. Methods Protoc. 2021, 6, bpab021. [Google Scholar] [CrossRef]
- Olsen, T.H.; Yesiltas, B.; Marin, F.I.; Pertseva, M.; García-Moreno, P.J.; Gregersen, S.; Overgaard, M.T.; Jacobsen, C.; Lund, O.; Hansen, E.B.; et al. AnOxPePred: Using Deep Learning for the Prediction of Antioxidative Properties of Peptides. Sci. Rep. 2020, 10, 21471. [Google Scholar] [CrossRef]
- Charoenkwan, P.; Nantasenamat, C.; Hasan, M.M.; Moni, M.A.; Lio’, P.; Manavalan, B.; Shoombuatong, W. StackDPPIV: A Novel Computational Approach for Accurate Prediction of Dipeptidyl Peptidase IV (DPP-IV) Inhibitory Peptides. Methods 2022, 204, 189–198. [Google Scholar] [CrossRef]
- Du, Z.; Ding, X.; Hsu, W.; Munir, A.; Xu, Y.; Li, Y. pLM4ACE: A Protein Language Model Based Predictor for Antihypertensive Peptide Screening. Food Chem. 2024, 431, 137162. [Google Scholar] [CrossRef]
- Kang, J.; Fang, Y.; Yao, P.; Li, N.; Tang, Q.; Huang, J. NeuroPP: A Tool for the Prediction of Neuropeptide Precursors Based on Optimal Sequence Composition. Interdiscip. Sci. Comput. Life Sci. 2019, 11, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Pinzi, L.; Rastelli, G. Molecular Docking: Shifting Paradigms in Drug Discovery. Int. J. Mol. Sci. 2019, 20, 4331. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, D.B.; Decornez, H.; Furr, J.R.; Bajorath, J. Docking and Scoring in Virtual Screening for Drug Discovery: Methods and Applications. Nat. Rev. Drug Discov. 2004, 3, 935–949. [Google Scholar] [CrossRef] [PubMed]
- Hennig, M.; Stihle, M.; Thoma, R.; Ruf, A. Crystal Structure of Human Dipeptidyl Peptidase IV (DPP-IV). 2008. Available online: https://www.rcsb.org/structure/1nu6 (accessed on 12 June 2024).
- Natesh, R.; Schwager, S.L.U.; Sturrock, E.D.; Acharya, K.R. Crystal Structure of Human Angiotensin Converting Enzyme (Native). Nature 2003, 421, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Bugnon, M.; Röhrig, U.F.; Goullieux, M.; Perez, M.A.S.; Daina, A.; Michielin, O.; Zoete, V. SwissDock 2024: Major Enhancements for Small-Molecule Docking with Attracting Cavities and AutoDock Vina. Nucleic Acids Res. 2024, 52, W324–W332. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Akkyra Systems Structures Molecular Viewer. 2023. Available online: https://apps.apple.com/us/app/structures-molecular-viewer/id1620788998?mt=12 (accessed on 14 June 2024).
- Hurley, W.L.; Theil, P.K. Perspectives on Immunoglobulins in Colostrum and Milk. Nutrients 2011, 3, 442–474. [Google Scholar] [CrossRef]
- Bardanzellu, F.; Fanos, V.; Reali, A. “Omics” in Human Colostrum and Mature Milk: Looking to Old Data with New Eyes. Nutrients 2017, 9, 843. [Google Scholar] [CrossRef]
- Weidemann, A.; Johnson, R.S. Biology of HIF-1α. Cell Death Differ. 2008, 15, 621–627. [Google Scholar] [CrossRef]
- Taylor, C.T.; McElwain, J.C. Ancient Atmospheres and the Evolution of Oxygen Sensing Via the Hypoxia-Inducible Factor in Metazoans. Physiology 2010, 25, 272–279. [Google Scholar] [CrossRef]
- Semenza, G.L. HIF-1: Mediator of Physiological and Pathophysiological Responses to Hypoxia. J. Appl. Physiol. 2000, 88, 1474–1480. [Google Scholar] [CrossRef] [PubMed]
- Ylioja, C.M.; Swartz, T.H.; Mamedova, L.K.; Bradford, B.J. Sodium Salicylate Reduced mRNA Abundance of Hypoxia-Associated Genes in MAC-T Cells. JDS Commun. 2021, 2, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Seagroves, T.N.; Hadsell, D.; McManaman, J.; Palmer, C.; Liao, D.; McNulty, W.; Welm, B.; Wagner, K.-U.; Neville, M.; Johnson, R.S. HIF1α Is a Critical Regulator of Secretory Differentiation and Activation, but Not Vascular Expansion, in the Mouse Mammary Gland. Development 2003, 130, 1713–1724. [Google Scholar] [CrossRef][Green Version]
- Sun, X.; Yu, Z.; Liang, C.; Xie, S.; Wang, H.; Wang, J.; Yang, Y.; Han, R. Comparative Analysis of Changes in Whey Proteins of Goat Milk throughout the Lactation Cycle Using Quantitative Proteomics. J. Dairy Sci. 2023, 106, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zheng, N.; Zhao, X.; Yang, J.; Zhang, Y.; Han, R.; Zhao, S.; Li, S.; Wen, F.; Wang, J. Changes in Whey Proteome with Lactation Stage and Parity in Dairy Cows Using a Label-Free Proteomics Approach. Food Res. Int. 2020, 128, 108760. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, A.; Lai, S.; Yuan, Q.; Jia, X.; Wang, P.; Zhang, Y. Longitudinal Changes in the Concentration of Major Human Milk Proteins in the First Six Months of Lactation and Their Effects on Infant Growth. Nutrients 2021, 13, 1476. [Google Scholar] [CrossRef]
- Vandooren, J.; Itoh, Y. Alpha-2-Macroglobulin in Inflammation, Immunity and Infections. Front. Immunol. 2021, 12, 803244. [Google Scholar] [CrossRef]
- Kościuczuk, E.M.; Lisowski, P.; Jarczak, J.; Strzałkowska, N.; Jóźwik, A.; Horbańczuk, J.; Krzyżewski, J.; Zwierzchowski, L.; Bagnicka, E. Cathelicidins: Family of Antimicrobial Peptides. A Review. Mol. Biol. Rep. 2012, 39, 10957–10970. [Google Scholar] [CrossRef]
- Wang, A.; Zhou, M.; Chen, Q.; Jin, H.; Xu, G.; Guo, R.; Wang, J.; Lai, R. Functional Analyses of Three Targeted DNA Antimicrobial Peptides Derived from Goats. Biomolecules 2023, 13, 1453. [Google Scholar] [CrossRef]
- Nishikawa, M.; Nii, T.; Yoshimura, Y.; Isobe, N. Investigation of the Binding of Goat Cathelicidin-7 to Lipopolysaccharide and Leucocidal Suppression of pro-Inflammatory Cytokines. Small Rumin. Res. 2018, 168, 101–106. [Google Scholar] [CrossRef]
- Haltiwanger, R.S.; Lowe, J.B. Role of Glycosylation in Development. Annu. Rev. Biochem. 2004, 73, 491–537. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhang, W.; Ma, C.; Pang, X.; Dai, Y.; Zhu, T.; Liu, J.; Xing, L.; Zhang, S.; Lv, J. Changes in Glycosylated Proteins in Colostrum and Mature Milk and Their Implication. Front. Nutr. 2023, 10, 1161310. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, C.; Sun, X.; Guo, M. Proteomic Analysis of Whey Proteins in the Colostrum and Mature Milk of Xinong Saanen Goats. J. Dairy Sci. 2020, 103, 1164–1174. [Google Scholar] [CrossRef]
- Sansi, M.S.; Iram, D.; Zanab, S.; Vij, S.; Puniya, A.K.; Singh, A.; Ashutosh; Meena, S. Antimicrobial Bioactive Peptides from Goat Milk Proteins: In Silico Prediction and Analysis. J. Food Biochem. 2022, 46, e14311. [Google Scholar] [CrossRef]
- Zhou, L.; Mendez, R.L.; Kwon, J.Y. In Silico Prospecting for Novel Bioactive Peptides from Seafoods: A Case Study on Pacific Oyster (Crassostrea gigas). Molecules 2023, 28, 651. [Google Scholar] [CrossRef]
- Vermeirssen, V.; Camp, J.V.; Verstraete, W. Bioavailability of Angiotensin I Converting Enzyme Inhibitory Peptides. Br. J. Nutr. 2004, 92, 357–366. [Google Scholar] [CrossRef]
- Xu, Q.; Hong, H.; Wu, J.; Yan, X. Bioavailability of Bioactive Peptides Derived from Food Proteins across the Intestinal Epithelial Membrane: A Review. Trends Food Sci. Technol. 2019, 86, 399–411. [Google Scholar] [CrossRef]
- Ralte, L.; Khiangte, L.; Thangjam, N.M.; Kumar, A.; Singh, Y.T. GC–MS and Molecular Docking Analyses of Phytochemicals from the Underutilized Plant, Parkia Timoriana Revealed Candidate Anti-Cancerous and Anti-Inflammatory Agents. Sci. Rep. 2022, 12, 3395. [Google Scholar] [CrossRef]
- Huang, Y.-P.; Dias, F.F.G.; Leite Nobrega De Moura Bell, J.M.; Barile, D. A Complete Workflow for Discovering Small Bioactive Peptides in Foods by LC-MS/MS: A Case Study on Almonds. Food Chem. 2022, 369, 130834. [Google Scholar] [CrossRef]
- Wu, J.; Aluko, R.E.; Nakai, S. Structural Requirements of Angiotensin I-Converting Enzyme Inhibitory Peptides: Quantitative Structure−Activity Relationship Study of Di- and Tripeptides. J. Agric. Food Chem. 2006, 54, 732–738. [Google Scholar] [CrossRef]
- Li, Y.-W.; Li, B.; He, J.; Qian, P. Quantitative Structure–Activity Relationship Study of Antioxidative Peptide by Using Different Sets of Amino Acids Descriptors. J. Mol. Struct. 2011, 998, 53–61. [Google Scholar] [CrossRef]
- Qiao, Q.; Chen, L.; Li, X.; Lu, X.; Xu, Q. Roles of Dietary Bioactive Peptides in Redox Balance and Metabolic Disorders. Oxidative Med. Cell. Longev. 2021, 2021, 5582245. [Google Scholar] [CrossRef] [PubMed]
- Jäkälä, P.; Vapaatalo, H. Antihypertensive Peptides from Milk Proteins. Pharmaceuticals 2010, 3, 251–272. [Google Scholar] [CrossRef] [PubMed]
- Forouzanfar, M.H.; Afshin, A.; Alexander, L.T.; Anderson, H.R.; Bhutta, Z.A.; Biryukov, S.; Brauer, M.; Burnett, R.; Cercy, K.; Charlson, F.J.; et al. Global, Regional, and National Comparative Risk Assessment of 79 Behavioural, Environmental and Occupational, and Metabolic Risks or Clusters of Risks, 1990–2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1659–1724. [Google Scholar] [CrossRef]
- Mulvihill, E.E.; Drucker, D.J. Pharmacology, Physiology, and Mechanisms of Action of Dipeptidyl Peptidase-4 Inhibitors. Endocr. Rev. 2014, 35, 992–1019. [Google Scholar] [CrossRef]
- Kim, W.; Egan, J.M. The Role of Incretins in Glucose Homeostasis and Diabetes Treatment. Pharmacol. Rev. 2008, 60, 470–512. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 Diabetes Mellitus. Nat. Rev. Dis. Primer 2015, 1, 15019. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, J.; Zhu, R.; Zhang, H.; Li, D.; Li, H.; Tang, H.; Chen, L.; Peng, X.; Xu, X.; et al. Mechanistic Study of Novel Dipeptidyl Peptidase IV Inhibitory Peptides from Goat’s Milk Based on Peptidomics and In Silico Analysis. Foods 2024, 13, 1194. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, R.; Ma, H.; Chen, S. Isolation and Identification of Dipeptidyl Peptidase IV-Inhibitory Peptides from Trypsin/Chymotrypsin-Treated Goat Milk Casein Hydrolysates by 2D-TLC and LC–MS/MS. J. Agric. Food Chem. 2015, 63, 8819–8828. [Google Scholar] [CrossRef]
- Du, X.; Jiang, C.; Wang, S.; Jing, H.; Mo, L.; Ma, C.; Wang, H. Preparation, Identification, and Inhibitory Mechanism of Dipeptidyl Peptidase IV Inhibitory Peptides from Goat Milk Whey Protein. J. Food Sci. 2023, 88, 3577–3593. [Google Scholar] [CrossRef]
- Lan, V.T.T.; Ito, K.; Ohno, M.; Motoyama, T.; Ito, S.; Kawarasaki, Y. Analyzing a Dipeptide Library to Identify Human Dipeptidyl Peptidase IV Inhibitor. Food Chem. 2015, 175, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Hrynkiewicz, M.; Iwaniak, A.; Minkiewicz, P.; Darewicz, M.; Płonka, W. Analysis of Structure–Activity Relationships of Food-Derived DPP IV-Inhibitory Di- and Tripeptides Using Interpretable Descriptors. Appl. Sci. 2023, 13, 12935. [Google Scholar] [CrossRef]
- De Gobba, C.; Tompa, G.; Otte, J. Bioactive Peptides from Caseins Released by Cold Active Proteolytic Enzymes from Arsukibacterium Ikkense. Food Chem. 2014, 165, 205–215. [Google Scholar] [CrossRef]
- Corrochano, A.R.; Ferraretto, A.; Arranz, E.; Stuknytė, M.; Bottani, M.; O’Connor, P.M.; Kelly, P.M.; De Noni, I.; Buckin, V.; Giblin, L. Bovine Whey Peptides Transit the Intestinal Barrier to Reduce Oxidative Stress in Muscle Cells. Food Chem. 2019, 288, 306–314. [Google Scholar] [CrossRef]
- De Espindola, J.S.; Ferreira Taccóla, M.; Da Silva, V.S.N.; Dos Santos, L.D.; Rossini, B.C.; Mendonça, B.C.; Pacheco, M.T.B.; Galland, F. Digestion-Resistant Whey Peptides Promote Antioxidant Effect on Caco-2 Cells. Food Res. Int. 2023, 173, 113291. [Google Scholar] [CrossRef]
- Basilicata, M.; Pepe, G.; Adesso, S.; Ostacolo, C.; Sala, M.; Sommella, E.; Scala, M.; Messore, A.; Autore, G.; Marzocco, S.; et al. Antioxidant Properties of Buffalo-Milk Dairy Products: A β-Lg Peptide Released after Gastrointestinal Digestion of Buffalo Ricotta Cheese Reduces Oxidative Stress in Intestinal Epithelial Cells. Int. J. Mol. Sci. 2018, 19, 1955. [Google Scholar] [CrossRef]
- Yang, F.; He, X.; Chen, T.; Liu, J.; Luo, Z.; Sun, S.; Qin, D.; Huang, W.; Tang, Y.; Liu, C.; et al. Peptides Isolated from Yak Milk Residue Exert Antioxidant Effects through Nrf2 Signal Pathway. Oxidative Med. Cell. Longev. 2021, 2021, 9426314. [Google Scholar] [CrossRef]
- Lennicke, C.; Cochemé, H.M. Redox Metabolism: ROS as Specific Molecular Regulators of Cell Signaling and Function. Mol. Cell 2021, 81, 3691–3707. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petre, M.L.; Kontouli Pertesi, A.N.; Boulioglou, O.E.; Sarantidi, E.; Korovesi, A.G.; Kozei, A.; Katsafadou, A.I.; Tsangaris, G.T.; Trichopoulou, A.; Anagnostopoulos, A.K. Bioactive Peptides in Greek Goat Colostrum: Relevance to Human Metabolism. Foods 2024, 13, 3949. https://doi.org/10.3390/foods13233949
Petre ML, Kontouli Pertesi AN, Boulioglou OE, Sarantidi E, Korovesi AG, Kozei A, Katsafadou AI, Tsangaris GT, Trichopoulou A, Anagnostopoulos AK. Bioactive Peptides in Greek Goat Colostrum: Relevance to Human Metabolism. Foods. 2024; 13(23):3949. https://doi.org/10.3390/foods13233949
Chicago/Turabian StylePetre, Maria Louiza, Anna Nefeli Kontouli Pertesi, Olympia Eirini Boulioglou, Eleana Sarantidi, Artemis G. Korovesi, Athina Kozei, Angeliki I. Katsafadou, George T. Tsangaris, Antonia Trichopoulou, and Athanasios K. Anagnostopoulos. 2024. "Bioactive Peptides in Greek Goat Colostrum: Relevance to Human Metabolism" Foods 13, no. 23: 3949. https://doi.org/10.3390/foods13233949
APA StylePetre, M. L., Kontouli Pertesi, A. N., Boulioglou, O. E., Sarantidi, E., Korovesi, A. G., Kozei, A., Katsafadou, A. I., Tsangaris, G. T., Trichopoulou, A., & Anagnostopoulos, A. K. (2024). Bioactive Peptides in Greek Goat Colostrum: Relevance to Human Metabolism. Foods, 13(23), 3949. https://doi.org/10.3390/foods13233949







