Evaluation of Anticancer and Immunomodulatory Effects of Microwave-Extracted Polysaccharide from Ruditapes philippinarum
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation of MCP
2.3. Cell Culture
2.4. Anticancer Activity of MCP
2.4.1. Tumor Cytotoxicity Experiment
2.4.2. Colony Formation
2.4.3. Wound Healing Assay
2.4.4. Analysis of Cell Apoptosis
2.4.5. Detection of Mitochondrial Membrane Potential (Δψm)
2.4.6. Quantitative Real-Time Reverse Transcription Polymerase Chain Reaction (RT-qPCR)
2.5. Immunomodulatory Activity of MCP
2.5.1. Immune Cell Viability of MCP
2.5.2. Phagocytosis Ability Effect of MCP
2.5.3. Secretion of Macrophage M1 and M2 Markers
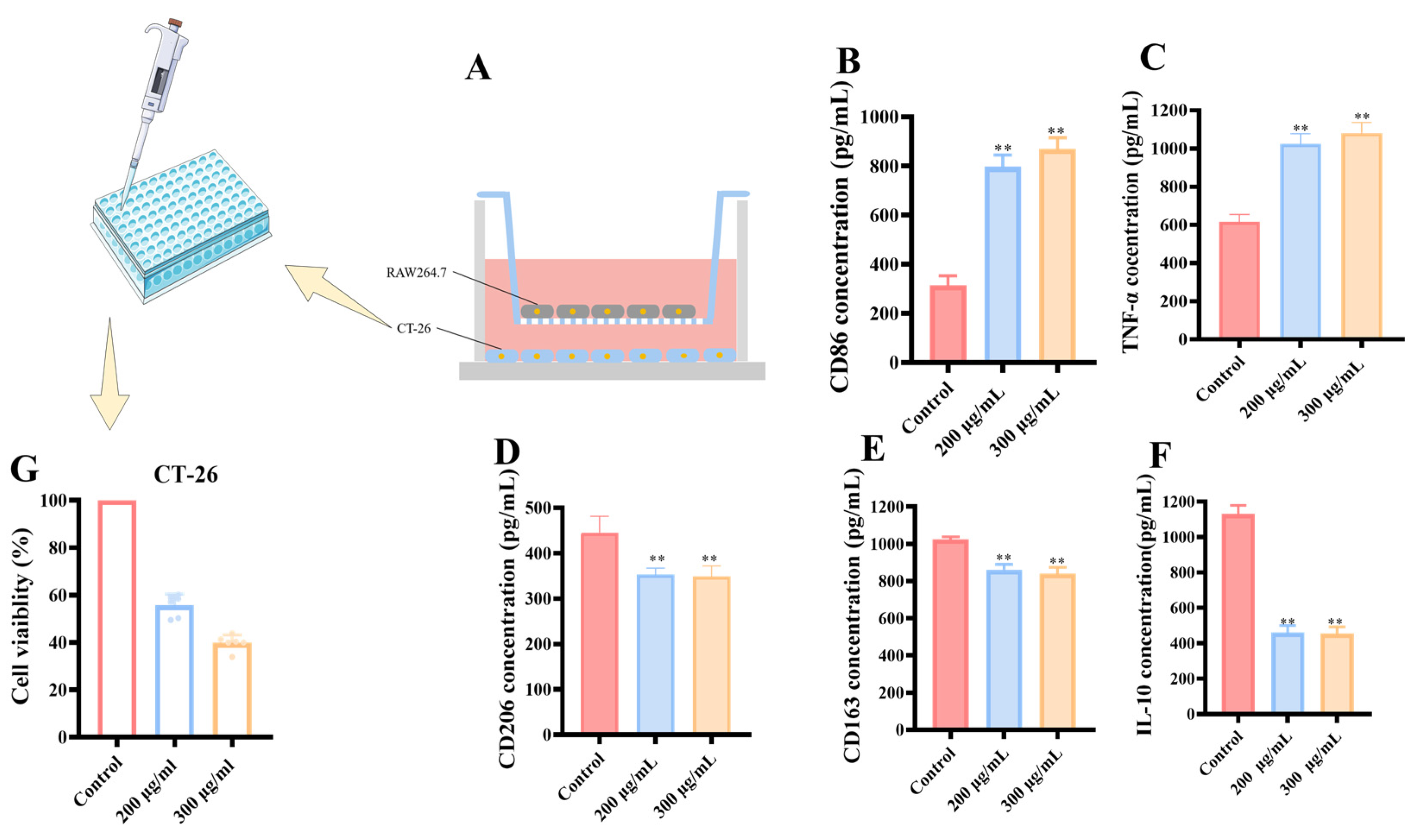
2.5.4. Transwell Co-Culture System of RAW 264.7 and CT-26
2.6. Antioxidant Activities
2.6.1. ABTS•+, DPPH•, and Hydroxyl Free Radical Scavenging Analysis
2.6.2. Intracellular Reactive Oxygen Species (ROS) Detection
2.7. Statistical Analysis
3. Results
3.1. Antitumor Activity of MCP In Vitro
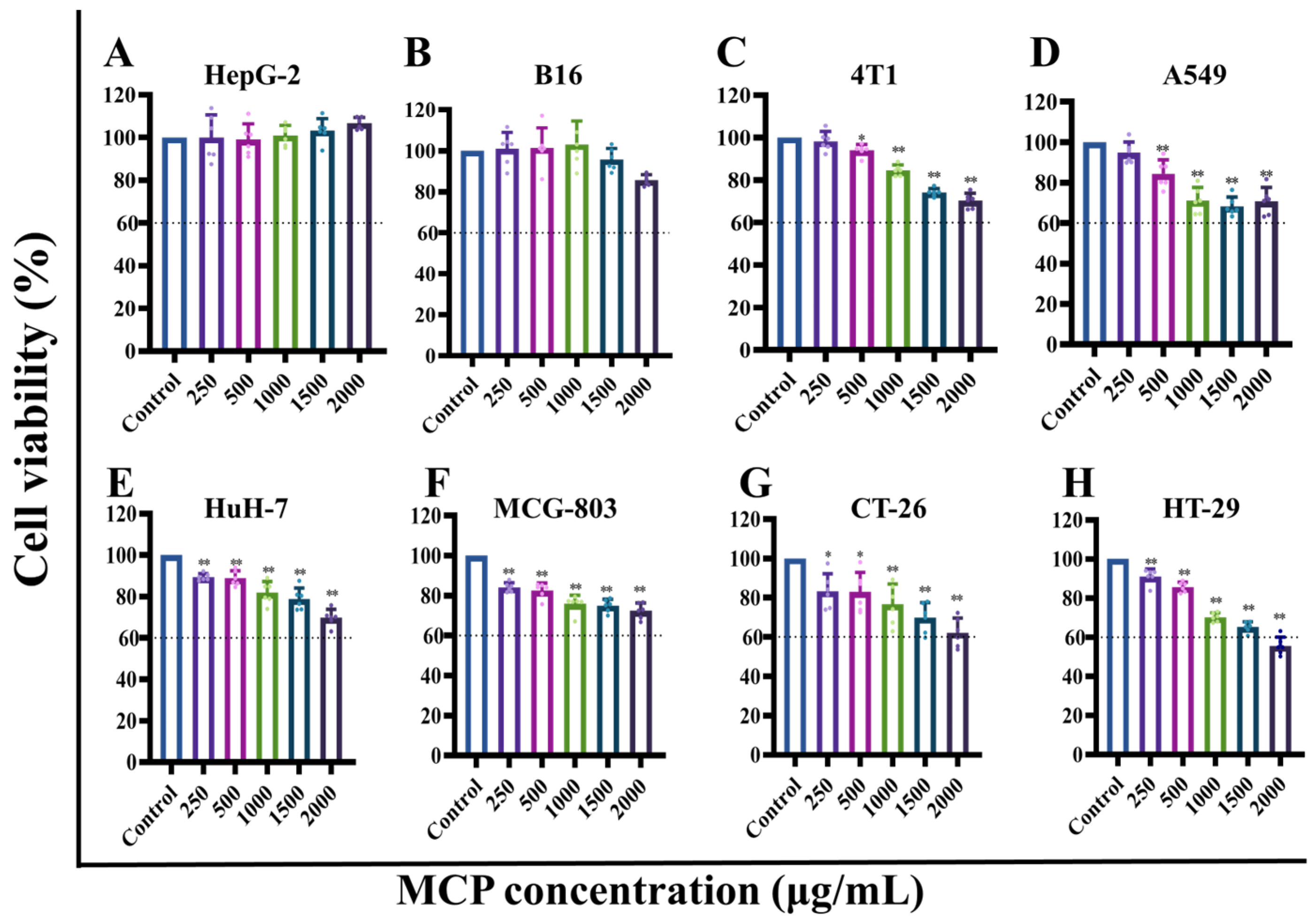
3.1.1. Effect of MCP on the Viability of Different Cells
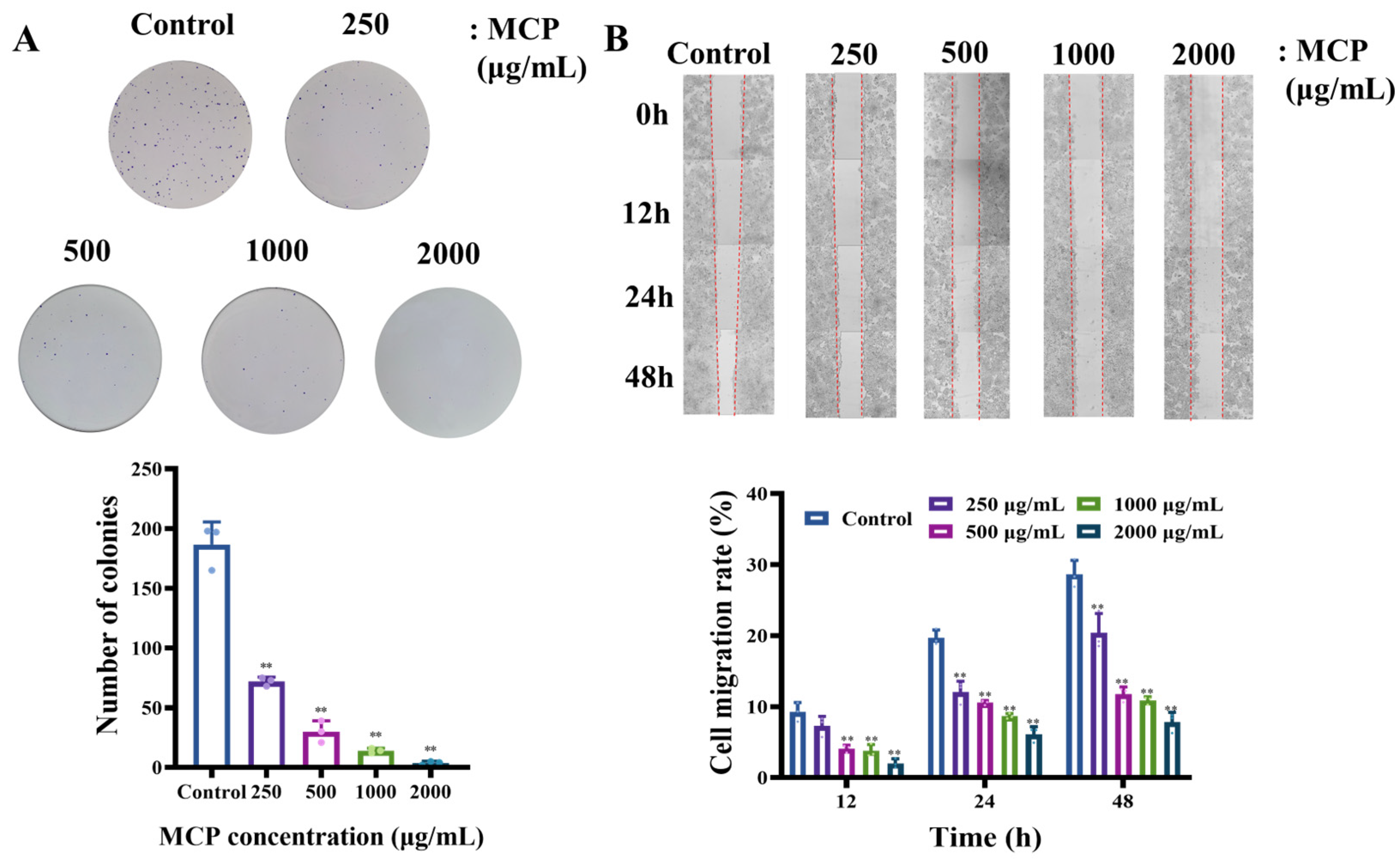
3.1.2. MCP-Attenuated Colony Formation and Migration Capacity in HT-29 Cells
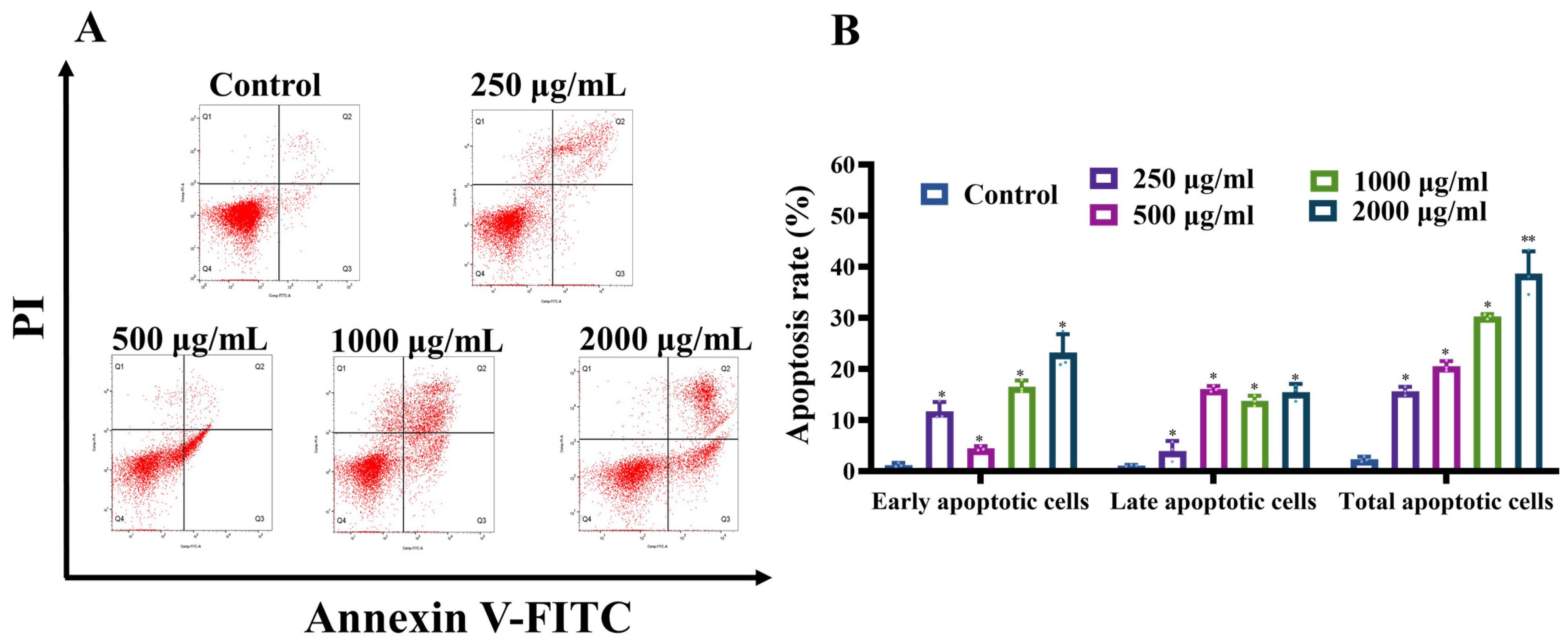
3.1.3. MCP Induced Apoptosis in HT-29 Cells
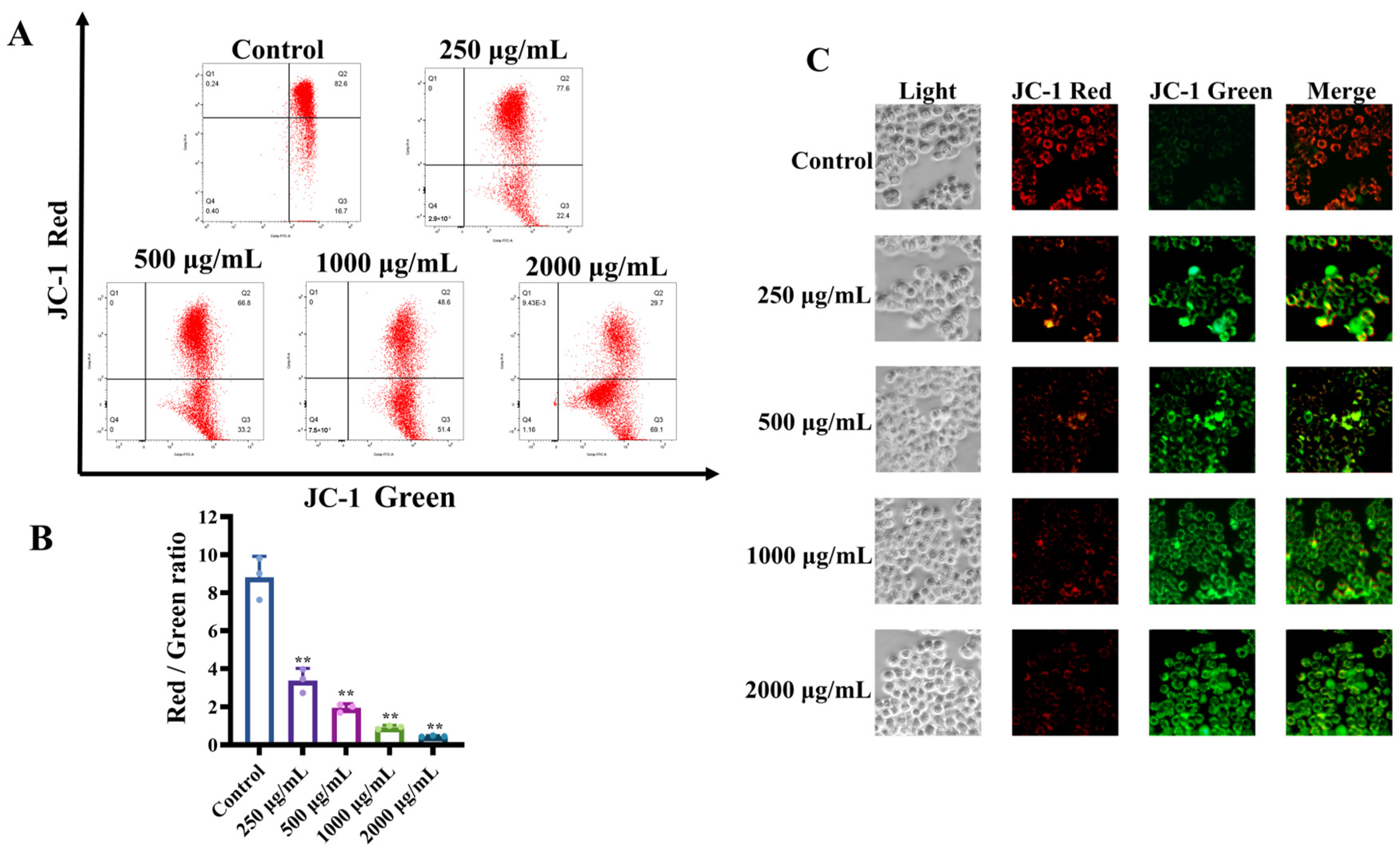
3.1.4. MCP-Induced Loss of Mitochondrial Membrane Potential (Δψm) in HT-29 Cells
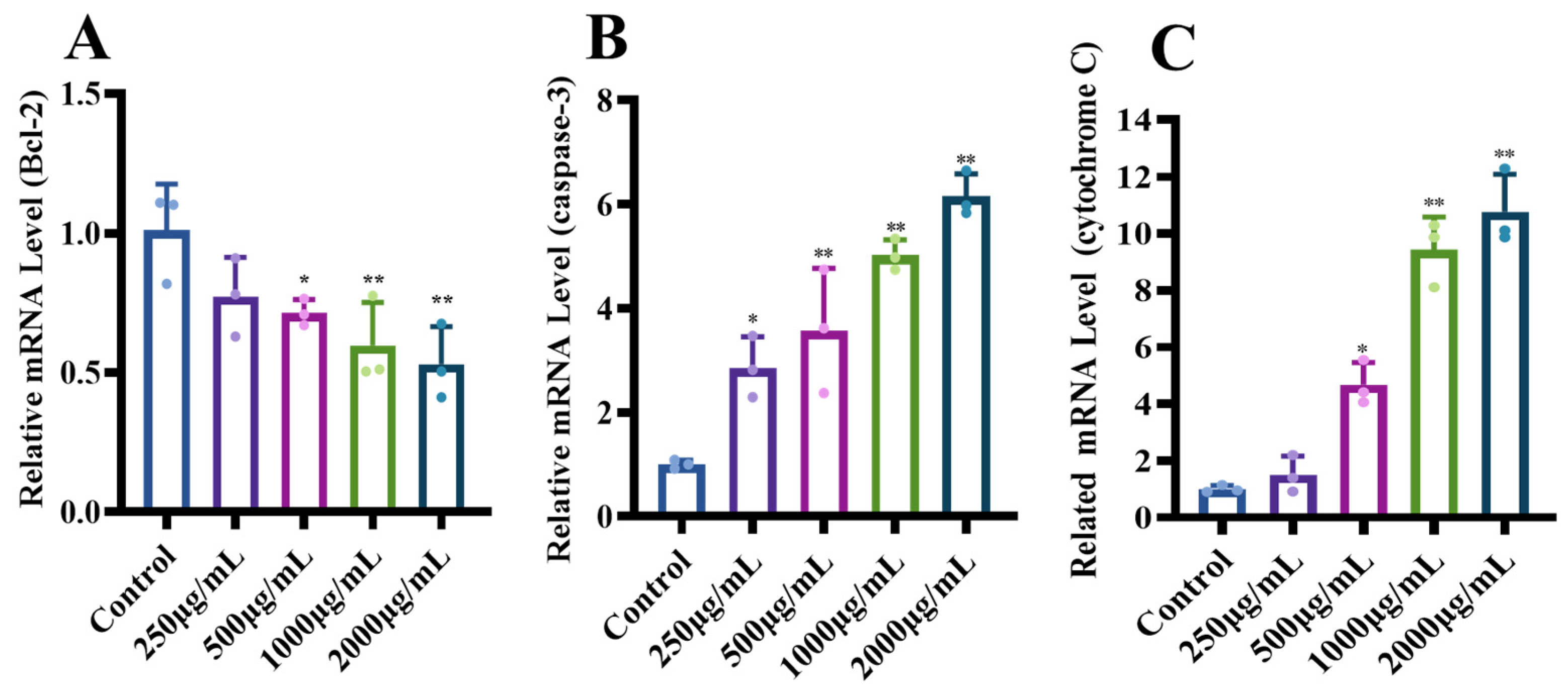
3.1.5. Effects of MCP on the Expression of Genes with the Apoptotic Pathway
3.2. Immunomodulatory of MCP in RAW 264.7 Macrophages
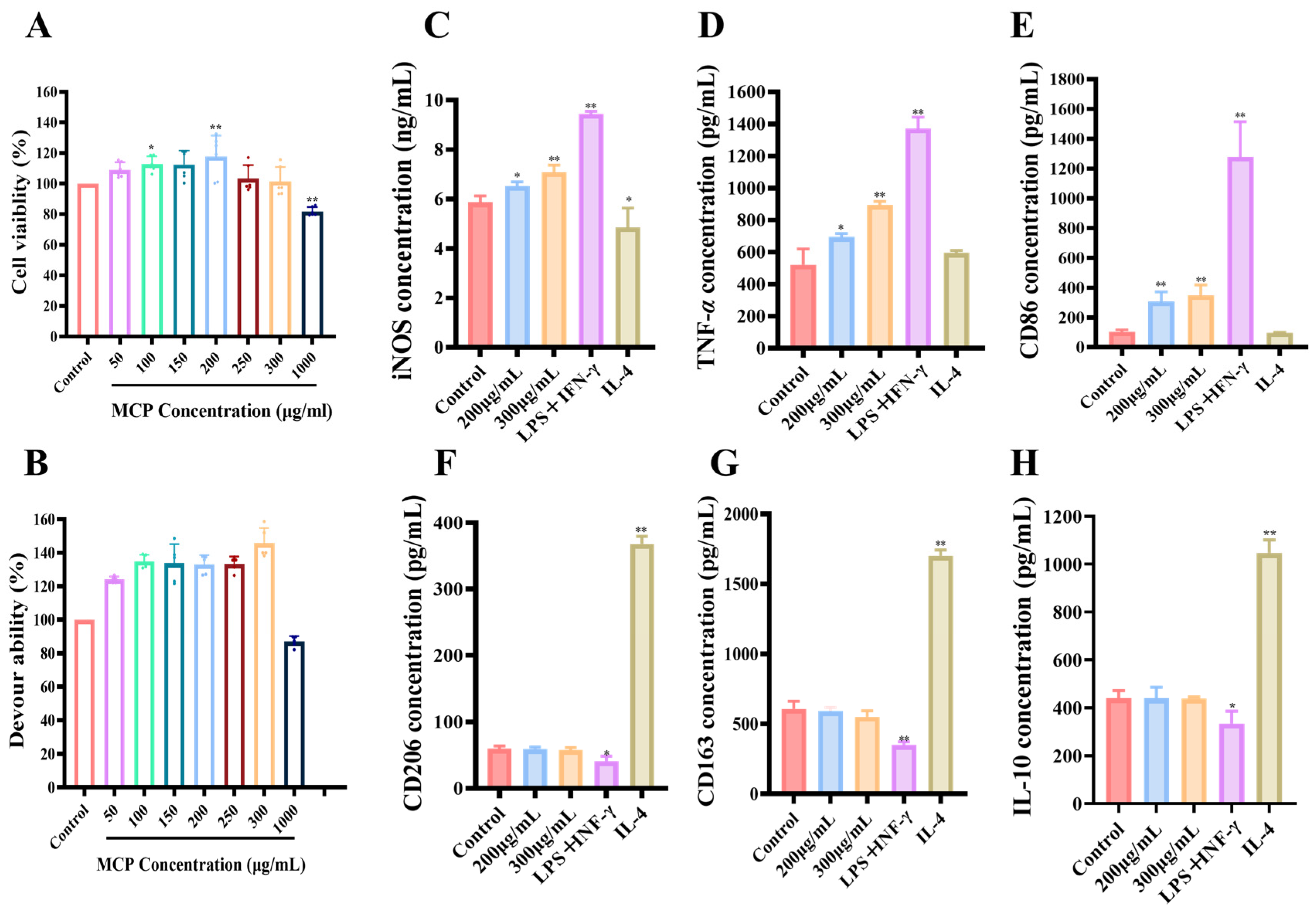
3.2.1. Impacts of MCP on RAW 264.7 Macrophages
3.2.2. Impacts of MCP on Phagocytosis Assay of RAW 264.7 Macrophages
3.2.3. Impact of MCP on Polarization of Original RAW 264.7 Macrophages
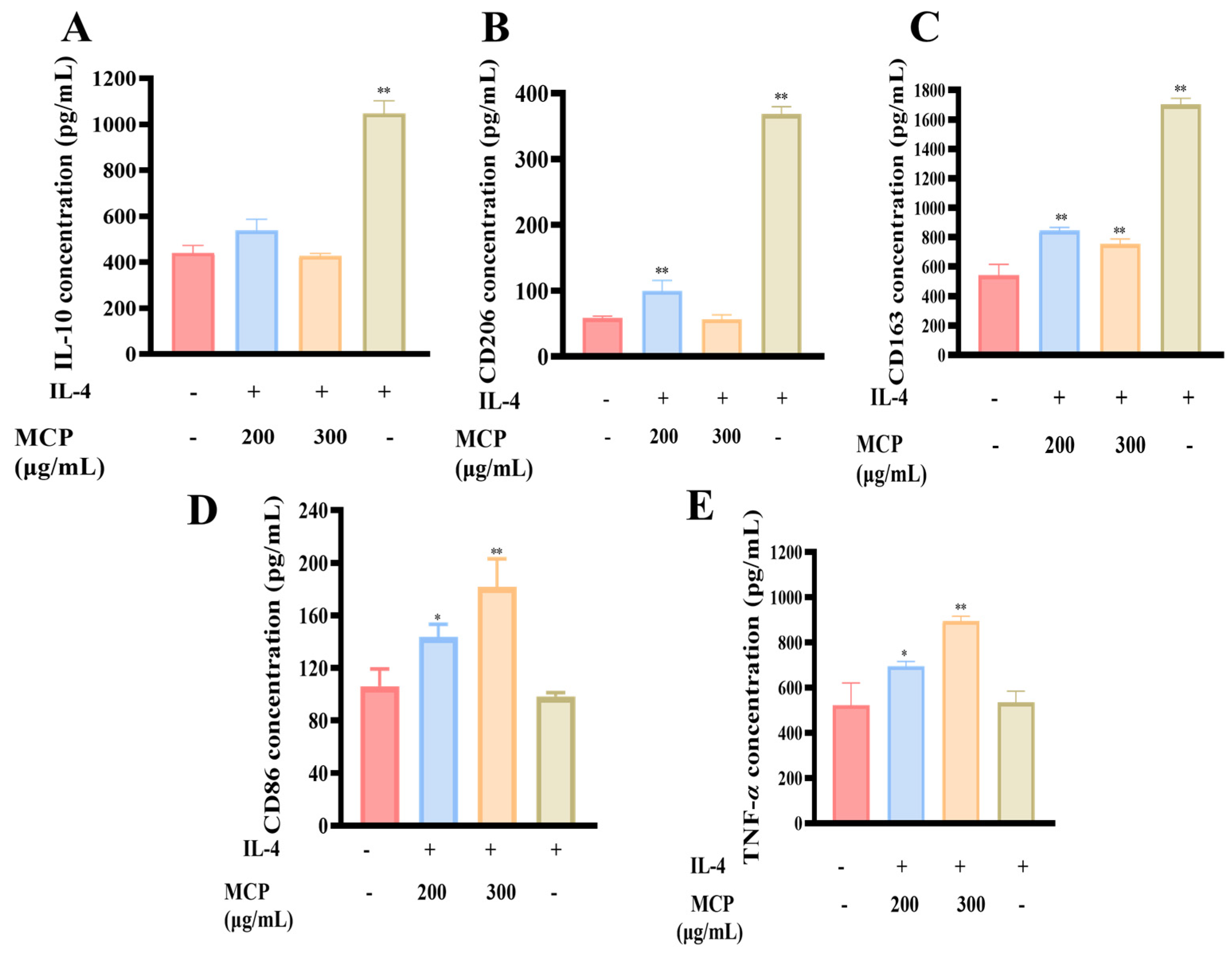
3.2.4. Impacts of MCP on M2 RAW 264.7 Macrophages
3.2.5. Transwell Co-Culture System of RAW 264.7 and CT-26
3.3. Antioxidant Activities of MCP
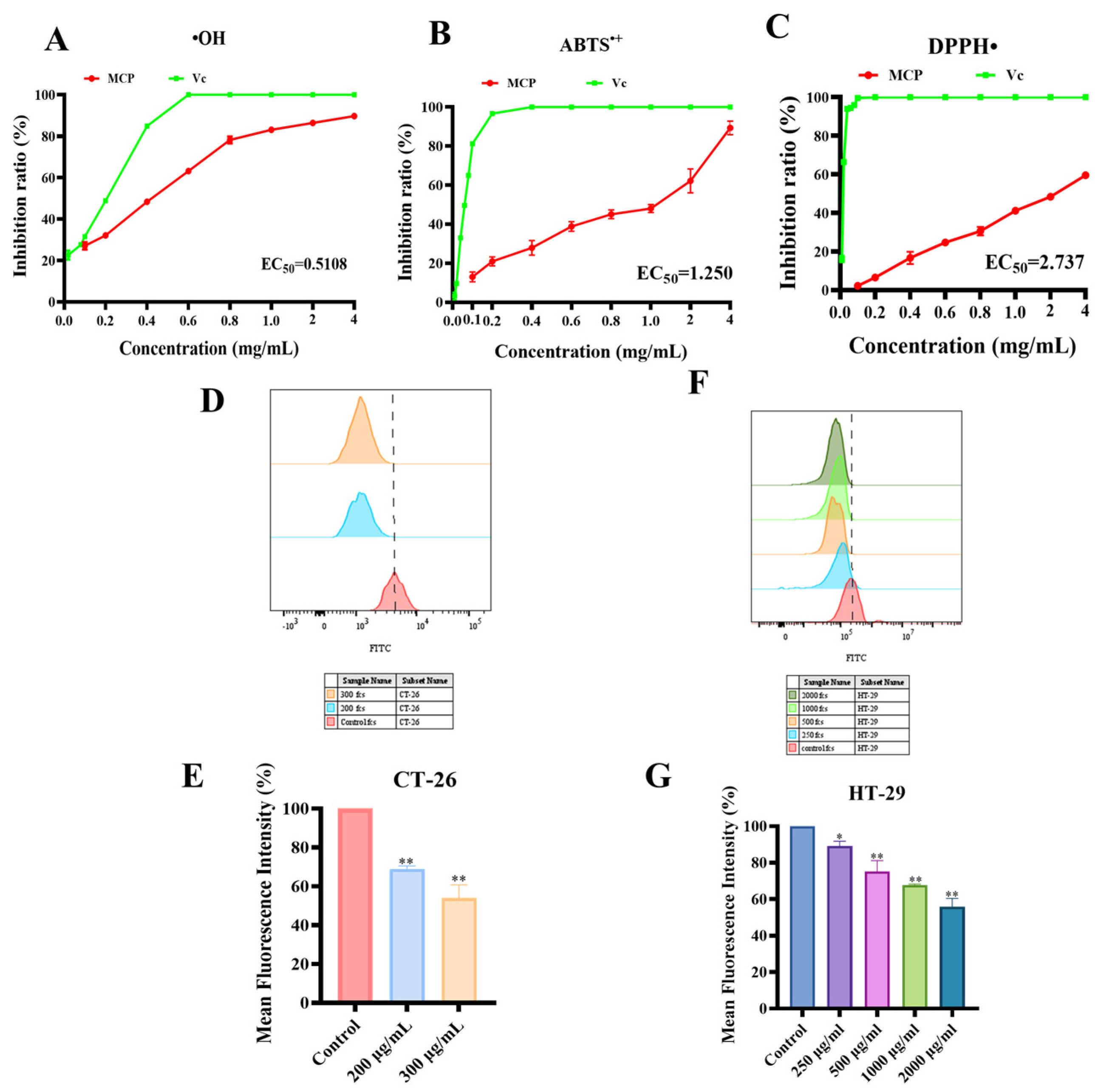
3.3.1. ABTS•+, DPPH• and Hydroxyl Free Radical Scavenging Analysis
3.3.2. Effect of MCP on Intracellular ROS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Byrne, R.M.; Ruhl, R.; Lanciault, C.; Anand, S.; Nellore, A.; Tsikitis, V.L. Age-related differences in gene expression in colorectal cancer (CRC). J. Clin. Oncol. 2018, 36, 1. [Google Scholar] [CrossRef]
- Killock, D. Early MRD predicts disease recurrence and benefit from adjuvant chemotherapy in CRC. Nat. Rev. Clin. Oncol. 2023, 20, 137. [Google Scholar] [CrossRef] [PubMed]
- Reilley, M.; Blando, J.M.; Katkhuda, R.; Menter, D.; Sharma, P.; Allison, J.P.; Kopetz, S.; Maru, D.M.; Overman, M.J. Immunologic profiling of consensus molecular subtype (CMS) stratified colorectal cancer (CRC) primary and liver metastectomy specimens: Implications for immune targeting of proficient mismatch repair CRC. J. Clin. Oncol. 2016, 34, 2. [Google Scholar] [CrossRef]
- Nusrat, M.; Roszik, J.; Katkhuda, R.; Menter, D.; Raghav, K.P.S.; Morris, V.K.; Sharma, P.; Allison, J.P.; Blando, J.M.; Maru, D.M.; et al. Association of PIK3CA mutations (mut) with immune engagement and clinical benefit from immunotherapy in microsatellite stable (MSS) colorectal cancer (CRC) patients (pts). J. Clin. Oncol. 2019, 37, 2. [Google Scholar] [CrossRef]
- He, R.; Lao, Y.F.; Yu, W.Y.; Zhang, X.H.; Jiang, M.; Zhu, C.R. Progress in the Application of Immune Checkpoint Inhibitor-Based Immunotherapy for Targeting Different Types of Colorectal Cancer. Front. Oncol. 2021, 11, 16. [Google Scholar] [CrossRef]
- Liu, W.; Li, N.; Hou, J.; Cao, R.; Jia, L.; Guo, Y.; Xu, J. Structure and antitumor activity of a polysaccharide from Rosa roxburghii. Int. J. Biol. Macromol. 2024, 273, 132807. [Google Scholar] [CrossRef]
- Dong, Y.W.; Liao, M.L.; Han, G.D.; Somero, G.N. An integrated, multi-level analysis of thermal effects on intertidal molluscs for understanding species distribution patterns. Biol. Rev. 2022, 97, 554–581. [Google Scholar] [CrossRef]
- Yan, L.F.; Zhu, M.S.; Wang, D.L.; Tao, W.Y.; Liu, D.H.; Zhang, F.M.; Linhardt, R.J.; Ye, X.Q.; Chen, S.G. Oral Administration of Fucosylated Chondroitin Sulfate Oligomers in Gastro-Resistant Microcapsules Exhibits a Safe Antithrombotic Activity. Thromb. Haemost. 2021, 121, 15–26. [Google Scholar] [CrossRef]
- Cheong, K.L.; Xia, L.X.; Liu, Y. Isolation and Characterization of Polysaccharides from Oysters (Crassostrea gigas) with Anti-Tumor Activities Using an Aqueous Two-Phase System. Mar. Drugs 2017, 15, 12. [Google Scholar] [CrossRef]
- Zhang, R.J.; Shi, Y.; Zheng, J.; Mao, X.M.; Liu, Z.Y.; Chen, Q.X.; Wang, Q. Effects of polysaccharides from abalone viscera (Haliotis discus hannai Ino) on MGC 803 cells proliferation. Int. J. Biol. Macromol. 2018, 106, 587–595. [Google Scholar] [CrossRef]
- Wang, Q.C.; Wei, M.S.; Yue, Y.; Wu, N.; Wang, J.; Zhang, Q.B. Structural characterization and immunostimulatory activity in vitro of a glycogen from sea urchin-Strongylocentyotus internedius. Carbohydr. Polym. 2021, 258, 14. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Cao, C.Y.; Ai, C.Q.; Wen, C.R.; Wang, L.L.; Zhao, J.; Han, Y.H.; Song, S.; Xiao, H. Structural characterization and immunostimulatory activity of a glucan from Cyclina sinensis. Int. J. Biol. Macromol. 2020, 161, 779–786. [Google Scholar] [CrossRef]
- Zhang, W.J.; Wang, J.; Jin, W.H.; Zhang, Q.B. The antioxidant activities and neuroprotective effect of polysaccharides from the starfish Asterias rollestoni. Carbohydr. Polym. 2013, 95, 9–15. [Google Scholar] [CrossRef]
- Hu, W.C.; Zhao, Y.N.; Yang, Y.; Zhang, H.F.; Ding, C.B.; Hu, C.; Zhou, L.J.; Zhang, Z.W.; Yuan, S.; Chen, Y.E.; et al. Microwave-assisted extraction, physicochemical characterization and bioactivity of polysaccharides from Camptotheca acuminata fruits. Int. J. Biol. Macromol. 2019, 133, 127–136. [Google Scholar] [CrossRef]
- Li, H.; Zhao, Z.Y.; Xiouras, C.; Stefanidis, G.D.; Li, X.G.; Gao, X. Fundamentals and applications of microwave heating to chemicals separation processes. Renew. Sust. Energ. Rev. 2019, 114, 16. [Google Scholar] [CrossRef]
- Tan, Y.; Fang, L.; Qiu, M.; Huo, Z.M.; Yan, X.W. Population genetics of the Manila clam (Ruditapes philippinarum) in East Asia. Sci. Rep. 2020, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, D.D.; Nie, H.T.; Dong, S.S.; Huo, Z.M.; Yan, X.W. Molecular cloning and expression analysis of C-type lectin (RpCTL) in Manila clam Ruditapes philippinarum after lipopolysaccharide challenge. Fish Shellfish Immunol. 2019, 86, 981–993. [Google Scholar] [CrossRef]
- Lv, M.B.; Liu, M.Y.; Zou, S.C.; Yin, D.L.; Lv, C.H.; Li, F.; Wei, Y.X. Immune Enhancement of Clam Peptides on Immunosuppressed Mice Induced by Hydrocortisone. Molecules 2023, 28, 15. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, F.J.; Wang, X.M.; Zhao, G.H.; Cai, D.; Yu, J.H.; Yin, F.W.; Zhou, D.Y. Preparation and Hepatoprotective Activities of Peptides Derived from Mussels (Mytilus edulis) and Clams (Ruditapes philippinarum). Mar. Drugs 2022, 20, 19. [Google Scholar] [CrossRef]
- Jin, H.; Zhao, H.S.; Shi, R.; Fan, F.J.; Cheng, W.J. Unlocking the Therapeutic Potential of a Manila Clam-Derived Antioxidant Peptide: Insights into Mechanisms of Action and Cytoprotective Effects against Oxidative Stress. Foods 2024, 13, 14. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Nie, H.T.; Yan, X.W. Metabolomic analysis provides new insights into the heat-hardening response of Manila clam (Ruditapes philippinarum) to high temperature stress. Sci. Total Environ. 2023, 857, 11. [Google Scholar] [CrossRef] [PubMed]
- Li, F.L.; Gong, X.Q.; Zhou, Y.; Geng, Q.Q.; Jiang, Y.H.; Yao, L.; Qu, M.; Tan, Z.J. Integrated evidence of transcriptional, metabolic, and intestinal microbiota changes in Ruditapes philippinarum due to perfluorooctanoic acid-induced immunotoxicity. Sci. Total Environ. 2024, 916, 13. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Liu, W.Z.; Jiang, H.; Chen, F.Y.; Li, Y.P.; Gardea-Torresdey, J.L.; Zhou, X.X.; Yan, B. Surface-Charge-Driven Ferroptosis and Mitochondrial Dysfunction Is Involved in Toxicity Diversity in the Marine Bivalve Exposed to Nanoplastics. ACS Nano 2024, 18, 2370–2383. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.J.; Vazquez, M.; Rodriguez-Lorenzo, L.; Moreda-Pineiro, A.; Fonseca, E.; Mallo, N.; Pinheiro, I.; Quarato, M.; Bigorra-Ferre, E.; Matos, A.; et al. Diving into the metabolic interactions of titanium dioxide nanoparticles in “Sparus aurata” and “Ruditapes philippinarum”. Environ. Pollut. 2024, 360, 124665. [Google Scholar] [CrossRef]
- Franken, N.A.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef]
- Bobadilla, A.V.P.; Arévalo, J.; Sarró, E.; Byrne, H.M.; Maini, P.K.; Carraro, T.; Balocco, S.; Meseguer, A.; Alarcón, T. In vitro cell migration quantification method for scratch assays. J. R. Soc. Interface 2019, 16, 11. [Google Scholar] [CrossRef]
- Tsujimoto, Y.; Shimizu, S. Role of the mitochondrial membrane permeability transition in cell death. Apoptosis 2007, 12, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Kroemer, G. Mitochondrial apoptosis without VDAC. Nat. Cell Biol. 2007, 9, 487–489. [Google Scholar] [CrossRef]
- Yuan, P.C.; Fang, F.; Shao, T.L.; Li, P.; Hu, W.; Zhou, Y.Y.; Wang, G.D.; Han, J.; Chen, K.S. Structure and Anti-Tumor Activities of Exopolysaccharides from Alternaria mali Roberts. Molecules 2019, 24, 14. [Google Scholar] [CrossRef]
- Abu-Qare, A.W.; Abou-Donia, M.B. Biomarkers of apoptosis:: Release of cytochrome, activation of caspase-3, induction of 8-hydroxy-2′-deoxyguanosine, increased 3-nitrotyrosine, and alteration of p53 gene. J. Toxicol. Environ. Health-Part B-Crit. Rev. 2001, 4, 313–332. [Google Scholar] [CrossRef]
- Cheng, X.D.; Wu, Q.X.; Zhao, J.; Su, T.; Lu, Y.M.; Zhang, W.N.; Wang, Y.; Chen, Y. Immunomodulatory effect of a polysaccharide fraction on RAW 264.7 macrophages extracted from the wild Lactarius deliciosus. Int. J. Biol. Macromol. 2019, 128, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.I.; Kim, M.R.; Jeong, H.Y.; Jeong, H.C.; Jeong, M.H.; Yoon, S.H.; Kim, Y.S.; Ahn, Y. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp. Mol. Med. 2014, 46, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.M.; Pan, L.C.; Zhang, L.J.; Yin, Y.; Zhu, Z.Y.; Sun, H.Q.; Liu, C.Y. Chemical structure and antioxidant activity of a polysaccharide from Siraitia grosvenorii. Int. J. Biol. Macromol. 2020, 165, 1900–1910. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, M.; Aydogmus-Öztürk, F.; Duru, M.E.; Topcu, G. Antioxidant activity of stem and root extracts of Rhubarb (Rheum ribes): An edible medicinal plant. Food Chem. 2007, 103, 623–630. [Google Scholar] [CrossRef]
- Zhang, X.H.; Liu, L.N.; Lin, C.W. Isolation, structural characterization and antioxidant activity of a neutral polysaccharide from Sisal waste. Food Hydrocoll. 2014, 39, 10–18. [Google Scholar] [CrossRef]
- Hatakeyama, M.; Nozawa, H. Hallmarks of Cancer: After the next generation. Cancer Sci. 2022, 113, 885. [Google Scholar]
- Chen, Y.Y.; Xue, Y.T. Optimization of microwave assisted extraction, chemical characterization and antitumor activities of polysaccharides from porphyra haitanensis. Carbohydr. Polym. 2019, 206, 179–186. [Google Scholar] [CrossRef]
- Wu, J.; Gao, W.P.; Song, Z.Y.; Xiong, Q.P.; Xu, Y.T.; Han, Y.; Yuan, J.; Zhang, R.; Cheng, Y.B.; Fang, J.S.; et al. Anticancer activity of polysaccharide from Glehnia littoralis on human lung cancer cell line A549. Int. J. Biol. Macromol. 2018, 106, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Li, W.F.; Hu, X.Y.; Wang, S.P.; Jiao, Z.R.; Sun, T.Y.; Liu, T.Q.; Song, K.D. Characterization and anti-tumor bioactivity of astragalus polysaccharides by immunomodulation. Int. J. Biol. Macromol. 2020, 145, 985–997. [Google Scholar] [CrossRef]
- Hata, A.N.; Engelman, J.A.; Faber, A.C. The BCL2 Family: Key Mediators of the Apoptotic Response to Targeted Anticancer Therapeutics. Cancer Discov. 2015, 5, 475–487. [Google Scholar] [CrossRef]
- Lee, K.S.; Shin, J.S.; Nam, K.S. Inhibitory effect of starfish polysaccharides on metastasis in HT-29 human colorectal adenocarcinoma. Biotechnol. Bioprocess Eng. 2012, 17, 764–769. [Google Scholar] [CrossRef]
- Sahayanathan, G.J.; Padmanaban, D.; Raja, K.; Chinnasamy, A. Anticancer effect of purified polysaccharide from marine clam Donax variabilis on A549 cells. J. Food Biochem. 2020, 44, 10. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity 2014, 41, 339–340. [Google Scholar] [CrossRef]
- Zhong, Q.W.; Wei, B.; Wang, S.J.; Ke, S.Z.; Chen, J.W.; Zhang, H.W.; Wang, H. The Antioxidant Activity of Polysaccharides Derived from Marine Organisms: An Overview. Mar. Drugs 2019, 17, 34. [Google Scholar] [CrossRef]
- Harris, I.S.; DeNicola, G.M. The Complex Interplay between Antioxidants and ROS in Cancer. Trends Cell Biol. 2020, 30, 440–451. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, X.; Wang, Y.; Jin, W.; Guo, Y. The immunoenhancement effects of starfish Asterias rollestoni polysaccharides in macrophages and cyclophosphamide-induced immunosuppression mouse models. Food Funct. 2020, 11, 10700–10708. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K. Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals. Cancer Lett. 2017, 387, 95–105. [Google Scholar] [CrossRef]
- Yang, X.L.; Wei, S.Q.; Lu, X.M.; Qiao, X.G.; Simal-Gandara, J.; Capanoglu, E.; Wozniak, L.; Zou, L.; Cao, H.; Xiao, J.B.; et al. A neutral polysaccharide with a triple helix structure from ginger: Characterization and immunomodulatory activity. Food Chem. 2021, 350, 10. [Google Scholar] [CrossRef]
- Zeng, D.; Zhu, S.M. Purification, characterization, antioxidant and anticancer activities of novel polysaccharides extracted from Bachu mushroom. Int. J. Biol. Macromol. 2018, 107, 1086–1092. [Google Scholar] [CrossRef]
| Gene | Primer Sequence | |
|---|---|---|
| Bcl-2 | F | ATCGCCCTGTGGATGACTGAGT |
| R | GCCAGGAGAAATCAAACAGAGGC | |
| Caspase-3 | F | AGAGGGGATCGTTGTAGAAGTC |
| R | ACAGTCCAGTTCTGTACCACG | |
| Cytochrome C | F | CTTTGGGCGGAAGACAGGTC |
| R | TTATTGGCGGCTGTGTAAGAG | |
| β-actin | F | GGGACCTGACTGACTACCTC |
| R | TCATACTCCTGCTTGCTGAT | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Li, F.; Feng, S.; Guo, J.; Yu, J.; Zou, S.; Gao, X.; Wei, Y. Evaluation of Anticancer and Immunomodulatory Effects of Microwave-Extracted Polysaccharide from Ruditapes philippinarum. Foods 2024, 13, 3552. https://doi.org/10.3390/foods13223552
Liu M, Li F, Feng S, Guo J, Yu J, Zou S, Gao X, Wei Y. Evaluation of Anticancer and Immunomodulatory Effects of Microwave-Extracted Polysaccharide from Ruditapes philippinarum. Foods. 2024; 13(22):3552. https://doi.org/10.3390/foods13223552
Chicago/Turabian StyleLiu, Mengyue, Fei Li, Shuang Feng, Jiamin Guo, Jia Yu, Shengcan Zou, Xiang Gao, and Yuxi Wei. 2024. "Evaluation of Anticancer and Immunomodulatory Effects of Microwave-Extracted Polysaccharide from Ruditapes philippinarum" Foods 13, no. 22: 3552. https://doi.org/10.3390/foods13223552
APA StyleLiu, M., Li, F., Feng, S., Guo, J., Yu, J., Zou, S., Gao, X., & Wei, Y. (2024). Evaluation of Anticancer and Immunomodulatory Effects of Microwave-Extracted Polysaccharide from Ruditapes philippinarum. Foods, 13(22), 3552. https://doi.org/10.3390/foods13223552






