Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry-Based Metabolic Characterization of Mango Ripened by Different Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Fruit Material and Treatments
2.3. Sample Preparation
2.4. UPLC-Q-TOF Untargeted Analysis
2.5. Data Processing and Statistical Analyses
3. Results and Discussion
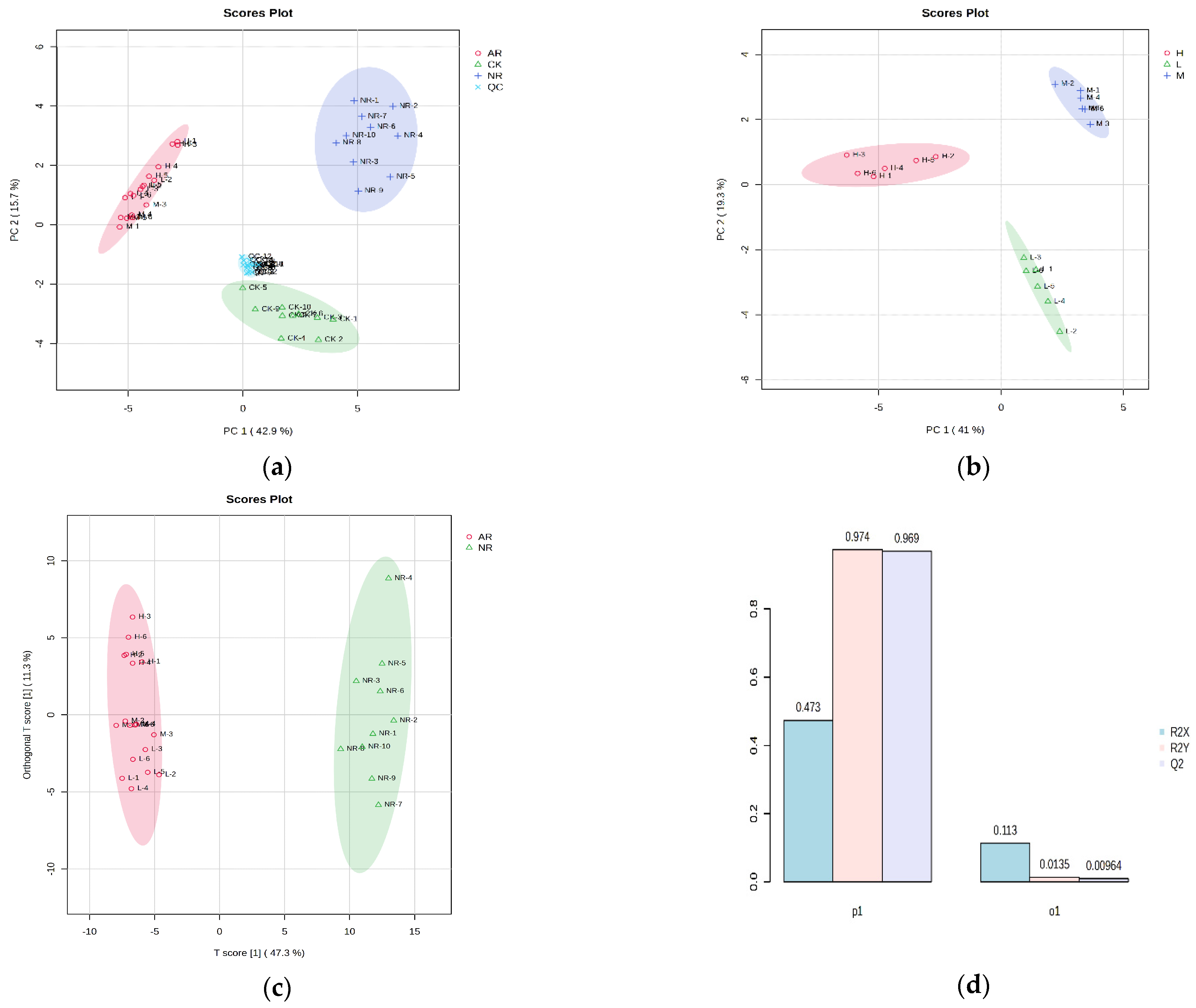
3.1. Untargeted Metabolomics Analysis
3.2. Potential Biomarkers Identification
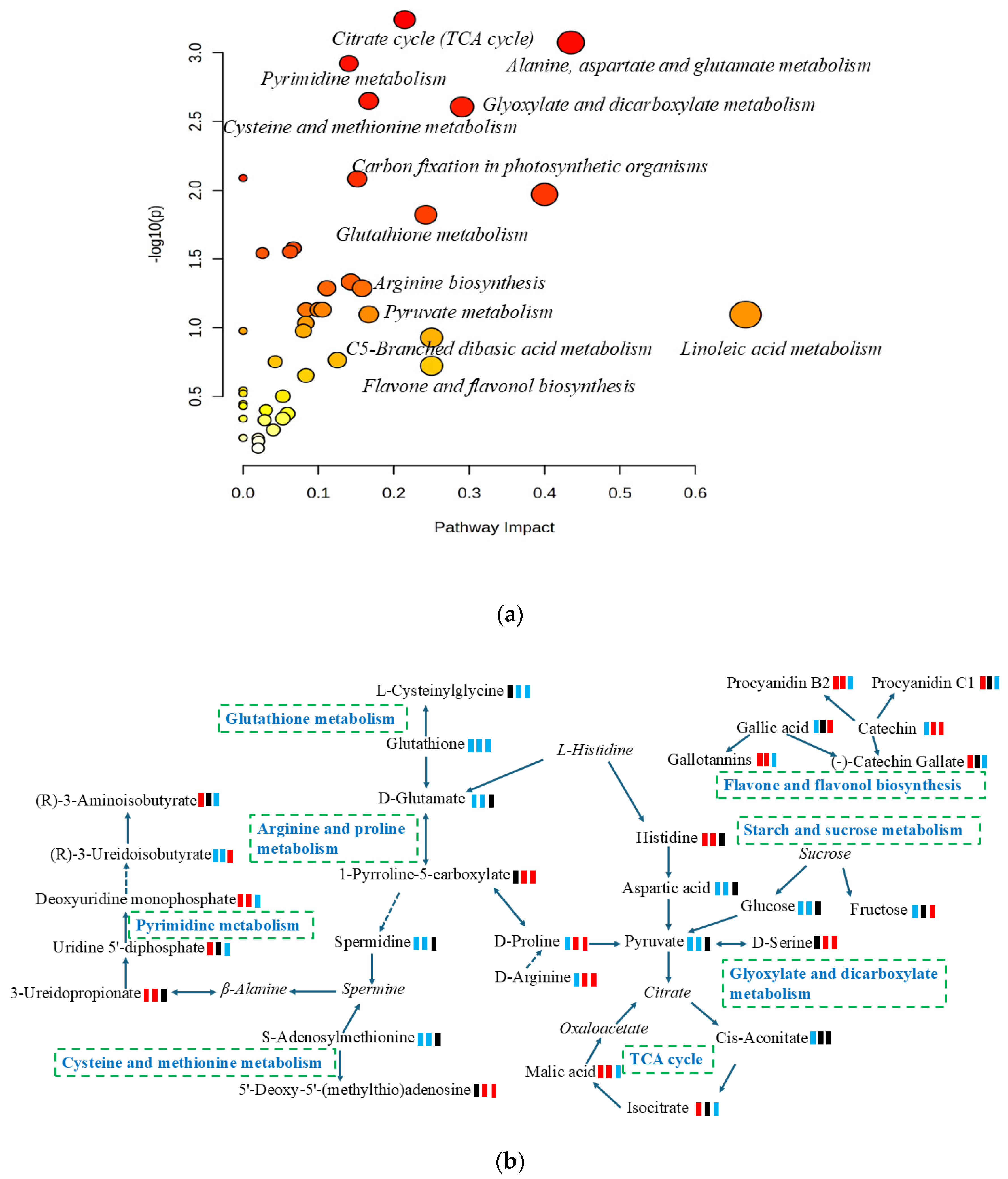
3.3. Metabolic Pathway Analysis
3.4. Elucidating the Biological Functions of Biomarkers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sivakumar, D.; Jiang, Y.; Yahia, E.M. Maintaining mango (Mangifera indica L.) fruit quality during the export chain. Food Res. Int. 2011, 44, 1254–1263. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Q.; Hu, M.; Gao, Z.; An, F.; Li, M.; Jiang, Y. Low-temperature conditioning induces chilling tolerance in stored mango fruit. Food Chem. 2017, 219, 76–84. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Data Collection. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 29 October 2024).
- Joas, J.; Vulcain, E.; Desvignes, C.; Morales, E.; Léchaudel, M. Physiological age at harvest regulates the variability in postharvest ripening, sensory and nutritional characteristics of mango (Mangifera indica L.) cv. Coghshall due to growing conditions. J. Sci. Food Agric. 2011, 92, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Cortés, V.; Ortiz, C.; Aleixos, N.; Blasco, J.; Cubero, S.; Talens, P. A new internal quality index for mango and its prediction by external visible and near-infrared reflection spectroscopy. Postharvest Biol. Technol. 2016, 118, 148–158. [Google Scholar] [CrossRef]
- Ramayya, N.; Niranjan, K.; Duncan, E. Effects of modified atmosphere packaging on quality of ‘Alphonso’ Mangoes. J. Food Sci. Technol. 2012, 49, 721–728. [Google Scholar] [CrossRef]
- Ullah, H.; Ahmad, S.; Thompson, A.K.; Anwar, R.; Nafees, M. Effect of ‘oxygen and carbon-dioxide’ on the post-harvest management in tree-ripe mango storage. J. Chem. Soc. Pakistan. 2010, 32, 485–491. [Google Scholar]
- Singh, Z.; Singh, R.K.; Sane, V.A.; Nath, P. Mango-Postharvest Biology and Biotechnology. Crit. Rev. Plant Sci. 2013, 32, 217–236. [Google Scholar] [CrossRef]
- Paul, V.; Pandey, R.; Srivastava, G.C. The fading distinctions between classical patterns of ripening in climacteric and non-climacteric fruit and the ubiquity of ethylene—An overview. J. Food Sci. Technol. 2011, 49, 1–21. [Google Scholar] [CrossRef]
- Liu, B.; Xin, Q.; Zhang, M.; Chen, J.; Lu, Q.; Zhou, X.; Li, X.; Zhang, W.; Feng, W.; Pei, H.; et al. Research progress on mango post-harvest ripening physiology and the regulatory technologies. Foods 2022, 12, 173. [Google Scholar] [CrossRef]
- Ho, B.T.; Hofman, P.J.; Joyce, D.C.; Bhandari, B.R. Uses of an innovative ethylene-α-cyclodextrin inclusion complex powder for ripening of mango fruit. Postharvest Biol. Technol. 2016, 113, 77–86. [Google Scholar] [CrossRef]
- Razzaq, K.; Singh, Z.; Khan, A.S.; Khan, S.A.K.U.; Ullah, S. Role of 1-MCP in regulating ‘Kensington Pride’ mango fruit softening and ripening. Plant Growth Regul. 2016, 78, 401–411. [Google Scholar] [CrossRef]
- Pino, J.A.; Mesa, J. Contribution of volatile compounds to mango (Mangifera indica L.) aroma. Flavour Fragr. J. 2006, 21, 207–213. [Google Scholar] [CrossRef]
- Zaharah, S.S.; Singh, Z.; Symons, G.M.; Reid, J.B. Mode of action of abscisic acid in triggering ethylene biosynthesis and softening during ripening in mango fruit. Postharvest Biol. Technol. 2013, 75, 37–44. [Google Scholar] [CrossRef]
- Scalbert, A.; Brennan, L.; Fiehn, O.; Hankemeier, T.; Kristal, B.S.; van Ommen, B.; Pujos-Guillot, E.; Verheij, E.; Wishart, D.; Wopereis, S. Mass-spectrometry-based metabolomics: Limitations and recommendations for future progress with particular focus on nutrition research. Metabolomics 2009, 5, 435–458. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Yi, X.; Sun, Y.; Bi, X.; Du, J.; Zhang, C.; Quan, S.; Zhang, F.; Sun, R.; Qian, L.; et al. Proteomic and Metabolomic Characterization of COVID-19 Patient Sera. Cell 2020, 182, 1–14. [Google Scholar] [CrossRef]
- He, Z.; Wang, Y.; Zhang, Y.; Cheng, H.; Liu, X. Stereoselective bioaccumulation of chiral PCB 91 in earthworm and its metabolomic and lipidomic responses. Environ Pollut. 2018, 238, 421–430. [Google Scholar] [CrossRef]
- Dudka, I.; Kossowska, B.; Senhadri, H.; Latajka, R.; Hajek, J.; Andrzejak, R.; Antonowicz-Juchniewicz, J.; Gancarz, R. Metabonomic analysis of serum of workers occupationally exposed to arsenic, cadmium and lead for biomarker research: A preliminary study. Environ Int. 2014, 68, 71–81. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, L.; Liu, X.; He, Z. Stereoselective effects of chiral epoxiconazole on the metabolomic and lipidomic profiling of leek. Food Chem. 2022, 405, 134962. [Google Scholar] [CrossRef] [PubMed]
- Broeckling, C.D.; Hoyes, E.; Richardson, K.; Brown, J.M.; Prenni, J.E. Comprehensive Tandem-Mass-Spectrometry Coverage of Complex Samples Enabled by Data-Set-Dependent Acquisition. Anal. Chem. 2018, 90, 8020–8027. [Google Scholar] [CrossRef]
- He, G.; Chen, X.; Hou, X.; Yu, X.; Han, M.; Qiu, S.; Li, Y.; Qin, S.; Wang, F. UPLC-Q-TOF/MS-based metabolomic analysis reveals the effects of asomate on the citrus fruit. Curr. Res. Food Sci. 2023, 6, 100523. [Google Scholar] [CrossRef]
- Li, M.; Li, L.; Kong, Z.; Gregoire, N.; Quan, R.; Luo, Z.; Lin, X.; Simal-Gandara, J.; Fan, B.; Wang, F. Integrative analysis of metabolome and genome-wide transcriptome reveal the flavor changes in apple (Malus pumila Mill) after the novel acaricide cyflumetofen application. LWT-Food Sci. Technol. 2023, 184, 114942. [Google Scholar] [CrossRef]
- Zhang, X.; Andrew, P.B.; Mishchuk, D.O.; Fake, C.E.; O’Mahony, M.A.; Slupsky, C.M. Fertilisation and pesticides affect mandarin orange nutrient composition. Food Chem. 2012, 134, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, R.; Yu, S.; Diao, J.; Deng, Y.; Zheng, M.; Nie, Y.; Li, J.Q.; Pan, C.; Zhou, Z. Insights into the Mechanism of Flavor Loss in Strawberries Induced by Two Fungicides Integrating Transcriptome and Metabolome Analysis. J. Agric. Food Chem. 2023, 71, 3906–3919. [Google Scholar] [CrossRef] [PubMed]
- Gika, H.G.; Theodoridis, G.A.; Wingate, J.E.; Wilson, I.D. Within-Day Reproducibility of an HPLC?MS-Based Method for Metabonomic Analysis:? Application to Human Urine. J. Proteome Res. 2007, 6, 3291–3303. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, P.; Masuero, D.; Vrhovsek, U.; Mattivi, F.; Wehrens, R. A benchmark spike-in data set for biomarker identification in metabolomics. J Chemometr. 2012, 26, 16–24. [Google Scholar] [CrossRef]
- Klamt, S.; Stelling, J. Two approaches for metabolic pathway analysis? Trends Biotechnol. 2003, 21, 64–69. [Google Scholar] [CrossRef]
- Stein, O.; Granot, D. An overview of sucrose synthases in plants. Front. Plant Sci. 2019, 10, 95. [Google Scholar] [CrossRef]
- Suh, J.H.; Madden, R.T.; Sung, J.; Chambers, A.H.; Crane, J.; Wang, Y. Pathway-Based Metabolomics Analysis Reveals Biosynthesis of Key Flavor Compounds in Mango. J. Agric. Food Chem. 2022, 70, 10389–10399. [Google Scholar] [CrossRef] [PubMed]
- Nordey, T.; Léchaudel, M.; Génard, M.; Joas, J. Spatial and temporal variations in mango colour, acidity, and sweetness in relation to temperature and ethylene gradients within the fruit. J. Plant Physiol. 2014, 171, 1555–1563. [Google Scholar] [CrossRef]
- Qiao, H.; Zhang, H.; Wang, Z.; Shen, Y. Fig fruit ripening is regulated by the interaction between ethylene and abscisic acid. J. Integr. Plant Biol. 2021, 63, 553–569. [Google Scholar] [CrossRef]
- Wu, S.; Wu, D.; Song, J.; Zhang, Y.; Tan, Q.; Yang, T.; Yang, J.; Wang, S.; Xu, J.; Xu, W.; et al. Metabolomic and transcriptomic analyses reveal new insights into the role of abscisic acid in modulating mango fruit ripening. Hortic. Res.-England 2022, 9, uhac102. [Google Scholar] [CrossRef] [PubMed]
- Dellapenna, D.; Pogson, B.J. Vitamin synthesis in plants: Tocopherols and carotenoids. Annu. Rev. Plant Biol. 2006, 57, 711–738. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, T.M.; Nesi, A.N.; Araújo, W.L.; Braun, H.P. Amino acid catabolism in plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Ali, S.A.; Nath, P.; Sane, V.A. Activation of ethylene-responsive p-hydroxyphenylpyruvate dioxygenase leads to increased tocopherol levels during ripening in mango. J. Exp. Bot. 2011, 44, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Aslam, J.; Mujib, A.; Fatima, Z.; Sharma, M.P. Variations in vinblastine production at different stages of somatic embryogenesis, embryo, and field-grown plantlets of Catharanthus roseus L. (G) Don, as revealed by HPLC. Vitr. Cell. Dev. Biol. Plant 2010, 46, 348–353. [Google Scholar] [CrossRef]
- Maldonado-Celis, M.E.; Yahia, E.M.; Bedoya, R.; Patricia, L.; Loango, N.; Johanny, A.; Restrepo, B.; Ospina, J.C.G. Chemical composition of mango (Mangifera indica L.) fruit: Nutritional and phytochemical compounds. Front. Plant Sci. 2019, 10, 1073. [Google Scholar] [CrossRef]
- Imran, M.; Arshad, M.S.; Butt, M.S.; Kwon, J.-H.; Arshad, M.U.; Sultan, M.T. Mangiferin: A natural miracle bioactive compound against lifestyle related disorders. Lipids Health Dis. 2017, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Tuo, X.; Wang, L.; Tundis, R.; Portillo, M.P.; Gandara, J.S.; Yu, Y.; Zou, L.; Xiao, J.; Deng, J. Bioactive procyanidins from dietary sources: The relationship between bioactivity and polymerization degree. Trends Food Sci Technol. 2021, 111, 114–127. [Google Scholar] [CrossRef]
- Engels, C.; Gänzle, M.G.; Schieber, A. Fast LC–MS analysis of gallotannins from mango (Mangifera indica L.) kernels and effects of methanolysis on their antibacterial activity and iron binding capacity. Food Res. Int. 2012, 45, 422–426. [Google Scholar] [CrossRef]
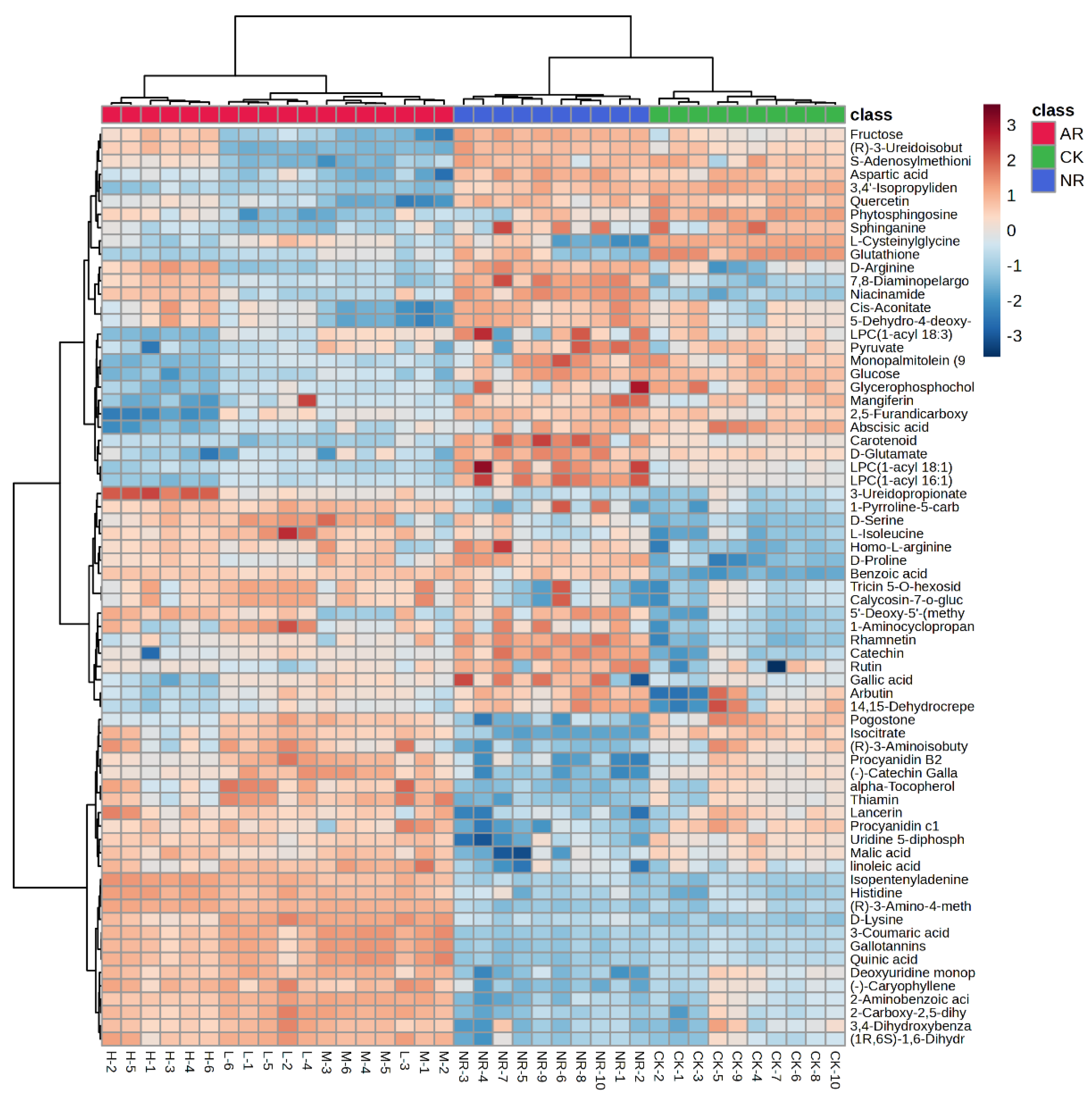
| Category | Compound | AR/NR | AR/CK | NR-CK | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FC | p Values | VIP | Up or Down | FC | p Values | VIP | Up or Down | FC | p Values | VIP | Up or Down | ||
| amino acids and derivatives | (R)-3-Amino-4-methylpentanoic acid a | 7.62 | 7.22 × 10−24 | 1.31 | ↑↑ | 5.4 | 8.26 × 10−19 | 1.38 | ↑↑ | 0.71 | 1.22 × 10−2 | 0.83 | - |
| (R)-3-Aminoisobutyrate a | 2.53 | 8.42 × 10−7 | 1.04 | ↑↑ | 1.09 | 6.87 × 10−1 | 0.11 | - | 0.43 | 2.31 × 10−6 | 1.22 | ↓↓ | |
| (R)-3-Ureidoisobutyrate a | 0.3 | 4.51 × 10−5 | 0.9 | ↓↓ | 0.51 | 2.05 × 10−3 | 0.71 | ↓ | 1.69 | 1.67 × 10−4 | 1.14 | ↑↑ | |
| 1-Aminocyclopropane-1-carboxylate | 0.95 | 6.52 × 10−1 | 0.09 | - | 1.99 | 1.27 × 10−4 | 0.9 | ↑ | 2.1 | 5.23 × 10−5 | 1.16 | ↑↑ | |
| 1-Pyrroline-5-carboxylate | 1.31 | 3.71 × 10−3 | 0.71 | - | 3.01 | 8.13 × 10−12 | 1.31 | ↑↑ | 2.3 | 1.57 × 10−1 | 0.53 | ↑↑ | |
| 2-Aminobenzoic acid a | 3.4 | 2.49 × 10−15 | 1.27 | ↑↑ | 2.25 | 4.21 × 10−11 | 1.27 | ↑↑ | 0.66 | 3.95 × 10−3 | 0.91 | ↓ | |
| 3-Ureidopropionate | 4 | 3.02 × 10−4 | 0.86 | ↑↑ | 4.39 | 1.16 × 10−4 | 1.01 | ↑↑ | 1.1 | 3.56 × 10−1 | 0.41 | - | |
| 7,8-Diaminopelargonic acid a | 0.43 | 2.64 × 10−6 | 0.99 | ↓↓ | 1.58 | 1.20 × 10−2 | 0.72 | ↑ | 3.67 | 2.34 × 10−9 | 1.4 | ↑↑ | |
| Aspartic acid | 0.27 | 3.02 × 10−8 | 1.1 | ↓↓ | 0.32 | 4.27 × 10−7 | 1.1 | ↓↓ | 1.21 | 8.22 × 10−2 | 0.61 | - | |
| D-Arginine a | 0.39 | 2.60 × 10−4 | 0.84 | ↓↓ | 1.64 | 5.62 × 10−1 | 0.2 | ↑ | 4.24 | 6.20 × 10−5 | 1.16 | ↑↑ | |
| D-Glutamate a | 0.41 | 4.61 × 10−8 | 1.1 | ↓↓ | 0.6 | 4.60 × 10−5 | 1 | ↓ | 1.44 | 2.76 × 10−5 | 1.2 | - | |
| D-Lysine a | 3.35 | 1.24 × 10−11 | 1.21 | ↑↑ | 4.86 | 1.29 × 10−14 | 1.3 | ↑↑ | 1.45 | 1.51 × 10−5 | 1.17 | - | |
| D-Proline | 0.71 | 1.73 × 10−3 | 0.76 | - | 2.9 | 4.42 × 10−9 | 1.2 | ↑↑ | 4.12 | 2.76 × 10−9 | 1.38 | ↑↑ | |
| D-Serine | 1.48 | 3.26 × 10−2 | 0.51 | - | 2.97 | 1.60 × 10−7 | 1.13 | ↑↑ | 2.01 | 2.89 × 10−5 | 1.22 | ↑↑ | |
| Histidine | 2.65 | 1.11 × 10−12 | 1.23 | ↑↑ | 3.27 | 2.69 × 10−16 | 1.37 | ↑↑ | 1.24 | 1.05 × 10−1 | 0.61 | - | |
| Homo-L-arginine | 0.82 | 9.22 × 10−2 | 0.43 | - | 1.88 | 6.84 × 10−8 | 1.18 | ↑ | 2.3 | 1.84 × 10−6 | 1.31 | ↑↑ | |
| L-Isoleucine | 1.9 | 3.46 × 10−3 | 0.68 | ↑ | 3.97 | 1.27 × 10−7 | 1.13 | ↑↑ | 2.08 | 4.34 × 10−4 | 1.09 | ↑↑ | |
| S-Adenosylmethionine | 0.5 | 3.55 × 10−6 | 1.08 | ↓↓ | 0.54 | 3.26 × 10−5 | 0.95 | ↓ | 1.05 | 5.57 × 10−1 | 0.17 | - | |
| carbohydrates and derivatives | Fructose | 0.43 | 2.23 × 10−5 | 1.03 | ↓↓ | 0.73 | 2.53 × 10−2 | 0.53 | - | 1.69 | 3.86 × 10−7 | 1.34 | ↑ |
| Glucose | 0.11 | 7.27 × 10−10 | 1.17 | ↓↓ | 0.13 | 1.90 × 10−11 | 1.3 | ↓↓ | 1.23 | 7.05 × 10−1 | 0.06 | - | |
| lipid compounds | 14,15-Dehydrocrepenynic acid | 0.4 | 1.55 × 10−7 | 1.1 | ↓↓ | 0.5 | 4.88 × 10−1 | 0.15 | ↓↓ | 1.25 | 8.74 × 10−2 | 0.64 | - |
| linoleic acid | 3.34 | 6.01 × 10−7 | 1.07 | ↑↑ | 2.3 | 4.47 × 10−6 | 1.03 | ↑↑ | 0.69 | 6.66 × 10−2 | 0.58 | - | |
| Monopalmitolein (9c) | 0.27 | 3.01 × 10−7 | 1.08 | ↓↓ | 0.35 | 5.99 × 10−9 | 1.25 | ↓↓ | 1.29 | 5.18 × 10−1 | 0.13 | - | |
| Phytosphingosine | 0.79 | 3.87 × 10−2 | 0.48 | - | 0.5 | 2.05 × 10−7 | 1.09 | ↓↓ | 0.65 | 3.08 × 10−5 | 1.2 | ↓ | |
| Sphinganine | 0.5 | 6.83 × 10−5 | 0.88 | ↓↓ | 0.5 | 3.60 × 10−7 | 1.09 | ↓↓ | 0.93 | 5.20 × 10−1 | 0.22 | - | |
| nucleosides | 5’-Deoxy-5’-(methylthio)adenosine a | 0.8 | 4.97 × 10−2 | 0.47 | - | 1.66 | 3.53 × 10−4 | 0.92 | ↑ | 2.07 | 8.03 × 10−7 | 1.3 | ↑↑ |
| Deoxyuridine monophosphate | 3.8 | 4.26 × 10−14 | 1.26 | ↑↑ | 1.8 | 8.13 × 10−8 | 1.19 | ↑ | 0.47 | 4.43 × 10−5 | 1.16 | ↓↓ | |
| Uridine 5-diphosphate | 2.98 | 1.71 × 10−8 | 1.13 | ↑↑ | 1.2 | 4.92 × 10−3 | 0.71 | - | 0.4 | 1.09 × 10−4 | 1.15 | ↓↓ | |
| organic acids | 2,5-Furandicarboxylic acid | 0.38 | 1.17 × 10−4 | 1.03 | ↓↓ | 0.5 | 2.55 × 10−3 | 0.86 | ↓↓ | 1.36 | 1.91 × 10−3 | 1.21 | - |
| 2-Carboxy-2,5-dihydro-5-oxofuran-2-acetate | 4.33 | 4.82 × 10−15 | 1.26 | ↑↑ | 2.91 | 3.13 × 10−9 | 1.21 | ↑↑ | 0.67 | 7.35 × 10−2 | 0.6 | - | |
| 3-Coumaric acid | 21.5 | 8.31 × 10−17 | 1.28 | ↑↑ | 14.22 | 2.63 × 10−15 | 1.34 | ↑↑ | 0.66 | 3.47 × 10−5 | 1.17 | ↓ | |
| Benzoic acid a | 1.07 | 1.97 × 10−1 | 0.37 | - | 4.67 | 7.73 × 10−23 | 1.38 | ↑↑ | 4.37 | 3.53 × 10−12 | 1.39 | ↑↑ | |
| Cis-Aconitate | 0.5 | 3.73 × 10−4 | 0.83 | ↓↓ | 0.84 | 1.34 × 10−1 | 0.39 | - | 1.4 | 1.42 × 10−3 | 1 | - | |
| Isocitrate | 32.98 | 1.77 × 10−12 | 1.23 | ↑↑ | 0.95 | 4.88 × 10−1 | 0.25 | - | 0.03 | 4.62 × 10−12 | 1.41 | ↓↓ | |
| Malic acid | 6.08 | 6.18 × 10−7 | 1.05 | ↑↑ | 1.6 | 1.00 × 10−2 | 0.72 | ↑ | 0.26 | 6.65 × 10−4 | 1.03 | ↓↓ | |
| Pyruvate | 0.5 | 2.08 × 10−3 | 1.06 | ↓↓ | 0.69 | 7.80 × 10−3 | 0.7 | - | 1.29 | 3.53 × 10−1 | 0.31 | - | |
| Quinic acid | 27.34 | 1.24 × 10−16 | 1.28 | ↑↑ | 14.23 | 1.47 × 10−14 | 1.34 | ↑↑ | 0.52 | 4.54 × 10−7 | 1.28 | ↓ | |
| others | (1R,6S)-1,6-Dihydroxycyclohexa-2,4-diene-1-carboxylate | 3.86 | 5.17 × 10−13 | 1.24 | ↑↑ | 2.8 | 2.99 × 10−10 | 1.26 | ↑↑ | 0.73 | 8.18 × 10−2 | 0.53 | - |
| 5-Dehydro-4-deoxy-D-glucarate | 0.5 | 1.97 × 10−4 | 0.86 | ↓↓ | 0.8 | 7.91 × 10−2 | 0.46 | - | 1.4 | 1.12 × 10−3 | 1.02 | - | |
| Abscisic acid | 0.5 | 4.24 × 10−5 | 0.91 | ↓↓ | 0.44 | 1.16 × 10−7 | 1.16 | ↓↓ | 0.81 | 7.75 × 10−2 | 0.57 | - | |
| Isopentenyladenine-9-N-glucoside | 22.32 | 5.61 × 10−17 | 1.29 | ↑↑ | 30.21 | 5.00 × 10−19 | 1.39 | ↑↑ | 1.35 | 1.73 × 10−1 | 0.51 | - | |
| peptides | Glutathione | 0.11 | 1.79 × 10−1 | 0.34 | ↓↓ | 0.02 | 3.74 × 10−14 | 1.34 | ↓↓ | 0.19 | 3.69 × 10−4 | 1.08 | ↓↓ |
| L-Cysteinylglycine | 0.85 | 1.45 × 10−1 | 0.38 | - | 0.12 | 1.69 × 10−7 | 1.18 | ↓↓ | 0.15 | 1.69 × 10−4 | 1.11 | ↓↓ | |
| phospholipids | Glycerophosphocholine | 0.62 | 5.26 × 10−4 | 0.87 | ↓ | 0.5 | 7.64 × 10−9 | 1.26 | ↓↓ | 0.93 | 3.48 × 10−1 | 0.34 | - |
| LPC(1-acyl 16:1) | 0.16 | 1.14 × 10−12 | 1.25 | ↓↓ | 0.59 | 1.49 × 10−6 | 1.12 | ↓ | 3.65 | 2.18 × 10−7 | 1.28 | ↑↑ | |
| LPC(1-acyl 18:1) | 0.3 | 2.03 × 10−10 | 1.21 | ↓↓ | 0.71 | 1.86 × 10−7 | 1.15 | - | 2.4 | 4.32 × 10−5 | 1.15 | ↑↑ | |
| LPC(1-acyl 18:3) | 0.69 | 1.70 × 10−2 | 0.63 | - | 0.83 | 2.12 × 10−2 | 1.08 | - | 1.2 | 4.27 × 10−1 | 0.29 | - | |
| polyphenols | (-)-Catechin Gallate | 3.92 | 3.80 × 10−10 | 1.17 | ↑↑ | 1.35 | 3.27 × 10−2 | 0.53 | - | 0.34 | 2.05 × 10−7 | 1.29 | ↓↓ |
| 3,4-Dihydroxybenzaldehyde | 3.4 | 1.35 × 10−9 | 1.16 | ↑↑ | 1.95 | 4.43 × 10−5 | 1 | ↑ | 0.57 | 9.33 × 10−2 | 0.5 | ↓ | |
| 3,4’-Isopropylidenediphenol | 0.21 | 8.57 × 10−16 | 1.27 | ↓↓ | 0.16 | 4.11 × 10−19 | 1.37 | ↓↓ | 0.76 | 9.07 × 10−4 | 1.02 | - | |
| Arbutin | 0.49 | 7.38 × 10−5 | 1.03 | ↓↓ | 0.84 | 4.34 × 10−1 | 0.25 | - | 1.53 | 2.70 × 10−2 | 0.78 | ↑ | |
| Calycosin-7-o-glucoside | 1.48 | 5.90 × 10−3 | 0.66 | - | 3.08 | 3.43 × 10−6 | 1.07 | ↑↑ | 2.08 | 4.73 × 10−1 | 0.36 | ↑↑ | |
| Catechin | 0.3 | 4.89 × 10−6 | 1.39 | ↓↓ | 2.14 | 2.71 × 10−3 | 0.77 | ↑↑ | 7.1 | 2.63 × 10−8 | 1.34 | ↑↑ | |
| Gallic acid | 0.41 | 7.49 × 10−2 | 0.42 | ↓↓ | 1.17 | 6.14 × 10−1 | 0.09 | - | 2.85 | 9.38 × 10−2 | 1.08 | ↑↑ | |
| Gallotannins | 24.27 | 6.45 × 10−17 | 1.28 | ↑↑ | 14.35 | 2.84 × 10−15 | 1.34 | ↑↑ | 0.59 | 5.25 × 10−5 | 1.14 | ↓ | |
| Lancerin | 4.17 | 2.13 × 10−7 | 1.1 | ↑↑ | 1.35 | 8.50 × 10−2 | 0.55 | - | 0.32 | 7.78 × 10−4 | 0.99 | ↓↓ | |
| Mangiferin | 0.37 | 7.00 × 10−5 | 1.01 | ↓↓ | 0.66 | 6.20 × 10−3 | 0.76 | ↓ | 1.79 | 3.04 × 10−2 | 0.74 | ↑ | |
| Procyanidin B2 | 3.48 | 1.36 × 10−7 | 1.08 | ↑↑ | 1.57 | 5.34 × 10−3 | 0.69 | ↑ | 0.45 | 5.70 × 10−4 | 1.01 | ↓↓ | |
| Procyanidin c1 | 4.27 | 4.55 × 10−8 | 1.11 | ↑↑ | 1.03 | 8.75 × 10−1 | 0.07 | - | 0.24 | 9.86 × 10−6 | 1.14 | ↓↓ | |
| Quercetin | 0.44 | 1.82 × 10−5 | 0.94 | ↓↓ | 0.42 | 6.40 × 10−6 | 1.02 | ↓↓ | 0.95 | 5.33 × 10−1 | 0.27 | - | |
| Rhamnetin | 0.39 | 3.86 × 10−8 | 1.09 | ↓↓ | 2.61 | 3.77 × 10−7 | 1.13 | ↑↑ | 6.61 | 3.68 × 10−11 | 1.43 | ↑↑ | |
| Rutin | 0.4 | 5.11 × 10−4 | 0.8 | ↓↓ | 1.12 | 9.90 × 10−2 | 0.51 | - | 2.81 | 8.06 × 10−3 | 1.1 | ↑↑ | |
| Tricin 5-O-hexoside | 1.45 | 8.48 × 10−3 | 0.64 | - | 3.01 | 8.29 × 10−6 | 1.05 | ↑↑ | 2.08 | 4.73 × 10−1 | 0.36 | ↑↑ | |
| terpenoids | Carotenoid | 0.27 | 7.19 × 10−11 | 1.18 | ↓↓ | 0.6 | 1.27 × 10−8 | 1.2 | ↓ | 2.22 | 1.00 × 10−4 | 1.08 | ↑↑ |
| Pogostone | 1.92 | 1.55 × 10−7 | 1.08 | ↑ | 0.87 | 1.18 × 10−1 | 0.46 | - | 0.45 | 4.44 × 10−7 | 1.29 | ↓↓ | |
| (-)-Caryophyllene oxide | 3.44 | 6.02 × 10−13 | 1.24 | ↑↑ | 2.07 | 7.18 × 10−9 | 1.21 | ↑↑ | 0.6 | 3.75 × 10−4 | 1.03 | ↓ | |
| vitamins with cofactors | alpha-Tocopherol | 7.43 | 5.29 × 10−9 | 1.15 | ↑↑ | 2.25 | 6.22 × 10−3 | 0.7 | ↑↑ | 0.3 | 9.03 × 10−6 | 1.23 | ↓↓ |
| Niacinamide a | 0.39 | 1.66 × 10−5 | 0.93 | ↓↓ | 2.55 | 5.76 × 10−4 | 0.9 | ↑↑ | 6.57 | 8.77 × 10−12 | 1.38 | ↑↑ | |
| Thiamin | 11.16 | 1.22 × 10−9 | 1.16 | ↑↑ | 2.8 | 1.80 × 10−3 | 0.78 | ↑↑ | 0.25 | 6.13 × 10−6 | 1.26 | ↓↓ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Ren, C.; Wang, J.; Fu, J.; Yin, Q.; Huang, Y.; He, Z. Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry-Based Metabolic Characterization of Mango Ripened by Different Methods. Foods 2024, 13, 3548. https://doi.org/10.3390/foods13223548
Wang J, Ren C, Wang J, Fu J, Yin Q, Huang Y, He Z. Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry-Based Metabolic Characterization of Mango Ripened by Different Methods. Foods. 2024; 13(22):3548. https://doi.org/10.3390/foods13223548
Chicago/Turabian StyleWang, Jishi, Chaoqi Ren, Jiafu Wang, Jiqiang Fu, Qingchun Yin, Yongping Huang, and Zeying He. 2024. "Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry-Based Metabolic Characterization of Mango Ripened by Different Methods" Foods 13, no. 22: 3548. https://doi.org/10.3390/foods13223548
APA StyleWang, J., Ren, C., Wang, J., Fu, J., Yin, Q., Huang, Y., & He, Z. (2024). Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry-Based Metabolic Characterization of Mango Ripened by Different Methods. Foods, 13(22), 3548. https://doi.org/10.3390/foods13223548





