Evaluating the Impact of Pre-Fermentative and Post-Fermentative Vinification Technologies on Bioactive Compounds and Antioxidant Activity of Teran Red Wine By-Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Grape Material
2.3. Minivinification
2.4. By-Product Sample Preparation
2.5. Extraction
2.6. Analysis of Individual Phenolic Compounds
2.7. Analysis of Total Phenolic Content
2.8. Analysis of Antioxidant Activity
2.9. Statistical Data Analysis
3. Results and Discussion
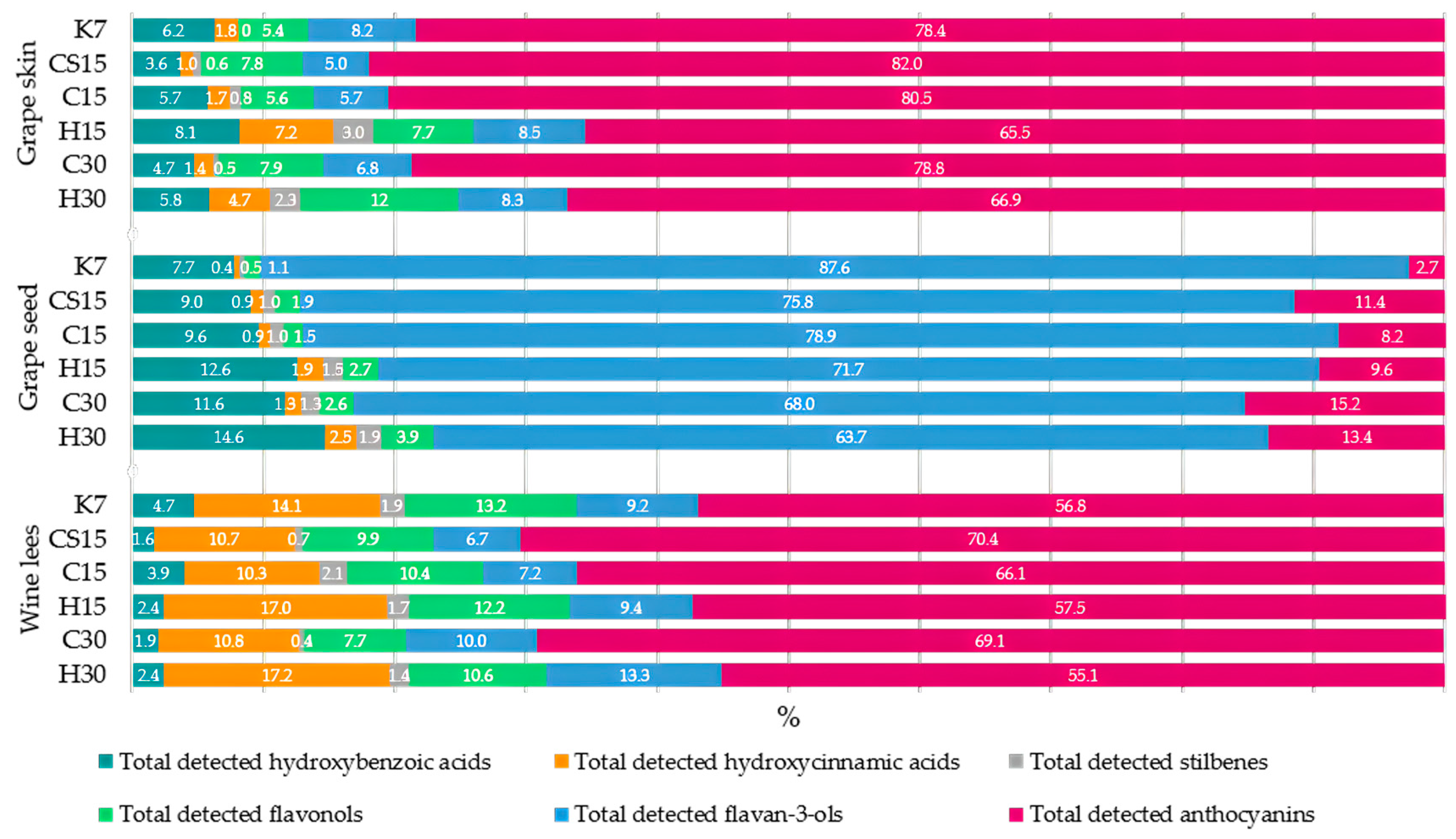
3.1. Individual Phenolic Compounds in Grape Skin Extracts
| Phenolic Compounds | Treatments | |||||
|---|---|---|---|---|---|---|
| K7 | CS15 | C15 | H15 | C30 | H30 | |
| Phenolic acids | ||||||
| Gallic acid | 9.83 ± 0.55 e | 14.60 ± 0.60 d | 16.80 ± 0.21 c | 19.97 ± 0.53 b | 17.33 ± 0.61 c | 23.15 ± 0.38 a |
| Protocatehuic acid | 3.79 ± 0.23 c | 4.69 ± 0.50 ab | 4.59 ± 0.18 ab | 4.35 ± 0.14 b | 5.06 ± 0.39 a | 3.61 ± 0.12 c |
| Vanillic acid | 8.83 ± 0.55 a | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b | 0 ± 0 b |
| Syringic acid | 10.51 ± 0.25 d | 15.46 ± 0.14 c | 25.48 ± 0.41 a | 20.63 ± 1.07 b | 16.20 ± 1.11 c | 9.82 ± 0.09 d |
| Total detected hydroxybenzoic acids | 32.95 ± 0.94 f | 34.76 ± 0.99 e | 46.86 ± 0.49 a | 44.94 ± 1.26 b | 38.59 ± 0.95 c | 36.58 ± 0.22 d |
| Caftaric acid | 9.45 ± 0.35 d | 9.79 ± 0.14 d | 14.33 ± 0.21 c | 40.05 ± 0.61 a | 11.25 ± 0.22 c | 29.80 ± 0.43 b |
| Total detected hydroxycinnamic acids | 9.45 ± 0.35 d | 9.79 ± 0.14 d | 14.33 ± 0.21 c | 40.05 ± 0.61 a | 11.25 ± 0.22 c | 29.80 ± 0.43 b |
| Stilbenes | ||||||
| trans-piceid | 0 ± 0 e | 1.99 ± 0.10 c | 1.93 ± 0.09 c | 9.08 ± 0.10 a | 1.10 ± 0.11 d | 8.61 ± 0.07 b |
| trans-resveratrol | 0 ± 0 e | 4.27 ± 0.46 c | 4.43 ± 0.27 c | 7.36 ± 0.08 a | 3.23 ± 0.15 d | 6.17 ± 0.06 b |
| Total detected stilbenes | 0 ± 0 e | 6.26 ± 0.48 c | 6.37 ± 0.25 c | 16.44 ± 0.18 a | 4.33 ± 0.25 d | 14.78 ± 0.12 b |
| Flavonols | ||||||
| Quercetin-3-O-glucoside | 12.84 ± 0.20 e | 17.33 ± 0.12 c | 15.10 ± 0.05 d | 22.56 ± 0.41 a | 9.65 ± 0.08 f | 19.13 ± 0.28 b |
| Quercetin | 10.85 ± 0.08 e | 52.73 ± 2.96 a | 24.14 ± 0.95 c | 14.40 ± 0.84 d | 48.84 ± 0.23 b | 48.42 ± 1.69 b |
| Kaempferol | 5.37 ± 0.11 e | 6.57 ± 0.37 c | 7.23 ± 0.11 b | 5.87 ± 0.05 d | 7.30 ± 0.16 b | 8.01 ± 0.28 a |
| Total detected flavonols | 29.06 ± 0.13 e | 76.63 ± 3.29 a | 46.47 ± 0.91 c | 42.83 ± 1.13 d | 65.79 ± 0.21 b | 75.56 ± 2.13 a |
| Flavan-3-ols | ||||||
| (+)-Catechin | 18.36 ± 3.27 a | 14.35 ± 1.42 b | 14.85 ± 1.51 ab | 14.66 ± 1.02 b | 16.87 ± 1.38 ab | 13.65 ± 2.74 b |
| (−)-Epicatechin | 2.79 ± 0.09 c | 6.67 ± 0.56 b | 6.61 ± 0.33 b | 6.93 ± 0.29 ab | 8.14 ± 0.42 a | 7.55 ± 0.39 a |
| Procyanidin B1 | 10.35 ± 0.69 d | 13.60 ± 0.42 ab | 12.44 ± 0.32 c | 12.56 ± 0.67 bc | 14.90 ± 0.29 a | 14.56 ± 0.99 a |
| Procyanidin B2 | 2.61 ± 0.15 d | 7.61 ± 0.42 b | 6.85 ± 0.07 c | 6.54 ± 0.40 c | 9.08 ± 0.14 a | 8.58 ± 0.4 a |
| Procyanidin B3 | 3.30 ± 0.11 c | 4.68 ± 0.17 b | 4.50 ± 0.09 b | 4.33 ± 0.41 b | 5.20 ± 0.28 a | 5.59 ± 0.17 a |
| Procyanidin C1 | 6.30 ± 1.19 a | 1.73 ± 0.02 b | 1.80 ± 0.10 b | 1.88 ± 0.13 b | 1.80 ± 0.22 b | 2.44 ± 0.07 b |
| Total detected flavan-3-ols | 43.71 ± 5.25 c | 48.63 ± 2.67 bc | 47.05 ± 2.20 bc | 46.90 ± 2.87 bc | 55.99 ± 2.12 a | 52.37 ± 3.24 ab |
| Anthocyanins | ||||||
| Delphinidin-3-O-glucoside | 12.31 ± 0.15 f | 45.91 ± 0.71 a | 29.58 ± 0.58 c | 11.54 ± 0.23 e | 31.34 ± 0.07 b | 18.60 ± 0.42 d |
| Cyanidin-3-O-glucoside | 1.23 ± 0.04 d | 2.86 ± 0.08 b | 2.55 ± 0.02 a | 0.74 ± 0.02 e | 1.60 ± 0.06 c | 0.83 ± 0.03 f |
| Petunidin-3-O-glucoside | 17.67 ± 0.03 d | 43.46 ± 0.16 a | 34.48 ± 0.30 b | 15.76 ± 0.23 f | 32.26 ± 0.28 c | 19.87 ± 0.40 e |
| Peonidin-3-O-glucoside | 14.40 ± 0.40 d | 27.71 ± 0.33 a | 20.34 ± 0.31 b | 11.34 ± 0.20 e | 17.15 ± 0.34 c | 11.19 ± 0.20 e |
| Malvidin-3-O-glucoside | 206.93 ± 5.97 d | 415.33 ± 3.94 a | 352.97 ± 2.77 b | 183.87 ± 3.47 f | 342.12 ± 4.16 c | 213.87 ± 3.42 d |
| Peonidin-3-O-acetilglucoside | 3.27 ± 0.25 e | 9.95 ± 0.35 a | 5.77 ± 0.10 c | 3.01 ± 0.18 e | 7.93 ± 0.95 b | 4.86 ± 0.08 d |
| Malvidin-3-O-acetilglucoside | 40.08 ± 0.64 c | 76.80 ± 2.02 a | 61.60 ± 1.03 b | 34.96 ± 0.57 d | 63.64 ± 3.40 b | 40.49 ± 0.66 c |
| Peonidin-3-O-coumarylglucoside | 13.04 ± 0.2 b | 16.97 ± 0.19 a | 13.77 ± 0.46 b | 9.46 ± 0.25 c | 12.89 ± 1.11 b | 10.30 ± 0.31 c |
| Malvidin-3-O-coumarylglucoside | 109.70 ± 1.69 c | 163.48 ± 3.35 a | 142.23 ± 1.43 b | 92.44 ± 4.88 e | 143.92 ± 0.83 b | 102.73 ± 3.43 d |
| Total detected anthocyanins | 418.63 ± 8.27 c | 802.47 ± 10.24 a | 663.29 ± 5.95 b | 363.11 ± 8.91 d | 652.86 ± 9.07 b | 422.74 ± 8.84 c |
| Total detected phenolic compounds | 533.80 ± 3.57 e | 978.54 ± 14.96 a | 824.37 ± 4.43 b | 554.27 ± 13.67 d | 828.81 ± 9.96 b | 631.82 ± 14.28 c |
3.2. Individual Phenolic Compounds in Grape Seed Extracts
| Phenolic Compounds | Treatments | |||||
|---|---|---|---|---|---|---|
| K7 | CS15 | C15 | H15 | C30 | H30 | |
| Phenolic acids | ||||||
| Gallic acid | 26.00 ± 0.90 a | 24.50 ± 2.13 a | 25.68 ± 1.38 a | 24.14 ± 0.91 a | 24.98 ± 0.81 a | 24.17 ± 0.94 a |
| Protocatehuic acid | 2.41 ± 0.04 ab | 2.21 ± 0.27 b | 2.64 ± 0.01 a | 2.52 ± 0.04 a | 2.14 ± 0.06 b | 1.89 ± 0.07 c |
| Syringic acid | 4.68 ± 0.77 a | 3.29 ± 0.08 b | 3.32 ± 0.34 b | 2.96 ± 0.53 c | 2.31 ± 0.31 d | 1.87 ± 0.13 e |
| Total detected hydroxybenzoic acids | 33.09 ± 1.54 a | 30.01 ± 2.30 bc | 31.64 ± 1.49 ab | 29.62 ± 0.81 bc | 29.43 ± 1.09 c | 27.93 ± 1.14 c |
| Caftaric acid | 1.63 ± 0.71 e | 2.89 ± 0.04 d | 2.87 ± 1.02 d | 4.49 ± 0.76 b | 3.19 ± 0.75 c | 4.81 ± 0.23 a |
| Total detected hydroxycinnamic acids | 1.63 ± 0.71 e | 2.89 ± 0.04 d | 2.87 ± 1.02 d | 4.49 ± 0.76 b | 3.19 ± 0.75 c | 4.81 ± 0.23 a |
| Stilbenes | ||||||
| trans-piceid | 1.06 ± 0.04 c | 1.13 ± 0.07 bc | 1.05 ± 0.25 c | 1.53 ± 0.38 a | 0.91 ± 0.26 d | 1.20 ± 0.04 b |
| trans-resveratrol | 1.19 ± 0.51 d | 2.05 ± 0.15 b | 2.11 ± 0.11 b | 2.10 ± 0.11 b | 2.40 ± 0.14 a | 2.35 ± 0.02 a |
| Total detected stilbenes | 2.25 ± 0.55 d | 3.17 ± 0.20 b | 3.16 ± 0.16 b | 3.63 ± 0.28 a | 3.31 ± 0.11 b | 3.55 ± 0.05 a |
| Flavonols | ||||||
| Quercetin-3-O-glucoside | 0.98 ± 0.37 e | 1.66 ± 0.26 c | 1.20 ± 0.56 d | 2.14 ± 0.69 a | 1.00 ± 0.39 e | 1.75 ± 0.03 b |
| Quercetin | 3.43 ± 0.10 c | 3.86 ± 0.6 b | 3.06 ± 0.04 c | 3.39 ± 0.51 c | 4.68 ± 0.24 a | 4.87 ± 0.06 a |
| Kaempferol | 0.44 ± 0.23 c | 0.86 ± 0.14 a | 0.70 ± 0.04 b | 0.86 ± 0.08 a | 0.81 ± 0.03 a | 0.86 ± 0.01 a |
| Total detected flavonols | 4.85 ± 0.70 c | 6.38 ± 0.99 b | 4.96 ± 0.63 c | 6.38 ± 0.27 b | 6.49 ± 0.19 b | 7.47 ± 0.04 a |
| Flavan-3-ols | ||||||
| (+)-Catechin | 142.18 ± 30.20 a | 92.51 ± 2.77 b | 93.94 ± 25.56 b | 57.36 ± 3.01 c | 62.91 ± 16.12 c | 43.72 ± 0.33 d |
| (−)-Epicatechin | 99.65 ± 24.42 a | 59.00 ± 1.36 b | 62.61 ± 18.48 b | 34.72 ± 1.17 d | 39.28 ± 10.04 c | 25.28 ± 0.26 e |
| Procyanidin B1 | 42.11 ± 5.57 a | 31.14 ± 0.81 b | 30.91 ± 3.70 b | 24.69 ± 2.09 c | 21.86 ± 3.09 d | 17.43 ± 0.64 e |
| Procyanidin B2 | 48.17 ± 5.26 a | 37.07 ± 2.27 b | 38.92 ± 5.53 b | 29.19 ± 1.67 c | 26.74 ± 4.32 d | 19.99 ± 0.57 e |
| Procyanidin B3 | 37.25 ± 6.11 a | 25.83 ± 0.8 b | 26.61 ± 6.41 b | 17.23 ± 1.62 c | 15.74 ± 2.61 c | 11.65 ± 0.29 d |
| Procyanidin C1 | 9.53 ± 1.30 a | 7.10 ± 0.21 c | 7.57 ± 1.39 b | 5.49 ± 0.35 d | 5.34 ± 1.10 d | 3.86 ± 0.11 e |
| Total detected flavan-3-ols | 378.89 ± 72.53 a | 252.65 ± 3.73 b | 260.55 ± 60.87 b | 168.67 ± 7.59 c | 171.88 ± 37.22 c | 121.93 ± 1.02 d |
| Anthocyanins | ||||||
| Delphinidin-3-O-glucoside | 2.20 ± 0.84 e | 3.65 ± 0.35 a | 3.04 ± 0.15 c | 2.83 ± 0.19 d | 3.30 ± 0.35 b | 2.82 ± 0.14 d |
| Cyanidin-3-O-glucoside | 0.11 ± 0.06 c | 0.21 ± 0.03 a | 0.16 ± 0.03 b | 0.12 ± 0.02 c | 0.15 ± 0.03 b | 0.10 ± 0 d |
| Petunidin-3-O-glucoside | 1.19 ± 0.74 d | 2.50 ± 0.34 a | 1.94 ± 0.08 bc | 1.77 ± 0.02 c | 2.20 ± 0.35 b | 1.86 ± 0.02 c |
| Peonidin-3-O-glucoside | 0.45 ± 0.62 d | 1.51 ± 0.31 a | 0.97 ± 0.03 c | 0.91 ± 0.13 c | 1.20 ± 0.18 b | 0.96 ± 0.05 c |
| Malvidin-3-O-glucoside | 5.11 ± 8.61 d | 20.05 ± 3.68 a | 14.00 ± 1.41 b | 11.47 ± 4.61 c | 20.87 ± 4.76 a | 13.81 ± 0.09 b |
| Malvidin-3-O-acetilglucoside | 1.15 ± 2.01 d | 4.11 ± 0.55 a | 2.96 ± 0.44 b | 2.20 ± 1.57 c | 4.50 ± 1.00 a | 2.68 ± 0.04 b |
| Malvidin-3-O-coumarylglucoside | 1.51 ± 2.67 d | 6.11 ± 1.27 a | 3.93 ± 0.41 b | 3.22 ± 1.38 c | 6.22 ± 1.82 a | 3.49 ± 0.08 c |
| Total detected anthocyanins | 11.72 ± 15.55 d | 38.15 ± 6.51 a | 27.02 ± 2.54 b | 22.50 ± 7.91 c | 38.44 ± 8.50 a | 25.72 ± 0.42 b |
| Total detected phenolic compounds | 432.42 ± 56.34 a | 333.25 ± 5.75 b | 330.19 ± 62.18 b | 235.3 ± 1.79 d | 252.73 ± 45.5 c | 191.41 ± 2.37 e |
3.3. Individual Phenolic Compounds in Wine Lees Extracts
| Phenolic Compounds | Treatments | |||||
|---|---|---|---|---|---|---|
| K7 | CS15 | C15 | H15 | C30 | H30 | |
| Phenolic acids | ||||||
| Gallic acid | 3.93 ± 0.25 e | 7.70 ± 0.13 c | 6.96 ± 0.32 d | 11.15 ± 0.43 b | 11.08 ± 0.44 b | 14.06 ± 0.56 a |
| Syringic acid | 5.89 ± 0.16 b | 0 ± 0 c | 7.22 ± 0.30 a | 0 ± 0 c | 0 ± 0 c | 0 ± 0 c |
| Vanillic acid | 5.00 ± 0.61 b | 0 ± 0 c | 5.52 ± 0.31 a | 0 ± 0 c | 0 ± 0 c | 0 ± 0 c |
| Total detected hydroxybenzoic acids | 14.82 ± 0.98 b | 7.70± 0.13 d | 19.70 ± 0.97 a | 11.15 ± 0.43 c | 11.08 ± 0.44 c | 14.06 ± 0.56 b |
| Caftaric acid | 44.52 ± 3.70 e | 53.00 ± 0.48 d | 52.22 ± 0.48 d | 80.12 ± 0.49 b | 62.97 ± 1.49 c | 102.47 ± 1.99 a |
| Total detected hydroxycinnamic acids | 44.52 ± 3.70 e | 53.00 ± 0.48 d | 52.22 ± 0.48 d | 80.12 ± 0.49 b | 62.97 ± 1.49 c | 102.47 ± 1.99 a |
| Stilbenes | ||||||
| trans-piceid | 2.61 ± 0.10 f | 3.67 ± 0.04 c | 4.15 ± 0.05 d | 7.81 ± 0.38 b | 2.49 ± 0.06 e | 8.56 ± 0.22 a |
| trans-resveratrol | 3.46 ± 0.21 b | 0 ± 0 c | 6.29 ± 0.48 a | 0 ± 0 c | 0 ± 0 c | 0 ± 0 c |
| Total detected stilbenes | 6.07 ± 0.30 d | 3.67 ± 0.04 e | 10.44 ± 0.45 a | 7.81 ± 0.38 c | 2.49 ± 0.06 f | 8.56 ± 0.22 b |
| Flavanols | ||||||
| Quercetin-3-O-glucoside | 3.93 ± 0.65 d | 7.48 ± 0.10 c | 6.85 ± 0.14 c | 15.66 ± 1.61 b | 4.37 ± 0.05 d | 19.68 ± 0.49 a |
| Myricetin | 5.58 ± 0.51 c | 7.31 ± 0.87 b | 10.66 ± 1.04 a | 10.33 ± 0.87 a | 7.94 ± 0.21 b | 9.80 ± 0.26 a |
| Quercetin | 28.76 ± 1.59 a | 29.71 ± 2.47 a | 31.52 ± 2.51 a | 27.59 ± 2.44 a | 28.44 ± 0.22 a | 28.73 ± 4.46 a |
| Kaempferol | 3.49 ± 0.12 e | 4.37 ± 0.14 b | 3.68 ± 0.19 de | 3.85 ± 0.02 cd | 4.07 ± 0.29 bc | 5.10 ± 0.27 a |
| Total detected flavonols | 41.75 ± 2.88 e | 48.88 ± 3.5 cd | 52.71 ± 3.82 bc | 57.43 ± 4.48 ab | 44.82 ± 0.34 de | 63.3 ± 5.36 a |
| Flavan-3-ols | ||||||
| (+)-Catechin | 17.89 ± 0.75 c | 15.31 ± 0.45 d | 15.74 ± 0.55 d | 19.84 ± 0.23 b | 20.05 ± 0.67 b | 29.46 ± 0.54 a |
| (−)-Epicatechin | 1.53 ± 0.47 d | 3.48 ± 0.13 c | 4.44 ± 0.24 c | 4.33 ± 2.54 c | 8.48 ± 0.33 b | 12.02 ± 1.27 a |
| Procyanidin B1 | 4.40 ± 0.52 e | 6.41 ± 0.38 d | 6.99 ± 0.86 d | 8.83 ± 0.06 c | 13.06 ± 0.31 b | 17.83 ± 0.68 a |
| Procyanidin B2 | 2.22 ± 0.35 f | 3.54 ± 0.02 e | 4.37 ± 0.21 d | 5.01 ± 0.08 c | 9.78 ± 0.22 b | 11.69 ± 0.19 a |
| Procyanidin B3 | 2.32 ± 0.29 d | 3.39 ± 0.28 c | 3.59 ± 0.07 c | 3.69 ± 0.03 c | 4.53 ± 0.12 b | 6.16 ± 0.27 a |
| Procyanidin C1 | 0.60 ± 0.05 e | 1.05 ± 0.04 d | 1.21 ± 0.11 c | 2.56 ± 2.16 ab | 2.36 ± 0.06 ab | 2.16 ± 0.05 b |
| Total detected flavan-3-ols | 28.95 ± 2.41 e | 33.18 ± 1.2 de | 36.34 ± 1.72 d | 44.26 ± 3.80 c | 58.25 ± 1.69 b | 79.33 ± 2.78 a |
| Anthocyanins | ||||||
| Delphinidin-3-O-glucoside | 5.26 ± 0.35 d | 14.61 ± 0.33 a | 14.65 ± 0.48 a | 12.68 ± 0.32 c | 13.6 ± 0.6 b | 13.31 ± 0.31 bc |
| Cyanidin-3-O-glucoside | 0.33 ± 0.02 e | 1.09 ± 0.01 a | 0.79 ± 0.06 b | 0.56 ± 0.03 d | 0.11 ± 0.01 f | 0.71 ± 0.03 c |
| Petunidin-3-O-glucoside | 6.68 ± 0.26 e | 16.65 ± 0.35 b | 15.29 ± 0.16 c | 12.78 ± 0.17 d | 17.35 ± 0.52 a | 15.63 ± 0.31 c |
| Peonidin-3-O-glucoside | 4.30 ± 0.10 e | 10.84 ± 0.18 a | 8.59 ± 0.05 c | 7.36 ± 0.17 d | 10.16 ± 0.22 b | 8.75 ± 0.17 c |
| Malvidin-3-O-glucoside | 89.00 ± 1.73 e | 179.35 ± 4.05 b | 168.78 ± 0.98 c | 137.23 ± 0.80 d | 207.12 ± 3.82 a | 171.41 ± 2.95 c |
| Peonidin-3-O-acetilglucoside | 2.14 ± 0.03 c | 3.87 ± 0.32 b | 4.53 ± 0.16 a | 3.49 ± 0.18 b | 4.38 ± 0.43 ab | 4.63 ± 0.48 a |
| Malvidin-3-O-acetilglucoside | 17.70 ± 0.63 e | 32.95 ± 0.13 b | 32.42 ± 0.3 b | 22.52 ± 0.92 d | 37.21 ± 0.93 a | 29.19 ± 0.23 c |
| Peonidin-3-O-coumarylglucoside | 4.90 ± 0.07 d | 4.68 ± 0.45 d | 8.28 ± 1.70 bc | 9.48 ± 0.58 b | 11.87 ± 1.09 a | 7.71 ± 0.51 c |
| Malvidin-3-O-coumarylglucoside | 48.88 ± 0.87 e | 84.12 ± 2.01 b | 81.25 ± 4.88 bc | 65.27 ± 6.89 d | 100.62 ± 1.35 a | 76.77 ± 1.49 c |
| Total detected anthocyanins | 179.19 ± 3.94 e | 348.16 ± 6.96 b | 334.60 ± 8.10 c | 271.38 ± 9.94 d | 402.43 ± 6.49 a | 328.11 ± 5.30 c |
| Total detected phenolic compounds | 334.96 ± 9.97 d | 494.58 ± 11.29 b | 506.01 ± 10.17 b | 472.15 ± 15.84 c | 582.04 ± 9.61 a | 595.83 ± 9.07 a |
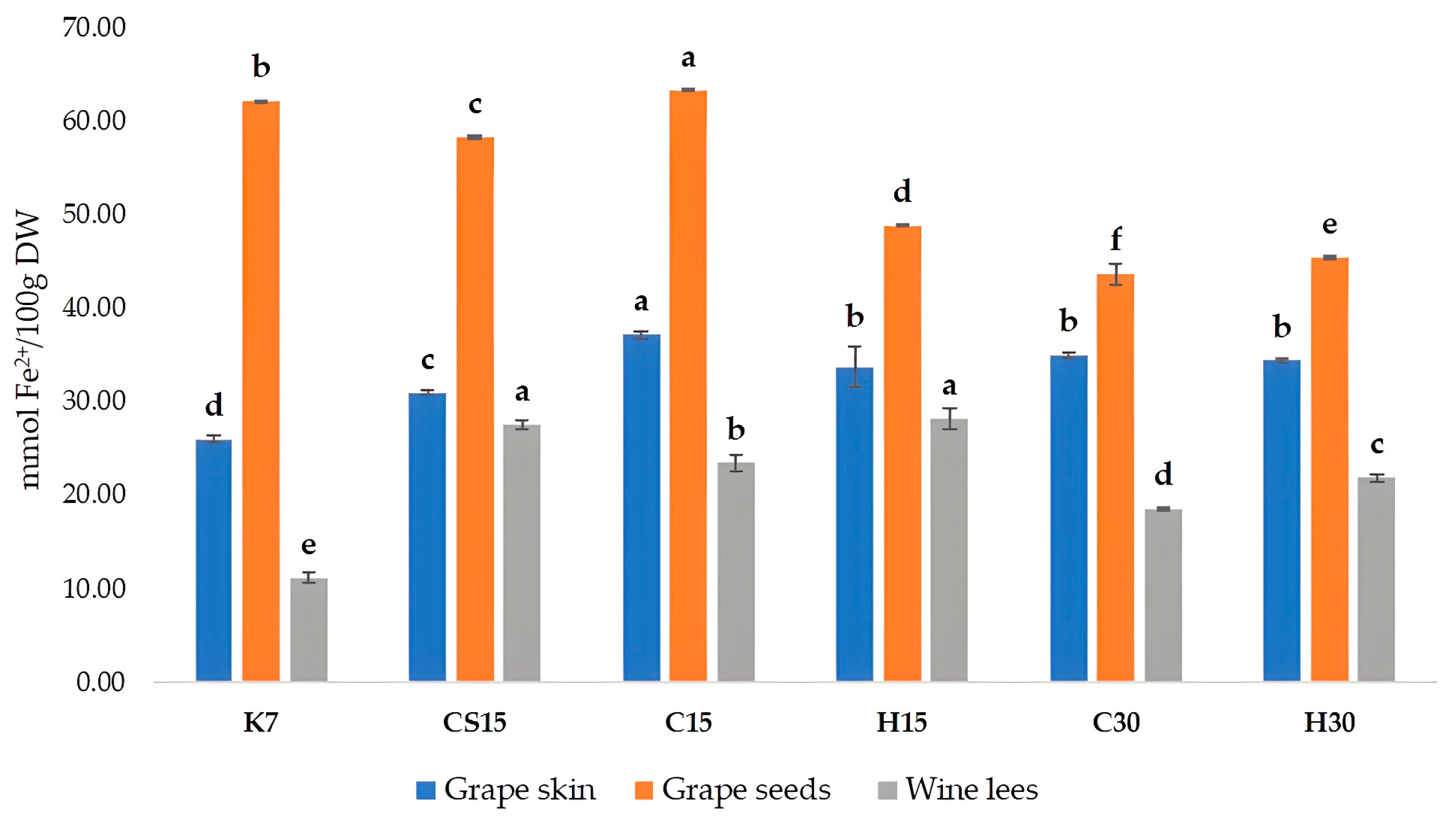
3.4. Total Phenolic Content and Antioxidant Activity of Grape Skin, Seed, and Wine Lees Extracts
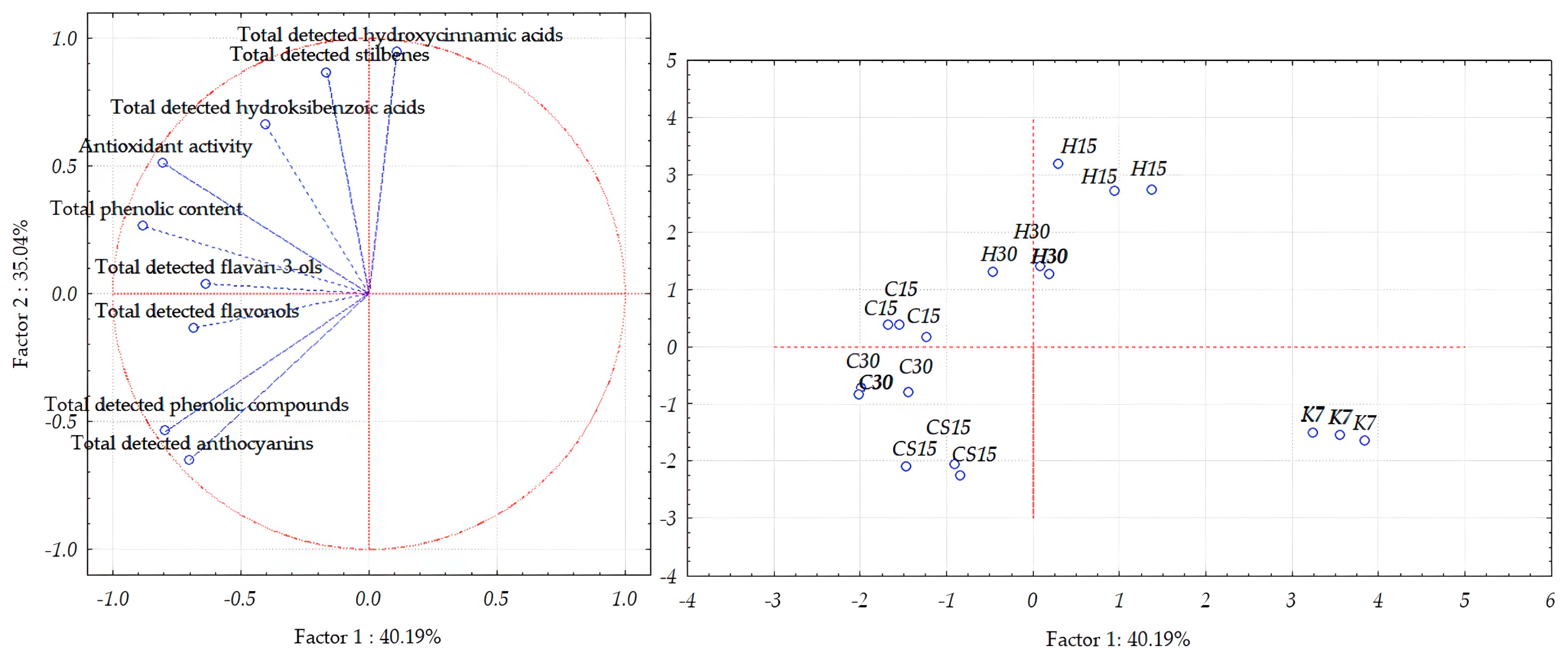
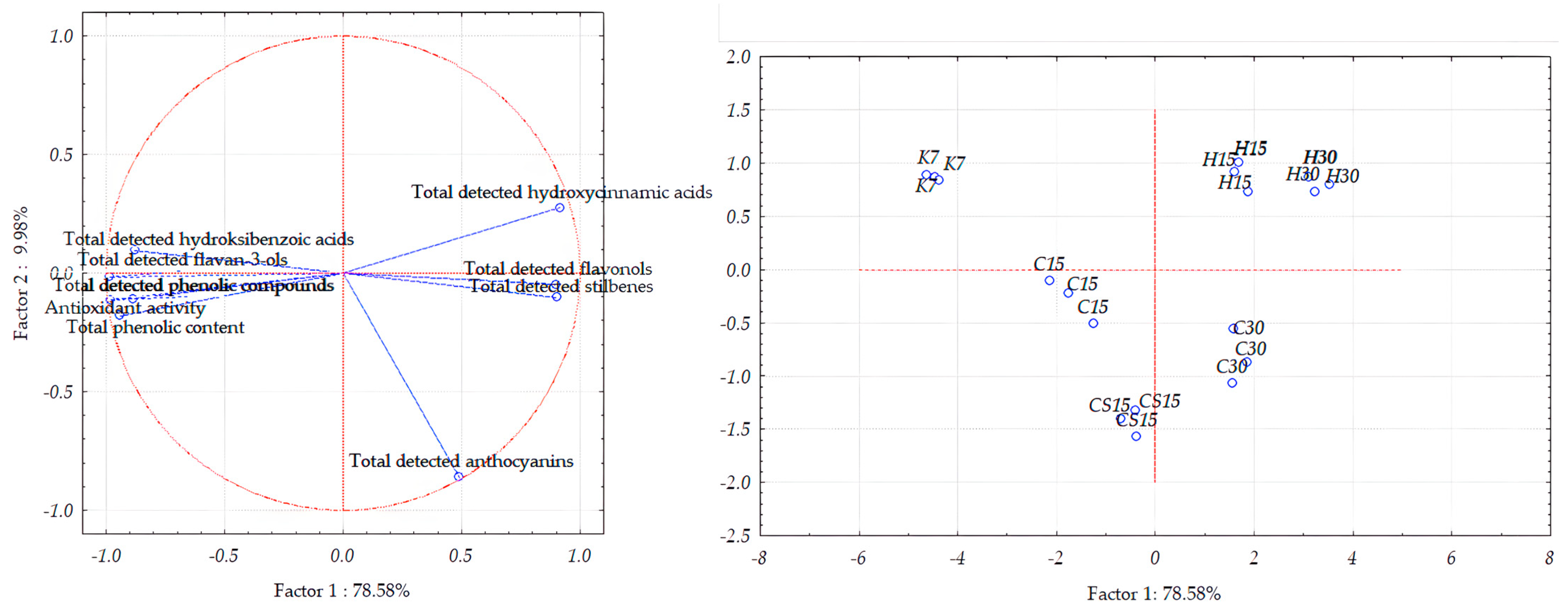
3.5. Principal Component Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gómez-Brandón, M.; Lores, M.; Insam, H.; Domínguez, J. Strategies for Recycling and Valorization of Grape Marc. Crit. Rev. Biotechnol. 2019, 39, 437–450. [Google Scholar] [CrossRef]
- Constantin, O.E.; Stoica, F.; Rațu, R.N.; Stănciuc, N.; Bahrim, G.E.; Râpeanu, G. Bioactive Components, Applications, Extractions, and Health Benefits of Winery By-from a Circular Bioeconomy Perspective: A Review. Antioxidants 2024, 13, 100. [Google Scholar] [CrossRef]
- Gambacorta, G.; Trani, A.; Fasciano, C.; Paradiso, V.M.; Faccia, M. Effects of Prefermentative Cold Soak on Polyphenols and Volatiles of Aglianico, Primitivo and Nero Di Troia Red Wines. Food Sci. Nutr. 2019, 7, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Gallego, R.; Silva, P. The Wine Industry By-Products: Applications for Food Industry and Health Benefits. Antioxidants 2022, 11, 2025. [Google Scholar] [CrossRef]
- Devesa-Rey, R.; Vecino, X.; Varela-Alende, J.L.; Barral, M.T.; Cruz, J.M.; Moldes, A.B. Valorization of Winery Waste vs. the Costs of Not Recycling. Waste Manag. 2011, 31, 2327–2335. [Google Scholar] [CrossRef]
- Chakka, A.K.; Babu, A.S. Bioactive Compounds of Winery By-Products: Extraction Techniques and Their Potential Health Benefits. Appl. Food Res. 2022, 2, 100058. [Google Scholar] [CrossRef]
- Tapia-Quirós, P.; Montenegro-Landívar, M.F.; Reig, M.; Vecino, X.; Cortina, J.L.; Saurina, J.; Granados, M. Recovery of Polyphenols from Agri-Food By-Products: The Olive Oil and Winery Industries Cases. Foods 2022, 11, 362. [Google Scholar] [CrossRef]
- Chassagne, D.; Guilloux-Benatier, M.; Alexandre, H.; Voilley, A. Sorption of Wine Volatile Phenols by Yeast Lees. Food Chem. 2005, 91, 39–44. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards Integral Utilization of Grape Pomace from Winemaking Process: A Review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef]
- Pérez-Bibbins, B.; Torrado-Agrasar, A.; Salgado, J.M.; Oliveira, R.P.D.S.; Domínguez, J.M. Potential of Lees from Wine, Beer and Cider Manufacturing as a Source of Economic Nutrients: An Overview. Waste Manag. 2015, 40, 72–81. [Google Scholar] [CrossRef]
- Castilla, P.; Echarri, R.; Dávalos, A.; Cerrato, F.; Ortega, H.; Teruel, J.L.; Lucas, M.F.; Gómez-Coronado, D.; Ortuño, J.; Lasunción, M.A. Concentrated Red Grape Juice Exerts Antioxidant, Hypolipidemic, and Antiinflammatory Effects in Both Hemodialysis Patients and Healthy Subjects. Am. J. Clin. Nutr. 2006, 84, 252–262. [Google Scholar] [CrossRef]
- Ky, I.; Lorrain, B.; Kolbas, N.; Crozier, A.; Teissedre, P.-L. Wine By-Products: Phenolic Characterization and Antioxidant Activity Evaluation of Grapes and Grape Pomaces from Six Different French Grape Varieties. Molecules 2014, 19, 482–506. [Google Scholar] [CrossRef]
- Otero-Pareja, M.; Casas, L.; Fernández-Ponce, M.; Mantell, C.; Ossa, E. Green Extraction of Antioxidants from Different Varieties of Red Grape Pomace. Molecules 2015, 20, 9686–9702. [Google Scholar] [CrossRef]
- Rondeau, P.; Gambier, F.; Jolibert, F.; Brosse, N. Compositions and Chemical Variability of Grape Pomaces from French Vineyard. Ind. Crops Prod. 2013, 43, 251–254. [Google Scholar] [CrossRef]
- Venkata Mohan, S.; Nikhil, G.N.; Chiranjeevi, P.; Nagendranatha Reddy, C.; Rohit, M.V.; Kumar, A.N.; Sarkar, O. Waste Biorefinery Models towards Sustainable Circular Bioeconomy: Critical Review and Future Perspectives. Bioresour. Technol. 2016, 215, 2–12. [Google Scholar] [CrossRef]
- Petrović, A.; Lisov, N.; Čakar, U.; Marković, N.; Matijašević, S.; Cvejić, J.; Atanacković, M.; Gojković-Bukarica, L. The Effects of Prokupac Variety Clones and Vinification Method on the Quantity of Resveratrol in Wine. Food Feed Res. 2019, 46, 189–198. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Understanding Wine Chemistry, 1st ed.; Wiley: New York, NY, USA, 2016; ISBN 978-1-118-62780-8. [Google Scholar]
- Heredia, F.J.; Escudero-Gilete, M.L.; Hernanz, D.; Gordillo, B.; Meléndez-Martínez, A.J.; Vicario, I.M.; González-Miret, M.L. Influence of the Refrigeration Technique on the Colour and Phenolic Composition of Syrah Red Wines Obtained by Pre-Fermentative Cold Maceration. Food Chem. 2010, 118, 377–383. [Google Scholar] [CrossRef]
- Sacchi, K.L.; Bisson, L.F.; Adams, D.O. A Review of the Effect of Winemaking Techniques on Phenolic Extraction in Red Wines. Am. J. Enol. Vitic. 2005, 56, 197–206. [Google Scholar] [CrossRef]
- Radeka, S.; Herjavec, S.; Peršurić, Đ.; Lukić, I.; Sladonja, B. Effect of Different Maceration Treatments on Free and Bound Varietal Aroma Compounds in Wine of Vitis vinifera L. Cv. Malvazija Istarska Bijela. Food Technol. Biotechnol. 2008, 46, 86–92. [Google Scholar]
- Radeka, S.; Lukić, I.; Peršurić, Đ. Influence of Different Maceration Treatments on the Aroma Profile of Rosé and Red Wines from Croatian Aromatic Cv. Muškat Ruža Porečki (Vitis vinifera L.). Food Technol. Biotechnol. 2012, 50, 442–453. [Google Scholar]
- Piccardo, D.; González-Neves, G. Extracción de polifenoles y composición de vinos tintos Tannat elaborados por técnicas de maceración prefermentativa. Agrociencia 2013, 17, 36–44. [Google Scholar] [CrossRef]
- de Beer, D.; Joubert, E.; Marais, J.; Manley, M. Maceration Before and During Fermentation: Effect on Pinotage Wine Phenolic Composition, Total Antioxidant Capacity and Objective Colour Parameters. S. Afr. J. Enol. Vitic. 2017, 27, 137–150. [Google Scholar] [CrossRef][Green Version]
- Maza, M.; Álvarez, I.; Raso, J. Thermal and Non-Thermal Physical Methods for Improving Polyphenol Extraction in Red Winemaking. Beverages 2019, 5, 47. [Google Scholar] [CrossRef]
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine Polyphenol Content and Its Influence on Wine Quality and Properties: A Review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef]
- Ivanova, V.; Vojnoski, B.; Stefova, M. Effect of Winemaking Treatment and Wine Aging on Phenolic Content in Vranec Wines. J. Food Sci. Technol. 2012, 49, 161–172. [Google Scholar] [CrossRef]
- Gómez-Plaza, E.; Gil-Muñoz, R.; López-Roca, J.M.; Martínez-Cutillas, A.; Fernández-Fernández, J.I. Maintenance of Colour Composition of a Red Wine During Storage. Influence of Prefermentative Practices, Maceration Time and Storage. LWT Food Sci. Technol. 2002, 35, 46–53. [Google Scholar] [CrossRef]
- Rossi, S.; Bestulić, E.; Horvat, I.; Plavša, T.; Lukić, I.; Bubola, M.; Ganić, K.K.; Ćurko, N.; Jagatić Korenika, A.-M.; Radeka, S. Comparison of Different Winemaking Processes for Improvement of Phenolic Composition, Macro- and Microelemental Content, and Taste Sensory Attributes of Teran (Vitis vinifera L.) Red Wines. LWT 2022, 154, 112619. [Google Scholar] [CrossRef]
- Orbanić, F.; Rossi, S.; Bestulić, E.; Budić-Leto, I.; Kovačević Ganić, K.; Horvat, I.; Plavša, T.; Bubola, M.; Lukić, I.; Jeromel, A.; et al. Applying Different Vinification Techniques in Teran Red Wine Production: Impact on Bioactive Compounds and Sensory Attributes. Foods 2023, 12, 3838. [Google Scholar] [CrossRef]
- Rossi, S.; Bestulić, E.; Orbanić, F.; Horvat, I.; Lukić, I.; Ilak Peršurić, A.S.; Bubola, M.; Plavša, T.; Radeka, S. Comprehensive Analysis of Teran Red Wine Aroma and Sensory Profiles: Impacts of Maceration Duration, Pre-Fermentation Heating Treatment, and Barrel Aging. Appl. Sci. 2024, 14, 8729. [Google Scholar] [CrossRef]
- Rusjan, D.; Bubola, M.; Janjanin, D.; Užila, Z.; Radeka, S.; Poljuha, D.; Pelengić, R.; Javornik, D.; Štajner, N. Ampelographic Characterisation of Grapevine Accessions Denominated ‘Refošk’, ‘Refosco’, ‘Teran’ and ‘Terrano’ (Vitis vinifera L.) from Slovenia, Croatia and Italy. VITIS J. Grapevine Res. 2015, 54, 77–80. [Google Scholar] [CrossRef]
- Bubola, M.; Sivilotti, P.; Janjanin, D.; Poni, S. Early Leaf Removal Has a Larger Effect than Cluster Thinning on Grape Phenolic Composition in Cv. Teran. Am. J. Enol. Vitic. 2017, 68, 234–242. [Google Scholar] [CrossRef]
- Orbanić, F.; Rossi, S.; Bestulić, E.; Kovačević Ganić, K.; Ćurko, N.; Tomašević, M.; Plavša, T.; Jeromel, A.; Radeka, S. Total Phenolic Content and Antioxidant Capacity of Teran Red Wine: Influence of Pre-Fermentative Mash Procedures. In Proceedings of the 58th Croatian & 18th International Symposium on Agriculture, Dubrovnik, Croatia, 11–17 February 2023; pp. 195–201. [Google Scholar]
- Šikuten, I.; Štambuk, P.; Tomaz, I.; Marchal, C.; Kontic, J.K.; Lacombe, T.; Maletic, E.; Preiner, D. Discrimination of Genetic and Geographical Groups of Grape Varieties (Vitis vinifera L.) Based on Their Polyphenolic Profiles. J. Food Compos. Anal. 2021, 102, 104062. [Google Scholar] [CrossRef]
- Tomaz, I.; Maslov, L.; Domagoj, D.; Preiner, D.; Ašperger, D.; Karoglan Kontić, J. Solid-Liquid Extraction of Phenolics from Red Grape Skins. Acta Chim. Slov. 2016, 63, 287–297. [Google Scholar] [CrossRef]
- Tomaz, I.; Maslov, L. Simultaneous Determination of Phenolic Compounds in Different Matrices Using Phenyl-Hexyl Stationary Phase. Food Anal. Methods 2016, 9, 401–410. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Casassa, L.F.; Larsen, R.C.; Harbertson, J.F. Effects of Vineyard and Winemaking Practices Impacting Berry Size on Evolution of Phenolics during Winemaking. Am. J. Enol. Vitic. 2016, 67, 257–268. [Google Scholar] [CrossRef]
- Aleixandre-Tudo, J.L.; du Toit, W. Cold Maceration Application in Red Wine Production and Its Effects on Phenolic Compounds: A Review. LWT 2018, 95, 200–208. [Google Scholar] [CrossRef]
- Katalinić, V.; Možina, S.S.; Skroza, D.; Generalić, I.; Abramovič, H.; Miloš, M.; Ljubenkov, I.; Piskernik, S.; Pezo, I.; Terpinc, P.; et al. Polyphenolic Profile, Antioxidant Properties and Antimicrobial Activity of Grape Skin Extracts of 14 Vitis vinifera Varieties Grown in Dalmatia (Croatia). Food Chem. 2010, 119, 715–723. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Gonzaga, L.V.; Rizelio, V.M.; Gonçalves, A.E.D.S.S.; Genovese, M.I.; Fett, R. Phenolic Compounds and Antioxidant Activity of Seed and Skin Extracts of Red Grape (Vitis vinifera and Vitis labrusca) Pomace from Brazilian Winemaking. Food Res. Int. 2011, 44, 897–901. [Google Scholar] [CrossRef]
- Casassa, L.F.; Harbertson, J.F. Extraction, Evolution, and Sensory Impact of Phenolic Compounds During Red Wine Maceration. Annu. Rev. Food Sci. Technol. 2014, 5, 83–109. [Google Scholar] [CrossRef]
- Bai, B.; He, F.; Yang, L.; Chen, F.; Reeves, M.J.; Li, J. Comparative Study of Phenolic Compounds in Cabernet Sauvignon Wines Made in Traditional and Ganimede Fermenters. Food Chem. 2013, 141, 3984–3992. [Google Scholar] [CrossRef]
- Poklar Ulrih, N.; Opara, R.; Skrt, M.; Košmerl, T.; Wondra, M.; Abram, V. Part I. Polyphenols Composition and Antioxidant Potential during ‘Blaufränkisch’ Grape Maceration and Red Wine Maturation, and the Effects of Trans-Resveratrol Addition. Food Chem. Toxicol. 2020, 137, 111122. [Google Scholar] [CrossRef]
- Rockenbach, I.; Rodrigues, E.; Gonzaga, L.; Caliari, V.; Genovese, M.; Gonçalves, A.E.; Fett, R. Phenolic Compounds Content and Antioxidant Activity in Pomace from Selected Red Grapes (Vitis vinifera L. and Vitis labrusca L.) Widely Produced in Brazil. Food Chem. 2011, 127, 174–179. [Google Scholar] [CrossRef]
- Milinčić, D.D.; Stanisavljević, N.S.; Kostić, A.Ž.; Soković Bajić, S.; Kojić, M.O.; Gašić, U.M.; Barać, M.B.; Stanojević, S.P.; Lj Tešić, Ž.; Pešić, M.B. Phenolic Compounds and Biopotential of Grape Pomace Extracts from Prokupac Red Grape Variety. LWT 2021, 138, 110739. [Google Scholar] [CrossRef]
- Rodríguez Montealegre, R.; Romero Peces, R.; Chacón Vozmediano, J.L.; Martínez Gascueña, J.; García Romero, E. Phenolic Compounds in Skins and Seeds of Ten Grape Vitis vinifera Varieties Grown in a Warm Climate. J. Food Compos. Anal. 2006, 19, 687–693. [Google Scholar] [CrossRef]
- Casassa, L.; Beaver, C.W.; Mireles, M.S.; Harbertson, J.F. Effect of Extended Maceration and Ethanol Concentration on the Extraction and Evolution of Phenolics, Colour Components and Sensory Attributes of Merlot Wines. Aust. J. Grape Wine Res. 2013, 19, 25–39. [Google Scholar] [CrossRef]
- Krasteva, D.; Ivanov, Y.; Chengolova, Z.; Godjevargova, T. Antimicrobial Potential, Antioxidant Activity, and Phenolic Content of Grape Seed Extracts from Four Grape Varieties. Microorganisms 2023, 11, 395. [Google Scholar] [CrossRef]
- Piccardo, D.; González-Neves, G.; Favre, G.; Pascual, O.; Canals, J.M.; Zamora, F. Impact of Must Replacement and Hot Pre-Fermentative Maceration on the Color of Uruguayan Tannat Red Wines. Fermentation 2019, 5, 80. [Google Scholar] [CrossRef]
- Fia, G. Wine Lees: Traditional and Potential Innovative Techniques for Their Exploitation in Winemaking. In Grape and Wine Biotechnology; IntechOpen: London, UK, 2016; ISBN 978-953-51-2693-5. [Google Scholar]
- De Iseppi, A.; Lomolino, G.; Marangon, M.; Curioni, A. Current and Future Strategies for Wine Yeast Lees Valorization. Food Res. Int. 2020, 137, 109352. [Google Scholar] [CrossRef]
- Bustamante, M.A.; Moral, R.; Paredes, C.; Pérez-Espinosa, A.; Moreno-Caselles, J.; Pérez-Murcia, M.D. Agrochemical Characterisation of the Solid By-Products and Residues from the Winery and Distillery Industry. Waste Manag. 2008, 28, 372–380. [Google Scholar] [CrossRef]
- Romero-Díez, R.; Rodríguez-Rojo, S.; Cocero, M.J.; Duarte, C.M.M.; Matias, A.A.; Bronze, M.R. Phenolic Characterization of Aging Wine Lees: Correlation with Antioxidant Activities. Food Chem. 2018, 259, 188–195. [Google Scholar] [CrossRef]
- Kalli, E.; Lappa, I.; Bouchagier, P.; Tarantilis, P.A.; Skotti, E. Novel Application and Industrial Exploitation of Winery By-Products. Bioresour. Bioprocess. 2018, 5, 46. [Google Scholar] [CrossRef]
- Landeka, I.; Jurčević; Dora, M.; Guberović, I.; Petras, M.; Rimac, S.; Brnčić; Đikić, D. Polyphenols from Wine Lees as a Novel Functional Bioactive Compound in the Protection Against Oxidative Stress and Hyperlipidaemia. Food Technol. Biotechnol. 2017, 55, 109–116. [Google Scholar] [CrossRef]
- Morata, A.; Gómez-Cordovés, M.C.; Suberviola, J.; Bartolomé, B.; Colomo, B.; Suárez, J.A. Adsorption of Anthocyanins by Yeast Cell Walls during the Fermentation of Red Wines. J. Agric. Food Chem. 2003, 51, 4084–4088. [Google Scholar] [CrossRef]
- Arcena, M.R.; Leong, S.Y.; Hochberg, M.; Sack, M.; Mueller, G.; Sigler, J.; Silcock, P.; Kebede, B.; Oey, I. Evolution of Volatile and Phenolic Compounds during Bottle Storage of Merlot Wines Vinified Using Pulsed Electric Fields-Treated Grapes. Foods 2020, 9, 443. [Google Scholar] [CrossRef]
- Canals, R.; Llaudy, M.C.; Valls, J.; Canals, J.M.; Zamora, F. Influence of Ethanol Concentration on the Extraction of Color and Phenolic Compounds from the Skin and Seeds of Tempranillo Grapes at Different Stages of Ripening. J. Agric. Food Chem. 2005, 53, 4019–4025. [Google Scholar] [CrossRef]
- Koyama, K.; Goto-Yamamoto, N.; Hashizume, K. Influence of Maceration Temperature in Red Wine Vinification on Extraction of Phenolics from Berry Skins and Seeds of Grape (Vitis vinifera). Biosci. Biotechnol. Biochem. 2007, 71, 958–965. [Google Scholar] [CrossRef]
- Razungles, A. Extraction Technologies and Wine Quality. In Managing Wine Quality: Oenology and Wine Quality; Woodhead Publishing: Sawston, UK, 2010; pp. 589–630. ISBN 978-1-84569-798-3. [Google Scholar]
- Setford, P.C.; Jeffery, D.W.; Grbin, P.R.; Muhlack, R.A. Factors Affecting Extraction and Evolution of Phenolic Compounds during Red Wine Maceration and the Role of Process Modelling. Trends Food Sci. Technol. 2017, 69, 106–117. [Google Scholar] [CrossRef]
- Cheng, V.J.; Bekhit, A.E.-D.A.; McConnell, M.; Mros, S.; Zhao, J. Effect of Extraction Solvent, Waste Fraction and Grape Variety on the Antimicrobial and Antioxidant Activities of Extracts from Wine Residue from Cool Climate. Food Chem. 2012, 134, 474–482. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant Activity of Dietary Polyphenols as Determined by a Modified Ferric Reducing/Antioxidant Power Assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [PubMed]


| Treatment | Pre-Fermentative Maceration Procedure | Fermentation and Maceration | Pre-Fermentative Maceration Procedure + Maceration Duration | |||
|---|---|---|---|---|---|---|
| Vinification Technology—Maceration | Fermentation/Maceration Temperature | Maceration Duration | ||||
| K7 | / | Standard maceration | 24 °C | 7 days | / | |
| CS15 | Cooling at 8 °C, 48 h (cryomaceration) | Saignée | Fermentation/maceration + prolonged post-fermentative maceration | 13 days | 15 days | |
| C15 | 13 days | 15 days | ||||
| C30 | 28 days | 30 days | ||||
| H15 | Heating at 50 °C, 48 h (hot pre-fermentative maceration) | 13 days | 15 days | |||
| H30 | 28 days | 30 days | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radeka, S.; Orbanić, F.; Rossi, S.; Bestulić, E.; Horvat, I.; Ilak Peršurić, A.S.; Lukić, I.; Plavša, T.; Bubola, M.; Jeromel, A. Evaluating the Impact of Pre-Fermentative and Post-Fermentative Vinification Technologies on Bioactive Compounds and Antioxidant Activity of Teran Red Wine By-Products. Foods 2024, 13, 3493. https://doi.org/10.3390/foods13213493
Radeka S, Orbanić F, Rossi S, Bestulić E, Horvat I, Ilak Peršurić AS, Lukić I, Plavša T, Bubola M, Jeromel A. Evaluating the Impact of Pre-Fermentative and Post-Fermentative Vinification Technologies on Bioactive Compounds and Antioxidant Activity of Teran Red Wine By-Products. Foods. 2024; 13(21):3493. https://doi.org/10.3390/foods13213493
Chicago/Turabian StyleRadeka, Sanja, Fumica Orbanić, Sara Rossi, Ena Bestulić, Ivana Horvat, Anita Silvana Ilak Peršurić, Igor Lukić, Tomislav Plavša, Marijan Bubola, and Ana Jeromel. 2024. "Evaluating the Impact of Pre-Fermentative and Post-Fermentative Vinification Technologies on Bioactive Compounds and Antioxidant Activity of Teran Red Wine By-Products" Foods 13, no. 21: 3493. https://doi.org/10.3390/foods13213493
APA StyleRadeka, S., Orbanić, F., Rossi, S., Bestulić, E., Horvat, I., Ilak Peršurić, A. S., Lukić, I., Plavša, T., Bubola, M., & Jeromel, A. (2024). Evaluating the Impact of Pre-Fermentative and Post-Fermentative Vinification Technologies on Bioactive Compounds and Antioxidant Activity of Teran Red Wine By-Products. Foods, 13(21), 3493. https://doi.org/10.3390/foods13213493











