Small-Sized Tomato Pomace: Source of Bioactive Compounds and Ingredient for Sustainable Production of Functional Bread
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Tomato Pomace Flour (TPF)
2.1.1. TPF Characterization
2.1.2. TPF Fatty Acid Profiles
2.1.3. Extraction of TPF Polyphenols
2.1.4. Total Polyphenol Content and Antioxidant Activity of TPF Extracts
2.1.5. HPLC/DAD and HPLC/ESI/MS Analyses of TPF Extracts
2.2. Preparation of Functional Bread Samples Using TPF as Partial Wheat Flour Substitute
2.2.1. Physico-Chemical Analyses of Functional Bread Samples
2.2.2. Sensory Evaluation of Functional Bread Samples
2.3. Statistical Analysis
3. Results
3.1. Physico-Chemical, Compositional, and Functional Characterization of TPF and TPF Extracts
3.2. Characterization of Bread with TPF
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arena, E.; Brighina, S.; Mazzaglia, A.; Spina, A.; Muccilli, S.; Giannone, V.; Fallico, B. Use of a natural low Na salt in durum wheat bread. Ital. J. Food Sci. 2015, 77–81. [Google Scholar]
- Arena, E.; Muccilli, S.; Mazzaglia, A.; Giannone, V.; Brighina, S.; Rapisarda, P.; Fallico, B.; Allegra, M.; Spina, A. Development of Durum Wheat Breads Low in Sodium Using a Natural Low-Sodium Sea Salt. Foods 2020, 9, 752. [Google Scholar] [CrossRef] [PubMed]
- Spina, A.; Brighina, S.; Muccilli, S.; Mazzaglia, A.; Rapisarda, P.; Fallico, B.; Arena, E. Partial Replacement of NaCl in Bread from Durum Wheat (Triticum turgidum L. subsp. durum (Desf.)) with KCl and Yeast Extract: Evaluation of Quality Parameters During Long Storage. Food Bioprocess Technol. 2015, 8, 1089–1101. [Google Scholar] [CrossRef]
- Dahdah, P.; Cabizza, R.; Farbo, M.G.; Fadda, C.; Del Caro, A.; Montanari, L.; Hassoun, G.; Piga, A. Effect of partial substitution of wheat flour with freeze-dried olive pomace on the technological, nutritional, and sensory properties of bread. Front. Sustain. Food Syst. 2024, 8, 1400339. [Google Scholar] [CrossRef]
- Johnston, C.; Leong, S.Y.; Teape, C.; Liesaputra, V.; Oey, I. In vitro digestion properties and use of automatic image analysis to assess the quality of wheat bread enriched with whole faba bean (Vicia faba L.) flour and its protein-rich fraction. Food Res. Int. 2023, 174, 113630. [Google Scholar] [CrossRef]
- Nouska, C.; Irakli, M.; Palakas, P.; Lytou, A.E.; Bouloumpasi, E.; Biliaderis, C.G.; Lazaridou, A. Influence of sesame cake on physicochemical, antioxidant and sensorial characteristics of fortified wheat breads. Food Res. Int. 2024, 178, 113980. [Google Scholar] [CrossRef]
- Parafati, L.; Restuccia, C.; Palmeri, R.; Fallico, B.; Arena, E. Characterization of prickly pear peel flour as bioactive and functional ingredient in bread preparation. Foods 2020, 9, 1189. [Google Scholar] [CrossRef]
- Hasan, M.M.; Islam, M.R.; Haque, A.R.; Kabir, M.R.; Hasan, S.K. Fortification of bread with mango peel and pulp as a source of bioactive compounds: A comparison with plain bread. Food Chem. Adv. 2024, 5, 100783. [Google Scholar] [CrossRef]
- Spina, A.; Brighina, S.; Muccilli, S.; Mazzaglia, A.; Fabroni, S.; Fallico, B.; Rapisarda, P.; Arena, E. Wholegrain durum wheat bread fortified with citrus fibres: Evaluation of quality parameters during long storage. Front. Nutr. 2019, 6, 422996. [Google Scholar] [CrossRef]
- ISMEA, Report n.1/2022. Available online: https://www.ismeamercati.it/flex/cm/pages/ServeBLOB.php/L/IT/IDPagina/12391 (accessed on 11 December 2022).
- Azabou, S.; Louati, I.; Ben Taheur, F.; Nasri, M.; Mechichi, T. Towards sustainable management of tomato pomace through the recovery of valuable compounds and sequential production of low-cost biosorbent. Environ. Sci. Pollut. Res. 2020, 27, 39402–39412. [Google Scholar] [CrossRef]
- Farinon, B.; Felli, M.; Sulli, M.; Diretto, G.; Savatin, D.V.; Mazzucato, A.; Merendino, N.; Costantini, L. Tomato pomace food waste from different variants as a high antioxidant potential resource. Food Chem. 2024, 452, 139509. [Google Scholar] [CrossRef] [PubMed]
- Chabi, I.B.; Zannou, O.; Dedehou, E.S.; Ayegnon, B.P.; Odouaro, O.B.O.; Maqsood, S.; Galanakis, C.M.; Kayodé, A.P.P. Tomato pomace as a source of valuable functional ingredients for improving physicochemical and sensory properties and extending the shelf life of foods: A review. Heliyon 2024, 10, e25261. [Google Scholar] [CrossRef] [PubMed]
- Navarro-González, I.; García-Valverde, V.; García-Alonso, J.; Jesús Periago, M. Chemical profile, functional and antioxidant properties of tomato peel fiber. Food Res. Int. 2011, 44, 1528–1535. [Google Scholar] [CrossRef]
- Coelho, M.C.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M.E. Integral valorisation of tomato by-products towards bioactive compounds recovery: Human health benefits. Food Chem. 2023, 410, 135319. [Google Scholar] [CrossRef]
- Salem, B.R. Use of tomato pomace, mango seeds kernel and pomegranate peels powders for the production of functional biscuits. Zagazig J. Agric. Res. 2020, 47, 1011–1023. [Google Scholar] [CrossRef]
- Betrouche, A.; Estivi, L.; Colombo, D.; Pasini, G.; Benatallah, L.; Brandolini, A.; Hidalgo, A. Antioxidant Properties of Gluten-Free Pasta Enriched with Vegetable By-Products. Molecules 2022, 27, 8993. [Google Scholar] [CrossRef]
- Mehta, D.; Prasad, P.; Sangwan, R.S.; Yadav, S.K. Tomato processing byproduct valorization in bread and muffin: Improvement in physicochemical properties and shelf life stability. J. Food Sci. Technol. 2018, 55, 2560–2568. [Google Scholar] [CrossRef]
- Nakov, G.; Brandolini, A.; Estivi, L.; Bertuglia, K.; Ivanova, N.; Jukić, M.; Komlenić, D.K.; Lukinac, J.; Hidalgo, A. Effect of Tomato Pomace Addition on Chemical, Technological, Nutritional, and Sensorial Properties of Cream Crackers. Antioxidants 2022, 11, 2087. [Google Scholar] [CrossRef]
- Alazb, B.R.; El-Sahy, K.M.; Sulieman, A.E.R.M.; Youssif, M.R. Physicochemical and organoleptic characteristics of cakes supplemented with tomato pomace, mango seeds kernel and pomegranate peels powders. Plant Arch. 2021, 21, 432–439. [Google Scholar] [CrossRef]
- Mironeasa, S.; Codină, G.G.; Mironeasa, C. Effect of composite flour made from tomato seed and wheat of 650 type of a strong quality for bread making on bread quality and alveograph rheological properties. Int. J. Food Eng. 2018, 4, 22–26. [Google Scholar] [CrossRef]
- Bhat, M.A.; Hafiza Ahsan, H.A. Physico-chemical characteristics of cookies prepared with tomato pomace powder. J. Food Process. Technol. 2016, 7, 543. [Google Scholar] [CrossRef]
- Padalino, L.; Conte, A.; Lecce, L.; Likyova, D.; Sicari, V.; Pellicanò, T.M.; Poiana, M.; Del Nobile, M.A. Functional pasta with tomato by-product as a source of antioxidant compounds and dietary fibre. Czech J. Food Sci. 2017, 35, 48–56. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2007. [Google Scholar]
- Jaeger de Carvalho, L.M.; Barros Gomes, P.; Luiz de Oliveira Godoy, R.; Pacheco, S.; Fernandes do Monte, P.H.; Nutti Regini, M.; Ramalho Ramos, S.R. Total carotenoid content, α-carotene and β-carotene, of landrace pumpkins (Cucurbita moschata Duch): A preliminary study. Food Res. Int. 2012, 47, 337–340. [Google Scholar] [CrossRef]
- European Parliament & Council of the European Union. Regulation (EC) No. 2105/2022 of the European Parliament and of the Council of 4th november 2022 on rules on conformity checks of marketing standards for olive oil and methods of analysis of the characteristics of olive oil. Off. J. Eur. Union (OJEU) 2022, L284, 23–48. [Google Scholar]
- Singleton, V.L.; Rossi, J.A., Jr. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Siracusa, L.; Patané, C.; Rizzo, V.; Cosentino, S.L.; Ruberto, G. Targeted secondary metabolic and physico-chemical traits analysis to assess genetic variability within a germplasm collection of “long storage” tomatoes. Food Chem. 2018, 244, 275–283. [Google Scholar] [CrossRef]
- Lefebvre, D.; Gabriel, V.; Vayssier, Y.; Fontagné-Faucher, C. Simultaneous HPLC Determination of Sugars, Organic Acids and Ethanol in Sourdough Process. LWT Food Sci. Technol. 2002, 35, 407–414. [Google Scholar] [CrossRef]
- UNI EN ISO 8589; Analisi Sensoriale—Guida Generale per la Progettazione di Locali di Prova. Ente Italiano di Normazione. ISO: Geneva, Switzerland, 2014.
- UNI EN ISO 13299; Analisi Sensoriale—Metodologia—Guida Generale per la Definizione del Profilo Sensoriale. Ente Italiano di Normazione. ISO: Geneva, Switzerland, 2016.
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef]
- Wootton-Beard, P.C.; Moran, A.; Ryan, L. Stability of the total antioxidant capacity and total polyphenol content of 23 commercially available vegetable juices before and after in vitro digestion measured by FRAP, DPPH, ABTS and Folin–Ciocalteu methods. Food Res. Int. 2011, 44, 217–224. [Google Scholar] [CrossRef]
- Manzo, N.; Pizzolongo, F.; Meca, G.; Aiello, A.; Marchetti, N.; Romano, R. Comparative Chemical Compositions of Fresh and Stored Vesuvian PDO “Pomodorino Del Piennolo” Tomato and the Ciliegino Variety. Molecules 2018, 23, 2871. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, L.; Patanè, C.; Avola, G.; Ruberto, G. Polyphenols as chemotaxonomic markers in Italian “long storage” tomato genotypes. J. Agric. Food Chem. 2012, 60, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, J.I.; Sperlinga, E.; Siracusa, L.; Spagna, G.; Parafati, L.; Todaro, A.; Palmeri, R. Water as a Solvent of Election for Obtaining Oleuropein-Rich Extracts from Olive (Olea europaea) Leaves. Agronomy 2021, 11, 465. [Google Scholar] [CrossRef]
- Khedr, A.A.; Abdelgaleel, M.A.; Bessar, B.A.; Salama, A.A. Effect of using tomato peels as a fat replacer on the sensory, nutritional and physical properties of beef burger and sausages. J. Sustain. Agric. Sci. 2016, 42, 469–490. [Google Scholar] [CrossRef]
- Nour, V.; Ionica, M.E.; Trandafir, I. Bread enriched in lycopene and other bioactive compounds by addition of dry tomato waste. J. Food Sci. Technol. 2015, 52, 8260–8267. [Google Scholar] [CrossRef]
- European Parliament & Council of the European Union. Regulation (EC) No. 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutrition and health claims made on foods. Off. J. Eur. Union (OJEU) 2006, L404, 9–25. [Google Scholar]

| Sample Code | Durum Wheat Semolina (g) | TPF (g) | Water (g) | Compressed Yeast (g) | NaCl (g) | EVO (g) |
|---|---|---|---|---|---|---|
| Control | 500 | − | 350 | 7 | 12 | 10 |
| TPB 1 | 450 | 50 | 350 | 7 | 12 | 10 |
| TPB 2 | 400 | 100 | 350 | 7 | 12 | 10 |
| Attribute | Definition | Scale Anchors | |
|---|---|---|---|
| 1 | 9 | ||
| Visual appearance | |||
| Crumb colour | Strength of colour from light to dark | Light yellow | Brown |
| Alveolation uniformity (crumb) | Porosity and homogeneity of the size of the holes | Fine and very homogeneous | Heterogeneous |
| Odour/flavours | |||
| Bread | Intensity of the characteristic odour/flavour of freshly baked bread | Extremely weak | Extremely intense |
| Yeasty (crumb) | Intensity of the characteristic odour/flavour associated with yeast used as a leaving agent | Extremely weak | Extremely intense |
| Fresh tomato | Intensity of the characteristic odour/flavour associated with tomato | Extremely weak | Extremely intense |
| Dried tomato | Intensity of the characteristic odour/flavour of dried tomato | Extremely weak | Extremely intense |
| Off-odour/off-flavour | Odour/flavour unpleasant, not characteristic of bread | Extremely weak | Extremely intense |
| Taste | |||
| Sweet | Primary sensation produced by sugars | Extremely weak | Extremely intense |
| Salty | Primary sensation produced by sodium chloride | Extremely weak | Extremely intense |
| Sour | Primary sensation produced by citric acid | Extremely weak | Extremely intense |
| Bitter | Primary sensation produced by caffeine | Extremely weak | Extremely intense |
| Astringency | Sensations of shrinking, puckering, or roughing in the mouth | Extremely weak | Extremely intense |
| Texture | |||
| Surface moistness | Degree of moistness perceived on the surface of the product when in contact with the lips | Dry | Wet |
| Softness | Degree of softness in the mouth | Soft | Hard |
| Cohesiveness | Degree to which the chewed sample holds together | Extremely weak | Extremely strong (cohesive mass) |
| Dryness | Degree of drying effect, amount of saliva absorbed by the sample during chewing | Extremely weak | Extremely strong |
| Coarse/grittiness | Degree of the presence of small insoluble particles in the mouth after ingesting the sample | Extremely weak | Extremely strong |
| Chewiness | Number of chews required before swallowing | Few (N ≤ 3) | Several (N ≥ 7) |
| Overall acceptance | Degree of overall acceptance considering all attributes | Low | High |
| Parameters | |
|---|---|
| Moisture (%) | 3.53 ± 0.85 |
| Lipid (%) | 12.3 ± 0.66 |
| Total fibre (%) | 52.3 ± 0.65 |
| Soluble fibre (%) | 5.3 ± 0.5 |
| Insoluble fibre (%) | 47 ± 0.58 |
| Carotenoids (mg/kg) | 30.9 ± 4.82 |
| Fatty Acids (%) a | |
| Lauric acid (C12:0) | <0.1 |
| Myristic acid (C14:0) | 0.48 |
| Palmitic acid (C16:0) | 18.45 |
| Palmitoleic acid (C16:1 | 0.28 |
| Heptadecanoic acid(C17:0) | 0.18 |
| Stearic acid (C18:0) | 5.94 |
| Oleic acid (C18:1n-9) | 20.67 |
| Linoleic acid (C18:2n-2) | 49.90 |
| Arachidic acid (C20:0) | 0.52 |
| cis−11Eicosenoic acid (C20:1n-11) | 0.13 |
| Linolenic (C18:3n-3) | 2.11 |
| W | WE | WEF | ||
|---|---|---|---|---|
| Spectrophotometric | ||||
| Total Polyphenols (µg GAE/g) | 1488.4 ± 62.95c | 1599.4 ± 30.12b | 7913.7 ± 112.3a | |
| Antioxidant Activity (DPPH %) | 6.3 ± 0.22c | 69.4 ± 0.12a | 51.4 ± 0.08b | |
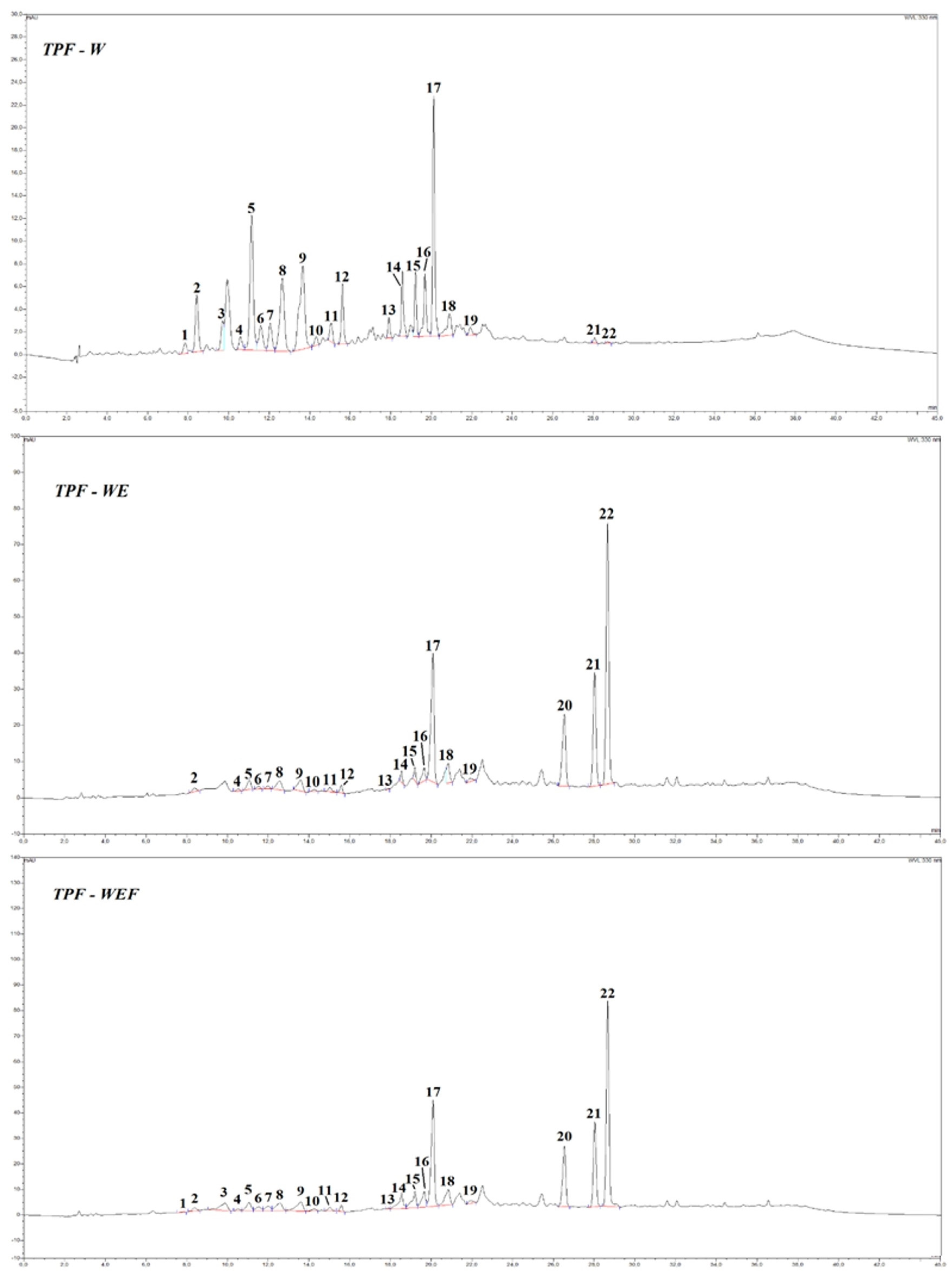
| Individual compounds 1–22, from HPLC-DAD/MS (µg/g) | ||||
| 1 | p-coumaroyl hexose isomer 1 | 0.77 ± 0.12a | n.d. | 0.53 ± 0.01ab |
| 2 | p-coumaroyl hexose isomer 2 | 4.39 ± 0.27a | 1.20 ± 0.19b | 0.79 ± 0.20bc |
| 3 | p-coumaroyl hexose isomer 3 | 2.18 ± 0.12a | n.d | 0.16 ± 0.01b |
| 4 | caffeoyl hexose isomer 1 | 1.07 ± 0.07b | 0.58 ± 0.02c | 1.56 ± 0.01a |
| 5 | p-coumaroyl hexose isomer 4 | 11.76 ± 0.64a | 2.94 ± 0.55b | 2.79 ± 0.11b |
| 6 | caffeoyl hexose isomer 2 | 2.35 ± 0.53a | 0.84 ± 0.06b | 0.76 ± 0.20b |
| 7 | caffeoyl hexose isomer 3 | 2.40 ± 0.23a | 0.87 ± 0.04b | 1.84 ± 0.10ab |
| 8 | caffeoyl hexose isomer 4 | 9.00 ± 0.41a | 3.87 ± 0.44c | 4.93 ± 0.16a |
| 9 | neo chlorogenic acid | 12.68 ± 0.55a | 4.56 ± 0.58bc | 6.15 ± 0.81b |
| 10 | caffeoyl quinic acid isomer 2 | 0.75 ± 0.04b | 0.61 ± 0.07b | 1.29 ± 0.18a |
| 11 | chlorogenic acid | 1.56 ± 0.08a | 1.58 ± 0.29a | 0.67 ± 0.37ab |
| 12 | quercetin di-hexoside derivative | 93.18 ± 6.03a | 41.54 ± 3.21b | 53.20 ± 7.52b |
| 13 | feruloyl quinic acid 1 | 1.31 ± 0.09c | 1.59 ± 0.01b | 3.35 ± 0.07a |
| 14 | quercetin derivative | 125.17 ± 5.59c | 130.47 ± 7.37b | 203.90 ± 20.24a |
| 15 | rutin-O-pentoside | 21.56 ± 0.60a | 10.49 ± 2.73c | 19.10 ± 1.26b |
| 16 | kaempferol di-O-hexoside | 99.72 ± 2.22b | 92.27 ± 16.33b | 141.96 ± 22.41a |
| 17 | rutin | 93.40 ± 4.64b | 151.72 ± 21.84a | 146.28 ± 27.52a |
| 18 | feruloyl quinic acid 2 | 3.01 ± 0.10b | 5.73 ± 0.34a | 5.88 ± 0.97a |
| 19 | feruloyl quinic acid 3 | 1.02 ± 0.04b | 1.19 ± 0.11b | 4.54 ± 0.04a |
| 20 | ferulic acid derivative | n.d | 12.06 ± 0.90a | 6.85 ± 2.12b |
| 21 | naringenin | 8.29 ± 1.86b | 410.78 ± 25.99a | 8.29 ± 1.86b |
| 22 | quercetin | 1.29 ± 0.39c | 1510.48 ± 83.80a | 1478.98 ± 195.64a |
| Sample | Weight (g) | Height of Bread (cm) | Colour Parameters | |||||
|---|---|---|---|---|---|---|---|---|
| Crumb | Crust | |||||||
| L* | a* | b* | L* | a* | b* | |||
| Control | 341.7 ± 0.22b | 5.9 ± 0.14a | 73.1 ± 3.07a | −2.0 ± 1.04c | 22.4 ± 1.14c | 72.0 ± 2.1a | 5.1 ± 2.05c | 30.5 ± 1.60c |
| 10% TPF | 355.1 ± 4.12ab | 6.3 ± 0.57ab | 59.4 ± 1.81b | 6.7 ± 1.28b | 36.8 ± 1.02b | 63.9 ± 1.51b | 9.2 ± 1.50b | 37.7 ± 2.24b |
| 20% TPF | 358.9 ± 2.00a | 5.9 ± 0.14a | 53.0 ± 1.38c | 10.5 ± 0.95a | 39.4 ± 1.39a | 57.4 ± 3.34c | 12.3 ± 3.12a | 40.0 ± 2.63a |
| Sample | Moisture (%) | pH | Acidity a | Fibre (%) | Carotenoids (mg/kg) | Total Polyphenols (mg/kg) | DPPH (%) |
|---|---|---|---|---|---|---|---|
| Control | 31.5 ± 0.52b | 6.2 ± 0.04a | 3.5 ± 0.16c | 2.9 ± 0.12c | 0.6 ± 0.13c | 292.6 ± 45.67c | 6.8 ± 4.12c |
| 10% TPF | 33.7 ± 0.83a | 5.7 ± 0.02b | 5.2 ± 0.29b | 6.2 ± 0.24b | 2.4 ± 0.28b | 552.8 ± 92.48b | 12.4 ± 4.85b |
| 20% TPF | 33.9 ± 0.85a | 5.4 ± 0.01c | 6.9 ± 0.24a | 9.6 ± 0.28a | 5.0 ± 0.57a | 708.9 ± 106.69a | 16.9 ± 6.25a |
| Attribute | Bread Samples | ||
|---|---|---|---|
| Control | 10%TPF | 20%TPF | |
| Visual appearance | |||
| Crumb colour | 1.36 ± 0.50c | 5.21 ± 0.98b | 6.79 ± 0.70a |
| Alveolation uniformity | 4.07 ± 1.07ab | 4.93 ± 0.92a | 3.93 ± 0.92b |
| Odour | |||
| Bread | 5.64 ± 0.84a | 4.64 ± 0.75b | 3.86 ± 0.95c |
| Yeasty | 3.79 ± 0.70a | 3.50 ± 0.86a | 3.57 ± 0.85a |
| Fresh tomato | 1.07 ± 0.27b | 2.64 ± 0.93a | 2.71 ± 0.73a |
| Dried tomato | 1.00 ± 0.00c | 2.86 ± 0.77b | 4.21 ± 0.80a |
| Off-odour | 1.14 ± 0.36a | 1.29 ± 0.61a | 1.57 ± 0.85a |
| Flavour | |||
| Bread | 6.07 ± 0.99a | 4.64 ± 0.84b | 3.79 ± 0.89c |
| Yeasty | 3.79 ± 0.80a | 2.21 ± 0.70b | 2.14 ± 0.86b |
| Fresh tomato | 1.64 ± 1.33b | 2.36 ± 0.93ab | 2.79 ± 0.89a |
| Dried tomato | 1.14 ± 0.36c | 3.79 ± 0.80b | 5.14 ± 0.77a |
| Off-flavour | 1.21 ± 0.43a | 1.29 ± 0.61a | 1.57 ± 0.85a |
| Taste | |||
| Sweet | 3.00 ± 0.96a | 2.79 ± 0.98a | 2.71 ± 0.99a |
| Salty | 3.93 ± 0.83a | 3.64 ± 0.93a | 3.57 ± 0.76a |
| Sour | 1.64 ± 0.93b | 1.93 ± 0.82b | 3.43 ± 0.85a |
| Bitter | 1.21 ± 0.43a | 1.36 ± 0.63a | 1.86 ± 0.95a |
| Astringency | 1.43 ± 0.76a | 1.64 ± 0.93a | 2.21 ± 0.98a |
| Texture | |||
| Surface moistness | 4.43 ± 1.02b | 5.43 ± 0.94a | 5.36 ± 1.01a |
| Softness | 3.64 ± 1.08b | 4.57 ± 0.94a | 5.29 ± 0.99a |
| Cohesiveness | 5.14 ± 0.95a | 5.21 ± 1.12a | 5.14 ± 0.77a |
| Dryness | 3.29 ± 0.99a | 3.14 ± 0.95a | 3.14 ± 0.66a |
| Coarse/grittiness | 2.29 ± 0.61c | 3.43 ± 0.94b | 4.64 ± 0.93a |
| Chewiness | 5.07 ± 1.07a | 4.57 ± 1.09a | 4.86 ± 0.95a |
| Overall acceptance | 7.00 ± 1.04a | 6.57 ± 1.01a | 6.71 ± 1.38a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brighina, S.; Pulvirenti, L.; Siracusa, L.; Arena, E.; Faulisi, M.V.; Restuccia, C. Small-Sized Tomato Pomace: Source of Bioactive Compounds and Ingredient for Sustainable Production of Functional Bread. Foods 2024, 13, 3492. https://doi.org/10.3390/foods13213492
Brighina S, Pulvirenti L, Siracusa L, Arena E, Faulisi MV, Restuccia C. Small-Sized Tomato Pomace: Source of Bioactive Compounds and Ingredient for Sustainable Production of Functional Bread. Foods. 2024; 13(21):3492. https://doi.org/10.3390/foods13213492
Chicago/Turabian StyleBrighina, Selina, Luana Pulvirenti, Laura Siracusa, Elena Arena, Maria Veronica Faulisi, and Cristina Restuccia. 2024. "Small-Sized Tomato Pomace: Source of Bioactive Compounds and Ingredient for Sustainable Production of Functional Bread" Foods 13, no. 21: 3492. https://doi.org/10.3390/foods13213492
APA StyleBrighina, S., Pulvirenti, L., Siracusa, L., Arena, E., Faulisi, M. V., & Restuccia, C. (2024). Small-Sized Tomato Pomace: Source of Bioactive Compounds and Ingredient for Sustainable Production of Functional Bread. Foods, 13(21), 3492. https://doi.org/10.3390/foods13213492






