Antimicrobial Efficacy of GS-2 on Reusable Food Packaging Materials for Specialty Crops
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Microbial Inocula
2.2. Evaluation of Efficacy of GS-2 Against A. niger, E. coli O157:H7, L. monocytogenes, and S. enterica Agona on Plastic and Cardboard Coupons at Different Exposure Times
2.3. Evaluation of Efficacy of GS-2 Against MNV on Plastic and Cardboard Coupons at Different Exposure Times
2.4. Evaluation of Different GS-2 Concentrations Against A. niger, E. coli O157:H7, L. monocytogenes, and S. enterica Agona on Plastic and Cardboard Coupons
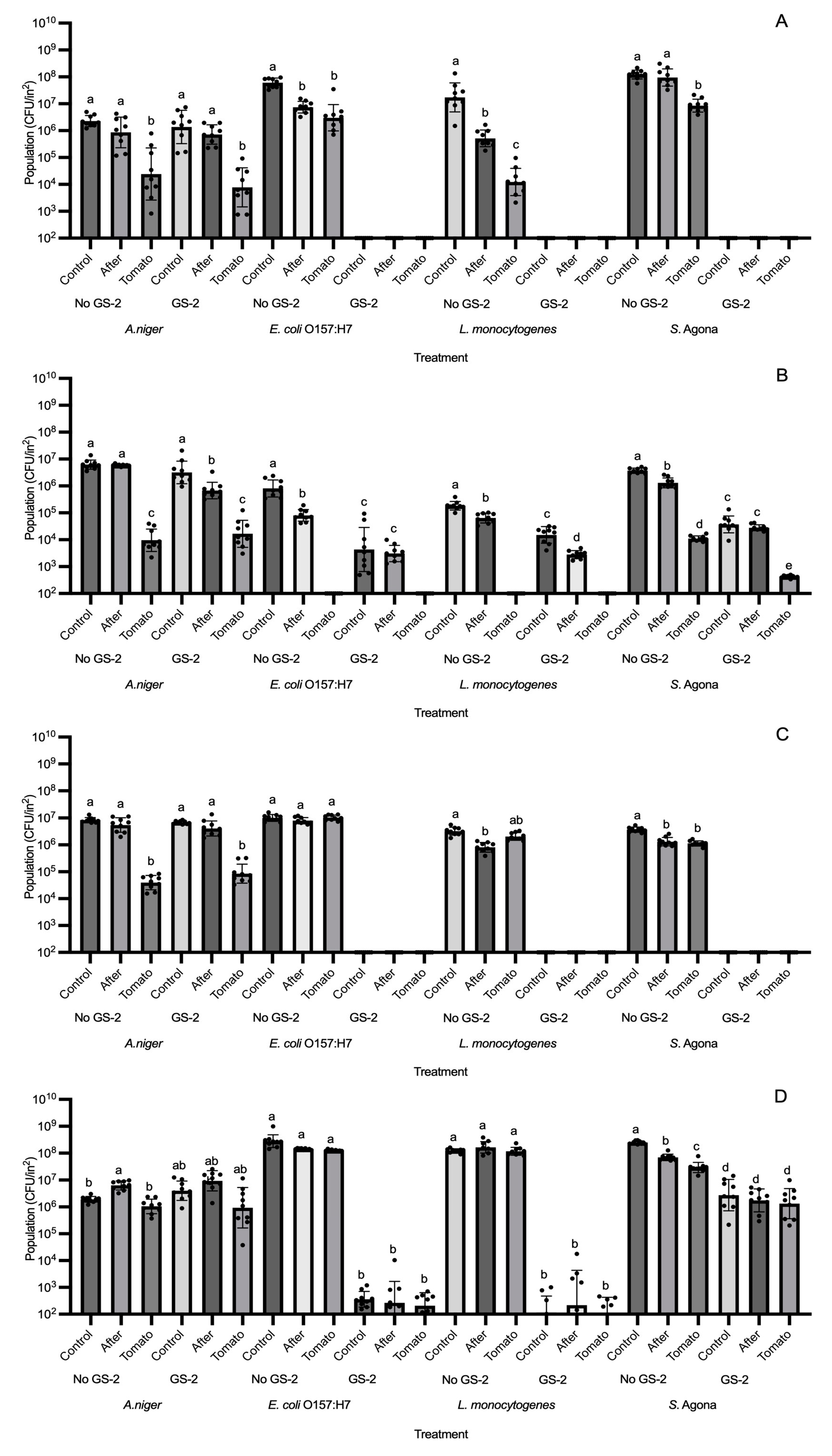
2.5. Evaluation of A. niger, E. coli O157:H7, L. monocytogenes, and S. enterica Agona Transfer from GS-2 Treated Plastic and Cardboard to Grape Tomatoes
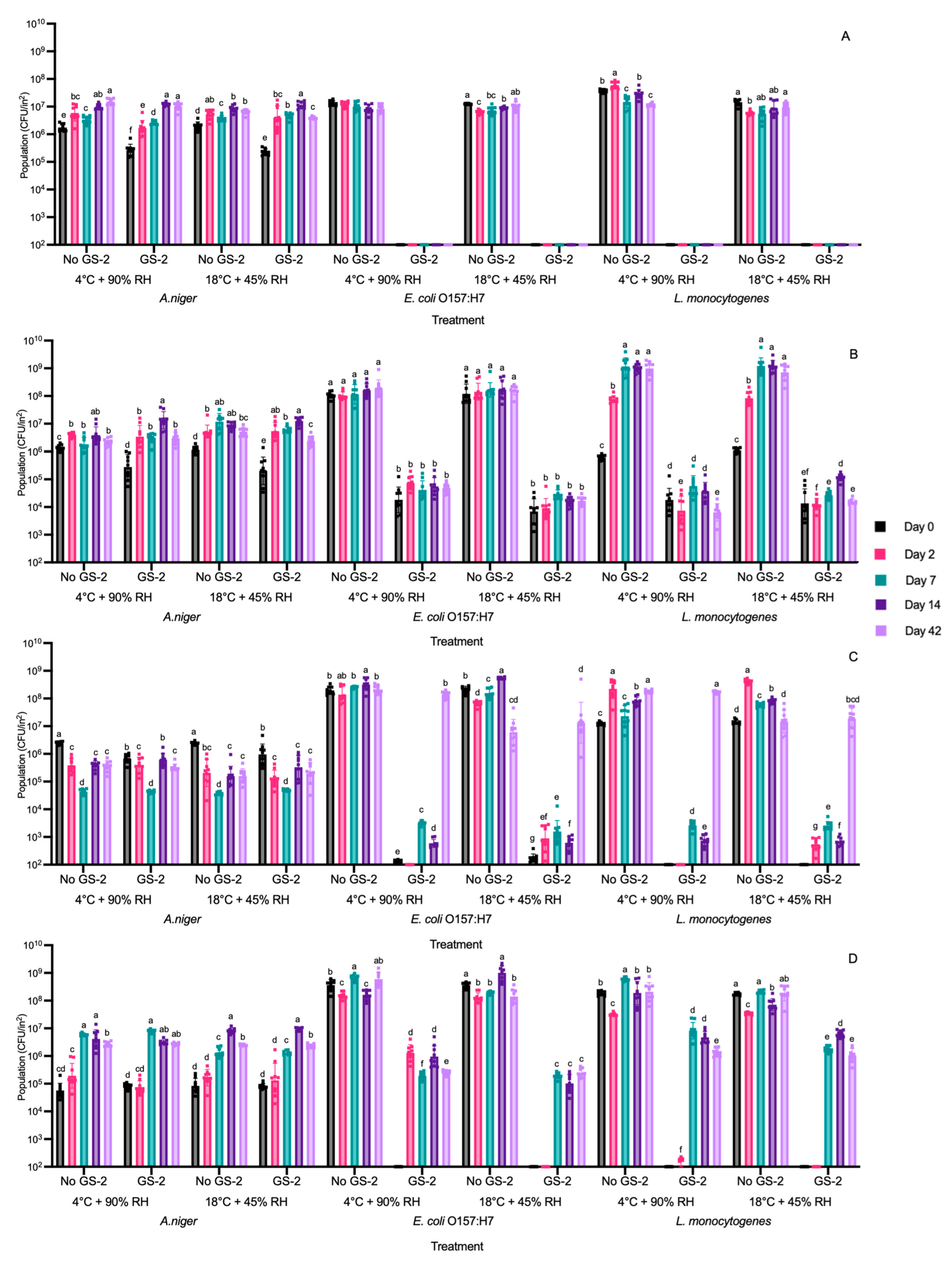
2.6. Efficacy of GS-2 Against A. niger, E. coli O157:H7, and L. monocytogenes on Plastic and Cardboard Coupons After Storage
2.7. Quantification of MNV Titre by Plaque-Forming Assay
2.8. Statistical Analysis
3. Results
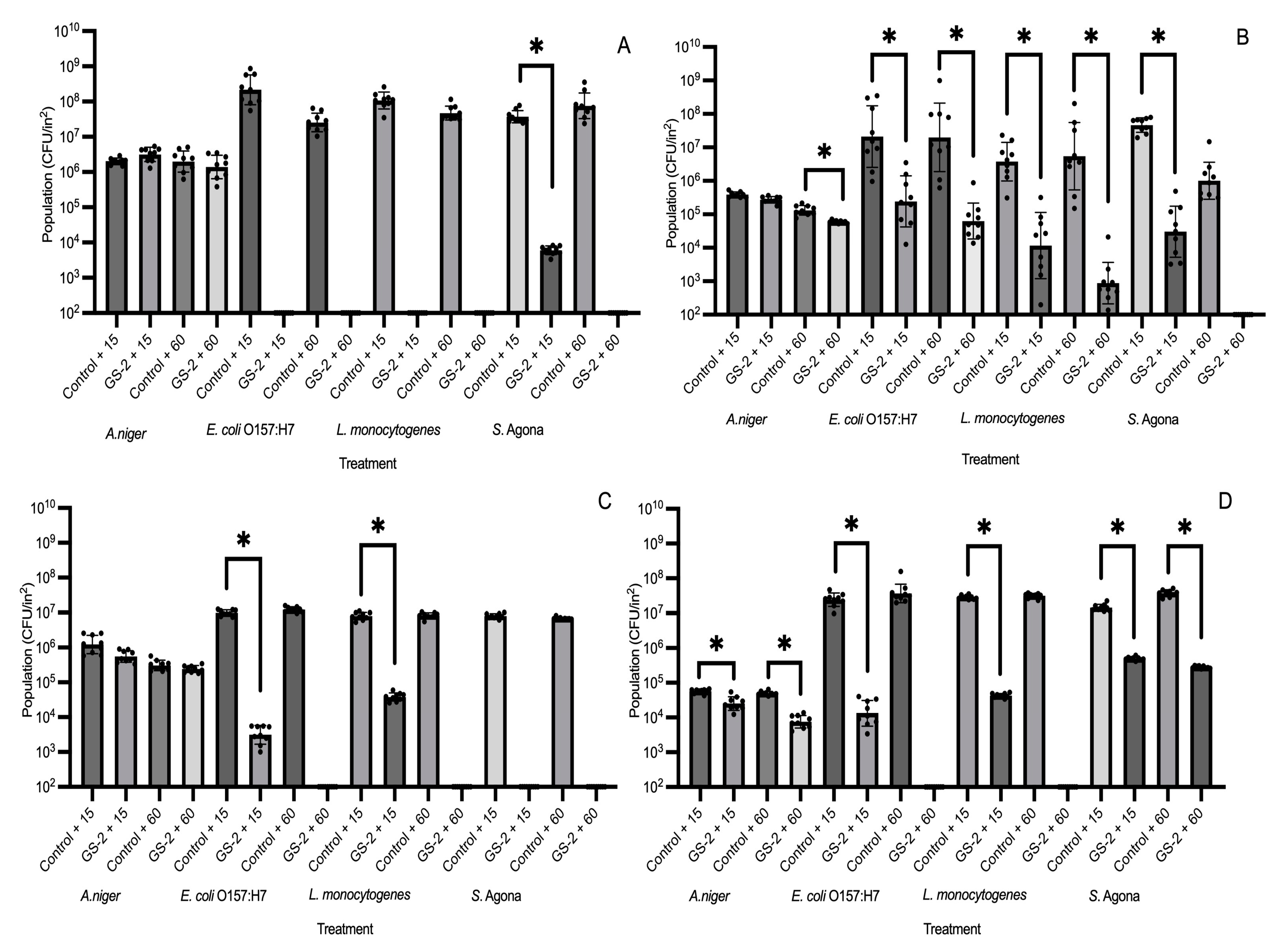
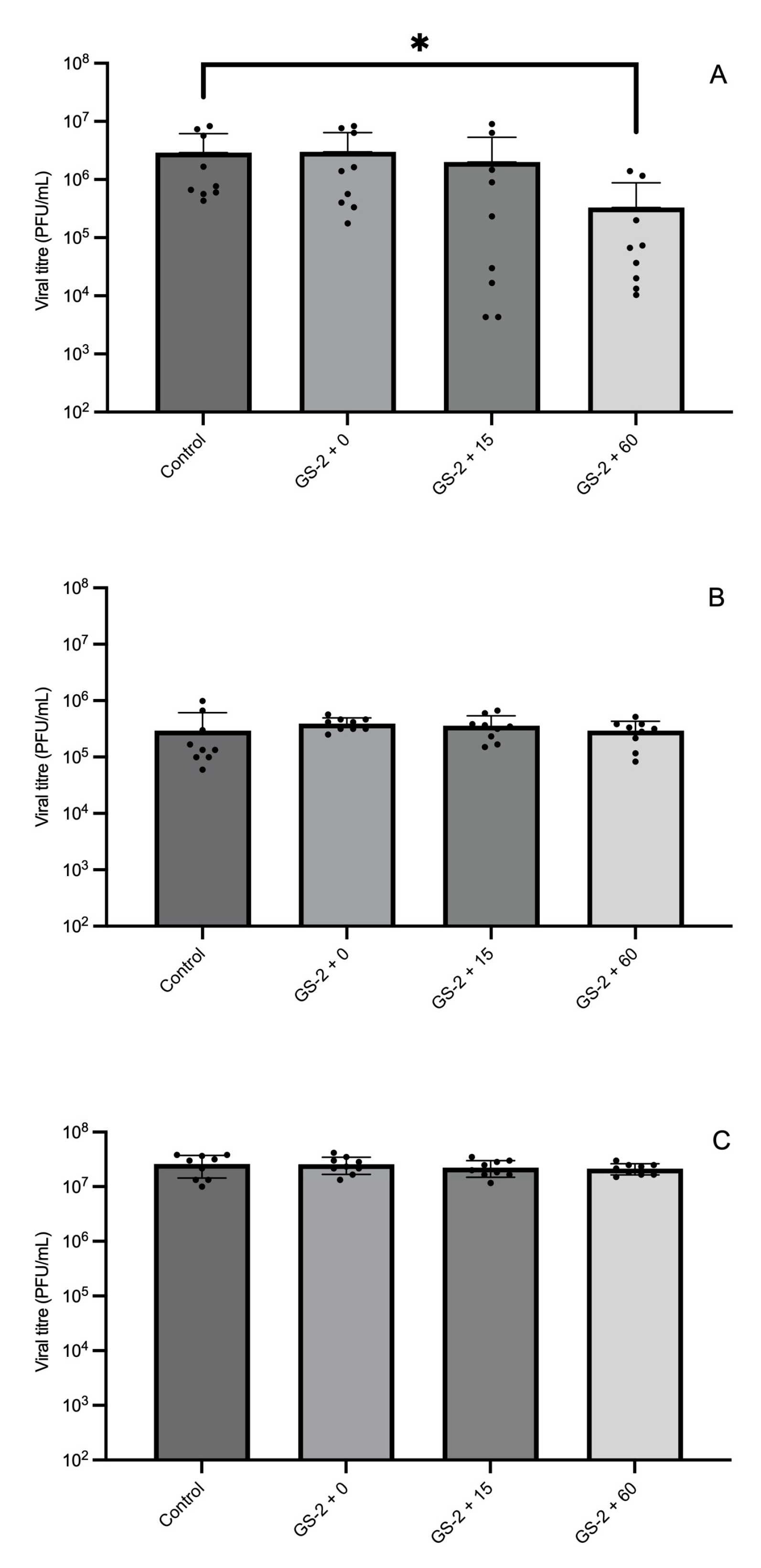
3.1. Efficacy of GS-2 Against Bacterial, Fungal, and Viral Populations After 15 and 60 min Exposure
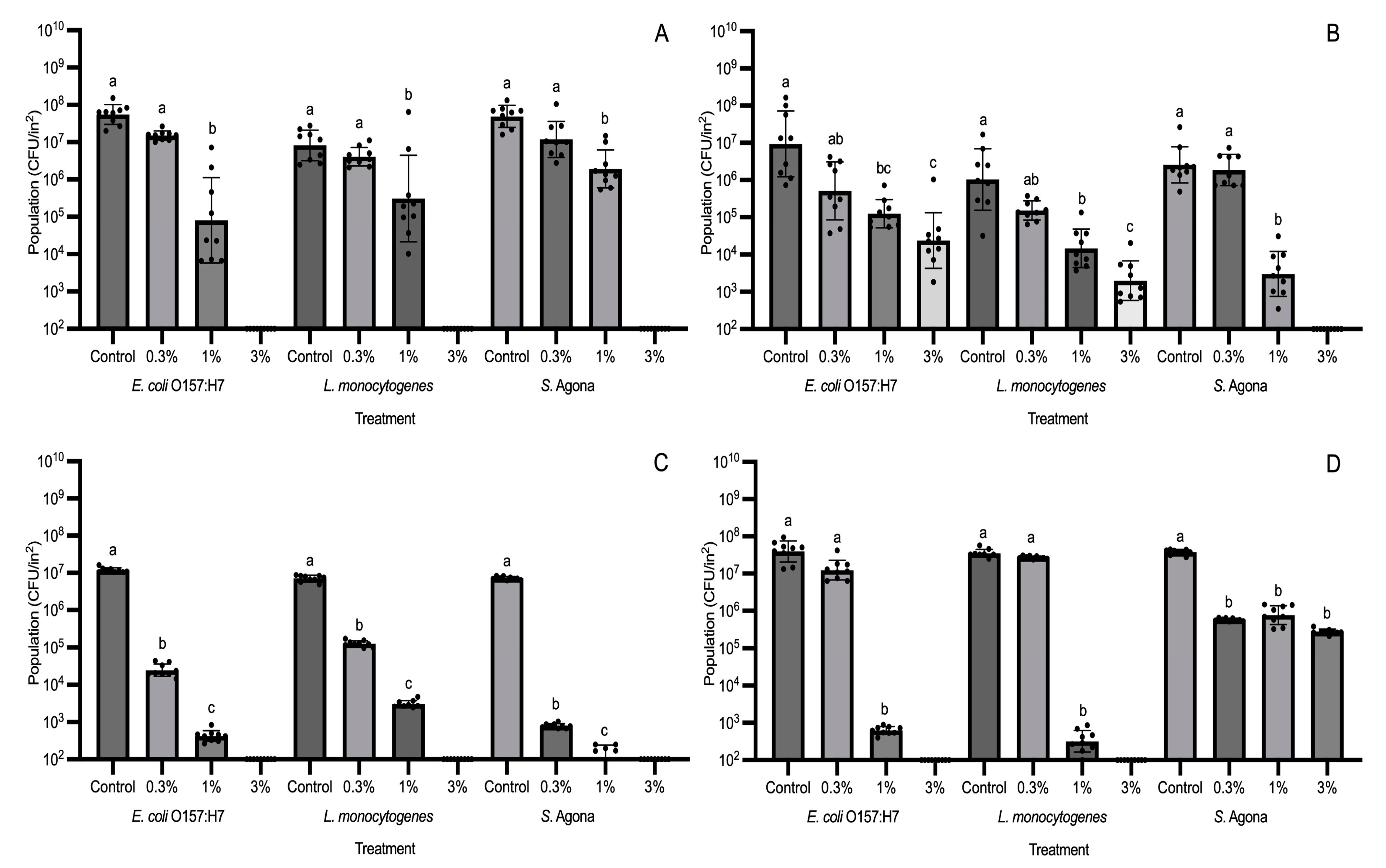
3.2. Evaluation of Different GS-2 Concentrations for Efficacy in Reducing Bacterial Populations
Evaluation of Effective GS-2 Concentrations for Reducing Fungal Populations
3.3. Efficacy of GS-2 Against Bacterial and Fungal Population Transfers to Grape Tomatoes
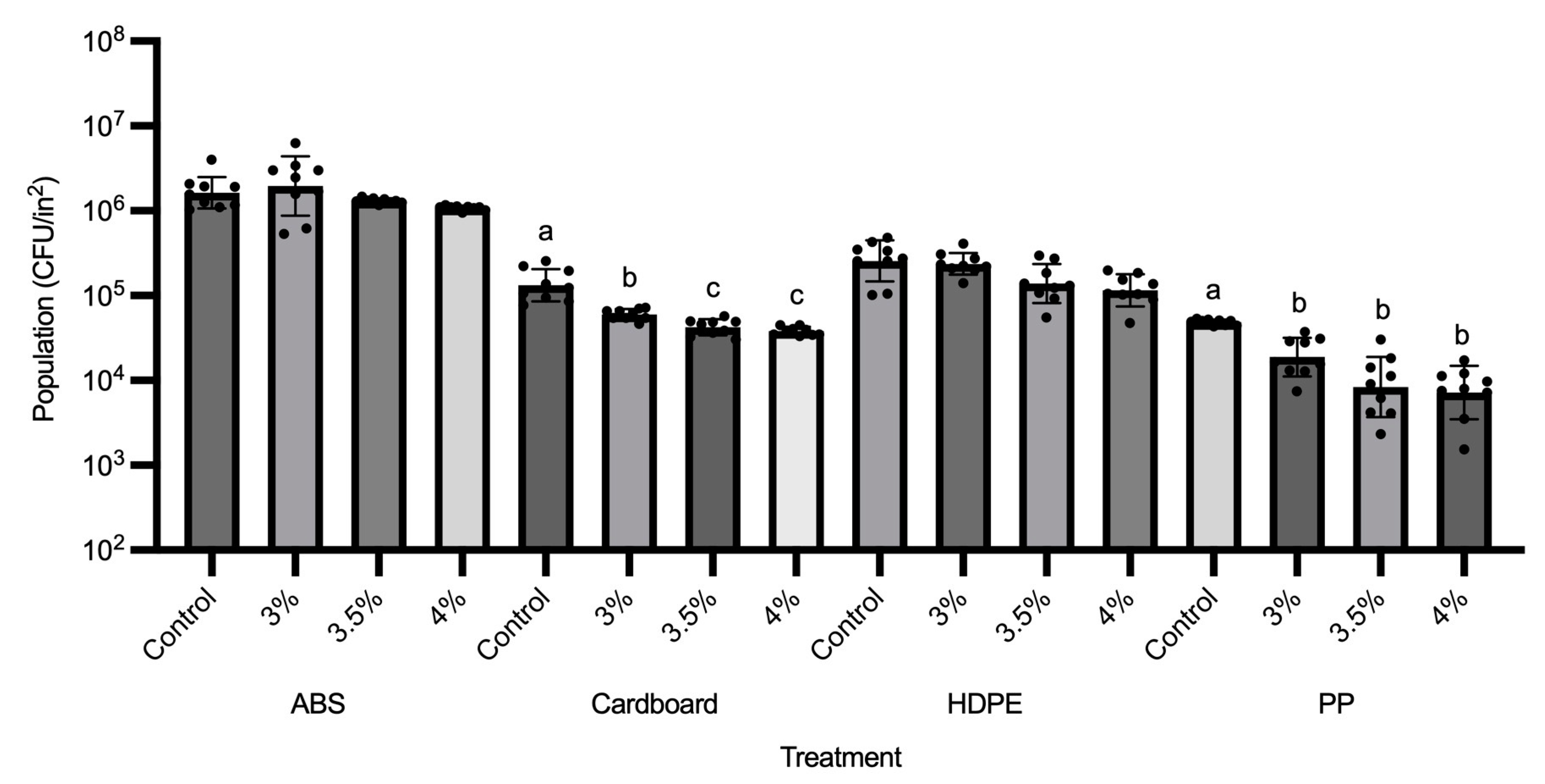
3.4. Efficacy of GS-2 During 42 Days of Storage at 4 °C and 90% RH and 18 °C and 45% RH Against Bacterial and Fungal Populations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- European Parliament. Directive 94/62/EC on Packaging and Packaging Waste; European Parliament: Strasbourg, France, 1994. [Google Scholar]
- European Commission. A European Strategy for Plastics in a Circular Economy; European Commission: Brussels, Belgium, 2018. [Google Scholar]
- Van Eygen, E.; Laner, D.; Fellner, J. Circular Economy of Plastic Packaging: Current Practice and Perspectives in Austria. Waste Manag. 2018, 72, 55–64. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture Global Agricultural Trade System (GATS) Data. Available online: https://apps.fas.usda.gov/gats/ExpressQuery1.aspx (accessed on 11 August 2024).
- U.S. Centers for Disease Control and Prevention Multistate Foodborne Outbreak Notices. Available online: https://www.cdc.gov/foodborne-outbreaks/active-investigations/all-foodborne-outbreak-notices.html (accessed on 27 August 2024).
- Economic Research Service U.S. Department of Agriculture Cost Estimates of Foodborne Illnesses. Available online: https://www.ers.usda.gov/data-products/cost-estimates-of-foodborne-illnesses.aspx (accessed on 28 August 2024).
- Ranjbar, A.; Ramezanian, A.; Shekarforoush, S.; Niakousari, M.; Eshghi, S. Antifungal Activity of Thymol against the Main Fungi Causing Pomegranate Fruit Rot by Suppressing the Activity of Cell Wall Degrading Enzymes. LWT 2022, 161, 113303. [Google Scholar] [CrossRef]
- Agrios, G.N. Plant Diseases Caused by Fungi. In Plant Pathology; Elsevier: Amsterdam, The Netherlands, 2005; pp. 385–614. [Google Scholar]
- Olaimat, A.N.; Taybeh, A.O.; Al-Nabulsi, A.; Al-Holy, M.; Hatmal, M.M.; Alzyoud, J.; Aolymat, I.; Abughoush, M.H.; Shahbaz, H.; Alzyoud, A.; et al. Common and Potential Emerging Foodborne Viruses: A Comprehensive Review. Life 2024, 14, 190. [Google Scholar] [CrossRef]
- O’Shea, H.; Blacklaws, B.A.; Collins, P.J.; McKillen, J.; Fitzgerald, R. Viruses Associated with Foodborne Infections. Ref. Modul. Life Sci. 2019. [Google Scholar] [CrossRef]
- Hall, A.J.; Lopman, B.A.; Payne, D.C.; Patel, M.M.; Gastañaduy, P.A.; Vinjé, J.; Parashar, U.D. Norovirus Disease in the United States. Emerg. Infect. Dis. 2013, 19, 1198–1205. [Google Scholar] [CrossRef]
- Burton, T.D.; Carrera Montoya, J.; Frota, T.; Mackenzie, J.M. Human Norovirus Cultivation Models, Immune Response and Vaccine Landscape. In Advances in Virus Research; Academic Press: Cambridge, MA, USA, 2024. [Google Scholar]
- Iwu, C.D.; Okoh, A.I. Preharvest Transmission Routes of Fresh Produce Associated Bacterial Pathogens with Outbreak Potentials: A Review. Int. J. Environ. Res. Public Health 2019, 16, 4407. [Google Scholar] [CrossRef] [PubMed]
- Alegbeleye, O.O.; Singleton, I.; Sant’Ana, A.S. Sources and Contamination Routes of Microbial Pathogens to Fresh Produce during Field Cultivation: A Review. Food Microbiol. 2018, 73, 177–208. [Google Scholar] [CrossRef]
- Beauchat, L.R.; Ryu, J.-H. Produce Handling and Processing Practices. Emerg. Infect. Dis. 1997, 3, 459–465. [Google Scholar] [CrossRef] [PubMed]
- López-Gálvez, F.; Rasines, L.; Conesa, E.; Gómez, P.A.; Artés-Hernández, F.; Aguayo, E. Reusable Plastic Crates (RPCs) for Fresh Produce (Case Study on Cauliflowers): Sustainable Packaging but Potential Salmonella Survival and Risk of Cross-Contamination. Foods 2021, 10, 1254. [Google Scholar] [CrossRef]
- Ohman, E.; Kilgore, S.; Waite-Cusic, J.; Kovacevic, J. Before and After: Evaluation of Microbial and Organic Loads in Produce Handling and Packing Operations with Diverse Cleaning and Sanitizing Procedures. J. Food Prot. 2023, 86, 100185. [Google Scholar] [CrossRef]
- Ohman, E.; Waite-Cusic, J.; Kovacevic, J. Cleaning and Sanitizing in Produce Facilities: Identifying Compliance Gaps and Associated Training Needs, Opportunities and Preferences. Food Prot. Trends 2023, 43, 409–418. [Google Scholar] [CrossRef]
- Simões, M.; Simões, L.C.; Vieira, M.J. A Review of Current and Emergent Biofilm Control Strategies. LWT 2010, 43, 573–583. [Google Scholar] [CrossRef]
- Mayfosh, A.J.; Day, Z.I.; Unsworth, N.B.; Liu, C.Q.; Gupta, R.; Haynes, S.; Abraham, R.; Abraham, S.; Shaw, Z.L.; Walia, S.; et al. GS-2: A Novel Broad-Spectrum Agent for Environmental Microbial Control. Biomolecules 2022, 12, 1293. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug Administration CFR—Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=172.785 (accessed on 26 September 2023).
- U.S. Food & Drug Administration CFR—Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=172.860 (accessed on 27 August 2024).
- U.S. Food & Drug Administration CFR—Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=582.5145 (accessed on 27 August 2024).
- U.S. Food & Drug Administration CFR—Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=172.515&SearchTerm=thymol (accessed on 27 August 2024).
- Yoon, B.K.; Jackman, J.A.; Valle-González, E.R.; Cho, N.J. Antibacterial Free Fatty Acids and Monoglycerides: Biological Activities, Experimental Testing, and Therapeutic Applications. Int. J. Mol. Sci. 2018, 19, 1114. [Google Scholar] [CrossRef]
- Min, D.B.; Ellefson, W.C. Food Analysis; Nielsen, S.S., Ed.; Food Science Texts Series; Springer: Boston, MA, USA, 2010; ISBN 978-1-4419-1477-4. [Google Scholar]
- Patra, C.N.; Sahu, K.; Singha, R.; Jena, G.K.; Jammula, S.; Das, N.R. Multifaceted Applications of Solid Lipid: A Comprehensive Review. Biomed. Mater. Devices 2024, 2, 834–860. [Google Scholar] [CrossRef]
- McCollum, J.T.; Cronquist, A.B.; Silk, B.J.; Jackson, K.A.; O’Connor, K.A.; Cosgrove, S.; Gossack, J.P.; Parachini, S.S.; Jain, N.S.; Ettestad, P.; et al. Multistate Outbreak of Listeriosis Associated with Cantaloupe. N. Engl. J. Med. 2013, 369, 944–953. [Google Scholar] [CrossRef]
- Grant, J.; Wendelboe, A.M.; Wendel, A.; Jepson, B.; Torres, P.; Smelser, C.; Rolfs, R.T. Spinach-Associated Escherichia coli O157:H7 Outbreak, Utah and New Mexico, 2006. Emerg. Infect. Dis. 2008, 14, 1633–1636. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention Multistate Outbreak of Salmonella Serotype Agona Infections Linked to Toasted Oats Cereal—United States, April–May, 1998. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/00053368.htm (accessed on 8 March 2024).
- Hwang, S.; Alhatlani, B.; Arias, A.; Caddy, S.L.; Christodoulou, C.; Cunha, J.; Emmott, E.; Gonzalez-Hernandez, M.; Kolawole, A.; Lu, J.; et al. Murine Norovirus: Propagation, Quantification and Genetic Manipulation. Curr. Protoc. Microbiol. 2014, 33, 15K.2.1–15K.2.61. [Google Scholar] [CrossRef]
- Guimarães, A.; Venâncio, A. The Potential of Fatty Acids and Their Derivatives as Antifungal Agents: A Review. Toxins 2022, 14, 188. [Google Scholar] [CrossRef]
- Liang, N.; Cai, P.; Wu, D.; Pan, Y.; Curtis, J.M.; Gänzle, M.G. High-Speed Counter-Current Chromatography (HSCCC) Purification of Antifungal Hydroxy Unsaturated Fatty Acids from Plant-Seed Oil and Lactobacillus Cultures. J. Agric. Food Chem. 2017, 65, 11229–11236. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Liang, N.Y.; Curtis, J.M.; Gänzle, M.G. Characterization of Linoleate 10-Hydratase of Lactobacillus Plantarum and Novel Antifungal Metabolites. Front. Microbiol. 2016, 7, 1561. [Google Scholar] [CrossRef]
- Black, B.A.; Zannini, E.; Curtis, J.M.; Gänzle, M.G. Antifungal Hydroxy Fatty Acids Produced during Sourdough Fermentation: Microbial and Enzymatic Pathways, and Antifungal Activity in Bread. Appl. Environ. Microbiol. 2013, 79, 1866–1873. [Google Scholar] [CrossRef]
- Martin-Arjol, I.; Bassas-Galia, M.; Bermudo, E.; Garcia, F.; Manresa, A. Identification of Oxylipins with Antifungal Activity by LC-MS/MS from the Supernatant of Pseudomonas 42A2. Chem. Phys. Lipids 2010, 163, 341–346. [Google Scholar] [CrossRef]
- McCarthy, M.W.; Walsh, T.J. Amino Acid Metabolism and Transport Mechanisms as Potential Antifungal Targets. Int. J. Mol. Sci. 2018, 19, 909. [Google Scholar] [CrossRef]
- Kim, C.; Jung, H.; Kim, Y.O.; Shin, C.S. Antimicrobial Activities of Amino Acid Derivatives of Monascus Pigments. FEMS Microbiol. Lett. 2006, 264, 117–124. [Google Scholar] [CrossRef]
- Tian, J.; Ban, X.; Zeng, H.; He, J.; Huang, B.; Wang, Y. Chemical Composition and Antifungal Activity of Essential Oil from Cicuta Virosa L. Var. Latisecta Celak. Int. J. Food Microbiol. 2011, 145, 464–470. [Google Scholar] [CrossRef]
- Bernardos, A.; Marina, T.; Žáček, P.; Pérez-Esteve, É.; Martínez-Mañez, R.; Lhotka, M.; Kouřimská, L.; Pulkrábek, J.; Klouček, P. Antifungal Effect of Essential Oil Components against Aspergillus Niger When Loaded into Silica Mesoporous Supports. J. Sci. Food Agric. 2015, 95, 2824–2831. [Google Scholar] [CrossRef]
- Yoshimi, A.; Miyazawa, K.; Abe, K. Cell Wall Structure and Biogenesis in Aspergillus Species. Biosci. Biotechnol. Biochem. 2016, 80, 1700–1711. [Google Scholar] [CrossRef]
- Ahmadipour, S.; Field, R.A.; Miller, G.J. Prospects for Anti-Candida Therapy through Targeting the Cell Wall: A Mini-Review. Cell Surf. 2021, 7, 100063. [Google Scholar] [CrossRef]
- Barthel, L.; Cairns, T.; Duda, S.; Müller, H.; Dobbert, B.; Jung, S.; Briesen, H.; Meyer, V. Breaking down Barriers: Comprehensive Functional Analysis of the Aspergillus Niger Chitin Synthase Repertoire. Fungal Biol. Biotechnol. 2024, 11, 3. [Google Scholar] [CrossRef]
- Miramón, P.; Pountain, A.W.; Lorenz, M.C. Candida Auris-Macrophage Cellular Interactions and Transcriptional Response. Infect. Immun. 2023, 91, e00274-23. [Google Scholar] [CrossRef]
- Greenway, D.L.A.; Dyke, K.G.H. Mechanism of the Inhibitory Action of Linoleic Acid on the Growth of Staphylococcus Aureus. J. Gen. Microbiol. 1979, 115, 233–245. [Google Scholar] [CrossRef]
- Chamberlain, N.R.; Mehrtens, B.G.; Xiong, Z.; Kapral, F.A.; Boardman, J.L.; Rearick4, J.I. Correlation of Carotenoid Production, Decreased Membrane Fluidity, and Resistance to Oleic Acid Killing in Staphylococcus Aureus 18Z. Infect. Immun. 1991, 59, 4332–4337. [Google Scholar] [CrossRef]
- Westergren, I.; Johansson, B.B. Altering the Blood-brain Barrier in the Rat by Intracarotid Infusion of Polycations: A Comparison between Protamine, poly-L-lysine and poly-L-arginine. Acta Physiol. Scand. 1993, 149, 99–104. [Google Scholar] [CrossRef]
- Nemoto, E.; Takahashi, H.; Kobayashi, D.; Ueda, H.; Morimoto, Y. Effects of Poly-L-Arginine on the Permeation of Hydrophilic Compounds through Surface Ocular Tissues. Biol. Pharm. Bull. 2006, 29, 155–160. [Google Scholar] [CrossRef]
- Li, L.; Vorobyov, I.; Allen, T.W. The Different Interactions of Lysine and Arginine Side Chains with Lipid Membranes. J. Phys. Chem. B 2013, 117, 11906–11920. [Google Scholar] [CrossRef]
- Jalal, R.; Sepahi, M.; Mashreghi, M. Antibacterial Activity of Poly-l-Arginine under Different Conditions. Iran. J. Microbiol. 2017, 9, 103–111. [Google Scholar]
- Ram, A.F.J.; Arentshorst, M.; Damveld, R.A.; vanKuyk, P.A.; Klis, F.M.; van den Hondel, C.A.M.J.J. The Cell Wall Stress Response in Aspergillus Niger Involves Increased Expression of the Glutamine: Fructose-6-Phosphate Amidotransferase-Encoding Gene (gfaA) and Increased Deposition of Chitin in the Cell Wall. Microbiology 2004, 150, 3315–3326. [Google Scholar] [CrossRef]
- Hingston, P.A.; Piercey, M.J.; Hansen, L.T. Genes Associated with Desiccation and Osmotic Stress in Listeria Monocytogenes as Revealed by Insertional Mutagenesis. Appl. Environ. Microbiol. 2015, 81, 5350–5362. [Google Scholar] [CrossRef]
- Kragh, M.L.; Truelstrup Hansen, L. Initial Transcriptomic Response and Adaption of Listeria Monocytogenes to Desiccation on Food Grade Stainless Steel. Front. Microbiol. 2020, 10, 3132. [Google Scholar] [CrossRef]
- Welsh, D.T.; Herbert, R.A. Osmotically Induced Intracellular Trehalose, but Not Glycine Betaine Accumulation Promotes Desiccation Tolerance in Escherichia coli. FEMS Microbiol. Lett. 1999, 174, 57–63. [Google Scholar] [CrossRef]
- Manzanera, M.; Vilchez, S.; Tunnacliffe, A. High Survival and Stability Rates of Escherichia Coli Dried in Hydroxyectoine. FEMS Microbiol. Lett. 2004, 233, 347–352. [Google Scholar] [CrossRef]
- Maserati, A.; Fink, R.C.; Lourenco, A.; Julius, M.L.; Diez-Gonzalez, F. General Response of Salmonella enterica Serovar Typhimurium to Desiccation: A New Role for the Virulence Factors sopD and sseD in Survival. PLoS ONE 2017, 12, e0187692. [Google Scholar] [CrossRef]
- Suehr, Q.J.; Chen, F.; Anderson, N.M.; Keller, S.E. Effect of pH on Survival of Escherichia coli O157, Escherichia coli O121, and Salmonella enterica During Desiccation and Short-Term Storage. J. Food Prot. 2020, 83, 211–220. [Google Scholar] [CrossRef]
- Silhavy, T.J.; Kahne, D.; Walker, S. The Bacterial Cell Envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef]
- Kazmierczak, M.J.; Mithoe, S.C.; Boor, K.J.; Wiedmann, M. Listeria Monocytogenes σB Regulates Stress Response and Virulence Functions. J. Bacteriol. 2003, 185, 5722–5734. [Google Scholar] [CrossRef]
- Patrignani, F.; Siroli, L.; Gardini, F.; Lanciotti, R. Contribution of Two Different Packaging Material to Microbial Contamination of Peaches: Implications in Their Microbiological Quality. Front. Microbiol. 2016, 7, 938. [Google Scholar] [CrossRef]
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Chiavari, C.; Benevelli, M.; Grazia, L.; Lanciotti, R. Survival of Spoilage and Pathogenic Microorganisms on Cardboard and Plastic Packaging Materials. Front. Microbiol. 2017, 8, 2606. [Google Scholar] [CrossRef]
- Hadiyanto, H.; Khoironi, A.; Dianratri, I.; Suherman, S.; Muhammad, F.; Vaidyanathan, S. Interactions Between Polyethylene and Polypropylene Microplastics and Spirulina sp. Microalgae in Aquatic Systems. Heliyon 2021, 7, e07676. [Google Scholar] [CrossRef]
- Trudel-Ferland, M.; Goetz, C.; Girard, M.; Curt, S.; Mafu, A.A.; Fliss, I.; Jean, J. Physicochemical Parameters Affecting Norovirus Adhesion to Ready-To-Eat Foods. Appl. Environ. Microbiol. 2021, 87, e01396-21. [Google Scholar] [CrossRef]
- Song, Y.; Dunleavy, M.; Li, L. How to Make Plastic Surfaces Simultaneously Hydrophilic/Oleophobic? ACS Appl. Mater. Interfaces 2023, 15, 31092–31099. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, C.W.Y.; Burton, T.; Carrera Montoya, J.; Birje, N.; Zhou, X.; Salazar, J.K.; Mackenzie, J.M.; Rau, T.F.; Teplitski, M.; Zhang, W. Antimicrobial Efficacy of GS-2 on Reusable Food Packaging Materials for Specialty Crops. Foods 2024, 13, 3490. https://doi.org/10.3390/foods13213490
Wong CWY, Burton T, Carrera Montoya J, Birje N, Zhou X, Salazar JK, Mackenzie JM, Rau TF, Teplitski M, Zhang W. Antimicrobial Efficacy of GS-2 on Reusable Food Packaging Materials for Specialty Crops. Foods. 2024; 13(21):3490. https://doi.org/10.3390/foods13213490
Chicago/Turabian StyleWong, Catherine W. Y., Thomas Burton, Julio Carrera Montoya, Nupoor Birje, Xinyi Zhou, Joelle K. Salazar, Jason M. Mackenzie, Thomas F. Rau, Max Teplitski, and Wei Zhang. 2024. "Antimicrobial Efficacy of GS-2 on Reusable Food Packaging Materials for Specialty Crops" Foods 13, no. 21: 3490. https://doi.org/10.3390/foods13213490
APA StyleWong, C. W. Y., Burton, T., Carrera Montoya, J., Birje, N., Zhou, X., Salazar, J. K., Mackenzie, J. M., Rau, T. F., Teplitski, M., & Zhang, W. (2024). Antimicrobial Efficacy of GS-2 on Reusable Food Packaging Materials for Specialty Crops. Foods, 13(21), 3490. https://doi.org/10.3390/foods13213490







