Effects of Genotype and Modified Atmosphere Packaging on the Quality of Fresh-Cut Melons
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Fruit Processing
2.2. Packaging and Storage
2.3. In-Package Atmosphere Composition Analysis
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
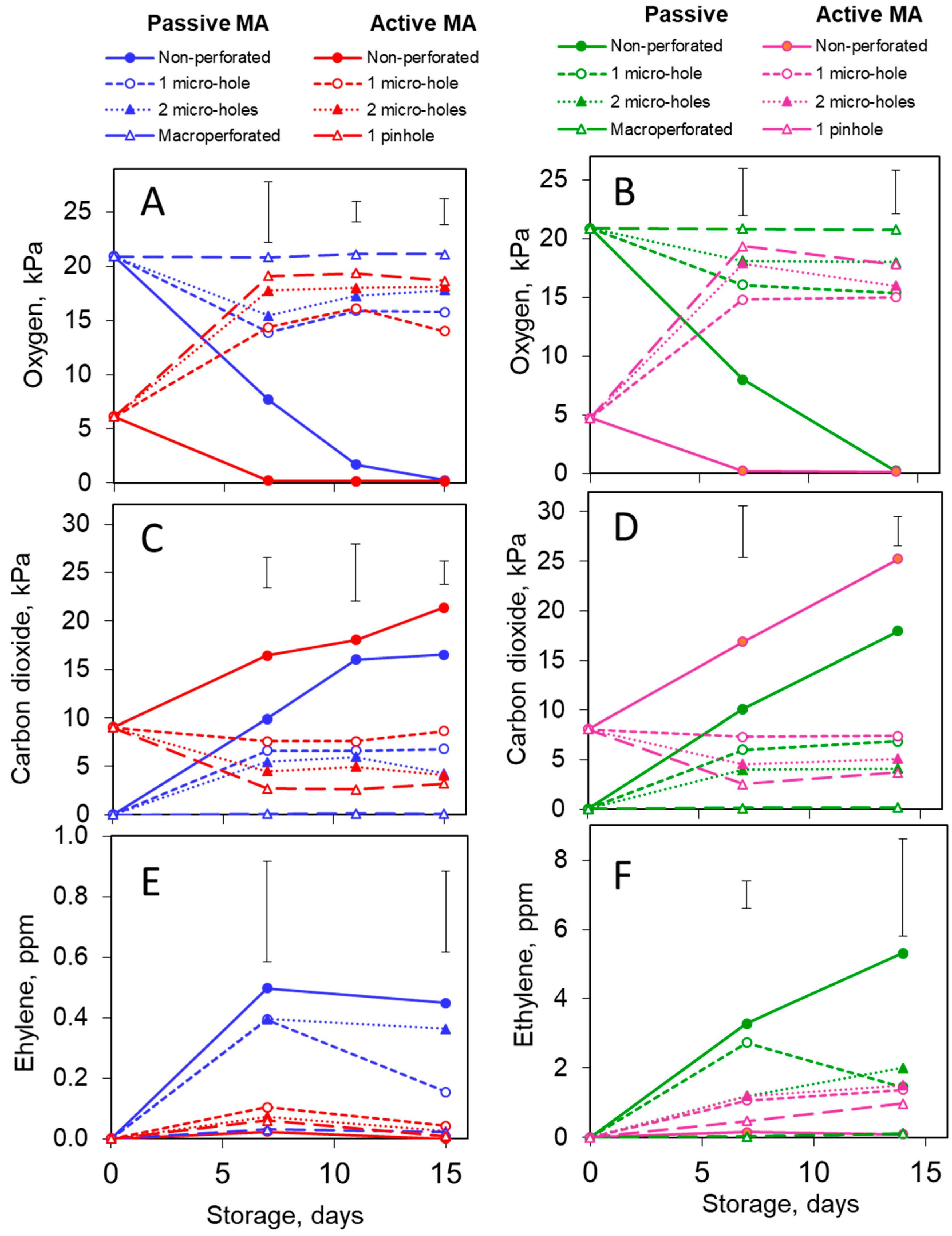
3.1. Headspace Atmosphere Composition
3.1.1. Oxygen
3.1.2. Carbon Dioxide
3.1.3. Ethylene
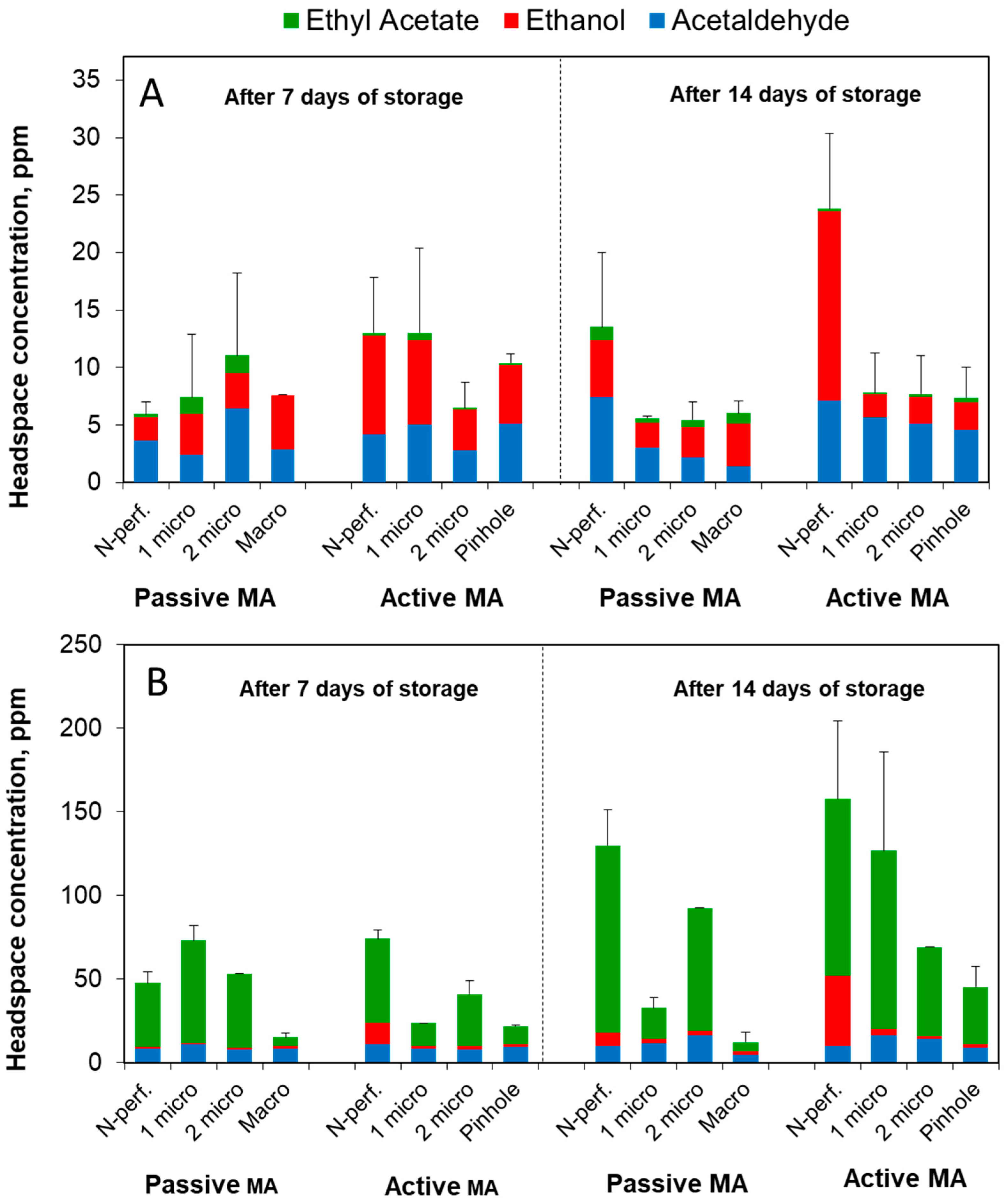
3.1.4. Fermentation Volatiles
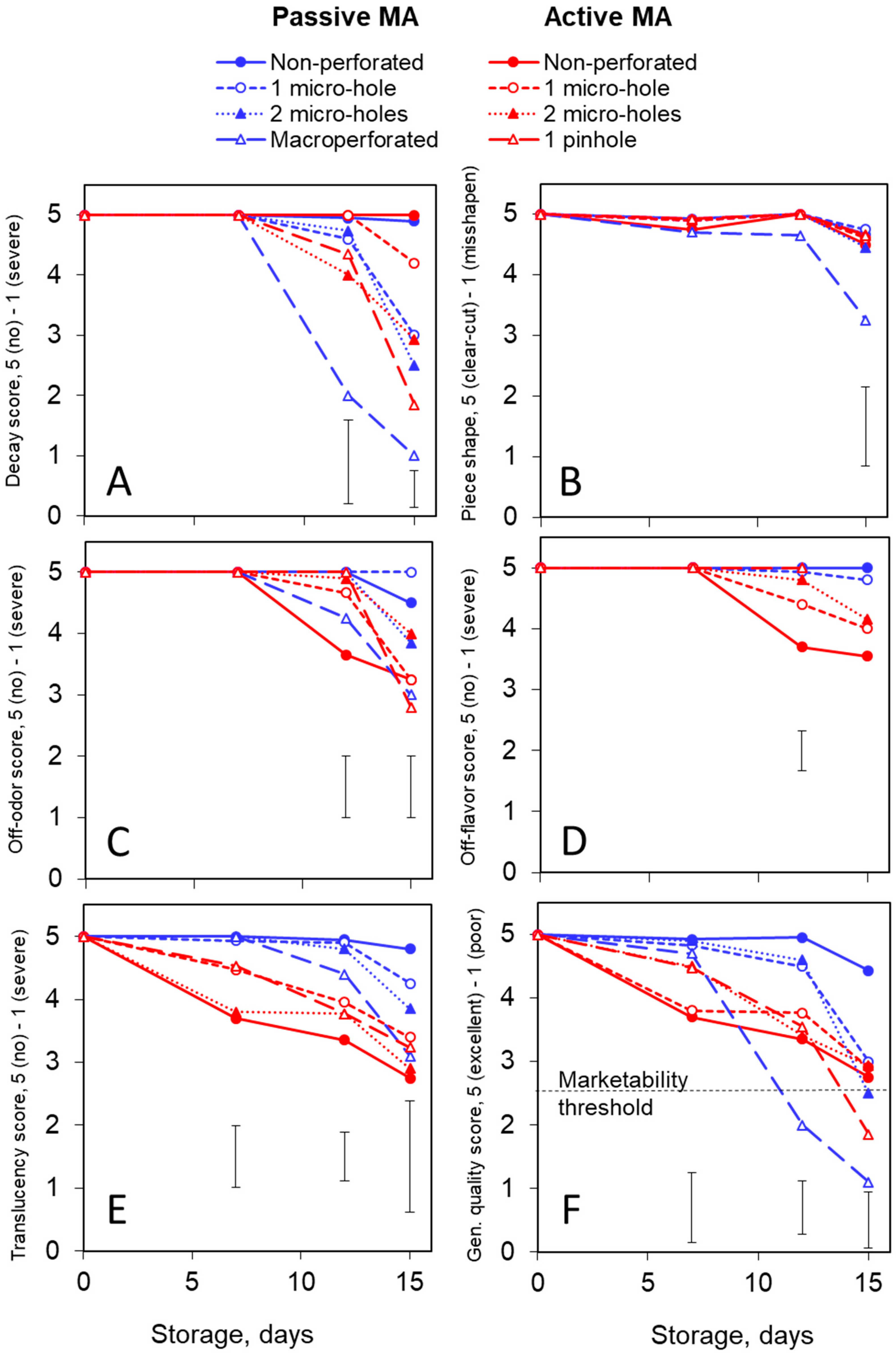
3.2. Quality: Inodorus Melons
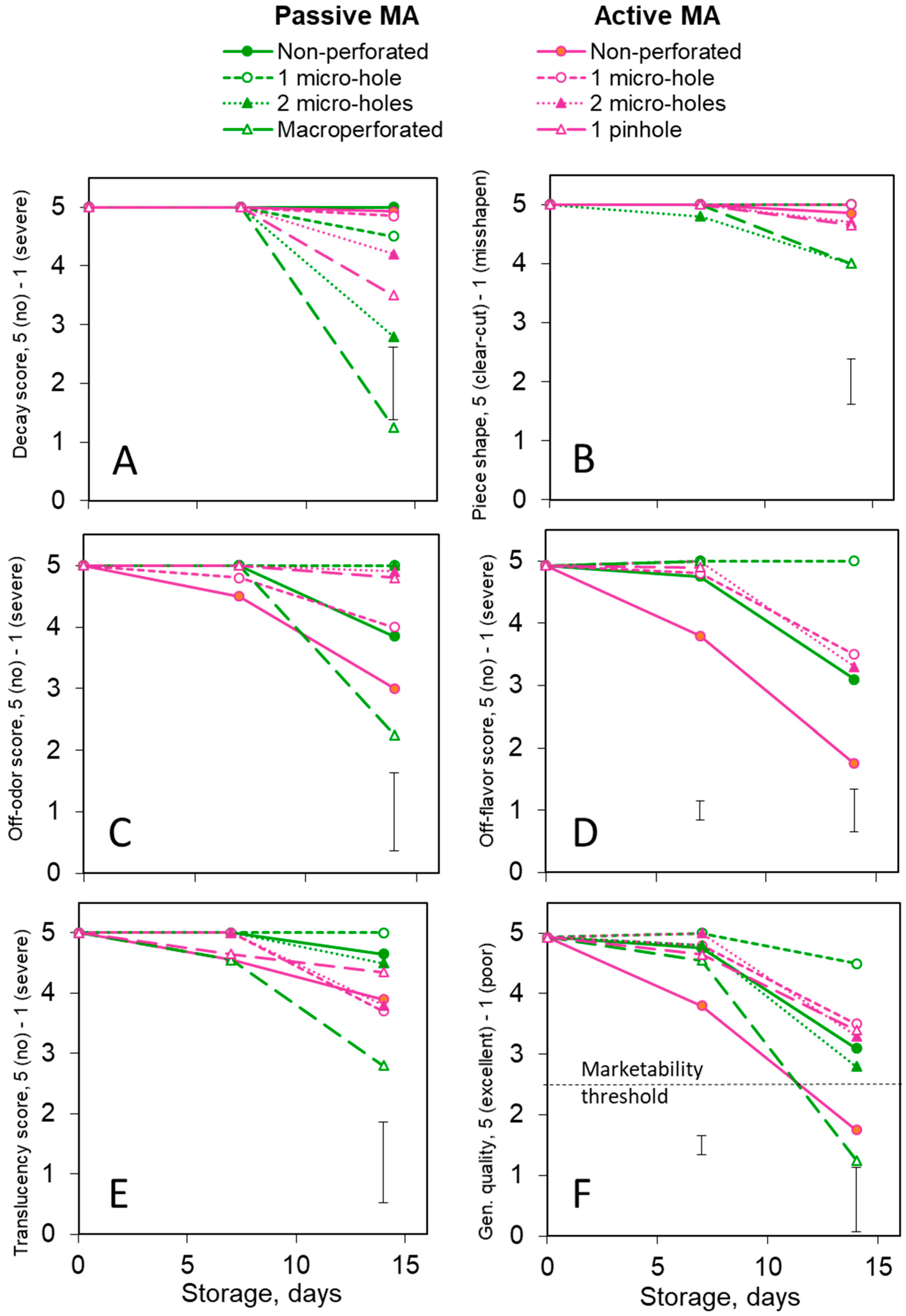
3.3. Quality: Cantalupensis Melons
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torres, A.; Langenhoven, P.; Behe, B.K. Characterizing the US melon market. HortScience 2020, 55, 795–803. [Google Scholar] [CrossRef]
- Amaro, A.L.; Beaulieu, J.C.; Grimm, C.C.; Stein, R.E.; Almeida, D.P. Effect of oxygen on aroma volatiles and quality of fresh-cut cantaloupe and honeydew melons. Food Chem. 2012, 130, 49–57. [Google Scholar] [CrossRef]
- Iturralde-García, R.D.; Cinco-Moroyoqui, F.J.; Martínez-Cruz, O.; Ruiz-Cruz, S.; Wong-Corral, F.J.; Borboa-Flores, J.; Cornejo-Ramírez, Y.I.; Bernal-Mercado, A.T.; Del-Toro-Sánchez, C.L. Emerging technologies for prolonging fresh-cut fruits’ quality and safety during storage. Horticulturae 2022, 8, 731. [Google Scholar] [CrossRef]
- Portela, S.I.; Cantwell, M.I. Quality changes of minimally processed honeydew melons stored in air or controlled atmosphere. Postharvest Biol. Technol. 1998, 14, 351–357. [Google Scholar] [CrossRef]
- Rodov, V.; Shinde, R. Fresh-cut fruits: Melons. In Controlled and Modified Atmospheres for Fresh and Fresh-Cut Produce; Academic Press: Cambridge, MA, USA, 2020; pp. 501–509. [Google Scholar]
- Aguayo, E.; Escalona, V.; Artés, F. Quality of minimally processed Cucumis melo var. saccharinus as improved by controlled atmosphere. Eur. J. Hortic. Sci. 2007, 72, 39–45. [Google Scholar]
- Beaulieu, J.C. Effect of cutting and storage on acetate and nonacetate esters in convenient, ready-to-eat fresh-cut melons and apples. HortScience 2006, 41, 65–73. [Google Scholar] [CrossRef]
- Beaulieu, J.C. Volatile changes in cantaloupe during growth, maturation, and in stored fresh-cuts prepared from fruit harvested at various maturities. J. Am. Soc. Hortic. Sci. 2006, 131, 127–139. [Google Scholar] [CrossRef]
- Bai, J.H.; Saftner, R.A.; Watada, A.E. Characteristics of fresh-cut honeydew (Cucumis melo L.) available to processors in winter and summer and its quality maintenance by modified atmosphere packaging. Postharvest Biol. Technol. 2003, 28, 349–359. [Google Scholar] [CrossRef]
- Saftner, R.A.; Lester, G.E. Sensory and analytical characteristics of a novel hybrid muskmelon fruit intended for the fresh-cut industry. Postharvest Biol. Technol. 2009, 51, 327–333. [Google Scholar] [CrossRef]
- Silveira, A.C.; Aguayo, E.; Artés, F. The suitability of three Galia melon cultivars and different types of cuts for the fresh-cut industry. J. Sci. Food Agric. 2013, 93, 3826–3831. [Google Scholar] [CrossRef]
- Oms-Oliu, G.; Soliva-Fortuny, R.; Martín-Belloso, O. Effect of ripeness on the shelf-life of fresh-cut melon preserved by modified atmosphere packaging. Eur. Food Res. Technol. 2007, 225, 301–311. [Google Scholar] [CrossRef]
- Sapers, G.; Miller, R.; Pilizota, V.; Mattrazzo, A. Antimicrobial treatments for minimally processed cantaloupe melon. J. Food Sci. 2001, 66, 345–349. [Google Scholar] [CrossRef]
- Portela, S.I.; Cantwell, M.I. Cutting blade sharpness affects appearance and other quality attributes of fresh-cut cantaloupe melon. J. Food Sci. 2001, 66, 1265–1270. [Google Scholar] [CrossRef]
- Silveira, A.C.; Conesa, A.; Aguayo, E.; Artes, F. Alternative sanitizers to chlorine for use on fresh-cut “Galia”(Cucumis melo var. cantalupensis) melon. J. Food Sci. 2008, 73, M405–M411. [Google Scholar] [CrossRef] [PubMed]
- Bett-Garber, K.L.; Lamikanra, O.; Lester, G.E.; Ingram, D.A.; Watson, M.A. Influence of soil type and storage conditions on sensory qualities of fresh-cut cantaloupe (Cucumis melo). J. Sci. Food Agric. 2005, 85, 825–830. [Google Scholar] [CrossRef]
- Amaro, A.L.; Spadafora, N.D.; Pereira, M.J.; Dhorajiwala, R.; Herbert, R.J.; Müller, C.T.; Rogers, H.J.; Pintado, M. Multitrait analysis of fresh-cut cantaloupe melon enables discrimination between storage times and temperatures and identifies potential markers for quality assessments. Food Chem. 2018, 241, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.H.; Saftner, R.A.; Watada, A.E.; Lee, Y. Modified atmosphere maintains quality of fresh-cut cantaloupe (Cucumis melo L.). J. Food Sci. 2001, 66, 1207–1211. [Google Scholar] [CrossRef]
- Wilson, M.D.; Stanley, R.A.; Eyles, A.; Ross, T. Innovative processes and technologies for modified atmosphere packaging of fresh and fresh-cut fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2019, 59, 411–422. [Google Scholar] [CrossRef]
- Saltveit, M.E. Biological basis for CA and MA. In Controlled and Modified Atmospheres for Fresh and Fresh-Cut Produce; Academic Press: Cambridge, MA, USA, 2020; pp. 3–22. [Google Scholar]
- Oms-Oliu, G.; Raybaudi-Massilia Martínez, R.; Soliva-Fortuny, R.; Martín-Belloso, O. Effect of superatmospheric and low oxygen modified atmospheres on shelf-life extension of fresh-cut melon. Food Control 2008, 19, 191–199. [Google Scholar] [CrossRef]
- Amodio, M.L.; Pati, S.; Derossi, A.; Mastrandrea, L.; Colelli, G. Reaction mechanisms for volatiles responsible of off-odors of fresh cut melons. Acta Hortic. 2021, 1311, 15–22. [Google Scholar] [CrossRef]
- Rodov, V.; Horev, B.; Goldman, G.; Vinokur, Y.; Fishman, S. Model-driven development of microperforated active modified-atmosphere packaging for fresh-cut produce. In Proceedings of the International Conference on Quality Management of Fresh Cut Produce, Bangkok, Thailand, 6–8 August 2007; Volume 746, pp. 83–88. [Google Scholar]
- Shinde, R.; Rodov, V.; Krishnakumar, S.; Subramanian, J. Active and intelligent packaging for reducing postharvest losses of fruits and vegetables. In Postharvest Biology and Nanotechnology; Paliyath, G., Subramanian, J., Lim, L.T., Subramanian, K.S., Handa, A.K., Mattoo, A.K., Eds.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2019; pp. 171–189. [Google Scholar]
- Stepansky, A.; Kovalski, I.; Perl-Treves, R. Intraspecific classification of melons (Cucumis melo L.) in view of their phenotypic and molecular variation. Plant Syst. Evol. 1999, 217, 313–332. [Google Scholar] [CrossRef]
- Robinson, R.W.; Decker-Walters, D. Cucurbits; CAB International: Wallingford, UK, 1997; 226p. [Google Scholar]
- Mitchell, J.M.; Cantliffe, D.J.; Sargent, S.A.; Datnoff, L.E.; Stoffella, P.J. Fruit yield, quality variables, and powdery mildew susceptibility of Galia melon cultivars grown in a passively ventilated greenhouse. Fla. State Hortic. Soc. 2007, 120, 162–167. [Google Scholar]
- Poverenov, E.; Danino, S.; Horev, B.; Granit, R.; Vinokur, Y.; Rodov, V. Layer-by-layer electrostatic deposition of edible coating on fresh cut melon model: Anticipated and unexpected effects of alginate–chitosan combination. Food Bioprocess Technol. 2014, 7, 1424–1432. [Google Scholar] [CrossRef]
- Freiman, Z.E.; Rodov, V.; Yablovitz, Z.; Horev, B.; Flaishman, M.A. Preharvest application of 1-methylcyclopropene inhibits ripening and improves keeping quality of ‘Brown Turkey’figs (Ficus carica L.). Sci. Hortic. 2012, 138, 266–272. [Google Scholar] [CrossRef]
- Van Oirschot, Q.E.; Tomlins, K.I. Applying analytical sensory evaluation techniques, which translate qualitative perceptions to numerical data to research on development issues. In Proceedings of the Conference on Combining Qualitative and Quantitative Methods in Development Research, Centre for Development Studies, University of Wales, Swansea, UK, 1–2 July 2002; pp. 1–30. [Google Scholar]
- Hintlian, C.B.; Hotchkiss, J.H. The safety of modified atmosphere packaging: A review. Food Technol. 1986, 40, 70–76. [Google Scholar]
- Soltani, M.; Alimardani, R.; Mobli, H.; Mohtasebi, S.S. Modified atmosphere packaging: A progressive technology for shelf-life extension of fruits and vegetables. J. Appl. Packag. Res. 2015, 7, 33–59. [Google Scholar]
- Gorris, L.G.; Peppelenbos, H.W. Modified-atmosphere packaging of produce. In Handbook of Food Preservation; CRC Press: Boca Raton, FL, USA, 2020; pp. 349–362. [Google Scholar]
- McMillin, K.W. Modified atmosphere packaging. In Food Safety Engineering; Demirci, A., Feng, H., Krishnamurthy, K., Eds.; Springer: Cham, Switzerland, 2020; pp. 693–718. [Google Scholar]
- Exama, A.; Arul, J.; Lencki, R.W.; Lee, L.Z.; Toupin, C. Suitability of plastic films for modified atmosphere packaging of fruits and vegetables. J. Food Sci. 1993, 58, 1365–1370. [Google Scholar] [CrossRef]
- Oliveira, J.C.; Ramos, A.V.; Sousa-Gallagher, M.J. A meta-study of the permeance of perforated packaging films to oxygen and carbon dioxide. Food Eng. Rev. 2022, 14, 328–352. [Google Scholar] [CrossRef]
- Beaudry, R.M. Responses of horticultural commodities to low oxygen: Limits to the expanded use of modified atmosphere packaging. HortTechnology 2000, 10, 491–500. [Google Scholar] [CrossRef]
- Hussein, Z.; Caleb, O.J.; Opara, U.L. Perforation-mediated modified atmosphere packaging of fresh and minimally processed produce—A review. Food Packag. Shelf Life 2015, 6, 7–20. [Google Scholar] [CrossRef]
- Falagán, N.; Terry, L.A. Recent advances in controlled and modified atmosphere of fresh produce. Johns. Matthey Technol. Rev. 2018, 62, 107–117. [Google Scholar] [CrossRef]
- Bishop, D.; Schaefer, J.; Beaudry, R. Industrial advances of CA/MA technologies: Innovative storage systems. In Controlled and Modified Atmospheres for Fresh and Fresh-Cut Produce; Academic Press: Cambridge, MA, USA, 2020; pp. 265–276. [Google Scholar]
- Belay, Z.A.; Caleb, O.J.; Opara, U.L. Influence of initial gas modification on physicochemical quality attributes and molecular changes in fresh and fresh-cut fruit during modified atmosphere packaging. Food Packag. Shelf Life 2019, 21, 100359. [Google Scholar] [CrossRef]
- Hu, W.Z.; Jiang, A.L.; Pang, K.; Qi, H.P. Atmospheric compositions, respiration rate and quality of fresh-cut cabbages in active modified atmosphere packaging. In Proceedings of the International Conference on Quality Management of Fresh Cut Produce, Bangkok, Thailand, 6–8 August 2007; Volume 746, pp. 69–76. [Google Scholar]
- De Reuck, K.; Sivakumar, D.; Korsten, L. Effect of passive and active modified atmosphere packaging on quality retention of two cultivars of litchi (Litchi chinensis Sonn.). J. Food Qual. 2010, 33, 337–351. [Google Scholar] [CrossRef]
- Hadjilouka, A.; Polychronopoulou, M.; Paramithiotis, S.; Tzamalis, P.; Drosinos, E.H. Effect of lemongrass essential oil vapors on microbial dynamics and Listeria monocytogenes survival on rocket and melon stored under different packaging conditions and temperatures. Microorganisms 2015, 3, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Kale, P.M.; Patil, S.S.; Palghadmal, U.B. Effect of different modified atmosphere packaging on physico-chemical, microbiological and sensorial attributes of fresh-cut muskmelon. Int. J. Environ. Agric. Biotechnol. 2020, 5, 1226–1233. [Google Scholar] [CrossRef]
- Fallik, E.; Shalom, Y.; Alkalai-Tuvia, S.; Larkov, O.; Brandeis, E.; Ravid, U. External, internal and sensory traits in Galia-type melon treated with different waxes. Postharvest Biol. Technol. 2005, 36, 69–75. [Google Scholar] [CrossRef]
- Shalit, M.; Katzir, N.; Tadmor, Y.; Larkov, O.; Burger, Y.; Shalekhet, F.; Lastochkin, E.; Ravid, U.; Amar, O.; Edelstein, M.; et al. Acetyl-CoA: Alcohol acetyltransferase activity and aroma formation in ripening melon fruits. J. Agric. Food Chem. 2001, 49, 794–799. [Google Scholar] [CrossRef]
- Burger, Y.; Sa’ar, U.; Paris, H.S.; Lewinsohn, E.; Katzir, N.; Tadmor, Y.; Schaffer, A.A. Genetic variability for valuable fruit quality traits in Cucumis melo. Isr. J. Plant Sci. 2006, 54, 233–242. [Google Scholar] [CrossRef]
- Aguayo, E.; Allende, A.; Artés, F. Keeping quality and safety of minimally fresh processed melon. Eur. Food Res. Technol. 2003, 216, 494–499. [Google Scholar] [CrossRef]
- Gorny, J.R. A summary of CA and MA requirements and recommendations for fresh-cut (minimally processed) fruits and vegetables. In Proceedings of the VIII International Controlled Atmosphere Research Conference, Rotterdam, The Netherlands, 10 March 2003; Volume 600, pp. 609–614. [Google Scholar]
- Mathooko, F.M. Regulation of ethylene biosynthesis in higher plants by carbon dioxide. Postharvest Biol. Technol. 1996, 7, 1–26. [Google Scholar] [CrossRef]
- Jin, Y.Z.; Liu, W.W.; Qi, H.Y.; Bai, X.H. Ethanol vapor treatment maintains postharvest storage quality and inhibits internal ethylene biosynthesis during storage of oriental sweet melons. Postharvest Biol. Technol. 2013, 86, 372–380. [Google Scholar] [CrossRef]
- Ergun, M.; Jeong, J.; Huber, D.J.; Cantliffe, D.J. Physiology of fresh-cut ‘Galia’ (Cucumis melo var. reticulatus) from ripe fruit treated with 1-methylcyclopropene. Postharvest Biol. Technol. 2007, 44, 286–292. [Google Scholar] [CrossRef]
- Supapvanich, S.; Tucker, G.A. The effect of 1-methylcyclopropene (1-MCP) on quality and cell wall hydrolases activities of fresh-cut muskmelon (Cucumis melo var. reticulatus L.) during storage. Food Bioprocess Technol. 2013, 6, 2196–2201. [Google Scholar] [CrossRef]
- Denoya, G.I.; Vaudagna, S.R.; Polenta, G. Effect of high pressure processing and vacuum packaging on the preservation of fresh-cut peaches. LWT-Food Sci. Technol. 2015, 62, 801–806. [Google Scholar] [CrossRef]
- Fundo, J.F.; Amaro, A.L.; Madureira, A.R.; Carvalho, A.; Feio, G.; Silva, C.L.; Quintas, M.A. Fresh-cut melon quality during storage: An NMR study of water transverse relaxation time. J. Food Eng. 2015, 167, 71–76. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Cai, W.; Ma, Y.; Xu, Y.; Zhao, X.; Zhang, C. Visible light exposure reduces the drip loss of fresh-cut watermelon. J. Food Sci. Technol. 2018, 55, 1816–1822. [Google Scholar] [CrossRef]
- Pesis, E. The role of the anaerobic metabolites, acetaldehyde and ethanol, in fruit ripening, enhancement of fruit quality and fruit deterioration. Postharvest Biol. Technol. 2005, 37, 1–19. [Google Scholar] [CrossRef]
- Liu, X.; Ma, H.; Liu, J.; Liu, D.; Wang, C. The γ-Aminobutyric Acid (GABA) Synthesis Gene Regulates the Resistance to Water Core-Induced Hypoxia Stress for Pear Fruits. Agronomy 2023, 13, 1062. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shinde, R.; Vinokur, Y.; Fallik, E.; Rodov, V. Effects of Genotype and Modified Atmosphere Packaging on the Quality of Fresh-Cut Melons. Foods 2024, 13, 256. https://doi.org/10.3390/foods13020256
Shinde R, Vinokur Y, Fallik E, Rodov V. Effects of Genotype and Modified Atmosphere Packaging on the Quality of Fresh-Cut Melons. Foods. 2024; 13(2):256. https://doi.org/10.3390/foods13020256
Chicago/Turabian StyleShinde, Ranjeet, Yakov Vinokur, Elazar Fallik, and Victor Rodov. 2024. "Effects of Genotype and Modified Atmosphere Packaging on the Quality of Fresh-Cut Melons" Foods 13, no. 2: 256. https://doi.org/10.3390/foods13020256
APA StyleShinde, R., Vinokur, Y., Fallik, E., & Rodov, V. (2024). Effects of Genotype and Modified Atmosphere Packaging on the Quality of Fresh-Cut Melons. Foods, 13(2), 256. https://doi.org/10.3390/foods13020256





