Nutritional and Phytochemical Composition and Antioxidant Activity of Edible Stems of Smooth Cordgrass (Spartina alterniflora)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nutritional Composition Analysis
2.3. Determination of Macro Elements, Trace Elements and Heavy Metals
2.4. Determination of Contents of Total Phenols, Total Flavonoids, Total Alkaloids, Total Nitrite, and Total Nitrate
2.5. Determination of Antioxidative Activities
2.6. Statistical Analyses
3. Results and Discussions
3.1. The Nutritional Composition of Tender Stems of S. alterniflora
3.2. Amino Acid Profiles
3.3. Vitamins
3.4. Minerals
3.5. Total Alkaloids, Total Flavonoids, and Total Phenols
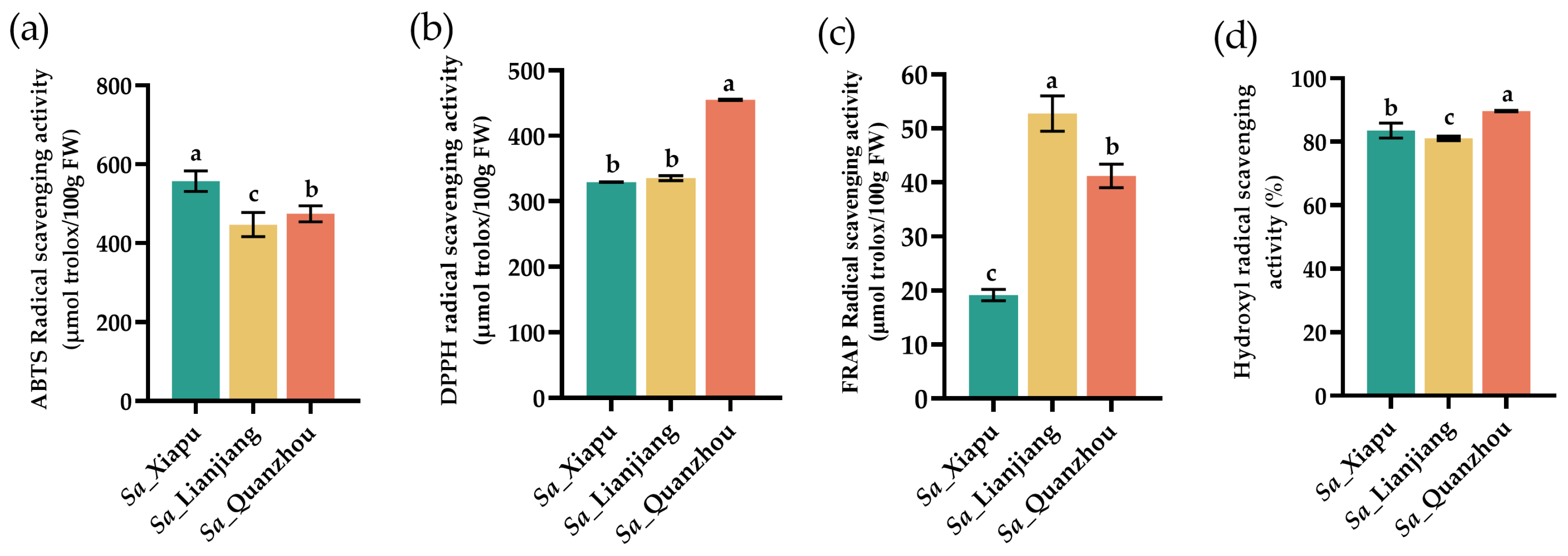
3.6. Antioxidant Activities
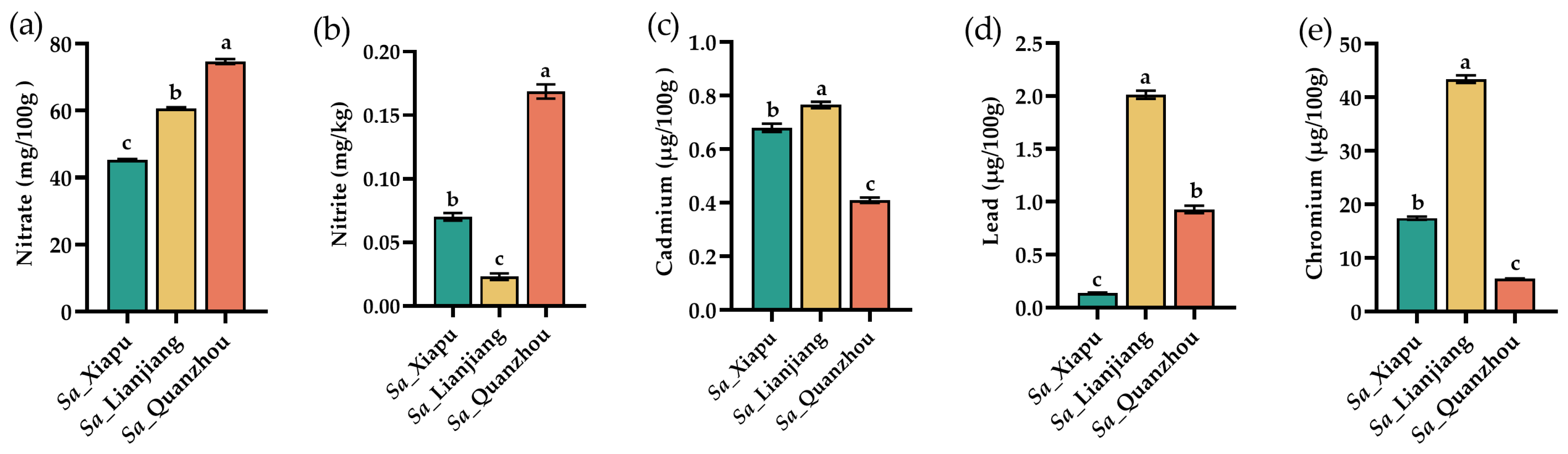
3.7. Food Safety Evaluations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wasowski, S.; Wasowsk, A. Gardening with Native Plants of the South, reprint edition; Taylor Trade Publishing: Lanham, MD, USA, 2009. [Google Scholar]
- Broome, S.W.; Seneca, E.D.; Woodhouse, W.W. Long-term growth and development of transplants of the salt-marsh grass Spartina alterniflora. Estuaries 1986, 9, 63–74. [Google Scholar] [CrossRef]
- Vernberg, F.J. Salt-marsh processes: A review. Environ. Toxicol. Chem. 1993, 12, 2167–2195. [Google Scholar] [CrossRef]
- Egerova, J.; Proffitt, C.E.; Travis, S.E. Facilitation of survival and growth of Baccharis halimifolia L. by Spartina alterniflora Loisel. In a created Louisiana salt marsh. Wetlands 2003, 23, 250–256. [Google Scholar] [CrossRef]
- Edwards, K.R.; Mills, K.P. Aboveground and belowground productivity of Spartina alterniflora (Smooth Cordgrass) in natural and created Louisiana salt marshes. Estuaries 2005, 28, 252–265. [Google Scholar] [CrossRef]
- Teal, J.M.; Howes, B.L. Salt marsh values: Retrospection from the end of the century. In Concepts and Controversies in Tidal Marsh Ecology; Weinstein, M.P., Kreeger, D.A., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 9–19. [Google Scholar]
- Teixeira, A.; Duarte, B.; Caçador, I. Salt marshes and biodiversity. In Tasks for Vegetation Science; Khan, M.A., Böer, B., Öztürk, M., Al Abdessalaam, T.Z., Clüsener-Godt, M., Gul, B., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 47, pp. 283–298. [Google Scholar]
- Sharma, S.K. Living Shorelines and Hybrid Designs for Coastal Restoration: Impacts on Water Quality, Submerged Aquatic Vegetation, Salt Marsh Flora and Associated Organisms. Doctoral Dissertation, University of South Alabama, Mobile, AL, USA, 2016. [Google Scholar]
- Burden, A.; Garbutt, R.A.; Evans, C.D.; Jones, D.L.; Cooper, D.M. Carbon sequestration and biogeochemical cycling in a saltmarsh subject to coastal managed realignment. Estuar. Coast. Shelf. 2013, 120, 12–20. [Google Scholar] [CrossRef]
- Zhao, W.; Li, X.; Xue, L.; Lin, S.; Ma, Y.; Su, L.; Li, Z.; Gong, L.; Yan, Z.; Macreadie, P.I. Mapping trade-offs among key ecosystem functions in tidal marsh to inform spatial management policy for exotic Spartina alterniflora. J. Environ. Manag. 2023, 348, 119216. [Google Scholar] [CrossRef]
- Qin, P.; Xie, M.; Jiang, Y.; Chung, C.H. Estimation of the ecological-economic benefits of two Spartina alterniflora plantations in North Jiangsu, China. Ecol. Eng. 1997, 1, 5–17. [Google Scholar] [CrossRef]
- Qin, P.; Xie, M.; Jiang, Y. Spartina green food ecological engineering. Ecol. Eng. 1998, 11, 147–156. [Google Scholar] [CrossRef]
- Chung, C.H. Thirty years of ecological engineering with Spartina plantations in China. Ecol. Eng. 1993, 2, 261–289. [Google Scholar] [CrossRef]
- Chung, C.H. Forty years of ecological engineering with Spartina plantations in China. Ecol. Eng. 2006, 27, 49–57. [Google Scholar] [CrossRef]
- Ma, Z.; Gan, X.; Cai, Y.; Chen, J.; Li, B. Effects of exotic Spartina alterniflora on the habitat patch associations of breeding saltmarsh birds at Chongming Dongtan in the Yangtze River estuary, China. Biol. Invasions 2011, 13, 673–1686. [Google Scholar] [CrossRef]
- Lu, J.B.; Ying, Z. Spatial distribution of an invasive plant Spartina alterniflora and its potential as biofuels in China. Ecol. Eng. 2013, 52, 175–181. [Google Scholar] [CrossRef]
- Zheng, X.; Javed, Z.; Liu, B.; Zhong, S.; Cheng, Z.; Rehman, A.; Du, D.; Li, J. Impact of Spartina alterniflora invasion in coastal wetlands of China: Boon or Bane? Biology 2023, 12, 1057. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Chen, J.; Jing, C.; Ye, G.; Wu, J.; Huang, Z.; Zhou, C. Monitoring the invasion of Spartina alterniflora from 1993 to 2014 with landsat TM and SPOT 6 satellite data in Yueqing Bay, China. PLoS ONE 2015, 10, e0135538. [Google Scholar] [CrossRef]
- Li, N.; Li, L.; Zhang, Y.; Wu, M. Monitoring of the invasion of Spartina alterniflora from 1985 to 2015 in Zhejiang Province, China. BMC Ecol. 2020, 20, 7. [Google Scholar] [CrossRef]
- Mao, D.; Liu, M.; Wang, Z.; Li, L.; Man, W.; Jia, M.; Zhang, Y. Rapid invasion of Spartina alterniflora in the coastal zone of mainland China: Spatiotemporal patterns and human prevention. Sensors. 2019, 19, 2308. [Google Scholar] [CrossRef]
- Liu, L.; Wang, H.J.; Yue, Q. China’s coastal wetlands: Ecological challenges, restoration, and management suggestions. Reg. Stud. Mar. Sci. 2020, 37, 101337. [Google Scholar] [CrossRef]
- Nie, M.; Liu, W.; Pennings, S.C.; Li, B. Lessons from the invasion of Spartina alterniflora in coastal China. Ecology 2023, 104, e3874. [Google Scholar] [CrossRef]
- Xie, B.H.; Han, G.X. Control of invasive Spartina alterniflora: A review. Chin. J. Appl. Ecol. 2018, 29, 3464–3476. [Google Scholar] [CrossRef]
- Xie, B.; Han, G.; Qiao, P.; Mei, B.; Wang, Q.; Zhou, Y.; Zhang, A.; Song, W.; Guan, B. Effects of mechanical and chemical control on invasive Spartina alterniflora in the Yellow River Delta, China. Peer J. 2019, 7, e7655. [Google Scholar] [CrossRef]
- Guo, Q.Y. Control for Spartina alterniflora in beach. Chin. Prot. For. Sci. Technol. 2011, 2, 3–5. [Google Scholar]
- Meng, K.H. Study on the treatment and long-term management mechanism of Spartina alterniflora in Xiangshan harbor. For. Sci. Technol. 2023, 1, 20–24. [Google Scholar]
- Yuan, L.; Zhang, L.; Xiao, D.; Huang, H. The application of cutting plus waterlogging to control Spartina alterniflora on saltmarshes in the Yangtze Estuary, China. Estuar. Coast. Shelf Sci. 2011, 92, 103–110. [Google Scholar] [CrossRef]
- Ji, Z.Y.; Yang, J.J.; Ju, J.H.; Cheng, X.M. The applied research on biomineral liquid to fish culture. In Applied Studies on Spartina; Qin, P., Chung, C.H., Eds.; Sea and Ocean Press: Beijing, China, 1992; pp. 145–148. [Google Scholar]
- Ji, Z.Y.; Qin, P.; Xie, M. The research on biomineral liquid applied to pearl culture. In Applied Studies on Spartina; Qin, P., Chung, C.H., Eds.; Sea and Ocean Press: Beijing, China, 1992; pp. 141–144. [Google Scholar]
- Qin, F.; Tang, B.; Zhang, H.; Shi, C.; Zhou, W.; Ding, L.; Qin, P. Potential use of Spartina alterniflora as forage for dairy cattle. Ecol. Eng. 2016, 92, 173–180. [Google Scholar] [CrossRef]
- Qin, P.; Zhang, H.S.; Qin, F.F. Spartina Alterniflora Ecological Engineering; Chemical Industry Press: Beijing, China, 2019. [Google Scholar]
- Lu, H.F.; Zhang, H.S.; Qin, P.; Li, X.Z.; Campbell, D.E. Integrated emergy and economic evaluation of an ecological engineering system for the utilization of Spartina alterniflora. J. Clean. Prod. 2020, 247, 119592. [Google Scholar] [CrossRef]
- Milião, G.L.; de Oliveira, A.P.H.; de Souza Soares., L.; Arruda, T.R.; Érica Nascif Rufino Vieira, E.N.R.; de Castro Leite Junior, B.R. Unconventional food plants: Nutritional aspects and perspectives for industrial applications. Future Foods 2022, 5, 100124. [Google Scholar] [CrossRef]
- ASTM D1102-21; Standard Test Method for Ash in Wood. ASTM International: West Conshohocken, PA, USA, 2021. [CrossRef]
- Horwitz, W.; Latimer, G.W. Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Fan, L.; Hu, J.; Guo, Z.; Chen, S.; He, Q. Shoot nutrition and flavor variation in two Phyllostachys species: Does the quality of edible bamboo shoot diaphragm and flesh differ? Foods 2023, 12, 1180. [Google Scholar] [CrossRef]
- Ferjančič, B.; Skrt, M.; Korošec, M.; Bertoncelj, J. Comparative analysis of dietary fibre determination by AOAC 991.43 and AOAC 2011.25 for frequently consumed foods in Slovenia. Food Chem. 2022, 397, 133753. [Google Scholar] [CrossRef]
- Nielsen, S.S.; Carpenter, C. Total carbohydrate by phenol-sulfuric acid method. In Food Analysis Laboratory Manual; Springer: Cham, Switzerland, 2017; pp. 137–141. [Google Scholar]
- Teixeira, R.S.S.; Da Silva, A.S.; Ferreira-Leitão, V.S.; Bon, E.P.D.S. Amino acids interference on the quantification of reducing sugars by the 3,5-dinitrosalicylic acid assay mislead carbohydrase activity measurements. Carbohydr. Res. 2012, 363, 33–37. [Google Scholar] [CrossRef]
- Nielsen, S.S.; Carpenter, C. Fat content determination. In Food Analysis Laboratory Manual; Springer: Cham, Switzerland, 2017; pp. 121–129. [Google Scholar]
- Sun, S.W.; Lin, Y.C.; Weng, Y.M.; Chen, M.J. Efficiency improvements on ninhydrin method for amino acid quantification. J. Food Compos. Anal. 2006, 19, 112–117. [Google Scholar] [CrossRef]
- Attard, E. A rapid microtitre plate Folin-Ciocalteu method for the assessment of polyphenols. Open Life Sci. 2013, 8, 48–53. [Google Scholar] [CrossRef]
- Ma, Z.; Ma, Y.; Liu, Y.; Zhou, B.; Zhao, Y.; Wu, P.; Zhang, D.; Li, D. Effects of maturity and processing on the volatile components, phytochemical profiles and antioxidant activity of lotus (Nelumbo nucifera) leaf. Foods 2023, 12, 198. [Google Scholar] [CrossRef] [PubMed]
- Ajanal, M.; Gundkalle, M.B.; Nayak, S.U. Estimation of total alkaloid in chitrakadivati by UV-spectrophotometer. Anc. Sci. Life 2012, 31, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Chetty, A.A. Nitrate-N determination in leafy vegetables: Study of the effects of cooking and freezing. Food Chem. 2008, 106, 772–780. [Google Scholar] [CrossRef]
- USDA. Food Data Central (Bamboo Shoot). 2018. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/169210/nutrients (accessed on 17 September 2024).
- Pegiou, E.; Mumm, R.; Acharya, P.; de Vos, R.C.H.; Hall, R.D. Green and white asparagus (Asparagus officinalis): A source of developmental, chemical and Urinary intrigue. Metabolites 2019, 10, 17. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, N.; Liu, H.; Li, Z.; Lu, L.; Wang, C. The bioactive compounds and biological functions of Asparagus offcinalis L.—A review. J. Funct. Foods. 2020, 65, 103727. [Google Scholar] [CrossRef]
- Qian, B.; Luo, Y.; Deng, Y.; Cao, L.; Yang, H.; Shen, Y.; Ping, J. Chemical composition, angiotensin-converting enzyme-inhibitory activity and antioxidant activities of few-flower wild rice (Zizania latifolia Turcz.). J. Sci. Food Agric. 2012, 92, 159–164. [Google Scholar] [CrossRef]
- Yan, N.; Du, Y.; Liu, X.; Chu, C.; Shi, J.; Zhang, H.; Liu, Y.; Zhang, Z. Morphological characteristics, nutrients, and bioactive compounds of Zizania latifolia, and health benefits of its seeds. Molecules 2018, 23, 1561. [Google Scholar] [CrossRef]
- Wu, W.; Han, Y.; Niu, B.; Yang, B.; Liu, R.; Fang, X.; Chen, H.; Xiao, S.; Farag, M.A.; Zheng, S.; et al. Recent advances in Zizania latifolia: A comprehensive review on phytochemical, health benefits and applications that maximize its value. Crit. Rev. Food Sci. Nutr. 2023, 64, 7535–7549. [Google Scholar] [CrossRef]
- Tyler, A.C.; Lambrinos, J.G.; Grosholz, E.D. Nitrogen inputs promote the spread of an invasive marsh grass. Ecol. Appl. 2007, 17, 1886–1898. [Google Scholar] [CrossRef]
- Hu, G.; Li, X.; Lai, A.; Liu, Y.; Zhang, Y.; Wang, J.; Sun, S.; Zhu, J.; Yang, M. Comparative analysis of the nutritional quality of Zizania latifolia cultivars harvested in different growing seasons. Foods 2023, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, L.; Dosi, R.; Di Maro, A.; Guida, V.; Cefarelli, G.; Pacifico, S.; Mastellone, C.; Fiorentino, A.; Rosati, A.; Parente, A. Nutritional values, metabolic profile and radical scavenging capacities of wild asparagus (A. acutifolius L.). J. Food Compos. Anal. 2010, 3, 326–333. [Google Scholar] [CrossRef]
- USDA. Food Data Central (Leek). 2019. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/169246/nutrients (accessed on 17 September 2024).
- USDA. Food Data Central (Asparagus). 2022. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/2345287/nutrients (accessed on 17 September 2024).
- USDA. Food Data Central (Celery). 2022. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/2346405/nutrients (accessed on 17 September 2024).
- Kang, S.; Kim, S.; Ha, S.; Lee, C.; Nam, S. Biochemical components and physiological activities of Ice plant (Mesembryanthemum crystallinum). J. Korean Soc. Food Sci. Nutr. 2016, 45, 1732–1739. [Google Scholar] [CrossRef]
- Nirmala, C.; David, E.; Sharma, M.L. Changes in nutrient components during ageing of emerging juvenile bamboo shoots. Int. J. Food Sci. Nutr. 2007, 58, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Cuenca, A.; García-Alonso, A.; Rodríguez-Arcos, R.; Castro, I.; Alba, C.; Miguel-Rodríguez, J.; Goñi, I. Nutritional composition of green asparagus (Asparagus officinalis L.), edible part and by-products, and assessment of their effect on the growth of human gut-associated bacteria. Food Res. Int. 2023, 163, 112284. [Google Scholar] [CrossRef]
- Dey, P.; Kundu, A.; Kumar, A.; Gupta, M.; Lee, B.M.; Bhakta, T.; Dash, S.; Kim, H.S. Analysis of alkaloids (indole alkaloids, isoquinoline alkaloids, tropane alkaloids). In Recent Advances in Natural Products Analysis; Silva, A.S., Nabavi, S.F., Saeedi, M., Nabavi, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 505–567. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and oher phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Belazougui, L.; Mesrouk, S.; Mohammedi, H.; Akcha, S.; Aïnouz, L.; Mecherara-Idjeri, F. Phytochemical analysis, mineral composition, assessment of antioxidant properties and cytotoxic potential of Ephedra alata. subsp. Alenda secondary metabolites. Food Biosci. 2023, 53, 102657. [Google Scholar] [CrossRef]
- Schiff, P.L. Ergot and its alkaloids. Am. J. Pharm. Educ. 2006, 70, 98. [Google Scholar] [CrossRef]
- Ren, W.; Ye, X.; Li, T.; Zheng, J. Analysis of the volatile components in polyphenol compounds extracted from Dendrocalamus latiflorus shoots. Chin. Food Sci. 2014, 35, 120–123. [Google Scholar] [CrossRef]
- Ren, Y.; Ma, Y.; Zhang, Z.; Qiu, L.; Zhai, H.; Gu, R.; Xie, Y. Total alkaloids from bamboo shoots and bamboo shoot shells of Pleioblastus amarus (Keng) Keng f. and their anti-inflammatory activities. Molecules 2019, 24, 2699. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ionita, P. The chemistry of DPPH free radical and congeners. Int. J. Mol. Sci. 2021, 22, 1545. [Google Scholar] [CrossRef] [PubMed]
- Tiveron, A.P.; Melo, P.S.; Bergamaschi, K.B.; Vieira, T.M.F.S.; Regitano-d’Arce, M.A.B.; Alencar, S.M. Antioxidant activity of Brazilian vegetables and its relation with phenolic composition. Int. J. Mol. Sci. 2012, 13, 8943–8957. [Google Scholar] [CrossRef] [PubMed]
- Ilić, T.; Dodevska, M.; Marčetić, M.; Božić, D.; Kodranov, I.; Vidović, B. Chemical characterization, antioxidant and antimicrobial properties of goji berries cultivated in Serbia. Foods 2020, 9, 1614. [Google Scholar] [CrossRef] [PubMed]
- Iammarino, M.; Berardi, G.; Vita, V.; Elia, A.; Conversa, G.; Di Taranto, A. Determination of nitrate and nitrite in Swiss chard (Beta vulgaris L. subsp. vulgaris) and wild rocket (Diplotaxis tenuifolia (L.) DC.) and food safety evaluations. Foods 2022, 11, 2571. [Google Scholar] [CrossRef]
- European Commission. Commission regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. 2006. Available online: https://www.legislation.gov.uk/eur/2006/1881 (accessed on 17 September 2024).
- China. GB 2762-2022 National Food Safety Standard Food Contaminant Limits. 2022. Available online: http://wjw.nmg.gov.cn/zzb/hybz/202210/P020220824606851329551.pdf (accessed on 17 September 2024).
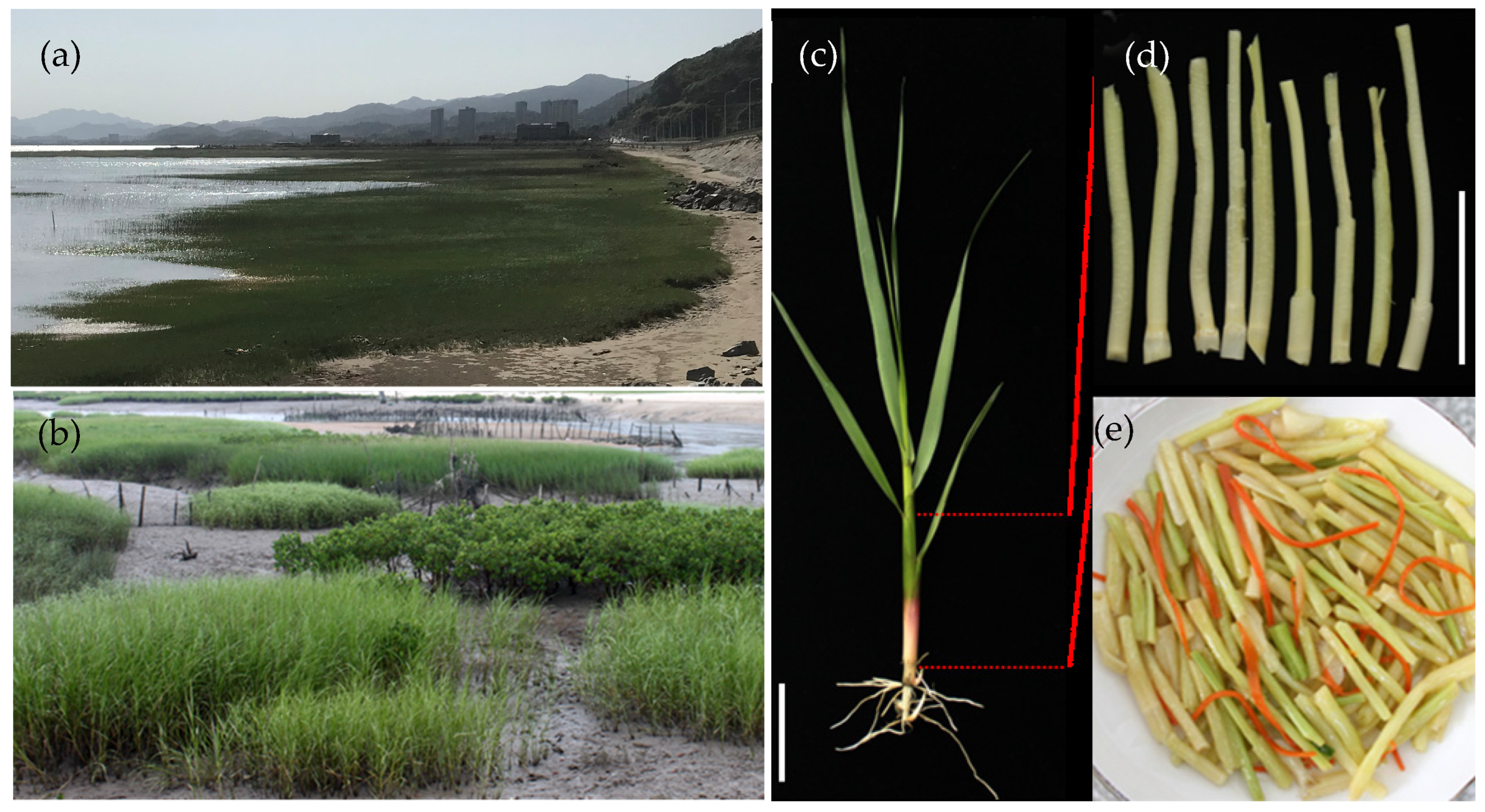
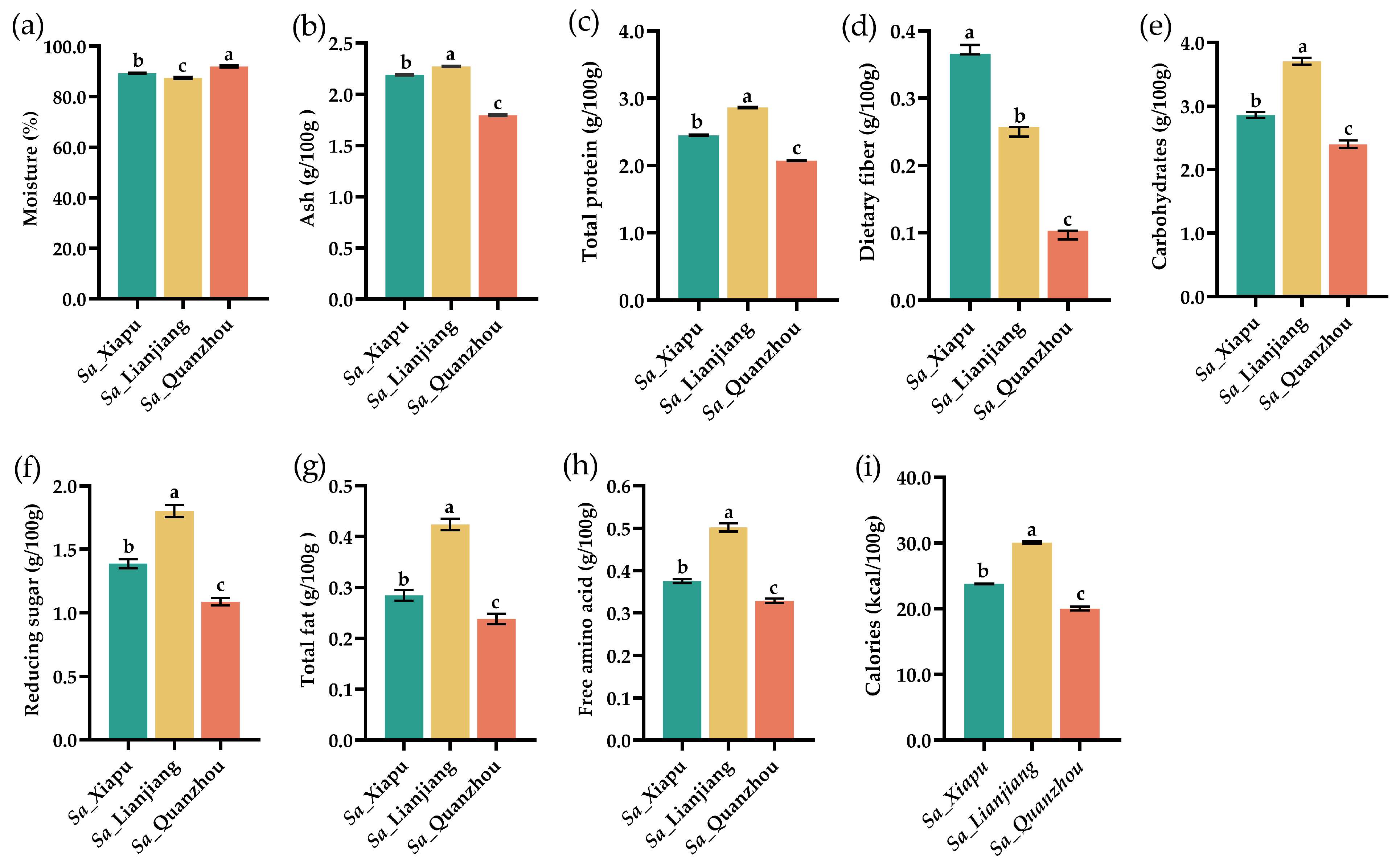
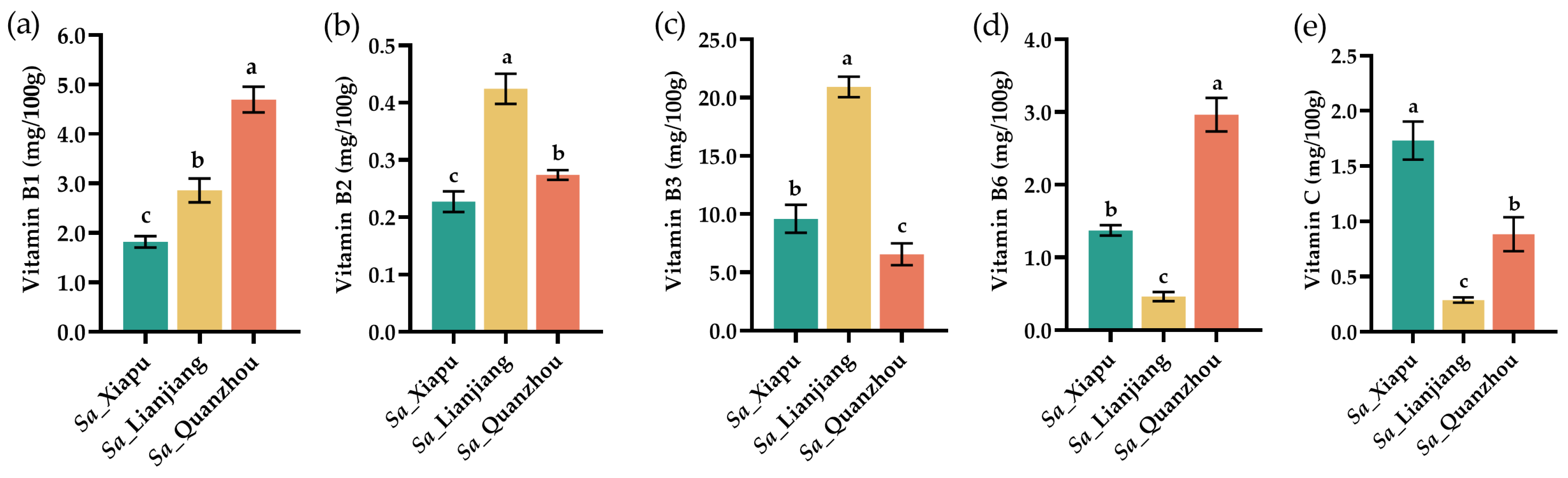
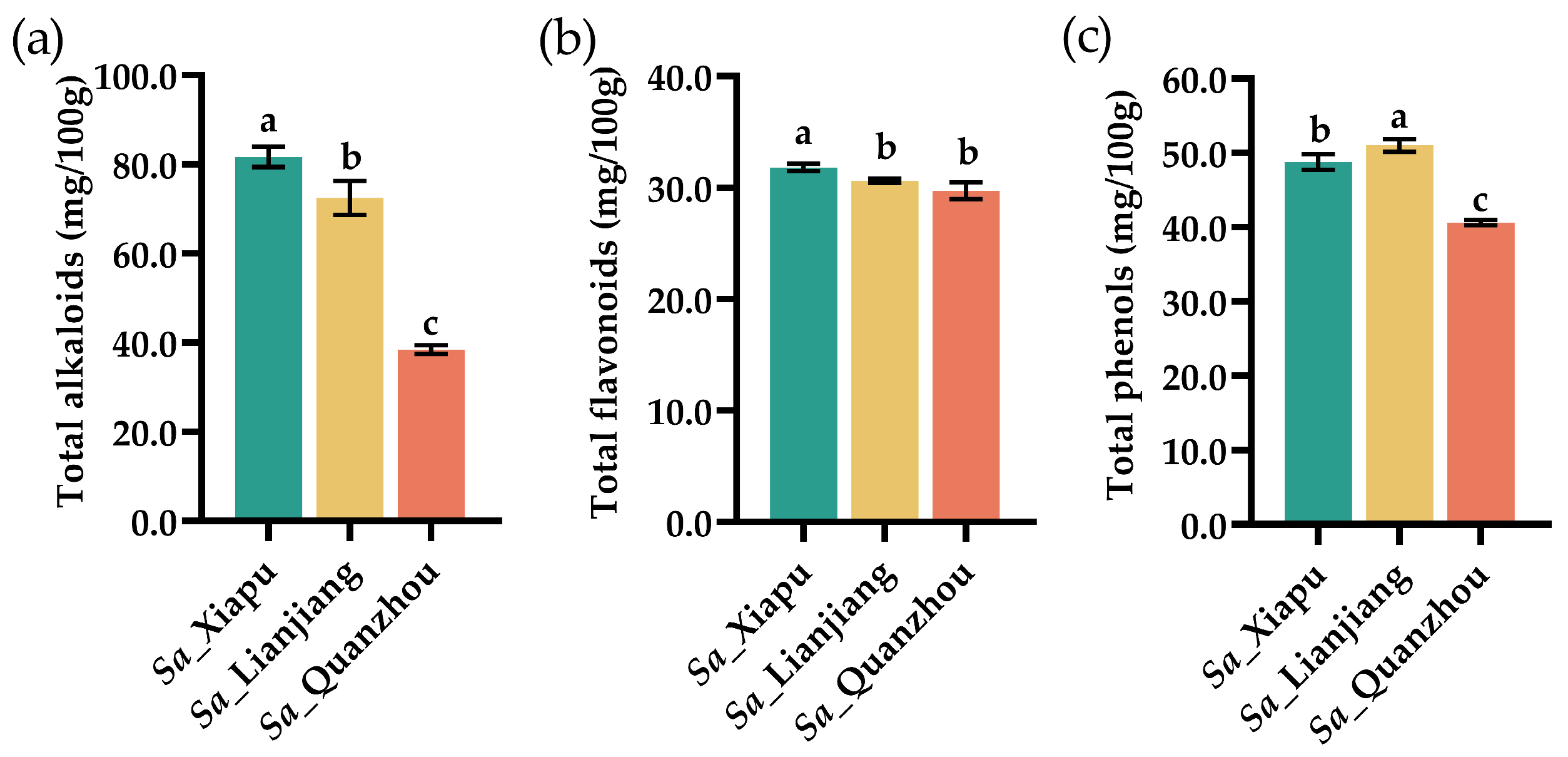
| Amino Acid | Sa_Xiapu | Sa_Lianjiang | Sa_Quanzhou |
|---|---|---|---|
| Essential amino acids | |||
| Histidine (His, H) | 10.07 ± 0.57 b | 2.8 ± 0.28 c | 34.28 ± 2.53 a |
| Isoleucine (Ile, I) | 6.08 ± 0.28 a | 4.18 ± 0.23 b | 3.36 ± 0.35 c |
| Leucine (Leu, L) | 3.00 ± 0.05 b | 1.90 ± 0.08 c | 3.81 ± 0.34 a |
| Lysine (Lys, K) | 5.72 ± 0.06 a | 1.79 ± 0.18 b | 1.53 ± 0.06 c |
| Methionine (Met, M) | 0.66 ± 0.04 b | 1.14 ± 0.07 a | 1.13 ± 0.04 a |
| Phenylalanine (Phe, F) | 6.97 ± 0.09 a | 2.1 ± 0.07 b | 2.61 ± 0.16 b |
| Threonine (Thr, T) | 3.31 ± 0.16 c | 4.34 ± 0.05 a | 3.75 ± 0.11 b |
| Tryptophan (Trp, W) | 5.19 ± 0.39 a | 2.19 ± 0.23 b | 1.94 ± 0.15 c |
| Valine (Val, V) | 28.61 ± 1.01 c | 41.36 ± 1.26 a | 39.94 ± 0.7 b |
| Non-essential amino acids | |||
| Alanine (Ala, A) | 11.39 ± 0.32 c | 15.23 ± 0.61 a | 14.16 ± 0.72 a |
| Arginine (Arg, R) | 6.33 ± 0.34 a | 2.11 ± 0.19 c | 5.99 ± 0.08 b |
| Asparagine (Asn, N) | 116.45 ± 2.97 b | 201.08 ± 3.56 a | 61.4 ± 2.34 c |
| Aspartic acid (Asp, D) | 32.91 ± 1.12 a | 27.37 ± 1.1 b | 10.58 ± 0.61 c |
| Cysteine (Cys, C) | 7.60 ± 0.67 a | 4.39 ± 0.15 c | 5.27 ± 0.17 b |
| Glutamic acid (Glu, E) | 38.29 ± 3.16 a | 31.9 ± 2.32 b | 23.34 ± 1.45 c |
| Glutamine (Gln, Q) | 3.94 ± 0.07 c | 7.15 ± 0.14 b | 10.45 ± 0.07 a |
| Glycine (Gly, G) | 0.58 ± 0.04 c | 1.24 ± 0.04 b | 2.75 ± 0.18 a |
| Proline (Pro, P) | 38.94 ± 0.78 b | 26.67 ± 0.9 c | 42.24 ± 2.74 a |
| Serine (Ser, S) | 19.36 ± 1.60 b | 26.47 ± 0.43 a | 8.81 ± 0.28 c |
| Tyrosine (Tyr, Y) | 11.25 ± 0.82 b | 13.34 ± 0.63 a | 7.72 ± 0.32 c |
| TEAA 1 | 69.61 ± 1.49 b | 61.79 ± 1.79 c | 92.36 ± 1.95 a |
| TNEAA 2 | 287.04 ± 7.87 b | 356.95 ± 6.16 a | 192.71 ± 4.29 c |
| TUAA 3 | 71.21 ± 3.87 a | 59.28 ± 1.73 b | 33.92 ± 1.07 c |
| TBAA 4 | 82.71 ± 1.78 b | 74.21 ± 2.12 c | 90.55 ± 3.26 a |
| TAA 5 | 356.65 ± 9.17 b | 418.73 ± 7.82 a | 285.07 ± 2.66 c |
| Mineral Elements | Sa_Xiapu | Sa_Lianjiang | Sa_Quanzhou |
|---|---|---|---|
| Macromineral elements (mg/100 g): | |||
| Sodium, Na | 549.62 ± 10.08 a | 518.52 ± 1.57 b | 549.06 ± 2.28 a |
| Potassium, K | 210.68 ± 2.7 c | 589.57 ± 3.41 a | 535.77 ± 4.52 b |
| Calcium, Ca | 27.24 ± 1.36 c | 41.73 ± 1.03 a | 38.7 ± 0.38 b |
| Magnesium, Mg | 37.4 ± 0.61 b | 41.62 ± 1.09 a | 36.55 ± 0.51 b |
| Phosphorus, P | 58.57 ± 1.25 b | 50.51 ± 0.19 c | 63.8 ± 1.25 a |
| Trace elements (μg/100 g): | |||
| Iron, Fe | 658.16 ± 35.67 c | 2371.98 ± 6.65 a | 894.42 ± 0.71 b |
| Manganese, Mn | 1247.66 ± 10.08 b | 606.46 ± 2.85 c | 1718.38 ± 25.79 a |
| Zinc, Zn | 506.19 ± 36.23 a | 506.32 ± 2.42 a | 479.75 ± 2.11 b |
| Copper, Cu | 83.84 ± 0.39 a | 58.05 ± 0.80 c | 67.48 ± 0.68 b |
| Boron, B | 124.00 ± 3.55 a | 72.82 ± 0.14 c | 100.67 ± 2.67 b |
| Selenium, Se | 2.01 ± 0.01 a | 1.12 ± 0.03 b | 0.8 ± 0.02 c |
| Iodine, I | 4.81 ± 0.05 a | 1.97 ± 0.04 b | 1.98 ± 0.02 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Chen, H.; Lai, M.; Lin, Z.; Huang, Y.; Tang, W.; Zhu, Y.; Zhang, Y.; Wang, Z.; Ni, H.; et al. Nutritional and Phytochemical Composition and Antioxidant Activity of Edible Stems of Smooth Cordgrass (Spartina alterniflora). Foods 2024, 13, 3150. https://doi.org/10.3390/foods13193150
Han Y, Chen H, Lai M, Lin Z, Huang Y, Tang W, Zhu Y, Zhang Y, Wang Z, Ni H, et al. Nutritional and Phytochemical Composition and Antioxidant Activity of Edible Stems of Smooth Cordgrass (Spartina alterniflora). Foods. 2024; 13(19):3150. https://doi.org/10.3390/foods13193150
Chicago/Turabian StyleHan, Yijuan, Huiquan Chen, Meiling Lai, Zhongyuan Lin, Yongji Huang, Weiqi Tang, Yanbing Zhu, Yange Zhang, Zonghua Wang, Hui Ni, and et al. 2024. "Nutritional and Phytochemical Composition and Antioxidant Activity of Edible Stems of Smooth Cordgrass (Spartina alterniflora)" Foods 13, no. 19: 3150. https://doi.org/10.3390/foods13193150
APA StyleHan, Y., Chen, H., Lai, M., Lin, Z., Huang, Y., Tang, W., Zhu, Y., Zhang, Y., Wang, Z., Ni, H., Chen, X., & Chen, S. (2024). Nutritional and Phytochemical Composition and Antioxidant Activity of Edible Stems of Smooth Cordgrass (Spartina alterniflora). Foods, 13(19), 3150. https://doi.org/10.3390/foods13193150








