Golden Flower Tibetan Tea Polysaccharides Alleviate Constipation in Mice by Regulating Aquaporins-Mediated Water Transport System and Gut Microbiota
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Golden Flower Tibetan Tea Polysaccharides (GFTTP)
2.3. Chemical Characterization of GFTTPs
2.3.1. Determination of Molecular Weight
2.3.2. Measurement of Total Sugar, Phenols, and Proteins
2.3.3. Evaluation of Monosaccharide Composition
2.3.4. Fourier-Transform Infrared (FT-IR) Spectroscopy
2.3.5. X-ray Diffraction (XRD) Analysis
2.3.6. Thermogravimetric Analysis (TGA)
2.3.7. Scanning Electron Microscopy (SEM)
2.4. Evaluation of Relieving Effect of GFTTPs in Constipated Mice
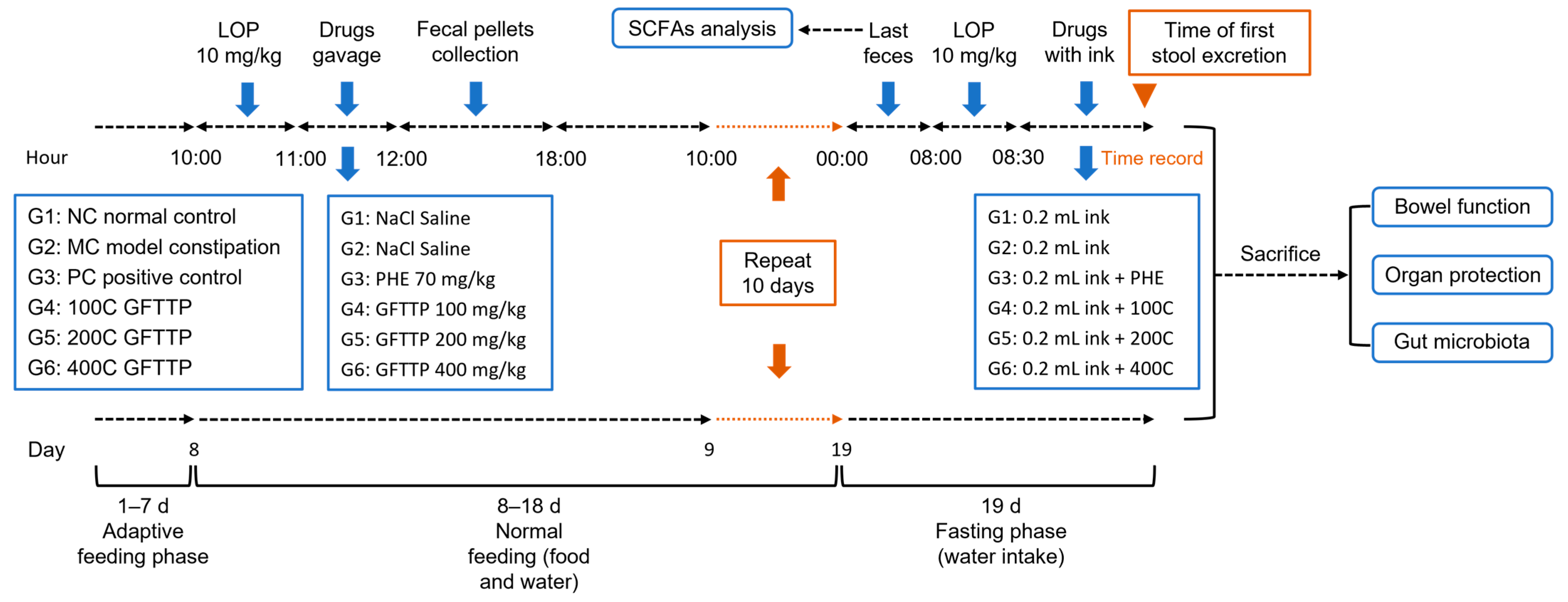
2.4.1. Animal Experiments
2.4.2. Assessment of Neurotransmitters
2.4.3. Real-Time Quantitative PCR (RT-qPCR) Analysis
2.4.4. Histopathological Analysis
2.4.5. Gut Microbiota Analysis
2.4.6. Determination of Short-Chain Fatty Acids (SCFAs)
2.5. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties of GFTTP
3.1.1. Basic Structures of GFTTP
3.1.2. FT-IR Spectroscopy
3.1.3. XRD Analysis
3.1.4. TGA Analysis
3.1.5. SEM Analysis
3.2. GFTTPs Relieve LOP-Induced Constipation in Mice
3.2.1. Effect of GFTTPs on Body Weight, Stool-Related Parameters, and GI Transit Rate
3.2.2. Effect of GFTTPs on Serum Levels of Gastrointestinal Regulatory-Related Peptides
3.2.3. Effect of GTTTP on the Expression-Related Genes in the Colon
3.2.4. Effect of GFTTPs on Histopathological Observation of Colon
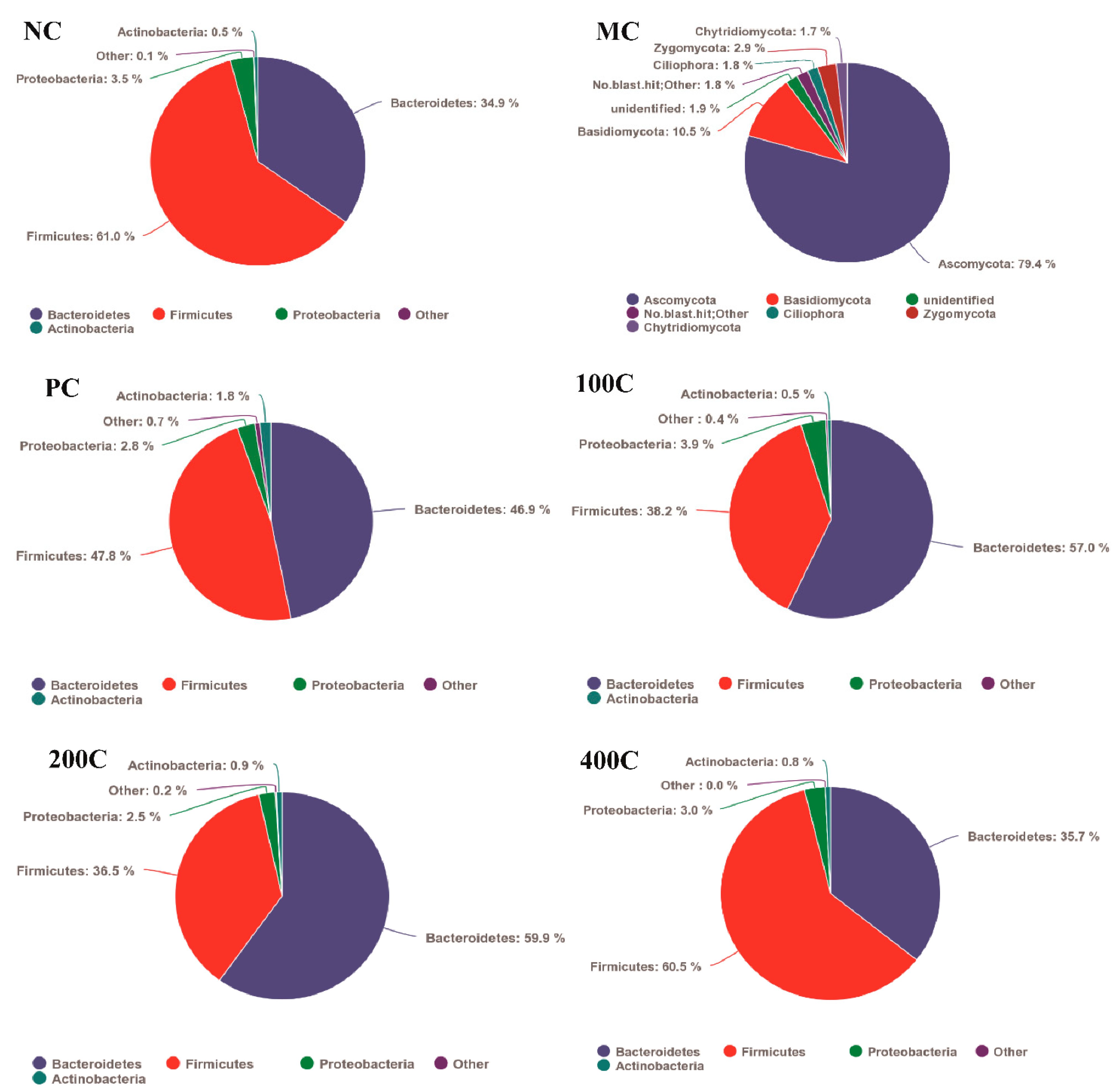
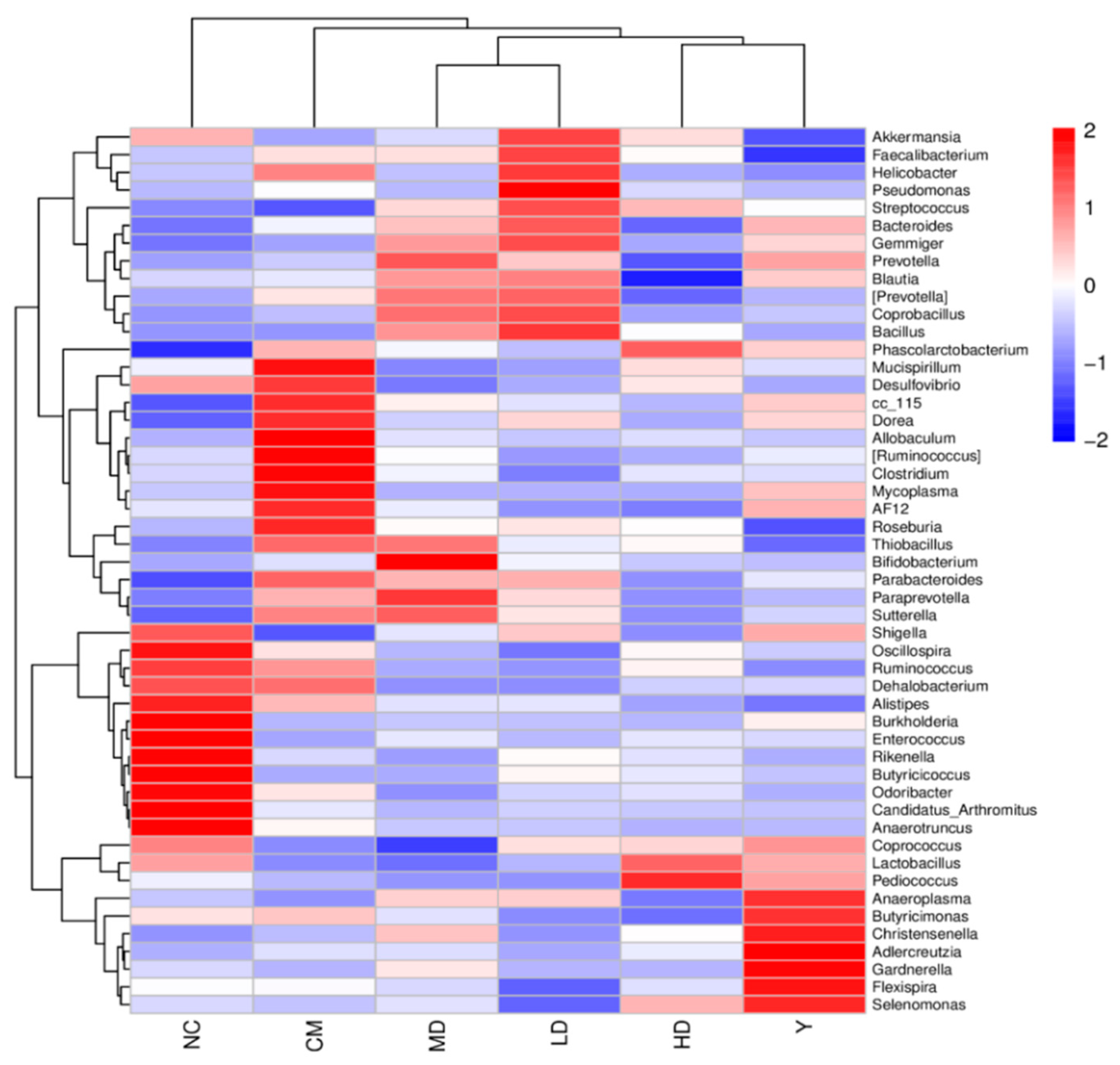
3.2.5. Effect of GFTTPs on Gut Microbiota
3.2.6. Effect of GFTTPs on SCFA Content
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, M.; Zhao, G.; Wang, Y.; Wang, D.; Sun, F.; Ning, J.; Wan, X.; Zhang, J. Safety and anti-hyperglycemic efficacy of various tea types in mice. Sci. Rep. 2016, 6, 31703. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Yang, Z.; He, L.; Lu, X.; Tang, J.; Laghi, L. The Longer the Storage Time, the Higher the Price, the Better the Quality? A 1H-NMR Based Metabolomic Investigation of Aged Ya’an Tibetan Tea (Camellia sinensis). Foods 2022, 11, 2986. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Li, Y.; Li, M.; Wang, Y.; Zhang, L.; Wan, X.; Yang, X. Tea aroma formation from six model manufacturing processes. Food Chem. 2019, 285, 347–354. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.-Z.; Zhou, Y.-B.; Ling, T.-J.; Wan, X.-C. Chinese dark teas: Postfermentation, chemistry and biological activities. Food Res. Int. 2013, 53, 600–607. [Google Scholar] [CrossRef]
- Zheng, Q.; Li, W.; Zhang, H.; Gao, X.; Tan, S. Optimizing synchronous extraction and antioxidant activity evaluation of polyphenols and polysaccharides from Ya’an Tibetan tea (Camellia sinensis). Food Sci. Nutr. 2020, 8, 489–499. [Google Scholar] [CrossRef]
- Xie, H.; Li, X.; Ren, Z.; Qiu, W.; Chen, J.; Jiang, Q.; Chen, B.; Chen, D. Antioxidant and Cytoprotective Effects of Tibetan Tea and Its Phenolic Components. Molecules 2018, 23, 179. [Google Scholar] [CrossRef]
- Li, J.; Wu, J.; Tan, X.; Lin, L.; Guo, C.; Xu, J.; He, C. Protective effects of Ya’an Tibetan tea polysaccharide on mice antioxidant function and the hematopoietic system induced by 60Co-γ ray radiation. J. Nucl. Agric. Sci. 2017, 31, 1509–1514. [Google Scholar]
- Wang, N.; Li, L.; Zhang, P.; Mehmood, M.A.; Lan, C.; Gan, T.; Li, Z.; Zhang, Z.; Xu, K.; Mo, S.; et al. In-silico annotation of the chemical composition of Tibetan tea and its mechanism on antioxidant and lipid-lowering in mice. Nutr. Res. Pract. 2023, 17, 682–697. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shi, M.; Salerno, S.; Tang, M.; Guo, F.; Liu, J.; Feng, Y.; Fu, M.; Huang, Q.; Ma, L.; et al. Microbial and metabolomic remodeling by a formula of Sichuan dark tea improves hyperlipidemia in apoE-deficient mice. PLoS ONE 2019, 14, e0219010. [Google Scholar] [CrossRef]
- Wang, N.; Mo, S.; Wu, T.; Mehmood, M.A.; Sun, H.; Tang, Y.; Mei, J.; Mei, Y.; Fang, W.; Xiao, X.; et al. Metabolomic Analysis of Fermented Tibetan Tea Using Bacillus circulans and Their Biological Activity on Mice via the Intestine–Hepatic Axis. Probiotics Antimicrob. Proteins 2023, 15, 1653–1664. [Google Scholar] [CrossRef]
- Zhao, C.-N.; Tang, G.-Y.; Liu, Q.; Xu, X.-Y.; Cao, S.-Y.; Gan, R.-Y.; Zhang, K.-Y.; Meng, S.-L.; Li, H.-B. Five-Golden-Flowers Tea: Green Extraction and Hepatoprotective Effect against Oxidative Damage. Molecules 2018, 23, 2216. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chen, F.; Liu, J.; Shao, Y.; Wang, X.; Zhou, Y. Genome Mining and Analysis of PKS Genes in Eurotium cristatum E1 Isolated from Fuzhuan Brick Tea. J. Fungi 2022, 8, 193. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Long, R.; Kreuzer, M.; Ding, L.; Shang, Z.; Zhang, Y.; Yang, Y.; Cui, G. Importance of Functional Ingredients in Yak Milk-Derived Food on Health of Tibetan Nomads Living Under High-Altitude Stress: A Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; He, Y.; Quan, B.; Xia, T.; Zhang, X.; Wang, Y.; Zheng, Y.; Wang, M. Physicochemical properties, structure, and ameliorative effects of insoluble dietary fiber from tea on slow transit constipation. Food Chem. X 2022, 14, 100340. [Google Scholar] [CrossRef]
- Qi, B.; Zhang, Y.; Ren, D.; Qin, X.; Wang, N.; Yang, X. Fu Brick Tea Alleviates Constipation via Regulating the Aquaporins-Mediated Water Transport System in Association with Gut Microbiota. J. Agric. Food Chem. 2023, 71, 3862–3875. [Google Scholar] [CrossRef]
- Wang, H.; Chen, J.; Ren, P.; Zhang, Y.; Onyango, S.O. Ultrasound irradiation alters the spatial structure and improves the antioxidant activity of the yellow tea polysaccharide. Ultrason. Sonochemistry 2021, 70, 105355. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Li, M.; Kong, K.; Xie, Y.; Zeng, Z.; Fang, Z.; Li, C.; Hu, B.; Hu, X.; Wang, C.; et al. In vitro simulated digestion of and microbial characteristics in colonic fermentation of polysaccharides from four varieties of Tibetan tea. Food Res. Int. 2023, 163, 112255. [Google Scholar] [CrossRef]
- Liu, Y.; Lei, S.; Hou, R.; Li, D.; Wan, X.; Cai, H.; Chen, G. Tea polysaccharides from Taiping Houkui may serve as a potential candidate for regulation of lipid metabolism: Roles of gut microbiota and metabolite in vitro. J. Funct. Foods 2023, 102, 105469. [Google Scholar] [CrossRef]
- Oh, J.-H.; Chung, J.-O.; Lee, C.-Y.; Yun, Y.; Park, M.-Y.; Hong, Y.-D.; Kim, W.-G.; Cha, H.-Y.; Shin, K.-S.; Hong, G.-P.; et al. Characterized Polysaccharides from Green Tea Inhibited Starch Hydrolysis and Glucose Intestinal Uptake by Inducing Microstructural Changes of Wheat Starch. J. Agric. Food Chem. 2021, 69, 14075–14085. [Google Scholar] [CrossRef]
- Wang, T.; Han, J.; Dai, H.; Sun, J.; Ren, J.; Wang, W.; Qiao, S.; Liu, C.; Sun, L.; Liu, S.; et al. Polysaccharides from Lyophyllum decastes reduce obesity by altering gut microbiota and increasing energy expenditure. Carbohydr. Polym. 2022, 295, 119862. [Google Scholar] [CrossRef]
- Du, L.-L.; Fu, Q.-Y.; Xiang, L.-P.; Zheng, X.-Q.; Lu, J.-L.; Ye, J.-H.; Li, Q.-S.; Polito, C.A.; Liang, Y.-R. Tea Polysaccharides and Their Bioactivities. Molecules 2016, 21, 1449. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Shan, S.; Gao, X.; Shi, Y.; Lu, W. The effect of sweet tea polysaccharide on the physicochemical and structural properties of whey protein isolate gels. Int. J. Biol. Macromol. 2023, 240, 124344. [Google Scholar] [CrossRef]
- Hu, T.; Wu, P.; Zhan, J.; Wang, W.; Shen, J.; Wang, M.; Ho, C.-T.; Li, S. Structure variety and its potential effects on biological activity of tea polysaccharides. Food Sci. Hum. Wellness 2022, 11, 587–597. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Zhang, L.; Xu, M. Construction of self-assembled polyelectrolyte complex hydrogel based on oppositely charged polysaccharides for sustained delivery of green tea polyphenols. Food Chem. 2020, 306, 125632. [Google Scholar] [CrossRef] [PubMed]
- Sa’aedi, A.; Akl, A.A.; Hassanien, A.S. Effective role of Rb doping in controlling the crystallization, crystal imperfections, and microstructural and morphological features of ZnO-NPs synthesized by the sol–gel approach. CrystEngComm 2022, 24, 4661–4678. [Google Scholar] [CrossRef]
- Singh, J.; Vennapusa, J.R.; Chattopadhyay, S. Protein-polysaccharide based microencapsulated phase change material composites for thermal energy storage. Carbohydr. Polym. 2020, 229, 115531. [Google Scholar] [CrossRef]
- Chen, Y.; Xue, Y. Purification, chemical characterization and antioxidant activities of a novel polysaccharide from Auricularia polytricha. Int. J. Biol. Macromol. 2018, 120, 1087–1092. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, N.; Jiang, H.; Li, H.; Cui, X.; Tang, S.; Jin, C.; Tian, J.; Li, B. Comparative study on alleviating effect of kiwi berry (Actinidia arguta) polysaccharide and polyphenol extracts on constipated mice. Food Res. Int. 2022, 162, 112037. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lin, B.; Zhang, Y.; Xie, Z.; Zheng, Y.; Wang, Q.; Xiao, H. Bamboo shavings derived O-acetylated xylan alleviates loperamide-induced constipation in mice. Carbohydr. Polym. 2022, 276, 118761. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, F.; Wang, Z.; Cao, J.; Li, C. Effect and mechanism of functional compound fruit drink on gut microbiota in constipation mice. Food Chem. 2023, 401, 134210. [Google Scholar] [CrossRef]
- Yang, H.; Wu, C.; Chen, L.; Chang, X.; Luo, G.; Wu, K.; Tian, W. A. A. macrocephala polysaccharide induces alterations to gut microbiome and serum metabolome in constipated mice. Microb. Pathog. 2023, 178, 106084. [Google Scholar] [CrossRef]
- Guzel, T.; Mirowska-Guzel, D. The Role of Serotonin Neurotransmission in Gastrointestinal Tract and Pharmacotherapy. Molecules 2022, 27, 1680. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ye, S.; Xu, Z.; Su, H.; Tian, X.; Han, B.; Shen, B.; Liao, Q.; Xie, Z.; Hong, Y. Dietary synbiotic ameliorates constipation through the modulation of gut microbiota and its metabolic function. Food Res. Int. 2021, 147, 110569. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Chen, M.; Chen, Y.; Mao, G.; Liu, S. Expression and clinical significance of 5-HT and 5-HT3R in the intestinal mucosa of patient with diarrhea-type irritable bowel syndrome. Exp. Ther. Med. 2019, 17, 3077–3082. [Google Scholar] [CrossRef] [PubMed]
- Keef, K.D.; Ward, S.M.; Stevens, R.J.; Frey, B.W.; Sanders, K.M. Electrical and mechanical effects of acetylcholine and substance P in subregions of canine colon. Am. J. Physiol.-Gastrointest. Liver Physiol. 1992, 262, G298–G307. [Google Scholar] [CrossRef]
- Ma, T.; Verkman, A.S. Aquaporin water channels in gastrointestinal physiology. J. Physiol. 1999, 517, 317–326. [Google Scholar] [CrossRef]
- Zhu, C.; Chen, Z.; Jiang, Z. Expression, Distribution and Role of Aquaporin Water Channels in Human and Animal Stomach and Intestines. Int. J. Mol. Sci. 2016, 17, 1399. [Google Scholar] [CrossRef]
- Masyuk, A.I.; Marinelli, R.A.; LaRusso, N.F. Water transport by epithelia of the digestive tract. Gastroenterology 2002, 122, 545–562. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shi, Y.; Zeng, S.; Zheng, Y.; Wang, H.; Liao, H.; Song, J.; Zhang, X.; Cao, J.; Li, C. Polysaccharides from Holothuria leucospilota Relieve Loperamide-Induced Constipation Symptoms in Mice. Int. J. Mol. Sci. 2023, 24, 2553. [Google Scholar] [CrossRef]
- Ma, H.; Xiong, H.; Zhu, X.; Ji, C.; Xue, J.; Li, R.; Ge, B.; Cui, H. Polysaccharide from Spirulina platensis ameliorates diphenoxylate-induced constipation symptoms in mice. Int. J. Biol. Macromol. 2019, 133, 1090–1101. [Google Scholar] [CrossRef]
- Wang, J.; Liang, Q.; Zhao, Q.; Tang, Q.; Ahmed, A.F.; Zhang, Y.; Kang, W. The effect of microbial composition and proteomic on improvement of functional constipation by Chrysanthemum morifolium polysaccharide. Food Chem. Toxicol. 2021, 153, 112305. [Google Scholar] [CrossRef]
- Urita, Y.; Goto, M.; Watanabe, T.; Matsuzaki, M.; Gomi, A.; Kano, M.; Miyazaki, K.; Kaneko, H. Continuous consumption of fermented milk containing Bifidobacterium bifidum YIT 10347 improves gastrointestinal and psychological symptoms in patients with functional gastrointestinal disorders. Biosci. Microbiota Food Health 2015, 34, 37–44. [Google Scholar] [CrossRef]
- Coutinho, C.M.L.M.; Coutinho-Silva, R.; Zinkevich, V.; Pearce, C.B.; Ojcius, D.M.; Beech, I. Sulphate-reducing bacteria from ulcerative colitis patients induce apoptosis of gastrointestinal epithelial cells. Microb. Pathog. 2017, 112, 126–134. [Google Scholar] [CrossRef]
- Fu, J.; Li, J.; Sun, Y.; Liu, S.; Song, F.; Liu, Z. In-depth investigation of the mechanisms of Schisandra chinensis polysaccharide mitigating Alzheimer’s disease rat via gut microbiota and feces metabolomics. Int. J. Biol. Macromol. 2023, 232, 123488. [Google Scholar] [CrossRef]
- Dubey, V.; Ghosh, A.R.; Bishayee, K.; Khuda-Bukhsh, A.R. Probiotic Pediococcus pentosaceus strain GS4 alleviates azoxymethane-induced toxicity in mice. Nutr. Res. 2015, 35, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.-F.; Cao, H.; Ma, Y.-L.; Zhang, C.; Ma, F.; Wang, C.-X.; Ni, X.-L.; Lee, W.-J.; Wei, Z.-J. Effect of lactic acid bacteria fermentation on tannins removal in Xuan Mugua fruits. Food Chem. 2019, 274, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Vinolo, M.A.R.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of Inflammation by Short Chain Fatty Acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhong, D.; Sun, R.; Zhang, Y.; Pegg, R.B.; Zhong, G. Prevention of loperamide induced constipation in mice by KGM and the mechanisms of different gastrointestinal tract microbiota regulation. Carbohydr. Polym. 2021, 256, 117418. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Hu, J.; Geng, F.; Nie, S. Bacteroides utilization for dietary polysaccharides and their beneficial effects on gut health. Food Sci. Hum. Wellness 2022, 11, 1101–1110. [Google Scholar] [CrossRef]
- Zhang, C.-Q.; Chen, X.; Ding, K. Structural characterization of a galactan from Dioscorea opposita Thunb. and its bioactivity on selected Bacteroides strains from human gut microbiota. Carbohydr. Polym. 2019, 218, 299–306. [Google Scholar] [CrossRef]






| GFTTP | Content |
|---|---|
| Chemical composition (%) | |
| total sugar | 17.83 ± 0.34 |
| total phenols | 9.24 ± 0.32 |
| total protein | 8.22 ± 0.48 |
| Molecular weight (Da) | |
| Mw | 70,989 |
| Mn | 24,227 |
| Mp | 66,395 |
| Monosaccharide composition (mol, %) | |
| guluronic acid | 2.138 |
| mannuronic acid | 0.054 |
| mannose | 6.786 |
| ribose | 0.486 |
| rhamnose | 4.389 |
| glucuronic acid | 1.086 |
| galacturonic acid | 17.177 |
| glucose | 1.875 |
| galactose | 32.212 |
| arabinose | 32.498 |
| L-fucose | 1.299 |
| SCFAs | NC (μg/g) | MC (μg/g) | PC (μg/g) | 100C (μg/g) | 200C (μg/g) | 400C (μg/g) |
|---|---|---|---|---|---|---|
| Acetic Acid | 1537.655 ± 139.809 a | 916.917 ± 202.936 b | 1329.511 ± 149.902 a | 1239.255 ± 141.321 * | 1348.105 ± 182.197 *# | 1347.185 ± 183.901 *# |
| Propionic Acid | 304.11 ± 66.245 | 198.07 ± 53.606 ** | 352.194 ± 70.846 | 312.844 ± 99.978 | 314.882 ± 178.344 | 403.203 ± 136.307 |
| Isobutyric Acid | 28.106 ± 11.662 | 15.331 ± 4.865 ** | 22.034 ± 7.971 ** | 23.915 ± 4.512 ** | 28.412 ± 10.181 ** | 16.356 ± 5.037 ** |
| Butyric Acid | 477.131 ± 187.69 | 237.539 ± 94.926 | 460.924 ± 205.229 | 352.185 ± 85.688 | 397.063 ± 117.726 | 411.467 ± 141.245 |
| Isovaleric Acid | 25.575 ± 9.303 | 18.428 ± 3.489 * | 17.469 ± 6.622 * | 19.968 ± 3.777 * | 22.088 ± 6.716 * | 14.821 ± 4.209 * |
| Caproic Acid | 1.757 ± 0.592 | 2.374 ± 3.623 | 1.186 ± 0.197 | 1.21 ± 0.169 | 1.128 ± 0.171 | 1.199 ± 0.374 |
| Valeric Acid | 52.772 ± 16.175 | 9.136 ± 4.397 *** | 18.679 ± 5.237 *** | 17.443 ± 5.422 *** | 24.395 ± 7.224 *** | 17.9 ± 17.306 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, M.; Zhao, J.; Xie, Q.; Deng, J.; Zhu, Y.; Chen, J.; Xiang, Z.; Zhang, T.; Liu, G.; Xia, C.; et al. Golden Flower Tibetan Tea Polysaccharides Alleviate Constipation in Mice by Regulating Aquaporins-Mediated Water Transport System and Gut Microbiota. Foods 2024, 13, 2749. https://doi.org/10.3390/foods13172749
Yu M, Zhao J, Xie Q, Deng J, Zhu Y, Chen J, Xiang Z, Zhang T, Liu G, Xia C, et al. Golden Flower Tibetan Tea Polysaccharides Alleviate Constipation in Mice by Regulating Aquaporins-Mediated Water Transport System and Gut Microbiota. Foods. 2024; 13(17):2749. https://doi.org/10.3390/foods13172749
Chicago/Turabian StyleYu, Manyou, Jiayuan Zhao, Qingling Xie, Junlin Deng, Yongqing Zhu, Jian Chen, Zhuoya Xiang, Ting Zhang, Gang Liu, Chen Xia, and et al. 2024. "Golden Flower Tibetan Tea Polysaccharides Alleviate Constipation in Mice by Regulating Aquaporins-Mediated Water Transport System and Gut Microbiota" Foods 13, no. 17: 2749. https://doi.org/10.3390/foods13172749
APA StyleYu, M., Zhao, J., Xie, Q., Deng, J., Zhu, Y., Chen, J., Xiang, Z., Zhang, T., Liu, G., Xia, C., Shi, L., Wu, B., Gouvinhas, I., & Barros, A. N. (2024). Golden Flower Tibetan Tea Polysaccharides Alleviate Constipation in Mice by Regulating Aquaporins-Mediated Water Transport System and Gut Microbiota. Foods, 13(17), 2749. https://doi.org/10.3390/foods13172749









