A Comprehensive Review on the Isolation, Bioactivities, and Structure–Activity Relationship of Hawthorn Pectin and Its Derived Oligosaccharides
Abstract
1. Introduction
2. Extraction Methods
2.1. Solvent Extraction Method
2.2. Physical Extraction Method
2.3. Enzymatic Hydrolysis Extraction Method
2.4. Combined Extraction Method
| Methods | Principles | Materials | Extraction Conditions | Extraction Rate | Physicochemical Indexes | References |
|---|---|---|---|---|---|---|
| Hot water extraction | Effectively disrupts plant cell walls and promotes chemical component release | hawthorn pectin | solid–liquid ratio of 1:10, temperature of 95 °C, time of 4 h | 8.7% | total sugar 91.7%, GalA 63.4%, protein 4.9%, methoxyl 2.5% | [23,27] |
| hawthorn pectin | solid–liquid ratio of 1:20, temperature of 90 °C, time of 3 h | 10.1%~13.7% | total sugar 47.8~76.5%, GalA 41.5~60.1%, DE 81.5~83.1% | [28] | ||
| hawthorn pectin | solid–liquid ratio of 1:20, temperature of 90 °C, time of 2 h | 9.14%~14.48% | protein 2.56~2.69%, GalA 69.1~93.3%, DE 30.9~76.7% | [29,31] | ||
| hawthorn pectin | solid–liquid ratio of 1:10, temperature of 90 °C, time of 4 h | - | GalA 81.72%, DE 29.34% | [30] | ||
| hawthorn pectin | solid–liquid ratio of 1:20, temperature of 70 °C, time of 3 h | - | GalA 28.95%, DE 32.52% | [32] | ||
| hawthorn pectin | solid–liquid ratio of 1:15, temperature of 90 °C, time of 2 h | 0.11%~3.29% | protein 1.27~1.68%, GalA 31.83~73.09%, DE 23.08~62.21% | [33] | ||
| Acid extraction | Polysaccharides containing acidic groups can be treated with acid and then precipitated with ethanol or insoluble complexes | hawthorn pectin | citric acid (4% w/v), solid–liquid ratio of 1:10, temperature of 85 °C, time of 2 h | 19.8%~20.9% | protein 2.8~3.5%, GalA 86.0~86.7%, DE 78.1~78.2% | [34] |
| hawthorn pectin | citric acid/lemon juice (7% v/v), solid–liquid ratio of 1:10, temperature of 60/90 °C, time of 2 h | 16.75%/7.32% | anhydrouronic acid 122.90%/86.21%, DE 54.16%/53.34%, acetyl 0.68%/0.76% | [35] | ||
| hawthorn pectin | citric acid (2 mol/L), solid–liquid ratio of 1:30, temperature of 85 °C, time of 2 h | - | protein 0.73~2.11%, GalA 82.43~90.27%, DE 37.67~39.80% | [36] | ||
| hawthorn pectin | hydrochloric acid/citric acid (2 mol/L), solid–liquid ratio of 1:6, temperature of 85 °C, time of 2 h | 61.05%/67.81% | protein 0.11%/0.12%, GalA 67.21%/72.24%, DE 37.95%/25.50% | [37] | ||
| hawthorn pectin | hydrochloric/sulfuric/nitric/phosphoric acids, solid–liquid ratio of 1:40, temperature of 80 °C, time of 80 min | 4.25%/4.40%/ 2.40%/3.80% | - | [38] | ||
| hawthorn pectin | sulfuric acid (1 mol/L), solid–liquid ratio of 1:10, temperature of 85 °C, time of 25 min | 0.71%~4.11% | total sugar 19.05~50.20%, GalA 51.26~55.47% | [39] | ||
| hawthorn pectin | hydrochloric acid (1.5 mol/L), solid–liquid ratio of 1:15, temperature of 70 °C, time of 40 min | 3.07% | GalA 78.06%, DE 63.46% | [40] | ||
| Ultrasound extraction | Accelerate the release, diffusion, and dissolution of effective substances in the cell | hawthorn pectin | ultrasound (20 kHz, 130 W), solid–liquid ratio of 1:6, temperature of 85 °C, time of 10 min | - | GalA 70.85~79.91%, DE 13.88~24.66% | [41] |
| hawthorn pectin | ultrasound (500 W), solid–liquid ratio of 1:15, temperature of 80 °C, time of 10 min | 6.0% | total sugar 85.0%, GalA 75.1%, DE 86.2% | [42] | ||
| hawthorn pectin | ultrasound (425 W), solid–liquid ratio of 1:15, temperature of 74 °C, time of 55 min | 6.15% | - | [43] | ||
| Microwave extraction | High-frequency electromagnetic waves penetrate the extraction medium, causing the cell to rupture and the active ingredient to dissolve | hawthorn pectin | microwave (440 W), solid–liquid ratio of 1:9, time of 80 s | 72.89% | protein 0.03%, GalA 68.94%, DE 31.65% | [37] |
| hawthorn pectin | microwave (700 W), solid–liquid ratio of 1:30, time of 120 s | 5.7% | - | [38] | ||
| hawthorn pectin | microwave (700 W, 2450 Hz), solid–liquid ratio of 1:124, time of 2.11 min | 6.59% | - | [44] | ||
| hawthorn pectin | microwave (440 W), solid–liquid ratio of 1:45, time of 80 s | - | total neutral sugars 4.73%, GalA 58.32%, DE 42.96% | [45] | ||
| Enzymatic hydrolysis extraction | Cell wall components were hydrolyzed, causing a reduction in the resistance to mass transfer of active ingredients from the extracellular medium | hawthorn pectin | cellulase (80 U/g), solid–liquid ratio of 1:8, temperature of 60 °C, time of 4 h | 62.29% | protein 0.24%, GalA 65.93%, DE 8.84% | [37] |
| hawthorn pectin | xylanase (70 U/g), solid–liquid ratio of 1:15, temperature of 50.5 °C, time of 3 h | 16.8% | - | [42] | ||
| hawthorn pectin | pectinase (0.9% w/v), solid–liquid ratio of 1:10, temperature of 48 °C, time of 4 h | - | - | [46] | ||
| pectin oligosacchari-des from hawthorn | endo-polygalacturonase containing pectinesterase from Aspergillus niger (0.2 U/mL), temperature of 50 °C, time of 2 h | 4.9–13.3% | total sugar 98.4~99.3%, uronic acid 97.1~99.7%, DP 2–11 | [26] | ||
| pectin oligosacchari-des from hawthorn | pectinase (0.2 U/mL), temperature of 45 °C, time of 3 h | - | total sugar 99.7%, uronic acid 93.6%, MW 200~6000 | [48] | ||
| pectin oligosacchari-des from hawthorn | endo-polygalacturonase (0.2 U/mL), temperature of 50 °C, time of 3 h | 11.4% | uronic acid 99.2%, DP 5, molecular weight 898 | [49,50,51] | ||
| Combined extraction | In order to improve the extraction rate or obtain hawthorn pectin with high purity and low molecular weight, enzyme, physical, and chemical methods are often used in combination | hawthorn pectin | hydrochloric acid (1.5 mol/L); ultrasound, solid–liquid ratio of 1:15, temperature of 70 °C, time of 40 min | 3.32% | GalA 83.66%, DE 65.51% | [40] |
| hawthorn pectin | hydrochloric acid (1 mol/L); ultrasound (240 W), solid–liquid ratio of 1:40, temperature of 80 °C, time of 1 h | - | total neutral sugars 6.17%, GalA 80.00%, DE 51.96% | [45] | ||
| hawthorn pectin | acetic acid (0.2 mol/L); pectinase (12 U/mL), solid–liquid ratio of 1:100, temperature of 40 °C, time of 4 h | - | GalA 77.81~82.81% | [52] | ||
| hawthorn pectin | hydrochloric acid (0.05 mol/L); microwave (800 W), solid–liquid ratio of 1:15, time of 50 s | 5.87% | - | [53] | ||
| hawthorn pectin | ultrasound (288 W); pectinesterase activator (12.28%), solid–liquid ratio of 1:26, temperature of 80 °C, time of 119 min | 25.84% | GalA 66.78%, DE 18.34% | [54] | ||
| hawthorn pectin | hawthorn juice (1:7 g/mL), high-pressure (300/600 MPa), temperature of 24/33 °C, time of 2/6 min | 37.4%~40.3% | GalA 43.85~45.17%, DE 34.56~39.51% | [57] | ||
| pectin oligosacchari-des from hawthorn | endo-polygalacturonase (0.2 U/mL), temperature of 50 °C, time of 3 h; ultrasound power of 800 W, frequency of 20 kHz, time of 20 min | low, medium, and high molecular weights were 12.6%, 80.9%, and 6.9%, respectively | carbohydrate 99.4~99.7%, uronic acid 56.6~86.3% | [58] |
3. Functional Activities
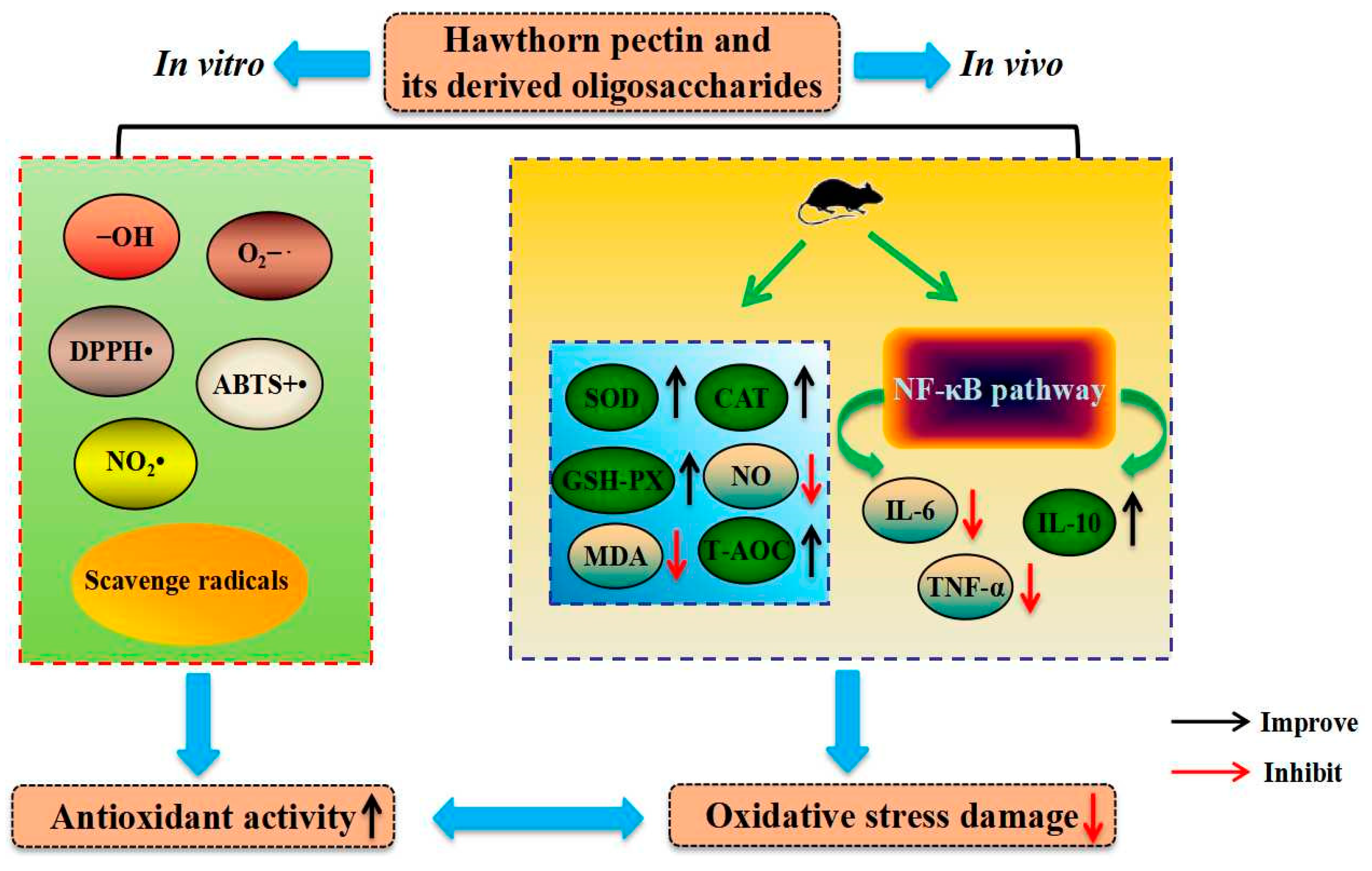
3.1. Improvement of Antiglycation and Antioxidant Properties
3.2. Regulation of Lipid Metabolism
3.3. Presence of Antimicrobial Activity
3.4. Other Functions
| Functional Properties | Materials | Testing Subjects | Result/Mechanism | References |
|---|---|---|---|---|
| Antiglycation and antioxidant properties | hawthorn pectin | in vitro | scavenge radicals and increase inhibition rate of glycosylation | [37,61,63,64] |
| hawthorn pectin | in vivo, 50/150/300 mg/kg·bw for 10 weeks, Kunming mice | increase the activities of antioxidant enzymes, as well as reduce content of malondialdehyde in liver | [65] | |
| pectin oligosaccharides from hawthorn | in vitro; in vivo, 50/150/300 mg/kg·bw for 6 weeks, Kunming mice | scavenge radicals and increase antioxidant enzyme activities | [13] | |
| pectin oligosaccharides from hawthorn | in vivo, 50/150/300 mg/kg·bw for 6 weeks, Kunming mice | increase antioxidant enzyme activities | [48] | |
| pectin oligosaccharides from hawthorn | in vitro | inhibit the formation of advanced glycation end-products | [58,59] | |
| Regulation of lipid metabolism | hawthorn pectin | in vitro | possesses a good inhibitory effect on lipid digestion | [70] |
| hawthorn pectin | in vitro | wrap cholesterol micelles to form complexes that accelerate the consumption of cholesterol | [72] | |
| hawthorn pectin | in vivo, 250 mg/kg·bw for 6 weeks, Sprague Dawley rats | enhance the excretion of cholesterol and bile acid and restore the imbalance of intestinal microbiota | [73] | |
| pectin pentasaccharide from hawthorn | in vivo, 50/150/300 mg/kg·bw, 4/10 weeks, Kunming mice | increase the content of serum high-density lipoprotein cholesterol, fecal bile acids, and the gene expressions of cholesterol 7α-hydroxylase | [49] | |
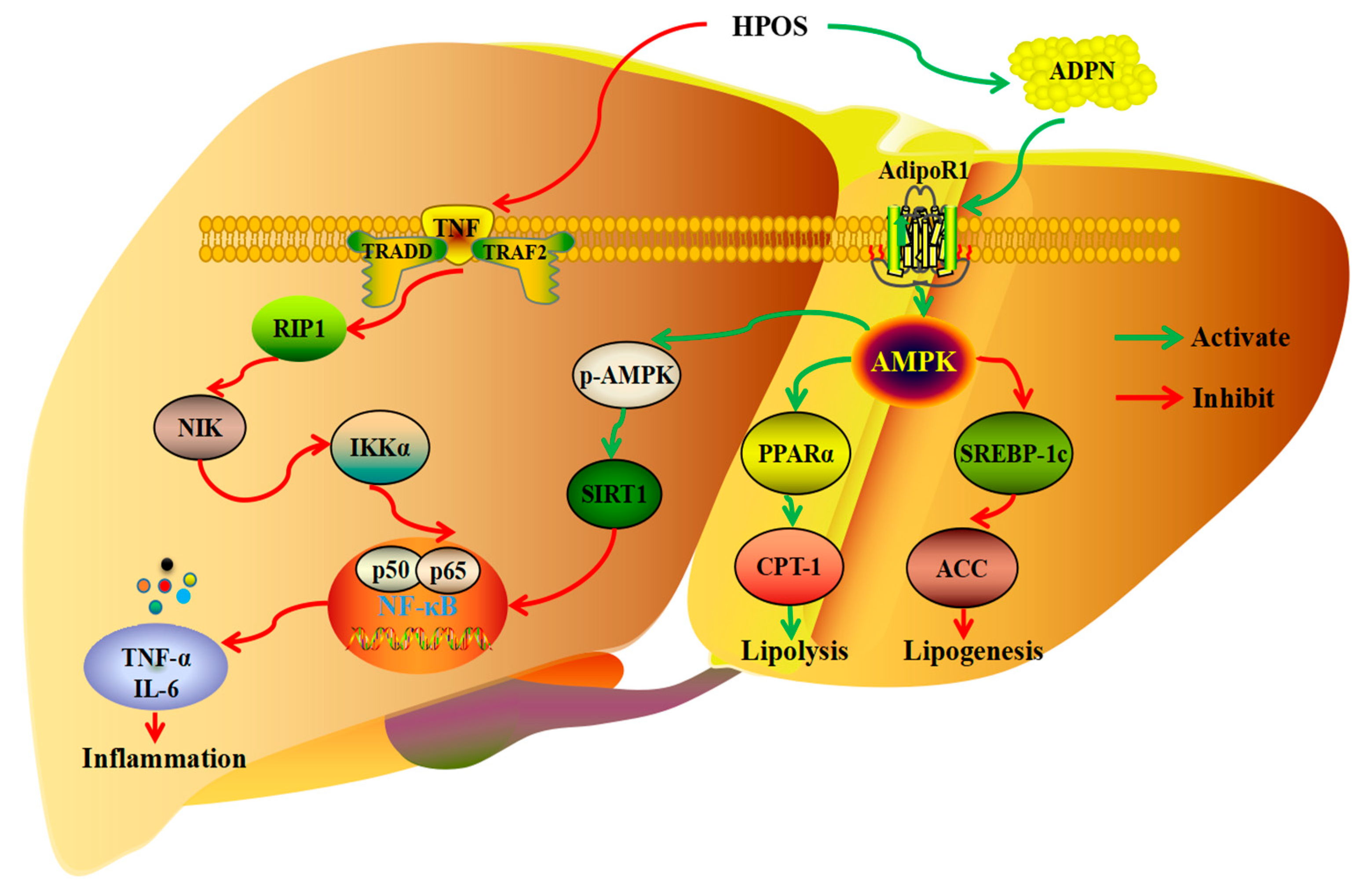
| pectin oligosaccharides from hawthorn | in vivo, 0.25/0.75/1.5 g/kg·bw for 10 weeks, Kunming mice | ameliorate hepatic inflammation via NF-κB inactivation | [50] | |
| pectin oligogalacturonide from hawthorn | in vivo, 0.25/0.75/1.5 g/kg·bw for 10 weeks, Kunming mice | facilitate the synthesis and activation of adiponectin to improve hepatic lipid oxidation | [51] | |
| hawthorn pectin and its hydrolyzates | in vivo, 300 mg/kg·bw for 4 weeks, hamsters | prevent high-fat diet-induced hypercholesterolemia, improve hepatic lipid accumulation, and promote fecal bile acid excretion | [71] | |
| pectin oligosaccharides from hawthorn | in vivo, 0.25/0.75/1.5 g/kg·bw for 10 weeks, Kunming mice | activate adiponectin-mediated AdipoR1/AMPK/PPARα signaling path in white adipose tissue | [74] | |
| pectin pentaglaracturonide from hawthorn | in vivo, 50/150/300 mg/kg·bw, 4/10 weeks, Kunming mice | inhibit fatty acid synthesis and improve insulin sensitivity | [75] | |
| pectin penta-oligogalacturonide from hawthorn | in vivo, 300 mg/kg·bw for 4 weeks, Kunming mice | suppress intestinal bile acids absorption and downregulate the FXR-FGF15 axis | [76] | |
| pectin pentaoligosaccharide from hawthorn | in vivo, 150 mg/kg·bw, 10 weeks, Kunming mice | increase the hepatic fatty acid oxidation-related enzyme activities and mRNA levels | [77] | |
| Antimicrobial activity | pectin oligosaccharides from hawthorn | in vitro | inhibit bacterial growth of Escherichia coli, Bacillus subtilis, and Staphylococcus aureus | [79,80] |
| pectin oligosaccharides from hawthorn | in vitro | maintain the quality characteristics of shiitake mushrooms by inhibiting the growth of mold | [81] | |
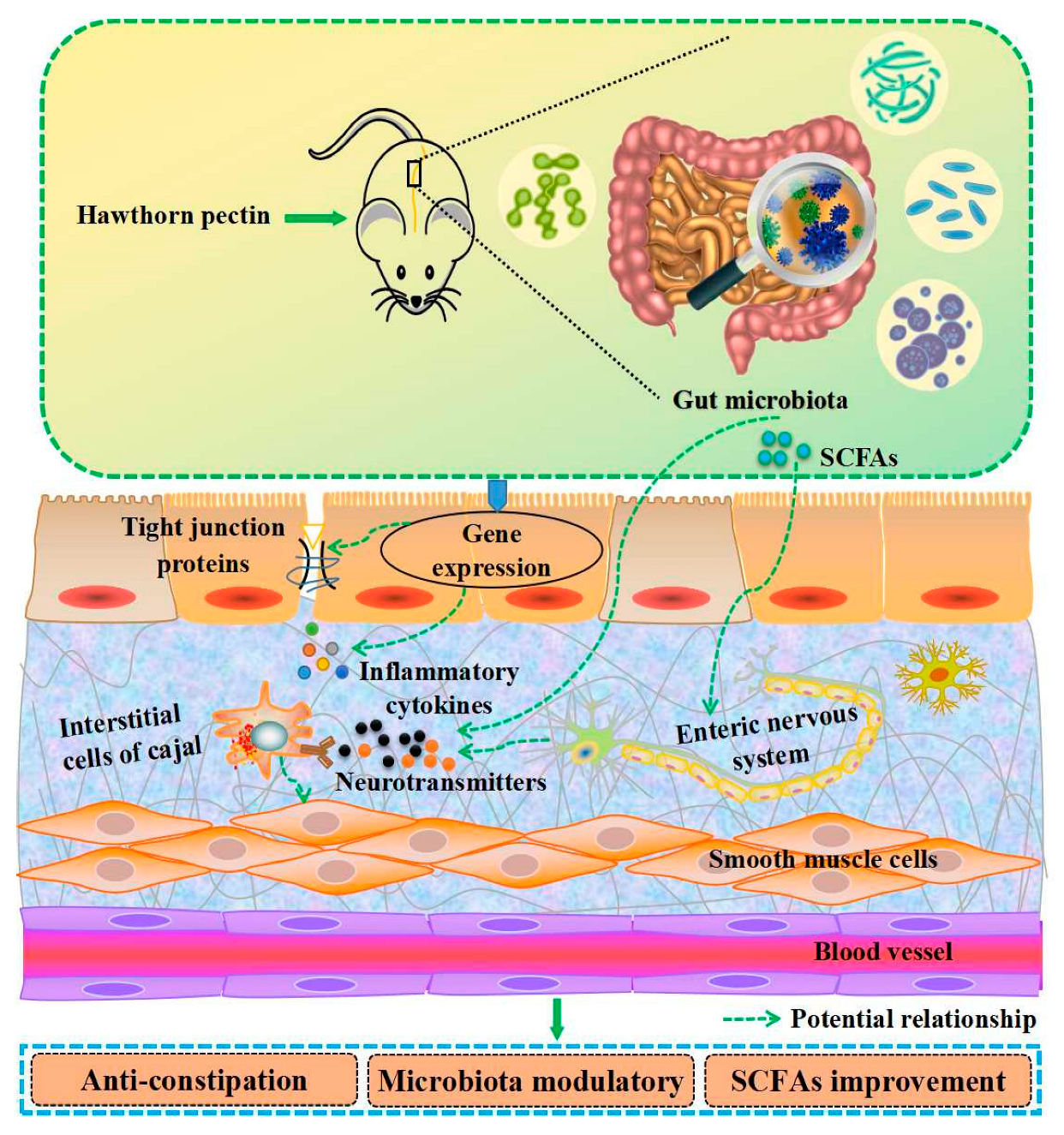
| Improvement of intestinal function | hawthorn pectin | in vivo, 250/500/1000 mg/kg·bw for 40 d, ICR mice | promote the rate of small intestinal propulsion and increase the volume of defecation in constipated mice | [84] |
| hawthorn pectin | in vivo, 1/2/4 g/kg·bw for 6 weeks, Kunming mice | promote the growth of probiotic bacteria and accumulation of short-chain fatty acids | [85] | |
| hawthorn pectin | in vitro | increase the relative abundance of Bacteroides and Faecalibacterium | [86,87] | |
| Anti-fatigue activity | hawthorn pectin | in vivo, 1/2/4 g/kg·bw for 6 weeks, Kunming mice | prolong the time of normobaric hypoxia tolerance and increase the time of weight-bearing swimming | [88] |
| Immunomodulatory activity | hawthorn pectin | in vitro | promote the proliferation of spleen lymphocytes | [90] |
| Anti-cancer activity | hawthorn pectin | in vitro | inhibit proliferation of HCT116 cells | [92] |
| Anti-photoaging activity | hawthorn pectin | in vitro | protect against oxidative damage and photoaging induced by ultraviolet B | [94] |
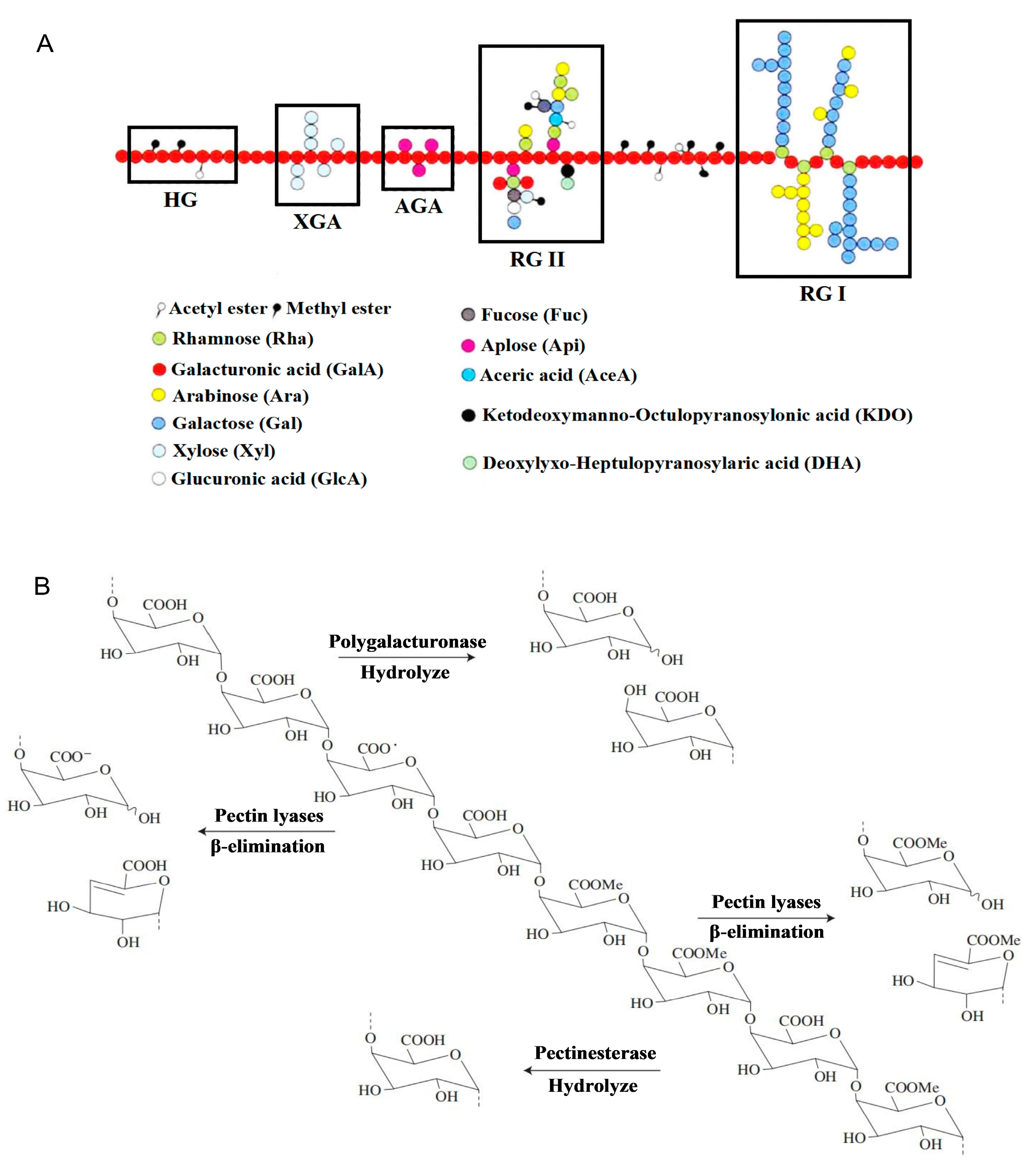
4. Structure–Activity Relationship
5. Processing and Application
5.1. Emulsifiers and Gelling Agents
5.2. Food Packaging Film
5.3. Modification
5.4. Current Products
5.4.1. Traditional and Recent Food Applications
5.4.2. Nutraceutical and Medicinal Applications
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, Y.; Meng, X.W.; Chen, M.J.; Li, D.; Rui, L.R.; Sun, T.D. Isolation, structural properties and bioactivities of polysaccharides from Crataegus pinnatifida. J. Ethnopharmacol. 2024, 323, 117688. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Lv, Z.; Huang, Z.; Ye, N.; Li, S.; Jiang, J.; Cheng, Y.; Shi, F. Dietary hawthorn-leaves flavonoids improve ovarian function and liver lipid metabolism in aged breeder hens. Poultry Sci. 2021, 100, 101499. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.F.; Wang, S.W.; Shu, L.M.; Cao, Q.Y.; Hu, H.M.; Zhu, Y.C.; Chen, C. Hawthorn leaf flavonoids alleviate the deterioration of atherosclerosis by inhibiting SCAP-SREBP2-LDLR pathway through sPLA2-IIA signaling in macrophages in mice. J. Ethnopharmacol. 2024, 327, 118006. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.W.; Yu, J.C.; Fu, M.F.; Wang, X.F.; Chang, X.D. Regulatory effects of hawthorn polyphenols on hyperglycemic, inflammatory, insulin resistance responses, and alleviation of aortic injury in type 2 diabetic rats. Food Res. Int. 2021, 142, 110239. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Li, X.; Fan, Y.; Zhao, C.C.; Mao, X.H.; Chen, X.T.; Huang, H.H.; Gao, W.Y. Effect of different solvents on the chemical composition, antioxidant activity and alpha-glucosidase inhibitory activity of hawthorn extracts. Int. J. Food Sci. Technol. 2016, 51, 1244–1251. [Google Scholar] [CrossRef]
- Martinelli, F.; Perrone, A.; Yousefi, S.; Papini, A.; Castiglione, S.; Guarino, F.; Cicatelli, A.; Aelaei, M.; Arad, N.; Gholami, M.; et al. Botanical, phytochemical, anti-microbial and pharmaceutical characteristics of hawthorn (Crataegusmonogyna Jacq.), rosaceae. Molecules 2021, 26, 7266. [Google Scholar] [CrossRef]
- Yu, Z.M. The effects of pectin on hawthorn processing. Hebei J. Forest. Orch. Res. 2019, 24, 309–310. (In Chinese) [Google Scholar]
- Cui, J.F.; Zhao, C.Y.; Feng, L.P.; Han, Y.H.; Du, H.J.; Xiao, H.; Zheng, J.K. Pectins from fruits: Relationships between extraction methods, structural characteristics, and functional properties. Trends Food Sci. Technol. 2021, 110, 39–54. [Google Scholar] [CrossRef]
- Marić, M.; Grassino, A.N.; Zhu, Z.; Barba, F.J.; Brnčić, M.; Brnčić, S.R. An overview of the traditional and innovative approaches for pectin extraction from plant food wastes and by-products: Ultrasound-, microwaves-, and enzyme-assisted extraction. Trends Food Sci. Technol. 2018, 76, 28–37. [Google Scholar] [CrossRef]
- Christiaens, S.; Van Buggenhout, S.; Houben, K.; Kermani, Z.J.; Moelants, K.R.N.; Ngouémazong, E.D.; Loey, A.V.; Hendrickx, M.E.G. Process-structure-function relations of pectin in food. Crit. Rev. Food Sci. 2016, 56, 1021–1042. [Google Scholar] [CrossRef]
- Abari, A.H.; Rourani, H.A.; Ghasemi, S.M.; Kim, H.; Kim, Y.G. Investigation of antioxidant and anticancer activities of unsaturated oligo-galacturonic acids produced by pectinase of Streptomyces hydrogenans YAM1. Sci. Rep. 2021, 11, 8491. [Google Scholar]
- Wang, Z.Y.; Xu, B.; Luo, H.Y.; Meng, K.; Wang, Y.; Liu, M.T.; Bai, Y.G.; Yao, B.; Tu, T. Production pectin oligosaccharides using Humicola insolens Y1-derived unusual pectate lyase. J. Biosci. Bioeng. 2020, 129, 16–22. [Google Scholar] [CrossRef]
- Li, T.P.; Li, S.H.; Dong, Y.P.; Zhu, R.G.; Liu, Y.H. Antioxidant activity of penta oligogalacturonide, isolated from haw pectin, suppresses triglyceride synthesis in mice fed with a high-fat diet. Food Chem. 2014, 145, 335–341. [Google Scholar] [CrossRef]
- Li, H.Y.; Li, Z.; Wang, P.W.; Liu, Z.; An, L.Z.; Zhang, X.M.; Xie, Z.Y.; Wang, Y.P.; Li, X.; Gao, W.Y. Evaluation of citrus pectin extraction methods: Synergistic enhancement of pectin’s antioxidant capacity and gel properties through combined use of organic acids, ultrasonication, and microwaves. Int. J. Biol. Macromol. 2024, 266, 131164. [Google Scholar] [CrossRef]
- Ma, X.B.; Wang, D.L.; Chen, W.J.; Ismail, B.B.; Wang, W.J.; Lv, R.L.; Ding, T.; Ye, X.Q.; Liu, D.H. Effects of ultrasound pretreatment on the enzymolysis of pectin: Kinetic study, structural characteristics and anti-cancer activity of the hydrolysates. Food Hydrocolloid. 2018, 79, 90–99. [Google Scholar] [CrossRef]
- Sun, Y.J.; Hou, S.T.; Song, S.; Zhang, B.; Ai, C.Q.; Chen, X.F.; Liu, N. Impact of acidic, water and alkaline extraction on structural features, antioxidant activities of Laminaria japonica polysaccharides. Int. J. Biol. Macromol. 2018, 112, 985–995. [Google Scholar] [CrossRef]
- Li, L.; Gao, X.L.; Liu, J.G.; Chitrakar, B.; Wang, B.; Wang, Y.C. Hawthorn pectin: Extraction, function and utilization. Cur. Res. Food Sci. 2021, 4, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Celis, M.E.; Yahia, E.M.; Bedoya, R.; Landázuri, P.; Loango, N.; Aguillón, J.; Restrepo, B.; Ospina, J.C.G. Chemical composition of Mango (Mangifera indica L.) fruit: Nutritional and phytochemical compounds. Front. Plant Sci. 2019, 10, 1073. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Kumar, M.; Rais, N.; Puri, S.; Sharma, K.; Natta, S.; Dhumal, S.; Damale, R.D.; Kumar, S.; Senapathy, M.; et al. Exploring apple pectic polysaccharides: Extraction, characterization, and biological activities-A comprehensive review. Int. J. Biol. Macromol. 2024, 255, 128011. [Google Scholar] [CrossRef]
- Podetti, C.; Riveros-Gomez, M.; Román, M.C.; Zalazar-García, D.; Fabani, M.P.; Mazza, G.; Rodríguez, R. Polyphenol-enriched pectin from pomegranate peel: Multi-objective optimization of the eco-friendly extraction process. Molecules 2023, 28, 7656. [Google Scholar] [CrossRef]
- Chandel, V.; Biswas, D.; Roy, S.; Vaidya, D.; Verma, A.; Gupta, A. Current advancements in pectin: Extraction, properties and multifunctional applications. Foods 2022, 11, 2683. [Google Scholar] [CrossRef] [PubMed]
- Linares-García, J.A.; Ramos-Ramírez, E.G.; Salazar-Montoya, J.A. Viscoelastic properties and textural characterisation of high methoxyl pectin of hawthorn (Crataegus pubescens) in a gelling system. Int. J. Food Sci. Technol. 2015, 50, 1484–1493. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, C.Y.; Qi, Y.D.; Li, T.P. Extraction and food chemical characterizations of haw pectins. Sci. Technol. Food Ind. 2007, 28, 87–89. (In Chinese) [Google Scholar]
- Granato, D.; Barba, F.J.; Kovačević, D.B.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Functional foods: Product development, technological trends, efficacy testing, and safety. Annu. Rev. Food Sci. Technol. 2020, 11, 93–118. [Google Scholar] [CrossRef]
- Shafie, M.H.; Gan, C.Y. Could choline chloride-citric acid monohydrate molar ratio in deep eutectic solvent affect structural, functional and antioxidant properties of pectin? Int. J. Biol. Macromol. 2020, 149, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H.; Li, T.P.; Jia, Y.F.; Zhu, R.G.; Wang, N.; Jin, S.; Guo, M. Fractionation and structural characterization of haw pectin oligosaccharides. Eur. Food Res. Technol. 2011, 233, 731–734. [Google Scholar] [CrossRef]
- Wang, N.; Li, J.; Jin, S.; Li, T.P. The conditions for the production of pectic oligosaccharides from haw pectin. Food Res. Dev. 2008, 29, 1–4. (In Chinese) [Google Scholar]
- Fang, Y.; Cui, N.; Dai, Y.Y.; Liu, S.W.; Chang, X.D. Effect of preparation methods on physicochemical properties of hawthorn pectin. Food Sci. 2021, 42, 130–136. (In Chinese) [Google Scholar]
- Li, Z.X.; Zhang, J.R.; Zhang, H.; Liu, Y.; Zhu, C.H. Effect of different processing methods of hawthorn on the properties and emulsification performance of hawthorn pectin. Carbohydr. Polym. 2022, 298, 120121. [Google Scholar] [CrossRef]
- Bu, K.X.; Wu, S.; Zhu, C.H.; Wei, M. Comparative study of HG-type low-ester hawthorn pectin as a promising material for the preparation of hydrogel. Carbohydr. Polym. 2022, 296, 119941. [Google Scholar] [CrossRef]
- Jiang, Y.; Du, J.H.; Zhang, L.G.; Li, W.Q. Properties of pectin extracted from fermented and steeped hawthorn wine pomace: A comparison. Carbohydr. Polym. 2018, 197, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.G.; Wang, C.Y.; Zhang, L.J.; Wang, Y.; Chen, G.; Fan, J.G.; Jia, Y.F.; Yan, F.W.; Ning, C. Pectin oligosaccharides from fruit of Actinidia arguta: Structure-activity relationship of prebiotic and antiglycation potentials. Carbohydr. Polym. 2019, 217, 90–97. [Google Scholar] [CrossRef]
- Li, Z.X.; Zhang, X.Y.; Zhu, C.H. Physicochemical properties and Pb2+ adsorption capacity of freeze-dried hawthorn pectin fractions by gradient ethanol precipitation. Int. J. Biol. Macromol. 2023, 245, 125581. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Bernardino, J.C.; Lobato-Calleros, C.; Román-Guerrero, A.; Alvarez-Ramirez, J.; Vernon-Carter, E.J. Physicochemical characterisation of hawthorn pectins and their performing in stabilising oil-in-water emulsions. React. Funct. Polym. 2016, 103, 63–71. [Google Scholar] [CrossRef]
- Uysal, S.Y.; Yildirim, E. Extraction and characterization of pectin from red hawthorn (Crataegus spp.) using citric acid and lemon juice. Asian J. Chem. 2014, 26, 6674–6678. [Google Scholar] [CrossRef]
- Wang, H.Y.; Zhu, Y.W.; Li, D.; Zhu, C.H. Characterization of hawthorn pectin gained via different ethanol concentrations. Food Sci. Nutr. 2023, 11, 2663–2676. [Google Scholar] [CrossRef]
- Sun, D.Y.; Chen, X.W.; Zhu, C.H. Physicochemical properties and antioxidant activity of pectin from hawthorn wine pomace: A comparison of different extraction methods. Int. J. Biol. Macromol. 2020, 158, 1239–1247. [Google Scholar] [CrossRef]
- Liu, X.L.; Jiang, S.J. Study on extraction technology of hawthorn pectin. Heilongjiang Agr. Sci. 2014, 10, 107–111. (In Chinese) [Google Scholar]
- Wu, Z.Y.; Liu, S.W.; Cui, L.X.; Chang, X.D. The physicochemical properties and antioxidation of hawthorn pectin by hydrochloric acid extraction. Sci. Technol. Food Ind. 2018, 39, 1–5. (In Chinese) [Google Scholar]
- Fu, M.L.; Sun, X.J.; Fei, C.X.; Li, D.D.; Zhang, D.; Tuo, X.Q.; Gao, S.; Han, X.; Xiu, J.H.; Li, Y. Optimization and characterization of pectin extracted from hawthorn by deep eutectic solvent. Int. J. Biol. Macromol. 2024, 256, 128688. [Google Scholar] [CrossRef]
- Chen, X.W.; Qi, Y.J.; Zhu, C.H.; Wang, Q. Effect of ultrasound on the properties and antioxidant activity of hawthorn pectin. Int. J. Biol. Macromol. 2019, 131, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.T.; Su, J.F.; Chen, S.Y.; Hu, F.Q.; Zhu, R.G. Effects of different extraction methods on physicochemical properties and anti-glycation activity of pectin extracted from hawthorn. Mod. Food Sci. Technol. 2018, 34, 159–166. (In Chinese) [Google Scholar]
- Wang, B.; Yao, L.G.; Lu, Y.F. Ultrasonic-assisted extraction process optimization and antioxidant activity of pectin from hawthorn peel dregs. Sci. Technol. Cereals Oils Foods 2023, 31, 78–86. (In Chinese) [Google Scholar]
- Wang, W.D.; Li, H.X.; Na, D.C. Optimization of the microwave assisted extraction process of pectin from Crataegus pinnatifida by response surface methodology. J. Anhui Agri. Sci. 2013, 41, 10405–10407. (In Chinese) [Google Scholar]
- Qin, C.G.; Yang, G.C.; Zhu, C.H.; Wei, M. Characterization of edible film fabricated with HG-type hawthorn pectin gained using different extraction methods. Carbohydr. Polym. 2022, 285, 119270. [Google Scholar] [CrossRef]
- Feng, J.W.; Wang, Y.; Wang, F.Y.; Liu, X.; Zhang, Y.X.; Ping, Y. Enzymolysis optimization of pectin degradation in hawthorn by pectinase. Food Ind. 2018, 39, 1–4. (In Chinese) [Google Scholar]
- Hou, Y.T.; Zhang, X.Y.; Su, J.F.; Fan, J.G.; Chen, G.; Hu, F.Q.; Zhu, R.G. Optimization of enzymatic-assisted extraction of haw pectin by response surface methodology and its anti-oxidation/glycation activities in vitro. Sci. Technol. Food Ind. 2018, 39, 180–186. (In Chinese) [Google Scholar]
- Li, T.P.; Li, S.H.; Du, L.J.; Wang, N.; Guo, M.; Zhang, J.W.; Yan, F.W.; Zhang, H.L. Effects of haw pectic oligosaccharide on lipid metabolism and oxidative stress in experimental hyperlipidemia mice induced by high-fat diet. Food Chem. 2010, 121, 1010–1013. [Google Scholar] [CrossRef]
- Zhu, R.G.; Li, T.P.; Dong, Y.P.; Liu, Y.H.; Li, S.H.; Chen, G.; Zhao, Z.S.; Jia, Y.F. Pectin pentasaccharide from hawthorn (Crataegus pinnatifida Bunge. Var. major) ameliorates disorders of cholesterol metabolism in high-fat diet fed mice. Food Res. Int. 2013, 54, 262–268. [Google Scholar] [CrossRef]
- Li, T.P.; Chen, X.J.; Huang, Z.; Xie, W.Y.; Tong, C.N.; Bao, R.W.; Sun, X.; Li, W.J.; Li, S.H. Pectin oligosaccharide from hawthorn fruit ameliorates hepatic inflammation via NF-κB inactivation in high-fat diet fed mice. J. Funct. Foods. 2019, 57, 345–350. [Google Scholar] [CrossRef]
- Yu, Q.H.; Chen, X.J.; Sun, X.; Li, W.J.; Liu, T.Z.; Zhang, X.S.; Li, Y.Q.; Li, T.P.; Li, S.H. Pectic oligogalacturonide facilitates the synthesis and activation of adiponectin to improve hepatic lipid oxidation. Mol. Nutr. Food Res. 2021, 65, 2100167. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.W.; Zhang, T.; Wang, H.Y.; Zhu, C.H.; Wei, M. Physicochemical properties, structure and biological activities of a novel low-molecular-weight hawthorn pectin. Process Biochem. 2022, 122, 282–291. [Google Scholar] [CrossRef]
- Tang, X.; Liu, Y.Y.; Zhang, Z.D. Orthogonal array design for the optimization of pectin extraction from hawthorn pulp by hydrochloric acid treatment, microwave heating and salting out. Food Sci. 2013, 34, 112–115. (In Chinese) [Google Scholar]
- Li, Z.W.; Ma, Y.; Chen, Y.; Pan, S.K. Optimization of extraction technology of low-ester hawthorn pectin by ultrasound and pectin esterase. Farm Prod. Process. 2024, 2, 53–58. (In Chinese) [Google Scholar]
- Qin, C.; Yang, G.C.; Wu, S.; Zhang, H.; Zhu, C.H. Synthesis, physicochemical characterization, antibacterial activity, and biocompatibility of quaternized hawthorn pectin. Int. J. Biol. Macromol. 2022, 213, 1047–1056. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, W.; Lan, X.; Gong, S.; Wu, J.; Wang, Z. Effects of high hydrostatic pressure and high pressure homogenization processing on characteristics of potato peel waste pectin. Carbohydr. Polym. 2018, 196, 474–482. [Google Scholar] [CrossRef]
- Tian, H.X.; Xiong, J.J.; Yu, H.Y.; Chen, C.; Xu, H.D.; Lou, X.M. Characterize the physicochemical properties and microstructure of pectin from high-pressure and thermal processed cloudy hawthorn (Crataegus pinnatifida) juice based on acid heating extraction. Food Chem. 2023, 407, 135199. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.G.; Zhang, X.Y.; Wang, Y.; Zhang, L.J.; Wang, C.Y.; Hu, F.Q.; Ning, C.; Chen, G. Pectin oligosaccharides from hawthorn (Crataegus pinnatifida Bunge. Var. major): Molecular characterization and potential antiglycation activities. Food Chem. 2019, 286, 129–135. [Google Scholar] [CrossRef]
- Zhu, R.G.; Hong, M.L.; Zhuang, C.Y.; Zhang, L.J.; Wang, C.Y.; Liu, J.L.; Duan, Z.H.; Shang, F.F.; Hu, F.Q.; Li, T.J.; et al. Pectin oligosaccharides from hawthorn (Crataegus pinnatifida Bunge. Var. major) inhibit the formation of advanced glycation end products in infant formula milk powder. Food Funct. 2019, 10, 8081–8093. [Google Scholar] [CrossRef]
- Wu, C.H.; Huang, S.M.; Lin, J.A.; Yen, G.C. Inhibition of advanced glycation endproduct formation by foodstuffs. Food Funct. 2021, 2, 224–234. [Google Scholar] [CrossRef]
- Shang, F.F.; Zhu, R.G.; Zhang, X.Y.; Wang, Y.; Wang, C. Extraction, isolation and purification of haw polysaccharide and its antioxidant and antiglycation activities in vitro. Mod. Food Sci. Technol. 2019, 35, 96–101. (In Chinese) [Google Scholar]
- Yang, J.; Chen, J.X.; Hao, Y.X.; Liu, Y.P. Identification of the DPPH radical scavenging reaction adducts of ferulic acid and sinapic acid and their structure-antioxidant activity relationship. LWT-Food Sci. Technol. 2021, 146, 111411. [Google Scholar] [CrossRef]
- Zheng, P.P.; Li, S.; Qi, L.R.; Yang, Z.T.; Zhang, T.; Ao, X.Y. Extraction and antioxidant activity of hawthorn polysaccharides. China Brew. 2015, 34, 107–113. (In Chinese) [Google Scholar]
- Wang, Y.R.; Jin, Y.L.; Piao, M.Z.; Ren, H. Isolation and chemical characterization of polysaccharide from hawthorn. China Brew. 2013, 32, 102–105. (In Chinese) [Google Scholar]
- Dong, Y.P.; Li, T.P. Antioxidant activity of hawthorn pectin. Food Sci. 2014, 35, 29–32. (In Chinese) [Google Scholar]
- Bonnin, E.; Garnier, C.; Ralet, M.C. Pectin-modifying enzymes and pectin-derived materials: Applications and impacts. Appl. Microbiol. Biot. 2014, 98, 519–532. [Google Scholar] [CrossRef]
- Ogutu, F.O.; Mu, T.H. Ultrasonic degradation of sweet potato pectin and its antioxidant activity. Ultrason. Sonochem. 2017, 38, 726–734. [Google Scholar] [CrossRef]
- Kumar, A.; Sundaram, K.; Mu, J.Y.; Dryden, G.W.; Sriwastva, M.K.; Lei, C.; Zhang, L.F.; Qiu, X.L.; Xu, F.Y.; Yan, J.; et al. High-fat diet-induced upregulation of exosomal phosphatidylcholine contributes to insulin resistance. Nat. Commun. 2021, 12, 213. [Google Scholar] [CrossRef] [PubMed]
- Frazier, K.; Kambal, A.; Zale, E.A.; Pierre, J.F.; Hubert, N.; Miyoshi, S.; Miyoshi, J.; Ringus, D.L.; Harris, D.; Yang, K.; et al. High-fat diet disrupts REG3γ and gut microbial rhythms promoting metabolic dysfunction. Cell Host Microbe 2022, 30, 809–823. [Google Scholar] [CrossRef]
- Zhou, M.; Bi, J.F.; Chen, J.X.; Wang, R.X.; Richel, A. Impact of pectin characteristics on lipid digestion under simulated gastrointestinal conditions: Comparison of water-soluble pectins extracted from different sources. Food Hydrocolloid. 2021, 112, 106350. [Google Scholar] [CrossRef]
- Zhu, R.G.; Sun, Y.D.; Li, T.P.; Chen, G.; Peng, X.; Duan, W.B.; Zheng, Z.Z.; Shi, S.L.; Xu, J.G.; Liu, Y.H.; et al. Comparative effects of hawthorn (Crataegus pinnatifida Bunge) pectin and pectin hydrolyzates on the cholesterol homeostasis of hamsters fed high-cholesterol diets. Chem-Biol. Interact. 2015, 238, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.J.; Zheng, Y.; Liu, M.Q.; Cao, Y.; Lou, J.Y.; Qu, Y. Structural characterization and in vitro lipid-lowering effect of pectin from raw and fried hawthorn. J. Food Saf. Qual. 2023, 14, 253–262. (In Chinese) [Google Scholar]
- Wang, Y.; Zheng, Y.; Liu, Y.; Shan, G.S.; Zhang, B.J.; Cai, Q.; Lou, J.Y.; Qu, Y. The lipid-lowering effects of fenugreek gum, hawthorn pectin, and burdock inulin. Front. Nutr. 2023, 10, 1149094. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.X.; Chen, X.J.; Liu, T.Z.; Yu, Q.H.; Song, Z.Q.; Wang, F.; Li, T.P. Pectin oligosaccharides improved lipid metabolism in white adipose tissue of high-fat diet fed mice. Food Sci. Biotechnol. 2022, 31, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H.; Huang, Z.; Dong, Y.P.; Zhu, R.G.; Li, T.P. Haw pectin pentaglaracturonide inhibits fatty acid synthesis and improves insulin sensitivity in high-fat-fed mice. J. Funct. Foods 2017, 34, 440–446. [Google Scholar] [CrossRef]
- Zhu, R.G.; Hou, Y.T.; Sun, Y.D.; Li, T.P.; Fan, J.G.; Chen, G.; Wei, J.X. Pectin penta-oligogalacturonide suppresses intestinal bile acids absorption and downregulates the FXR-FGF15 axis in high-cholesterol fed mice. Lipids 2017, 52, 489–498. [Google Scholar] [CrossRef]
- Li, T.P.; Zhu, R.G.; Dong, Y.P.; Liu, Y.H.; Li, S.H.; Chen, G. Effects of pectin pentaoligosaccharide from hawthorn (crataegus pinnatifida bunge. var. major) on the activity and mrna levels of enzymes involved in fatty acid oxidation in the liver of mice fed a high-fat diet. J. Agric. Food Chem. 2013, 61, 7599–7605. [Google Scholar] [CrossRef]
- Men’shikov, D.D.; Lazareva, E.B.; Popova, T.S.; Shramko, L.U.; Tokaev, I.S.; Zalogueva, G.V.; Gaponova, I.N. Antimicrobial properties of pectins and their effects on antibiotics. Antibiot. Khimioter. 1997, 42, 10–15. [Google Scholar]
- Wang, W.; Mou, D.H.; Li, D.D. Antibacterial activity and mechanism of hawthorn pectin oligosaccharides. Food Sci. 2018, 39, 110–116. (In Chinese) [Google Scholar]
- Li, T.P.; Li, S.H.; Song, Y.R.; Wu, J.; Zhu, R.G.; Zhao, Z.S.; Jia, Y.F. Inhibitory effect of haw pectic oligosaccharide and its compounds against Bacillus subtilis. Sci. Technol. Food Ind. 2012, 33, 154–156. (In Chinese) [Google Scholar]
- Tao, L.; Shen, K.; Liu, T.Z.; Sun, R.Y.; Song, Z.Q.; Wang, F.; Li, T.P. Effect of haw pectin oligosaccharide on the preservation of shiitake mushrooms. Food Ferment. Ind. 2022, 48, 226–230. (In Chinese) [Google Scholar]
- Wu, L.; Tang, C.H.; Chen, L.L.; Zhao, J.Y. Modified dietary fiber from soybean dregs by fermentation alleviated constipation in mice. Food Chem. X 2023, 19, 100810. [Google Scholar] [CrossRef]
- Cao, J.H.; Wang, K.; Li, N.X.; Zhang, L.P.; Qin, L.; He, Y.Y.; Wang, J.F.; Qu, C.F.; Miao, J.L. Soluble dietary fiber and cellulose from Saccharina japonica by-product ameliorate Loperamide-induced constipation via modulating enteric neurotransmitters, short-chain fatty acids and gut microbiota. Int. J. Biol. Macromol. 2023, 226, 1319–1331. [Google Scholar] [CrossRef]
- Chen, H.Y.; Ma, L.; Yang, J.Q.; Zhao, W.; Cui, T. Maythorn dietary fiber ameliorates functional constipation and prevents lead poisoning. Food Sci. 2013, 34, 232–235. (In Chinese) [Google Scholar]
- Yang, G.; Zhou, Q.; Dong, L.J.; Hao, H.W.; Liu, X.Y.; Zhao, W. Effect of increased dietary fiber in freeze-dried hawthorn on the intestinal health of mice. Mod. Food Sci. Technol. 2016, 32, 20–25. (In Chinese) [Google Scholar]
- Zhao, Y.Y.; Wang, D.; Wang, P.; Zhao, W.T.; Zhao, S.; Ma, Y.; Chang, H.; Wang, Y.B.; Liu, Y.; Zhao, X.Y. Microbiota response of pectin determined by its structural characteristics during in vitro fecal fermentation: A comparative study of various pectin sources. Food Hydrocolloid. 2024, 150, 109730. [Google Scholar] [CrossRef]
- Liao, Q.; Deng, Z.Y.; Liu, J.; Zhang, W.J.; Shao, S.J.; Wu, S.; Liu, J. Isolation and purification of polysaccharides from hawthorn fruit and the effects on fecal microbiota. Food Ferment. Ind. 2023, 49, 54–61. (In Chinese) [Google Scholar]
- Tang, L.K. Experimental study on anti-fatigue effect of hawthorn polysaccharides. Yunnan J. Tradit. Chin Med. 2008, 29, 32–33. (In Chinese) [Google Scholar]
- Zhang, Y.; Wang, D.P.; Tan, D.T.; Zou, A.Q.; Gong, H.; Yang, Y.; Sun, L.S.; Lin, X.L.; Liang, M.; Yu, Y.; et al. Immune-enhancing activity of compound polysaccharide on the inactivated influenza vaccine. Carbohydr. Polym. 2024, 336, 122080. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Gu, X.L.; Zhao, Z.S.; Shao, Y.B. Effect of compound polysaccharide of Chinese medicine and polysaccharide of Chinese medicine on lymphocyte proliferation in mice. Heilongjiang Anim. Sci. Vet. Med. 2008, 2, 89–90. (In Chinese) [Google Scholar]
- Li, Y.M.; Zhong, R.F.; Chen, J.; Luo, Z.G. Structural characterization, anticancer, hypoglycemia and immune activities of polysaccharides from Russula virescens. Int. J. Biol. Macromol. 2021, 184, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Xu, G.B.; Tang, X.; Zhang, C.; Zhao, W.; Wang, J.; Chen, H. Anti-cancer potential of polysaccharide extracted from hawthorn (Crataegus.) on human colon cancer cell line HCT116 via cell cycle arrest and apoptosis. J. Funct. Foods 2020, 64, 103677. [Google Scholar] [CrossRef]
- Hayden, M.S.; Sankar, G. Shared principles in NF-kappa B signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.W.; Wu, Z.Y.; Lu, Y.; Chang, X.D. Protective effect of hawthorn pectin oligogalacturonide extract against ultraviolet B-induced oxidative damage and photoaging in HaCaT cells. Food Sci. 2018, 39, 210–218. (In Chinese) [Google Scholar]
- Braünlich, P.M.; Inngjerdingen, K.T.; Inngjerdingen, M.; Johnson, Q.; Paulsen, B.S.; Mabusela, W. Polysaccharides from the South African medicinal plant Artemisia afra: Structure and activity studies. Fitoterapia 2018, 124, 182–187. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, Y.J.; Sun, P.L.; Zhang, F.M.; Linhardt, R.J.; Zhang, A.Q. Chemically modified polysaccharides: Synthesis, characterization, structure activity relationships of action. Int. J. Biol. Macromol. 2019, 132, 970–977. [Google Scholar] [CrossRef]
- Guo, R.; Chang, M.C.; Cheng, Y.F.; Meng, J.L.; Feng, C.P.; Geng, X.R.; Xu, L.Q.; Guo, D.D. Effects of different degradation methods on the physicochemical properties and antioxidant activity of polysaccharides from Clitocybe squamulosa fruiting body. Food Sci. 2023, 44, 123–131. (In Chinese) [Google Scholar]
- Xu, C.P.; Sun, Y.Y.; Bai, J.F.; Jia, X.W.; Ma, K.Y.; Wang, X.J.; Xiong, Y.M.; Liu, S.H. Study on the extraction, sulfation modification and antioxidant activity of yam polysaccharides. J. Henan Univ. Technol. Nat. Sci. Ed. 2019, 40, 50–55. (In Chinese) [Google Scholar]
- Huo, J.Y.; Lei, M.; Li, F.F.; Hou, J.J.; Zhang, Z.J.; Long, H.L.; Zhong, X.C.; Liu, Y.M.; Xie, C.; Wu, W.Y. Structural characterization of a polysaccharide from Gastrodia elata and its bioactivity on gut microbiota. Molecules 2021, 26, 4443. [Google Scholar] [CrossRef]
- Liu, D.; Wang, S.Y.; Wang, G.N.; Zheng, L.H.; Sun, Y.; Liu, L.; Bao, Y.L. Structural characterization and immunoregulatory activity of a neutral polysaccharide from the roots of Apocynum venetum L. Int. J. Biol. Macromol. 2022, 222, 90–100. [Google Scholar] [CrossRef]
- Liang, Z.; Li, L.; Fu, Q.; Zhang, X.; Xu, Z.; Li, B. Formation and elimination of pyrraline in the Maillard reaction in a saccharide-lysine model system. J. Sci. Food Agric. 2016, 96, 2555–2564. [Google Scholar] [CrossRef]
- Dickinson, E. Hydrocolloids at interfaces and the influence on the properties of dispersed systems. Food Hydrocolloid. 2003, 17, 25–39. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhu, Y.Z.; Li, F.; Du, J.H.; Huang, Q.R.; Sun-Waterhouse, D.X.; Li, D.P. Antioxidative pectin from hawthorn wine pomace stabilizes and protects Pickering emulsions via forming zein-pectin gel-like shell structure. Int. J. Biol. Macromol. 2020, 151, 193–203. [Google Scholar] [CrossRef]
- Yang, Z.K.; Li, M.R.; Li, Z.H.; Li, Y.X.; Shi, J.Y.; Huang, X.W.; Sun, Y.; Zhai, X.D.; Zou, X.B.; Xiao, J.B. Incorporation of hawthorn pectin/β-cyclodextrin-stabilized Pickering emulsion and Titanium dioxide nanoparticles for improving the physical, biological, and release properties of guar gum/agar/sodium alginate-based bilayer films. Ind. Crop. Prod. 2024, 212, 118302. [Google Scholar] [CrossRef]
- Lv, C.X.; Zhao, D.J.; Song, L.; Zhao, L.H.; Geng, S.S. Analysis of influence made by hawthorn pectin to physical index of yoghurt. Food Sci. 2007, 28, 302–307. (In Chinese) [Google Scholar]
- Wu, C.; Li, Y.; Du, Y.; Wang, L.; Tong, C.; Hu, Y.; Pang, J.; Yan, Z. Preparation and characterization of konjac glucomannan-based bionanocomposite film for active food packaging. Food Hydrocolloid. 2019, 89, 682–690. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Du, W.X.; de Jesús Avena-Bustillos, R.; Soares, N.D.F.F.; McHugh, T.H. Edible films from pectin: Physical-mechanical and antimicrobial properties-A review. Food Hydrocolloid. 2014, 35, 287–296. [Google Scholar] [CrossRef]
- Qin, C.; Li, Z.X.; Zhang, J.R.; Meng, H.M.; Zhu, C.H. Preparation, physicochemical properties, antioxidant, and antibacterial activities of quaternized hawthorn pectin films incorporated with thyme essential oil. Food. Packaging. Shelf. 2024, 41, 101235. [Google Scholar] [CrossRef]
- Zhang, X.; Han, Y.; Huang, W.; Jin, M.J.; Gao, Z.G. The influence of the gut microbiota on the bioavailability of oral drugs. Acta Pharm. Sin. B 2021, 11, 1789–1812. [Google Scholar] [CrossRef]
- Chen, J.; Liu, W.; Liu, C.M.; Li, T.; Liang, R.H.; Luo, S.J. Pectin modifications: A review. Crit. Rev. Food Sci. 2015, 55, 1684–1698. [Google Scholar] [CrossRef]
- Zhang, L.F.; Zhang, X.Z.; Liu, D.H.; Ding, T.; Ye, X.Q. Effect of degradation methods on the structural properties of citrus pectin. LWT-Food Sci. Technol. 2015, 61, 630–637. [Google Scholar] [CrossRef]
- Zhu, Y.W.; Wang, H.Y.; Zhang, T.; Zhang, X.Y.; Zhu, C.H. Characterization, antioxidant activity and in vitro digestion of hawthorn pectin prepared by gradient ethanol precipitation. Int. J. Biol. Macromol. 2024, 267, 131278. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.W.; Chen, X.W.; Zhao, L.W.; Zhu, C.H. Study on the hawthorn pectin modified by which response surface optimized ultrasonic with VC/H2O2. J. Shandong Agric. Univ. Nat. Sci. Ed. 2019, 50, 686–691. (In Chinese) [Google Scholar]
- Culum, D.; Copra-Janicijevic, A.; Vidic, D.; Klepo, L.; Tahirovic, A.; Basic, N.; Maksimovic, M. HPLC-ED analysis of phenolic compounds in three Bosnian Crataegus species. Foods 2018, 7, 66. [Google Scholar] [CrossRef]
- Li, T.; Xu, L.H.; Yan, Q.J.; Liu, J.; Jiang, Z.Q. Sucrose-free hawthorn leathers formulated with fructooligosaccharides and xylooligosaccharides ameliorate high-fat diet induced inflammation, glucose and lipid metabolism in liver of mice. Food Sci. Hum. Well. 2022, 11, 1064–1075. [Google Scholar] [CrossRef]
- Li, T.; Xu, L.H.; Liu, J.; Yang, Q.J.; Jiang, Z.Q. Preventive effects of sugar-free hawthorn rolls on constipation in mice. Sci. Technol. Food Ind. 2020, 41, 271–277. (In Chinese) [Google Scholar]
- Farzaliev, E.B.; Ökten, S. Production and characterization of fruit jam with activated pectin using wild hawthorn puree (Crataegus monogyna Jacq.). Nat. Prod. Res. 2023. [Google Scholar] [CrossRef]
- Lu, H.X.; Xuan, R.S. Study on the application of hawthorn and hawthorn pectin to produce soft confectionery in Changbai Mountain area. Ind. Technol. Econ. 1988, 6, 45–47. (In Chinese) [Google Scholar]
- Sun, X.; Liu, H.; Duan, C.Q.; Yan, G.L. Effects of mixed starters of plant- and wine-derived L. plantarum on hawthorn juice fermentation: Physicochemical properties, phenolic and volatile profiles. Food Biosci. 2023, 56, 103363. [Google Scholar] [CrossRef]
- Peng, S.Z.; Li, Z.Y.; Xu, H.; Zhu, Z.H.; Wu, B.J. Optimization of enzymolysis for clear concentrated hawthorn and tangerine peel juice. Bev. Ind. 2024, 27, 36–40. (In Chinese) [Google Scholar]
- Jia, R.J.; Zhang, H.; Jin, X.C.; Han, Y.Y.; Du, J.H. Effect of fermentation and soaking on hawthorn wine security. Liq. Mak. 2024, 51, 53–59. (In Chinese) [Google Scholar]
- Castro-Munoz, R.; Correa-Delgado, M.; Cordova-Almeida, R.; Lara-Nava, D.; ChavezMunoz, M.; Velasquez-Chavez, V.F.; Hernandez-Torres, C.E.; Gontarek-Castro, E.; Ahmad, M.Z. Natural sweeteners: Sources, extraction and current uses in foods and food industries. Food Chem. 2022, 370, 130991. [Google Scholar] [CrossRef]
- Raposo, A.; Saraiva, A.; Ramos, F.; Carrascosa, C.; Raheem, D.; Barbara, R.; Silva, H. The role of food supplementation in microcirculation–A comprehensive review. Biology 2021, 10, 616. [Google Scholar] [CrossRef]
- Dong, D.S. Development of health yoghurt mixed with pawpaw and hawthorn. Guizhou Agric. Sci. 2012, 40, 187–190. (In Chinese) [Google Scholar]
- Zeng, W.L.; Zhang, F.J.; Zhao, Y.G. Development of red-fleshed pitaya-hawthorn compound jelly. Storage Process. 2022, 22, 34–40. (In Chinese) [Google Scholar]
- Cheng, J.J.; Wang, J.; Zhang, X.W.; Lu, C.Y. Research on preparation of mixed beverage of hawthorn and green tea. J. Xuchang Univ. 2020, 39, 104–107. (In Chinese) [Google Scholar]
- You, L.; Zhao, Y.X.; Sui, Q.Q.; Liu, S.Q.; Liu, S.W.; Chang, X.D. Process optimization of hawthorn pectin oligogalacturonic acid (PGA) yogurt drinks and inhibitory effect on lactic acid bacteria by PGA. Sci. Technol. Food Ind. 2019, 40, 148–154. [Google Scholar]
- Cui, M.; Cheng, L.; Zhou, Z.Y.; Zhu, Z.M.; Liu, Y.L.; Li, C.H.; Liao, B.B.; Fan, M.; Duan, B.Z. Traditional uses, phytochemistry, pharmacology, and safety concerns of hawthorn (Crataegus genus): A comprehensive review. J. Ethnopharmacol. 2024, 319, 117229. [Google Scholar] [CrossRef]
- Bu, K.X.; Huang, D.J.; Zhang, H.; Xu, K.; Zhu, C.H. Ultrasonic-microwave technique promotes the physicochemical structure of hydrogel and its release characterization of curcumin in vitro. Food Chem. 2024, 451, 139389. [Google Scholar] [CrossRef]
- Sun, W.X.; Bu, K.X.; Meng, H.M.; Zhu, C.H. Hawthorn pectin/soybean isolate protein hydrogel bead as a promising ferrous ion-embedded delivery system. Colloid. Surface B 2024, 237, 113867. [Google Scholar] [CrossRef]
- Bu, K.X.; Huang, D.J.; Li, D.P.; Zhu, C.H. Encapsulation and sustained release of curcumin by hawthorn pectin and Tenebrio Molitor protein composite hydrogel. Int. J. Biol. Macromol. 2022, 222, 251–261. [Google Scholar] [CrossRef]
- Huang, X.; Li, T.P.; Li, S.H. Encapsulation of vitexin-rhamnoside based on zein/pectin nanoparticles improved its stability and bioavailability. Cur. Res. Food Sci. 2023, 6, 100419. [Google Scholar]
- Li, Z.X.; Geng, Y.X.; Bu, K.X.; Chen, Z.T.; Xu, K.; Zhu, C.H. Construction of a pectin/sodium alginate composite hydrogel delivery system for improving the bioaccessibility of phycocyanin. Int. J. Biol. Macromol. 2024, 269, 131969. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Ji, W.; Dong, H.; Wu, Y.; Guo, L.; Chen, L.; Wang, X. A Comprehensive Review on the Isolation, Bioactivities, and Structure–Activity Relationship of Hawthorn Pectin and Its Derived Oligosaccharides. Foods 2024, 13, 2750. https://doi.org/10.3390/foods13172750
Li T, Ji W, Dong H, Wu Y, Guo L, Chen L, Wang X. A Comprehensive Review on the Isolation, Bioactivities, and Structure–Activity Relationship of Hawthorn Pectin and Its Derived Oligosaccharides. Foods. 2024; 13(17):2750. https://doi.org/10.3390/foods13172750
Chicago/Turabian StyleLi, Tao, Wenhua Ji, Hongjing Dong, Yingqun Wu, Lanping Guo, Lei Chen, and Xiao Wang. 2024. "A Comprehensive Review on the Isolation, Bioactivities, and Structure–Activity Relationship of Hawthorn Pectin and Its Derived Oligosaccharides" Foods 13, no. 17: 2750. https://doi.org/10.3390/foods13172750
APA StyleLi, T., Ji, W., Dong, H., Wu, Y., Guo, L., Chen, L., & Wang, X. (2024). A Comprehensive Review on the Isolation, Bioactivities, and Structure–Activity Relationship of Hawthorn Pectin and Its Derived Oligosaccharides. Foods, 13(17), 2750. https://doi.org/10.3390/foods13172750







