Characterization of Flavor Compounds in Chinese Indigenous Sheep Breeds Using Gas Chromatography–Ion Mobility Spectrometry and Chemometrics
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Materials
2.3. GC-IMS Analysis
2.4. Statistical Analysis
3. Results
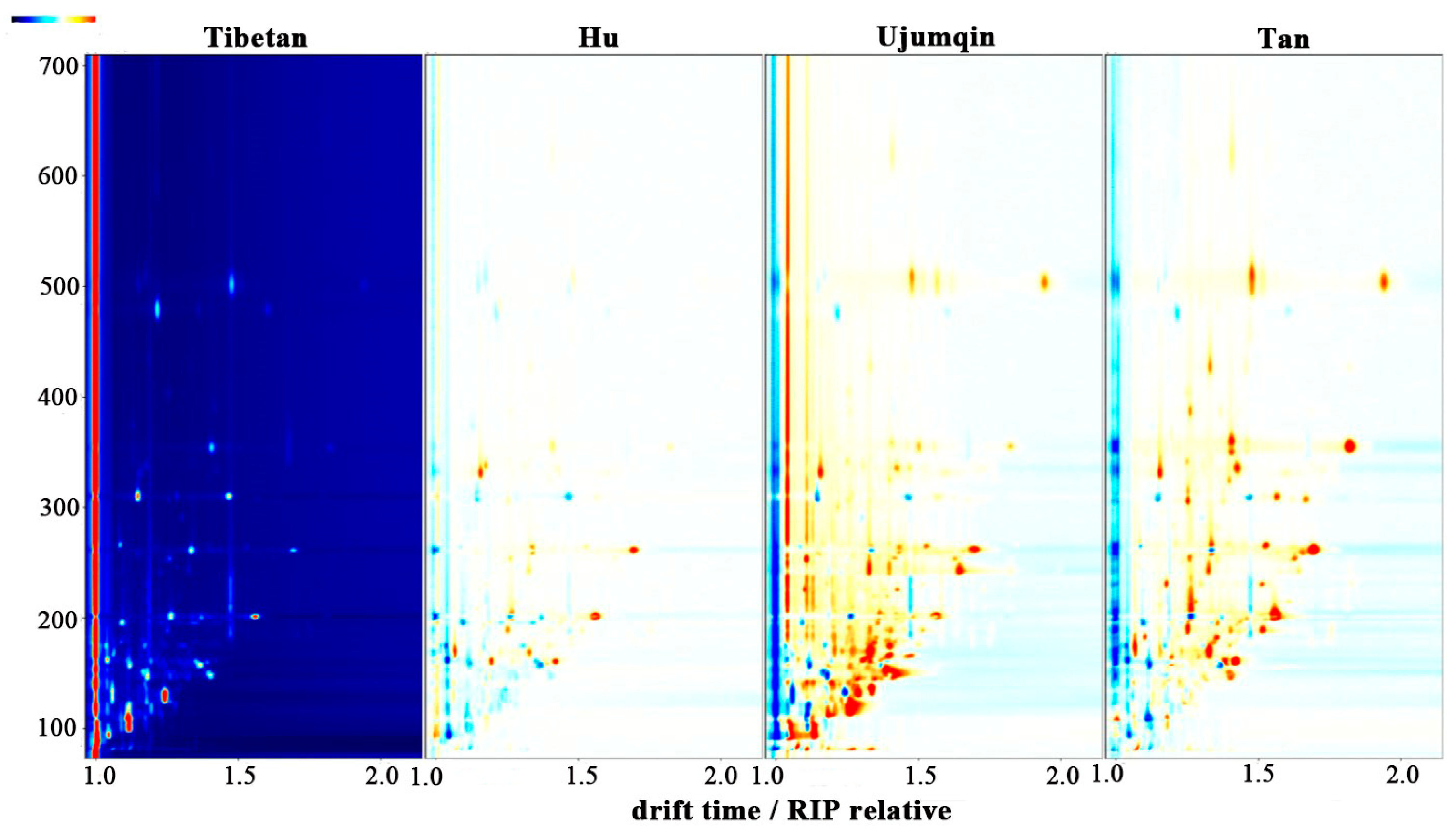
3.1. GC-IMS Maps of Meats of Different Breeds of Sheep
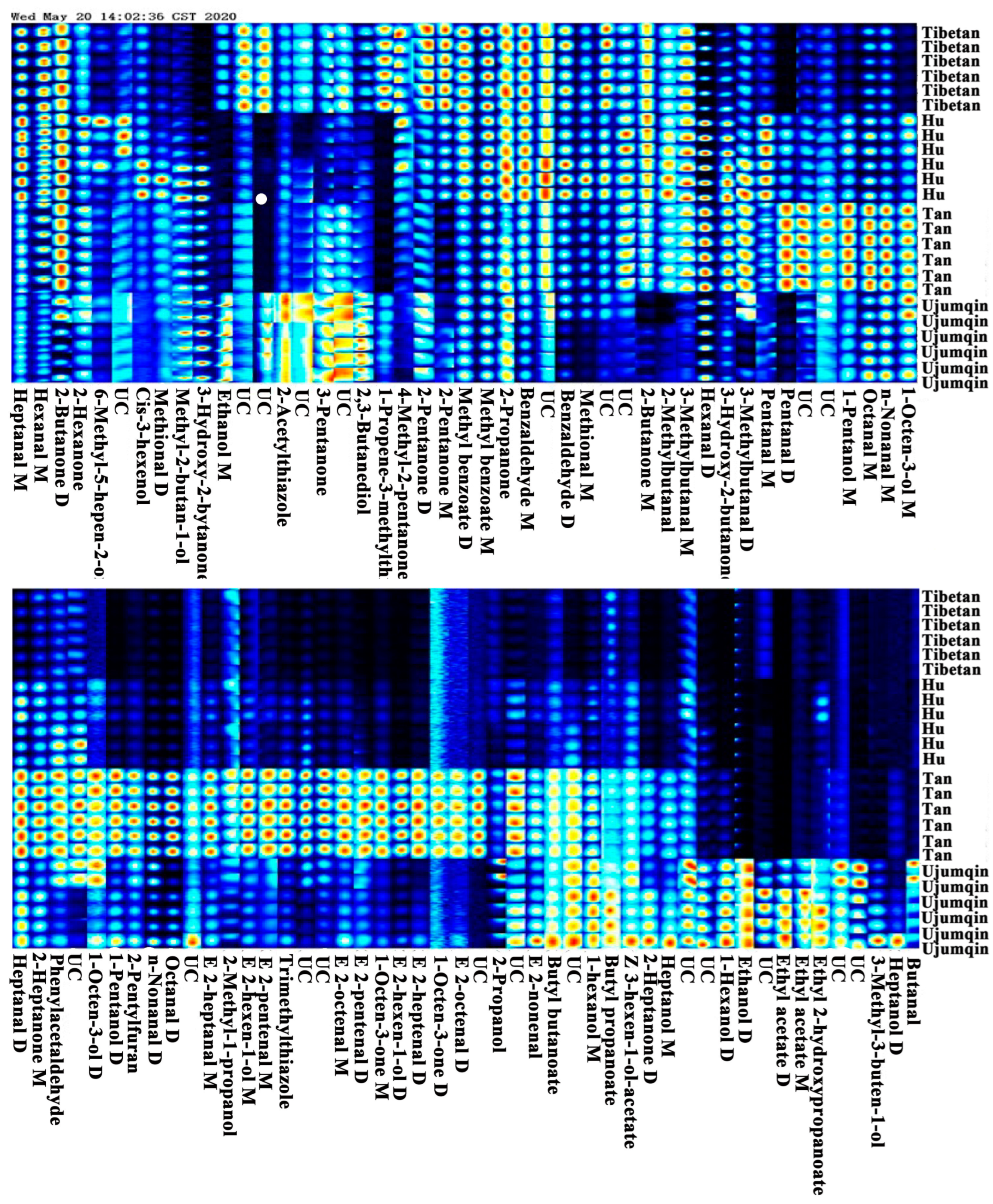
3.2. Volatile Compound Identification and Fingerprints Profile in Different Breeds’ Meat
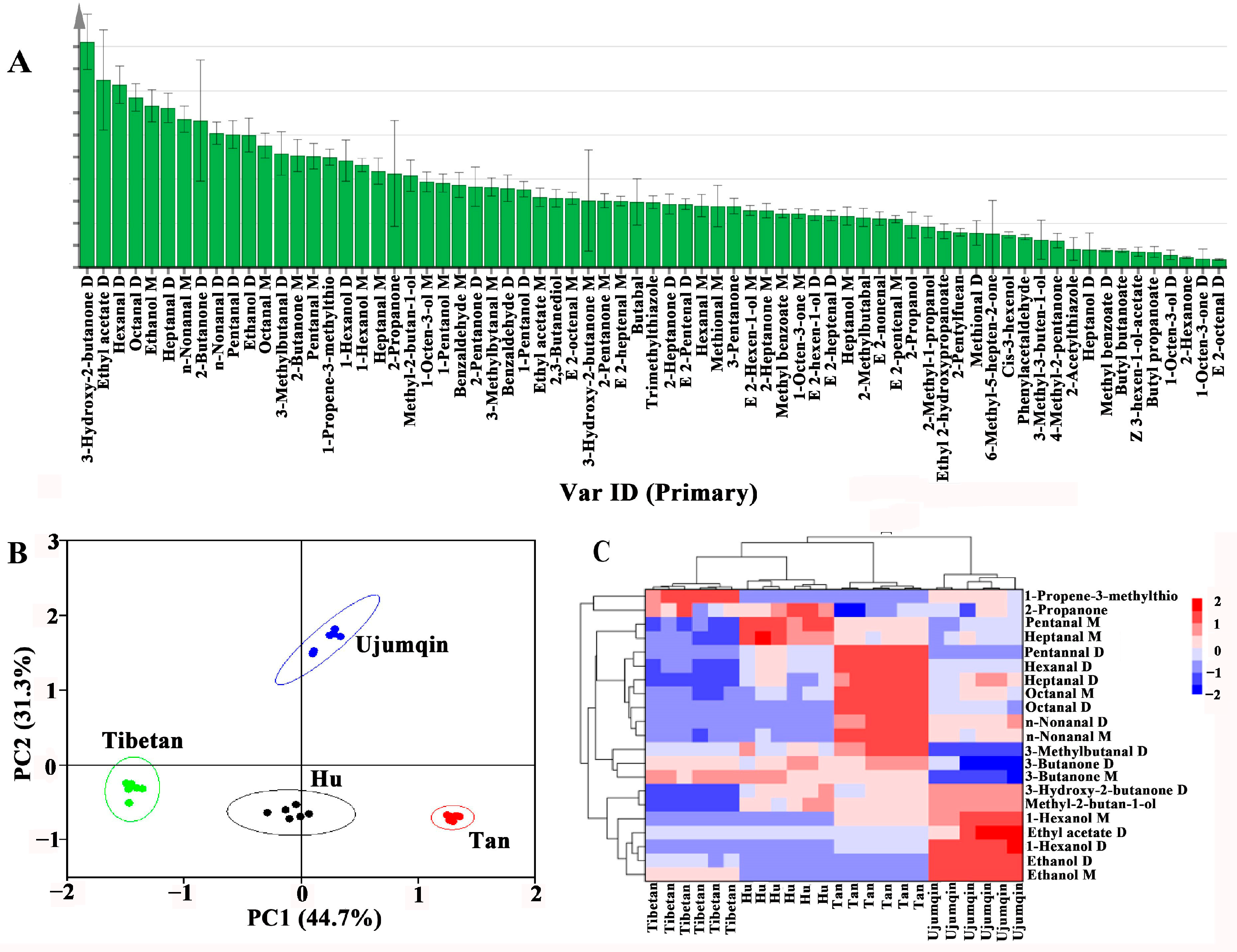
3.3. Analysis of Volatile Compounds in Sheep Meat of Different Breeds
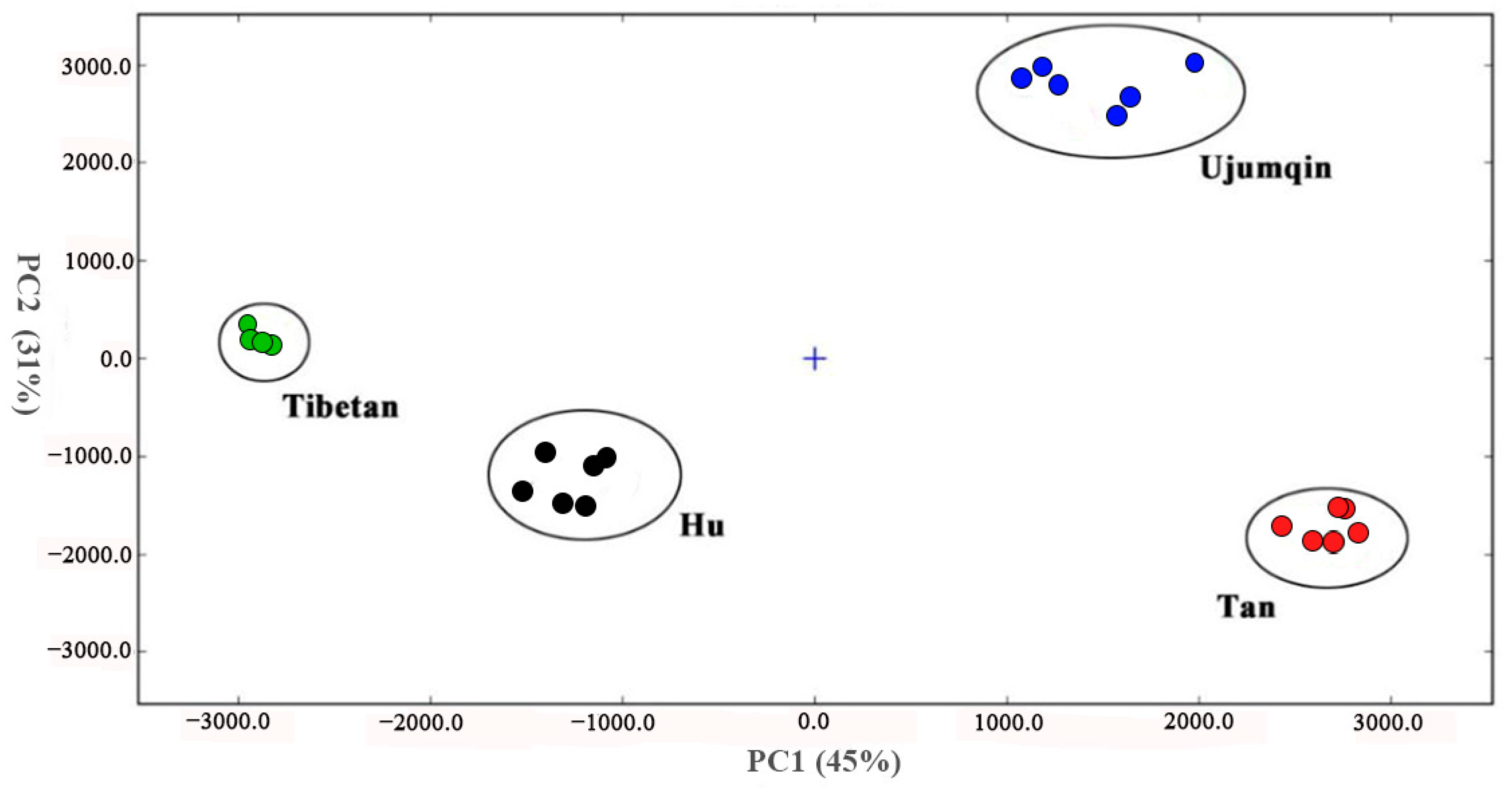
3.4. Principal Component Analysis (PCA) of Volatile Compounds
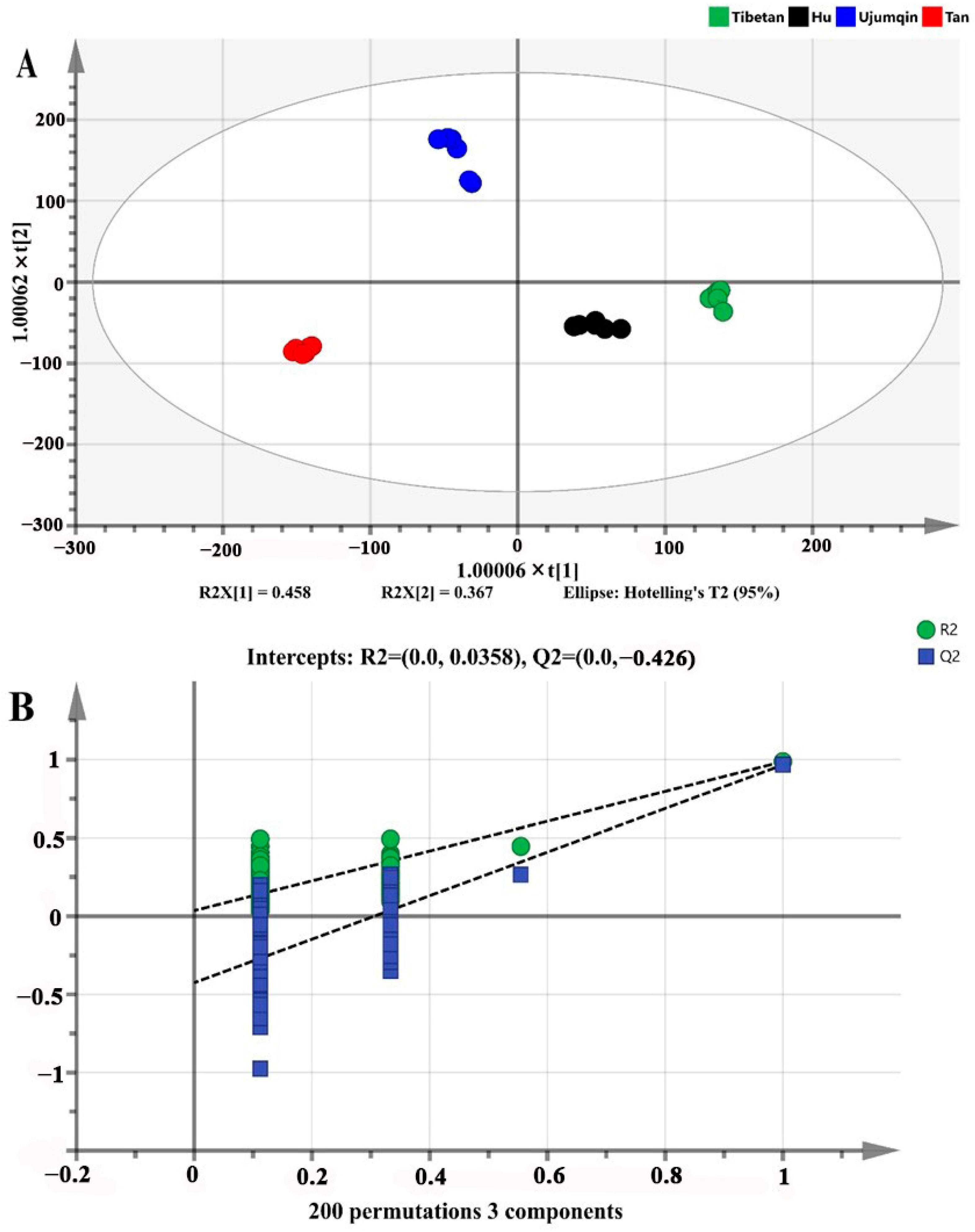
3.5. OPLS-DA of Volatile Compounds
3.6. Screening of Differential Volatile Components in Different Breeds of Sheep Meat
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, Y.; Matthew, C.; Li, F.; Nan, Z. Comparative effects of stovers of four varieties of common vetch on growth performance, ruminal fermentation, and nutrient digestibility of growing lambs. Animals 2020, 10, 596. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.; Li, S.; Zhang, D.; Huang, C.; Zhao, Y.; Zheng, X.; Wang, Z.; Chen, L. Characterization and discrimination of the flavor profiles of Chinese indigenous sheep breeds via electronic sensory, smart instruments and chemometrics. J. Food Compos. Anals. 2023, 122, 105458. [Google Scholar] [CrossRef]
- Miller, R. Drivers of consumer liking for beef, pork, and lamb: A review. Foods 2020, 9, 428. [Google Scholar] [CrossRef] [PubMed]
- Matsuishi, M.; Igeta, M.; Takeda, S.; Okitani, A. Sensory factors contributing to the identification of the animal species of meat. J. Food Sci. 2004, 69, S218–S220. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, J.; Dong, H.; Bai, W.; Yu, L.; Li, X. Variations of volatile flavour compounds in Cordyceps militaris chicken soup after enzymolysis pretreatment by SPME combined with GC-MS, GC × GC-TOF MS and GC-IMS. Int. J. Food Sci Tech. 2020, 55, 509–516. [Google Scholar] [CrossRef]
- Bueno, M.; Resconi, V.C.; Campo, M.M.; Cacho, J.; Ferreira, V.; Escudero, A. Effect of freezing method and frozen storage duration on odor-active compounds and sensory perception of lamb. Food Res. Int. 2013, 54, 772–780. [Google Scholar] [CrossRef]
- Caroprese, M.; Ciliberti, M.G.; Marino, R.; Napolitano, F.; Braghieri, A.; Sevi, A.; Albenzio, M. Effect of information on geographical origin, duration of transport and welfare condition on consumer’s acceptance of lamb meat. Sci. Rep. 2020, 10, 9754. [Google Scholar]
- Karpas, Z. Applications of ion mobility spectrometry (IMS) in the field of foodomics. Food Res. Int. 2013, 54, 1146–1151. [Google Scholar] [CrossRef]
- Zhu, H.; Zhu, D.; Sun, J. Application of GC-IMS coupled with chemometric analysis for the classification and authentication of geographical indication agricultural products and food. Front. Nutr. 2023, 10, 1247695. [Google Scholar] [CrossRef]
- Hong, H.; Liu, J. GC-O-MS technique and its applications in food flavor analysis. Food Res. Int. 2018, 114, 187–198. [Google Scholar]
- Grosch, W. Detection of potent odorants in foods by aroma extract dilution analysis. Trends Food Sci. Tech. 1993, 4, 68–73. [Google Scholar] [CrossRef]
- Peris, M.; Escuder-Gilabert, L. A 21st century technique for food control: Electronic noses. Anal. Chim. Acta 2009, 638, 1–15. [Google Scholar] [CrossRef] [PubMed]
- García-Nicolás, M.; Arroyo-Manzanares, N.; Arce, L.; Hernández-Córdoba, M.; Viñas, P. Headspace gas chromatography coupled to mass spectrometry and ion mobility spectrometry: Classification of virgin olive oils as a study case. Foods 2020, 9, 1288. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Lu, D.; Chen, B. Application of headspace/gas chromatography-tandem ion mobility spectrometry with chemometrics in classification of edible vegetable oils. J. Instrum Anal. 2017, 36, 1235–1239. [Google Scholar]
- Guo, Y.; Chen, D.; Dong, Y.; Ju, H.; Wu, C.; Lin, S. Characteristic volatiles fingerprints and changes of volatile compounds in fresh and dried Tricholoma matsutake Singer by HS-GC-IMS and HS-SPME-GC–MS. J. Chromatogr. B 2018, 1099, 46–55. [Google Scholar] [CrossRef]
- Zhang, X.; Dai, Z.; Fan, X.; Liu, M.; Ma, J.; Shang, W.; Liu, J.; Strappe, P.; Blanchard, C.; Zhou, Z. A study on volatile metabolites screening by HS-SPME-GC-MS and HS-GC-IMS for discrimination and characterization of white and yellowed rice. Cereal Chem. 2020, 97, 496–504. [Google Scholar] [CrossRef]
- Cavanna, D.; Zanardi, S.; Dall’Asta, C.; Suman, M. Ion mobility spectrometry coupled to gas chromatography: A rapid tool to assess eggs freshness. Food Chem. 2019, 271, 691–696. [Google Scholar] [CrossRef]
- Ge, S.; Chen, Y.; Ding, S.; Zhou, H.; Jiang, L.; Yi, Y.; Deng, F.; Wang, R. Changes in volatile flavor compounds of peppers during hot air drying process based on headspace-gas chromatography-ion mobility spectrometry (HS-GC-IMS). J. Sci. Food Agr. 2020, 100, 3087–3098. [Google Scholar] [CrossRef]
- Arroyo-Manzanares, N.; Martín-Gómez, A.; Jurado-Campos, N.; Garrido-Delgado, R.; Arce, C.; Arce, L. Target vs spectral fingerprint data analysis of Iberian ham samples for avoiding labelling fraud using headspace–gas chromatography–ion mobility spectrometry. Food Chem. 2018, 246, 65–73. [Google Scholar] [CrossRef]
- Triba, M.N.; Le Moyec, L.; Amathieu, R.; Goossens, C.; Bouchemal, N.; Nahon, P.; Rutledge, D.N.; Savarin, P. PLS/OPLS models in metabolomics: The impact of permutation of dataset rows on the K-fold cross-validation quality parameters. Mol. BioSyst. 2015, 11, 13–19. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Q.; Liu, S.; Hong, P.; Zhou, C.; Zhong, S. Characterization of the effect of different cooking methods on volatile compounds in fish cakes using a combination of GC–MS and GC-IMS. Food Chem. X 2024, 22, 101291. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wan, H.; Wu, X.; Yin, J.; Zhu, L.; Chen, H.; Song, X.; Han, L.; Yang, W.; Yu, H. Discrimination and characterization of the volatile organic compounds in Schizonepetae Spica from six regions of China using HS-GC-IMS and HS-SPME-GC-MS. Molecules 2022, 27, 4393. [Google Scholar] [CrossRef]
- Arroyo-Manzanares, N.; García-Nicolás, M.; Castell, A.; Campillo, N.; Viñas, P.; López-García, I.; Hernández-Córdoba, M. Untargeted headspace gas chromatography–Ion mobility spectrometry analysis for detection of adulterated honey. Talanta 2019, 205, 120123. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Maecker, R.; Vyhmeister, E.; Meisen, S.; Rosales Martinez, A.; Kuklya, A.; Telgheder, U. Identification of terpenes and essential oils by means of static headspace gas chromatography-ion mobility spectrometry. Anal. Bioanal. Chem. 2017, 409, 6595–6603. [Google Scholar] [CrossRef]
- d’Acampora Zellner, B.; Dugo, P.; Dugo, G.; Mondello, L. Gas chromatography–olfactometry in food flavour analysis. J. Chromatogr. A 2008, 1186, 123–143. [Google Scholar] [CrossRef] [PubMed]
- Larick, D.; Turner, B. Headspace volatiles and sensory characteristics of ground beef from forage-and grain-fed heifers. J. Food Sci. 1990, 55, 649–654. [Google Scholar] [CrossRef]
- Mottram, D.S. Flavour formation in meat and meat products: A review. Food Chem. 1998, 62, 415–424. [Google Scholar] [CrossRef]
- Lotfy, S.N.; Fadel, H.H.; El-Ghorab, A.H.; Shaheen, M.S. Stability of encapsulated beef-like flavourings prepared from enzymatically hydrolysed mushroom proteins with other precursors under conventional and microwave heating. Food Chem. 2015, 187, 7–13. [Google Scholar] [CrossRef]
- Nachtigall, F.M.; Vidal, V.A.; Pyarasani, R.D.; Domínguez, R.; Lorenzo, J.M.; Pollonio, M.A.; Santos, L.S. Substitution effects of NaCl by KCl and CaCl2 on lipolysis of salted meat. Foods 2019, 8, 595. [Google Scholar] [CrossRef]
- Leisner, J.J.; Laursen, B.G.; Prévost, H.; Drider, D.; Dalgaard, P. Carnobacterium: Positive and negative effects in the environment and in foods. FEMS Microbiol. Rev. 2007, 31, 592–613. [Google Scholar] [CrossRef]
- Li, J.; Tang, C.; Zhao, Q.; Yang, Y.; Li, F.; Qin, Y.; Liu, X.; Yue, X.; Zhang, J. Integrated lipidomics and targeted metabolomics analyses reveal changes in flavor precursors in psoas major muscle of castrated lambs. Food Chem. 2020, 333, 127451. [Google Scholar] [CrossRef] [PubMed]
- Popova, T. Effect of the rearing system on the fatty acid composition and oxidative stability of the M. longissimus lumborum and M. semimembranosus in lambs. Small Ruminant Res. 2007, 71, 150–157. [Google Scholar] [CrossRef]
- Selli, S.; Cayhan, G.G. Analysis of volatile compounds of wild gilthead sea bream (Sparus aurata) by simultaneous distillation–extraction (SDE) and GC–MS. Microchem. J. 2009, 93, 232–235. [Google Scholar] [CrossRef]
- Calkins, C.R.; Hodgen, J.M. A fresh look at meat flavor. Meat Sci. 2007, 77, 63–80. [Google Scholar] [CrossRef]
- Ma, Q.; Hamid, N.; Bekhit, A.; Robertson, J.; Law, T. Evaluation of pre-rigor injection of beef with proteases on cooked meat volatile profile after 1 day and 21 days post-mortem storage. Meat Sci. 2012, 92, 430–439. [Google Scholar] [CrossRef]
- Elmore, J.; Mottram, D. Flavour development in meat. In Improving the Sensory and Nutritional Quality of Fresh Meat; Woodhead Publishing Limited: Sawston, UK, 2009; Volume 4, pp. 111–146. [Google Scholar]
- Carrapiso, A.I.; Noseda, B.; García, C.; Reina, R.; Del Pulgar, J.S.; Devlieghere, F. SIFT-MS analysis of Iberian hams from pigs reared under different conditions. Meat Sci. 2015, 104, 8–13. [Google Scholar] [CrossRef]
- Salum, P.; Govce, G.; Kendirci, P.; Bas, D.; Erbay, Z. Composition, proteolysis, lipolysis, volatile compound profile and sensory characteristics of ripened white cheeses manufactured in different geographical regions of Turkey. Int. Dairy J. 2018, 87, 26–36. [Google Scholar] [CrossRef]
- Van Ba, H.; Amna, T.; Hwang, I. Significant influence of particular unsaturated fatty acids and pH on the volatile compounds in meat-like model systems. Meat Sci. 2013, 94, 480–488. [Google Scholar] [CrossRef]
- Hemmler, D.; Roullier-Gall, C.; Marshall, J.W.; Rychlik, M.; Taylor, A.J.; Schmitt-Kopplin, P. Evolution of complex maillard chemical reactions, resolved in time. Sci. Rep. 2017, 7, 3227. [Google Scholar] [CrossRef]
- Saison, D.; De Schutter, D.P.; Uyttenhove, B.; Delvaux, F.; Delvaux, F.R. Contribution of staling compounds to the aged flavour of lager beer by studying their flavour thresholds. Food Chem. 2009, 114, 1206–1215. [Google Scholar] [CrossRef]
- Van Boekel, M. Formation of flavour compounds in the Maillard reaction. Biotechnol. Adv. 2006, 24, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhan, J.; Tang, X.; Li, T.; Duan, S. Characterization and identification of pork flavor compounds and their precursors in Chinese indigenous pig breeds by volatile profiling and multivariate analysis. Food Chem. 2022, 385, 132543. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Zhang, Y.; Zhang, M.; Qi, J.; Zhao, W.; Gu, J.; Guo, W.; Li, Y. Screening of specific quantitative peptides of beef by LC–MS/MS coupled with OPLS-DA. Food Chem. 2022, 387, 132932. [Google Scholar] [CrossRef]
- Dou, X.; Zhang, L.; Yang, R.; Wang, X.; Yu, L.; Yue, X.; Ma, F.; Mao, J.; Wang, X.; Li, P. Adulteration detection of essence in sesame oil based on headspace gas chromatography-ion mobility spectrometry. Food Chem. 2022, 370, 131373. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, J.; Zhu, L. Tea quality evaluation by applying E-nose combined with chemometrics methods. J. Food Sci. Tech. 2021, 58, 1549–1561. [Google Scholar] [CrossRef]
| No. | Compounds | Retention Index Was | Retention Times (s) | Drift Times (ms) | Intensity (V) | p-Value | |||
|---|---|---|---|---|---|---|---|---|---|
| Tibetan | Hu | Ujumqin | Tan | ||||||
| Aldehydes | Aldehydes | ||||||||
| 1 | n-Nonanal(monomer) | 1111.6 | 503.873 | 1.4763 | 852.90 ± 157.8 d | 1228.2 ± 114.7 c | 2176.0 ± 211.5 b | 3484.9 ± 111.6 a | <0.001 |
| 2 | n-Nonanal(dimer) | 1111.6 | 503.873 | 1.9430 | – | – | 1009.5 ± 122.0 | 1804.3 ± 158.5 | – |
| 3 | Octanal(monomer) | 1001.5 | 356.663 | 1.4052 | 393.26 ± 72.23 d | 871.25 ± 119.5 c | 1158.2 ± 222.6 b | 2345.9 ± 28.70 a | <0.001 |
| 4 | Octanal(dimer) | 1000.3 | 355.394 | 1.8268 | – | 161.20 ± 30.43 | 801.52 ± 349.6 | 3087.3 ± 150.0 | – |
| 5 | Heptanal(monomer) | 896.6 | 262.930 | 1.3380 | 714.59 ± 69.54 d | 1194.8 ± 88.76 a | 890.21 ± 59.43 c | 980.08 ± 20.32 b | <0.001 |
| 6 | Heptanal(dimer) | 895.0 | 261.717 | 1.6961 | 274.53 ± 71.29 d | 1507.0 ± 431.4 c | 2325.0 ± 454.3 b | 3630.0 ± 79.62 a | <0.001 |
| 7 | 3-Methylbutanal(monomer) | 657.4 | 147.779 | 1.1822 | 1071.1 ± 25.77 c | 1169.2 ± 30.82 a | 448.21 ± 48.05 d | 1115.9 ± 19.20 b | <0.001 |
| 8 | 3-Methylbutanal(dimer) | 659.1 | 148.294 | 1.4031 | 745.22 ± 47.10 c | 1225.4 ± 269.2 b | 208.06 ± 21.69 d | 1883.3 ± 81.89 a | <0.001 |
| 9 | Hexanal(monomer) | 796.7 | 202.150 | 1.2638 | 874.95 ± 22.03 b | 952.69 ± 33.85 a | 586.19 ± 30.34 d | 663.80 ± 18.41 c | <0.001 |
| 10 | Hexanal(dimer) | 797.4 | 202.513 | 1.5595 | 1137.6 ± 161.8 d | 3018.3 ± 254.0 b | 2592.9 ± 156.3 c | 5392.6 ± 107.6 a | <0.001 |
| 11 | Pentanal(monomer) | 699.5 | 161.350 | 1.1953 | 257.04 ± 27.81 d | 884.52 ± 66.76 a | 433.57 ± 72.08 c | 594.56 ± 13.24 b | <0.001 |
| 12 | Pentanal(dimer) | 699.5 | 161.350 | 1.4224 | – | 698.19 ± 123.9 | 138.75 ± 18.14 | 2091.4 ± 26.58 | – |
| 13 | 2-Methylbutanal | 671.4 | 152.074 | 1.1669 | 297.12 ± 7.320 b | 378.64 ± 56.69 a | 93.190 ± 38.57 c | 306.62 ± 12.66 b | <0.001 |
| 14 | (E)-2-Octenal(monomer) | 1060.3 | 427.724 | 1.3303 | – | – | 60.160 ± 21.04 | 466.27 ± 29.40 | – |
| 15 | (E)-2-Octenal(dimer) | 1060.6 | 428.108 | 1.8179 | – | – | – | 5.7300 ± 0.3700 | – |
| 16 | (E)-2-Nonenal | 1176.0 | 619.851 | 1.4068 | – | – | 199.44 ± 43.18 | 224.92 ± 48.26 | – |
| 17 | (E)-2-Heptenal(monomer) | 951.5 | 307.387 | 1.2535 | – | 44.600 ± 11.79 | 185.27 ± 65.85 | 501.61 ± 14.21 | – |
| 18 | (E)-2-Heptenal(dimer) | 952.1 | 307.879 | 1.6690 | – | – | 16.530 ± 9.510 | 255.94 ± 19.08 | – |
| 19 | (E)-2-Pentenal(monomer) | 755.4 | 182.988 | 1.1027 | 20.150 ± 4.210 c | 34.840 ± 10.47 c | 128.13 ± 21.77 b | 273.50 ± 8.970 a | <0.001 |
| 20 | (E)-2-Pentenal(dimer) | 755.2 | 182.891 | 1.3552 | – | – | 164.65 ± 27.32 | 401.62 ± 24.71 | – |
| 21 | Methional(monomer) | 902.2 | 267.016 | 1.0886 | 249.87 ± 40.72 b | 402.23 ± 88.24 c | 41.190 ± 14.93 a | 219.02 ± 10.17 b | <0.001 |
| 22 | Methional(dimer) | 901.1 | 266.212 | 1.3970 | – | 82.540 ± 33.22 | 79.580 ± 16.08 | 91.040 ± 6.470 | – |
| 23 | Benzaldehyde(monomer) | 955.5 | 310.997 | 1.1478 | 1338.8 ± 54.49 a | 1308.5 ± 110.7 a | 630.29 ± 140.7 c | 995.33 ± 20.19 b | <0.001 |
| 24 | Benzaldehyde(dimer) | 956.5 | 311.847 | 1.4698 | 861.81 ± 110.0 b | 995.85 ± 114.7 a | 241.36 ± 106.0 d | 638.56 ± 28.82 c | <0.001 |
| 25 | Phenylacetaldehyde | 1042.8 | 404.911 | 1.2571 | – | – | – | 85.830 ± 1.570 | – |
| 26 | Butanal | 632.5 | 140.723 | 1.1124 | 46.070 ± 12.49 b | 51.040 ± 10.97 b | 541.50 ± 139.6 a | 21.980 ± 8.920 b | <0.001 |
| Alcohols | |||||||||
| 27 | 1-Octen-3-ol(monomer) | 978.2 | 332.525 | 1.1579 | 23.380 ± 3.270 d | 527.47 ± 92.48 c | 721.45 ± 172.5 b | 875.78 ± 29.51 a | <0.001 |
| 28 | 1-Octen-3-ol(dimer) | 977.4 | 331.738 | 1.5990 | – | – | – | 15.280 ± 5.180 | – |
| 29 | Ethanol(monomer) | 355.2 | 94.087 | 1.0473 | 1309.1 ± 20.07 b | 178.19 ± 64.75 d | 2799.2 ± 116.4 a | 509.92 ± 76.29 c | <0.001 |
| 30 | Ethanol(dimer) | 353.9 | 93.969 | 1.1330 | 57.740 ± 8.370 | 20.100 ± 5.530 | 1853.8 ± 57.72 | – | – |
| 31 | 1-Hexanol(monomer) | 870.0 | 244.461 | 1.3263 | – | 121.97 ± 53.86 | 1203.0 ± 95.83 | 744.45 ± 16.20 | – |
| 32 | 1-Hexanol(dimer) | 869.3 | 243.958 | 1.6447 | – | – | 1195.3 ± 193.5 | 66.860 ± 7.360 | – |
| 33 | Methyl-2-butan-1-ol | 722.3 | 169.615 | 1.2336 | – | 472.66 ± 72.18 | 578.06 ± 24.30 | 414.11 ± 23.15 | – |
| 34 | Heptanol(monomer) | 970.7 | 325.203 | 1.4007 | – | – | 265.75 ± 73.50 | 208.75 ± 10.02 | – |
| 35 | Heptanol(dimer) | 970.3 | 324.829 | 1.7668 | – | – | 39.870 ± 38.22 | – | – |
| 36 | (E)-2-Hexen-1-ol(monomer) | 849.0 | 231.146 | 1.1791 | 67.900 ± 5.440 d | 108.81 ± 22.80 c | 173.72 ± 17.84 b | 436.25 ± 13.92 a | <0.001 |
| 37 | (E)-2-Hexen-1-ol(dimer) | 849.0 | 231.146 | 1.5160 | – | – | – | 253.87 ± 21.38 | – |
| 38 | 1-Pentanol(monomer) | 769.4 | 189.159 | 1.2548 | 63.480 ± 21.04 d | 434.02 ± 57.26 c | 486.54 ± 50.54 b | 10008 ± 11.86 a | <0.001 |
| 39 | 1-Pentanol(dimer) | 769.5 | 189.191 | 1.5160 | – | 21.750 ± 6.060 | 267.17 ± 146.3 | 660.26 ± 32.92 | – |
| 40 | Cis-3-hexenol | 866.2 | 241.985 | 1.2291 | – | 44.930 ± 2.260 | – | – | – |
| 41 | 2-Methyl-1-propanol | 629.0 | 139.767 | 1.1732 | 200.34 ± 32.43 b | 223.72 ± 48.44 b | 176.87 ± 62.28 b | 372.14 ± 32.26 a | <0.001 |
| 42 | 2-Propanol | 486.8 | 110.348 | 1.1797 | 148.67 ± 13.74 b | 191.69 ± 49.32 b | 361.86 ± 120.9 a | 380.49 ± 35.08 a | <0.001 |
| 43 | 3-Methyl-3-buten-1-ol | 739.4 | 176.297 | 1.5005 | – | – | 86.540 ± 74.51 | – | – |
| 44 | 2,3-Butanediol | 796.1 | 201.865 | 1.3666 | 190.41 ± 36.31 c | 147.13 ± 66.19 c | 695.25 ± 52.24 a | 253.54 ± 7.260 b | <0.001 |
| Ketones | |||||||||
| 45 | 2-Pentanone(monomer) | 687.6 | 157.292 | 1.1198 | 635.89 ± 13.82 a | 339.10 ± 27.00 b | 275.64 ± 104.3 b | 73.940 ± 5.340 c | <0.001 |
| 46 | 2-Pentanone(dimer) | 689.5 | 157.941 | 1.3672 | 803.69 ± 58.28 a | 394.90 ± 49.01 b | 709.80 ± 110.2 a | 430.08 ± 14.15 b | <0.001 |
| 47 | 3-Pentanone | 700.2 | 161.577 | 1.3504 | 270.94 ± 25.81 c | 133.72 ± 11.57 d | 476.41 ± 42.06 a | 348.99 ± 12.20 b | <0.001 |
| 48 | 2-Heptanone(monomer) | 885.6 | 255.072 | 1.2622 | 59.490 ± 10.90 d | 210.50 ± 25.50 c | 252.10 ± 49.42 b | 496.72 ± 13.35 a | <0.001 |
| 49 | 2-Heptanone(dimer) | 884.4 | 254.232 | 1.6306 | – | – | 427.75 ± 79.73 | 254.21 ± 18.43 | – |
| 50 | 1-Octen-3-one(monomer) | 1029.4 | 388.481 | 1.2639 | – | – | – | 268.88 ± 9.850 | – |
| 51 | 1-Octen-3-one(dimer) | 1029.1 | 388.096 | 1.6851 | – | – | – | 9.4600 ± 1.010 | – |
| 52 | 3-Hydroxy-2-butanone(monomer) | 721.9 | 169.481 | 1.0655 | 1823.5 ± 857.3 a | 2115.7 ± 541.0 a | 847.44 ± 79.35 a | 1968.6 ± 38.99 a | 0.478 |
| 53 | 3-Hydroxy-2-butanone(dimer) | 722.3 | 169.626 | 1.3298 | – | 2787.35 ± 377.1 | 3898.6 ± 122.5 | 2541.7 ± 127.3 | – |
| 54 | 2-Hexanone | 787.2 | 197.492 | 1.1909 | 102.41 ± 4.460 b | 108.82 ± 16.81 ab | 127.09 ± 28.21 a | 115.72 ± 7.060 ab | 0.105 |
| 55 | 2-Butanone(monomer) | 591.0 | 130.333 | 1.0590 | 1666.1 ± 36.94 a | 1684.9 ± 86.22 a | 271.69 ± 45.84 c | 1381.3 ± 32.19 b | <0.001 |
| 56 | 2-Butanone(dimer) | 587.2 | 129.463 | 1.2448 | 4543.0 ± 290.1 a | 4637.8 ± 428.7 a | 2513.1 ± 262.0 b | 5002.0 ± 167.5 a | <0.001 |
| 57 | 2-Propanone | 481.7 | 109.558 | 1.1164 | 3151.0 ± 397.2 ab | 3378.9 ± 269.9 a | 2849.6 ± 271.0 bc | 2430.6 ± 502.7 c | 0.002 |
| 58 | 4-Methyl-2-pentanone | 740.3 | 176.683 | 1.1776 | 171.89 ± 14.80 a | 151.58 ± 23.41 a | 84.480 ± 13.38 b | 101.50 ± 11.93 b | <0.001 |
| 59 | 6-Methyl-5-hepten-2-one | 984.7 | 339.068 | 1.1773 | – | 104.24 ± 29.93 | 50.100 ± 27.64 | 37.020 ± 13.96 | – |
| Esters | |||||||||
| 60 | Ethyl acetate(monomer) | 620.2 | 137.471 | 1.0946 | 11.730 ± 2.600 | – | 506.50 ± 111.6 | 6.3400 ± 2.500 | – |
| 61 | Ethyl acetate (dimer) | 614.2 | 135.942 | 1.3345 | – | – | 3536.6 ± 324.6 | – | – |
| 62 | ethyl 2-hydroxypropanoate | 810.9 | 209.472 | 1.1442 | – | – | 134.89 ± 33.77 | 3.9900 ± 1.030 | – |
| 63 | Butyl propanoate | 873.9 | 247.025 | 1.2849 | – | – | 24.740 ± 12.12 | – | – |
| 64 | (Z)3-Hexen-1-ol-acetate | 984.3 | 338.625 | 1.3063 | – | – | 26.980 ± 7.070 | 3.3300 ± 1.620 | – |
| 65 | Methyl benzoate(monomer) | 1097.2 | 481.030 | 1.2179 | 1005.26 ± 20.60 a | 936.50 ± 19.08 b | 690.08 ± 22.35 c | 709.72 ± 15.68 c | <0.001 |
| 66 | Methyl benzoate(dimer) | 1096.8 | 480.396 | 1.6071 | 38.160 ± 4.010 | 26.630 ± 3.900 | 10.650 ± 2.700 | – | – |
| 67 | Butyl butanoate | 988.6 | 343.068 | 1.3363 | – | – | 18.420 ± 4.620 | 28.180 ± 170.0 | – |
| Furan | |||||||||
| 68 | 2-Pentylfuran | 988.5 | 342.924 | 1.2520 | – | – | 48.520 ± 5.390 | 123.94 ± 9.370 | – |
| Ether | |||||||||
| 69 | 1-Propene-3-methylthio | 701.6 | 162.080 | 1.0425 | 807.79 ± 26.87 a | 37.280 ± 11.45 c | 480.16 ± 78.12 b | 21.380 ± 1.260 c | <0.001 |
| Thiazole | |||||||||
| 70 | Trimethylthiazole | 955.3 | 310.770 | 1.5658 | – | 35.100 ± 7.700 | 80.390 ± 20.60 | 455.97 ± 21.06 | – |
| 71 | 2-Acetylthiazole | 1018.8 | 375.938 | 1.1294 | – | – | 45.160 ± 22.38 | – | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Wang, H.; Liang, Z.; Liu, J.; Yang, C.; Zhai, H.; Chen, A.; Lu, Z.; Gao, Y.; Ding, X.; et al. Characterization of Flavor Compounds in Chinese Indigenous Sheep Breeds Using Gas Chromatography–Ion Mobility Spectrometry and Chemometrics. Foods 2024, 13, 2647. https://doi.org/10.3390/foods13172647
Wang F, Wang H, Liang Z, Liu J, Yang C, Zhai H, Chen A, Lu Z, Gao Y, Ding X, et al. Characterization of Flavor Compounds in Chinese Indigenous Sheep Breeds Using Gas Chromatography–Ion Mobility Spectrometry and Chemometrics. Foods. 2024; 13(17):2647. https://doi.org/10.3390/foods13172647
Chicago/Turabian StyleWang, Fang, Hongbo Wang, Zeyi Liang, Jing Liu, Chen Yang, Huan Zhai, Anle Chen, Zengkui Lu, Yaqin Gao, Xuezhi Ding, and et al. 2024. "Characterization of Flavor Compounds in Chinese Indigenous Sheep Breeds Using Gas Chromatography–Ion Mobility Spectrometry and Chemometrics" Foods 13, no. 17: 2647. https://doi.org/10.3390/foods13172647
APA StyleWang, F., Wang, H., Liang, Z., Liu, J., Yang, C., Zhai, H., Chen, A., Lu, Z., Gao, Y., Ding, X., & Liu, J. (2024). Characterization of Flavor Compounds in Chinese Indigenous Sheep Breeds Using Gas Chromatography–Ion Mobility Spectrometry and Chemometrics. Foods, 13(17), 2647. https://doi.org/10.3390/foods13172647





