UV-B Radiation Exhibited Tissue-Specific Regulation of Isoflavone Biosynthesis in Soybean Cell Suspension Cultures
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Cell Suspension Cultures
2.3. Microscopic Observations
2.4. Cell Growth and Viability
2.5. Physiological Metabolism
2.6. Phenolics and Flavonoid Contents and Their Antioxidant Capacity
2.7. Identification and Quantification of Isoflavonoids
2.8. Gene Expression and Enzyme Activity in Isoflavone Synthesis
2.9. Statistical Analysis
3. Results
3.1. Physiological Indicators of Cell Suspension Cultures
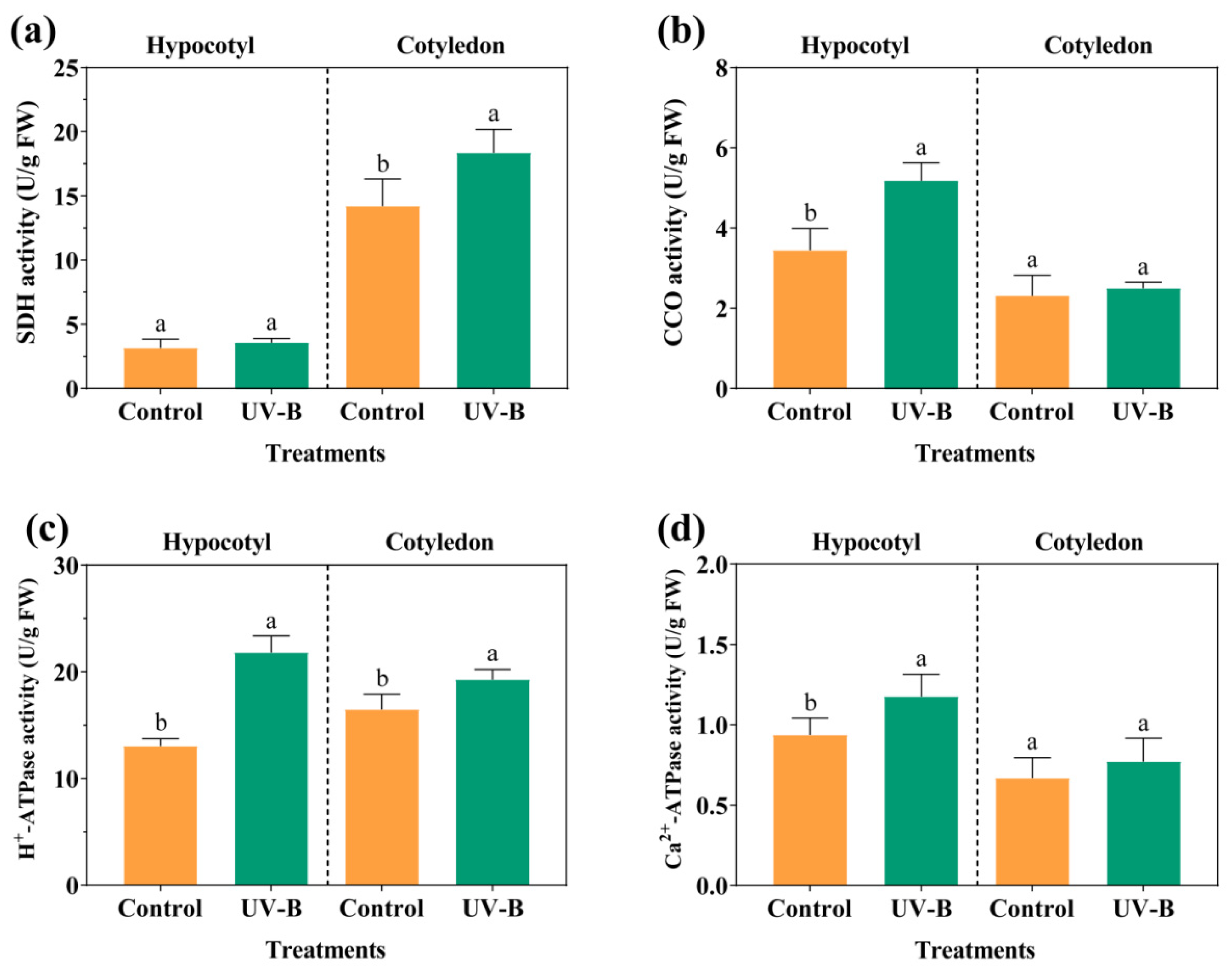
3.2. Energy Metabolism of Cell Suspension Cultures
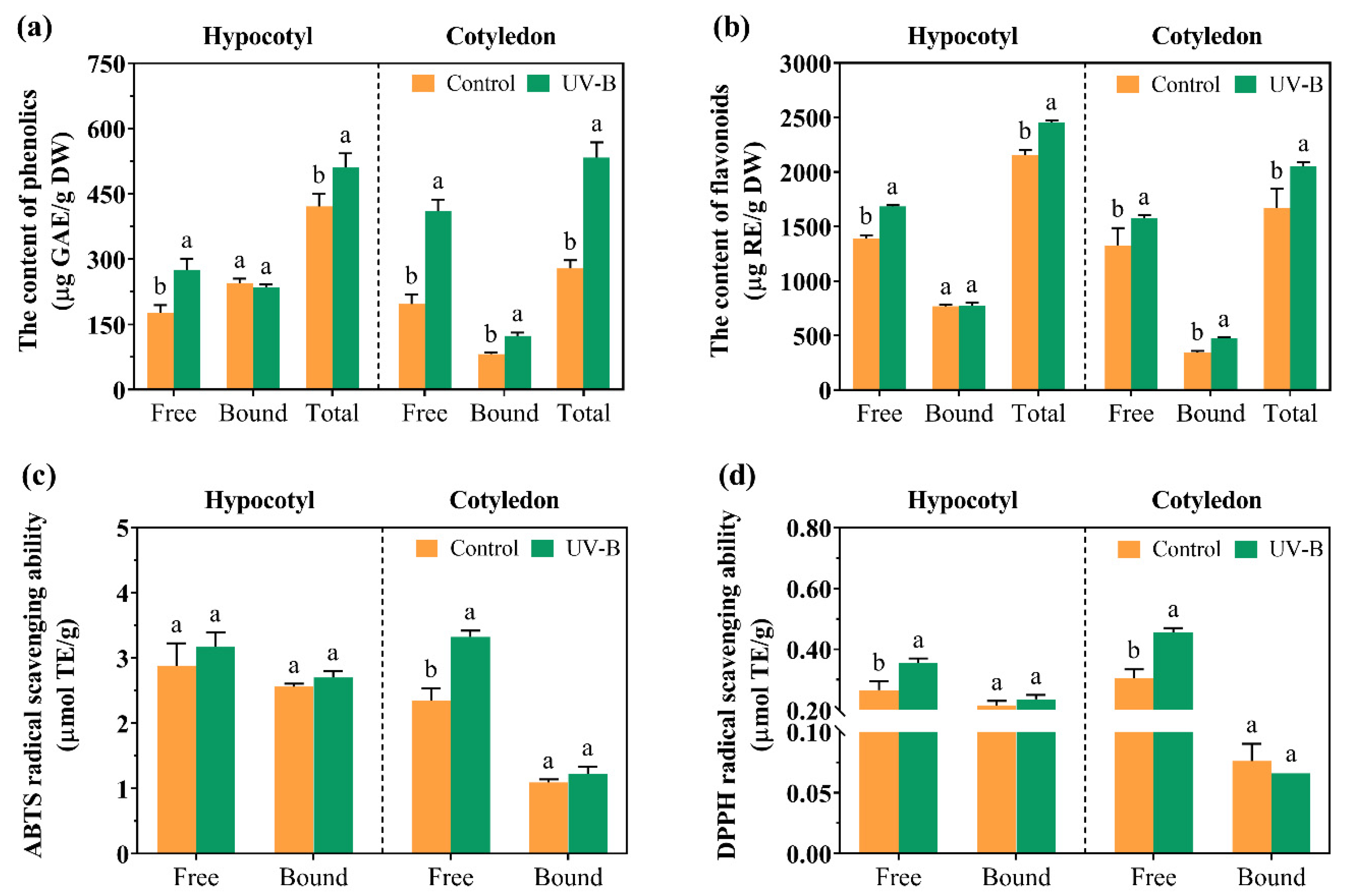
3.3. The Accumulation of Secondary Metabolites in Cell Suspension Cultures
3.4. Isoflavone Composition and Quantification of Cell Suspension Cultures
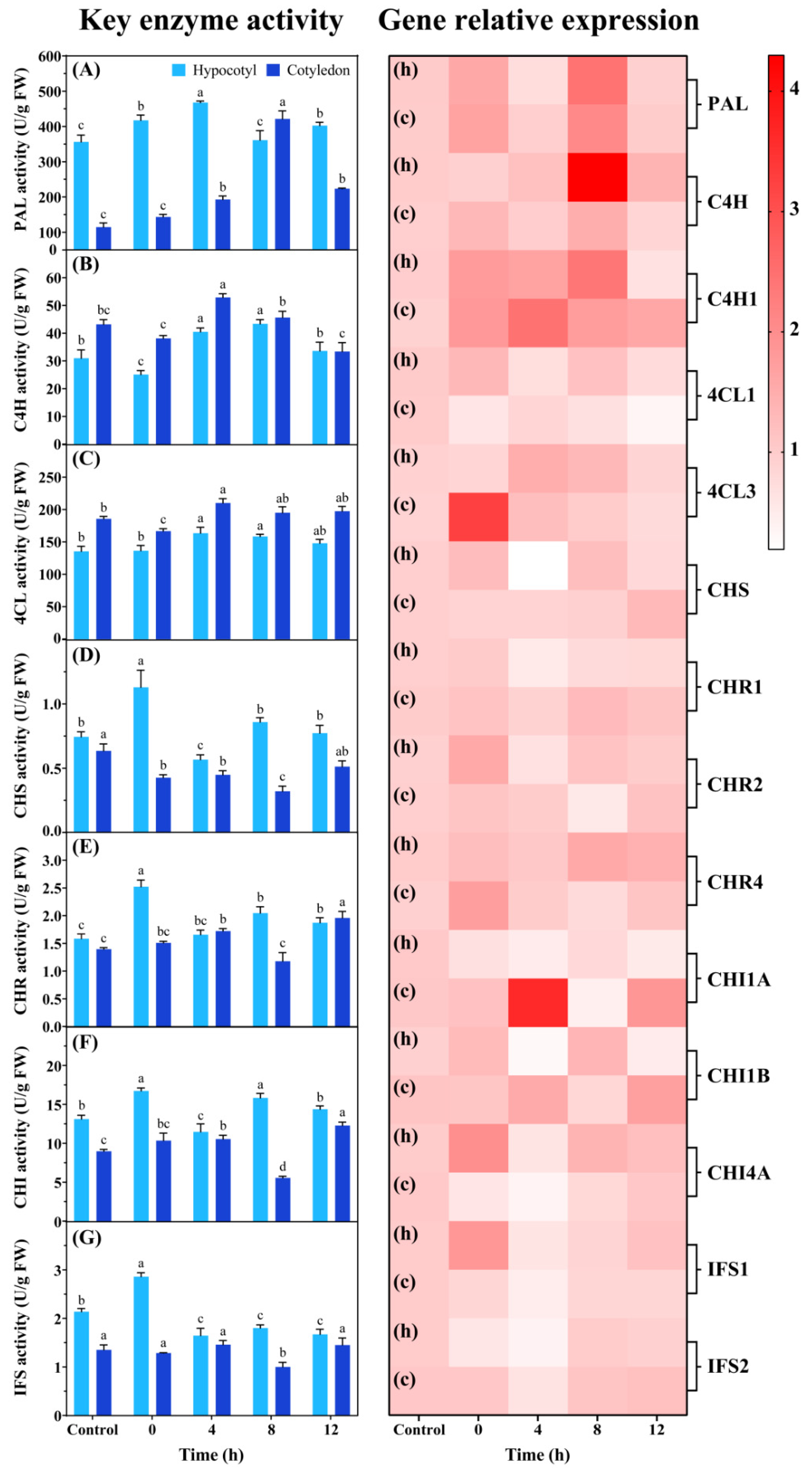
3.5. The Key Enzyme Activities and Relative Expression of Isoflavone Synthesis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Phenolic compounds: Structure, classification, and antioxidant power. In Bioactive Compounds; Woodhead Publishing: Sawston, UK, 2019; pp. 33–50. [Google Scholar]
- David, C.K.; John, A.E. Phytoestrogen-a short review. Maturitas 1995, 22, 167–175. [Google Scholar]
- Chen, L.R.; Nai, Y.; Kuo, H. Isoflavone supplements for menopausal women: A systematic review. Nutrients 2019, 11, 2649. [Google Scholar] [CrossRef] [PubMed]
- Thoma, F.; Somborn-Schulz, A.; Schlehuber, D.; Keuter, V.; Deerberg, G. Effects of light on secondary metabolites in selected leafy greens: A review. Front. Plant Sci. 2020, 11, 497. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Wang, P.; Yang, R.Q.; Gu, Z.X. Effects of UV-B radiation on the isoflavone accumulation and physiological-biochemical changes of soybean during germination Physiological-biochemical change of germinated soybean induced by UV-B. Food Chem. 2018, 250, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.J.; Kwon, S.J.; Eom, S.H. Red and blue light-specific metabolic changes in soybean seedlings. Front. Plant Sci. 2023, 14, 1128001. [Google Scholar] [CrossRef]
- Yin, Y.Q.; Tian, X.; Yang, J.; Yang, Z.F.; Tao, J.; Fang, W.M. Melatonin mediates isoflavone accumulation in germinated soybeans (Glycine max L.) under ultraviolet-B stress. Plant Physiol. Biochem. 2022, 175, 23–32. [Google Scholar] [CrossRef]
- Eum, H.L.; Park, Y.; Yi, T.G.; Lee, J.W.; Ha, K.S.; Choi, I.Y.; Park, N.I.L. Effect of germination environment on the biochemical compounds and anti-inflammatory properties of soybean cultivars. PLoS ONE 2020, 15, e0232159. [Google Scholar] [CrossRef]
- Occhialini, A.; Nguyen, M.-A.; Dice, L.; Pfotenhauer, A.C.; Sultana, M.S.; Neal Stewart, C., Jr.; Lenaghan, S.C. High-throughput transfection and analysis of soybean (Glycine max) protoplasts. Methods Mol. Biol. 2022, 2464, 245–259. [Google Scholar]
- Huckelhoven, R.; Schuphan, I.; Thiede, B.; Schmidt, B. Biotransformation of pyrene by cell cultures of soybean (Glycine max L), wheat (Triticum aestivum L), jimsonweed (Datura stramonium L), and purple foxglove (Digitalis purpurea L). J. Agric. Food Chem. 1997, 4, 263–269. [Google Scholar] [CrossRef]
- Rani, D.; Vimolmangkang, S. Trends in the biotechnological production of isoflavonoids in plant cell suspension cultures. Phytochem. Rev. 2022, 21, 1843–1862. [Google Scholar] [CrossRef]
- Jeong, Y.J.; An, C.H.; Park, S.C.; Pyun, J.W.; Lee, J.; Kim, S.W.; Kim, H.S.; Kim, H.R.; Jeong, J.C.; Kim, C.Y. Methyl jasmonate increases isoflavone production in soybean cell cultures by activating structural genes involved in isoflavonoid biosynthesis. J. Agric. Food Chem. 2018, 66, 4099–4105. [Google Scholar] [CrossRef] [PubMed]
- Devi, M.K.A.; Kumar, G.; Giridhar, P. Effect of biotic and abiotic elicitors on isoflavone biosynthesis during seed de-velopment and in suspension cultures of soybean (Glycine max L.). 3 Biotech. 2020, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Veremeichik, G.N.; Grigorchuk, V.P.; Silanteva, S.A.; Shkryl, Y.N.; Bulgakov, D.V.; Brodovskaya, E.V.; Bulgakov, V.P. Increase in isoflavonoid content in Glycine max cells transformed by the constitutively active Ca2+ independent form of the AtCPK1 gene. Phytochemistry 2019, 157, 111–120. [Google Scholar] [CrossRef]
- Wang, M.; Liu, G.N.; Guo, T.W.; Xie, C.; Wang, P.; Yang, R.Q. UV-B radiation enhances isoflavone accumulation and antioxidant capacity of soybean calluses. Front. Nutr. 2023, 10, 1139698. [Google Scholar] [CrossRef] [PubMed]
- Towill, L.E.; Mazur, P. Studies on the reduction of 2,3,5-triphenyltetrazolium chloride as a viability assay for plant tissue cultures. Can. J. Bot. 1975, 53, 1097–1102. [Google Scholar] [CrossRef]
- Jin, P.; Zhang, Y.; Shan, T.M.; Huang, Y.P.; Xu, J.; Zheng, Y.H. Low-temperature conditioning alleviates chilling injury in loquat fruit and regulates glycine betaine content and energy status. J. Agric. Food Chem. 2015, 63, 3654–3659. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Leng, C.; Zhu, Y.; Wang, P.; Gu, Z.; Yang, R. UV-B treatment enhances phenolic acids accumulation and antioxidant capacity of barley seedlings. LWT 2022, 153, 112445. [Google Scholar] [CrossRef]
- Ma, M.; Xu, W.L.; Wang, P.; Gu, Z.X.; Zhang, H.Z.; Yang, R.Q. UV-B-triggered H2O2 production mediates isoflavones synthesis in germinated soybean. Food Chem. X 2022, 14, 100331. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, P.; Zhou, T.; Chen, Z.J.; Yang, R.Q. NaCl stress on physio-biochemical metabolism and antioxidant ca-pacity in germinated hulless barley (Hordeum vulgare L.). J. Sci. Food Agric. 2019, 99, 1755–1764. [Google Scholar] [CrossRef]
- Li, D.; Limwachiranon, J.; Li, L.; Du, R.X.; Luo, Z.S. Involvement of energy metabolism to chilling tolerance induced by hydrogen sulfide in cold-stored banana fruit. Food Chem. 2016, 208, 272–278. [Google Scholar] [CrossRef]
- Jin, P.; Zhu, H.; Wang, L.; Shan, T.M.; Zheng, Y.H. Oxalic acid alleviates chilling injury in peach fruit by regulating energy metabolism and fatty acid contents. Food Chem. 2014, 161, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Li, L.; Luo, Z.S.; Zeng, F.F.; Jiang, L.; Tang, K.C. Effect of brassinolide on energy status and proline me-tabolism in postharvest bamboo shoot during chilling stress. Postharvest Biol. Technol. 2016, 111, 240–246. [Google Scholar] [CrossRef]
- Nassarawa, S.S.; Bao, N.A.; Zhang, X.T.; Ru, Q.M.; Luo, Z.S. Evaluation of light irradiation on anthocyanins and energy metabolism of grape (Vitis vinifera L.) during storage. Food Chem. 2024, 431, 137141. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; Zhao, G.; Zhou, Y.; Xia, X.; Wang, J.; Wang, G.; Lu, S.; He, W.; Bi, T.; Li, J. Metabonomics analysis of fla-vonoids in seeds and sprouts of two Chinese soybean cultivars. Sci. Rep. 2022, 12, 5541. [Google Scholar] [PubMed]
- Liu, Y.; Liu, J.; Wang, Y.; Abozeid, A.; Tian, D.M.; Zhang, X.N.; Tang, Z.H. The different resistance of two Astragalus plants to UV-B stress is tightly associated with the organ-specific isoflavone metabolism. Photochem. Photobiol. 2018, 94, 115–125. [Google Scholar] [CrossRef]
- Santin, M.; Castagna, A.; Miras-Moreno, B.; Rocchetti, G.; Lucini, L.; Hauser, M.T.; Ranieri, A. Beyond the visible and below the peel: How UV-B radiation influences the phenolic profile in the pulp of peach fruit. A biochemical and molecular study. Front. Plant Sci. 2020, 11, 579063. [Google Scholar] [CrossRef] [PubMed]
- Nam, T.-G.; Lim, Y.J.; Eom, S.H. Flavonoid accumulation in common buckwheat (Fagopyrum esculentum) sprout tissues in response to light. Hortic. Environ. Biotechnol. 2018, 59, 19–27. [Google Scholar] [CrossRef]
- Kakade, M.L.; Simons, N.R.; Liener, I.E.; Lambert, J.W. Biochemical and nutritional assessment of different varieties of soybeans. J. Agric. Food Chem. 1972, 20, 87–90. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Pereira, A.G.; Lourenco-Lopes, C.; Garcia-Oliveira, P.; Cassani, L.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Main bioactive phenolic compounds in marine algae and their mechanisms of action supporting potential health benefits. Food Chem. 2020, 341, 128262. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Mori, T.; Nishizawa, T.; Saito, K. Temporal lag between gene expression and metabolite accumulation in flavonol biosynthesis of Arabidopsis roots. Phytochem Lett. 2017, 22, 44–48. [Google Scholar] [CrossRef]
- Newton, C.B.; Young, E.M.; Roberts, S.C. Targeted control of supporting pathways in paclitaxel biosynthesis with CRISPR-guided methylation. Front. Bioeng. Biotech. 2023, 11, 1272811. [Google Scholar]
- Bamneshin, M.; Mirjalili, M.H.; Naghavi, M.R.; Cusido, R.M.; Palazon, J. Gene expression pattern and taxane bio-synthesis in a cell suspension culture of Taxus baccata L. subjected to light and a phenylalanine ammonia lyase (PAL) in-hibitor. J. Photochem. Photobiol. B 2022, 234, 112532. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jeong, Y.J.; Park, S.H.; Park, S.C.; Lee, S.B.; Lee, J.; Kim, S.W.; Ha, B.K.; Kim, H.S.; Kim, H.; et al. The synergistic effect of co-treatment of methyl jasmonate and cyclodextrins on pterocarpan production in Sophora flavescens cell cultures. Int. J. Mol. Sci. 2020, 21, 3944. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Gao, M.; Kang, S.O.C.; Yang, C.M.; Meng, H.; Yang, Y.; Zhao, X.S.; Gao, Z.H.; Xu, Y.H.; Jin, Y.; et al. Molecular mechanism underlying mechanical wounding-induced flavonoid accumulation in Dalbergia odorifera T. Chen, an endangered tree that produces Chinese rosewood. Genes 2020, 11, 478. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primer Name | Primer Sequences (5′→3′) | Accession Number |
|---|---|---|---|
| PAL | Sense | GCTAAGAAGTTGCATGAGATTGA | NM_001357058.1 |
| Ant-sense | TCATTGACAAGCTCAGAGAATTG | ||
| C4H | Sense | CGATTTGGCCAAAAAATTCGGTG | NM_001250388.3 |
| Ant-sense | CCTTTCCGGTGAAGATGTCG | ||
| C4H1 | Sense | CGTAGAATTTGGCTCTCGCCC | NM_001371219.1 |
| Ant-sense | GCTGAAGCCGCCTTCTGAT | ||
| 4CL1 | Sense | CGGTGAAATTTGCATAAGAGGC | NM_001250821.2 |
| Ant-sense | AATCCTTTGTATTTGATCAATTCCT | ||
| 4CL3 | Sense | ATCTCCAACCACCTCCCT | NM_001250341.2 |
| Ant-sense | GGATTCCGAGGTTGGACAAT | ||
| CHS | Sense | GCTATTGATGGACACCTTCG | NM_001371381.1 |
| Ant-sense | ACCAGGGTGTGCAATCCA | ||
| CHI1A | Sense | CATTGGATGGTCGTGAATACGT | NM_001248290.2 |
| Ant-sense | TTGTAGAAAACAGTGGAGCCTG | ||
| CHI1B | Sense | CATTGGATGGTCGTGAATACGT | NM_001249826.2 |
| Ant-sense | TTGTAGAAAACAGTGGAGCCTG | ||
| CHI4A | Sense | ATCTTTGCTTGGCCATGGAAT | NM_001249853.2 |
| Ant-sense | AGCTGCCAACCTATCCCT | ||
| CHR1 | Sense | ACTTCCAAGCTTTGGGTCAC | NM_001249044.2 |
| Ant-sense | AGGCCAAGTTTCTGGCACT | ||
| CHR2 | Sense | GATTCATTGGCCAGTGAGGC | NM_001367003.1 |
| Ant-sense | TCCACCTGATTGACAGCAGGA | ||
| CHR4 | Sense | AGCAGGCTCTTGGAGAAG | NM_001367005.1 |
| Ant-sense | CCGAAGCGAATTTTGTAGAGCA | ||
| IFS1 | Sense | GAGAGCTGGCCTCACAGTTC | NM_001249093.2 |
| Ant-sense | TGCGATGGCAAGACACTACT | ||
| IFS2 | Sense | TGGAAGTTCGTGAGGAAG | NM_001251586.2 |
| Ant-sense | ATGGAGATGGTGCTGTTG | ||
| EF1b | Sense | CCACTGCTGAAGAAGATGATGATG | NM_001249608.2 |
| Ant-sense | AAGGACAGAAGACTTGCCACTC |
| Part | Treatment | Content (µg/g FW) | Energy Charge | ||
|---|---|---|---|---|---|
| ATP | ADP | AMP | |||
| Hypocotyl | Control | 30.59 ± 1.49 a | ND | 20.67 ± 0.43 b | 0.60 ± 0.02 a |
| UV-B | 30.45 ± 0.57 a | 11.12 ± 0.37 a | 25.77 ± 1.11 a | 0.54 ± 0.01 b | |
| Cotyledon | Control | 26.51 ± 1.41 b | ND | 32.40 ± 1.87 a | 0.45 ± 0.01 b |
| UV-B | 34.18 ± 1.07 a | 9.47 ± 1.16 a | 26.96 ± 1.63 b | 0.55 ± 0.01 a | |
| Isoflavone | Hypocotyl | Cotyledon | ||
|---|---|---|---|---|
| Control | UV-B | Control | UV-B | |
| Daidzin | 45.19 ± 2.05 b | 173.33 ± 12.66 a | ND | 74.22 ± 2.86 a |
| Glycitin | ND | ND | 25.78 ± 1.07 b | 36.59 ± 0.59 a |
| Genistin | 89.66 ± 1.49 b | 139.57 ± 8.36 a | ND | ND |
| Malonyl daidzin | 106.54 ± 8.64 b | 217.20 ± 5.43 a | 161.47 ± 12.17 b | 279.28 ± 10.90 a |
| Malonyl glycitin | 54.78 ± 4.92 b | 98.00 ± 9.08 a | ND | 34.94 ± 1.69 a |
| Malonyl genistin | 297.49 ± 9.48 a | 83.11 ± 5.99 b | 83.74 ± 0.62 a | 68.93 ± 6.13 b |
| Daidzein | ND | ND | 27.98 ± 2.07 b | 47.15 ± 2.54 a |
| Glycitein | ND | ND | ND | ND |
| Genistein | ND | ND | ND | 30.56 ± 0.65 a |
| Total | 593.65 ± 14.42 b | 711.21 ± 16.08 a | 298.97 ± 12.51 b | 571.65 ± 13.10 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Wang, Y.; Bilal, M.; Xie, C.; Wang, P.; Rui, X.; Yang, R. UV-B Radiation Exhibited Tissue-Specific Regulation of Isoflavone Biosynthesis in Soybean Cell Suspension Cultures. Foods 2024, 13, 2385. https://doi.org/10.3390/foods13152385
Wang M, Wang Y, Bilal M, Xie C, Wang P, Rui X, Yang R. UV-B Radiation Exhibited Tissue-Specific Regulation of Isoflavone Biosynthesis in Soybean Cell Suspension Cultures. Foods. 2024; 13(15):2385. https://doi.org/10.3390/foods13152385
Chicago/Turabian StyleWang, Mian, Yiting Wang, Muhammad Bilal, Chong Xie, Pei Wang, Xin Rui, and Runqiang Yang. 2024. "UV-B Radiation Exhibited Tissue-Specific Regulation of Isoflavone Biosynthesis in Soybean Cell Suspension Cultures" Foods 13, no. 15: 2385. https://doi.org/10.3390/foods13152385
APA StyleWang, M., Wang, Y., Bilal, M., Xie, C., Wang, P., Rui, X., & Yang, R. (2024). UV-B Radiation Exhibited Tissue-Specific Regulation of Isoflavone Biosynthesis in Soybean Cell Suspension Cultures. Foods, 13(15), 2385. https://doi.org/10.3390/foods13152385








