The Volatile Composition and the Potential Health Benefits of Different Microalgae Strains
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Methods
2.2.1. Culture Growth Conditions and Harvesting
2.2.2. Extraction
2.2.3. Nutritional Composition
2.2.4. Quantification of Chlorophyll a, b, and Carotenoids
2.2.5. Antioxidant Activity
FRAP Assay
DPPH Assay
2.2.6. Total Phenolic Content
2.2.7. Determination of Phenolic Compounds Composition
2.2.8. α-Amylase Inhibition
2.2.9. Volatile Organic Compounds Profile
2.2.10. Statistical Analysis
3. Results
3.1. Nutritional Composition
3.2. Chlorophyll and Carotenoid Quantification
3.3. Antioxidant Activity and Total Phenolic Content
3.4. Identification and Quantification of Phenolic Compounds
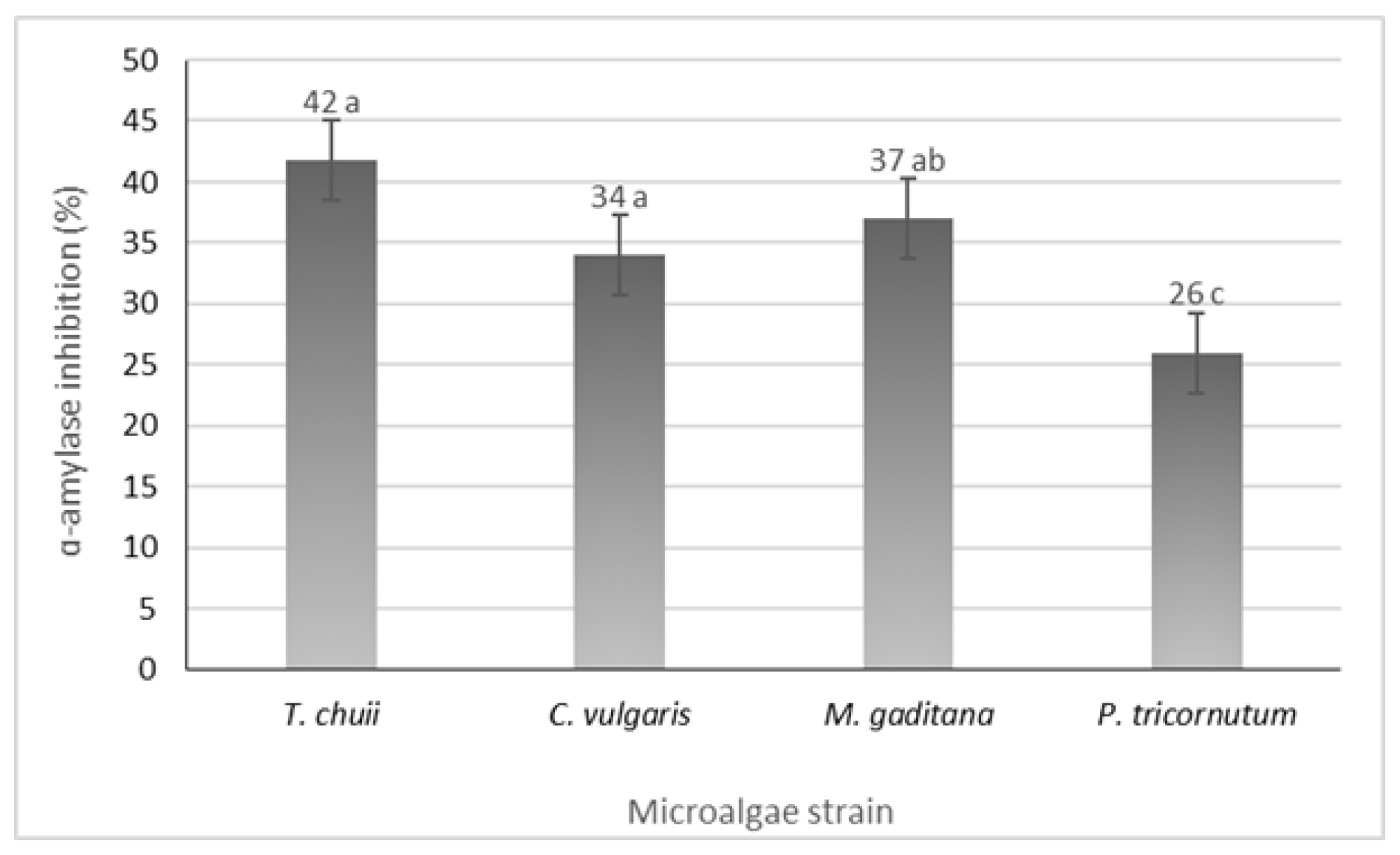
3.5. α-Amylase Inhibition Potential
3.6. Volatile Organic Compounds Profile
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torres-Tiji, Y.; Fields, F.J.; Mayfield, S.P. Microalgae as a future food source. Biotechnol. Adv. 2020, 41, 107536. [Google Scholar] [CrossRef] [PubMed]
- Qazi, M.W.; de Sousa, I.; Nunes, M.C.; Raymundo, A. Improving the Nutritional, Structural, and Sensory Properties of Gluten-Free Bread with Different Species of Microalgae. Foods 2022, 11, 397. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.C.; Ferreira, J.; Raymundo, A. Volatile fingerprint impact on the sensory properties of microalgae and development of mitigation strategies. Curr. Opin. Food Sci. 2023, 51, 101040. [Google Scholar] [CrossRef]
- AACC International. Approved Methods of the American Association of Cereal Chemists, 10th ed.; Method 08-01, 46-10, 10-10B, and 54-21; The Association: St. Paul, MN, USA, 2000. [Google Scholar]
- Norma Portuguesa. Cereais e Derivados-Determinação Do Teor de Matéria Gorda Total. 1991. Available online: http://www.Esa.Ipsantarem.Pt/Norma_IPQ_homepage_206.Pdf (accessed on 30 July 2023).
- Kirsten, W.J. Automatic Methods for the Simultaneous Determination of Carbon, Hydrogen, Nitrogen, and Sulfur, and for Sulfur Alone in Organic and Inorganic Materials. Anal. Chem. 1979, 51, 1173–1179. [Google Scholar] [CrossRef]
- Aher, R.; Kumar, B.; Sudalai, A. One-Pot Synthesis of Cyclic Carbonates from Aldehydes, Sulfur Ylide, and CO2. Synlett 2013, 25, 97–101. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A Procedure to Measure the Antiradical Efficiency of Polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Fukumoto, L.R.; Mazza, G. Assessing Antioxidant and Prooxidant Activities of Phenolic Compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef] [PubMed]
- Dvořáková, M.; Hulín, P.; Karabín, M.; Dostálek, P. Determination of Polyphenols in Beer by an Effective Method Based onSolid-Phase Extraction and High Performance Liquid Chromatography with Diode-Array Detection. Czech J. Food Sci. 2007, 25, 182–188. [Google Scholar] [CrossRef]
- Wickramaratne, M.N.; Punchihewa, J.C.; Wickramaratne, D.B.M. In-vitro alpha amylase inhibitory activity of the leaf extracts of Adenanthera pavonina. BMC Complement. Altern. Med. 2016, 16, 466. [Google Scholar] [CrossRef]
- Zanella, L.; Vianello, F. Microalgae of the genus Nannochloropsis: Chemical composition and functional implications for human nutrition. J. Funct. Foods 2020, 68, 103919. [Google Scholar] [CrossRef]
- Costa, M.M.; Spínola, M.P.; Prates, J.A.M. Microalgae as an Alternative Mineral Source in Poultry Nutrition. Vet. Sci. 2024, 11, 44. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; Abd El-Hack, M.E.; Arif, M.; Khafaga, A.F.; Taha, A.E. The application of the microalgae I spp. as a supplement in broiler feed. World’s Poult. Sci. J. 2019, 75, 305–318. [Google Scholar] [CrossRef]
- Mobin, S.M.A.; Chowdhury, H.; Alam, F. Commercially important bioproducts from microalgae and their current applications—A review. Energy Procedia 2019, 160, 752–760. [Google Scholar] [CrossRef]
- Canelli, G.; Tarnutzer, C.; Carpine, R.; Neutsch, L.; Bolten, C.J.; Dionisi, F.; Mathys, A. Biochemical and Nutritional Evaluation of Chlorella and Auxenochlorella Biomasses Relevant for Food Application. Front. Nutr. 2020, 7, 565996. [Google Scholar] [CrossRef]
- Khatoon, H.; Haris, H.; Rahman, N.A.; Zakaria, M.N.; Begum, H.; Mian, S. Growth, Proximate Composition and Pigment Production of Tetraselmis chuii Cultured with Aquaculture Wastewater. J. Ocean. Univ. China 2018, 17, 641–646. [Google Scholar] [CrossRef]
- Branco-Vieira, M.; San Martin, S.; Agurto, C.; Santos, M.; Freitas, M.; Mata, T.; Martins, A.; Caetano, N. Potential of Phaeodactylum tricornutum for Biodiesel Production under Natural Conditions in Chile. Energies 2017, 11, 54. [Google Scholar] [CrossRef]
- Sørensen, M.; Kousoulaki, K.; Hammerø, R.; Kokkali, M.; Kleinegris, D.; Marti-Quijal, F.J.; Barba, F.J.; Palihawadana, A.M.; Egeland, E.S.; Johnsen, C.A.; et al. Mechanical processing of Phaeodactylum tricornutum and Tetraselmis chui biomass affects phenolic and antioxidant compound availability, nutrient digestibility and deposition of carotenoids in Atlantic salmon. Aquaculture 2023, 569, 739395. [Google Scholar] [CrossRef]
- Vieira, M.V.; Turkiewicz, I.P.; Tkacz, K.; Fuentes-Grünewald, C.; Pastrana, L.M.; Fuciños, P.; Wojdyło, A.; Nowicka, P. Microalgae as a Potential Functional Ingredient: Evaluation of the Phytochemical Profile, Antioxidant Activity and In-Vitro Enzymatic Inhibitory Effect of Different Species. Molecules 2021, 26, 7593. [Google Scholar] [CrossRef]
- Safafar, H.; van Wagenen, J.; Møller, P.; Jacobsen, C. Carotenoids, Phenolic Compounds and Tocopherols Contribute to the Antioxidative Properties of Some Microalgae Species Grown on Industrial Wastewater. Mar. Drugs 2015, 13, 7339–7356. [Google Scholar] [CrossRef]
- Rahman, N.A.; Khatoon, H.; Yusuf, N.; Banerjee, S.; Haris, N.A.; Lananan, F.; Tomoyo, K. Tetraselmis chuii biomass as a potential feed additive to improve survival and oxidative stress status of Pacific white-leg shrimp Litopenaeus vannamei postlarvae. Int. Aquat. Res. 2017, 9, 235–247. [Google Scholar] [CrossRef]
- Suganya, T.; Varman, M.; Masjuki, H.H.; Renganathan, S. Macroalgae and microalgae as a potential source for commercial applications along with biofuels production: A biorefinery approach. Renew. Sustain. Energy Rev. 2016, 55, 909–941. [Google Scholar] [CrossRef]
- Li, H.; Cheng, K.; Wong, C.; Fan, K.; Chen, F.; Jiang, Y. Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem. 2007, 102, 771–776. [Google Scholar] [CrossRef]
- Coulombier, N.; Jauffrais, T.; Lebouvier, N. Antioxidant Compounds from Microalgae: A Review. Marine Drugs 2021, 19, 549. [Google Scholar] [CrossRef] [PubMed]
- Widowati, I.; Zainuri, M.; Kusumaningrum, H.P.; Susilowati, R.; Hardivillier, Y.; Leignel, V.; Bourgougnon, N.; Mouget, J.-L. Antioxidant activity of three microalgae Dunaliella salina, Tetraselmis chuii and Isochrysis galbana clone Tahiti. IOP Conf. Ser. Earth Environ. Sci. 2017, 55, 012067. [Google Scholar] [CrossRef]
- Nour, V.; Trandafir, I.; Cosmulescu, S. HPLC Determination of Phenolic Acids, Flavonoids and Juglone in Walnut Leaves. J. Chromatogr. Sci. 2013, 51, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Popović-Djordjević, J.B.; Katanić Stanković, J.S.; Mihailović, V.; Pereira, A.G.; Garcia-Oliveira, P.; Prieto, M.A.; Simal-Gandara, J. Algae as a Source of Bioactive Compounds to Prevent the Development of Type 2 Diabetes Mellitus. Curr. Med. Chem. 2021, 28, 4592–4615. [Google Scholar] [CrossRef]
- Moreira, C.; Ferreira-Santos, P.; Teixeira, J.A.; Rocha, C.M.R. Active aroma compounds assessment of processed and non-processed micro- and macroalgae by solid-phase microextraction and gas chromatography/mass spectrometry targeting seafood analogs. Front. Sustain. Food Syst. 2022, 6, 1011020. [Google Scholar] [CrossRef]



| Sample | T. chuii | C. vulgaris | M. gaditana | P. tricornutum |
|---|---|---|---|---|
| Na | 6652.1 ± 87.16 a | 1610.4 ± 45.13 b | 1418.9 ± 17.91 d | 7299.0 ± 211.96 e |
| K | 1227.1 ± 21.56 a | 374.5 ± 32.85 b | 1239.9 ± 97.33 d | 2532.1 ± 135.63 e |
| Ca | 2273.3 ± 238.36 a | 65.5 ± 1.69 b | 196.5 ± 41.63 d | 457.9 ± 82.13 e |
| Mg | 822.6 ± 42.33 a | 105.1 ± 23.77 b | 493.7 ± 17.87 d | 994.4 ± 63.79 e |
| P | 299.0 ± 47.13 a | 786.7 ± 6.90 b | 1036.5 ± 26.47 d | 562.1 ± 74.30 e |
| S | 888.3 ± 32.71 a | 224.7 ± 21.88 b | 108.8 ± 18.03 d | 819.6 ± 83.3 e |
| Fe | nd 1 | 18.1 ± 0.63 a | 36.6 ± 14.72 c | 8.2 ± 0.04 f |
| Mn | nd 1 | nd 1 | nd 1 | nd 1 |
| Polyphenol | T. chuii | C. vulgaris | M. gaditana | P. tricornutum |
|---|---|---|---|---|
| Gallic acid | 1.04 ± 0.034 a | 2.21 ± 0.140 b | 6.47 ± 0.560 c | 1.19 ± 0.103 a |
| Caffeic acid | 1.16 ± 0.052 a | nd | 0.47 ± 0.000 | 1.10 ± 0.002 |
| Catechin | 8.76 ± 0.400 a | 6.29 ± 0.302 b | 1.14 ± 0.113 c | 3.27 ± 0.007 d |
| Epicatechin | 3.01 ± 0.741 a | 28.71 ± 1.01 b | 3.15 ± 0.805 c | 12.56 ± 1.194 d |
| Vanillic acid | 1.31 ± 0.013 a | 1.16 ± 0.044 b | 14.16 ± 0.471 c | 10.14 ± 1.022 d |
| Syringic acid | nd 1 | 0.74 ± 0.012 a | nd 1 | nd 1 |
| Rutin | nd 1 | 0.79 ± 0.010 a | 1.65 ± 0.023 b | nd 1 |
| Ferulic acid | 0.11 ± 0.004 a | nd 1 | nd 1 | 0.89 ± 0.007 b |
| Total | 15.35 | 39.90 | 27.04 | 29.15 |
| Compound | T. chuii | C. vulgaris | M. gaditana | P. tricornutum |
|---|---|---|---|---|
| Aldehydes | 10.40 ± 1.032 | 17.20 ± 1.284 | 5.06 ± 1.351 | 8.63 ±2.033 |
| hexanal | 2.22 ± 0.001 | 4.40 ± 0.004 | - | - |
| heptanal | - | 1.76 ± 0.001 | - | 1.59 ± 0.002 |
| decanal | 0.11 ± 0.002 | 0.67 ± 0.003 | 0.84 ± 0.044 | 0.57 ± 0.001 |
| valeraldehyde | 2.22 ± 0.007 | 0.97 ± 0.000 | 0.17 ± 0.000 | - |
| benzaldehyde | 2.01 ± 0.013 | 1.82 ± 0.010 | 3.71 ± 0.007 | 5.68 ± 0.002 |
| 9,12-octadecadienal | 0.21 ± 0.004 | 0.73 ± 0.003 | - | 0.34 ± 0.001 |
| 2,6,6-trimethyl-1,3-cyclohexadiene-1-carboxaldehyde | 1.43 ± 0.001 | 1.46 ± 0.003 | 0.34 ± 0.016 | 0.45 ± 0.051 |
| Alcohols | 6.71 ± 0.801 | 11.47 ± 1.663 | 10.96 ± 2.033 | 31.41 ± 3.498 |
| benzenemethanol | 2.27 ± 0.021 | 1.40 ± 0.107 | 0.67 ± 0.004 | 5.24 ± 0.102 |
| 2-ethyl-1-hexanol | - | 0.49 ± 0.004 | 1.85 ± 0.033 | 5.11 ± 0.103 |
| 6-methyl-5-hepten-2-ol | - | 3.34 ± 0.016 | 0.84 ± 0.045 | 10.17 ± 0.400 |
| 1-undecanol | 0.74 ± 0.004 | 4.31 ± 0.214 | - | 1.02 ± 0.001 |
| 1-tetradecanol | - | 0.24 ± 0.004 | 0.84 ± 0.074 | 2.16 ± 0.001 |
| 1-nonanol | 0.37 ± 0.013 | 0.06 ± 0.017 | - | 1.48 ± 0.004 |
| 2-hexadecanol | 1.06 ± 0.006 | 0.24 ± 0.003 | - | - |
| 1-hexadecanol | - | - | 6.07 ± 0.142 | - |
| Ketones | 22.92 ± 9.475 | 2.25 ± 0.559 | 2.02 ± 0.955 | 4.09 ± 0.481 |
| 6-methyl-5-hepten-2-one | 18.16 ± 0.211 | 1.52 ± 0.097 | 1.69 ± 0.044 | 1.70 ± 0.411 |
| nona-3,5-dien-2-one | 4.76 ± 0.143 | 0.73 ± 0.007 | 0.34 ± 0.021 | 2.38 ± 0.063 |
| Alkanes | 1.16 ± 0.223 | 16.75 ± 4.001 | 57.62 ± 15.575 | 5.56 ± 1.340 |
| pentadecane | 0.21 ± 0.001 | 4.92 ± 0.031 | 34.69 ± 7.085 | 0.57 ± 0.102 |
| dodecane | 0.58 ± 0.340 | 0.24 ± 0.002 | 1.52 ± 0.021 | 3.29 ± 0.501 |
| nonadecane | 0.32 ± 0.047 | 10.56 ± 1.041 | 18.55 ± 2.140 | 1.36 ± 0.320 |
| docosane | 0.05 ± 0.004 | 1.03 ± 0.002 | 2.87 ± 0.047 | 0.34 ± 0.000 |
| Alkenes | 0.16 ± 0.031 | 22.09 ± 3.458 | 10.62 ± 0.320 | 0.68 ± 0.000 |
| 8-heptadecene | 0.16 ± 0.031 | 22.09 ± 3.458 | 10.62 ± 0.320 | 0.68 ±0.000 |
| Alkynes | 0.05 ± 0.000 | 11.96 ± 0.901 | 0.34 ± 0.041 | - |
| 7-octadecyne, 2-methyl | 0.05 ± 0.000 | 11.96 ± 0.901 | 0.34 ± 0.041 | - |
| S-based compounds | 3.01 ± 0.014 | 4.19 ± 0.307 | 0.67 ± 0.000 | - |
| 2-pentylfuran | 3.01 ± 0.014 | 4.19 ± 0.307 | 0.67 ± 0.000 | - |
| N-based compounds | 47.53 ± 6.575 | 9.41 ± 1.605 | 1.01 ± 0.198 | 32.37 ± 4.752 |
| 2-methylpyrazine | - | 3.28 ± 0.001 | 0.34 ± 0.103 | - |
| 2,5-dimethylpyrazine | 10.83 ± 0.036 | 4.19 ± 0.474 | - | 12.39 ± 2.166 |
| 2-ethyl-5-methylpyrazine | 9.72 ± 0.302 | 0.55 ± 0.025 | 0.51 ± 0.007 | 6.47 ± 1.252 |
| 2,5-dimethyl-3-ethyl-pyrazine | 5.65 ± 0.432 | 0.49 ± 0.002 | - | 2.50 ± 0.020 |
| 2,4,5,6-tetramethylpyrimidine | 18.22 ± 1.004 | 0.30 ± 0.201 | 0.17 ± 0.022 | 9.19 ± 1.140 |
| 4-ethyl-2,5,6-trimethylpyrimidine | 3.06 ± 0.026 | 0.06 ± 0.000 | - | 1.70 ± 0.002 |
| 1,2-dihydro-1,4-diphenylphtalazine | - | 0.24 ± 0.041 | - | - |
| cathine | 0.05 ± 0.000 | 0.30 ± 0.004 | - | 0.11 ± 0.004 |
| Terpenoids | 6.92 ± 0.889 | 2.06 ± 0.358 | 1.69 ± 0.266 | 12.94 ± 2.123 |
| nerolidol | - | - | 0.34 ± 0.003 | - |
| b-cyclocitral | 1.48 ± 0.003 | 0.97 ± 0.012 | 0.51 ± 0.007 | 1.70 ± 0.031 |
| a-ionone | 2.43 ± 0.122 | 0.30 ± 0.000 | - | 5.11 ± 0.007 |
| b-lonol | 1.27 ± 0.047 | 0.30 ± 0.001 | 0.17 ± 0.000 | 1.70 ± 0.611 |
| b-ionone | 1.74 ± 0.003 | 0.49 ± 0.025 | 0.67 ± 0.120 | 4.43 ± 0.102 |
| Other | 0.63 ± 0.445 | 0.42 ± 0.127 | 0.67 ± 0.474 | 0.57 ± 0.403 |
| guanidinosuccinimide | 0.63 ± 0.445 | 0.30 ± 0.007 | - | 0.57 ± 0.403 |
| 2’-o-methylguanosine | - | 0.12 ± 0.041 | 0.67 ± 0.474 | - |
| Total identified compounds | 99.40 ± 14.931 | 97.8 ± 7.439 | 90.66 ± 17.530 | 96.25 ± 12.465 |
| Non-identified compounds | 0.51 | 2.20 | 9.34 | 3.75 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grácio, M.; Ferreira, J.; Steinrücken, P.; Kleinegris, D.M.M.; Sousa, I.; Nunes, M.C.; Raymundo, A. The Volatile Composition and the Potential Health Benefits of Different Microalgae Strains. Foods 2024, 13, 2174. https://doi.org/10.3390/foods13142174
Grácio M, Ferreira J, Steinrücken P, Kleinegris DMM, Sousa I, Nunes MC, Raymundo A. The Volatile Composition and the Potential Health Benefits of Different Microalgae Strains. Foods. 2024; 13(14):2174. https://doi.org/10.3390/foods13142174
Chicago/Turabian StyleGrácio, Madalena, Joana Ferreira, Pia Steinrücken, Dorinde M. M. Kleinegris, Isabel Sousa, M. Cristiana Nunes, and Anabela Raymundo. 2024. "The Volatile Composition and the Potential Health Benefits of Different Microalgae Strains" Foods 13, no. 14: 2174. https://doi.org/10.3390/foods13142174
APA StyleGrácio, M., Ferreira, J., Steinrücken, P., Kleinegris, D. M. M., Sousa, I., Nunes, M. C., & Raymundo, A. (2024). The Volatile Composition and the Potential Health Benefits of Different Microalgae Strains. Foods, 13(14), 2174. https://doi.org/10.3390/foods13142174











