Harnessing Fermented Soymilk Production by a Newly Isolated Pediococcus acidilactici F3 to Enhance Antioxidant Level with High Antimicrobial Activity against Food-Borne Pathogens during Co-Culture
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Culture Conditions
2.3. Optimization of Fermentation Parameters by Response Surface Methodology (RSM)
2.4. Preparation of Fermented Soymilk Extract
2.5. Storage Stability
2.6. Antimicrobial Activity by Co-Culture Incubation
2.7. Analytical Methods
2.7.1. 2,2-Diphenyl-2-picryl-hydrazyl (DPPH) Assay
2.7.2. Phenolic Content
2.7.3. Organic Acid Measurement
2.8. Statistical Analysis
3. Results and Discussion
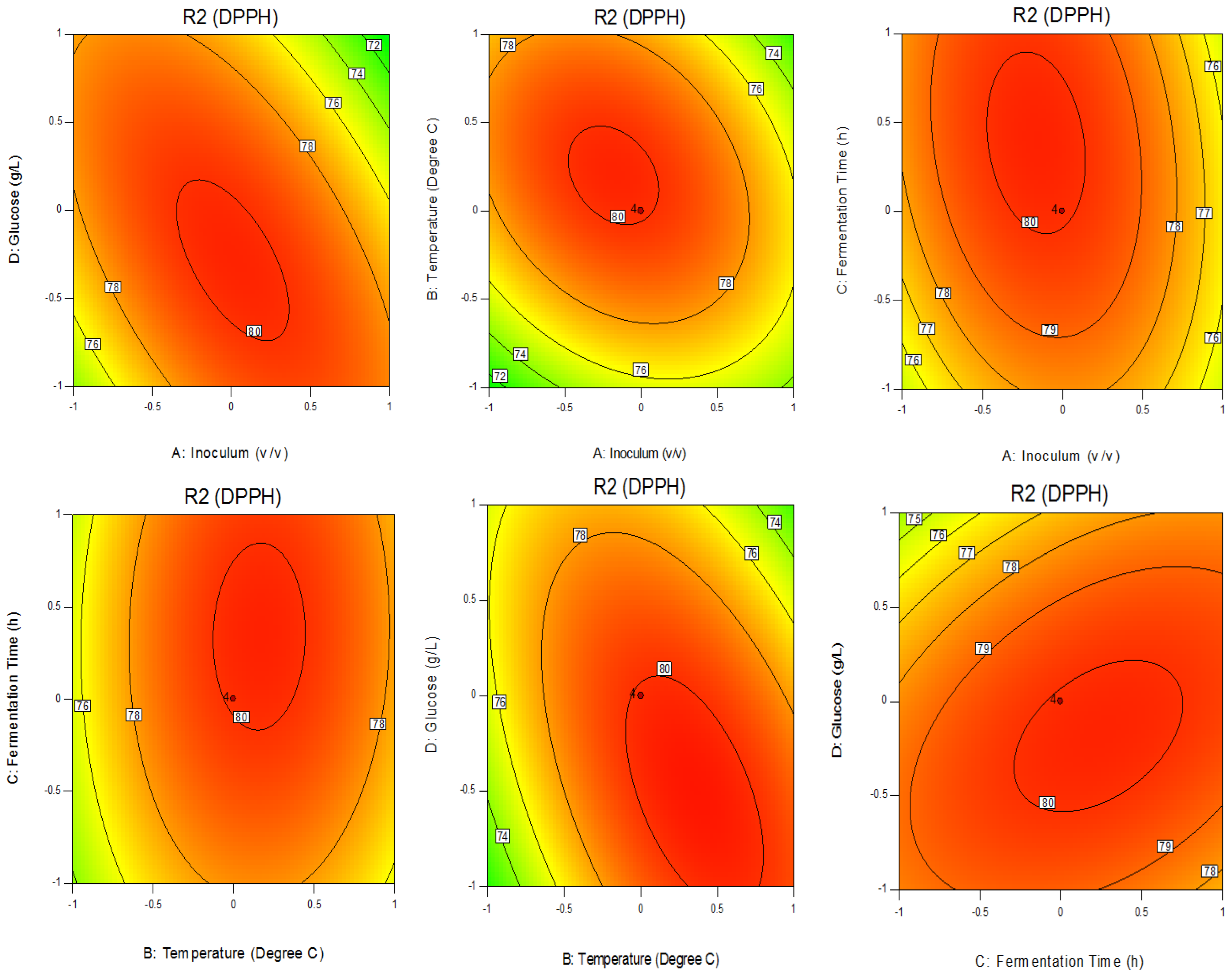
3.1. Optimization of Fermented Soymilk Enhancing Antioxidant Level by P. acidilactici F3
2.72 × BD + 1.21 × CD − 3.08 × A2 − 3.50 × B2 − 1.11 × C2 − 1.88 × D2
3.2. Validation of the Mathematical Model
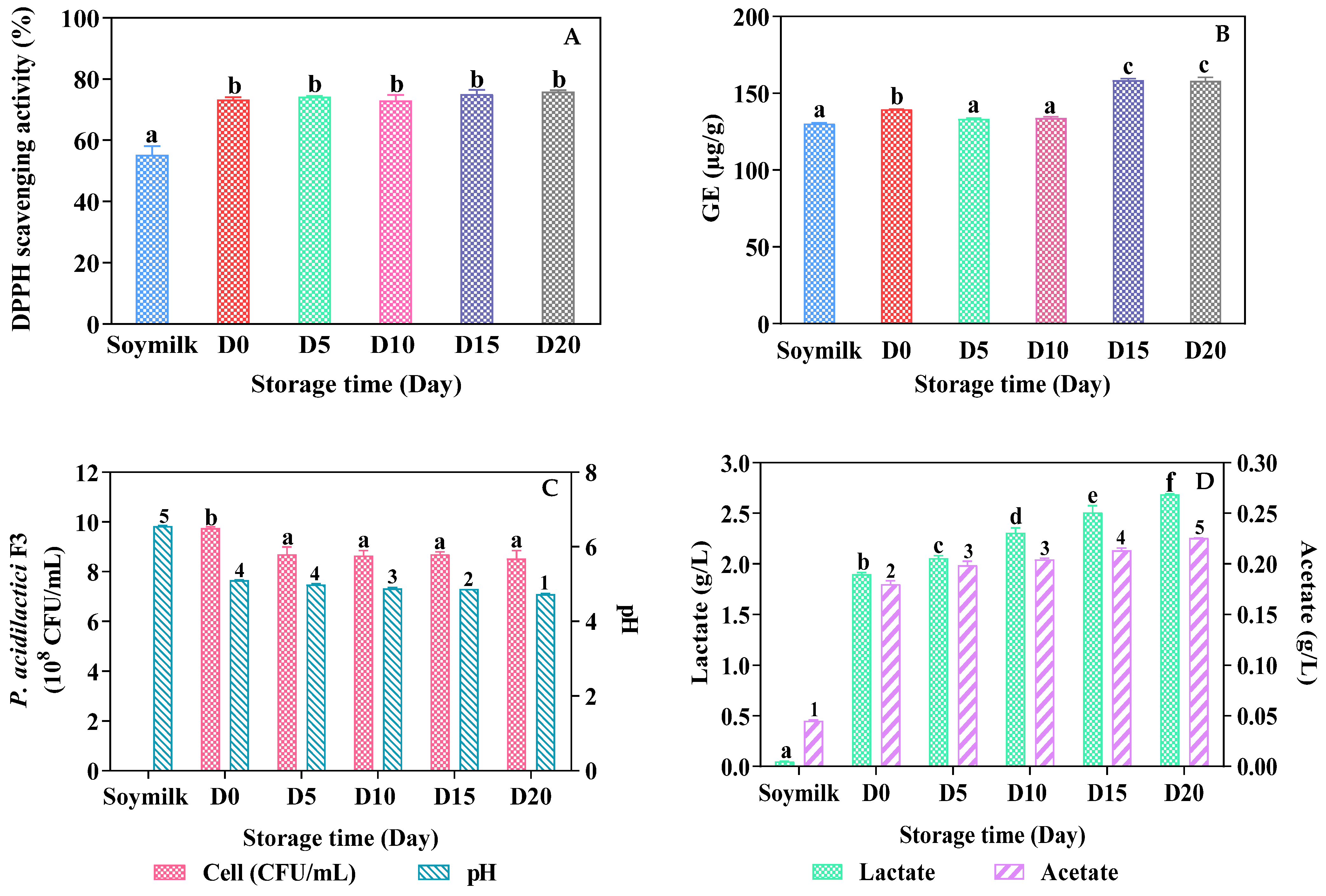
3.3. Product Storage and Stability
3.3.1. DPPH Scavenging Activity and Total Phenolic Content (TPC)
3.3.2. Viable Cell Counts and Post Acidification
3.3.3. Organic Acid Production
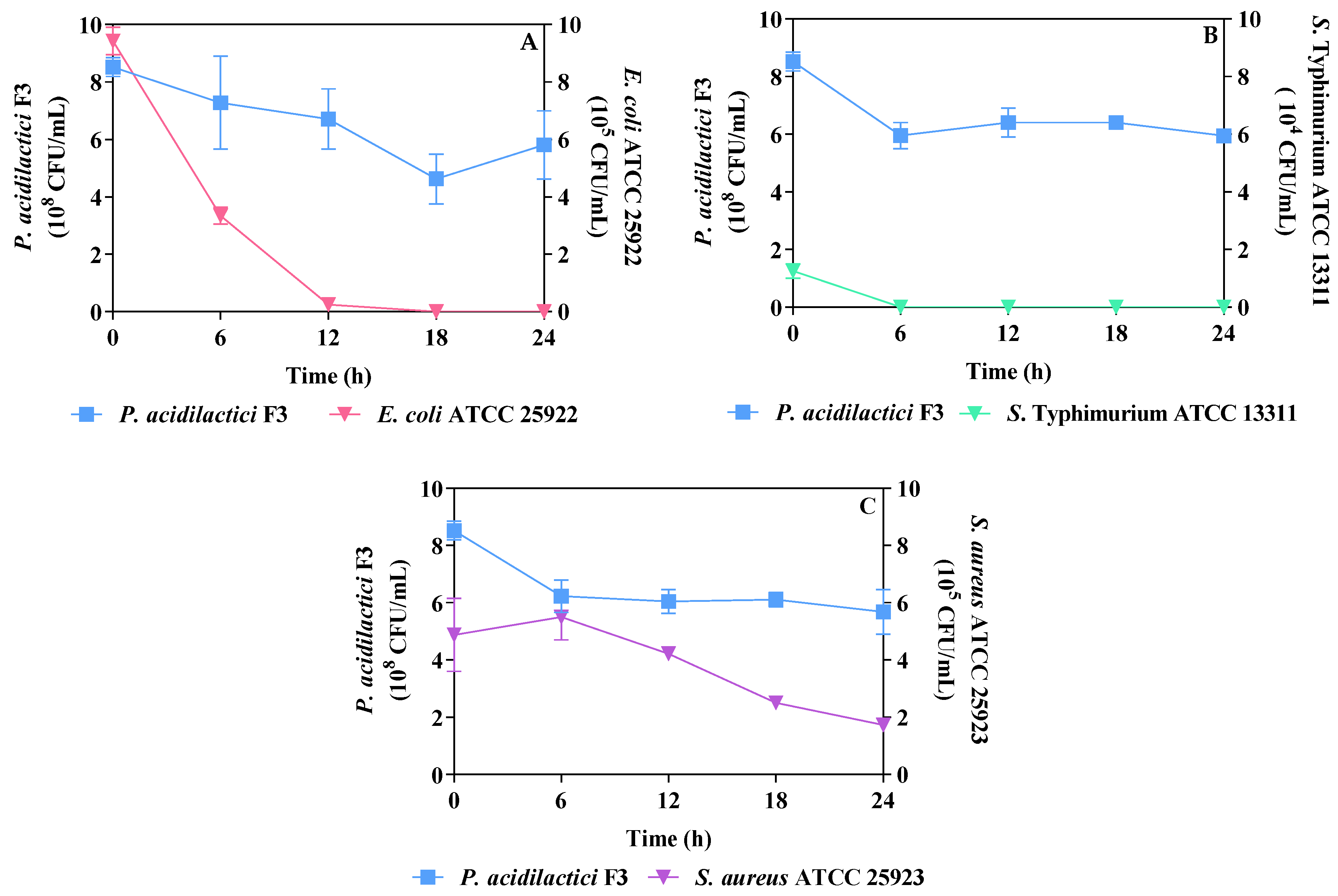
3.4. Antibacterial Activity in Fermented Soymilk during Storage
3.5. Dynamic Inhibition of Pathogens Co-Cultured with Refrigerated Fermented Soymilk
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mascaraque, M. Trends to Watch in Plant-Based Milk. Euromonitor International. 2021. Available online: https://www.euromonitor.com/article/trends-to-watch-in-plant-based-milk (accessed on 3 September 2022).
- Mukherjee, R.; Chakraborty, R.; Dutta, A. Role of fermentation in improving nutritional quality of soybean meal—A Review. Asian Australas. J. Anim. Sci. 2016, 29, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Haakensen, M.; Vickers, D.M.; Ziola, B. Susceptibility of Pediococcus isolates to antimicrobial compounds in relation to hop-resistance and beer-spoilage. BMC Microbiol. 2009, 9, 190. [Google Scholar] [CrossRef] [PubMed]
- Klare, I.; Konstabel, C.; Werner, G.; Huy, S.G.; Vankerckhoven, V.; Kahlmeter, G.; Hildebrandt, B.; Müller-Bertling, S.; Witte, W.; Goossens, H. Antimicrobial susceptibilities of Lactobacillus, Pediococcus and Lactococcus human isolates and cultures intended for probiotic or nutritional use. J. Antimicrob. Chemother. 2007, 59, 900–912. [Google Scholar] [CrossRef] [PubMed]
- Papagianni, M.; Anastasiadou, S. Encapsulation of Pediococcus acidilactici cells in corn and olive oil microcapsules emulsified by peptides and stabilized with xanthan in oil-in-water emulsions: Studies on cell viability under gastro-intestinal simulating conditions. Enzym. Microb. Technol. 2009, 45, 514–522. [Google Scholar] [CrossRef]
- Devi, S.M.; Ramaswamy, A.M.; Halami, P.M. In situ production of pediocin PA-1 like bacteriocin by different genera of lactic acid bacteria in soymilk fermentation and evaluation of sensory properties of the fermented soy curd. J. Food Sci. Technol. 2014, 51, 3325–3332. [Google Scholar] [CrossRef]
- Sharma, A.; Noda, M.; Sugiyama, M.; Ahmad, A.; Kaur, B. Production of Functional Buttermilk and Soymilk Using Pediococcus acidilactici BD16 (alaD+). Molecules 2021, 26, 4671. [Google Scholar] [CrossRef] [PubMed]
- Yuenyaow, L.; Wansutha, S.; Jantama, K.; Jantama, S.S. Probiotic potential of lactic acid bacteria isolated from fermented fish. In Proceedings of the 7th International Graduate Research Conference (IGRC 7), Ubon Ratchathani, Thailand, 19–20 October 2017. [Google Scholar]
- Chan, S.; Pimphong, K.; Prasitpuriprecha, C.; Jantama, K.; Jantama, S.S. Enhancement of antioxidant properties in soymilk fermented by lactic acid bacteria. In Proceedings of the 11th UBU Research Conference “Microbial Diversity: Literacy and Applications”, Ubon Ratchathani, Thailand, 13–14 July 2017. [Google Scholar]
- Heo, S.J.; Park, E.J.; Lew, K.W.; Jeon, Y.J. Antioxidant activities of enzyme extracts from brown seaweeds. Bioresour. Technol. 2005, 96, 1613–1623. [Google Scholar] [CrossRef]
- Livinska, O.; Ivaschenko, O.; Garmasheva, I.; Kovalenko, N. The screening of lactic acid bacteria with antioxidant properties. AIMS Microbiol. 2016, 2, 447–459. [Google Scholar] [CrossRef]
- Khoshayand, F.; Goodarzi, S.; Shahverdi, A.R.; Khoshayand, M.R. Optimization of culture conditions for fermentation of soymilk using Lactobacillus casei by Response surface methodology. Probiotics Antimicrob. Proteins 2011, 3, 159–167. [Google Scholar] [CrossRef]
- Rui, X.; Wang, M.; Zhang, Y.; Chen, X.; Li, L.; Liu, Y.; Dong, M. Optimization of soy solid-state fermentation with selected lactic acid bacteria and the effect on the anti-nutritional components. J. Food Process. Preserv. 2017, 41, e13290. [Google Scholar] [CrossRef]
- Hlahla, L.N.; Mudau, F.N.; Mariga, I.K. Effect of fermentation temperature and time on the chemical composition of bush tea (Athrixia phylicoides DC.). J. Med. Plants Res. 2010, 4, 824–829. [Google Scholar]
- Réblová, Z. Effect of temperature on the antioxidant activity of phenolic acids. Czech J. Food Sci. 2012, 30, 171–177. [Google Scholar] [CrossRef]
- Wang, Y.C.; Yu, R.C.; Chou, C.C. Antioxidative activities of soymilk fermented with lactic acid bacteria and bifidobacteria. Food Microbiol. 2006, 23, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Shah, N.P. Changes in antioxidant capacity, isoflavone profile, phenolic and vitamin contents in soymilk during extended fermentation. LWT-Food Sci. Technol. 2014, 58, 454–462. [Google Scholar] [CrossRef]
- Sheih, I.C.; Wu, H.Y.; Lai, Y.J.; Lin, C.F. Preparation of high free radical scavenging tempeh by a newly isolated Rhizopus sp. R-96 from Indonesia. Food Sci. Agric. Chem. 2000, 2, 35–40. [Google Scholar]
- Shori, A.B.; Baba, A.S. Cinnamomum verum improved the functional properties of bioyogurts made from camel and cow milks. J. Saudi Soc. Agric. Sci. 2011, 10, 101–107. [Google Scholar] [CrossRef]
- Hur, S.J.; Lee, S.Y.; Kim, Y.C.; Choi, I.; Kim, G.B. Review: Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.D.S.; Lima, F.S.; Rodrigues, D.; Guelfi, C.H.M.; Garcia, S.; Ida, E.I. Evaluation of the isoflavone and total phenolic contents of kefir-fermented soymilk storage and after the in vitro digestive system simulation. Food Chem. 2017, 229, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Marazza, J.A.; Nazareno, M.A.; de Giori, G.S.; Marisa, S.; Garro, M.S. Enhancement of the antioxidant capacity of soymilk by fermentation with Lactobacillus rhamnosus. J. Funct. Foods 2012, 4, 594–601. [Google Scholar] [CrossRef]
- Fakri, E.M.; Lim, S.; Musa, N.; Hasan, M.H.; Adam, A.; Ramasamy, K. Lactobacillus fermentum LAB 9-fermented soymilk with enriched isoflavones and antioxidants improved memory in vivo. Sains Malays. 2016, 45, 1289–1297. [Google Scholar]
- Song, Y.-R.; Kim, Y.-E.; Kim, J.-H.; Song, N.-E.; Jeong, D.-Y.; Baik, S.-H. Preparation of fermented sugar-soaked black soybean snacks (FSBSS) and characterization of their quality changes. Food Sci. Biotechnol. 2011, 20, 1547–1553. [Google Scholar] [CrossRef]
- Marazza, J.A.; LeBlanc, J.G.; de Giori, G.S.; Garro, M.S. Soymilk fermented with Lactobacillus rhamnosus CRL981 ameliorates hyperglycemia, lipid profiles and increases antioxidant enzyme activities in diabetic mice. J. Funct. Foods 2013, 5, 1848–1853. [Google Scholar] [CrossRef]
- Rekha, C.R.; Vijayalakshmi, G. Isoflavone phytoestrogens in soymilk fermented with β-glucosidase producing probiotic lactic acid bacteria. Int. J. Food Sci. Nutr. 2011, 62, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Fiocco, D.; Capozzi, V.; Goffin, P.; Hols, P.; Spano, G. Improved adaptation to heat, cold, and solvent tolerance in Lactobacillus plantarum. Appl. Microbiol. Biotechnol. 2007, 77, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Abd EI-Gawad, I.A.; EI-Sayed, E.M.; Zeini, H.M.; Hafez, S.A.; Saleh, F.A. Antibacterial activity of probiotic yoghurt and soy yoghurt against Escherichia coli and Staphylococcus aureus. J. Nutr. Food Sci. 2014, 4, 1000303. [Google Scholar]
- Lourens-Hattingh, A.; Viljoen, C.B. Yogurt as probiotic carrier food. Int. Dairy J. 2001, 11, 1–17. [Google Scholar] [CrossRef]
- Barbosa, J.; Borges, S.; Teixeira, P. Pediococcus acidilactici as a potential probiotic to be used in food industry. Int. J. Food Sci. Technol. 2015, 50, 1151–1157. [Google Scholar] [CrossRef]
- Ribeiro, M.C.d.O.; Vandenberghe, L.P.d.S.; Spier, M.R.; Paludo, K.S.; Soccol, C.R.; Soccol, V.T. Evaluation of probiotic properties of Pediococcus acidilactici B14 in association with Lactobacillus acidophilus ATCC 4356 for application in a soy based aerated symbiotic dessert. Braz. Arch. Biol. Technol. 2014, 57, 755–765. [Google Scholar] [CrossRef]
- Marazza, J.A.; Garro, M.S.; de Giori, G.S. Aglycone production by Lactobacillus rhamnosus CRL981 during soymilk fermentation. Food Microbiol. 2009, 26, 333–339. [Google Scholar] [CrossRef]
- Abbasiliasi, S.; Tan, J.S.; Bashokouh, F.; Ibrahim, T.A.T.; Mustafal, S.; Vakhshiteh, F.; Sivasamboo, S.; Ariff, A.B. In vitro assessment of Pediococcus acidilactici Kp10 for its potential use in the food industry. BMC Microbiol. 2017, 17, 121. [Google Scholar] [CrossRef]
- Oh, D.H.; Park, J.H.; Park, B.K. Effect of organic acids on the survival of Escherichia coli 0157:H7. J. Food Sci. Nutr. 2000, 5, 131–135. [Google Scholar]
- Reda, F.M. Antibacterial and anti-adhesive efficiency of Pediococcus acidilactici against foodborne biofilm producer Bacillus cereus attached on different food processing surfaces. Food Sci. Biotechnol. 2019, 28, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Tankoano, A.; Diop, M.B.; Sawadogo-Lingani, H.; Mbengue, M.; Kaboré, D.; Traoré, Y.; Savadogo, A. Isolation and characterization of Lactic Acid Bacteria producing bacteriocin like inhibitory substance (BLIS) from “Gappal”, a dairy product from Burkina Faso. Adv. Microbiol. 2019, 9, 343–358. [Google Scholar] [CrossRef]
- Diguță, C.F.; Nițoi, G.D.; Matei, F.; Luță, G.; Cornea, C.P. The biotechnological potential of Pediococcus spp. isolated from Kombucha microbial consortium. Foods 2020, 9, 1780. [Google Scholar] [CrossRef] [PubMed]
- Kaya, H.İ.; Şimşek, Ö. Characterization of Pediococcus acidilactici PFC69 and Lactococcus lactis PFC77 bacteriocins and their antimicrobial activities in Tarhana fermentation. Microorganisms 2020, 8, 1083. [Google Scholar] [CrossRef] [PubMed]
- Rwubuzizi, R.; Carneiro, K.O.; Holzapfel, W.H.; Vaz-Vello, M.; Todorov, S.D. Bacteriocin and antioxidant production, a beneficial property of Lactic Acid Bacteria isolated from fermented vegetables of Northwest Bulgaria. Probiotics Antimicrob. Proteins 2023. [Google Scholar] [CrossRef]
- Kumari, M.; Kokkiligadda, A.; Dasriya, V.; Naithani, H. Functional relevance and health benefits of soymilk fermented by lactic acid bacteria. J. Appl. Microbiol. 2022, 133, 104–119. [Google Scholar] [CrossRef]
| Parameters | Level | ||||
|---|---|---|---|---|---|
| −1.4142 | −1 | 0 | 1 | 1.4142 | |
| Cell inoculum (%, v/v) | 0.58 | 1 | 2 | 3 | 3.41 |
| Temperature (°C) | 15.85 | 20 | 30 | 40 | 44.14 |
| Fermentation time (h) | 4.68 | 8 | 16 | 24 | 27.31 |
| Glucose (g/L) | 1.46 | 2.5 | 5 | 7.5 | 8.53 |
| Run | Parameters | %DPPH Activity | ||||
|---|---|---|---|---|---|---|
| A: Inoculum (%, v/v) | B: Temperature (°C) | C: Time (h) | D: Glucose (g/L) | Observed (%) | Predicted (%) | |
| 1 | 2 | 15.85 | 16 | 5 | 71.6 ± 1.6 | 71.6 |
| 2 | 2 | 30 | 16 | 1.46 | 77.7 ± 0.8 | 77.2 |
| 3 | 1 | 40 | 8 | 7.5 | 72.8 ± 0.9 | 70.6 |
| 4 | 2 | 30 | 27.31 | 5 | 81.1 ± 0.2 | 78.9 |
| 5 | 0.58 | 30 | 16 | 5 | 74.9 ± 0.4 | 73.3 |
| 6 | 2 | 44.14 | 16 | 5 | 72.7 ± 0.8 | 74.6 |
| 7 | 3 | 40 | 24 | 2.5 | 72.7 ± 0.1 | 74.4 |
| 8 | 3 | 40 | 8 | 2.5 | 76.8 ± 0.3 | 76.6 |
| 9 | 3.41 | 30 | 16 | 5 | 72.9 ± 0.3 | 72.9 |
| 10 | 1 | 40 | 24 | 7.5 | 75.9 ± 1.2 | 76.3 |
| 11 | 3 | 20 | 24 | 7.5 | 66.9 ± 1.2 | 70.6 |
| 12 | 1 | 20 | 8 | 2.5 | 61.7 ± 0.5 | 63.4 |
| 13 | 3 | 20 | 8 | 7.5 | 68.6 ± 0.8 | 68.4 |
| 14 | 2 | 30 | 16 | 7.5 | 77.1 ± 0.9 | 77.2 |
| 15 | 2 | 30 | 4.68 | 5 | 76.3 ± 1.6 | 76.8 |
| 16 | 2 | 30 | 16 | 8.53 | 72.0 ± 1.1 | 74.9 |
| 17 | 2 | 30 | 16 | 7.5 | 77.1 ± 0.9 | 77.2 |
| 18 | 1 | 20 | 24 | 2.5 | 63.9 ± 0.1 | 63.7 |
| Treatment | Viability Count after 24 h Co-Incubation Time at 37 °C (CFUs/mL) | |||||
|---|---|---|---|---|---|---|
| Soymilk | D0 | D5 | D10 | D15 | D20 | |
| Anti-E. coli P. acidilactici F3 E. coli ATCC 25922 | ND 2.80 × 108 | 7.26 × 108 1.01 × 105 | 5.25 × 108 3.65 × 104 | 4.73 × 108 ND | 5.10 × 108 ND | 5.81 × 108 ND |
| Anti-S. Typhimurium P. acidilactici F3 S. Typhimurium ATCC 13311 | ND 4.04 × 108 | 7.50 × 108 ND | 4.12 × 108 ND | 6.10 × 108 ND | 5.10 × 108 ND | 6.00 × 108 ND |
| Anti-S. aureus P. acidilactici F3 S. aureus ATCC 25923 | ND 1.97 × 108 | 3.25 × 108 4.40 × 105 | 8.87 × 108 7.07 × 105 | 6.56 × 108 1.97 × 105 | 5.10 × 108 4.40 × 105 | 5.68 × 108 1.73 × 105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, S.; Jantama, K.; Prasitpuriprecha, C.; Wansutha, S.; Phosriran, C.; Yuenyaow, L.; Cheng, K.-C.; Jantama, S.S. Harnessing Fermented Soymilk Production by a Newly Isolated Pediococcus acidilactici F3 to Enhance Antioxidant Level with High Antimicrobial Activity against Food-Borne Pathogens during Co-Culture. Foods 2024, 13, 2150. https://doi.org/10.3390/foods13132150
Chan S, Jantama K, Prasitpuriprecha C, Wansutha S, Phosriran C, Yuenyaow L, Cheng K-C, Jantama SS. Harnessing Fermented Soymilk Production by a Newly Isolated Pediococcus acidilactici F3 to Enhance Antioxidant Level with High Antimicrobial Activity against Food-Borne Pathogens during Co-Culture. Foods. 2024; 13(13):2150. https://doi.org/10.3390/foods13132150
Chicago/Turabian StyleChan, Sitha, Kaemwich Jantama, Chutinun Prasitpuriprecha, Supasson Wansutha, Chutchawan Phosriran, Laddawan Yuenyaow, Kuan-Chen Cheng, and Sirima Suvarnakuta Jantama. 2024. "Harnessing Fermented Soymilk Production by a Newly Isolated Pediococcus acidilactici F3 to Enhance Antioxidant Level with High Antimicrobial Activity against Food-Borne Pathogens during Co-Culture" Foods 13, no. 13: 2150. https://doi.org/10.3390/foods13132150
APA StyleChan, S., Jantama, K., Prasitpuriprecha, C., Wansutha, S., Phosriran, C., Yuenyaow, L., Cheng, K.-C., & Jantama, S. S. (2024). Harnessing Fermented Soymilk Production by a Newly Isolated Pediococcus acidilactici F3 to Enhance Antioxidant Level with High Antimicrobial Activity against Food-Borne Pathogens during Co-Culture. Foods, 13(13), 2150. https://doi.org/10.3390/foods13132150






