Recent Advances and Perspectives on Food-Grade Immobilisation Systems for Enzymes
Abstract
1. Introduction
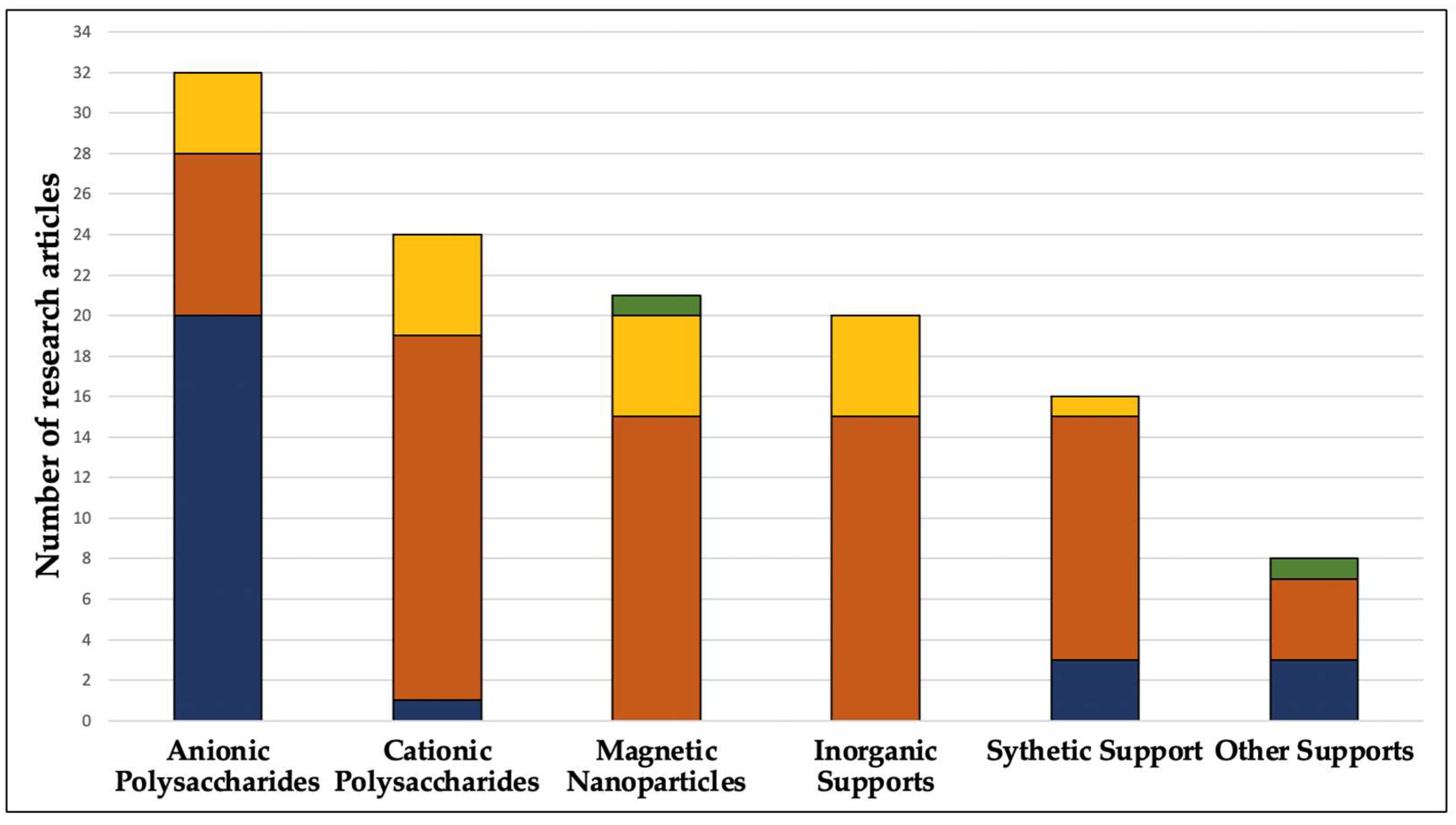
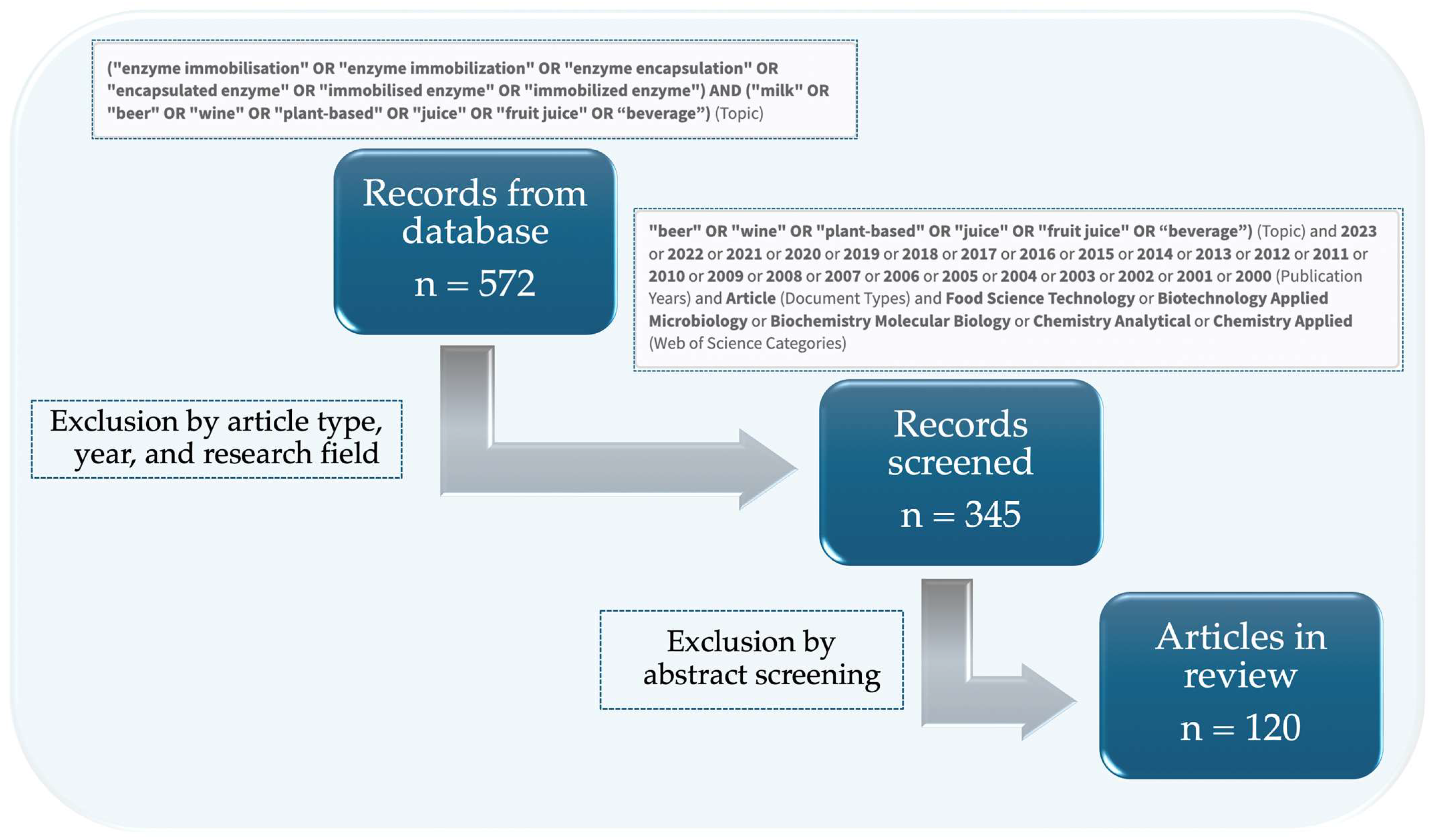
2. Methodology for Collecting and Screening the Literature
3. Immobilisation to Change Enzyme Properties
3.1. Optimum pH and pH Stability
3.2. Optimum Temperature and Thermal Stability
3.3. Kinetic Properties
| Enzyme and Source | Method | Carrier/Support | Substrate | KM | VMAX | Application | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|
| FE | IE | FE | IE | ||||||
| Alkaline protease (Bacillus licheniformis) | Covalent attachment | Eupergit CM | Casein | 26.53 g/L | 37.59 g/L | 2.84 g/L.min | 3.31 g/L.min | Lactose hydrolysis. | [67] |
| β-Galactosidase (Kluyveromyces lactis) | Adsorption | Polyvinyl-alcohol-functionalised gold nanoparticles | ONPG * | 3.56 mmol/L | 3.74 mmol/L | 2.8 mmol/L.min | 2.07 mmol/L.min | Lactose hydrolysis. | [68] |
| β-Galactosidase (Kluyveromyces lactis) | Covalent attachment | Collagen–glutaraldehyde | ONPG * | 3.86 mmol/L | 7.50 mmol/L | 42.92 mmol/L.min | 32.37 mmol/L.min | Lactose hydrolysis. | [71] |
| Pectinase and cellulase (commercial) | Covalent attachment | Glutaraldehyde-activated magnetic particles | Pectin | 17.60 mmol/L | 25.65 mmol/L | 40.58 μmol/min | 26.66 μmol/min | Grape juice clarification. | [44] |
| Pectinase and cellulase (commercial) | Cross-linking | Magnetic CLEA | Pectin | 17.60 mmol/L | 33.83 mmol/L | 40.58 μmol/min | 26.87 μmol/min | Grape juice clarification. | [44] |
| Polygalacturonase (Aspergillus niger) | Adsorption | Calcium alginate microspheres | Pectin | 4.472 mg/mL | 5.041 mg/mL | 0.214 U | 0.112 U | Apple juice clarification. | [73] |
| β-Glucosidase (Bacillus subtilis) | Covalent attachment | Functionalised silicon oxide nanoparticles | pNPG * | 0.9 mmol/L | 1.074 mmol/L | 3.5 U/mg | 1.513 U/mg | Sugarcane juice clarification. | [74] |
| Pectinase (commercial) | Covalent attachment | Polyaldehyde-pullulan-activated glass beads | Pectin | - | 11.2 mg/mL | - | 2.2 μmol/min | Barberry juice clarification. | [38] |
| Pectinase (commercial) | Covalent attachment | Glutaraldehyde-activated glass beads | Pectin | - | 10.1 mg/mL | - | 2.9 μmol/min | Barberry juice clarification. | [38] |
| Naringinase (Penicillium decumbens) | Covalent attachment | Glutaraldehyde-activated chitosan beads | Naringin | 2.56 mmol/L | 6.59 mmol/L | 1.21 μmol/L.min | 0.19 μmol/L.min | Debittering grape juice. | [48] |
| Protease (Penaeus vannamei) | Adsorption | Chitosan nanoparticles | Casein | 2.5 μmol/L | 2.7 μmol/L | 87 μmol/L.min | 83 μmol/L.min | Pomegranate juice clarification. | [32] |
| Pectinase | Covalent attachment | Trichlorotriazine-functionalised polyethylene-glycol-grafted magnetic nanoparticles | Polygalacturonic acid | 14.89 mg/mL | 10.5 mg/mL | 0.578 U/mL | 1.190 U/mL | Pineapple juice clarification. | [75] |
| β-Galactosidase (Aspergillus oryzae) | Adsorption | Nanosilver-reduced graphene oxide nanocomposite | ONPG * | 0.5 mmol/L | 0.44 mmol/L | 0.031 mmol/L.min | 0.039 mmol/L.min | Lactose hydrolysis. | [57] |
| Pectinase (Aspergillus aculeatus) | Covalent attachment | Montmorillonite support activated with glutaraldehyde | Pectin | 11.49 mg/mL | 6.06 mg/mL | 2.93 mmol/L.min | 1.73 mmol/L.min | Pineapple juice clarification. | [76] |
| Invertase (bakery yeast) | Entrapment | Poly(VP-co-BAc-co-NHMAAm) film | Sucrose | 29.6 mmol/L | 4.5 mmol/L | 13.43 μmol/min | 13.04 μmol/min | Sucrose determination in fruit juice. | [77] |
| Papain (Carica papaya) | Covalent attachment | Glutaraldehyde-poly(HEMA)–chitosan cryogels | Casein | 4.255 mg/mL | 1.544 mg/mL | 0.554 mmol/min | 0.199 mmol/min | Apple juice clarification. | [43] |
| Xylanase (Thermomyces lanuginosus) | Covalent attachment | Trichlorotriazine-functionalised polyethylene-glycol-grafted magnetic nanoparticles | Xylan | 25.51 mg/mL | 40.42 mg/mL | 2.69 U/mL | 6.01 U/mL | Pineapple juice clarification. | [25] |
| Laccase (Pleurotus ostreatus) | Adsorption | Poly(methacrylate) beads | ABTS | 0.063 mmol/L | 0.032 mmol/L | - | - | Fruit juice clarification. | [78] |
| β-glucosidase (commercial) | Entrapment | Calcium–alginate beads | Cellobiose | 0.22 mmol/L | 0.21 mmol/L | 2.52 μmol/mg.min | 3.35 μmol/mg.min | Wine aroma enhancement. | [79] |
| Pectinase (Aspergillus aculeatus) | Covalent attachment | Polyethyleneimine-based cryogel | Starch | 40 mg/mL | 31.25 mg/mL | 66.6 mg/mLmin | 1 mg/mLmin | Apple juice clarification. | [80] |
| β-Glucosidase (Laminaria hyperborea) | Covalent attachment | Chitosan glutaraldehyde beads | Cellobiose | 0.18 mmol/L | 0.21 mmol/L | 3.6 μmol/min.mg | 3.5 μmol/mg.min | Aroma hydrolysis in grape must. | [49] |
| β-galactosidase (Escherichia coli) | Covalent attachment | Magnetic graphene oxide nanocomposites | ONPG * | 6.99 mmol/L | 8.50 mmol/L | 47.8 mmol/L.min | 38.2 mmol/L.min | Lactose hydrolysis. | [34] |
| Invertase (Baking yeast) | Covalent attachment | Poly(N-vinylpyrrolidone-co-butylacrylate-co-N hydroxymethylacrylamide) terpolymer membranes | Sucrose | 29.41 mM | 8.33 mM | 13.4 μM/min | 12.2 μM/min | Hydrolysis of sucrose in peach juice and orange juice. | [81] |
3.4. Thermodynamic Properties
3.5. Inhibition Resistance
4. Enzyme Immobilisation to Enhance Enzyme Recovery and Reuse
| Enzyme and Source | Method | Carrier/Support | Substrate | Operational Stability | Ref. |
|---|---|---|---|---|---|
| Pectinase (Aspergillus tamari) | Adsorption | Zr-treated pumice | Pectin | 72% enzymatic activity after 11 cycles of reuse. | [26] |
| Pectinase (Penicillium oxalicum) | Adsorption | Magnetic cornstarch microspheres | Pectin | 60% enzymatic activity after 8 cycles of juice clarification. | [92] |
| α-Galactosidase (Debaryomyces hansenii) | Adsorption | Cellulose film | p-nitrophenyl α-D-galactopyranoside | 100 and 80% enzymatic activity after 7 and 10 cycles of reuse, respectively. | [31] |
| β-Galactosidases (Aspergillus oryzae) | Adsorption | Chitosan beads | Fresh milk | 80% enzymatic activity after 5 cycles of reuse. | [28] |
| β-Glucosidase (Melaleuca pulchella) | Adsorption | Monoaminoethyl–N- ethyl-agarose ionic support | p-nitrophenyl α-D-galactopyranoside | 50% enzymatic activity after 20 cycles of reuse. | [41] |
| Pullulanase | Adsorption | Chitosan | Pullulan | 70.8% enzymatic activity after 10 cycles of reuse. | [22] |
| Phytase (Aspergillus niger) | Adsorption | Zeolite modified with iron (II) | Soymilk | 50% enzymatic activity after 6 cycles of hydrolysis. | [37] |
| Tannase (Penicillium rolfsii) | Entrapment | Calcium alginate beads | Pectin | Above 50% enzymatic activity after 6 cycles of reuse. | [21] |
| Tannase (Escherichia coli) | Entrapment + cross-linking | Calcium alginate beads cross-linked with glutaraldehyde | Propyl gallate | 100, 80, and 40% enzymatic activity after 10, 26, and 46 cycles of reuse, respectively. | [90] |
| β-Glucosidase (commercial solution) | Entrapment | Sodium alginate gel beads | Cellobiose | 96.5% enzymatic activity after 7 cycles of hydrolysis. | [79] |
| α-Galactosidase (Aspergillus oryzae) | Entrapment | Calcium alginate beads | Fresh milk | 60% enzymatic activity after 5 cycles of reuse. | [28] |
| α-Acetolactate decarboxylase | Entrapment | Alginate gel beads | Z-Gly-Pro-pNA | Above 80% enzymatic activity after 6 cycles of reuse. | [27] |
| Naringinase (Aspergillus niger) | Entrapment | Calcium alginate beads | Naringin | Above 70% enzymatic activity after 7 cycles of reuse. | [64] |
| Pectinase (commercial preparation) | Covalent attachment | Polyaldehyde pullulan-activated glass beads | Apple pectin or galacturonic acid | 80% enzymatic activity after 15 cycles of reuse. | [38] |
| Pectinase (Aspergillus aculeatus) | Covalent attachment | Dextran-aldehyde-cross-linked polyethyleneimine | Apple juice | Above 95% enzymatic activity after 10 cycles of reuse. | [93] |
| Pectinase (Rhizopus sp.) | Covalent attachment | Glutaraldehyde-activated bentonite | Pectin | Full and 87% enzymatic activity after 18 and 25 cycles of reuse, respectively. | [88] |
| Naringinase (Penicillium decumbens) | Covalent attachment | Glutaraldehyde-activated chitosan beads | Grapefruit juice | 88.1% enzymatic activity after 10 cycles of reuse. | [48] |
| Naringinase (Aspergillus niger) | Covalent attachment | Dextran aldehyde-cross-linked magnetic polysaccharide carrier | Grapefruit juice | 82.8% enzymatic activity after 10 cycles. | [94] |
| α-Galactosidase (Citrullus vulgaris) | Covalent attachment | Sepabeads EC-EP | p-nitrophenyl α-D-galactopyranoside | 74 and 41% enzymatic activity after 18 and 30 cycles of reuse, respectively. | [95] |
| β-Galactosidase (Bacillus circulans) | Covalent attachment | Glutaraldehyde-activated chitosan beads | ONPG * | 91% lactose conversion after 17 cycles of 165 min at 40 °C. | [53] |
| β-Galactosidase (Kluyveromyces sp.) | Covalent attachment | Magnetic cellulose | ONPG * | Hydrolytic efficiency of 50% after 15 cycles of reuse. | [96] |
| β-Galactosidase (Aspergillus oryzae) | Covalent attachment | Eupergit CM | Lactose | 99.3% enzymatic activity after 20 cycles of reuse. | [20] |
| β-Galactosidase (Kluyveromyces lactis) | Covalent attachment | Glutaraldehyde-modified Immobead 150 supports (resin) | Cow’s milk | 52% enzymatic activity after 15 cycles of reuse. | [97] |
| β-Galactosidase | Covalent attachment | Modified collagen supports | Cow’s milk | 50% enzymatic activity after 17 cycles of reuse. | [71] |
| Papain (Carica papaya) | Covalent attachment | Poly(HEMA)–chitosan cryogels cross-linked with glutaraldehyde | Apple juice | 86% enzymatic activity after 5 cycles of reuse. | [43] |
| Alkaline protease (Bacillus licheniformis) | Covalent attachment | Eupergit CM | Casein | Full enzymatic activity after 20 cycles of reuse. | [67] |
| Lactase (Escherichia coli) | Covalent attachment | Glutaraldehyde-activated magnetic nanocomposite | OPNG * | 83.1% enzymatic activity after 20 cycles of reuse. | [34] |
| Laccase (Trametes versicolor) | Covalent attachment | Green coconut fiber activated with glyoxyl or glutaraldehyde | Apple juice | Above 80% enzymatic activity after 10 cycles of reuse. | [50] |
| α-Amylase (Rhizoctonia solani) | Covalent attachment | Chitosan beads cross-linked with glutaraldehyde | Soluble starch | 80% enzymatic activity after 7 cycles of reuse. | [42] |
| β-Mannanase (Aspergillus quadrilineatus) | Covalent attachment | Glutaraldehyde-activated aluminum oxide pellets | Locust bean gum | Full enzymatic activity up to 10 cycles of hydrolysis. | [98] |
| β-Glucosidase (Bacillus subtilis) | Covalent attachment | Functionalised silicon oxide nanoparticles | Sugarcane juice | 60% enzymatic activity after 10 cycles of reuse. | [74] |
| α-Acetolactate decarboxylase (Brevibacillus brevis) | Covalent attachment | Glutaraldehyde-activated chitosan beads | α-acetolactate | 80% enzymatic activity after 12 cycles of reuse. | [63] |
| Xylanase (Thermomyces lanuginosus) | Covalent attachment | Trichlorotriazine-functionalised polyethylene-glycol-grafted magnetic nanoparticles | Pineapple juice | 60% enzymatic activity after 9 cycles of hydrolysis. | [25] |
| Xylanase (Trichoderma longibrachiatum) | Covalent attachment | Glutaraldehyde-activated silica gel supports | Orange juice | 77% enzymatic activity after 10 cycles of reuse. | [36] |
| Pectinase (commercial preparation) | Covalent attachment | Genipin-activated chitosan particles | Orange/grape juice | 50 and 40% relative clarification after 10 cycles for the orange juice and grape juice, respectively. | [99] |
5. Enzyme Immobilisation to Facilitate Continuous Reaction Treatment
6. Enzyme Immobilisation and Its Application in the Beverage Sector
6.1. Application in Winemaking
6.2. Application in Brewing
6.3. Application in Vegetable and Fruit Juice Production
| Enzyme/Source | Method | Carrier/Support | Turbidity Reduction (%) | Viscosity Reduction (%) | Operational Stability | Juice Type | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|
| FE | IE | FE | IE | ||||||
| Tannase (Penicillium rolfsii) | Entrapment | Calcium alginate beads | 73.0 | 78.0 | No change | 44.0 | Above 50% activity after 6 reuse cycles. | Apple | [21] |
| Polygalacturonase (Aspergillus niger) | Physical adsorption | Calcium alginate beads | 94.5 | 96.8 | - | - | 20% activity after 10 reuse cycles. | Apple | [73] |
| Pectin Lyase (Acinetobacter calcoaceticus) | Covalent attachment | Magnetic carboxymethyl cellulose nanoparticles | 49.2 | 54.4 | 12.5 | 28.6 | - | Plum | [65] |
| Pectinmethylesterase (Lycopersicon esculentum) | Entrapment | Calcium alginate beads | - | 98.0 | - | 55.0 | 55% activity after 10 reuse cycles. | Orange | [60] |
| Alkyne-pectinase (Aspergillus aculeatus) | Covalent attachment | Polyethyleneimine cryogel support | 100.0 | 55.0 | - | - | 61% activity after 12 reuse cycles. | Apple | [80] |
| Pectinase (Aspergillus aculeatus) | Cross-linking | Epoxy polymer support | 99.5 | 99.5 | 27.9 | 27.8 | Above 95% activity after 10 reuse cycles. | Apple | [93] |
| Exopolygalacturonase (Penicillium paxilli) | Covalent attachment | Chitosan magnetic nanosupport | 93.0 | 93.6 | 51.6 | 55.0 | 63% activity after 4 reuse cycles. | Grape | [33] |
| Xylanase (Thermomyces lanuginosus) | Covalent attachment | Magnetic polyethylene trichlorotriazine nanoparticles | 52.8 | 42.0 | - | - | 50% activity after 9 reuse cycles. | Pineapple | [25] |
| Pectinase (Aspergillus aculeatus) | Covalent attachment | Cross-linked alginate–montmorillonite beads | 77.0 | 80.50 | 39.10 | 40.0 | 53% activity after 6 reuse cycles. | Pineapple | [29] |
| Pectinase (Aspergillus aculeatus) | Covalent attachment | Magnetic polyethylene trichlorotriazine nanoparticles | 48.0 | 59.0 | - | - | 60% activity after 9 reuse cycles. | Pineapple | [75] |
| Xylanase (Aspergillus flavus) | Entrapment | Calcium alginate beads | 51.6 | 52.8 | 5.2 | 17.8 | 63% and 22% activity after 8 and 12 reuse cycles, respectively. | Pineapple | [61] |
| Pectinase (Aspergillus aculeatus) | Entrapment | Calcium alginate beads | - | 97.2 | - | 20.8 | 80% and 30% activity after 3 and 8 reuse cycles, respectively. | Apple | [19] |
| Xylanase (Trichoderma longibrachiatum) | Covalent attachment | Activated silica gel supports | 74.7 | 73.6 | - | - | 77% activity after 10 reuse cycles. | Orange | [36] |
| Pectinase (Rhizopus sp.) | Covalent attachment | Activated bentonite clay | - | 61.6 | - | 63.9 | 87% activity after 25 reuse cycles | Orange | [88] |
| Pectinase (Aspergillus niger) | Entrapment | Synthetic polyvinyl alcohol sponge | Hazed sample | Cleared sample | 69 | 75 | 97.5% and 91% activity after 10 and 12 reuse cycles, respectively. | Orange | [134] |
| Xylanase (Bacillus pumilus) | Covalent attachment | Activated aluminum oxide pellets | 1.0 | 2.0 | 80.0 | 79.8 | 55% activity after 5 reuse cycles. | Papaya | [45] |
| Xylanase (Bacillus pumilus) | Covalent attachment | Activated aluminium oxide pellets | 27.0 | 30.0 | 35.0 | 60.0 | 85% and 58% activity after 5 and 10 reuse cycles, respectively. | Grape | [39] |
| Pectinase (Pectinex® Ultra Color) | Cross-linking | Glass beads | 33.3 | 39.8 | 96.5 | 96.5 | 80% activity after 15 reuse cycles. | Barberry | [72] |
| Pectinase (Penicillium crustosum) | Covalent attachment | Amino-functionalised magnetic core–shell nanoparticles | 64.0 | 62.0 | - | - | 85% activity after 5 reuse cycles | Orange | [135] |
6.4. Application in Dairy Beverages
6.5. Application in the Production of Plant-Based Dairy Alternatives
7. Conclusions
8. Future and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Taheri-Kafrani, A.; Kharazmi, S.; Nasrollahzadeh, M.; Soozanipour, A.; Ejeian, F.; Etedali, P.; Mansouri-Tehrani, H.A.; Razmjou, A.; Yek, S.M.; Varma, R.S. Recent developments in enzyme immobilization technology for high-throughput processing in food industries. Crit. Rev. Food Sci. Nutr. 2021, 61, 3160–3196. [Google Scholar] [CrossRef]
- Espejo, F. Role of commercial enzymes in wine production: A critical review of recent research. J. Food Sci. Technol. 2021, 58, 9–21. [Google Scholar] [CrossRef]
- Uzuner, S.; Cekmecelioglu, D. Enzymes in the Beverage Industry. In Enzymes in Food Biotechnology; Academic Press: Cambridge, MA, USA, 2019; pp. 29–43. [Google Scholar]
- Bilal, M.; Iqbal, H.M.N. Sustainable bioconversion of food waste into high-value products by immobilized enzymes to meet bio-economy challenges and opportunities—A review. Food Res. Int. 2019, 123, 226–240. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, M.; Fang, Y.; Wu, Y.; Liu, Y.; Zhao, Y.; Xu, J. Recent advancements in encapsulation of chitosan-based enzymes and their applications in food industry. Crit. Rev. Food Sci. Nutr. 2022, 63, 11044–11062. [Google Scholar] [CrossRef]
- Dal Magro, L.; Pessoa, J.P.S.; Klein, M.P.; Fernandez-Lafuente, R.; Rodrigues, R.C. Enzymatic clarification of orange juice in continuous bed reactors: Fluidized-bed versus packed-bed reactor. Catal. Today 2021, 362, 184–191. [Google Scholar] [CrossRef]
- Pervez, S.; Aman, A.; Ul Qader, S.A. Role of two polysaccharide matrices on activity, stability and recycling efficiency of immobilized fungal amyloglucosidase of GH15 family. Int. J. Biol. Macromol. 2017, 96, 70–77. [Google Scholar] [CrossRef]
- Chalella Mazzocato, M.; Jacquier, J.-C. Encapsulation of Amyloglucosidase in Chitosan-SDS Coacervates as a Means to Control Starch Hydrolysis in Plant-Based Beverages. Beverages 2023, 9, 83. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Guisan, J.M.; Bolivar, J.M.; López-Gallego, F.; Rocha-Martín, J. Immobilization of Enzymes and Cells: Methods and Protocols, 4th ed.; Humana Press: Totowa, NJ, USA, 2020. [Google Scholar]
- Ashly, P.C.; Mohanan, P.V. Preparation and characterization of Rhizopus amyloglucosidase immobilized on poly(o-toluidine). Process Biochem. 2010, 45, 1422–1426. [Google Scholar] [CrossRef]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme immobilization by adsorption: A review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef]
- Vijayalakshmi, S.; Anand, M.; Ranjitha, J. Microalgae-Based Biofuel Production Using Low-Cost Nanobiocatalysts. In Microalgae Cultivation for Biofuels Production; Academic Press: Cambridge, MA, USA, 2020; pp. 251–263. [Google Scholar]
- Attique, S.A.; Qurat ul, A.; Hussain, N.; Bilal, M.; Iqbal, H.M.N. Enzyme immobilization approaches. In Biocatalyst Immobilization; Academic Press: Cambridge, MA, USA, 2023; pp. 37–54. [Google Scholar]
- Yamaguchi, H.; Kiyota, Y.; Miyazaki, M. Techniques for Preparation of Cross-Linked Enzyme Aggregates and Their Applications in Bioconversions. Catalysts 2018, 8, 174. [Google Scholar] [CrossRef]
- Datta, S.; Christena, L.R.; Rajaram, Y.R. Enzyme immobilization: An overview on techniques and support materials. 3 Biotech 2013, 3, 1–9. [Google Scholar] [CrossRef]
- Coelho, A.L.S.; Orlandelli, R.C. Immobilized microbial lipases in the food industry: A systematic literature review. Crit. Rev. Food Sci. Nutr. 2021, 61, 1689–1703. [Google Scholar] [CrossRef]
- Guzik, U.; Hupert-Kocurek, K.; Wojcieszynska, D. Immobilization as a strategy for improving enzyme properties-application to oxidoreductases. Molecules 2014, 19, 8995–9018. [Google Scholar] [CrossRef]
- de Oliveira, R.L.; Dias, J.L.; da Silva, O.S.; Porto, T.S. Immobilization of pectinase from Aspergillus aculeatus in alginate beads and clarification of apple and umbu juices in a packed bed reactor. Food Bioprod. Process. 2018, 109, 9–18. [Google Scholar] [CrossRef]
- Aslan, Y.; Taher, A.Y.; Cavidoğlu, I. Improved catalytic activity of Aspergillus oryzae β-galactosidase by covalent immobilization on Eupergit CM. J. Anim. Plant Sci. 2018, 28, 1648–1655. [Google Scholar]
- Andrade, P.M.L.; Baptista, L.; Bezerra, C.O.; Peralta, R.M.; Góes-Neto, A.; Uetanabaro, A.P.T.; Costa, A.M.d. Immobilization and characterization of tannase from Penicillium rolfsii CCMB 714 and its efficiency in apple juice clarification. J. Food Meas. Charact. 2021, 15, 1005–1013. [Google Scholar] [CrossRef]
- Zhang, H.R.; Ma, W.X.; Han, X.Y.; Chen, G.E.; Xu, Z.L.; Mao, H.F. Self-adhesive PMIA membranes with chitosan porous beads immobilized pullulanase for efficient biological aging of beer. Colloids Surf. B Biointerfaces 2022, 218, 112720. [Google Scholar] [CrossRef]
- Wang, C.; Chen, P.X.; Xiao, Q.; Yang, Q.M.; Weng, H.F.; Zhang, Y.H.; Xiao, A.F. Chitosan Activated with Genipin: A Nontoxic Natural Carrier for Tannase Immobilization and Its Application in Enhancing Biological Activities of Tea Extract. Mar. Drugs 2021, 19, 166. [Google Scholar] [CrossRef]
- Ionata, E.; Marcolongo, L.; Cara, F.L.; Cetrangolo, G.P.; Febbraio, F. Improvement of functional properties of a thermostable β-glycosidase for milk lactose hydrolysis. Biopolymers 2018, 109, e23234. [Google Scholar] [CrossRef]
- Kharazmi, S.; Taheri-Kafrani, A.; Soozanipour, A.; Nasrollahzadeh, M.; Varma, R.S. Xylanase immobilization onto trichlorotriazine-functionalized polyethylene glycol grafted magnetic nanoparticles: A thermostable and robust nanobiocatalyst for fruit juice clarification. Int. J. Biol. Macromol. 2020, 163, 402–413. [Google Scholar] [CrossRef]
- Sahin, S.; Ozmen, I. Immobilization of pectinase on Zr-treated pumice for fruit juice industry. J. Food Process. Preserv. 2020, 44, e14661. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, Q.; Dong, J.; Xian, M.; Yu, J.; Yin, H.; Chang, Z.; Mu, X.; Hou, T.; Wang, J. Enzyme-inorganic nanoflowers/alginate microbeads: An enzyme immobilization system and its potential application. Process Biochem. 2017, 57, 87–94. [Google Scholar] [CrossRef]
- Katrolia, P.; Liu, X.; Li, G.; Kopparapu, N.K. Enhanced Properties and Lactose Hydrolysis Efficiencies of Food-Grade beta-Galactosidases Immobilized on Various Supports: A Comparative Approach. Appl. Biochem. Biotechnol. 2019, 188, 410–423. [Google Scholar] [CrossRef]
- Mohammadi, M.; Rezaei Mokarram, R.; Shahvalizadeh, R.; Sarabandi, K.; Lim, L.-T.; Hamishehkar, H. Immobilization and stabilization of pectinase on an activated montmorillonite support and its application in pineapple juice clarification. Food Biosci. 2020, 36, 100625. [Google Scholar] [CrossRef]
- Ruiz, E.; Busto, M.D.; Ramos-Gómez, S.; Palacios, D.; Pilar-Izquierdo, M.C.; Ortega, N. Encapsulation of glucose oxidase in alginate hollow beads to reduce the fermentable sugars in simulated musts. Food Biosci. 2018, 24, 67–72. [Google Scholar] [CrossRef]
- Baffa Júnior, J.C.; Viana, P.A.; de Rezende, S.T.; Soares, N.d.F.F.; Guimarães, V.M. Immobilization of an alpha-galactosidase from Debaryomyces hansenni UFV-1 in cellulose film and its application in oligosaccharides hydrolysis. Food Bioprod. Process. 2018, 111, 30–36. [Google Scholar] [CrossRef]
- Shojaei, F.; Homaei, A.; Taherizadeh, M.R.; Kamrani, E. Characterization of biosynthesized chitosan nanoparticles from Penaeus vannameifor the immobilization of P. vannameiprotease: An eco-friendly nanobiocatalyst. Int. J. Food Prop. 2017, 20 (Suppl. 2), 1413–1423. [Google Scholar] [CrossRef]
- Amin, F.; Asad, S.A.; Nazli, Z.-i.-H.; Kalsoom, U.; Bhatti, H.N.; Bilal, M. Immobilization, biochemical, thermodynamic, and fruit juice clarification properties of lignocellulosic biomass–derived exo-polygalacturonase from Penicillium paxilli. Biomass Convers. Biorefinery 2022, 13, 13181–13196. [Google Scholar] [CrossRef]
- Li, Y.; Wang, B.; Wu, M.; Huan, W.; Li, J. Magnetic graphene oxide nanocomposites as an effective support for lactase immobilization with improved stability and enhanced photothermal enzymatic activity. New J. Chem. 2021, 45, 5939–5948. [Google Scholar] [CrossRef]
- Wang, L.L.; Fan, M.; Xing, X.; Liu, Y.; Sun, S. Immobilization of glyceraldehyde-3-phosphate dehydrogenase on Fe3O4 magnetic nanoparticles and its application in histamine removal. Colloids Surf. B Biointerfaces 2021, 205, 111917. [Google Scholar] [CrossRef] [PubMed]
- Alagöz, D.; Varan, N.E.; Toprak, A.; Yildirim, D.; Tukel, S.S.; Fernandez-Lafuente, R. Immobilization of xylanase on differently functionalized silica gel supports for orange juice clarification. Process Biochem. 2022, 113, 270–280. [Google Scholar] [CrossRef]
- Lopes, M.M.; Coutinho, T.C.; Malafatti, J.O.D.; Paris, E.C.; Sousa, C.P.d.; Farinas, C.S. Immobilization of phytase on zeolite modified with iron(II) for use in the animal feed and food industry sectors. Process Biochem. 2021, 100, 260–271. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Khodaiyan, F.; Mousavi, S.M.E.; Kennedy, J.F.; Azimi, S.Z. A Health-Friendly Strategy for Covalent-Bonded Immobilization of Pectinase on the Functionalized Glass Beads. Food Bioprocess. Technol. 2021, 14, 177–186. [Google Scholar] [CrossRef]
- Kumar, L.; Nagar, S.; Mittal, A.; Garg, N.; Gupta, V.K. Immobilization of xylanase purified from Bacillus pumilus VLK-1 and its application in enrichment of orange and grape juices. J. Food Sci. Technol. 2014, 51, 1737–1749. [Google Scholar] [CrossRef]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Computational modelling approach for the optimization of apple juice clarification using immobilized pectinase and xylanase enzymes. Curr. Res. Food Sci. 2020, 3, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, L.M.O.; Pereira, M.G.; Vici, A.C.; Heinen, P.R.; Buckeridge, M.S.; Polizeli, M. Efficient hydrolysis of wine and grape juice anthocyanins by Malbranchea pulchella beta-glucosidase immobilized on MANAE-agarose and ConA-Sepharose supports. Int. J. Biol. Macromol. 2019, 136, 1133–1141. [Google Scholar] [CrossRef]
- Uzun, U.; Yildirim Akatin, M. Immobilization and some application of α-amylase purified from Rhizoctonia solani AG-4 strain ZB-34. Turk. J. Biochem. 2019, 44, 397–407. [Google Scholar] [CrossRef]
- Yavaser, R.; Karagozler, A.A. Covalent immobilization of papain onto poly(hydroxyethyl methacrylate)-chitosan cryogels for apple juice clarification. Food Sci. Technol. Int. 2020, 26, 629–641. [Google Scholar] [CrossRef]
- Dal Magro, L.; Silveira, V.C.C.; de Menezes, E.W.; Benvenutti, E.V.; Nicolodi, S.; Hertz, P.F.; Klein, M.P.; Rodrigues, R.C. Magnetic biocatalysts of pectinase and cellulase: Synthesis and characterization of two preparations for application in grape juice clarification. Int. J. Biol. Macromol. 2018, 115, 35–44. [Google Scholar] [CrossRef]
- Tanwar, E.; Nagar, S.; Kumari, K.; Mallesh, G.; Goyal, S.; Sonu. Enrichment of papaya juice using covalently immobilized xylanase from Bacillus pumilus SV-85S. Biomass Convers. Biorefinery 2022, 1–17. [Google Scholar] [CrossRef]
- Zhang, D.-H.; Yuwen, L.-X.; Peng, L.-J. Parameters Affecting the Performance of Immobilized Enzyme. J. Chem. 2013, 2013, 946248. [Google Scholar] [CrossRef]
- Katrolia, P.; Liu, X.; Li, J.; Kopparapu, N.K. Enhanced elimination of non-digestible oligosaccharides from soy milk by immobilized alpha-galactosidase: A comparative analysis. J. Food Biochem. 2019, 43, e13005. [Google Scholar] [CrossRef]
- Bodakowska-Boczniewicz, J.; Garncarek, Z. Immobilization of Naringinase from Penicillium decumbens on Chitosan Microspheres for Debittering Grapefruit Juice. Molecules 2019, 24, 4234. [Google Scholar] [CrossRef] [PubMed]
- Romo-Sánchez, S.; Arévalo-Villena, M.; García Romero, E.; Ramirez, H.L.; Briones Pérez, A. Immobilization of β-Glucosidase and its application for enhancement of aroma precursors in Muscat wine. Food Bioprocess. Technol. 2014, 7, 1381–1392. [Google Scholar] [CrossRef]
- Bezerra, T.M.d.S.; Bassan, J.C.; Santos, V.T.d.O.; Ferraz, A.; Monti, R. Covalent immobilization of laccase in green coconut fiber and use in clarification of apple juice. Process Biochem. 2015, 50, 417–423. [Google Scholar] [CrossRef]
- Chen, K.I.; Lo, Y.C.; Liu, C.W.; Yu, R.C.; Chou, C.C.; Cheng, K.C. Enrichment of two isoflavone aglycones in black soymilk by using spent coffee grounds as an immobiliser for beta-glucosidase. Food Chem. 2013, 139, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.J.; Woo, S.H.; Jeong, H.M.; Kim, J.S.; Ko, D.S.; Jeong, D.W.; Lee, J.H.; Shim, J.H. Characterization of novel alpha-galactosidase in glycohydrolase family 97 from Bacteroides thetaiotaomicron and its immobilization for industrial application. Int. J. Biol. Macromol. 2020, 152, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Hackenhaar, C.R.; Spolidoro, L.S.; Flores, E.E.E.; Klein, M.P.; Hertz, P.F. Batch synthesis of galactooligosaccharides from co-products of milk processing using immobilized β-galactosidase from Bacillus circulans. Biocatal. Agric. Biotechnol. 2021, 36, 102136. [Google Scholar] [CrossRef]
- de Freitas, M.F.M.; Hortencio, L.C.; de Albuquerque, T.L.; Rocha, M.V.P.; Goncalves, L.R.B. Simultaneous hydrolysis of cheese whey and lactulose production catalyzed by beta-galactosidase from Kluyveromyces lactis NRRL Y1564. Bioprocess. Biosyst. Eng. 2020, 43, 711–722. [Google Scholar] [CrossRef]
- Girigowda, K.; Mulimani, V.H. Hydrolysis of Galacto-oligosaccharides in Soymilk by κ-carrageenan-entrapped α-galactosidase from Aspergillus Oryzae. World J. Microbiol. Biotechnol. 2006, 22, 437–442. [Google Scholar] [CrossRef]
- Husain, Q.; Ansari, S.A.; Alam, F.; Azam, A. Immobilization of Aspergillus oryzae beta galactosidase on zinc oxide nanoparticles via simple adsorption mechanism. Int. J. Biol. Macromol. 2011, 49, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Shafi, A.; Ahmed, F.; Husain, Q. beta-Galactosidase mediated synthesized nanosupport for the immobilization of same enzyme: Its stability and application in the hydrolysis of lactose. Int. J. Biol. Macromol. 2021, 184, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, T.; Saman, T.; Irfan, M.; Anwar, F.; Ikram, M.S.; Tabassam, Q. Pectinase Production from Schizophyllum commune Through Central Composite Design Using Citrus Waste and Its Immobilization for Industrial Exploitation. Waste Biomass Valorization 2018, 10, 2527–2536. [Google Scholar] [CrossRef]
- Rajan, A.; Nair, G.R. Production of soya milk containing low flatulence-causing oligosaccharides in a packed bed reactor using immobilised α-galactosidase. Int. J. Food Sci. Technol. 2010, 45, 2023–2031. [Google Scholar] [CrossRef]
- Bogra, P.; Kumar, A.; Kuhar, K.; Panwar, S.; Singh, R. Immobilization of tomato (Lycopersicon esculentum) pectinmethylesterase in calcium alginate beads and its application in fruit juice clarification. Biotechnol. Lett. 2013, 35, 1895–1900. [Google Scholar] [CrossRef]
- Bhushan, B.; Pal, A.; Kumar, S.; Jain, V. Biochemical characterization and kinetic comparison of encapsulated haze removing acidophilic xylanase with partially purified free xylanase isolated from Aspergillus flavus MTCC 9390. J. Food Sci. Technol. 2015, 52, 191–200. [Google Scholar] [CrossRef]
- Dwevedi, A.; Kayastha, A.M. Optimal immobilization of beta-galactosidase from Pea (PsBGAL) onto Sephadex and chitosan beads using response surface methodology and its applications. Bioresour. Technol. 2009, 100, 2667–2675. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.P.; Spolidoro, L.S.; Manfroi, V.; Rodrigues, R.C.; Hertz, P.F. alpha-Acetolactate decarboxylase immobilized in chitosan: A highly stable biocatalyst to prevent off-flavor in beer. Biotechnol. Prog. 2022, 38, e3295. [Google Scholar] [CrossRef]
- Gupta, A.K.; Yumnam, M.; Medhi, M.; Koch, P.; Chakraborty, S.; Mishra, P. Isolation and characterization of naringinase enzyme and its application in debittering of Pomelo juice (Citrus grandis): A comparative study with macroporous resin. J. Food Process. Preserv. 2021, 45, e15380. [Google Scholar] [CrossRef]
- Tasgin, E.; Babagil, A.; Nadaroglu, H.; Allegretti, P.E. Immobilization of Purified Pectin Lyase from Acinetobacter calcoaceticus onto Magnetic Carboxymethyl Cellulose Nanoparticles and Its Usability in Food Industry. J. Chem. 2020, 2020, 4791408. [Google Scholar] [CrossRef]
- Benucci, I.; Lombardelli, C.; Cacciotti, I.; Liburdi, K.; Nanni, F.; Esti, M. Chitosan beads from microbial and animal sources as enzyme supports for wine application. Food Hydrocoll. 2016, 61, 191–200. [Google Scholar] [CrossRef]
- Aslan, Y.; Ömerosmanoğlu, D.; Koç, E.Ö. Covalent immobilization of an alkaline protease from Bacillus licheniformis. Turk. J. Biochem. 2018, 43, 595–604. [Google Scholar] [CrossRef]
- Alshanberi, A.M.; Ansari, S.A. Validation and Optimization of Polyvinyl Alcohol-Functionalized Gold Nanoparticles for Producing Lactose-Free Dairy Products. Orient. J. Chem. 2021, 37, 643–647. [Google Scholar] [CrossRef]
- Vaz, R.P.; Vici, A.C.; Teixeira de Moraes Polizeli, M.L.; Magalhaes, P.O.; Filho, E.X.F. Immobilization studies of a pectinase produced by Aspergillus terreus. Biotechnol. Appl. Biochem. 2021, 68, 197–208. [Google Scholar] [CrossRef]
- Neira, H.D.; Herr, A.E. Kinetic Analysis of Enzymes Immobilized in Porous Film Arrays. Anal. Chem. 2017, 89, 10311–10320. [Google Scholar] [CrossRef]
- Gennari, A.; Mobayed, F.H.; Catto, A.L.; Benvenutti, E.V.; Volpato, G.; Volken de Souza, C.F. Kluyveromyces lactis β-galactosidase immobilized on collagen: Catalytic stability on batch and packed-bed reactor hydrolysis. React. Kinet. Mech. Catal. 2019, 127, 583–599. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Khodaiyan, F.; Mousavi, S.M.; Azimi, S.Z. Continuous Clarification of Barberry Juice with Pectinase Immobilised by Oxidized Polysaccharides. Food Technol. Biotechnol. 2021, 59, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Wang, F.; Zhou, B.; Li, J.; Li, B.; Liang, H. Immobilization of pectinases into calcium alginate microspheres for fruit juice application. Food Hydrocoll. 2019, 89, 691–699. [Google Scholar] [CrossRef]
- Agrawal, R.; Srivastava, A.; Verma, A.K. Immobilization of beta-glucosidase onto silicon oxide nanoparticles and augment of phenolics in sugarcane juice. J. Food Sci. Technol. 2016, 53, 3002–3012. [Google Scholar] [CrossRef]
- Kharazmi, S.; Taheri-Kafrani, A.; Soozanipour, A. Efficient immobilization of pectinase on trichlorotriazine-functionalized polyethylene glycol-grafted magnetic nanoparticles: A stable and robust nanobiocatalyst for fruit juice clarification. Food Chem. 2020, 325, 126890. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Khakbaz Heshmati, M.; Sarabandi, K.; Fathi, M.; Lim, L.T.; Hamishehkar, H. Activated alginate-montmorillonite beads as an efficient carrier for pectinase immobilization. Int. J. Biol. Macromol. 2019, 137, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Hakkoymaz, O.; Mazi, H. An immobilized invertase enzyme for the selective determination of sucrose in fruit juices. Anal. Biochem. 2020, 611, 114000. [Google Scholar] [CrossRef]
- Lettera, V.; Pezzella, C.; Cicatiello, P.; Piscitelli, A.; Giacobelli, V.G.; Galano, E.; Amoresano, A.; Sannia, G. Efficient immobilization of a fungal laccase and its exploitation in fruit juice clarification. Food Chem. 2016, 196, 1272–1278. [Google Scholar] [CrossRef]
- Fernandez-Pacheco, P.; Garcia-Bejar, B.; Briones Perez, A.; Arevalo-Villena, M. Free and Immobilised beta-Glucosidases in Oenology: Biotechnological Characterisation and Its Effect on Enhancement of Wine Aroma. Front. Microbiol. 2021, 12, 723815. [Google Scholar] [CrossRef]
- Oktay, B.; Demir, S.; Kayaman-Apohan, N. Immobilization of pectinase on polyethyleneimine based support via spontaneous amino-yne click reaction. Food Bioprod. Process. 2020, 122, 159–168. [Google Scholar] [CrossRef]
- Hakkoymaz, O.; Mazi, H. Termostable and effective immobilized invertase for sucrose determination in fruit juices. Anal. Biochem. 2024, 690, 115515. [Google Scholar] [CrossRef]
- Muley, A.B.; Mulchandani, K.H.; Singhal, R.S. Immobilization of enzymes on iron oxide magnetic nanoparticles: Synthesis, characterization, kinetics and thermodynamics. Methods Enzym. 2020, 630, 39–79. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Saleh, S.A.A.; Abdel-Hameed, S.A.M.; Fayad, A.M. Catalytic, kinetic and thermodynamic properties of free and immobilized caseinase on mica glass-ceramics. Heliyon 2019, 5, e01674. [Google Scholar] [CrossRef] [PubMed]
- Tavares, A.P.; Silva, C.G.; Drazic, G.; Silva, A.M.; Loureiro, J.M.; Faria, J.L. Laccase immobilization over multi-walled carbon nanotubes: Kinetic, thermodynamic and stability studies. J. Colloid. Interface Sci. 2015, 454, 52–60. [Google Scholar] [CrossRef]
- Karam, E.A.; Abdel Wahab, W.A.; Saleh, S.A.A.; Hassan, M.E.; Kansoh, A.L.; Esawy, M.A. Production, immobilization and thermodynamic studies of free and immobilized Aspergillus awamori amylase. Int. J. Biol. Macromol. 2017, 102, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Zhao, Y.; Noreen, S.; Shah, S.Z.H.; Bharagava, R.N.; Iqbal, H.M.N. Modifying bio-catalytic properties of enzymes for efficient biocatalysis: A review from immobilization strategies viewpoint. Biocatal. Biotransform. 2019, 37, 159–182. [Google Scholar] [CrossRef]
- Kishore, D.; Kayastha, A.M. Optimisation of immobilisation conditions for chick pea beta-galactosidase (CpGAL) to alkylamine glass using response surface methodology and its applications in lactose hydrolysis. Food Chem. 2012, 134, 1650–1657. [Google Scholar] [CrossRef] [PubMed]
- Alsoufi, M.A. Use of immobilized Pectinase for Fruit Juice Clarification. Biosci. Res. 2021, 202118, 1480–1487. [Google Scholar]
- de Lima, J.S.; Cabrera, M.P.; de Souza Motta, C.M.; Converti, A.; Carvalho, L.B., Jr. Hydrolysis of tannins by tannase immobilized onto magnetic diatomaceous earth nanoparticles coated with polyaniline. Food Res. Int. 2018, 107, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Chen, Q.; Zhong, G.; Cao, W.; Yu, A.; Liu, Y. Immobilization and characterization of tannase from a metagenomic library and its use for removal of tannins from green tea infusion. J. Microbiol. Biotechnol. 2014, 24, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Soria, F.; Ellenrieder, G.; Oliveira, G.B.; Cabrera, M.; Carvalho, L.B., Jr. alpha-L-rhamnosidase of Aspergillus terreus immobilized on ferromagnetic supports. Appl. Microbiol. Biotechnol. 2012, 93, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Cheng, F.; Lu, Y.; Ge, W.; Zhang, M.; Yue, B. Immobilization of pectinase from Penicillium oxalicum F67 onto magnetic cornstarch microspheres: Characterization and application in juice production. J. Mol. Catal. B Enzym. 2013, 97, 137–143. [Google Scholar] [CrossRef]
- Rajdeo, K.; Harini, T.; Lavanya, K.; Fadnavis, N.W. Immobilization of pectinase on reusable polymer support for clarification of apple juice. Food Bioprod. Process. 2016, 99, 12–19. [Google Scholar] [CrossRef]
- Bodakowska-Boczniewicz, J.; Garncarek, Z. Immobilization of Naringinase from Aspergillus niger on a Magnetic Polysaccharide Carrier. Molecules 2020, 25, 2731. [Google Scholar] [CrossRef]
- Çelem, E.B.; Önal, S. Sepabeads EC-EP immobilized α-galactosidase: Immobilization, characterization and application in the degradation of raffinose-type oligosaccharides. Process Biochem. 2022, 116, 136–147. [Google Scholar] [CrossRef]
- Gennari, A.; Simon, R.; Sperotto, N.D.M.; Bizarro, C.V.; Basso, L.A.; Machado, P.; Benvenutti, E.V.; Da Cas Viegas, A.; Nicolodi, S.; Renard, G.; et al. One-step purification of a recombinant beta-galactosidase using magnetic cellulose as a support: Rapid immobilization and high thermal stability. Bioresour. Technol. 2022, 345, 126497. [Google Scholar] [CrossRef]
- Gennari, A.; Mobayed, F.H.; Rafael, R.d.S.; Catto, A.L.; Benvenutti, E.V.; Rodrigues, R.C.; Sperotto, R.A.; Volpato, G.; Souza, C.F.V.d. Stabilization study of tetrameric Kluyveromyces lactis β-galactosidase by immobilization on immobead: Thermal, physico-chemical, textural and catalytic properties. Braz. J. Chem. Eng. 2019, 36, 1403–1417. [Google Scholar] [CrossRef]
- Suryawanshi, R.K.; Jana, U.K.; Prajapati, B.P.; Kango, N. Immobilization of Aspergillus quadrilineatus RSNK-1 multi-enzymatic system for fruit juice treatment and mannooligosaccharide generation. Food Chem. 2019, 289, 95–102. [Google Scholar] [CrossRef]
- Zimmermann, V.; Esparza-Flores, E.E.; Partichelli, C.P.; da Silva, E.F.C.; Rodrigues, R.C. Genipin-activated chitosan particles as support of pectinase immobilization and their application as stable biocatalyst for fruit juice clarification. Process Biochem. 2024, 139, 1–9. [Google Scholar] [CrossRef]
- Shafi, A.; Khan, M.; Khan, M.Z.; Husain, Q. Ameliorating the activity and stability of β galactosidase by tailoring potential nanobiocatalyst on functionalized nanographene: Headway to lactose hydrolysis. LWT 2019, 112, 108260. [Google Scholar] [CrossRef]
- Su, E.; Xia, T.; Gao, L.; Dai, Q.; Zhang, Z. Immobilization and Characterization of Tannase and its Haze-removing. Food Sci. Technol. Int. 2010, 15, 545–552. [Google Scholar] [CrossRef]
- Gennari, A.; Mobayed, F.H.; da Silva Rafael, R.; Rodrigues, R.C.; Sperotto, R.A.; Volpato, G.; Volken de Souza, C.F. Modification of Immobead 150 support for protein immobilization: Effects on the properties of immobilized Aspergillus oryzae beta-galactosidase. Biotechnol. Prog. 2018, 34, 934–943. [Google Scholar] [CrossRef]
- Dal Magro, L.; de Moura, K.S.; Backes, B.E.; de Menezes, E.W.; Benvenutti, E.V.; Nicolodi, S.; Klein, M.P.; Fernandez-Lafuente, R.; Rodrigues, R.C. Immobilization of pectinase on chitosan-magnetic particles: Influence of particle preparation protocol on enzyme properties for fruit juice clarification. Biotechnol. Rep. 2019, 24, e00373. [Google Scholar] [CrossRef]
- Poppe, J.K.; Fernandez-Lafuente, R.; Rodrigues, R.C.; Ayub, M.A. Enzymatic reactors for biodiesel synthesis: Present status and future prospects. Biotechnol. Adv. 2015, 33, 511–525. [Google Scholar] [CrossRef]
- Sen, P.; Nath, A.; Bhattacharjee, C. Packed-Bed Bioreactor and Its Application in Dairy, Food, and Beverage Industry. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 235–277. [Google Scholar]
- Azimi, S.Z.; Hosseini, S.S.; Khodaiyan, F. Continuous clarification of grape juice using a packed bed bioreactor including pectinase enzyme immobilized on glass beads. Food Biosci. 2021, 40, 100877. [Google Scholar] [CrossRef]
- Carceller, J.M.; Martínez Galán, J.P.; Monti, R.; Bassan, J.C.; Filice, M.; Yu, J.; Climent, M.J.; Iborra, S.; Corma, A. Covalent Immobilization of Naringinase over Two-Dimensional 2D Zeolites and its Applications in a Continuous Process to Produce Citrus Flavonoids and for Debittering of Juices. ChemCatChem 2020, 12, 4502–4511. [Google Scholar] [CrossRef]
- Ko, C.-Y.; Liu, J.-M.; Chen, K.-I.; Hsieh, C.-W.; Chu, Y.-L.; Cheng, K.-C. Lactose-Free Milk Preparation by Immobilized Lactase in Glass Microsphere Bed Reactor. Food Biophys. 2018, 13, 353–361. [Google Scholar] [CrossRef]
- Roy, I.; Gupta, M.N. Lactose hydrolysis by Lactozym™ immobilized on cellulose beads in batch and fluidized bed modes. Process Biochem. 2003, 39, 325–332. [Google Scholar] [CrossRef]
- Benucci, I.; Caso, M.C.; Bavaro, T.; Masci, S.; Keršienė, M.; Esti, M. Prolyl endopeptidase from Aspergillus niger immobilized on a food-grade carrier for the production of gluten-reduced beer. Food Control 2020, 110, 106987. [Google Scholar] [CrossRef]
- Benucci, I.; Mazzocchi, C.; Lombardelli, C.; Cacciotti, I.; Esti, M. Multi-enzymatic Systems Immobilized on Chitosan Beads for Pomegranate Juice Treatment in Fluidized Bed Reactor: Effect on Haze-Active Molecules and Chromatic Properties. Food Bioprocess. Technol. 2019, 12, 1559–1572. [Google Scholar] [CrossRef]
- Patil, A.G.; Kote, N.V.; Mulimani, V. Enzymatic removal of flatulence-inducing sugars in chickpea milk using free and polyvinyl alcohol immobilized alpha-galactosidase from Aspergillus oryzae. J. Ind. Microbiol. Biotechnol. 2009, 36, 29–33. [Google Scholar] [CrossRef]
- González-Temiño, Y.; Ruíz, M.O.; Ortega, N.; Ramos-Gómez, S.; Busto, M.D. Immobilization of naringinase on asymmetric organic membranes: Application for debittering of grapefruit juice. Innov. Food Sci. Emerg. Technol. 2021, 73, 102790. [Google Scholar] [CrossRef]
- Fidaleo, M.; Tavilli, E. Urea removal in rosé and red wines by immobilised acid urease in a packed bed reactor. Food Bioprod. Process. 2021, 126, 42–50. [Google Scholar] [CrossRef]
- Mandity, I.M.; Otvos, S.B.; Fulop, F. Strategic Application of Residence-Time Control in Continuous-Flow Reactors. ChemistryOpen 2015, 4, 212–223. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Q.Z.K.; Chen, X.D. Pilot-scale lactose hydrolysis using β-galactosidase immobilized on cotton fabric. Chem. Eng. Process. Process Intensif. 2007, 46, 497–500. [Google Scholar] [CrossRef]
- Ansari, S.A.; Husain, Q. Lactose hydrolysis from milk/whey in batch and continuous processes by concanavalin A-Celite 545 immobilized Aspergillus oryzae β galactosidase. Food Bioprod. Process. 2012, 90, 351–359. [Google Scholar] [CrossRef]
- Ibrahim, O.A.; Fathy, H.M.; Ibrahim, G.A.; Barakat, O.S.; El-Hofi, M.A.; Hassanein, H.A. Application of immobilized bioagents in lactose hydrolysis. Biosci. Res. 2018, 15, 2218–2227. [Google Scholar]
- Shankar, S.K.; Kumar, S.K.; Mulimani, V.H. Calcium alginate entrapped preparation of alpha-galactosidase: Its stability and application in hydrolysis of soymilk galactooligosaccharides. J. Ind. Microbiol. Biotechnol. 2011, 38, 1399–1405. [Google Scholar] [CrossRef]
- Oluwaseun, G.E.; Fredrick, O.T.; Anthony, O.A. Characteristics of Luffa sponge-immobilized pectinase from Aspergillus niger FTR 002 and its use in the continuous clarification of orange juice in packed-bed column bioreactor. Appl. Food Biotechnol. 2023, 10, 4. [Google Scholar] [CrossRef]
- Benucci, I.; Lombardelli, C.; Liburdi, K.; Acciaro, G.; Zappino, M.; Esti, M. Immobilised native plant cysteine proteases: Packed-bed reactor for white wine protein stabilisation. J. Food Sci. Technol. 2016, 53, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Ahumada, K.; Urrutia, P.; Illanes, A.; Wilson, L. Production of combi-CLEAs of glycosidases utilized for aroma enhancement in wine. Food Bioprod. Process. 2015, 94, 555–560. [Google Scholar] [CrossRef]
- de Ovalle, S.; Brena, B.; Gonzalez-Pombo, P. Influence of beta glucosidases from native yeast on the aroma of Muscat and Tannat wines. Food Chem. 2021, 346, 128899. [Google Scholar] [CrossRef]
- Tavernini, L.; Ottone, C.; Illanes, A.; Wilson, L. Entrapment of enzyme aggregates in chitosan beads for aroma release in white wines. Int. J. Biol. Macromol. 2020, 154, 1082–1090. [Google Scholar] [CrossRef]
- Benucci, I.; Liburdi, K.; Cacciotti, I.; Lombardelli, C.; Zappino, M.; Nanni, F.; Esti, M. Chitosan/clay nanocomposite films as supports for enzyme immobilization: An innovative green approach for winemaking applications. Food Hydrocoll. 2018, 74, 124–131. [Google Scholar] [CrossRef]
- Liburdi, K.; Benucci, I.; Esti, M. Study of Two Different Immobilized Acid Proteases for Wine Application. Food Biotechnol. 2010, 24, 282–292. [Google Scholar] [CrossRef]
- Serra, I.; Benucci, I.; Robescu, M.S.; Lombardelli, C.; Esti, M.; Calvio, C.; Pregnolato, M.; Terreni, M.; Bavaro, T. Developing a Novel Enzyme Immobilization Process by Activation of Epoxy Carriers with Glucosamine for Pharmaceutical and Food Applications. Catalysts 2019, 9, 843. [Google Scholar] [CrossRef]
- Del-Bosque, D.; Vila-Crespo, J.; Ruiperez, V.; Fernandez-Fernandez, E.; Rodriguez-Nogales, J.M. Entrapment of Glucose Oxidase and Catalase in Silica-Calcium-Alginate Hydrogel Reduces the Release of Gluconic Acid in Must. Gels 2023, 9, 622. [Google Scholar] [CrossRef] [PubMed]
- Silva-Barbieri, D.; Salazar, F.N.; Lopez, F.; Brossard, N.; Escalona, N.; Perez-Correa, J.R. Advances in White Wine Protein Stabilization Technologies. Molecules 2022, 27, 1251. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhu, J.; Shi, J.; Liu, Y.; Shao, D.; Jiang, C. Immobilized enzymes from Geotrichum spp. improve wine quality. Appl. Microbiol. Biotechnol. 2017, 101, 6637–6649. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Qi, J.; Hinkley, T.C.; Nugen, S.R.; Goddard, J.M. Recombinant lactase with a cellulose binding domain permits facile immobilization onto cellulose with retained activity. Food Bioprod. Process. 2021, 126, 207–214. [Google Scholar] [CrossRef]
- Zhao, F.; Hou, T.; Wang, J.; Jiang, Y.; Huang, S.; Wang, Q.; Xian, M.; Mu, X. Immobilization of proline-specific endoprotease on nonporous silica nanoparticles functionalized with amino group. Bioprocess. Biosyst. Eng. 2017, 40, 1–7. [Google Scholar] [CrossRef] [PubMed]
- da Silva, P.M.; Esparza-Flores, E.E.; Virgili, A.H.; de Menezes, E.W.; Fernandez-Lafuente, R.; Dal Magro, L.; Rodrigues, R.C. Effect of Support Matrix and Crosslinking Agents on Nutritional Properties of Orange Juice during Enzyme Clarification: A Comparative Study. Foods 2023, 12, 3919. [Google Scholar] [CrossRef]
- Esawy, M.A.; Gamal, A.A.; Kamel, Z.; Ismail, A.M.; Abdel-Fattah, A.F. Evaluation of free and immobilized Aspergillus niger NRC1ami pectinase applicable in industrial processes. Carbohydr. Polym. 2013, 92, 1463–1469. [Google Scholar] [CrossRef]
- Nunez-Serrano, A.; Garcia-Reyes, R.B.; Solis-Pereira, S.; Garcia-Gonzalez, A. Production and immobilization of pectinases from Penicillium crustosum in magnetic core-shell nanostructures for juice clarification. Int. J. Biol. Macromol. 2024, 263, 130268. [Google Scholar] [CrossRef]
- Karim, M.R.; Hashinaga, F. Preparation and properties of immobilized pummelo limonoid glucosyltransferase. Process Biochem. 2002, 38, 809–814. [Google Scholar] [CrossRef]
- Imran, M.; Hussain, A.; Anwar, Z.; Zeeshan, N.; Yaseen, A.; Akmal, M.; Idris, M. Immobilization of Fungal Cellulase on Calcium Alginate and Xerogel Matrix. Waste Biomass Valorization 2020, 11, 1229–1237. [Google Scholar] [CrossRef]
- Xing, M.; Chen, Y.; Li, B.; Tian, S. Highly efficient removal of patulin using immobilized enzymes of Pseudomonas aeruginosa TF-06 entrapped in calcium alginate beads. Food Chem. 2022, 377, 131973. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gao, L.; Li, S.; Wang, L.; Cai, R.; Yue, T.; Yuan, Y.; Zhao, X.; Wang, Z. Cellulose-based magnetic nanomaterials immobilized esterases as a reusable and effective detoxification agent for patulin in apple juice. Food Control 2024, 160, 110381. [Google Scholar] [CrossRef]
- Li, C.; Li, L.; Feng, Z.; Guan, L.; Lu, F.; Qin, H.M. Two-step biosynthesis of d-allulose via a multienzyme cascade for the bioconversion of fruit juices. Food Chem. 2021, 357, 129746. [Google Scholar] [CrossRef] [PubMed]
- Tavano, O.L.; Berenguer-Murcia, A.; Secundo, F.; Fernandez-Lafuente, R. Biotechnological Applications of Proteases in Food Technology. Compr. Rev. Food Sci. Food Saf. 2018, 17, 412–436. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Y.; Fei, X.; Tian, J.; Xu, L.; Wang, Y. Synthesis of papain–polyacrylamide hydrogel microspheres and their catalytic application. New J. Chem. 2021, 45, 16696–16704. [Google Scholar] [CrossRef]
- Li, H.; Yang, J.; Qin, A.; Yang, F.; Liu, D.; Li, H.; Yu, J. Milk protein hydrolysates obtained with immobilized alcalase and neutrase on magnetite nanoparticles: Characterization and antigenicity study. J. Food Sci. 2022, 87, 3107–3116. [Google Scholar] [CrossRef] [PubMed]
- Atacan, K.; Cakiroglu, B.; Ozacar, M. Improvement of the stability and activity of immobilized trypsin on modified Fe3O4 magnetic nanoparticles for hydrolysis of bovine serum albumin and its application in the bovine milk. Food Chem. 2016, 212, 460–468. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Samaniego, N.; Talens-Perales, D.; Fabra, M.J.; Lopez-Rubio, A.; Polaina, J.; Marin-Navarro, J. Development of enzymatically-active bacterial cellulose membranes through stable immobilization of an engineered beta-galactosidase. Int. J. Biol. Macromol. 2018, 115, 476–482. [Google Scholar] [CrossRef]
- Lima, P.C.; Gazoni, I.; de Carvalho, A.M.G.; Bresolin, D.; Cavalheiro, D.; de Oliveira, D.; Rigo, E. β-galactosidase from Kluyveromyces lactis in genipin-activated chitosan: An investigation on immobilization, stability, and application in diluted UHT milk. Food Chem. 2021, 349, 129050. [Google Scholar] [CrossRef]
- Ricardi, N.C.; de Menezes, E.W.; Valmir Benvenutti, E.; da Natividade Schoffer, J.; Hackenhaar, C.R.; Hertz, P.F.; Costa, T.M.H. Highly stable novel silica/chitosan support for beta-galactosidase immobilization for application in dairy technology. Food Chem. 2018, 246, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.P.; Hackenhaar, C.R.; Lorenzoni, A.S.G.; Rodrigues, R.C.; Costa, T.M.H.; Ninow, J.L.; Hertz, P.F. Chitosan crosslinked with genipin as support matrix for application in food process: Support characterization and beta-D-galactosidase immobilization. Carbohydr. Polym. 2016, 137, 184–190. [Google Scholar] [CrossRef]
- Satar, R.; Ansari, S.A. Functionalized agarose as an effective and novel matrix for immobilizing Cicer arietinum β-galactosidase and its application in lactose hydrolysis. Braz. J. Chem. Eng. 2017, 34, 451–457. [Google Scholar] [CrossRef]
- Eskandarloo, H.; Abbaspourrad, A. Production of galacto-oligosaccharides from whey permeate using beta-galactosidase immobilized on functionalized glass beads. Food Chem. 2018, 251, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Li, Y.; Ren, X.H.; Lee, J.H. Immobilization of Lactase onto Various Polymer Nanofibers for Enzyme Stabilization and Recycling. J. Microbiol. Biotechnol. 2015, 25, 1291–1298. [Google Scholar] [CrossRef]
- Neri, D.F.M.; Balcão, V.M.; Carneiro-da-Cunha, M.G.; Carvalho, L.B., Jr.; Teixeira, J.A. Immobilization of β-galactosidase from Kluyveromyces lactis onto a polysiloxane–polyvinyl alcohol magnetic (mPOS–PVA) composite for lactose hydrolysis. Catal. Commun. 2008, 9, 2334–2339. [Google Scholar] [CrossRef]
- Wolf, M.; Belfiore, L.A.; Tambourgi, E.B.; Paulino, A.T. Production of low-dosage lactose milk using lactase immobilised in hydrogel. Int. Dairy J. 2019, 92, 77–83. [Google Scholar] [CrossRef]
- Vallath, A.; Shanmugam, A.; Rawson, A. Prospects of future pulse milk variants from other healthier pulses—As an alternative to soy milk. Trends Food Sci. Technol. 2022, 124, 51–62. [Google Scholar] [CrossRef]
- Naganagouda, K.; Prashanth, S.J.; Shankar, S.K.; Dhananjay, S.K.; Mulimani, V.H. Immobilization of Aspergillus oryzae α-galactosidase in gelatin and its application in removal of flatulence-inducing sugars in soymilk. World J. Microbiol. Biotechnol. 2007, 23, 1131–1137. [Google Scholar] [CrossRef]
- Ushasree, M.V.; Gunasekaran, P.; Pandey, A. Single-step purification and immobilization of MBP-phytase fusion on starch agar beads: Application in dephytination of soy milk. Appl. Biochem. Biotechnol. 2012, 167, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.I.; Chiang, C.Y.; Ko, C.Y.; Huang, H.Y.; Cheng, K.C. Reduction of Phytic Acid in Soymilk by Immobilized Phytase System. J. Food Sci. 2018, 83, 2963–2969. [Google Scholar] [CrossRef]
- Sirin, Y.; Akatin, M.Y.; Colak, A.; Saglam Ertunga, N. Dephytinization of food stuffs by phytase of Geobacillus sp. TF16 immobilized in chitosan and calcium-alginate. Int. J. Food Prop. 2017, 20, 2911–2922. [Google Scholar] [CrossRef]
- Celem, E.B.; Onal, S. Immobilization of avocado phytase on epoxy-activated Sepabead EC-EP and its application in soymilk phytate hydrolysis. Artif. Cells Blood Substit. Immobil. Biotechnol. 2009, 37, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.I.; Yao, Y.; Chen, H.J.; Lo, Y.C.; Yu, R.C.; Cheng, K.C. Hydrolysis of isoflavone in black soy milk using cellulose bead as enzyme immobilizer. J. Food Drug Anal. 2016, 24, 788–795. [Google Scholar] [CrossRef]
- Okabe, Y.; Shimazu, T.; Tanimoto, H. Higher bioavailability of isoflavones after a single ingestion of aglycone-rich fermented soybeans compared with glucoside-rich non-fermented soybeans in Japanese postmenopausal women. J. Sci. Food Agric. 2011, 91, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Grade, L.C.; Moreira, A.A.; Varea, G.d.S.; Mandarino, J.M.G.; Silva, J.B.d.; Ida, E.I.; Ribeiro, M.L.L. Soybean β-Glucosidase immobilisated on chitosan beads and its application in soy drink increase the aglycones. Braz. Arch. Biol. Technol. 2014, 57, 766–773. [Google Scholar] [CrossRef]
- Chen, K.I.; Lo, Y.C.; Su, N.W.; Chou, C.C.; Cheng, K.C. Enrichment of two isoflavone aglycones in black soymilk by immobilized beta-glucosidase on solid carriers. J. Agric. Food Chem. 2012, 60, 12540–12546. [Google Scholar] [CrossRef]
- de Ávila, A.R.A.; de Queirós, L.D.; Lopes, D.B.; Barin, C.G.; Ueta, T.M.; Ruiz, A.L.T.G.; Macedo, G.A.; Macedo, J.A. Enhanced estrogenic effects of biotransformed soy extracts. J. Funct. Foods 2018, 48, 117–124. [Google Scholar] [CrossRef]
- Neta, N.d.A.S.; Santos, J.C.S.d.; Sancho, S.d.O.; Rodrigues, S.; Gonçalves, L.R.B.; Rodrigues, L.R.; Teixeira, J.A. Enzymatic synthesis of sugar esters and their potential as surface-active stabilizers of coconut milk emulsions. Food Hydrocoll. 2012, 27, 324–331. [Google Scholar] [CrossRef]
- Sahoo, A.K.; Gaikwad, V.S.; Ranveer, R.C.; Dandge, P.B.; Waghmare, S.R. Improvement of shelf life of soymilk using immobilized protease of Oerskovia xanthineolytica NCIM 2839. 3 Biotech 2016, 6, 161. [Google Scholar] [CrossRef] [PubMed]
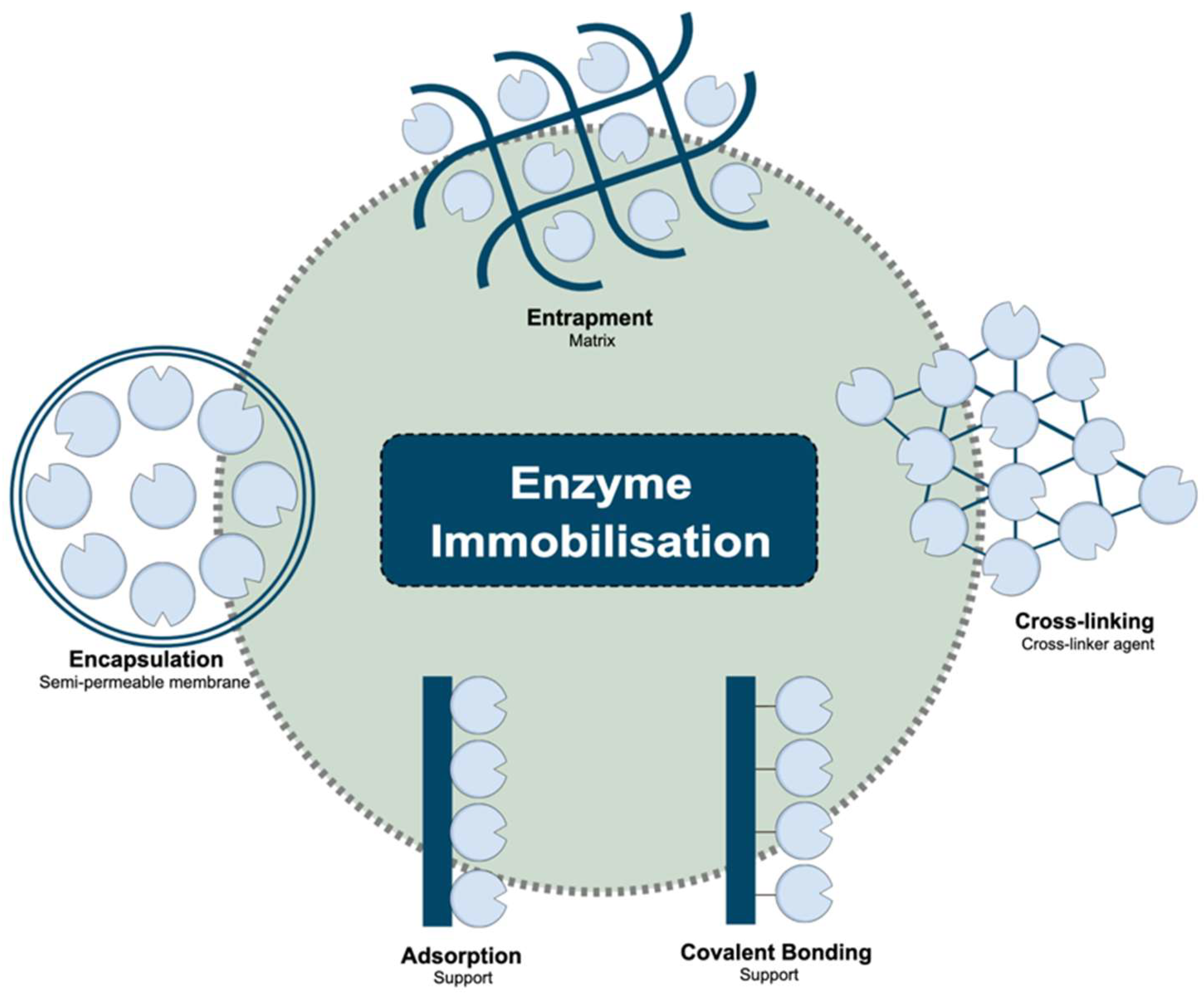

| Enzyme and Source | Method | Carrier/Support | Substrate | Optimum pH | pH Range/Stability | Application | Ref. |
|---|---|---|---|---|---|---|---|
| Tannase (Penicillium rolfsii) | Entrapment | Calcium alginate beads | Pectin | Increased from 4.0 to 4.3 | Over 50% activity at all pH values tested for up to 16 h. | Apple juice clarification. | [21] |
| α-Acetolactate decarboxylase | Entrapment | Alginate gel beads | Z-Gly-Pro-pNA * | Increased from 4.5 to 5.5 | 86% activity preserved over pH range of 3.5 to 7.0. | Reduce off flavour and shorten beer maturation time. | [27] |
| β-Galactosidases (Aspergillus oryzae) | Entrapment | Barium alginate beads | ONPG * | Decreased from 4.5 to 4.0 | Over 50% activity at pH range of 6.0 to 8.5. | Cow milk lactose hydrolysis. | [28] |
| Pectinase (Aspergillus aculeatus) | Covalent attachment | Glutaraldehyde-cross-linked alginate montmorillonite beads | Pectin | Decreased from 5.5 to 5.0 | Higher pH stability than free enzyme in acidic conditions. | Pineapple juice clarification. | [29] |
| Glucose oxidase (Aspergillus niger) | Encapsulation | Calcium alginate beads | Glucose | Decreased from 5.5 to 4–4.5 | Double activity remained compared to free enzyme at pH 3.0. | Reduce fermentable sugars in simulated wine musts. | [30] |
| Pectinase (Aspergillus aculeatus) | Entrapment | Calcium alginate beads | Pectin | Decreased from 5.0 to 3.0 | 95% and 79% activity at pH 4.0 and 7.0, respectively. | Apple and umbu juice clarification. | [19] |
| α-Galactosidase (Debaryomyces hansenii) | Adsorption | Cellulose film | pNPG * | Decreased from 5.0 to 4.0 | 60% activity over pH range of 4.0 to 6.5. | Soymilk RFO removal. | [31] |
| Protease (Penaeus vannamei) | Adsorption | Chitosan nanoparticles | Casein | Increased from 7.0 to 8.0 | 30% and 64% activity at pH 3.0 and 12.0, respectively. No activity for free enzyme. | Pomegranate juice clarification. | [32] |
| Exo-polygalacturonase (Penicillium paxilli) | Covalent attachment | Polyaldehyde dextran-cross-linked chitosan magnetic nanosupport | Pectin | Increased from 3.5 to 6.5 | pH stability over a very broad range of pH values, mainly in acidic conditions. | Fruit juice clarification (apple, pineapple, pomegranate, grapes). | [33] |
| Lactase (Escherichia coli) | Covalent attachment | Glutaraldehyde-activated functional magnetic nanocomposite | ONPG * | Increased from 4.5 to 5.0 | Higher activity levels than free lactase in both acidic and alkaline pH ranges. | Cow milk lactose hydrolysis. | [34] |
| Glyceraldehyde-3-phosphate dehydrogenase (Lactobacillus plantarum) | Covalent attachment | Amino-coated magnetic nanoparticles cross-linked with glutaraldehyde | Histamine | Increased from 6.5 to 7.5 | 80% enzymatic activity after 1 h of incubation over pH range of 4.5–8.5. | Histamine removal in winemaking process. | [35] |
| Xylanase (Trichoderma longibrachiatum) | Covalent attachment | Glutaraldehyde-activated silica gel support | Xylan | No change (pH 6.0) | Significant higher activity in acidic pH range. | Orange juice clarification. | [36] |
| Phytase (Aspergillus niger) | Adsorption | Zeolite modified with iron (II) | Phytate | No changes (pH 6.0) | Relative activity increased by 40% and 30% at pH 2 and 3, respectively. | Soymilk dephytination. | [37] |
| Pectinase (commercial preparation) | Covalent attachment | Polyaldehyde-pullulan-activated glass beads | Pectin or galacturonic acid | Increased from 5.0 to 5.5 | Over 95% activity at pH range of 3.0 to 5.5. | Barberry juice clarification. | [38] |
| Pectinase (Aspergillus tamari) | Adsorption | Zr-treated pumice | Pectin | Increased from 6.0 to 7.0 | Higher activity than free pectinase in the pH range of 7.0–9.0. | Fruit juice clarification. | [26] |
| Xylanase (Bacillus pumilus) | Covalent attachment | Glutaraldehyde-activated aluminum pellets | Xylan | Increased from 8.0 to 9.0 | At pH 11.0, 48% and 72% activity (free and immobilised enzymes, respectively). | Grape and orange juice clarification. | [39] |
| Xylanase (Mucor hiemalis) | Covalent attachment | Genipin-activated alginate beads | Xylan | No changes (pH 5.0) | Over 80% activity over a pH range of 3.0–7.0. | Apple juice clarification. | [40] |
| β-Glucosidase (Melaleuca pulchella) | Adsorption | Monoaminoethyl–N-ethyl-agarose ionic support | pNPG * | No changes (pH 6.0) | 70% activity at all acidic pH values and over 90% in neutral and basic pH ranges. | Grape juice and red wine clarification (anthocyanin hydrolysis). | [41] |
| α-Amylase (Rhizoctonia solani) | Covalent attachment | Chitosan beads cross-linked with glutaraldehyde | Soluble starch | Decreased from 5.5 to 4.5 | 70–80% activity after 5 days of tests at pH 5.5, 7.0, and 8.0. | Apple juice clarification. | [42] |
| Papain (Carica papaya) | Covalent attachment | Poly(HEMA) chitosan cryogels cross-linked with glutaraldehyde | Casein | No changes (pH 8.0) | A broader proteolytic activity profile than free enzyme. | Apple juice clarification via protein hydrolysis. | [43] |
| Pectinase and cellulase (commercial preparation) | Cross-linking | CLEA magnetic particles | Pectin | No changes (pH 4.0) | 80% activity at pH 8.0. | Grape juice clarification. | [44] |
| Xylanase (Bacillus pumilus) | Covalent attachment | Glutaraldehyde aluminum oxide pellets | Xylan | No changes (pH 7.0) | More than two-fold activity at pH 4.0, 5.0, and 10.0. | Papaya juice clarification | [45] |
| Enzyme and Source | Method | Carrier/Support | Reactor Type | Substrate | Flow (mL/h) | Conversion | Application | Ref. |
|---|---|---|---|---|---|---|---|---|
| β-Galactosidase (Kluyveromyces lactis) | Covalent attachment | Glutaraldehyde–cotton cloth | Packed-bed reactor (pilot-scale) | Whole milk | 840,000 | 60.5% | Whole milk lactose hydrolysis | [116] |
| 3,120,000 | 30.2% | |||||||
| Pectinex Ultra SP-L (Aspergillus aculeatus) | Entrapment | Alginate beads | Packed-bed reactor | Apple juice | 600 | 97.2% | Apple juice clarification | [19] |
| α-galactosidase (Aspergillus oryzae) | Entrapment | Glutaraldehyde–K-carrageenan | Fluidised-bed reactor | Soymilk | 25 | 92.0% | RFOs soymilk hydrolysis | [55] |
| 50 | 85.0% | |||||||
| α-Galactosidase (Aspergillus oryzae) | Entrapment | Glutaraldehyde–polyvinyl alcohol | Fluidised-bed reactor | Chickpea milk | 30 | 94.0% | RFOs chickpea milk hydrolysis | [112] |
| 90 | 65.0% | |||||||
| Naringinase (Penicillium decumbens) | Covalent attachment | Glutaraldehyde-modified zeolite | Packed-bed reactor | Grapefruit juices | 0.25 | 54.0% | Debittering grapefruit juice | [107] |
| Lactase (Pharmacopeia) | Covalent attachment | Glutaraldehyde–glass beads | Packed-bed reactor | Whole milk | 60 | 100.0% | Whole milk lactose hydrolysis | [108] |
| β-Galactosidase (Aspergillus oryzae) | Adsorption | Cross-linked concanavalin A-celite | Packed-bed reactor | Whole milk | 20 | 95.0% | Whole milk lactose hydrolysis | [117] |
| 30 | 81.0% | |||||||
| β-Galactosidase (Kluyveromyces lactis) | Covalent attachment | Modified collagen | Packed-bed reactor | Skimmed milk | 120 | 75.0% | Skimmed milk lactose hydrolysis | [71] |
| β-Galactosidase (Str. Thermophilus and L. bulgaricus) | Entrapment | Barium alginate | Packed-bed reactor | Skimmed milk | 6 | 92.9% | Skimmed milk lactose hydrolysis | [118] |
| 15 | 73.1% | |||||||
| β-Galactosidase (Kluyveromyces fragilis) | Covalent attachment | Epicholorohydrin-activated cellulose beads | Fluidised-bed reactor | Whole milk | 120 | 65.0% | Whole milk lactose hydrolysis | [109] |
| α-Galactosidase (Aspergillus terreus) | Entrapment | Cross-linked concanavalin A-calcium alginate | Fluidised-bed reactor | Soymilk | 40 | 85.0% | RFOs soymilk hydrolysis | [119] |
| 80 | 72.0% | |||||||
| Commercial enzyme cocktail for juice clarification | Covalent attachment | Glutaraldehyde-activated chitosan | Fluidised-bed reactor | Orange juice | 30 | 87.0% | Orange juice clarification | [6] |
| 60 | 57.0% | |||||||
| Commercial enzyme cocktail for juice clarification | Covalent attachment | Glutaraldehyde-activated chitosan | Packed-bed reactor | Orange juice | 30 | 83.0% | Orange juice clarification | [6] |
| 90 | 80.0% | |||||||
| Pectinase (Aspergillus niger) | Covalent attachment | Glutaraldehyde loofah sponge | Packed-bed reactor | Orange juice | 30 180 | 89.0% 30.0% | Orange juice clarification | [120] |
| Papain (Carica papaya) | Covalent attachment | Commercial chitosan beads | Packed-bed reactor | White wine | 360 | 59 to 96 | White wine turbidity removal | [121] |
| Papain (Carica papaya) | Covalent attachment | Commercial chitosan beads | Packed-bed reactor | White wine | 360 | 14 to 68 | White wine protein haze reduction | [121] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chalella Mazzocato, M.; Jacquier, J.-C. Recent Advances and Perspectives on Food-Grade Immobilisation Systems for Enzymes. Foods 2024, 13, 2127. https://doi.org/10.3390/foods13132127
Chalella Mazzocato M, Jacquier J-C. Recent Advances and Perspectives on Food-Grade Immobilisation Systems for Enzymes. Foods. 2024; 13(13):2127. https://doi.org/10.3390/foods13132127
Chicago/Turabian StyleChalella Mazzocato, Marcella, and Jean-Christophe Jacquier. 2024. "Recent Advances and Perspectives on Food-Grade Immobilisation Systems for Enzymes" Foods 13, no. 13: 2127. https://doi.org/10.3390/foods13132127
APA StyleChalella Mazzocato, M., & Jacquier, J.-C. (2024). Recent Advances and Perspectives on Food-Grade Immobilisation Systems for Enzymes. Foods, 13(13), 2127. https://doi.org/10.3390/foods13132127








