The Effect of Recycled Spent Coffee Grounds Fertilizer, Vermicompost, and Chemical Fertilizers on the Growth and Soil Quality of Red Radish (Raphanus sativus) in the United Arab Emirates: A Sustainability Perspective
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Design
2.1.1. Control (C)
2.1.2. SCGs
2.1.3. VC
2.1.4. CF
2.2. Greenhouse Experiments
2.3. Plant Growth Measurements
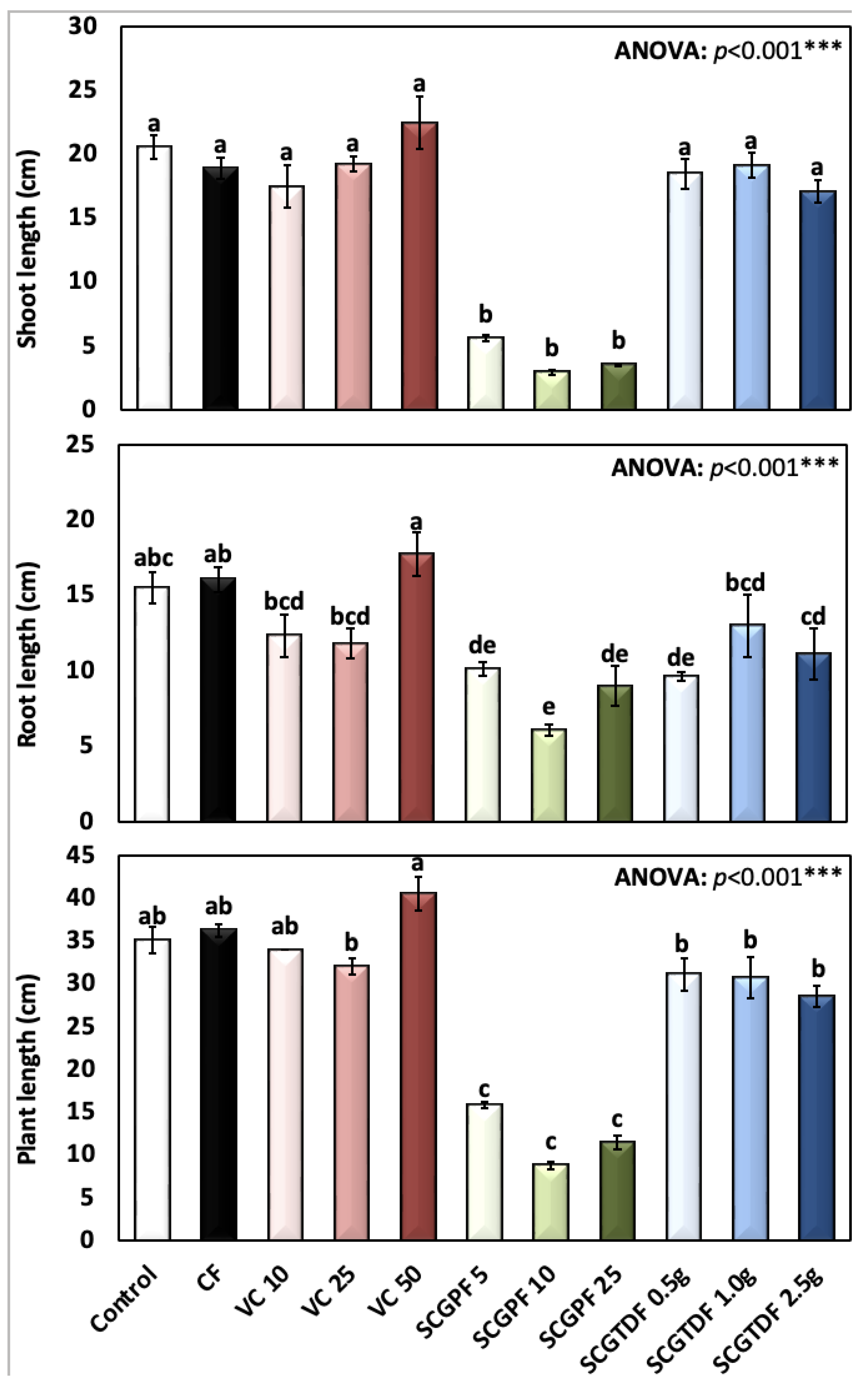
2.3.1. Radish Height
- The height of the whole fresh radish (including the shoot, leaves, and roots) using a tape measure with cm as the unit.
- The height of the shoot of the fresh radish by using a tape measure with cm as the unit.
- The height of the root of the fresh radish by using a tape measure with cm as the unit.
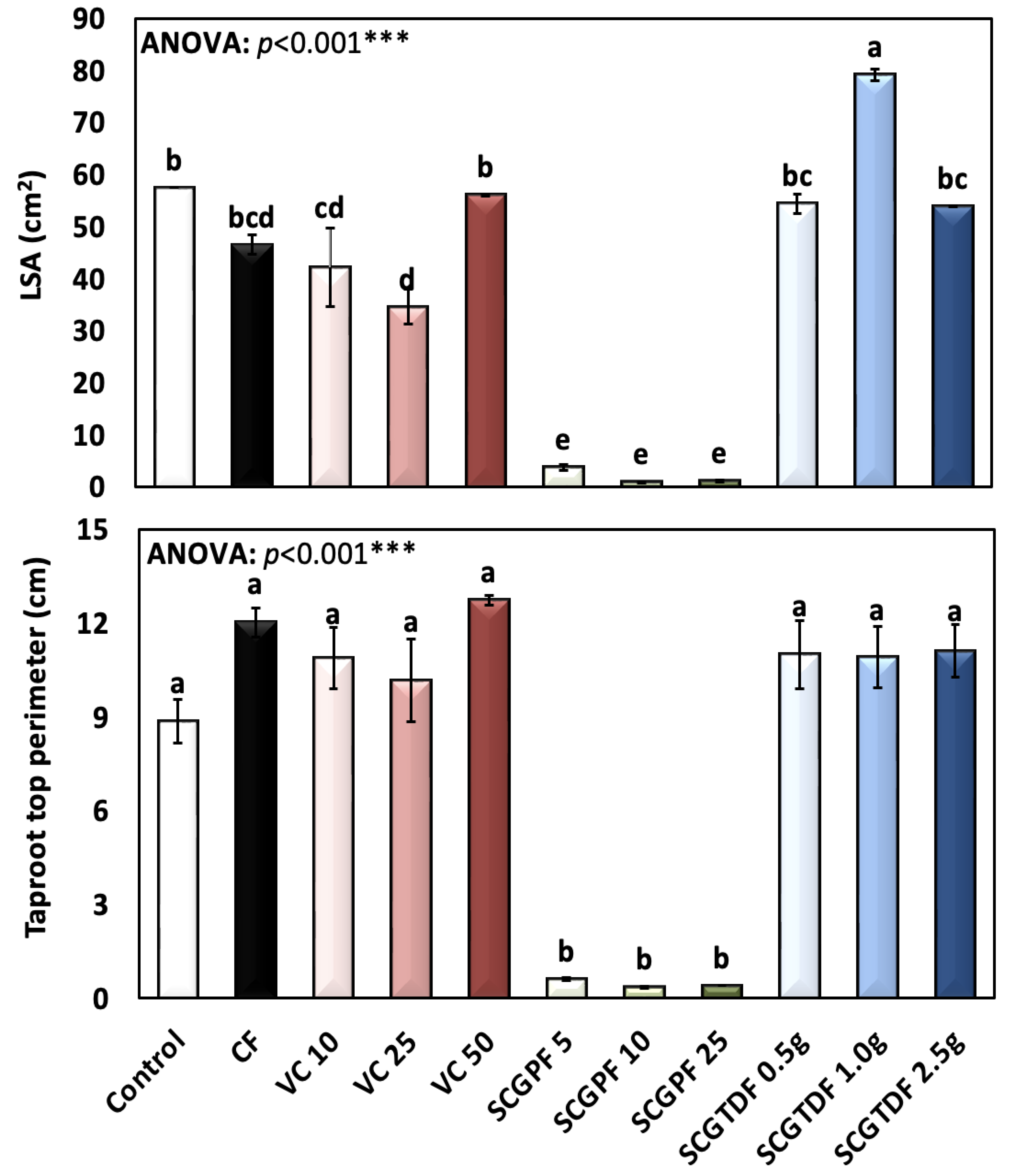
- The taproot top perimeter of the fresh radish root using a tape measure with cm as the unit.
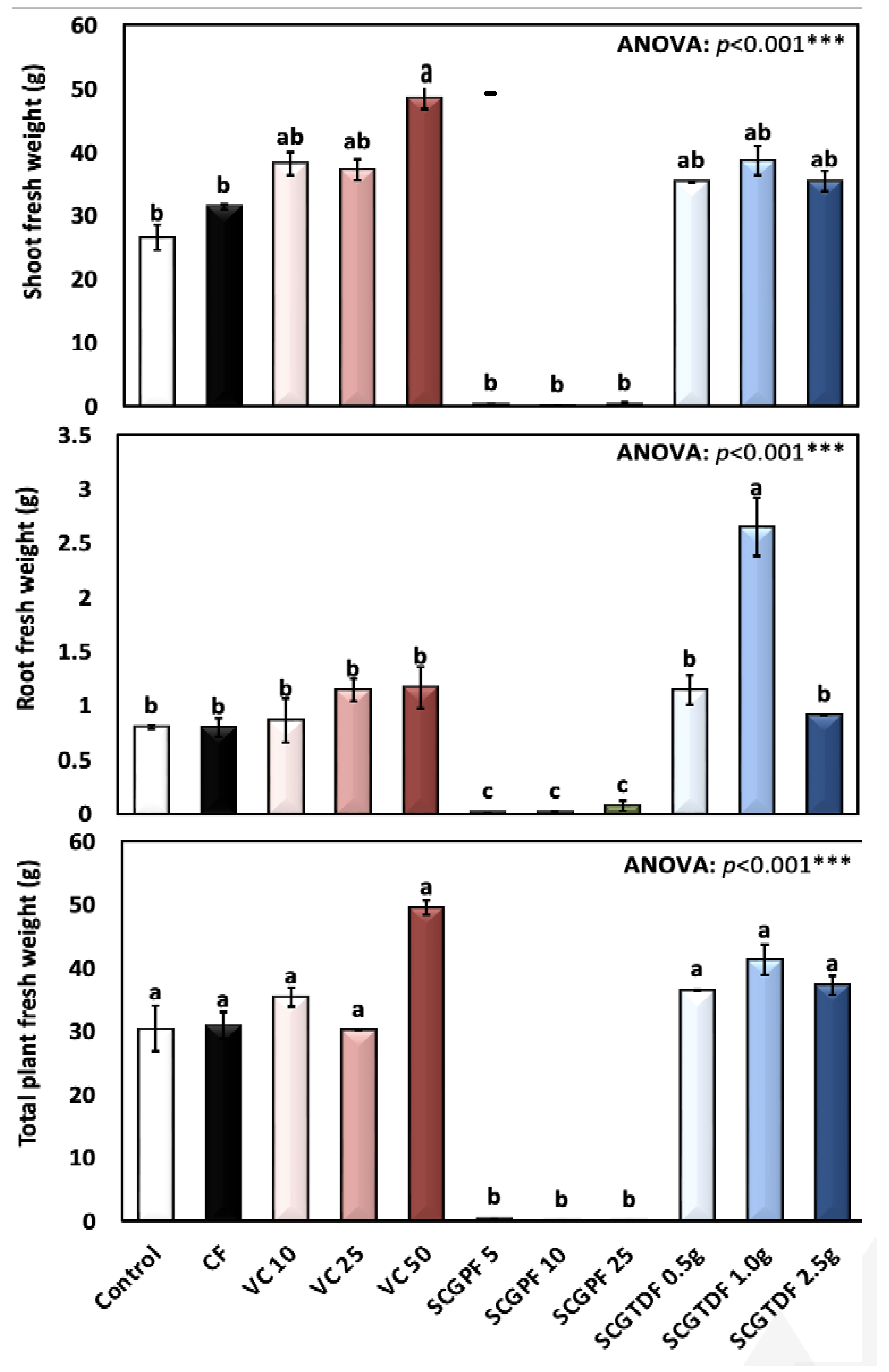
2.3.2. Radish Weight
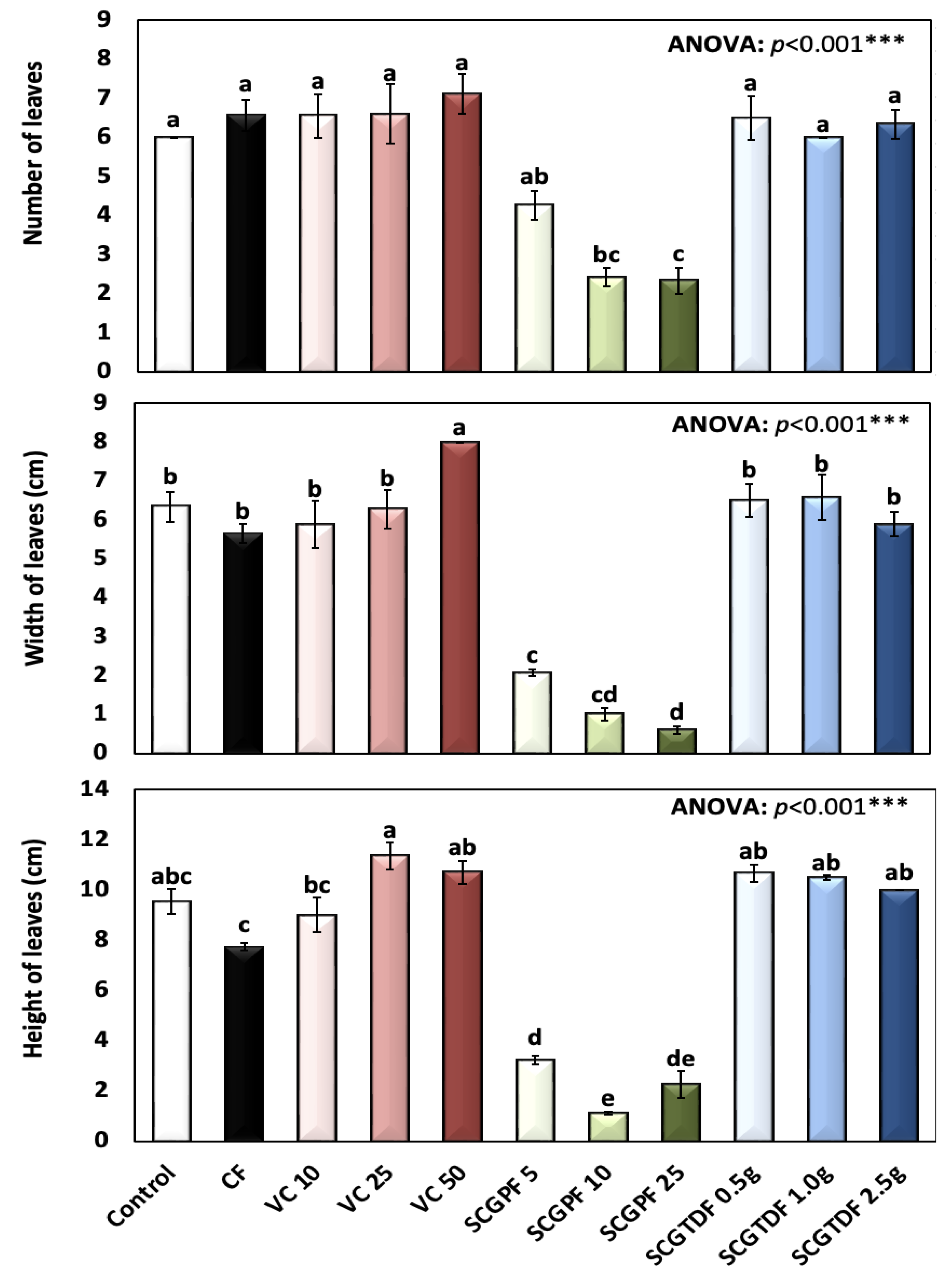
2.3.3. Leaf Surface Area (LSA)
2.4. Effect of Different Treatments on the Soil’s pH and EC
2.5. Determination of the Soil’s Macronutrients
2.6. Determination of the Total Bacterial Population in the Soil Samples
2.7. Statistical Analyses
3. Results
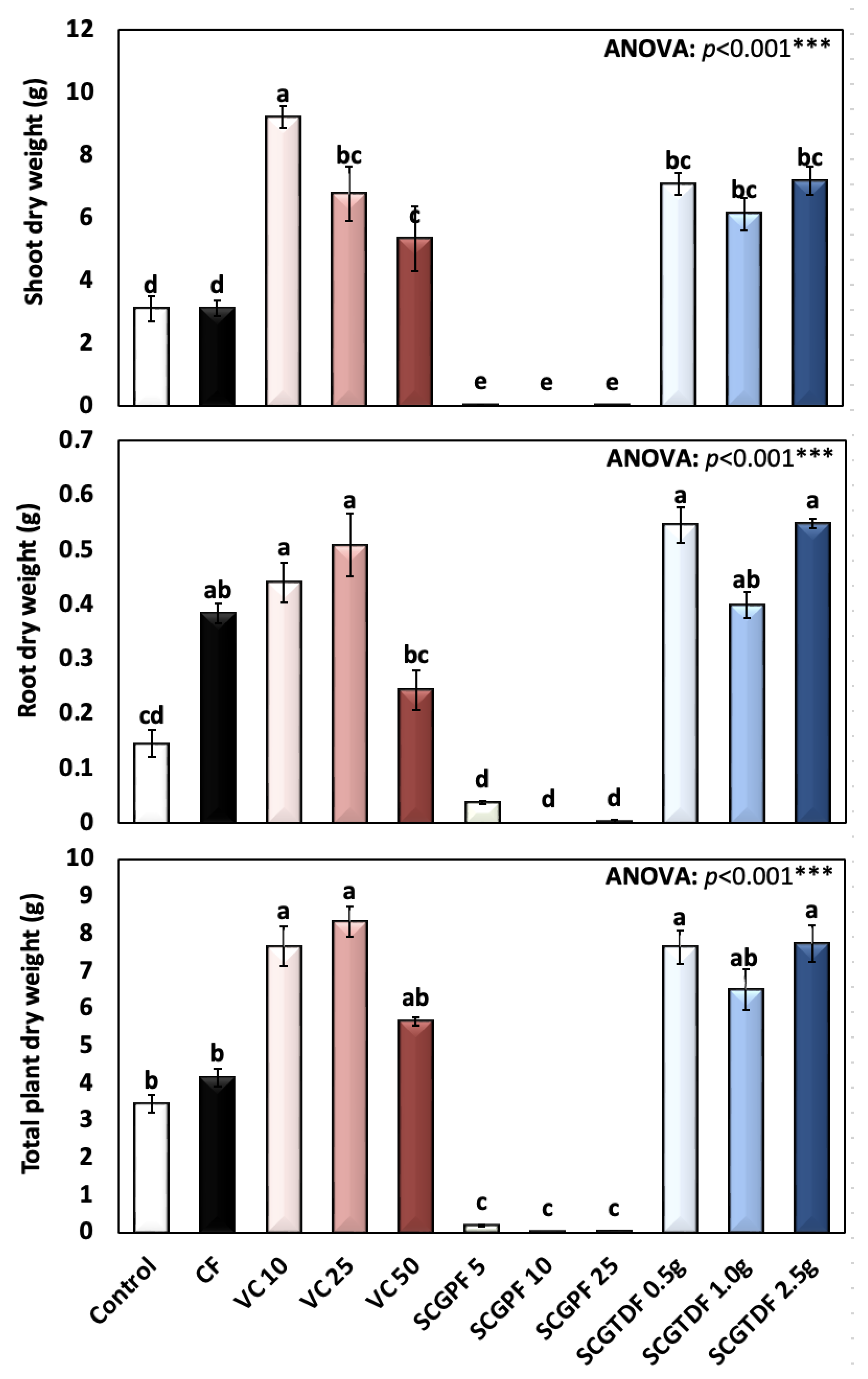
3.1. Effect of Different Treatments on R. sativus Growth Characteristics
| Experiment Replication | Treatments | SCGPF | SCGTDF | VC | CF | C | |||||||||||||||||||||||||||||||||||
| Day | Day 0 | Weeks 2 and 3 | Day 0 | Weeks 2 and 3 | Day 0 | ||||||||||||||||||||||||||||||||||||
| Amount | SCGPF 5% | 0.5 g | VC 10 | 1 g of CF | C | ||||||||||||||||||||||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| SCGPF 10% | 1.0 g | VC 25 | |||||||||||||||||||||||||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||||||||||||||||||
| SCGPF 25% | 2.5 g | VC 50 | |||||||||||||||||||||||||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||||||||||||||||||
| SCGPF 50% | |||||||||||||||||||||||||||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||||||||||||||||||||||||||||||||||
| Parameter | pH | EC (dS/m) | Bacterial Population (CFU) | C (%) | N (%) | P (mg/kg) | K (mg/kg) |
|---|---|---|---|---|---|---|---|
| Control | 6.4 ± 0.6 a | 0.5 ± 0.1 c | 9.0 ± 1.7 b | 30.5 ± 10.1 a | 1.1 ± 0.4 ab | 809.2 ± 62.1 abc | 2784.2 ± 698.7 abc |
| CF | 6.7 ± 0.7 a | 1.1 ± 0.1 c | 5.0 ± 2.3 b | 42.4 ± 15.2 a | 1.1 ± 0.3 ab | 549.0 ± 139.2 bc | 322.0 ± 44.4 c |
| VC-10% | 6.5 ± 0.7 a | 2.0 ± 0.2 b | 10.0 ± 4.0 b | 39.1 ± 11.4 a | 1.1 ± 0.5 ab | 730.4 ± 304.1 bc | 1986.9 ± 553.1 bc |
| VC-25% | 6.7 ± 0.7 a | 1.2 ± 0.1 c | 38.0 ± 9.5 b | 20.3 ± 3.7 a | 0.7 ± 0.2 b | 508.8 ± 130.5 bc | 2243.4 ± 979.0 bc |
| VC-50% | 6.8 ± 0.7 a | 2.7 ± 0.3 ab | 35.0 ± 14.7 b | 40.8 ± 13.0 a | 1.2 ± 0.2 ab | 915.5 ± 205.6 abc | 5157.1 ± 1956.1 a |
| SCGPF-5% | 5.6 ± 0.6 a | 2.5 ± 0.3 ab | 46.0 ± 5.3 b | 41.5 ± 15.1 a | 1.3 ± 0.4 ab | 1168.2 ± 369.6 abc | 2258.0 ± 1,054.9 bc |
| SCGPF-10% | 5.8 ± 0.6 a | 3.0 ± 0.3 a | 12.0 ± 4.0 b | 43.7 ± 12.2 a | 1.5 ± 0.4 ab | 1281.2 ± 454.2 abc | 2795.1 ± 798.7 abc |
| SCGPF-25% | 5.4 ± 0.5 a | 2.6 ± 0.3 ab | 101.3 ± 15.5 a | 43.8 ± 18.7 a | 1.6 ± 0.6 a | 1443.0 ± 613.6 a | 3752.7 ± 1577.3 ab |
| SCGTDF-0.5 g | 5.6 ± 0.6 a | 2.5 ± 0.3 ab | 4.0 ± 1.7 b | 41.2 ± 9.4 a | 1.1 ± 0.4 ab | 420.6 ± 114.4 c | 167.2 ± 75.9 c |
| SCGTDF-1.0 g | 5.8 ± 0.6 a | 3.0 ± 0.3 a | 4.0 ± 0.9 b | 42.8 ± 3.3 a | 1.2 ± 0.5 ab | 462.2 ± 203.0 c | 184.3 ± 29.7 c |
| SCGTDF-2.5 g | 5.4 ± 0.5 a | 2.6 ± 0.3 ab | 15.0 ± 3.1 b | 41.9 ± 14.7 a | 1.2 ± 0.4 ab | 469.9 ± 195.2 c | 200.0 ± 28.3 c |
| ANOVA | 0.023 * | <0.001 ** | <0.001 ** | 0.454 ns | 0.145 ns | <0.001 ** | <0.001 ** |
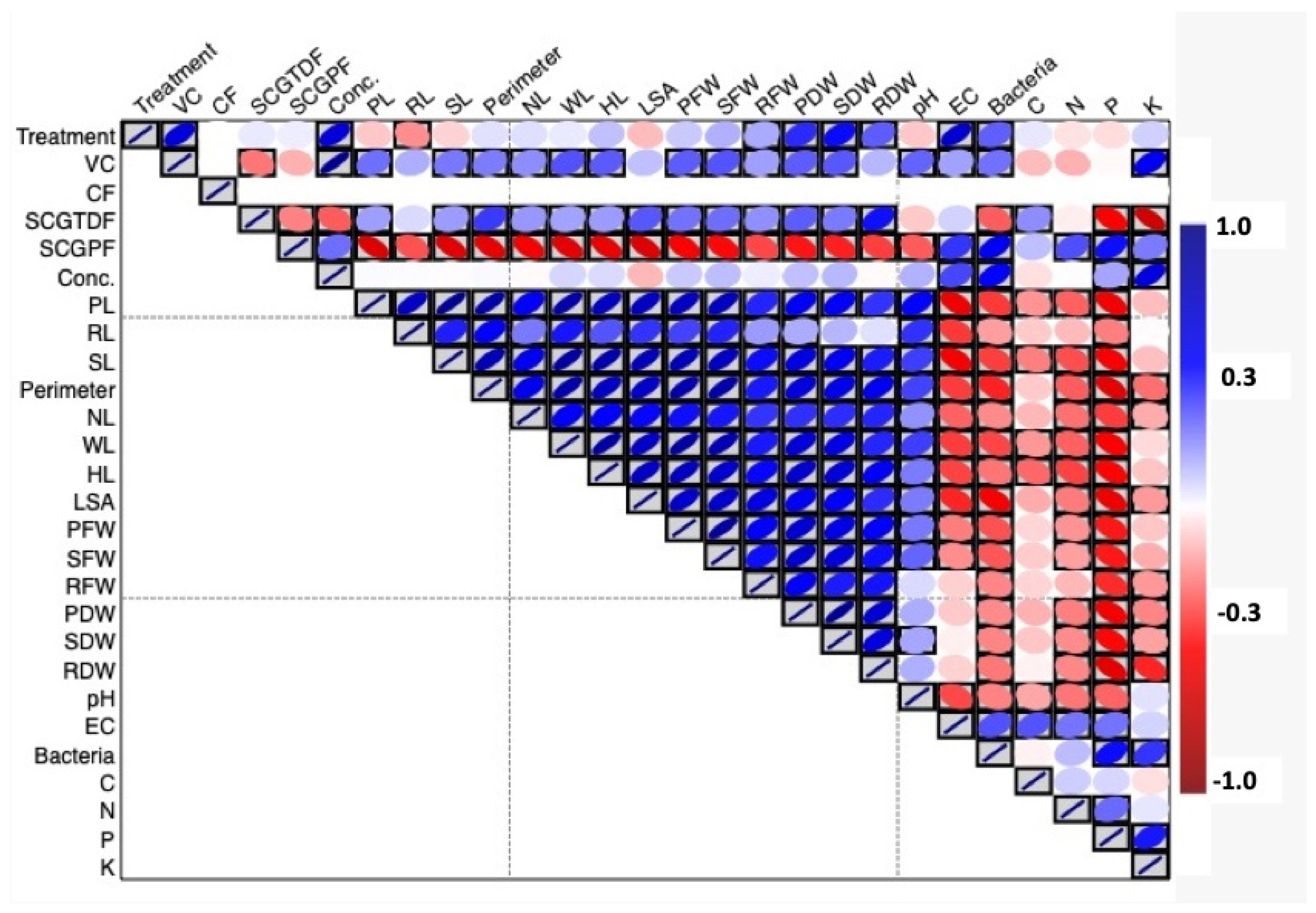
3.2. Effect of Different Treatments on the Soil Quality and Bacterial Population
4. Discussion
5. Conclusions
6. Future Studies
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cervera-Mata, A.; Delgado, G.; Fernández-Arteaga, A.; Fornasier, F.; Mondini, C. Spent coffee grounds by-products and their influence on soil C–N dynamics. J. Environ. Manag. 2022, 302, 114075. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Molinero-García, A.; Martín-García, J.M.; Delgado, G. Sequential effects of spent coffee grounds on soil physical properties. Soil Use Manag. 2023, 39, 286–297. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Mondini, C.; Fernández-Arteaga, A.; Sinicco, T.; Delgado, G. Influence of the application of charred and uncharred spent coffee grounds on soil carbon and nitrogen cycles. Arch. Agron. Soil Sci. 2023, 69, 3235–3251. [Google Scholar] [CrossRef]
- Mak, S.L.; Wu, M.Y.T.; Chak, W.Y.; Kwong, W.K.; Tang, W.F.; Li, C.H.; Lee, C.C.; Li, C.Y. A review of the feasibility of producing polylactic acid (PLA) polymers using spent coffee ground. Sustainability 2023, 15, 13498. [Google Scholar] [CrossRef]
- Alotaibi, K.D.; Alharbi, H.A.; Yaish, M.W.; Ahmed, I.; Alharbi, S.A.; Alotaibi, F.; Kuzyakov, Y. Date palm cultivation: A review of soil and environmental conditions and future challenges. Land Degrad. Dev. 2023, 34, 2431–2444. [Google Scholar] [CrossRef]
- Horgan, F.G.; Floyd, D.; Mundaca, E.A.; Crisol-Martínez, E. Spent coffee grounds applied as a top-dressing or incorporated into the soil can improve plant growth while reducing slug herbivory. Agriculture 2023, 13, 257. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Cervera-Mata, A.; Fernández-Arteaga, A.; Pastoriza, S.; Rufián-Henares, J.Á.; Delgado, G. Why should we be concerned with the use of spent coffee grounds as an organic amendment of soils? A narrative review. Agronomy 2022, 12, 2771. [Google Scholar] [CrossRef]
- Kekelis, P.; Argyropoulou, M.D.; Theofilidou, A.; Papatheodorou, E.M.; Aschonitis, V.; Monokrousos, N. The differentiations in the soil nematode community in an agricultural field after soil amendment using composted coffee waste in various concentrations. Agronomy 2023, 13, 2831. [Google Scholar] [CrossRef]
- Albattah, M.; Bande, L. Awareness and perception of the environmental sustainability of the UAE university campus: A case study of sustainability course. Int. J. Sustain. High. Educ. 2023, 24, 1610–1628. [Google Scholar] [CrossRef]
- Alnaqbi, S.A.; Alami, A.H. Sustainability and renewable energy in the UAE: A case study of Sharjah. Energies 2023, 16, 7034. [Google Scholar] [CrossRef]
- Ahmed, R.R.; Abdulla, A.I. Recycling of food waste to produce the plant fertilizer. Int. J. Eng. Technol. 2018, 7, 173–178. [Google Scholar] [CrossRef]
- Hamid, H.A.; Qi, L.P.; Harun, H.; Sunar, N.M.; Ahmad, F.H.; Muhamad, M.S.; Hamidon, N. Development of organic fertilizer from food waste by composting in UTHM campus Pagoh. J. Appl. Chem. Nat. Resour. 2019, 1, 1–6. [Google Scholar]
- Okareh, O.T.; Oyewole, S.A.; Taiwo, L.B. Conversion of food wastes to organic fertilizer: A strategy for promoting food security and institutional waste management in Nigeria. J. Res. Environ. Sci. Toxicol. 2014, 3, 66–72. [Google Scholar]
- Thi, N.B.D.; Kumar, G.; Lin, C.Y. An overview of food waste management in developing countries: Current status and future perspective. J. Environ. Manag. 2015, 157, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Yagoub, M.M.; Al Hosani, N.; AlSumaiti, T.; Kortbi, O.; Alshehhi, A.A.; Aldhanhani, S.R.; Albedwawi, S.A. University students’ perceptions of food waste in the UAE. Sustainability 2022, 14, 11196. [Google Scholar] [CrossRef]
- Zaman, B.; Hardyanti, N.; Purwono; Ramadan, B.S. An innovative thermal composter to accelerate food waste decomposition at the household level. Bioresour. Technol. Rep. 2022, 19, 101203. [Google Scholar] [CrossRef]
- Greco, C.; Comparetti, A.; Fascella, G.; Febo, P.; La Placa, G.; Saiano, F.; Mammano, M.M.; Orlando, S.; Laudicina, V.A. Effects of vermicompost, compost and digestate as commercial alternative peat-based substrates on qualitative parameters of Salvia officinalis. Agronomy 2021, 11, 98. [Google Scholar] [CrossRef]
- Arancon, N.Q.; Edwards, C.A. Effects of vermicomposts on plant growth. In Proceedings of the International Symposium Workshop on Vermi Technologies for Developing Countries (ISWVT 2005), Los Banos, Philippines, 16–18 November 2005; pp. 16–18. [Google Scholar]
- Cho, S.R.; Lee, Y.J.; Lee, C.W.; Hyun, B.G.; Song, Y.S.; Ryu, C.H.; Kim, Y.M.; Lee, S.B. The effect of livestock and food waste compost on rice yield and nutrient utilization efficiency according to different nitrogen fertilizer treatments rates. Korean J. Soil Sci. Fertil. 2021, 54, 558–566. [Google Scholar] [CrossRef]
- Kang, S.M.; Shaffique, S.; Kim, L.R.; Kwon, E.H.; Kim, S.H.; Lee, Y.H.; Kalsoom, K.; Aaqil Khan, M.; Lee, I.J. Effects of organic fertilizer mixed with food waste dry powder on the growth of Chinese cabbage seedlings. Environments 2021, 8, 86. [Google Scholar] [CrossRef]
- Kaur, R.; Choudhary, D.; Bali, S.; Bandral, S.S.; Singh, V.; Ahmad, M.A.; Rani, N.; Singh, T.G.; Chandrasekaran, B. Pesticides: An alarming detrimental to health and environment. Sci. Total Environ. 2024, 915, 170113. [Google Scholar] [CrossRef]
- Litskas, V.D. Environmental impact assessment for animal waste, organic and synthetic fertilizers. Nitrogen 2023, 4, 16–25. [Google Scholar] [CrossRef]
- Weerahewa, J.; Dayananda, D. Land use changes and economic effects of alternative fertilizer policies: A simulation analysis with a bio-economic model for a Tank Village of Sri Lanka. Agric. Syst. 2023, 205, 103563. [Google Scholar] [CrossRef]
- Podsędek, A. Natural antioxidants and antioxidant capacity of Brassica vegetables: A review. LWT-Food. Sci. Technol. 2007, 40, 1–11. [Google Scholar] [CrossRef]
- Mahmood, A.; Iguchi, R.; Kataoka, R. Multifunctional food waste fertilizer having the capability of Fusarium-growth inhibition and phosphate solubility: A new horizon of food waste recycle using microorganisms. Waste Manag. 2019, 94, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Melchini, A.; Traka, M.H. Biological Profile of Erucin: A new promising anticancer agent from cruciferous vegetables. Toxins 2010, 2, 593–612. [Google Scholar] [CrossRef]
- Vanghele, N.A.; Pruteanu, M.A.; Petre, A.A.; Matache, A.; Mihalache, D.B.; Stanciu, M.M. The influence of environmental factors and heavy metals in the soil on plants’ growth and development. E3S Web Conf. 2020, 180, 03014. [Google Scholar] [CrossRef]
- Mattos, D., Jr.; Kadyampakeni, D.M.; Oliver, A.Q.; Boaretto, R.M.; Morgan, K.T.; Quaggio, J.A. Soil and nutrition interactions. In The Genus Citrus; Talon, M., Caruso, M., Gmitter, F.G., Jr., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 311–331. [Google Scholar] [CrossRef]
- Kaushalya Madhavi, B.G.; Bhujel, A.; Kim, N.E.; Kim, H.T. Measurement of overlapping leaf area of ice plants using digital image processing technique. Agriculture 2022, 12, 1321. [Google Scholar] [CrossRef]
- Barros, J.; Machado, R.; Amaral, C.; Schiavo, D.; Nogueira, A.R.; Nobrega, J. Plant Nutrient Analysis Using the Agilent 5100 Synchronous Vertical Dual View ICP-OES. Application Note; Food Testing and Agriculture; Agilent Technologies: Santa Clara, CA, USA, 2015. [Google Scholar]
- United States Environmental Protection Agency (USEPA). 3015A Microwave Assisted Acid Digestion of Sediments, Sludge, and Oils, 2nd ed.; Revision 1; United States Environmental Protection Agency (USEPA): Washington, DC, USA, 1998.
- Page, A.I.; Miller, R.H.; Keeny, D.R. Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties; American Society of Agronomy, Madison Inc.: Madison, WI, USA, 1982; p. 1159. [Google Scholar]
- Harley, J.P.; Harley, J. Laboratory Exercises in Microbiology, 7th ed.; The McGraw-Hill/Higher Education: New York, NY, USA, 2008. [Google Scholar]
- Arancon, N.Q.; Edwards, C.A.; Bierman, P.; Metzger, J.D.; Lee, S.; Welch, C. Effects of vermicompost on growth and marketable fruits of field-grown tomatoes, peppers and strawberries: The 7th international symposium on earthworm ecology. Cardiff, Wales. 2002. Pedobiologia 2003, 47, 731–735. [Google Scholar] [CrossRef]
- Atiyeh, R.M.; Edwards, C.A.; Subler, S.; Metzger, J.D. Pig manure vermicompost as a component of a horticultural bedding plant medium: Effects on physicochemical properties and plant growth. Bioresour. Technol. 2001, 78, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Toor, M.D.; Kizilkaya, R.; Anwar, A.; Koleva, L.; Eldesoky, G.E. Effects of vermicompost on soil microbiological properties in lettuce rhizosphere: An environmentally friendly approach for sustainable green future. Environ. Res. 2024, 243, 117737. [Google Scholar] [CrossRef]
- González-Moreno, M.A.; García Gracianteparaluceta, B.; Marcelino Sádaba, S.; Zaratiegui Urdin, J.; Robles Domínguez, E.; Pérez Ezcurdia, M.A.; Seco Meneses, A. Feasibility of vermicomposting of spent coffee grounds and silverskin from coffee industries: A Laboratory Study. Agronomy 2020, 10, 1125. [Google Scholar] [CrossRef]
- Jiang, Z.; Lou, Y.; Liu, X.; Sun, W.; Wang, H.; Liang, J.; Guo, J.; Li, N.; Yang, Q. Combined application of coffee husk compost and inorganic fertilizer to improve the soil ecological environment and photosynthetic characteristics of arabica coffee. Agronomy 2023, 13, 1212. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, S.H.; Jeong, M.; Park, M.K.; Jo, Y.; Kang, G.U.; Jung, D.R.; Lee, C.I.; Shin, J.H. Fermented coffee grounds diminish livestock odors: A Microbiome Study. Agronomy 2021, 11, 1914. [Google Scholar] [CrossRef]
- Lorenzo, P.; Guilherme, R.; Barbosa, S.; Ferreira, A.J.; Galhano, C. Agri-Food waste as a method for weed control and soil amendment in crops. Agronomy 2022, 12, 1184. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Navarro-Alarcón, M.; Rufián-Henares, J.Á.; Pastoriza, S.; Montilla-Gómez, J.; Delgado, G. Phytotoxicity and chelating capacity of spent coffee grounds: Two contrasting faces in its use as soil organic amendment. Sci. Total Environ. 2020, 717, 137247. [Google Scholar] [CrossRef]
- Almaramah, S.B.; Abu-Elsaoud, A.M.; Alteneiji, W.A.; Albedwawi, S.T.; El-Tarabily, K.A.; Al Raish, S.M. The impact of food waste compost, vermicompost, and chemical fertilizers on the growth measurement of red radish (Raphanus sativus): A sustainability perspective in the United Arab Emirates. Foods 2024, 13, 1608. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mesmar, A.K.; Albedwawi, S.T.; Alsalami, A.K.; Alshemeili, A.R.; Abu-Elsaoud, A.M.; El-Tarabily, K.A.; Al Raish, S.M. The Effect of Recycled Spent Coffee Grounds Fertilizer, Vermicompost, and Chemical Fertilizers on the Growth and Soil Quality of Red Radish (Raphanus sativus) in the United Arab Emirates: A Sustainability Perspective. Foods 2024, 13, 1997. https://doi.org/10.3390/foods13131997
Mesmar AK, Albedwawi ST, Alsalami AK, Alshemeili AR, Abu-Elsaoud AM, El-Tarabily KA, Al Raish SM. The Effect of Recycled Spent Coffee Grounds Fertilizer, Vermicompost, and Chemical Fertilizers on the Growth and Soil Quality of Red Radish (Raphanus sativus) in the United Arab Emirates: A Sustainability Perspective. Foods. 2024; 13(13):1997. https://doi.org/10.3390/foods13131997
Chicago/Turabian StyleMesmar, Athari K., Shaikha T. Albedwawi, Aysha K. Alsalami, Alreem R. Alshemeili, Abdelghafar M. Abu-Elsaoud, Khaled A. El-Tarabily, and Seham M. Al Raish. 2024. "The Effect of Recycled Spent Coffee Grounds Fertilizer, Vermicompost, and Chemical Fertilizers on the Growth and Soil Quality of Red Radish (Raphanus sativus) in the United Arab Emirates: A Sustainability Perspective" Foods 13, no. 13: 1997. https://doi.org/10.3390/foods13131997
APA StyleMesmar, A. K., Albedwawi, S. T., Alsalami, A. K., Alshemeili, A. R., Abu-Elsaoud, A. M., El-Tarabily, K. A., & Al Raish, S. M. (2024). The Effect of Recycled Spent Coffee Grounds Fertilizer, Vermicompost, and Chemical Fertilizers on the Growth and Soil Quality of Red Radish (Raphanus sativus) in the United Arab Emirates: A Sustainability Perspective. Foods, 13(13), 1997. https://doi.org/10.3390/foods13131997








