Grains in a Modern Time: A Comprehensive Review of Compositions and Understanding Their Role in Type 2 Diabetes and Cancer
Abstract
1. Introduction
2. Material and Methods
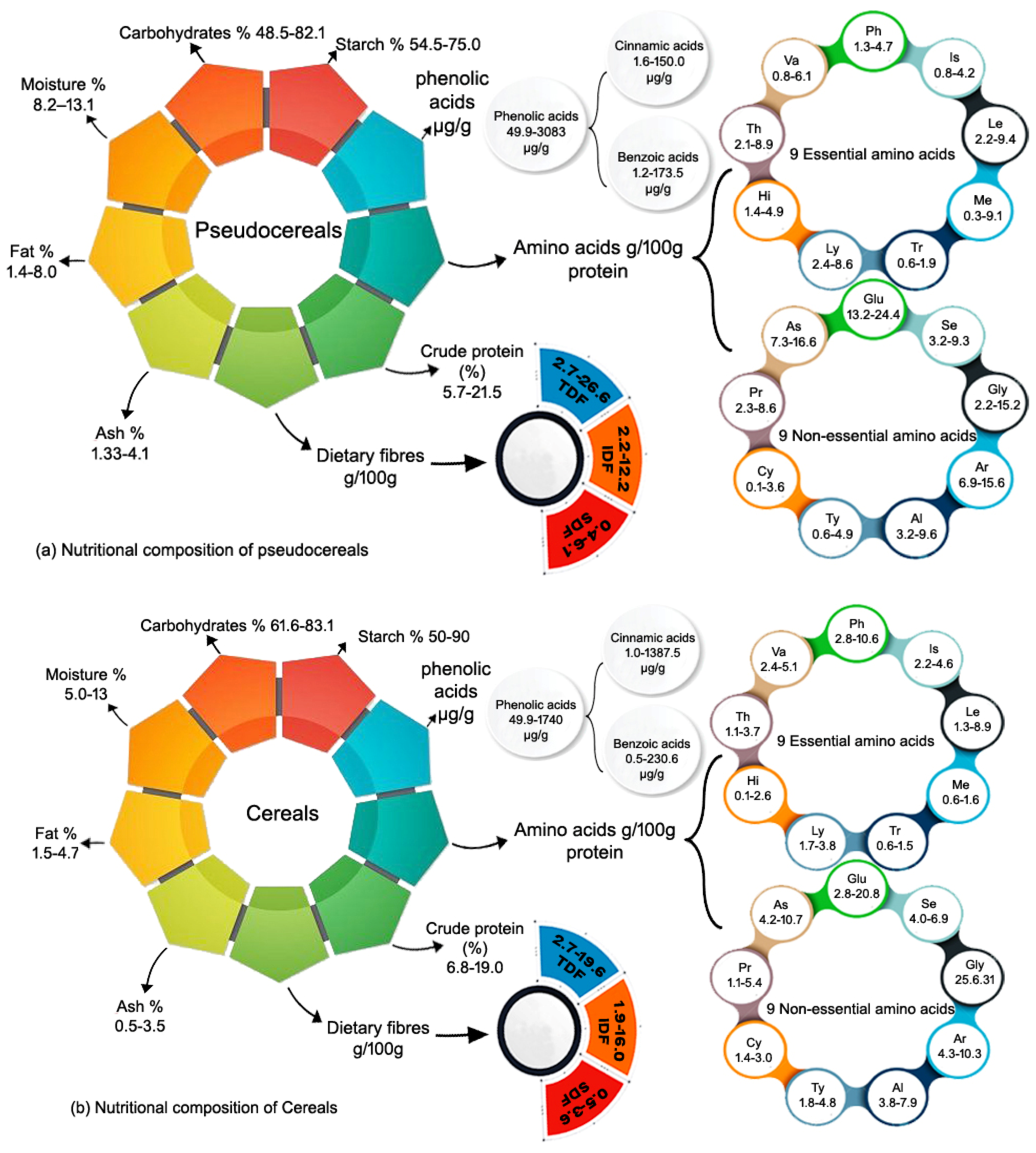
3. Nutritional Composition of Pseudo Cereals and Cereals
3.1. Fat in Pseudo Cereals and Cereals
3.2. Carbohydrates and Starch in Pseudo Cereals and Cereals
3.3. Protein in Pseudo Cereals and Cereals
| Pseudo Cereals | |||||||
|---|---|---|---|---|---|---|---|
| Whole Grains | Moisture (w.b) | Fat | Ash | Carbohydrates | Starch | Crude Protein | References |
| Quinoa | 8.2–13.1 | 4.9–7.5 | 4.1 | 48.5–77.0 | 58.1–64.2 | 9.1–16.7 | [35,37,38,60,62,76] |
| Amaranth | 8.9–9.4 | 6.4–8.0 | 3.3 | 63.1–70.0 | 65.0–75.0 | 13.1–21.5 | [34,35,36,50,60,77] |
| Buckwheat | 10–11 | 1.4–7.4 | 1.33–3.09 | 62.1–82.1 | 54.5–57.4 | 5.7–14.2 | [35,39,40,51,52,60,78] |
| Cereals | |||||||
| Wheat | 12–13 | 1.72–3.3 | 1.7–1.9 | 62.6–83.1 | 60–75 | 8–19.0 | [43,45,46,54,55,56,57,58,59] |
| Rice | 5.0–12.7 | 1.5–2.2 | 0.5–3.5 | 71.1–78.2 | 50–90 | 6.8–7.3 | [47,48,62] |
| Corn | 10.4 | 3.8–4.7 | 1.33–1.44 | 65.0–74.3 | 72.8 | 8.8–9.4 | [41,42] |
3.4. Amino Acids in Pseudo Cereals and Cereals
| Essential Amino Acids | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pseudo Cereals | ||||||||||
| Whole Grains | Th | Va | Ph | Is | Le | Me | Tr | Ly | Hi | References |
| Quinoa | 2.1–8.9 | 0.8–6.1 | 3.0–4.7 | 0.8–7.4 | 2.3–9.4 | 0.3–9.1 | 0.6–1.9 | 2.4–7.8 | 1.4–5.4 | [61] |
| Amaranth | 3.3–5.0 | 3.9–5.0 | 3.7–4.7 | 2.7–4.2 | 4.2–6.9 | 1.6–4.6 | 0.9–1.8 | 4.8–8.0 | 1.9–3.8 | [36,50] |
| Buckwheat | 3.9–4.0 | 2.3–6.1 | 1.3–7.2 | 1.1–4.1 | 2.2–7.6 | 0.5–2.5 | 0.7–1.8 | 4.2–8.6 | 1.8–4.9 | [52,63] |
| Cereals | ||||||||||
| Wheat | 1.8–2.7 | 2.4–4.1 | 2.8–8.1 | 2.2–4.1 | 4.1–6.3 | 0.9–1.2 | 0.7–1.2 | 1.7–2.6 | 0.2- 1.3 | [49,91,92] |
| Rice | 3.2–3.7 | 4.5–4.5 | 5.2–9.1 | 2.8–4.5 | 8.2–8.9 | 1.0–1.6 | 1.0–1.5 | 3.3–3.8 | 0.1–1.7 | [49,91,96] |
| Corn | 1.1–4.0 | 3.6–5.1 | 4.8–10.6 | 2.3–4.6 | 1.3–3.8 | 0.6–0.7 | 0.6- 1.1 | 2.6–1.9 | 2.3- 2.6 | [49,91,93,96] |
| Non-Essential Amino Acids | ||||||||||
| Pseudo cereals | ||||||||||
| Whole Grains | As | Glu | Se | Gly | Ar | Al | Ty | Cy | Pr | References |
| Quinoa | 8.0 | 13.2 | 3.4–5.7 | 2.2–6.1 | 6.9–13.6 | 3.2–5.7 | 2.5–3.7 | 0.1–2.7 | 2.3–5.5 | [61,64] |
| Amaranth | 7.3–10.7 | 14.4–17.7 | 4.9–9.3 | 6.7–15.2 | 8.7–15.6 | 3.5–6.2 | 3.3–3.7 | 2.1–3.6 | 2.82–4.6 | [33,55] |
| Buckwheat | 7.6–16.6 | 23.2–24.4 | 3.2–8.6 | 6.2–13.2 | 10.5–11.3 | 4.6–9.6 | 0.6–4.9 | 0.8–3.5 | 2.6–8.8 | [52,62] |
| Cereals | ||||||||||
| Wheat | 4.2–6.6 | 2.8–3.5 | 6.1–6.9 | 4.6–6.31 | 4.7–7.2 | 3.8–5.4 | 1.8–3.8 | 1.4–3.0 | 1.5–2.3 | [92,93,94,95] |
| Rice | 4.2–10.7 | 7.2–20.8 | 4.0–5.7 | 3.9–5.2 | 7.2–8.2 | 4.5–6.3 | 2.3–3.2 | 1.6–2.0 | 4.8–5.4 | [93,96] |
| Corn | 4.7–6.0 | 7.13–15.8 | 5.0–6.4 | 2.5–4.0 | 4.3–10.3 | 5.1–7.9 | 3.0–4.8 | 2.1–2.3 | 1.1–2.8 | [93,96,98] |
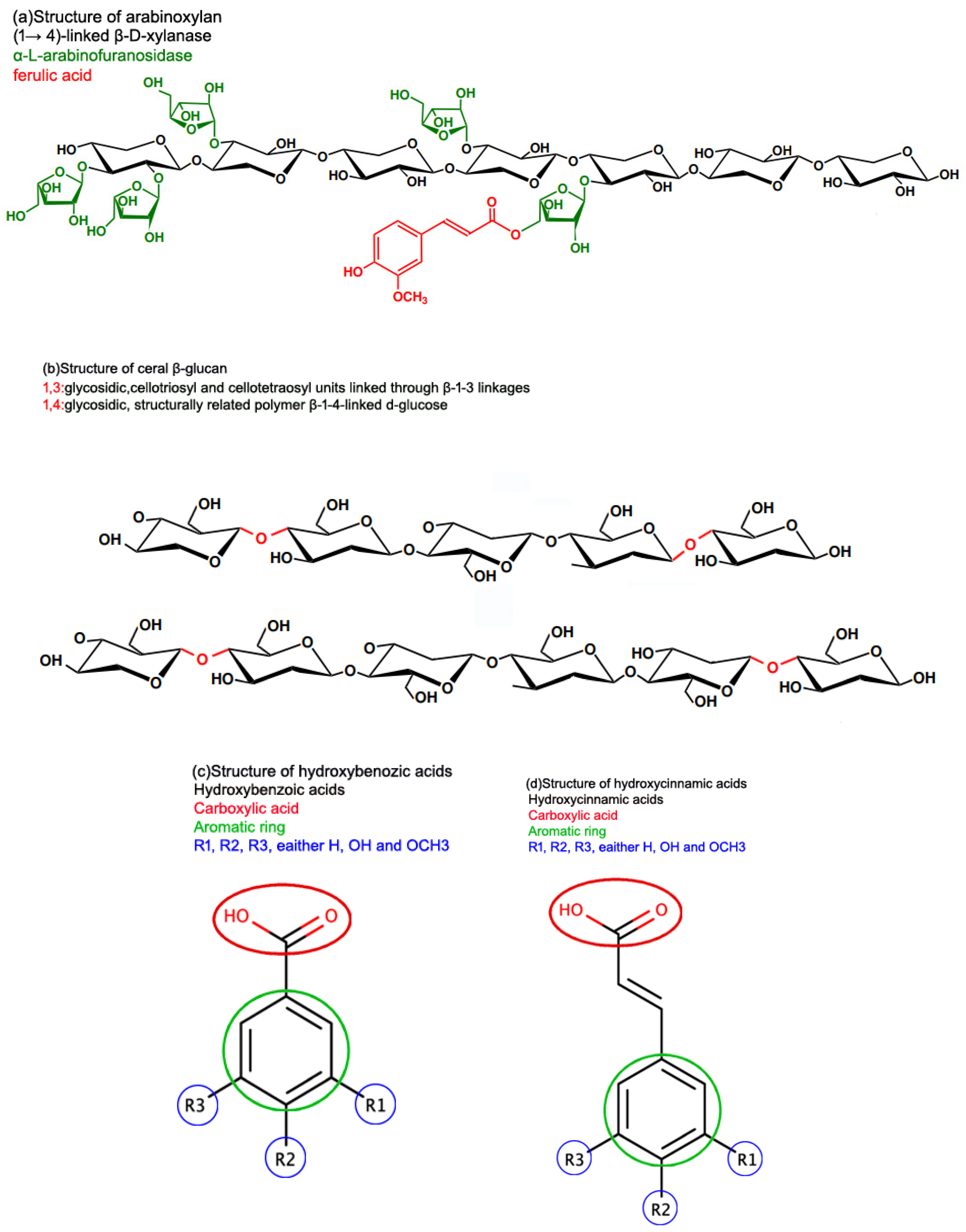
4. Dietary Fibers and Phenolic Acids in Pseudo Cereals and Cereals, and Their Antioxidant Properties
| Pseudo Cereals | |||||||
|---|---|---|---|---|---|---|---|
| Whole Grains | TDF | IDF | SDF | PA | HC | HB | References |
| Quinoa | 7.0–26.5 | 9.9–12.2 | 0.4–2.9 | 1672–3083 | 7.0–150.0 | 13.8–110.0 | [35,50,61,66,107,108,131,132] |
| Amaranth | 2.7–20.6 | 7.7–9.1 | 2.7–3.7 | 212–570 | 1.6–55.4 | 1.8–173.5 | [35,50,51,107,109,110,127,128] |
| Buckwheat | 10.3–19.0 | 2.2–5.8 | 4.8–6. | 49.9 | 1.7–122.8 | 1.2–118.0 | [33,35,51,111,120,133] |
| Cereals | |||||||
| Wheat | 9.2–17.0 | 7.2–14.7 | 1.4–2.9 | 658–1171 | 3.4–195.0 | 7.5–230.6 | [35,61,112,113,121,129,130] |
| Rice | 2.7–9.9 | 1.9–5.4 | 0.5–2.5 | 300–360 | 1.0–301.7 | 2.8–115.6 | [114,115,122,124,129,134] |
| Corn | 13.1–19.6 | 3.1–16.0 | 1.5–3.6 | 601–1740 | 5.7–1387.5 | 0.5–116.5 | [35,116,117,121,122,126,127] |
| Pseudo Cereals | ||||||
|---|---|---|---|---|---|---|
| Whole Grains | DPPH | FRAP | ABTS | TEAC | CUPRAC | References |
| Quinoa | 60.22 (mmol Trolox/100 g) | 58.7 (mg Trolox/100 g dw) | 8.61 (µmolTrolox/100 g) | 59.56 (µmolTrolox/100 g) | 4.968 (µmolTrolox/g) | [147,151,152,153] |
| Amaranth | 134.8 (mmol Trolox/100 g) | 147.4 (µmolTrolox/100 g) | 179.8 (µmolTrolox/100 g) | _ | 5.11 (µmol Trolox/g | [145,147,151] |
| Buckwheat | 80.80 (mmol Trolox/100 g) | 49.43 (µmolTrolox/100 g) | 58 (mmolTrolox/100 g) | 5.93 (µmol/100 g) | 4.14 (µmol Trolox/g) | [151,154,155,156] |
| Cereals | ||||||
| Wheat | 20.9 (mmol Trolox/100 g) | 70.0 (µmol TE/g dw) | 5.4 (mmolTroex/100 g) | 17.8 (mmol TEAC/g) | 13.44 (mmol Trolox/g) | [140,141,142,143,144] |
| Rice | 180.41 (mmol Trolox/100 g) | 46.5 (µmolTrolox/100 g) | 1.78 (mgTEAC/g extract) | 21.3 (mg Trolox equiv/100 g) | 3.21 (µmol Trolox/g) | [137,138,139,144] |
| Corn | 350.29 (mmol Trolox/100 g) | 30.56 (μmolTrolox/100 g) | 92.69 (μmol Trolox/100 g) | 59.6 (µg/mL) | 22.78 (mg/g) | [136,148,149,150] |
5. Grains in Modern Times
6. Intake of Whole Grains and Human Health
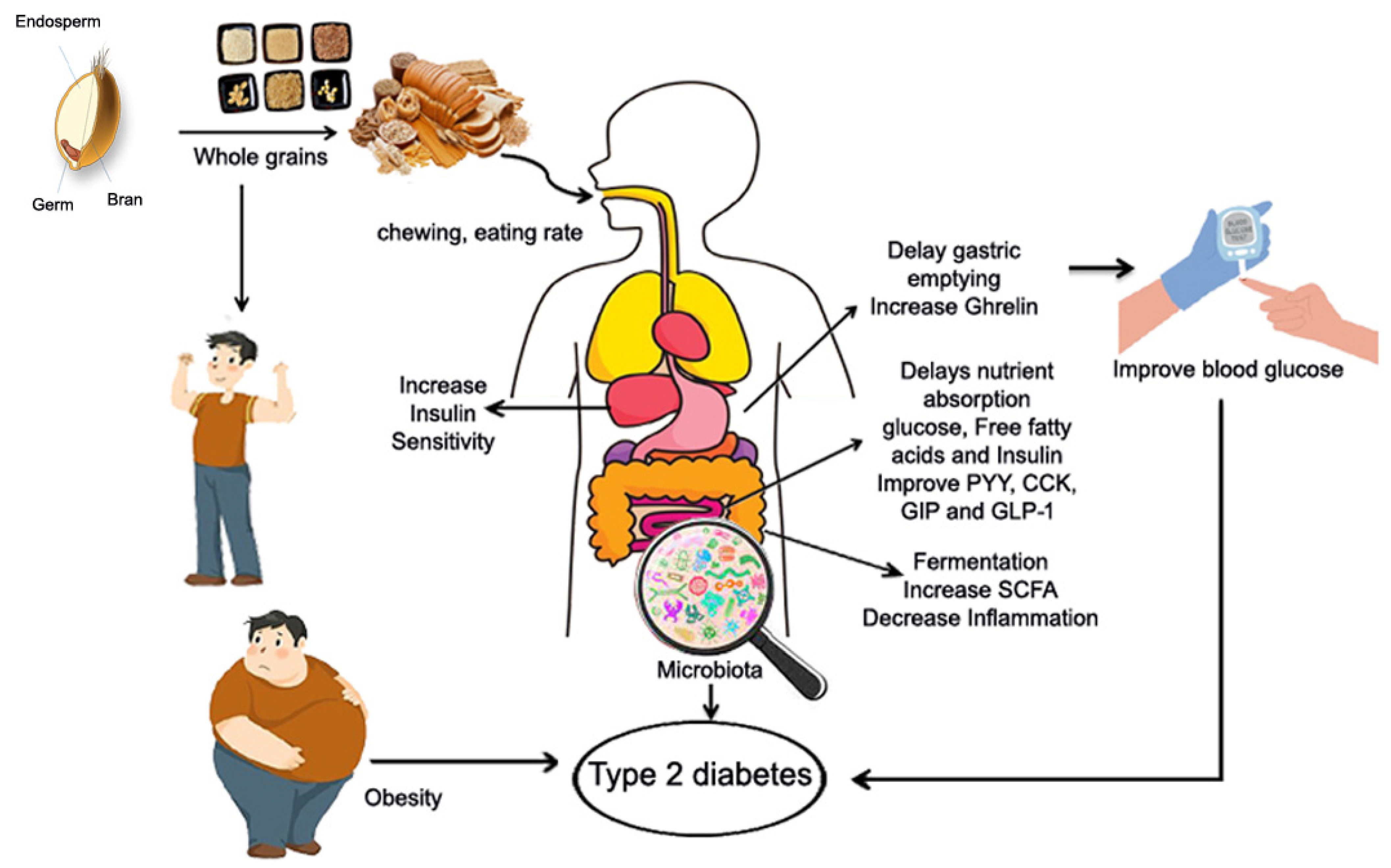
6.1. Relationship between Intake of Pseudo Cereals and Cereals, and T2D
6.2. Potential Mechanism of Whole Grains and T2D
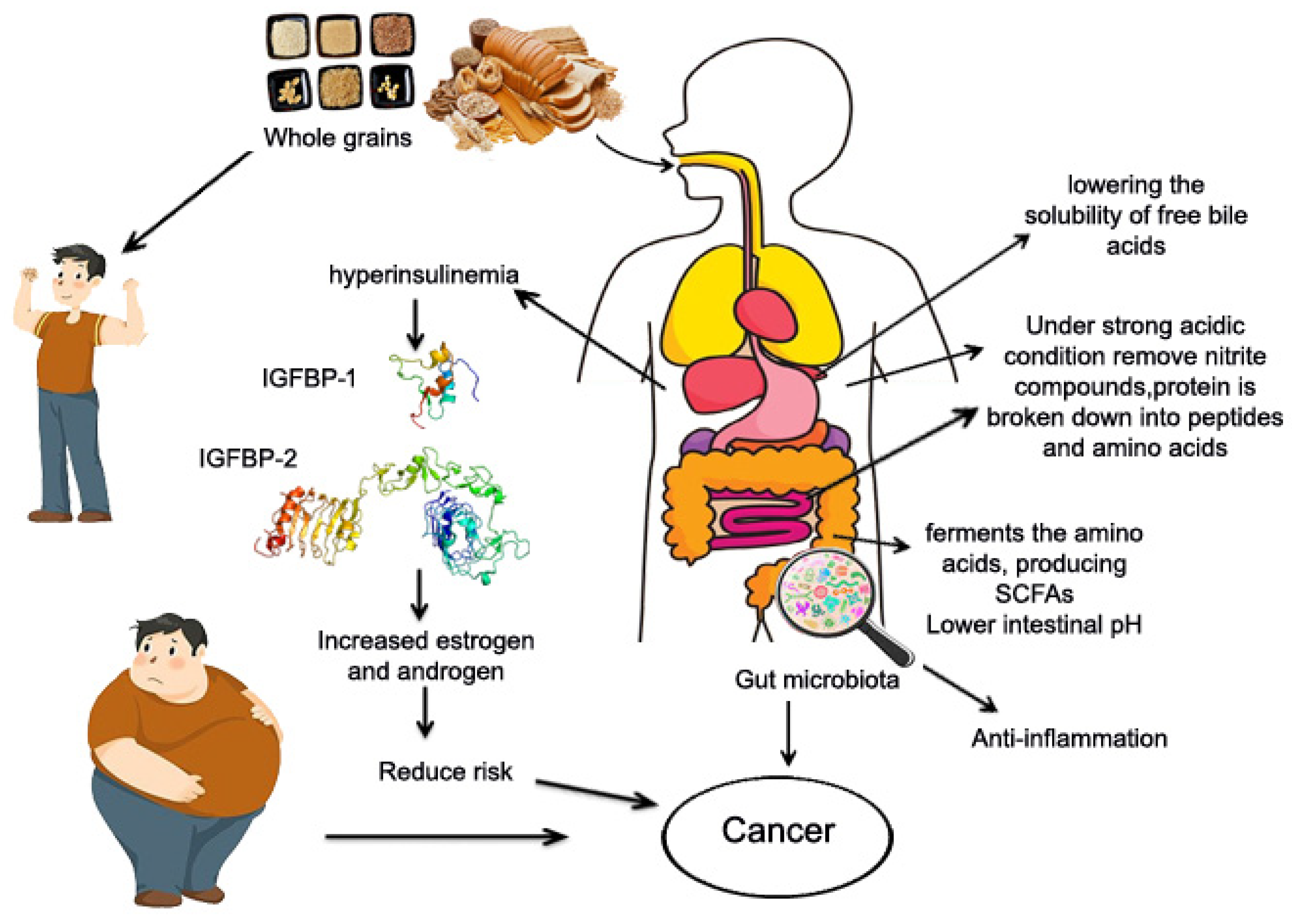
6.3. Relationship between Intake of Pseudo Cereals and Cereals, and Cancer
6.4. Potential Mechanism of Whole Grains and Cancer
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- World Health Organization Report (WHO) Diabetes Reports Predication by 2025. Available online: https://www.who.int/health-topics/diabetes (accessed on 14 May 2024).
- World Health Organization Report (WHO) Cancer Report 2023, Predication of Cancer by 2040. Available online: https://www.paho.org/en/campaigns/world-cancer-day-2023-close-care-gap#:~:text=Globally%2C%20there%20were%20an%20estimated,10%20million%20deaths%20from%20cancer (accessed on 14 May 2024).
- World Cancer Research Fund; American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective. Available online: http://dietandcancerreport.org (accessed on 14 May 2024).
- U.S. Department of Health and Human Services; U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans 8th Edition. Available online: http://health.gov/dietaryguidelines/2015/guidelines/ (accessed on 14 May 2024).
- Sastre, M.; Cimbalo, A.; Mañes, J.; Manyes, L. Gut Microbiota and Nutrition: Strategies for the Prevention and Treatment of Type 2 Diabetes. J. Med. Food 2024, 27, 97–109. [Google Scholar] [CrossRef]
- Ghanbari-Gohari, F.; Mousavi, S.M.; Esmaillzadeh, A. Consumption of whole grains and risk of type 2 diabetes: A comprehensive systematic review and dose–response meta-analysis of prospective cohort studies. Food Sci. Nutr. 2022, 10, 1950–1960. [Google Scholar] [CrossRef]
- Watling, C.Z.; Wojt, A.; Florio, A.A.; Butera, G.; Albanes, D.; Weinstein, S.J.; McGlynn, K.A. Fiber and whole grain intakes in relation to liver cancer risk: An analysis in two prospective cohorts and systematic review and meta-analysis of prospective studies. Hepatology 2024. [Google Scholar] [CrossRef]
- Song, X.; Feng, X.; Chen, S.; Dai, Y.; Huang, H.; Li, X.; Liu, L. Potential impact of time trend of whole grain intake on burden of major cancers in China. Prev. Med. 2023, 175, 107674. [Google Scholar] [CrossRef]
- Gaesser, G.A. Whole grains, refined grains, and cancer risk: A systematic review of meta-analyses of observational studies. Nutrients 2020, 12, 3756. [Google Scholar] [CrossRef]
- Aune, D.; Norat, T.; Romundstad, P.; Vatten, L.J. Whole grain and refined grain consumption and the risk of type 2 diabetes: A systematic review and dose–response meta-analysis of cohort studies. Eur. J. Epidemiol. 2013, 28, 845–858. [Google Scholar] [CrossRef]
- Gaesser, G.A. Refined grain intake and risk of type 2 diabetes. Mayo Clin. Proc. 2022, 97, 1428–1436. [Google Scholar] [CrossRef]
- Mahzari, M.; Mamun, A. Does Consumption of Refined Carbohydrates Predict the Incidence of Type 2 Diabetes Mellitus? A Systematic Review and Meta-Analysis. Rom. J. Diabetes Nutr. Metab. Dis. 2020, 27, 168–179. [Google Scholar] [CrossRef]
- Ernest, D.K.; Lemus, H.; Hsu, F.C.; Pierce, J.P.; Wu, T. The Independent and Joint Associations of Whole Grain and Refined Grain with Total Mortality among Breast Cancer Survivors: A Prospective Cohort Study. Nutrients 2022, 14, 3333. [Google Scholar] [CrossRef]
- AACCI. Definition of Whole Grain. 1999. Available online: http://www.aaccnet.org/definitions/wholegrain.asp (accessed on 19 May 2024).
- House, N.C.; Puthenparampil, D.; Malayil, D.; Narayanankutty, A. Variation in the polyphenol composition, antioxidant, and anticancer activity among different Amaranthus species. S. Afr. J. Bot. 2020, 135, 408–412. [Google Scholar] [CrossRef]
- Rao, V.; Poonia, A. Protein characteristics, amino acid profile, health benefits and methods of extraction and isolation of proteins from some pseudocereals—A review. Food Prod. Process. Nutr. 2023, 5, 37. [Google Scholar] [CrossRef]
- Joye, I.J. Dietary fibre from whole grains and their benefits on metabolic health. Nutrients 2020, 12, 3045. [Google Scholar] [CrossRef]
- Khan, J.; Khan, M.Z.; Ma, Y.; Meng, Y.; Mushtaq, A.; Shen, Q.; Xue, Y. Overview of the Composition of Whole Grains’ Phenolic Acids and Dietary Fibre and Their Effect on Chronic Non-Communicable Diseases. Int. J. Environ. Res. Public Health 2022, 19, 3042. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, X.; Liu, H.; Brown, M.A.; Qiao, S. Dietary Protein and Gut Microbiota Composition and Function. Curr. Protein Pept. Sci. 2019, 20, 145–154. [Google Scholar] [CrossRef]
- Bröer, S.; Fairweather, S.J. Amino Acid Transport across the Mammalian Intestine. Compr. Physiol. 2018, 9, 343–373. [Google Scholar]
- Sánchez-Alcoholado, L.; Ramos-Molina, B.; Otero, A.; Laborda-Illanes, A.; Ordóñez, R.; Medina, J.A.; Gómez-Millán, J.; Queipo-Ortuño, M.I. The role of the gut microbiome in colorectal cancer development and therapy response. Cancers 2020, 12, 1406. [Google Scholar] [CrossRef]
- Baky, M.H.; Salah, M.; Ezzelarab, N.; Shao, P.; Elshahed, M.S.; Farag, M.A. Insoluble dietary fibers: Structure, metabolism, interactions with human microbiome, and role in gut homeostasis. Crit. Rev. Food Sci. Nutr. 2024, 64, 1954–1968. [Google Scholar] [CrossRef]
- González Hernández, M.A.; Canfora, E.E.; Jocken, J.W.E.; Blaak, E.E. The Short-Chain Fatty Acid Acetate in Body Weight Control and Insulin Sensitivity. Nutrients 2019, 11, 1943. [Google Scholar] [CrossRef]
- Bach Knudsen, K.E. Microbial Degradation of Whole-Grain Complex Carbohydrates and Impact on Short-Chain Fatty Acids and Health. Adv. Nutr. 2015, 6, 206–213. [Google Scholar] [CrossRef]
- Idehen, E.; Tang, Y.; Sang, S. Bioactive Phytochemicals in Barley. J. Food Drug Anal. 2017, 25, 148–161. [Google Scholar] [CrossRef]
- Aune, D.; Chan, D.S.M.; Lau, R.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Dietary Fibre, Whole Grains, and Risk of Colorectal Cancer: Systematic Review and Dose–Response Meta-Analysis of Prospective Studies. BMJ 2011, 343, d6617. [Google Scholar] [CrossRef]
- Călinoiu, L.F.; Vodnar, D.C. Whole Grains and Phenolic Acids: A Review on Bioactivity, Functionality, Health Benefits and Bioavailability. Nutrients 2018, 10, 1615. [Google Scholar] [CrossRef]
- de Melo Ribeiro, P.V.; Andrade, P.A.; Hermsdorff, H.H.M.; Dos Santos, C.A.; Cotta, R.M.M.; Estanislau, J.D.A.S.G.; de Oliveira Campos, A.A.; Rosa, C.D.O.B. Dietary Non-Nutrients in the Prevention of Non-Communicable Diseases: Potentially Related Mechanisms. Nutrition 2019, 66, 22–28. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services; U.S. Department of Agriculture. 2020–2025 Dietary Guidelines for Americans. 2022. Available online: https://www.dietaryguidelines.gov (accessed on 19 May 2024).
- Public Health England. 2016. Available online: https://www.gov.uk/government/publications/the-eatwell-guide (accessed on 19 May 2024).
- Health Canada, Canada’s Food Guide. 2020. Available online: https://food-guide.canada.ca/en/healthy-eating-recommendations/limit-highly-processed-foods/choosing-healthy-menu-options/ (accessed on 19 May 2024).
- Mir, N.A.; Riar, C.S.; Singh, S. Nutritional Constituents of Pseudo Cereals and Their Potential Use in Food Systems: A Review. Trends Food Sci. Technol. 2018, 75, 170–180. [Google Scholar] [CrossRef]
- De Bock, P.; Daelemans, L.; Selis, L.; Raes, K.; Vermeir, P.; Eeckhout, M.; Van Bockstaele, F. Comparison of the Chemical and Technological Characteristics of Wholemeal Flours Obtained from Amaranth (Amaranthus sp.), Quinoa (Chenopodium quinoa) and Buckwheat (Fagopyrum sp.) Seeds. Foods 2021, 10, 651. [Google Scholar] [CrossRef]
- Alonso-Miravalles, L.; O’Mahony, J.A. Composition, Protein Profile and Rheological Properties of Pseudocereal-Based Protein-Rich Ingredients. Foods 2018, 7, 73. [Google Scholar] [CrossRef]
- Venskutonis, P.R.; Kraujalis, P. Nutritional Components of Amaranth Seeds and Vegetables: A Review on Composition, Properties and Uses. Compr. Rev. Food Sci. 2013, 12, 381–412. [Google Scholar] [CrossRef]
- Ando, H.; Chen, Y.C.; Tang, H.J.; Shimizu, M.; Watanabe, K.; Mitsunaga, T. Food Components in Fractions of Quinoa Seed. Food Sci. Technol. Res. 2002, 8, 80–84. [Google Scholar] [CrossRef]
- Marmouzi, I.; El Madani, N.; Charrouf, Z.; Cherrah, Y.; Faouzi, M.E.A. Proximate Analysis, Fatty Acids and Mineral Composition of Processed Moroccan Chenopodium quinoa Willd. and Antioxidant Properties According to the Polarity. Phytothérapie 2015, 13, 110–117. [Google Scholar] [CrossRef]
- Pirzadah, T.B.; Malik, B. Pseudocereals as Super Foods of 21st Century: Recent Technological Interventions. J. Agric. Food Res. 2020, 2, 100052. [Google Scholar] [CrossRef]
- Qin, P.; Wang, Q.; Shan, F.; Hou, Z.; Ren, G. Nutritional Composition and Flavonoids Content of Flour from Different Buckwheat Cultivars. Int. J. Food Sci. Technol. 2010, 45, 951–958. [Google Scholar] [CrossRef]
- Siyuan, S.; Tong, L.; Liu, R. Corn Phytochemicals and Their Health Benefits. Food Sci. Hum. Wellness 2018, 7, 185–195. [Google Scholar] [CrossRef]
- Ramashia, S.E.; Anyasi, T.A.; Gwata, E.T.; Meddows-Taylor, S.; Jideani, A.I.O. Processing, Nutritional Composition and Health Benefits of Finger Millet in Sub-Saharan Africa. Food Sci. Technol. 2019, 39, 253–266. [Google Scholar] [CrossRef]
- Oliveira, L.C.; Rosell, C.M.; Steel, C.J. Effect of the Addition of Whole-Grain Wheat Flour and of Extrusion Process Parameters on Dietary Fibre Content, Starch Transformation and Mechanical Properties of a Ready-to-Eat Breakfast Cereal. Int. J. Food Sci. Technol. 2015, 50, 1504–1514. [Google Scholar] [CrossRef]
- Huda, M.N.; Lu, S.; Jahan, T.; Ding, M.; Jha, R.; Zhang, K.; Zhou, M. Treasure from Garden: Bioactive Compounds of Buckwheat. Food Chem. 2021, 335, 127653. [Google Scholar] [CrossRef]
- Kulathunga, J.; Reuhs, B.L.; Zwinger, S.; Simsek, S. Comparative study on kernel quality and chemical composition of ancient and modern wheat species: Einkorn, emmer, spelt and hard red spring wheat. Foods 2021, 10, 761. [Google Scholar] [CrossRef]
- Shewry, P.R.; Hawkesford, M.J.; Piironen, V.; Lampi, A.M.; Gebruers, K.; Boros, D.; Ward, J.L. Natural Variation in Grain Composition of Wheat and Related Cereals. J. Agric. Food Chem. 2013, 61, 8295–8303. [Google Scholar] [CrossRef]
- Hirawan, R.; Ser, W.Y.; Arntfield, S.D.; Beta, T. Antioxidant Properties of Commercial, Regular- and Whole-Wheat Spaghetti. Food Chem. 2010, 119, 258–264. [Google Scholar] [CrossRef]
- Kumar, A.; Metwal, M.; Kaur, S.; Gupta, A.K.; Puranik, S.; Singh, S.; Gupta, S.S.; Babu, B.K.; Sood, S.; Yadav, R. Nutraceutical Value of Finger Millet [Eleusine coracana (L.) Gaertn.] and Their Improvement Using Omics Approaches. Front. Plant Sci. 2016, 7, 1–14. [Google Scholar] [CrossRef]
- Rastogi, A.; Shukla, S. Amaranth: A New Millennium Crop of Nutraceutical Values. Crit. Rev. Food Sci. Nutr. 2013, 53, 109–125. [Google Scholar] [CrossRef]
- Joshi, D.C.; Sood, S.; Hosahatti, R.; Kant, L.; Pattanayak, A.; Kumar, A.; Yadav, D.; Stetter, M.G. From Zero to Hero: The Past, Present and Future of Grain Amaranth Breeding. Theor. Appl. Genet. 2018, 131, 1807–1823. [Google Scholar] [CrossRef]
- Joshi, D.C.; Chaudhari, G.V.; Sood, S.; Kant, L.; Pattanayak, A.; Zhang, K.; Fan, Y.; Janovská, D.; Meglič, V.; Zhou, M. Revisiting the Versatile Buckwheat: Reinvigorating Genetic Gains through Integrated Breeding and Genomics Approach. Planta 2019, 250, 783–801. [Google Scholar] [CrossRef]
- Manzoor, M.; Hami, A.; Pakhtoon, M.M.; Batool, A.; Zaffar, A.; Sudan, J.; Zargar, S.M. Genetic variability of buckwheat (Fagopyrum spp.) genotypes for nutritional and nutraceutical traits. Nucleus 2023, 66, 1–9. [Google Scholar] [CrossRef]
- Seo, C.R.; Yi, B.; Oh, S.; Kwon, S.M.; Kim, S.; Song, N.J.; Cho, J.Y.; Park, K.M.; Ahn, J.Y.; Hong, J.W.; et al. Aqueous Extracts of Hulled Barley Containing Coumaric Acid and Ferulic Acid Inhibit Adipogenesis in Vitro and Obesity in Vivo. J. Funct. Foods 2015, 12, 208–218. [Google Scholar] [CrossRef]
- Podeszwa, T.; Harasym, J.; Czerniecki, P.; Kopacz, M. Congress Wort Analysis from Commercial Buckwheat Malt Mixtures with RSM. Nauk. Inz. I Technol. 2016, 3, 77–89. [Google Scholar]
- Aderibigbe, O.R.; Ezekiel, O.O.; Owolade, S.O.; Korese, J.K.; Sturm, B.; Hensel, O. Exploring the Potentials of Underutilized Grain Amaranth (Amaranthus spp.) along the Value Chain for Food and Nutrition Security: A Review. Crit. Rev. Food Sci. Nutr. 2020, 62, 656–669. [Google Scholar] [CrossRef]
- Gebremariam, M.M.; Zarnkow, M.; Becker, T. Teff (Eragrostis tef) as a Raw Material for Malting, Brewing and Manufacturing of Gluten-Free Foods and Beverages: A Review. J. Food Sci. Technol. 2014, 51, 2881–2895. [Google Scholar] [CrossRef]
- de Sousa, T.; Ribeiro, M.; Sabença, C.; Igrejas, G. The 10,000-Year Success Story of Wheat! Foods 2021, 10, 2124. [Google Scholar] [CrossRef]
- Kaplan Evlice, A.; Cetiner, B.; Pehlivan, A.; Kara, R. Wheat Quality. In Advances in Wheat Breeding: Towards Climate Resilience and Nutrient Security; Springer Nature: Berlin/Heidelberg, Germany, 2024; pp. 453–477. [Google Scholar]
- Zhygunov, D.; Sots, S.; Barkovska, Y.; Liu, J.; Wang, F.; Liu, X.; Wang, Z.; Li, X. Influence of grain quality indicators on the flour quality indicators at the laboratory milling. Grain Prod. Mix. Fodd. 2022, 22, 17. [Google Scholar] [CrossRef]
- Pereira, E.; Encina-Zelada, C.; Barros, L.; Gonzales-Barron, U.; Cadavez, V.; Ferreira, I.C.F.R. Chemical and Nutritional Characterization of Chenopodium quinoa Willd. (Quinoa) Grains: A Good Alternative to Nutritious Food. Food Chem. 2019, 280, 110–114. [Google Scholar] [CrossRef]
- Nowak, V.; Du, J.; Charrondière, U.R. Assessment of the Nutritional Composition of Quinoa (Chenopodium quinoa Willd.). Food Chem. 2016, 193, 47–54. [Google Scholar] [CrossRef]
- Thanh-Tien, N.N.; Trinh, L.N.D.; Inoue, N.; Morita, N.; Van Hung, P. Nutritional Composition, Bioactive Compounds, and Diabetic Enzyme Inhibition Capacity of Three Varieties of Buckwheat in Japan. Cereal Chem. 2018, 95, 615–624. [Google Scholar] [CrossRef]
- Guo, H.; Hao, Y.; Yang, X.; Ren, G.; Richel, A. Exploration on bioactive properties of quinoa protein hydrolysate and peptides: A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 2896–2909. [Google Scholar] [CrossRef]
- Dakhili, S.; Abdolalizadeh, L.; Hosseini, S.M.; Shojaee-Aliabadi, S.; Mirmoghtadaie, L. Quinoa Protein: Composition, Structure and Functional Properties. Food Chem. 2019, 299, 125121. [Google Scholar] [CrossRef]
- Fotschki, B.; Ju’skiewicz, J.; Jurgo’nski, A.; Amarowicz, R.; Opyd, P.; Bez, J.; Muranyi, I.; Petersen, I.L.; Llopis, M.L. Protein-Rich Flours from Quinoa and Buckwheat Favourably Affect the Growth Parameters, Intestinal Microbial Activity and Plasma Lipid Profile of Rats. Nutrients 2020, 12, 2781. [Google Scholar] [CrossRef]
- Abugoch, L.E.; Romero, N.; Tapia, C.A.; Silva, J.; Rivera, M. Study of Some Physicochemical and Functional Properties of Quinoa (Chenopodium quinoa Willd) Protein Isolates. J. Agric. Food Chem. 2008, 56, 4745–4750. [Google Scholar] [CrossRef]
- Vega-Galvez, A.; Miranda, M.; Vergara, J.; Uribe, E.; Puente, L.; Martinez, E.A. Nutrition Facts and Functional Potential of Quinoa (Chenopodium quinoa Willd.): An Ancient Andean Grain: A Review. J. Sci. Food Agric. 2010, 90, 2541–2547. [Google Scholar] [CrossRef]
- Okon, O.G. The nutritional applications of quinoa seeds. In Biology and Biotechnology of Quinoa: Super Grain for Food Security; Springer: Singapore, 2021; pp. 35–49. [Google Scholar] [CrossRef]
- Brinegar, C.; Sine, B.; Nwokocha, L. High-Cysteine 2S Seed Storage Proteins from Quinoa (Chenopodium quinoa). J. Agric. Food Chem. 1996, 44, 1621–1623. [Google Scholar] [CrossRef]
- Singh, A.; Punia, D. Characterization and nutritive values of amaranth seeds. Curr. J. Appl. Sci. Technol. 2020, 39, 27–33. [Google Scholar] [CrossRef]
- Manassero, C.A.; Añón, M.C.; Speroni, F. Development of a High Protein Beverage Based on Amaranth. Plant Foods Hum. Nutr. 2020, 75, 599–607. [Google Scholar] [CrossRef]
- Arslan-Tontul, S.; Uslu, C.C.; Mutlu, C.; Erba¸s, M. Expected Glycemic Impact and Probiotic Stimulating Effects of Whole Grain Flours of Buckwheat, Quinoa, Amaranth and Chia. J. Food Sci. Technol. 2021, 59, 1460–1467. [Google Scholar] [CrossRef]
- Olawoye, B.; Kadiri, O.; Oluwajuyitan, T.D. Grain Amaranth: Processing, Health Benefits and Applications. In Handbook of Cereals, Pulses, Roots, and Tubers; CRC Press: Boca Raton, FL, USA, 2021; pp. 221–234. [Google Scholar]
- Schmidt, D.; Verruma-Bernardi, M.R.; Forti, V.A.; Borges, M.T.M.R. Quinoa and Amaranth as Functional Foods: A Review. Food Rev. Int. 2021, 39, 2277–2296. [Google Scholar] [CrossRef]
- Malik, M.; Sindhu, R.; Dhull, S.B.; Bou-Mitri, C.; Singh, Y.; Panwar, S.; Khatkar, B.S. Nutritional composition, functionality, and processing technologies for Amaranth. J. Food Proc. Pres. 2023, 1, 1753029. [Google Scholar] [CrossRef]
- Pandya, A.; Thiele, B.; Köppchen, S.; Zurita-Silva, A.; Usadel, B.; Fiorani, F. Characterization of Bioactive Phenolic Compounds in Seeds of Chilean Quinoa (Chenopodium quinoa Willd.) Germplasm. Agronomy 2023, 13, 2170. [Google Scholar] [CrossRef]
- Orona-Tamayo, D.; Paredes-Lopez, O. Amaranth Part 2—Sustainable Crop for the 21st Century: Food Properties and Nutraceuticals for Improving Human Health. In Sustainable Protein Sources; Elsevier: Amsterdam, The Netherlands, 2024; pp. 413–441. [Google Scholar]
- Liu, T.; Hou, G.G. Trends in Whole Grain Processing Technology and Product Development. In Whole Grains; CRC Press: Boca Raton, FL, USA, 2019; pp. 257–279. [Google Scholar]
- Zhou, Y.; Jiang, Y.; Shi, R.; Chen, Z.; Li, Z.; Wei, Y.; Zhou, X. Structural and Antioxidant Analysis of Tartary Buckwheat (Fagopyrum tartaricum Gaertn.) 13S Globulin. J. Sci. Food Agric. 2019, 100, 1220–1229. [Google Scholar] [CrossRef]
- Taniya, M.S.; Reshma, M.V.; Shanimol, P.S.; Krishnan, G.; Priya, S. Bioactive Peptides from Amaranth Seed Protein Hydrolysates Induced Apoptosis and Antimigratory Effects in Breast Cancer Cells. Food Biosci. 2020, 35, 100588. [Google Scholar] [CrossRef]
- Fan, X.; Guo, H.; Teng, C.; Zhang, B.; Blecker, C.; Ren, G. Anti-Colon Cancer Activity of Novel Peptides Isolated from In Vitro Digestion of Quinoa Protein in Caco-2 Cells. Foods 2022, 11, 194. [Google Scholar] [CrossRef]
- Prabhakar, B.N.; Suneetha, W.J.; Devi, S.S.; Shreeja, K. Effect of germination on nutritional composition of common buckwheat (Fagopyrum esculentum Moench). Int. Res. J. Pure Appl. Chem. 2021, 22, 1–7. [Google Scholar] [CrossRef]
- Fabian, C.; Ju, Y.H. A Review on Rice Bran Protein: Its Properties and Extraction Methods. Crit. Rev. Food Sci. Nutr. 2011, 51, 816–827. [Google Scholar] [CrossRef]
- Deng, Y.; Lim, J.; Lee, G.H.; Nguyen, T.T.H.; Xiao, Y.; Piao, M.; Kim, D. Brewing Rutin-Enriched Lager Beer with Buckwheat Malt as Adjuncts. J. Microbiol. Biotechnol. 2019, 29, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Cadenas, R.; Caballero, I.; Nimubona, D.; Blanco, C.A. Brewing with Starchy Adjuncts: Its Influence on the Sensory and Nutritional Properties of Beer. Foods 2021, 10, 1726. [Google Scholar] [CrossRef] [PubMed]
- Chrungoo, N.K.; Chettry, U. Buckwheat: A Critical Approach towards Assessment of Its Potential as a Super Crop. Indian J. Genet. Plant Breed. 2021, 81, 1–23. [Google Scholar] [CrossRef]
- Dabija, A.; Ciocan, M.E.; Chetrariu, A.; Mîrzan, D. Comparative evaluation of the physico-chemical characteristics of buckwhweat malt and barley malt. Int. Multidiscip. Sci. 2021, 21, 51–58. [Google Scholar]
- Comino, I.; Moreno, M.D.; Real, A.; Rodriguez-Herrera, A.; Barro, F.; Sousa, C. The Gluten-Free Diet: Testing Alternative Cereals Tolerated by Celiac Patients. Nutrients 2013, 5, 4250–4268. [Google Scholar] [CrossRef] [PubMed]
- Filho, A.M.M.; Pirozi, M.R.; Borges, J.T.D.S.; Pinheiro Sant’Ana, H.M.; Chaves, J.B.P.; Coimbra, J.S.D.R. Quinoa: Nutritional, Functional, and Antinutritional Aspects. Crit. Rev. Food Sci. Nutr. 2017, 57, 1618–1630. [Google Scholar] [CrossRef] [PubMed]
- Chettry, U.; Chrungoo, N.K. Beyond the Cereal Box: Breeding Buckwheat as a Strategic Crop for Human Nutrition. Plant. Foods Hum. Nutr. 2021, 76, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Promising Das, S. Amaranths: The Crop of Great Prospect. In Amaranthus: A Promising Crop of Future; Springer: Singapore, 2016; pp. 13–48. [Google Scholar]
- Shobana, S.; Krishnaswamy, K.; Sudha, V.; Malleshi, N.G.; Anjana, R.M.; Palaniappan, L.; Mohan, V. Finger Millet (Ragi, Eleusine coracana L.): A Review of Its Nutritional Properties, Processing, and Plausible Health Benefits. Adv. Food Nutr. Res. 2013, 69, 1–39. [Google Scholar]
- Hou, Y.; He, W.; Hu, S.; Wu, G. Composition of Polyamines and Amino Acids in Plant-Source Foods for Human Consumption. Amino Acids 2019, 51, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Kabthymer, R.H.; Karim, M.N.; Hodge, A.M.; de Courten, B. High Cereal Fibre but Not Total Fibre Is Associated with a Lower Risk of Type 2 Diabetes: Evidence from the Melbourne Collaborative Cohort Study. Diabetes Obes. Metab. 2023, 25, 1911–1921. [Google Scholar] [CrossRef]
- Jovanovski, E.; Nguyen, M.; Kurahashi, Y.; Komishon, A.; Li, D.; Thanh, H.H.V.; Vuksan, V. Are All Fibres Created Equal with Respect to Lipid Lowering? Comparing the Effect of Viscous Dietary Fibre to Non-Viscous Fibre from Cereal Sources: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Br. J. Nutr. 2023, 129, 1180–1192. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Cavada, E.; Drago, S.R.; González, R.J.; Juan, R.; Pastor, J.E.; Alaiz, M.; Vioque, J. Effects of the Addition of Wild Legumes (Lathyrus annuus and Lathyrus clymenum) on the Physical and Nutritional Properties of Extruded Products Based on Whole Corn and Brown Rice. Food Chem. 2011, 128, 961–967. [Google Scholar] [CrossRef]
- Gong, K.; Chen, L.; Li, X.; Sun, L.; Liu, K. Effects of Germination Combined with Extrusion on the Nutritional Composition, Functional Properties and Polyphenol Profile and Related in Vitro Hypoglycemic Effect of Whole Grain Corn. J. Cereal Sci. 2018, 83, 1–8. [Google Scholar] [CrossRef]
- Schulze, M.B.; Schulz, M.; Heidemann, C.; Schienkiewitz, A.; Hoffmann, K.; Boeing, H. Fiber and Magnesium Intake and Incidence of Type 2 Diabetes: A Prospective Study and Meta-Analysis. Arch. Intern. Med. 2007, 167, 956–965. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Carbohydrates and Dietary Fibre. EFSA J. 2010, 8, 1462. [Google Scholar]
- Meghwal, M.; Kadeppagari, R.K. Dietary Fibers and Their Role as Functional Food for Human Health: Food Fibers and Human Health. In Examining the Development, Regulation, and Consumption of Functional Foods; IGI Global: Hershey, PA, USA, 2017; pp. 29–44. [Google Scholar]
- Zeng, Z.; Liu, C.; Luo, S.; Chen, J.; Gong, E. The Profile and Bioaccessibility of Phenolic Compounds in Cereals Influenced by Improved Extrusion Cooking Treatment. PLoS ONE 2016, 11, e0161086. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary Fibre in Foods: A Review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; El-Said, A.M.A.; Khalifa, S.A.M.; Göransson, U.; Bohlin, L.; Borg-Karlson, A.K.; Verpoorte, R. Biosynthesis, Natural Sources, Dietary Intake, Pharmacokinetic Properties, and Biological Activities of Hydroxycinnamic Acids. J. Agric. Food Chem. 2012, 60, 10877–10895. [Google Scholar] [CrossRef]
- Vitaglione, P.; Napolitano, A.; Fogliano, V. Cereal Dietary Fibre: A Natural Functional Ingredient to Deliver Phenolic Compounds into the Gut. Trends Food Sci. Technol. 2008, 19, 451–463. [Google Scholar] [CrossRef]
- Bunzel, M.; Ralph, J.; Marita, J.M.; Hatfield, R.D.; Steinhart, H. Diferulates as Structural Components in Soluble and Insoluble Cereal Dietary Fibre. J. Sci. Food Agric. 2001, 81, 653–660. [Google Scholar] [CrossRef]
- Malleshi, N.G.; Agarwal, A.; Tiwari, A.; Sood, S. Nutritional Quality and Health Benefits. In Millets and Pseudocereal; Elsevier: Amsterdam, The Netherlands, 2021; pp. 159–168. [Google Scholar]
- Srichuwong, S.; Curti, D.; Austin, S.; King, R.; Lamothe, L.; Gloria-Hernandez, H. Physicochemical Properties and Starch Digestibility of Whole Grain Sorghums, Millet, Quinoa and Amaranth Flours, as Affected by Starch and Non-Starch Constituents. Food Chem. 2017, 233, 1–10. [Google Scholar] [CrossRef]
- Pulvento, C.; Riccardi, M.; Lavini, A.; Iafelice, G.; Marconi, E.; D’Andria, R. Yield and Quality Characteristics of Quinoa Grown in Open Field Under Different Saline and Non-Saline Irrigation Regimes. J. Agron. Crop Sci. 2012, 198, 254–263. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Arendt, E.K.; Gallagher, E. Nutritive Value and Chemical Composition of Pseudocereals as Gluten-Free Ingredients. Int. J. Food Sci. Nutr. 2009, 60, 240–257. [Google Scholar] [CrossRef]
- Robin, F.; Théoduloz, C.; Srichuwong, S. Properties of Extruded Whole Grain Cereals and Pseudocereals Flours. Int. J. Food Sci. Technol. 2015, 50, 2152–2159. [Google Scholar] [CrossRef]
- Steadman, K.J.; Burgoon, M.S.; Lewis, B.A.; Edwardson, S.E.; Obendorf, R.L. Buckwheat Seed Milling Fractions: Description, Macronutrient Composition and Dietary Fibre. J. Cereal Sci. 2001, 33, 271–278. [Google Scholar] [CrossRef]
- De Santis, M.A.; Kosik, O.; Passmore, D.; Flagella, Z.; Shewry, P.R.; Lovegrove, A. Comparison of the Dietary Fibre Composition of Old and Modern Durum Wheat (Triticum turgidum spp. durum) Genotypes. Food Chem. 2018, 244, 304–310. [Google Scholar] [CrossRef]
- Rainakari, A.-I.; Rita, H.; Putkonen, T.; Pastell, H. New Dietary Fibre Content Results for Cereals in the Nordic Countries Using AOAC 2011.25 Method. J. Food Compos. Anal. 2016, 51, 1–8. [Google Scholar] [CrossRef]
- Amalraj, A.; Pius, A. Influence of Oxalate, Phytate, Tannin, Dietary Fiber, and Cooking on Calcium Bioavailability of Commonly Consumed Cereals and Millets in India. Cereal Chem. 2015, 92, 389–394. [Google Scholar] [CrossRef]
- Prasad, V.S.S.; Hymavathi, A.; Babu, V.R.; Longvah, T. Nutritional Composition in Relation to Glycemic Potential of Popular Indian Rice Varieties. Food Chem. 2018, 238, 29–34. [Google Scholar] [CrossRef]
- Prasanthi, P.S.; Naveena, N.; Vishnuvardhana Rao, M.; Bhaskarachary, K. Compositional Variability of Nutrients and Phytochemicals in Corn after Processing. J. Food Sci. Technol. 2017, 54, 1080–1090. [Google Scholar] [CrossRef]
- Gartaula, G.; Dhital, S.; Netzel, G.; Flanagan, B.M.; Yakubov, G.E.; Beahan, C.T.; Collins, H.M.; Burton, R.A.; Bacic, A.; Gidley, M.J. Quantitative Structural Organisation Model for Wheat Endosperm Cell Walls: Cellulose as an Important Constituent. Carbohydr. Polym. 2018, 196, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Dietary fiber polysaccharides of amaranth, buckwheat and quinoa grains: A review of chemical structure, biological functions and food uses. Carbohydr. Polym. 2020, 248, 116819. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, P.; Omar, L.S.; Kamal, H.; Kilari, B.P.; Maqsood, S. Multi-Functional Bioactive Properties of Intact and Enzymatically Hydrolysed Quinoa and Amaranth Proteins. LWT 2019, 110, 207–213. [Google Scholar] [CrossRef]
- Zhang, G.; Xu, Z.; Gao, Y.; Huang, X.; Zou, Y.; Yang, T. Effects of Germination on the Nutritional Properties, Phenolic Profiles, and Antioxidant Activities of Buckwheat. J. Food Sci. 2015, 80, H1111–H1119. [Google Scholar] [CrossRef]
- Adom, K.K.; Liu, R.H. Antioxidant Activity of Grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.; Pihlava, J.-M.; Hellstrom, J. Contents of Phenolic Acids, Alkyl- and Alkenylresorcinols, and Avenanthramides in Commercial Grain Products. J. Agric. Food Chem. 2005, 53, 8290–8295. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shewry, P.R.; Ward, J.L. Phenolic Acids in Wheat Varieties in the HEALTHGRAIN Diversity Screen. J. Agric. Food Chem. 2008, 56, 9732–9739. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; van der Hooft, J.J.; Crozier, A. Human Studies on the Absorption, Distribution, Metabolism, and Excretion of Tea Polyphenols. Am. J. Clin. Nutr. 2013, 98 (Suppl. 6), 1619S–1630S. [Google Scholar] [CrossRef]
- Sánchez-Patán, F.; Barroso, E.; van de Wiele, T.; Jiménez-Girón, A.; Martín-Alvarez, P.J.; Moreno-Arribas, M.V.; Martínez-Cuesta, M.C.; Peláez, C.; Requena, T.; Bartolomé, B. Comparative in Vitro Fermentations of Cranberry and Grape Seed Polyphenols with Colonic Microbiota. Food Chem. 2015, 183, 273–782. [Google Scholar] [CrossRef]
- Kandil, A.; Li, J.; Vasanthan, T.; Bressler, D.C. Phenolic Acids in Some Cereal Grains and Their Inhibitory Effect on Starch Liquefaction and Saccharification. J. Agric. Food Chem. 2012, 60, 8444–8449. [Google Scholar] [CrossRef]
- Žilic, S.; Serpen, A.; Akıllıoğlu, G.; Gökmeng, V.; Vancetovic, J. Phenolic Compounds, Carotenoids, Anthocyanins, and Antioxidant Capacity of Colored Maize (Zea mays L.) Kernels. J. Agric. Food Chem. 2012, 60, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.H.; Jo, I.H.; Sebastin, R.; Jeong, W.T.; Oh, S.; Heo, T.Y.; Sung, J.; Hyun, T.K.; So, Y.-S.; Yu, J.-K.; et al. Comparative Analysis of Polyphenolic Compounds in Different Amaranthus Species: Influence of Genotypes and Harvesting Year. Antioxidants 2024, 13, 501. [Google Scholar] [CrossRef] [PubMed]
- Irakli, M.N.; Samanidou, V.F.; Biliaderis, C.G.; Papadoyannis, I.N. Development and Validation of an HPLC-Method for Determination of Free and Bound Phenolic Acids in Cereals after Solid-Phase Extraction. Food Chem. 2012, 134, 1624–1632. [Google Scholar] [CrossRef] [PubMed]
- Hithamani, G.; Srinivasan, K. Bioaccessibility of Polyphenols from Wheat (Triticum aestivum), Sorghum (Sorghum bicolor), Green Gram (Vigna radiata), and Chickpea (Cicer arietinum) as Influenced by Domestic Food Processing. J. Agric. Food Chem. 2014, 62, 11170–11179. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Caravaca, A.M.; Iafelice, G.; Verardo, V.; Marconi, E.; Caboni, M.F. Influence of Pearling Process on Phenolic and Saponin Content in Quinoa (Chenopodium quinoa Willd). Food Chem. 2014, 157, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Repo-Carrasco-Valencia, R.; Hellström, J.K.; Pihlava, J.M.; Mattila, P.H. Flavonoids and Other Phenolic Compounds in Andean Indigenous Grains: Quinoa (Chenopodium quinoa), Kañiwa (Chenopodium pallidicaule), and Kiwicha (Amaranthus caudatus). Food Chem. 2010, 120, 128–133. [Google Scholar] [CrossRef]
- Sedej, I.; Sakač, M.; Mandić, A.; Mišan, A.; Tumbas, V.; Čanadanović-Brunet, J. Buckwheat (Fagopyrum esculentum Moench) Grain and Fractions: Antioxidant Compounds and Activities. J. Food Sci. 2012, 77, C954–C959. [Google Scholar] [CrossRef] [PubMed]
- Quan, N.T.; Xuan, T.D. Foliar Application of Vanillic and p-Hydroxybenzoic Acids Enhanced Drought Tolerance and Formation of Phytoalexin Momilactones in Rice. Arch. Agron. Soil Sci. 2018, 64, 1831–1846. [Google Scholar] [CrossRef]
- Patel, S. Cereal Bran: The Next Super Food with Significant Antioxidant and Anticancer Potential. Mediterr. J. Nutr. Metab. 2012, 5, 91–104. [Google Scholar]
- Feregrino-Pérez, A.A.; Mercado-Luna, A.; Murillo-Cárdenas, C.A.; González-Santos, R.; Chávez-Servín, J.L.; Vargas-Madriz, A.F.; Luna-Sánchez, E. Polyphenolic Compounds and Antioxidant Capacity in Native Maize of the Sierra Gorda of Querétaro. Agronomy 2024, 14, 142. [Google Scholar] [CrossRef]
- Imam, M.U.; Musa, S.N.A.; Azmi, N.H.; Ismail, M. Effects of White Rice, Brown Rice and Germinated Brown Rice on Antioxidant Status of Type 2 Diabetic Rats. Int. J. Mol. Sci. 2012, 13, 12952–12969. [Google Scholar] [CrossRef]
- Gong, E.S.; Luo, S.; Li, T.; Liu, C.; Zhang, G.; Chen, J.; Zeng, Z.; Liu, R.H. Phytochemical Profiles and Antioxidant Activity of Processed Brown Rice Products. Food Chem. 2017, 232, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Ranjkesh, N.; Daliri, M.S.; Mazloum, P.; Mousavi, A.; Rameeh, V. Evaluation of physicochemical characteristics and antioxidant properties of elite rice (Oryza sativa L.). Cer. Res. Communi. 2021, 49, 485–491. [Google Scholar] [CrossRef]
- Çelik, E.E.; Gökmen, V. Effects of Fermentation and Heat Treatments on Bound-Ferulic Acid Content and Total Antioxidant Capacity of Bread Crust-Like Systems Made of Different Whole Grain Flours. J. Cereal Sci. 2020, 93, 102978. [Google Scholar] [CrossRef]
- Halvorsen, B.L.; Holte, K.; Myhrstad, M.C.; Barikmo, I.; Hvattum, E.; Remberg, S.F.; Wold, A.B.; Haffner, K.; Baugerød, H.; Andersen, L.F.; et al. A Systematic Screening of Total Antioxidants in Dietary Plants. J. Nutr. 2002, 132, 461–471. [Google Scholar] [CrossRef]
- Serpen, A.; Gökmen, V.; Pellegrini, N.; Fogliano, V. Direct Measurement of the Total Antioxidant Capacity of Cereal Products. J. Cereal Sci. 2008, 48, 816–820. [Google Scholar] [CrossRef]
- Tufan, A.N.; Çelik, S.E.; Özyürek, M.; Güçlü, K.; Apak, R. Direct Measurement of Total Antioxidant Capacity of Cereals: QUENCHER-CUPRAC Method. Talanta 2013, 108, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Adebo, O.A.; Gabriela Medina-Meza, I. Impact of fermentation on the phenolic compounds and antioxidant activity of whole cereal grains: A mini review. Molecules 2020, 25, 927. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J.; Kita, A.; Pęksa, A.; Wawrzyniak, A.; Hamułka, J.; Jeznach, M.; Danilčenko, H.; Jariene, E. Analysis of the Content of Bioactive Compounds in Selected Flours and Enriched Extruded Corn Products. J. Food Compos. Anal. 2017, 64, 147–155. [Google Scholar] [CrossRef]
- Peñarrieta, J.M.; Alvarado, J.A.; Åkesson, B.; Bergenståhl, B. Total Antioxidant Capacity and Content of Flavonoids and Other Phenolic Compounds in Canihua (Chenopodium pallidicaule): An Andean Pseudocereal. Mol. Nutr. Food Res. 2008, 52, 708–717. [Google Scholar] [CrossRef]
- Gorinstein, S.; Lojek, A.; Číž, M.; Pawelzik, E.; Delgado-Licon, E.; Medina, O.J.; Moreno, M.; Arnao Salas, I.; Goshev, I. Comparison of Composition and Antioxidant Capacity of Some Cereals and Pseudocereals. Int. J. Food Sci. Technol. 2008, 43, 629–637. [Google Scholar] [CrossRef]
- Loarca-Piña, G.; Neri, M.; Figueroa, J.D.D.; Castaño-Tostado, E.; Ramos-Gómez, M.; Reynoso, R.; Mendoza, S. Chemical Characterization, Antioxidant and Antimutagenic Evaluations of Pigmented Corn. J. Food Sci. Technol. 2019, 56, 3177–3184. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhai, W. Identification and Antioxidant Activity of Anthocyanins Extracted from the Seed and Cob of Purple Corn (Zea mays L.). Innov. Food Sci. Emerg. Technol. 2010, 11, 169–176. [Google Scholar] [CrossRef]
- Kostić, A.Ž.; Milinčić, D.D.; Špirović Trifunović, B.; Nedić, N.; Gašić, U.M.; Tešić, Ž.L.; Stanojević, S.P.; Pešić, M.B. Monofloral Corn Poppy Bee-Collected Pollen—A Detailed Insight into Its Phytochemical Composition and Antioxidant Properties. Antioxidants 2023, 12, 1424. [Google Scholar] [CrossRef] [PubMed]
- Chlopicka, J.; Pasko, P.; Gorinstein, S.; Jedryas, A.; Zagrodzki, P. Total Phenolic and Total Flavonoid Content, Antioxidant Activity and Sensory Evaluation of Pseudocereal Breads. LWT-Food Sci. Technol. 2012, 46, 548–555. [Google Scholar] [CrossRef]
- Martínez-Villaluenga, C.; Peñas, E.; Hernández-Ledesma, B. Pseudocereal Grains: Nutritional Value, Health Benefits and Current Applications for the Development of Gluten-Free Foods. Food Chem. Toxicol. 2020, 137, 111178. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lietz, G.; Seal, C.J. Phenolic, Apparent Antioxidant and Nutritional Composition of Quinoa (Chenopodium quinoa Willd.) Seeds. Int. J. Food Sci. Technol 2021, 56, 3245–3254. [Google Scholar]
- Gallardo, C.; Jimenez, L.; Garcia-Conesa, M.T. Hydroxycinnamic Acid Composition and in Vitro Antioxidant Activity of Selected Grain Fractions. Food Chem. 2006, 99, 455–463. [Google Scholar] [CrossRef]
- Đorđević, T.M.; Šiler-Marinković, S.S.; Dimitrijević-Branković, S.I. Effect of Fermentation on Antioxidant Properties of Some Cereals and Pseudo Cereals. Food Chem. 2010, 119, 957–963. [Google Scholar] [CrossRef]
- Inglett, G.E.; Chen, D.; Berhow, M.; Lee, S. Antioxidant Activity of Commercial Buckwheat Flours and Their Free and Bound Phenolic Compositions. Food Chem. 2011, 125, 923–929. [Google Scholar] [CrossRef]
- Pruett, A.; Aramouni, F.M.; Bean, S.R.; Haub, M.D. Effect of Flour Particle Size on the Glycemic Index of Muffins Made from Whole Sorghum, Whole Corn, Brown Rice, Whole Wheat, or Refined Wheat Flours. Foods 2023, 12, 4188. [Google Scholar] [CrossRef] [PubMed]
- FAO. Food and Agriculture Organization. Available online: http://www.fao.org/alc/file/media/pubs/2011/cultivo_quinua_en.pdf (accessed on 19 May 2024).
- Bommer, C.; Heesemann, E.; Sagalova, V.; Manne-Goehler, J.; Atun, R.; Bärnighausen, T.; Vollmer, S. The Global Economic Burden of Diabetes in Adults Aged 20–79 Years: A Cost-of-Illness Study. Lancet Diabetes Endocrinol. 2017, 5, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.; Unadkat, S.; Patel, H.; Rathod, M. Dietary Practices among Type 2 Diabetes Patients Visiting a Non-Communicable Disease (NCD) Clinic in a District of Western India: A Cross-Sectional Study. Cureus 2024, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Duan, Y.; Zhu, L.; Fang, Z.; He, L.; Ai, D.; Jin, Y. Whole Grain and Cereal Fiber Intake and the Risk of Type 2 Diabetes: A Meta-Analysis. Int. J. Mol. Epidemiol. Genet. 2019, 10, 38–46. [Google Scholar] [PubMed]
- Pasini, F.; Marzocchi, S.; Caboni, M. Whole Grain: An Open Issue. In Proceedings of the Global Summit on Food Science and Technology, Kuala Lumpur, Malaysia, 22–24 August 2024; pp. 1–2. [Google Scholar]
- Xiao, Y.; Ke, Y.; Wu, S.; Huang, S.; Li, S.; Lv, Z.; Yeoh, E.-K.; Lao, X.; Wong, S.; Kim, J.H.; et al. Association between Whole Grain Intake and Breast Cancer Risk: A Systematic Review and Meta-Analysis of Observational Studies. Nutr. J. 2018, 17, 87. [Google Scholar] [CrossRef] [PubMed]
- Pasko, P.; Barton, H.; Zagrodzki, P.; Izewska, A.; Krosniak, M.; Gawlik, M.; Gorinstein, S. Effect of Diet Supplemented with Quinoa Seeds on Oxidative Status in Plasma and Selected Tissues of High Fructose-Fed Rats. Plant Foods Hum. Nutr. 2010, 65, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.A.R.; Ali, M.U.; Chand, S.; Razzaq, M.; Latif, M.U.; Amjid, M.; Afzal, I. Anti-diabetic properties of Chenopodium quinoa on human health; an update and future prospects. One Health Triad 2023, 2, 168–175. [Google Scholar] [CrossRef]
- Kasozi, K.I.; Namubiru, S.; Safiriyu, A.A.; Ninsiima, H.I.; Nakimbugwe, D.; Namayanja, M.; Valladares, M.B. Grain amaranth is associated with improved hepatic and renal calcium metabolism in type 2 diabetes mellitus of male wistar rats. Evid.-Based Complement. Altern. Med. 2018, 2018, 4098942. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Liu, Y.; Yue, Y.; Qin, Y.; Li, Z. Dietary Tartary Buckwheat Intake Attenuates Insulin Resistance and Improves Lipid Profiles in Patients with Type 2 Diabetes: A Randomized Controlled Trial. Nutr. Res. 2016, 36, 1392–1401. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, Z.; Qin, F.; Qiu, J. Anti-Diabetic Effects of the Soluble Dietary Fiber from Tartary Buckwheat Bran in Diabetic Mice and Their Potential Mechanisms. Food Nutr. Res. 2021, 65. [Google Scholar] [CrossRef]
- Kyrø, C.; Tjønneland, A.; Overvad, K.; Olsen, A.; Landberg, R. Higher Whole-Grain Intake Is Associated with Lower Risk of Type 2 Diabetes among Middle-Aged Men and Women: The Danish Diet, Cancer, and Health Cohort. J. Nutr. 2018, 148, 1434–1444. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qiu, J.; Yue, Y.; Li, K.; Ren, G. Dietary Black-Grained Wheat Intake Improves Glycemic Control and Inflammatory Profile in Patients with Type 2 Diabetes: A Randomized Controlled Trial. Ther. Clin. Risk Manag. 2018, 14, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ding, M.; Sampson, L.; Willett, W.C.; Manson, J.E.; Wang, M.; Rosner, B.; Hu, F.B.; Su, Q. Intake of Whole Grain Foods and Risk of Type 2 Diabetes: Results from Three Prospective Cohort Studies. BMJ 2020, 370, m2206. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Wang, Z.; Park, J.H.; Ryu, O.H.; Choi, M.K.; Lee, J.Y.; Kang, Y.-H.; Lim, S.S. Anti-Diabetic Effect of Purple Corn Extract on C57BL/KsJ db/db Mice. Nutr. Res. Pract. 2015, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.L.; Zhang, G.W.; Mu, H.Y.; Zhang, H.; Chen, Y.Q. The Mixture of Corn and Wheat Peptide Prevent Diabetes in NOD Mice. J. Diabetes Res. 2019, 56, 163–170. [Google Scholar] [CrossRef]
- Hu, E.A.; Pan, A.; Malik, V.; Sun, Q. White Rice Consumption and Risk of Type 2 Diabetes: Meta-Analysis and Systematic Review. BMJ 2012, 344, e1454. [Google Scholar] [CrossRef]
- Seah, J.Y.H.; Koh, W.P.; Yuan, J.M.; van Dam, R.M. Rice Intake and Risk of Type 2 Diabetes: The Singapore Chinese Health Study. Eur. J. Nutr. 2019, 58, 3349–3360. [Google Scholar] [CrossRef] [PubMed]
- Nanri, A.; Mizoue, T.; Noda, M.; Takahashi, Y.; Kato, M.; Inoue, M.; Tsugane, S. Fish Intake and Type 2 Diabetes in Japanese Men and Women: The Japan Public Health Center–Based Prospective Study. Am. J. Clin. Nutr. 2010, 92, 168–1477. [Google Scholar] [CrossRef]
- Bhavadharini, B.; Mohan, V.; Dehghan, M.; Rangarajan, S.; Swaminathan, S.; Rosengren, A.; Wielgosz, A.; Avezum, A.; Lopez-Jaramillo, P.; Lanas, F.; et al. White Rice Intake and Incident Diabetes: A Study of 132,373 Participants in 21 Countries. Diabetes Care 2020, 43, 2643–2650. [Google Scholar] [CrossRef]
- Schadow, A.M.; Revheim, I.; Spielau, U.; Dierkes, J.; Schwingshackl, L.; Frank, J.; Rosendahl-Riise, H. The effect of regular consumption of reformulated breads on glycemic control: A systematic review and meta-analysis of randomized clinical trials. Adv. Nutr. 2023, 14, 30–43. [Google Scholar] [CrossRef]
- Milesi, G.; Rangan, A.; Grafenauer, S. Whole Grain Consumption and Inflammatory Markers: A Systematic Literature Review of Randomized Control Trials. Nutrients 2022, 14, 374. [Google Scholar] [CrossRef]
- Khan, J.; Gul, P.; Rashid, M.T.; Li, Q.; Liu, K. Composition of Whole Grain Dietary Fiber and Phenolics and Their Impact on Markers of Inflammation. Nutrients 2024, 16, 1047. [Google Scholar] [CrossRef]
- Kanata, M.C.; Yanni, A.E.; Koliaki, C.; Pateras, I.; Anastasiou, I.A.; Kokkinos, A.; Karathanos, V.T. Effects of Wheat Biscuits Enriched with Plant Proteins Incorporated into an Energy-Restricted Dietary Plan on Postprandial Metabolic Responses of Women with Overweight/Obesity. Nutrients 2024, 16, 1229. [Google Scholar] [CrossRef] [PubMed]
- Akhlaghi, M. The Role of Dietary Fibers in Regulating Appetite, an Overview of Mechanisms and Weight Consequences. Crit. Rev. Food Sci. Nutr. 2024, 64, 3139–3150. [Google Scholar]
- Weickert, M.O.; Pfeiffer, A.F. Impact of Dietary Fiber Consumption on Insulin Resistance and the Prevention of Type 2 Diabetes. J. Nutr. 2018, 148, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Lattimer, J.M.; Haub, M.D. Effects of Dietary Fiber and Its Components on Metabolic Health. Nutrients 2010, 2, 1266–1289. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, B.T.; Shin, J.; Kim, K.N. Combined Effect of Serum Alanine Aminotransferase and Gamma-Glutamyltransferase on Incidence of Diabetes Mellitus: A Longitudinal Study. Medicine 2020, 99, 11. [Google Scholar] [CrossRef]
- Fardet, A. New Hypotheses for the Health-Protective Mechanisms of Whole-Grain Cereals: What Is beyond Fibre? Nutr. Res. Rev. 2010, 23, 65–134. [Google Scholar] [CrossRef]
- De Angelis, M.; Montemurno, E.; Vannini, L.; Cosola, C.; Cavallo, N.; Gozzi, G.; Maranzano, V.; Di Cagno, R.; Gobbetti, M.; Gesualdo, L. Effect of Whole-Grain Barley on the Human Fecal Microbiota and Metabolome. Appl. Environ. Microbiol. 2015, 81, 7945–7956. [Google Scholar] [CrossRef]
- Adom, K.K.; Sorrells, M.E.; Liu, R.H. Phytochemicals and Antioxidant Activity of Milled Fractions of Different Wheat Varieties. J. Agric. Food Chem. 2005, 53, 2297–2306. [Google Scholar] [CrossRef]
- Belobrajdic, D.P.; Bird, A.R. The Potential Role of Phytochemicals in Wholegrain Cereals for the Prevention of Type-2 Diabetes. Nutr. J. 2013, 12, 62. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhao, X.; Ran, L.; Wan, J.; Wang, X.; Qin, J.; Shu, F.; Gao, Y.; Yuan, L.; Zhang, Q.; et al. Resveratrol Improves Insulin Resistance, Glucose and Lipid Metabolism in Patients with Non-Alcoholic Fatty Liver Disease: A Randomized Controlled Trial. Dig. Liver Dis. 2015, 47, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Bozzetto, L.; Annuzzi, G.; Pacini, G.; Costabile, G.; Vetrani, V.; Vitale, M.; Griffo, G.; Giacco, A.; De Natale, C.; Cocozza, S.; et al. Polyphenol-Rich Diets Improve Glucose Metabolism in People at High Cardiometabolic Risk: A Controlled Randomized Intervention Trial. Diabetologia 2015, 58, 1551–1560. [Google Scholar] [CrossRef]
- De Carvalho, F.G.; Ovídio, P.P.; Padovan, G.J.; Jordão Junior, A.A.; Marchini, J.S.; Navarro, A.M. Metabolic Parameters of Postmenopausal Women after Quinoa or Corn Flakes Intake–A Prospective and Double-Blind Study. Int. J. Food Sci. Nutr. 2014, 65, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Vilcacundo, R.; Miralles, B.; Carrillo, W.; Hernández-Ledesma, B. In Vitro Chemopreventive Properties of Peptides Released from Quinoa (Chenopodium quinoa Willd.) Protein under Simulated Gastrointestinal Digestion. Food Res. Int. 2018, 105, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; Lin, J.Y. Immune Cell–Conditioned Media Suppress Prostate Cancer PC-3 Cell Growth Correlating with Decreased Proinflammatory/Anti-Inflammatory Cytokine Ratios in the Media Using 5 Selected Crude Polysaccharides. Integr. Cancer Ther. 2016, 15, NP13–NP25. [Google Scholar] [CrossRef] [PubMed]
- Tomotake, H.; Yamamoto, N.; Yanaka, N.; Ohinata, H.; Yamazaki, R.; Kayashita, J.; Kato, N. High Protein Buckwheat Flour Suppresses Hypercholesterolemia in Rats and Gallstone Formation in Mice by Hypercholesterolemic Diet and Body Fat in Rats because of Its Low Protein Digestibility. Nutrition 2006, 22, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Kyrø, C.; Olsen, A.; Landberg, R.; Skeie, G.; Loft, S.; Åman, P.; Bueno-de-Mesquita, H.A. Plasma Alkylresorcinols, Biomarkers of Whole-Grain Wheat and Rye Intake, and Incidence of Colorectal Cancer. J. Natl. Cancer Inst. 2014, 106, djt352. [Google Scholar] [CrossRef]
- Buescher, M.I.; Gallaher, D.D. Wheat Color (Class), Not Refining, Influences Colon Cancer Risk in Rats. Nutr. Cancer 2014, 66, 849–856. [Google Scholar] [CrossRef]
- Dilworth, L.; Stennett, D.; Omoruyi, F. Cellular and molecular activities of IP6 in disease prevention and therapy. Biomolecules 2023, 13, 972. [Google Scholar] [CrossRef]
- Khatiwada, J.; Verghese, M.; Davis, S.; Williams, L.L. Green Tea, Phytic Acid, and Inositol in Combination Reduced the Incidence of Azoxymethane-Induced Colon Tumors in Fisher 344 Male Rats. J. Med. Food 2011, 14, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Sivagami, G.; Karthikkumar, V.; Balasubramanian, T.; Nalini, N. The Modulatory Influence of p-Methoxycinnamic Acid, an Active Rice Bran Phenolic Acid, against 1,2-Dimethylhydrazine-Induced Lipid Peroxidation, Antioxidant Status and Aberrant Crypt Foci in Rat Colon Carcinogenesis. Chem. Biol. Interact. 2012, 196, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Reynoso Guerrero-Villanueva, G.; de Dios Figueroa, J.; Gallegos-Corona, M.A.; Mendoza, S.; Loarca-Pina, G.; Ramos-Gomez, M. Anticarcinogenic Effect of Corn Tortilla against 1,2-Dimethylhydrazine (DMH)-Induced Colon Carcinogenesis in Sprague–Dawley Rats. Plant Foods Hum. Nutr. 2015, 70, 146–152. [Google Scholar]
- Long, N.; Suzuki, S.; Sato, S.; Naiki-Ito, A.; Sakatani, K.; Shirai, T.; Takahashi, S. Purple Corn Color Inhibition of Prostate Carcinogenesis by Targeting Cell Growth Pathways. Cancer Sci. 2013, 104, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yang, J.; Du, L.; Li, K.; Zhou, Y. Association of Whole Grain, Refined Grain, and Cereal Consumption with Gastric Cancer Risk: A Meta-Analysis of Observational Studies. Foods 2019, 7, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.M.; Rigi, S.; Shayanfar, M.; Mohammad-Shirazi, M.; Sharifi, G.; Esmaillzadeh, A. Refined Grains Consumption Is Associated with a Greater Odds of Glioma. Nutrients 2022, 25, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Hao, Y.; Richel, A.; Everaert, N.; Chen, Y.; Liu, M.; Yang, X.; Ren, G. Antihypertensive Effect of Quinoa Protein under Simulated Gastrointestinal Digestion and Peptide Characterization. J. Sci. Food Agric. 2020, 100, 5569–5576. [Google Scholar] [PubMed]
- Fillería, S.G.; Tironi, V. Intracellular Antioxidant Activity and Intestinal Absorption of Amaranth Peptides Released Using Simulated Gastrointestinal Digestion with Caco-2 TC7 Cells. Food Biosci. 2021, 41, 101086. [Google Scholar] [CrossRef]
- Oh, H.; Kim, H.; Lee, D.H.; Lee, A.; Giovannucci, E.L.; Kang, S.-S.; Keum, N. Different Dietary Fibre Sources and Risks of Colorectal Cancer and Adenoma: A Dose–Response Meta-Analysis of Prospective Studies. Br. J. Nutr. 2019, 122, 605–615. [Google Scholar] [CrossRef]
- Makarem, N.; Nicholson, J.M.; Bandera, E.V.; McKeown, N.M.; Parekh, N. Consumption of Whole Grains and Cereal Fiber in Relation to Cancer Risk: A Systematic Review of Longitudinal Studies. Nutr. Rev. 2016, 74, 353–373. [Google Scholar] [CrossRef]
- Shikata, K.; Ninomiya, T.; Kiyohara, Y. Diabetes Mellitus and Cancer Risk: Review of the Epidemiological Evidence. Cancer Sci. 2013, 104, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Pajari, A.M.; Freese, R.; Kariluoto, S.; Lampi, A.M.; Piironen, V. Bioactive Compounds in Whole Grains and Their Implications for Health. In Whole Grains Health; John Wiley & Sons: Hoboken, NJ, USA, 2021; pp. 301–336. [Google Scholar]

| Pseudo Cereals | |||||
|---|---|---|---|---|---|
| Whole Grains | Action Mechanism | Model | Doses | Key Findings | References |
| Quinoa | Antidiabetic | 24 rats; 5 weeks | quinoa seeds in high-fructose diet | ↓ Blood glucose, ↓ blood triglycerides, ↓ low density lipoprotein, ↓ total cholesterol | [164] |
| Antidiabetic | 55 male albino rats; 6 weeks | quinoa seeds powder | ↓ glucose levels, ↓ thyroid hormones | [165] | |
| Amaranth | Antidiabetic | 30 rats; 5 weeks | Amaranth grains | ↑ Calcium in the diet and improved calcium signaling in blood, kidney, and liver of diabetic rats. ↑ expression of the s100a1 calcium transport proteins | [166] |
| Antidiabetic | 25 CDI mice; 4 weeks | Amaranth protein hydrolyzate | ↑ plasma insulin | [17] | |
| Buckwheat | Antidiabetic | 165 Diabetic Individuals; 4 weeks | Buckwheat | ↓ level of serum glucose, ↓ fast insulin, ↓ total cholesterol, ↓ LDL cholesterol | [167] |
| Antidiabetic | 50 C57BL/6 mice; weeks | Buckwheat soluble dietary fibers | ↓ levels of fasting blood glucose, ↑ oral glucose tolerance, ↑ levels of liver glycogen and insulin, ↑ lipid profiles in both the serum and liver. | [168] | |
| Cereals | |||||
| Wheat | Antidiabetic | 57,053 men and women aged 50–65 | Whole grain Wheat | 16 g of whole wheat per day lower risk of type 2 diabetes and high whole grain intake may have more benefits | [169] |
| Antidiabetic | 120 patients of T2D | Black wheat grains intake (>69 g/d) for 5 weeks | ↑ glycemia and the inflammatory profile in T2D patients, ↓ glycated albumin, and prevented the increase in TNF-α and IL-6 levels | [170] | |
| Rice | Antidiabetic | 4,618,796 men and women | Brown rice | higher consumption of total whole grains and the most commonly consumed whole grain foods was significantly associated with a lower risk of type 2 diabetes | [171] |
| diabatic | 45,411 male and female aged 45–74, 25,666 men 33,622 women age 45–75 y, 132,373 individuals age 35–70 y, 13,284 cases, 2,352,384 participants | White rice | intake of white rice is associated with an increased risk of type 2 diabetes | [174,175,176,177] | |
| Corn | Antidiabetic | 4 groups mice; 8 week | Purple corn | ↑ insulin secretion ↑ AMPK activation in the liver, ↑ phosphorylation of activated protein kinase, ↓ phosphoenolpyruvate carboxykinase, ↓ glucose 6-phosphatase | [172] |
| Antidiabetic | 6 mice; 1–6 week | corn | ↓ serum level ↓ pancreatic gene expression of IL-6 and insulitis, ↑ pancreatic β-cell areas, pancreatic gene, ↑ expression of IL-10 serum levels of serine and histidine | [173] | |
| Pseudo Cereals | |||||
|---|---|---|---|---|---|
| Whole Grains | Action Mechanism | Model | Doses | Key Findings | References |
| Quinoa | Cancer | 35 females; 2 years | quinoa flakes daily | ↓ interleukin-6, which is a marker of inflammation, ↓ Tumor, ↓ total cholesterol↓ serum triglyceride | [192] |
| Colon cancer | In vitro gastrointestinadigestion model | Quinoa protein | Large peptides responsible for the colon cancer cell viability inhibitory activitySmaller peptides < 5 kDa with antiproliferative activity i and was the main responsible for the radical scavenging activity while peptides > 5 kDa showed greater anticancer effects. | [193] | |
| Amaranth | Cancer | Animal model | Amaranth protein and polyphenols | Inhibitory effect on tumor cell proliferation inhibition of histone acetylation | [16] |
| Breast cancer | Human breast cells | Amaranth | The results indicated that the digested sample was capable of inhibiting cell growth and found that amaranth may be a good source of bioactive peptides with good antioxidant activity and promising anticancer activity. | [80] | |
| Buckwheat | Prostate cancer | 10 weeks old mice | crude polysaccharides buckwheat | negative correlation between PC-3 cell viabilities and (interleukin [IL]-6 + tumor necrosis factor [TNF]-α)/IL-10 level ratios in the corresponding MCM, implying that macrophages suppress PC-3 cell growth through decreasing secretion ratios of proinflammatory/anti-inflammatory cytokines in a tumor microenvironment. | [194] |
| Colon cancer | Mice weight 70 g and 28 g | High protein buckwheat flour | Strong activities against cancer, buckwheat protein significantly inhibited the growth of an artificially induced tumor | [195] | |
| Cereals | |||||
| Wheat | Colorectal cancer | Human study 1372 colorectal cancer cases | Wheat phenolic acids | High concentrations of phenolic acids were associated with a lower incidence of distal colon cancer but not with overall colorectal cancer, proximal colon cancer, and rectal cancer. | [196] |
| Colon cancer | Mice | Whole wheat vs. refined wheat | Red wheat had significantly fewer colonic precancerous lesions than soft white-fed, while refined grains had no reduction risk. Oxygen radical and fecal bile acid concentration were higher than refined grains | [197] | |
| Rice | Cancer | Rat model of liver cancer | Rice bran | ↑ glutathione-S-transferase activity, ↓ lipid peroxidation ↓ level of placental glutathione-S-transferase -positive foci, a marker of hepato-carcinogenesis, ↓ number of colon tumors | [198,199] |
| Colorectal cancer | Rat model of colorectal cancer | Rice bran phytochemicals | Reverse the effect of chemically-induced colorectal cancer in rats, by reducing level of lipid peroxidation and protein oxidation in liver, ↑ activity of superoxide dismutase, catalase and glutathione peroxidase↑ glutathione, vitamin E and vitamin C levels↓ number of aberrant crypt foci and colon tumors | [200] | |
| Corn | Cancer | 4–5 wk, male rats | corns | inhibition of β-glucuronidase activity, and induction of detoxifying enzymes in liver and colon, as well as a decrease in the expression of the two most important proliferative proteins (K-ras and β-catenin) involved in colon carcinogenesis | [201] |
| prostate cancer | 36 rats; 8 weeks | Purple corn | ↓ incidence of adenocarcinoma in the lateral prostate and slowed down the progression of prostate cancer.↓ expression cyclin-dependent kinases, downregulation of the activation growth factor and cytokines | [202] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, J.; Gul, P.; Liu, K. Grains in a Modern Time: A Comprehensive Review of Compositions and Understanding Their Role in Type 2 Diabetes and Cancer. Foods 2024, 13, 2112. https://doi.org/10.3390/foods13132112
Khan J, Gul P, Liu K. Grains in a Modern Time: A Comprehensive Review of Compositions and Understanding Their Role in Type 2 Diabetes and Cancer. Foods. 2024; 13(13):2112. https://doi.org/10.3390/foods13132112
Chicago/Turabian StyleKhan, Jabir, Palwasha Gul, and Kunlun Liu. 2024. "Grains in a Modern Time: A Comprehensive Review of Compositions and Understanding Their Role in Type 2 Diabetes and Cancer" Foods 13, no. 13: 2112. https://doi.org/10.3390/foods13132112
APA StyleKhan, J., Gul, P., & Liu, K. (2024). Grains in a Modern Time: A Comprehensive Review of Compositions and Understanding Their Role in Type 2 Diabetes and Cancer. Foods, 13(13), 2112. https://doi.org/10.3390/foods13132112







