Advances in the Preparation, Structure and Bioactivity of Polysaccharides from Lycium ruthenicum Murr.: A Review
Abstract
1. Introduction
2. Extraction and Purification
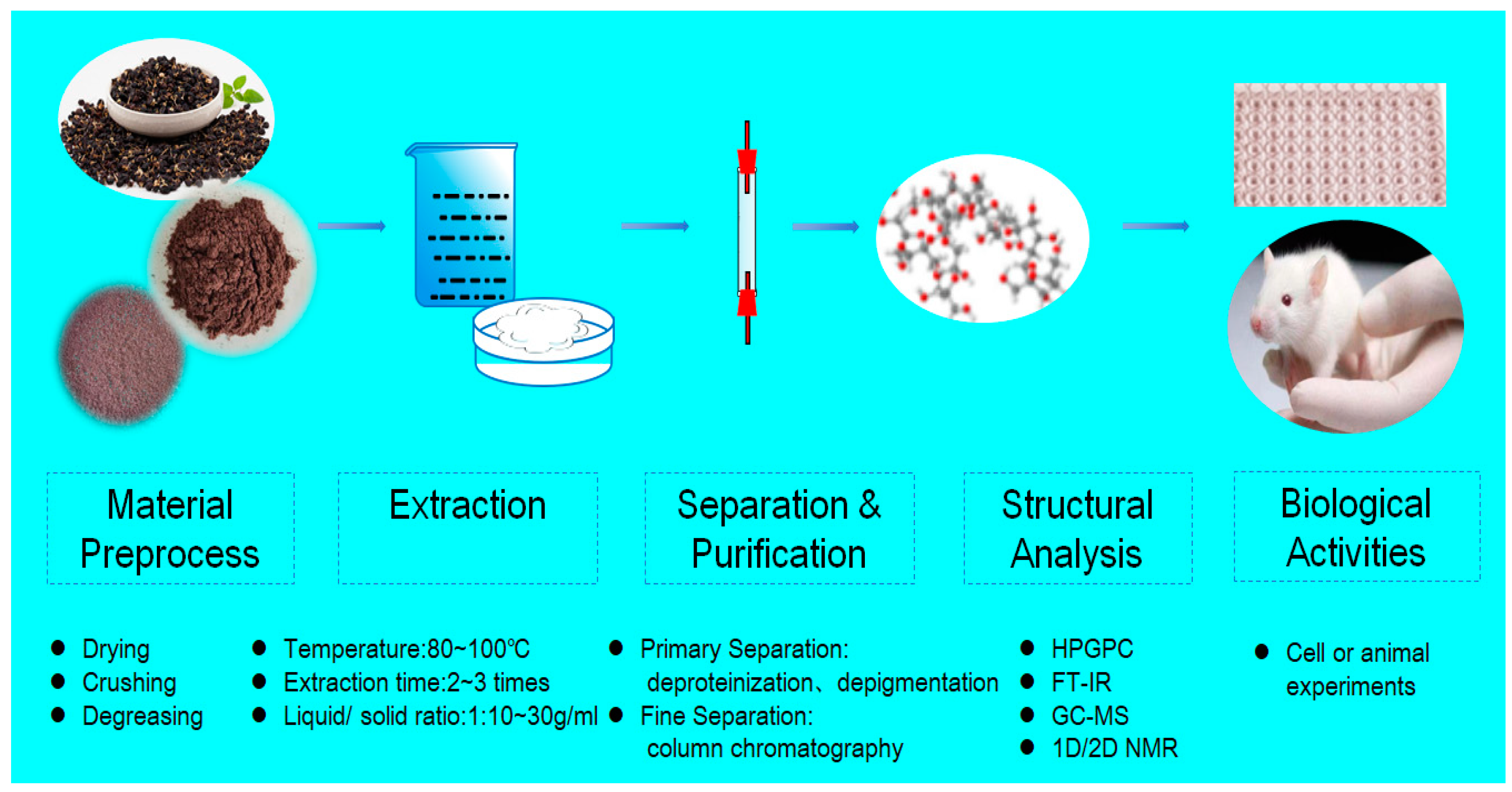
2.1. Extraction of Lycium ruthenicum Murr. Polysaccharides
2.2. Purification of LRPS
3. Structural Characteristics
3.1. The Molecular Weight of LRPS
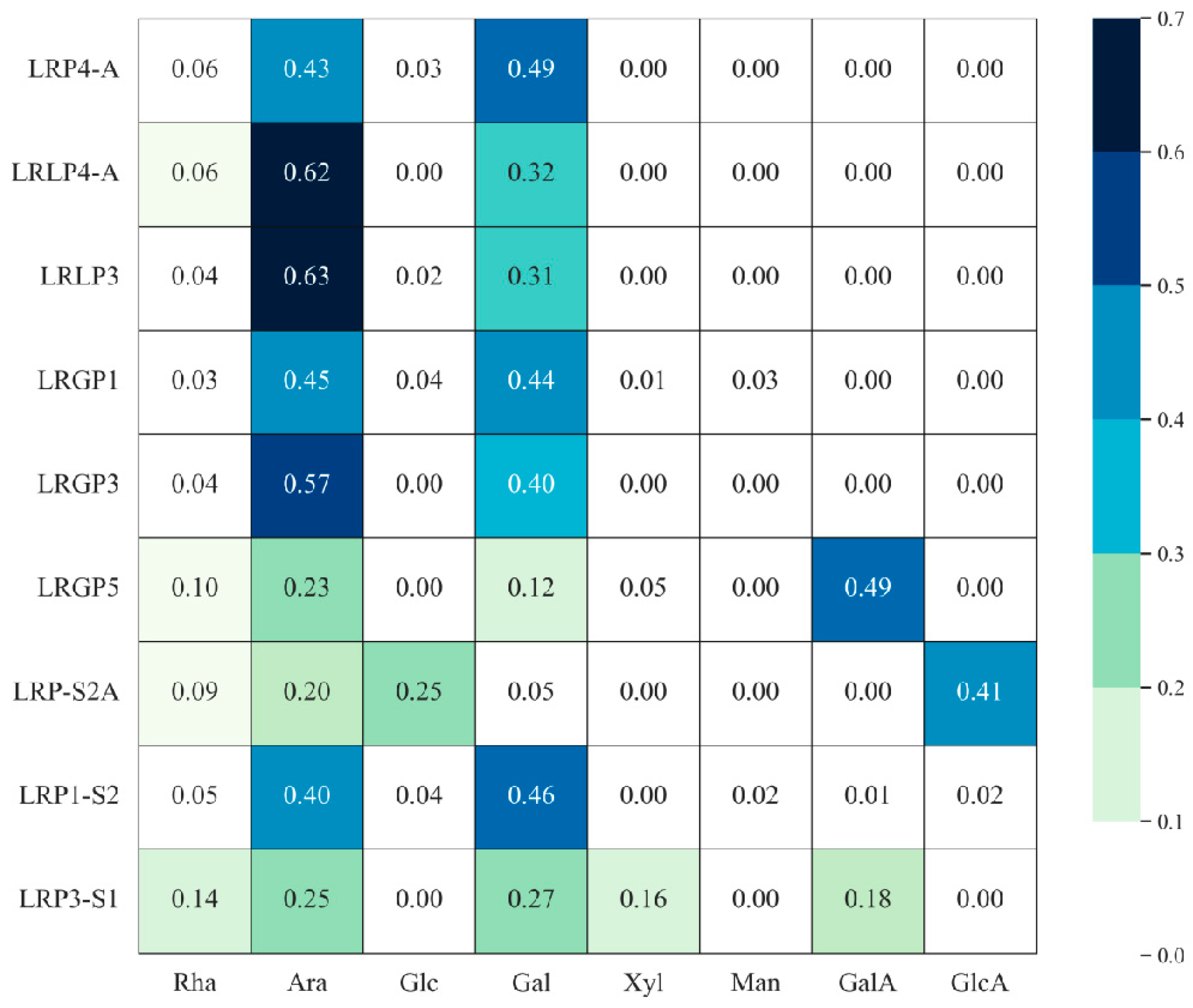
3.2. The Monosaccharide Composition of LRPS
3.3. The Primary Structure of LRPS
| Number | Designation of Polysaccharides | Separation and Purification Methods | Molecular Weight (kDa) | Monosaccharide Composition | Glycosidic Linkage Pattern | Branch Point | References |
|---|---|---|---|---|---|---|---|
| 1 | LRP4-A | DEAE-Cellulose-52 (0.5 M NaHCO3) Sephadex G-100 (0.1 M NaCl) | 105 | Rha:Ara:Glc:Gal =1:7.6:0.5:8.6 | →6)-Galp-(1→ | O-3 of Gal | [30] |
| 2 | LRLP4-A | DEAE-Cellulose-52 (0.5 M NaHCO3) Sephadex G-100 (0.1 M NaCl) | 135 | Rha:Ara:Gal =1:10.3:5.3 | →6)-β-Galp-(1→ | O-3 of Ara or Gal | [37] |
| 3 | LRLP3 | DEAE-Cellulose-52 (0.1 M NaHCO3) Sephadex G-100 (0.1 M NaCl) | 79.4 | Ara:Gal:Rha:Glc =2:1:0.12:0.06 | →3)-β-Galp-(1→ | / | [38] |
| 4 | LRGP1 | DEAE-Cellulose-52 (0.05 M NaHCO3) Sephadex G-100 (0.1 M NaCl) | 56.2 | Rha:Ara:Xyl:Man:Glc:Gal =0.65:10.71:0.33:0.67:1:10.41 | →3)-Galp-(1→ | O-6 of Ara and Gal | [33] |
| 5 | LRGP3 | DEAE-Cellulose-52 (0.15 M NaHCO3) Sephadex G-100 (0.1 M NaCl) | 75.6 | Rha:Ara:Gal =1.0:14.9:10.4 | →3)-β-D-Galp-(1→ | O-6 of Gal or Ara | [12,34] |
| 6 | LRGP5 | DEAE-Cellulose-52 (0.5 M NaHCO3) Sephadex G-100 (0.1 M NaCl) | 137 | Rha:Ara:Xyl:Gal:GalA =1.0:2.2:0.5:1.2:4.7 | →4)-GalA-(1→ →1)-Rha-(→2 | O-4 of Rha | [35] |
| 7 | LRP-S2A | DEAE Sepharose Fast Flow Sephacryl S-500 HR | 2650 | Rha:Ara:Gal:Glc:GlcA =1.00:2.07:0.57:2.59:4.33 | →4)-6-O-α-D-GlcpA-(1→ →4)-2-O-acetyl-α-D-Glcp-(1→ →2,4)-α-L-Rhap-(1→ →4)-β-D-GlcpA-(1→ | / | [11] |
| 8 | LRP1-S2 | DEAE Sepharose Fast Flow Sephacryl S-100 HR | 17.0 | Gal:Ara:Rha:Glc:GlcA: Man:GalA =46.2:40.2:5.1:4.0:2.3:1.7:0.5 | →3)-β-D-Galp-(1→ →6)-β-D-Galp-(1→ →3,6)-β-D-Galp-(1→ →3)-β-D-Manp-(1→ | / | [36] |
| 9 | LRP3-S1 | DEAE Sepharose Fast Flow (0.2 M NaCl) Sephacryl S-300 HR (0.2 M NaCl) | 114.8 | Rha:GalA:Gal:Xyl:Ara =14.4:17.7:26.6:16.4:24.9 | →2)-α-L-Rhap-(1→ →4)-α-D-GalA-(1→ | / | [39] |
4. Bioactivities
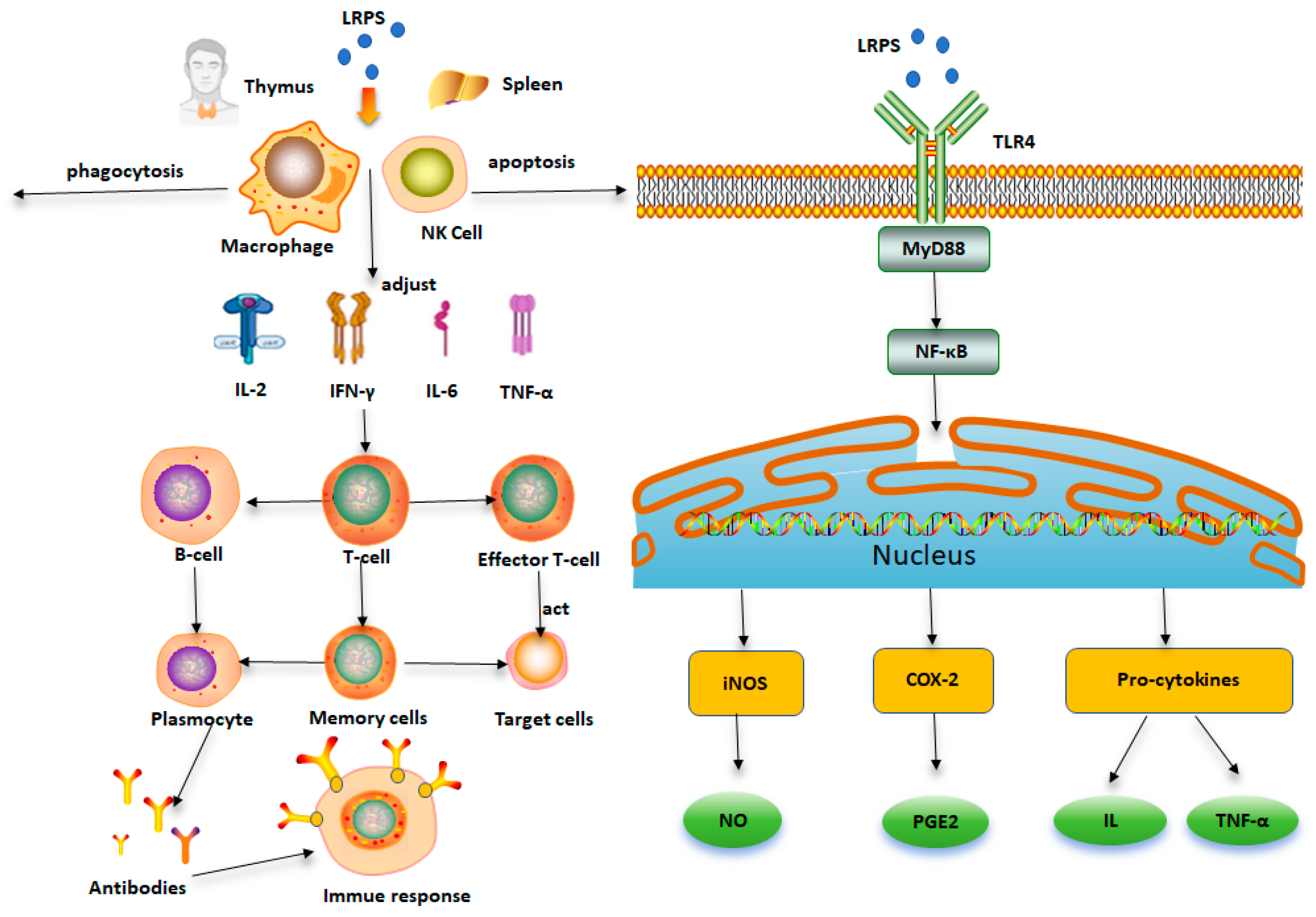
4.1. Immunomodulatory Activity
4.2. Antioxidant Activity
4.3. Anti-Osteoporosis Activity
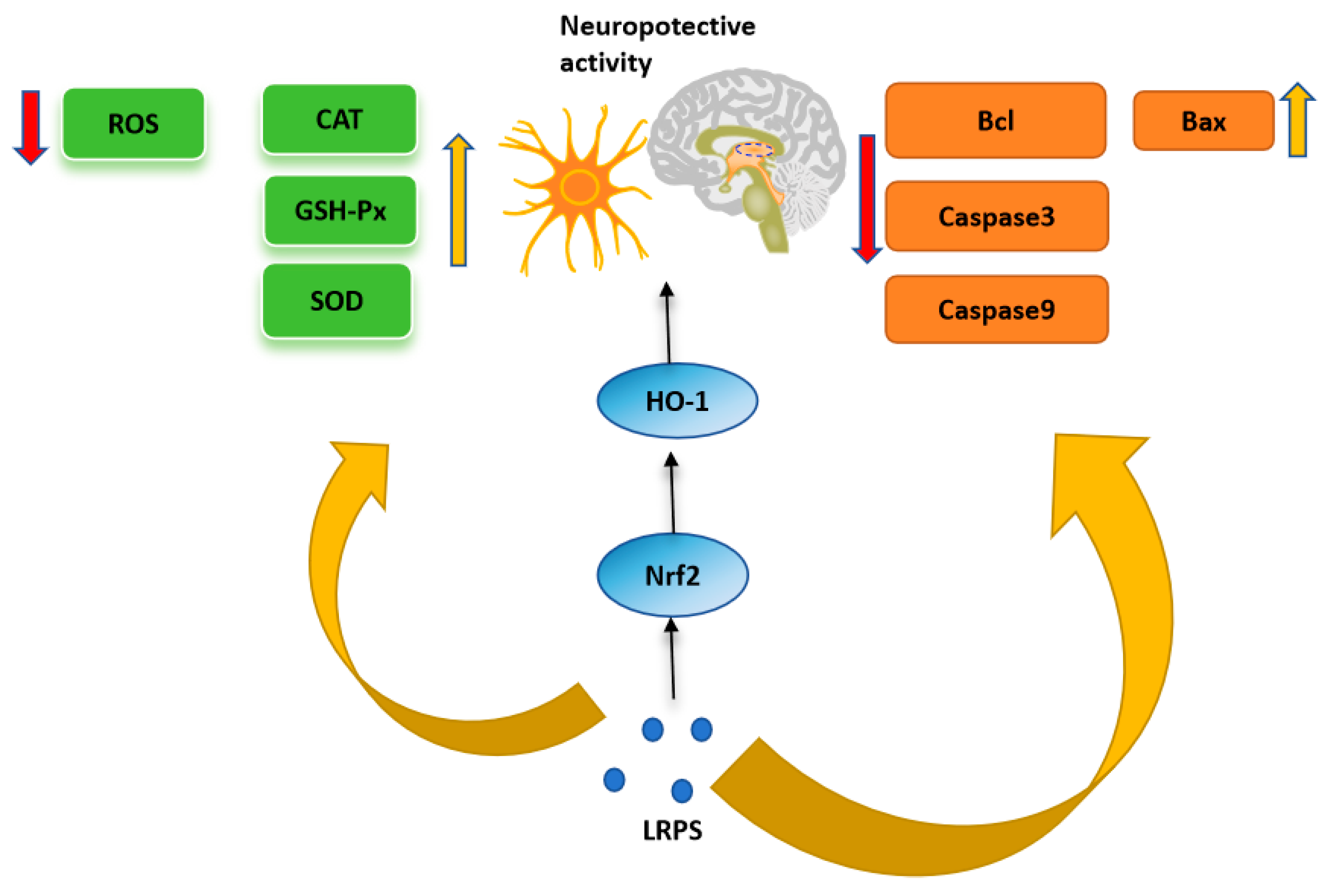
4.4. Neuroprotective Activity
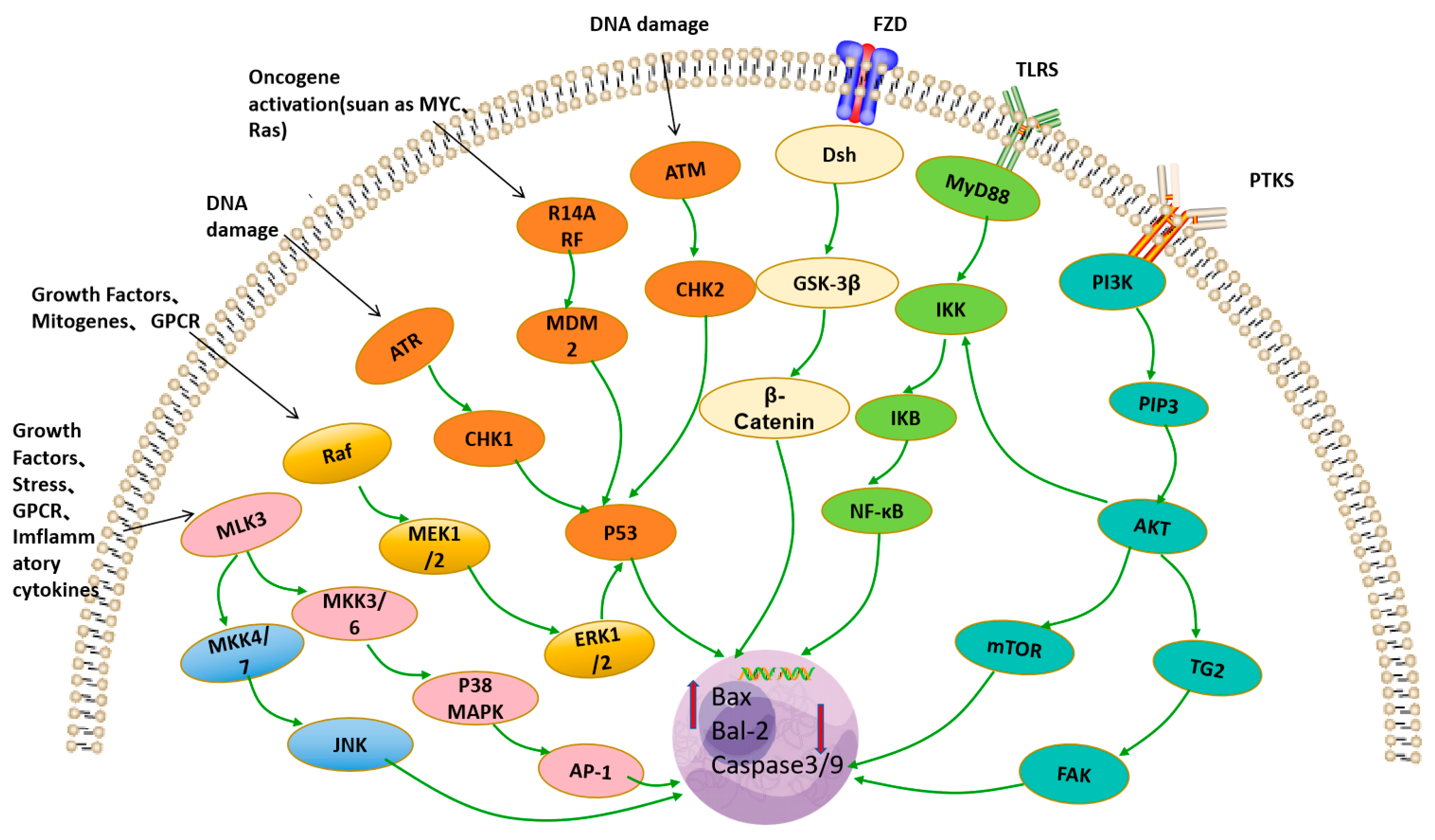
4.5. Anti-Tumor Activity
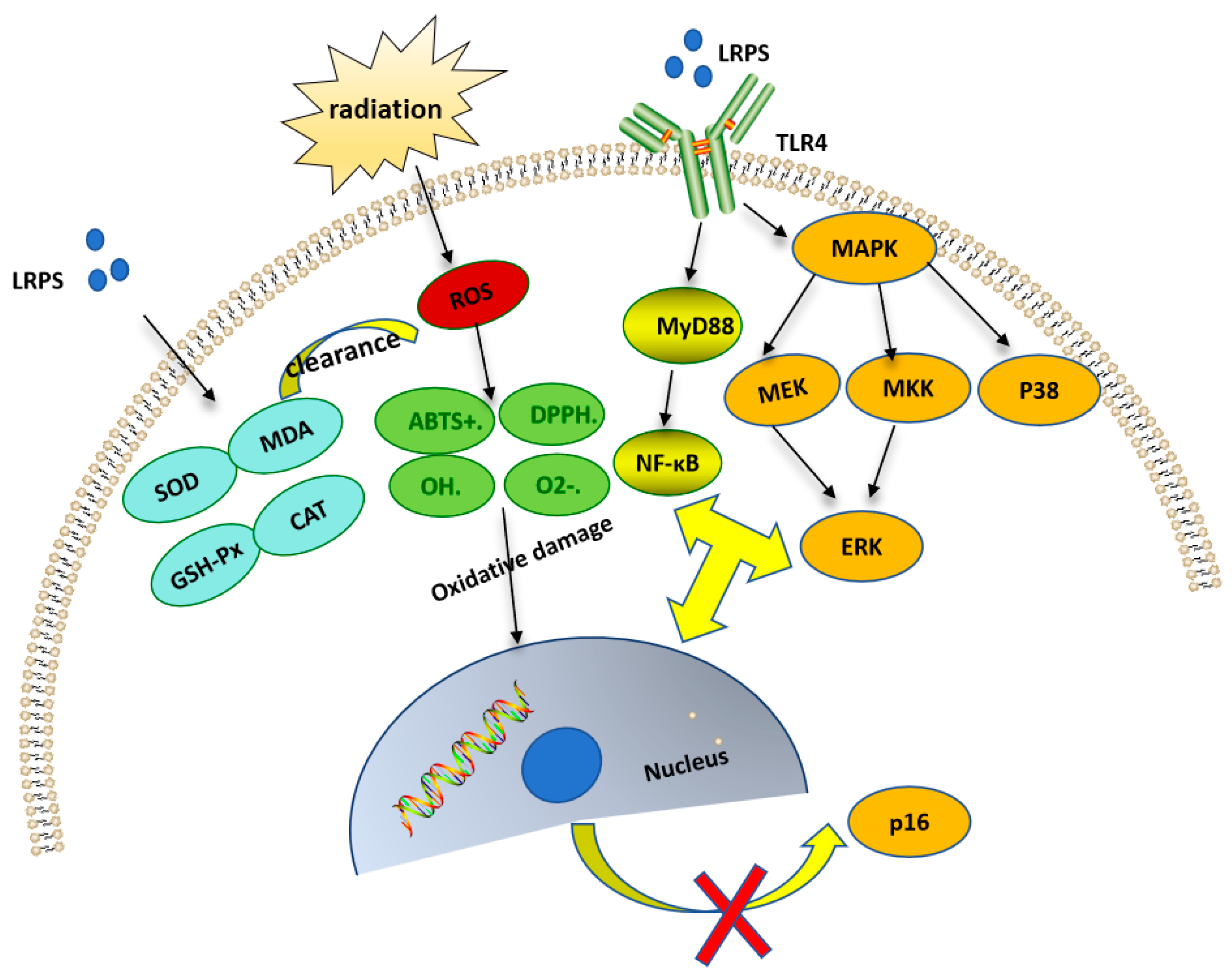
4.6. Anti-Radiation Activity
4.7. Anti-Fatigue Activity
4.8. Hypoglycemic Activity
4.9. Hepatoprotective Activity
4.10. Prebiotic Activity
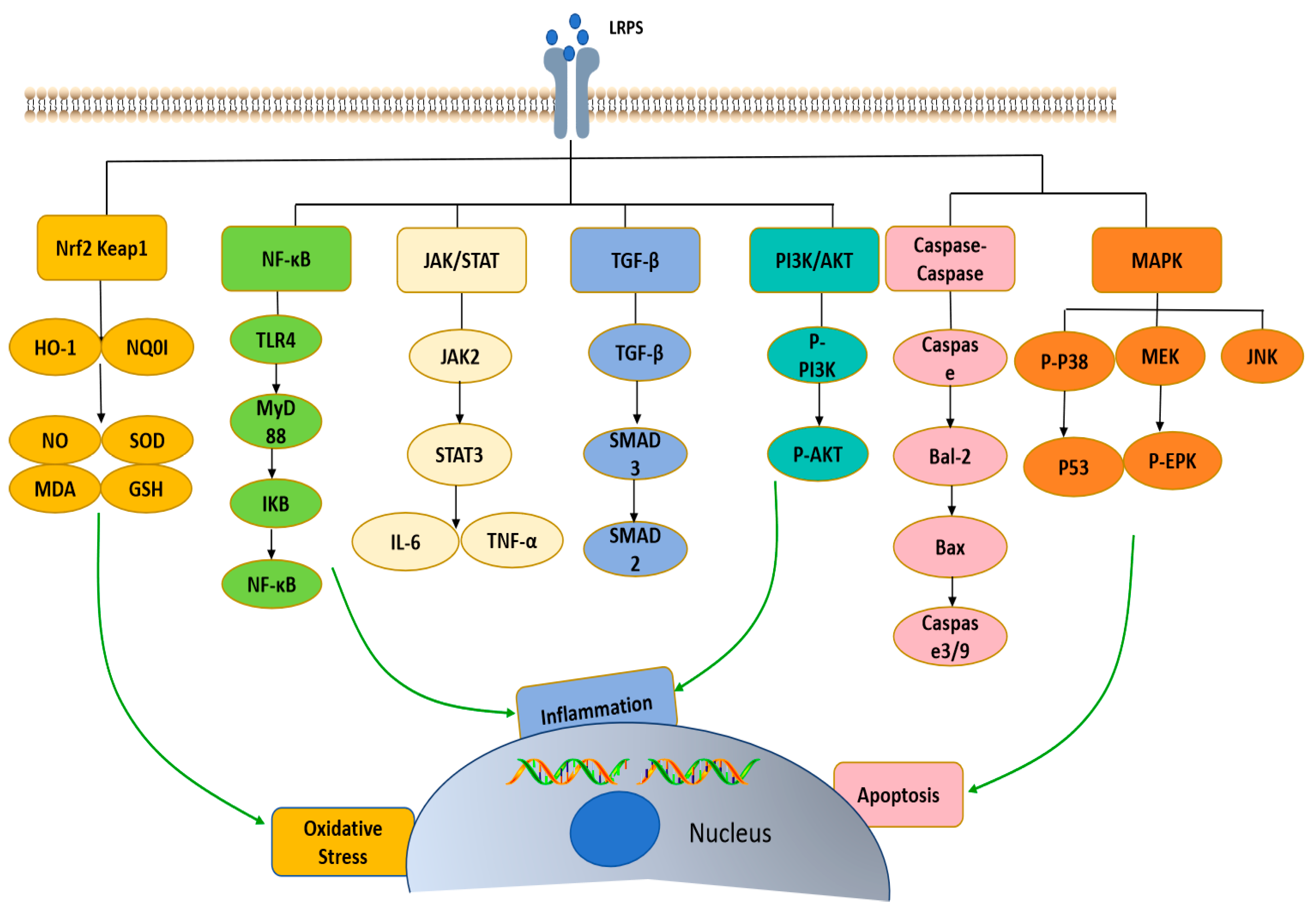
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gan, Q.M.; Zuo, Z.C.; Chang, Y.P. Research on the authentication and pharmacognostics of Tibetan medicine “Pang Ma”. Chin. J. Ethnomed. Ethnopharm. 1995, 1, 31–33. [Google Scholar]
- Dong, Y.H.; Hu, W.Z.; Lian, J.H. Research progress on active ingredients and pharmacological effects of Lycium ruthenicum. Guangdong Chem. Ind. 2020, 23, 48–54. [Google Scholar] [CrossRef]
- PengMao, D.J.; Jia, H.P.; Hu, Y. Research progress on extraction, purification, chemical composition, and functional activities of Lycium ruthenicum polysaccharides. Grain Oil 2023, 12, 34–43. [Google Scholar]
- Zhang, G.; Chen, S.S.; Zhou, W. Verification and research progress on the functions of Lycium ruthenicum. West China J. Pharm. Sci. 2019, 34, 638–642. [Google Scholar] [CrossRef]
- Ma, L.J.; Huo, P.C.; Sun, M.R. Research progress on chemical components and pharmacological activities of Lycium ruthenicum. Chin. Tradit. Herb. Drugs. 2020, 22, 5884–5893. [Google Scholar] [CrossRef]
- Tan, Y.Y.; Aikesahan, W.; Yizaetiguli, W.L. Protective effect of polysaccharides from Lycium ruthenicum on H2O2-induced ARPE-19 cell injury. Chin. J. Tradit. Chin. Med. 2022, 37, 2289–2294. [Google Scholar]
- Wang, J.H.; Chen, X.Q.; Zhang, W.J. Study on anti-fatigue bioactivity and mechanism of polysaccharides from Lycium ruthenicum Fruit. Food Sci. 2009, 34, 203–207. [Google Scholar] [CrossRef]
- Xing, L.J.; Zhang, X.L.; Wang, Y. Research progress on the anti-tumor effects of Lycium ruthenicum. Agric. Prod. Process. 2022, 3, 73–76. [Google Scholar] [CrossRef]
- Wang, R.Z.; Ren, L.C.; Jia, Y.E.; Yan, H.L. Effect of polysaccharides from Lycium ruthenicum on proliferative activity and P16 protein expression of HaCaT Cells induced by medium wave ultraviolet. J. Plateau Med. Biol. 2018, 3, 171–174. [Google Scholar] [CrossRef]
- Wang, J.H.; Chen, X.Q.; Zhang, W.J. Study on the hypoglycemic bioactivity and mechanism of polysaccharides from Lycium ruthenicum Fruit. Food Sci. 2009, 5, 244–248. [Google Scholar] [CrossRef]
- Wang, S.Q.; Liu, B.; Su, L. Structural features of an acidic polysaccharide with the potential of promoting osteoblast differentiation from Lycium ruthenicum Murr. Nat. Prod. Res. 2018, 16, 2249–2254. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Liu, H.; Shi, S. Lycium ruthenicum polysaccharide attenuates inflammation through inhibiting TLR4/NF-κB signaling pathway. Int. J. Biol. Macromol. 2014, 67, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.; Li, Y.; Xiao, M. Lycium ruthenicum Murr polysaccharide protects cortical neurons against oxygen-glucose deprivation/reperfusion in neonatal hypoxic-ischemic encephalopathy. Int. J. Biol. Macromol. 2020, 158, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ye, W.J.; Wu, H.J. Study on the hepatoprotective effect of polysaccharides from Lycium ruthenicum in rats. Food Ind. Sci. Technol. 2020, 41, 287–290. [Google Scholar] [CrossRef]
- Wang, Y. Study on the Isolation, Purification, and Immunomodulatory Mechanism of Lycium Polysaccharides Based on Regulation of Intestinal Flora. Ph.D. Thesis, Beijing University of Chinese Medicine, Beijing, China, 2020. [Google Scholar] [CrossRef]
- Qian, R.; An, J.X.; Xu, X.Q. Structural characterization of polysaccharides from Lycium ruthenicum and their effect on ethanol-induced gastric mucosal epithelial cell injury. Food Sci. 2024, 45, 109–115. [Google Scholar] [CrossRef]
- Shen, J.L. Study on the Extraction, Purification, Antioxidant Activity, and In Vitro Simulated Digestion and Fermentation of Polysaccharides from Lycium ruthenicum. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2017. [Google Scholar]
- Lu, X.J.; Feng, Y.B.; Chen, X.R. Optimization of extraction process of polysaccharides from Lycium ruthenicum by response surface methodology. China Brew. 2013, 32, 79–83. [Google Scholar] [CrossRef]
- Lou, T.T.; Jin, L.; Tuo, Y.L. Optimization of extraction process and colorimetric determination of polysaccharides from Lycium ruthenicum. Asia-Pacific Tradit. Med. 2017, 13, 16–20. [Google Scholar] [CrossRef]
- Cao, J.L. Optimization of ultrasonic-assisted extraction process of Lycium polysaccharides by response surface methodology. Anhui Chem. Ind. 2023, 49, 105–109. [Google Scholar] [CrossRef]
- Wu, C.Y.; Wang, H.B.; Li, W.F.; Bai, H.J. Study on the extraction process of polysaccharides from Black Goji Leaves. Food Res. Dev. 2009, 12, 1–5. [Google Scholar] [CrossRef]
- Xu, F.L.; Wang, D.F.; Liu, Z.B. Optimization of microwave-assisted extraction conditions for polysaccharides from Lycium ruthenicum. J. Shangluo Univ. 2023, 37, 69–74. [Google Scholar] [CrossRef]
- Wang, H.B.; Bai, H.J.; Wang, J.L. Study on microwave-assisted extraction of polysaccharides from Lycium ruthenicum by ultrasonic-microwave synergistic extraction method. J. Northwest A&F Univ. 2007, 16, 157–175. [Google Scholar] [CrossRef]
- Mei, L.; Shu, Y.; Fu, X.H. Study on enzyme-assisted extraction process of polysaccharides from Lycium ruthenicum in Haixi and its in vitro antioxidant activity analysis. J. Xichang Univ. Nat. Sci. Ed. 2021, 35, 15–22. [Google Scholar] [CrossRef]
- Yuan, X.T.; Qiu, X.Y.; Liu, Z.H.; Yin, J.; Han, N. Study on the enzyme-assisted ultrasonic extraction process of polysaccharides from Lycium ruthenicum and its hepatoprotective activity. J. Shenyang Pharm. Univ. 2023, 40, 1366–1374. [Google Scholar]
- Jin, X.Y.; Huang, L.P.; Wang, H.M. Optimization of high hydrostatic pressure-assisted extraction of polysaccharides from Lycium ruthenicum Murr. by response surface methodology and their antioxidant activity. J. Biobased Mater. Bioenergy 2022, 16, 715–720. [Google Scholar] [CrossRef]
- Shi, L. Bioactivities, isolation, and purification methods of polysaccharides from natural products: A Review. Int. J. Biol. Macromol. 2016, 92, 37–48. [Google Scholar] [CrossRef]
- Jin, M.; Huang, Q.; Zhao, K. Biological activities and potential health benefit effects of polysaccharides isolated from Lycium barbarum L. Int. J. Biol. Macromol. 2013, 54, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.L.; Cheng, Y.Q.; Tian, J.Y.; Zhang, S.Q.; Jing, Y.S.; Shi, M.M. Structural characterization of polysaccharide from jujube (Ziziphus jujuba Mill.) fruit. Chem. Biol. Technol. Agric. 2021, 54, 1–7. [Google Scholar] [CrossRef]
- Lv, X.; Wang, C.; Cheng, Y. Isolation and structural characterization of a polysaccharide LRP4-A from Lycium ruthenicum Murr. Carbohydr. Res. 2013, 365, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.L.; Peng, B.X.; Ding, H.H.; Cui, B.B.; Nie, H.; Yan, Y.Z. Purification, structure and biological activity of pumpkin polysaccharides: A review. Food Rev. Int. 2023, 39, 307–319. [Google Scholar] [CrossRef]
- Ji, X.L.; Guo, J.H.; Tian, J.Y.; Ma, K.; Liu, Y.Q. Research progress on degradation methods and product properties of plant polysaccharides. J. Light Ind. 2023, 38, 55–62. [Google Scholar] [CrossRef]
- Peng, Q.; Lv, X.; Xu, Q. Isolation and structural characterization of the polysaccharide LRGP1 from Lycium ruthenicum. Carbohydr. Polym. 2012, 90, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Song, J.; Lv, X. Structural characterization of an arabinogalactan-protein from the fruits of Lycium ruthenicum. J. Agric. Food Chem. 2012, 60, 9424. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Xu, Q.; Yin, H. Characterization of an immunologically active pectin from the fruits of Lycium ruthenicum. Int. J. Biol. Macromol. 2014, 64, 69–75. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Zhang, S.; Li, Y. The structure elucidation of novel arabinogalactan LRP1-S2 against pancreatic cancer cells growth in vitro and in vivo. Carbohydr. Polym. 2021, 267, 118172. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gong, G.; Sun, Y. Isolation, structural characterization, and immunological activity of a polysaccharide LRLP4-A from the leaves of Lycium ruthenicum. J. Carbohydr. Chem. 2016, 35, 40–56. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, L.; Gong, G.P. Structure, antioxidant activity, and immunological activity of polysaccharide LRLP3 from Lycium ruthenicum leaves. J. Chem. Educ. 2016, 37, 261–268. [Google Scholar] [CrossRef]
- Zhang, S.; He, F.; Chen, X. Isolation and structural characterization of a pectin from Lycium ruthenicum Murr and its anti-pancreatic ductal adenocarcinoma cell activity. Carbohydr. Polym. 2019, 223, 115104. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yan, B.; Wang, Z. Natural polysaccharides with immunomodulatory activities. Mini Rev. Med. Chem. 2020, 20, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.Y.K.; Liu, C.; Koon, J.C.M. Polysaccharide biological response modifiers. Immunol. Lett. 2006, 105, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.R.S.; Feitosa, J.P.A.; Freitas, A.L.P. Isolation and characterization of soluble sulfated polysaccharide from the Red Seaweed Gracilaria cornea. Carbohydr. Polym. 2002, 49, 491–498. [Google Scholar] [CrossRef]
- Peng, Q.; Liu, H.; Lei, H. Relationship between structure and immunological activity of an arabinogalactan from Lycium ruthenicum. Food Chem. 2016, 194, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, W.; Tang, Z. Research progress on the preventive and therapeutic effects of phycocyanin on diseases related to oxidative stress. Mar. Sci. 2017, 10, 132–138. [Google Scholar] [CrossRef]
- Hybertson, B.M.; Gao, B.; Bose, S.K. Oxidative stress in health and disease: The therapeutic potential of Nrf2 activation. Book Chapter 2011, 4–6, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Dang, J.; Wang, Q. Optimization of polysaccharides from Lycium ruthenicum fruit using RSM and its anti-oxidant activity. Int. J. Biol. Macromol. 2013, 61, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Zhao, L. Research on the antioxidant activity of functional components in Lycium ruthenicum and their protective effects on mitochondria. Food Ind. Sci. Technol. 2014, 35, 162–175. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, Z.; He, J. Extraction and antioxidant analysis of polysaccharides from Jingning Lycium ruthenicum. Food Ind. Sci. Technol. 2019, 40, 196–202. [Google Scholar] [CrossRef]
- Mirzadeh, M.; Arianejad, M.R.; Khedmat, L. Antioxidant, antiradical, and antimicrobial activities of polysaccharides obtained by microwave-assisted extraction method: A review. Carbohydr. Polym. 2020, 229, 115421. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Du, D.; Hua, J. Research on the isolation, purification, and antioxidant activity of polysaccharides from Lycium ruthenicum. Food Ind. 2016, 37, 134–137. [Google Scholar]
- Xu, Y.; Zhu, Y.; Xue, Y. Extraction of polysaccharides from Lycium ruthenicum and their effects on the growth characteristics and antioxidant capacity of Lactobacillus L9 and Thermophilic Streptococcus G2. Food Ferment. Ind. 2021, 47, 179–185. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, X.; Wen, H. Analysis of antioxidant components and antioxidant capacity of Lycium ruthenicum. Chin. J. Hosp. Pharm. 2011, 31, 1305–1306. [Google Scholar]
- Cui, X.; Cao, J.; Zhou, H. Effects of Lycium ruthenicum seeds on the expression of MAPK signaling pathway proteins in skeletal muscle of rats with excessive training and their ability to resist oxidative stress damage. Chin. J. Exp. Formulas 2017, 23, 122–127. [Google Scholar] [CrossRef]
- Arceo-Mendoza, R.M.; Camacho, P.M. Postmenopausal osteoporosis: Latest guidelines. Endocrinol. Metab. Clin. N. Am. 2021, 50, 167. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Mu, Y. Insulin-like growth factor and neurodegenerative diseases. Clin. Med. 2023, 32, 603–606. [Google Scholar] [CrossRef]
- Gao, Q.H.; Fu, X.; Zhang, R. Neuroprotective effects of plant polysaccharides: A review of the mechanisms. Int. J. Biol. Macromol. 2018, 106, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Shen, X.; Liu, Y. Radioprotective effects of Sipunculus nudus L. polysaccharide combined with WR-2721, rhIL-11 and rhG-CSF on radiation-injured mice. J. Radiat. Res. 2015, 56, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xue, C.; Mao, X. Radioprotective effects and mechanisms of animal, plant and microbial polysaccharides. Int. J. Biol. Macromol. 2020, 153, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Yao, X.; Wang, C. Study on the protective effect of Lycium ruthenicum on X-ray irradiated mice. Nat. Prod. Res. Dev. 2015, 27, 148–152. [Google Scholar] [CrossRef]
- Ni, B.; Li, X.; Zhang, C. Research progress on the molecular mechanism of the anti-fatigue activity of natural substances. Food Res. Dev. 2023, 44, 208–214. [Google Scholar] [CrossRef]
- Ni, W.; Gao, T.; Wang, H. Anti-fatigue activity of polysaccharides from the fruits of four Tibetan plateau indigenous medicinal plants. J. Ethnopharmacol. 2013, 150, 529–535. [Google Scholar] [CrossRef]
- Kavvoura, F.K.; Owen, K.R. Diagnosis and management of monogenic diabetes. Medicine 2022, 50, 632–637. [Google Scholar] [CrossRef]
- Ji, X.L.; Guo, J.H.; Cao, T.Z.; Zhang, T.T.; Liu, Y.Q.; Yan, Y.Z. Review on mechanisms and structure-activity relationship of hypoglycemic effects of polysaccharides from natural resources. Food Sci. Hum. Wellness. 2023, 12, 1969–1980. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Anti-diabetic effects and mechanisms of dietary polysaccharides. Molecules 2019, 24, 2556. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yan, H.T.; Che, J.X. Effects of neurolytic celiac plexus block on liver regeneration in rats with partial hepatectomy. Med. J. Natl. Defend. Forces Southwest China 2013, 8, e73101. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Huang, P.; Zhang, L. Hepatoprotective effect of plant polysaccharides from natural resources: A review of the mechanisms and structure-activity relationship. Int. J. Biol. Macromol. 2020, 161, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Luo, C.M.; Chen, J.Q. Differential expression of NF-κB signaling pathway in obstructive jaundice and exercise with intervention of Lycium ruthenicum Polysaccharide in mice. China Sport Sci. Technol. 2017, 53, 119–137. [Google Scholar] [CrossRef]
- Song, Q.; Wang, Y.; Huang, L. Review of the relationships among polysaccharides, gut microbiota, and human health. Food Res. Int. 2020, 140, 109858. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.L.; Hou, C.Y.; Gao, Y.G.; Xue, Y.Q.; Yan, Y.Z.; Guo, X.D. Metagenomic analysis of gut microbiota modulatory effects of jujube (Ziziphus jujuba Mill.) polysaccharides in a colorectal cancer mouse model. Food Funct. 2020, 11, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lin, Z.Y.; Pang, X.T. Study on the regulation of Lycium ruthenicum polysaccharide on intestinal microbiota of humanized mice. Anhui Agric. Sci. 2020, 48, 178–180. [Google Scholar] [CrossRef]

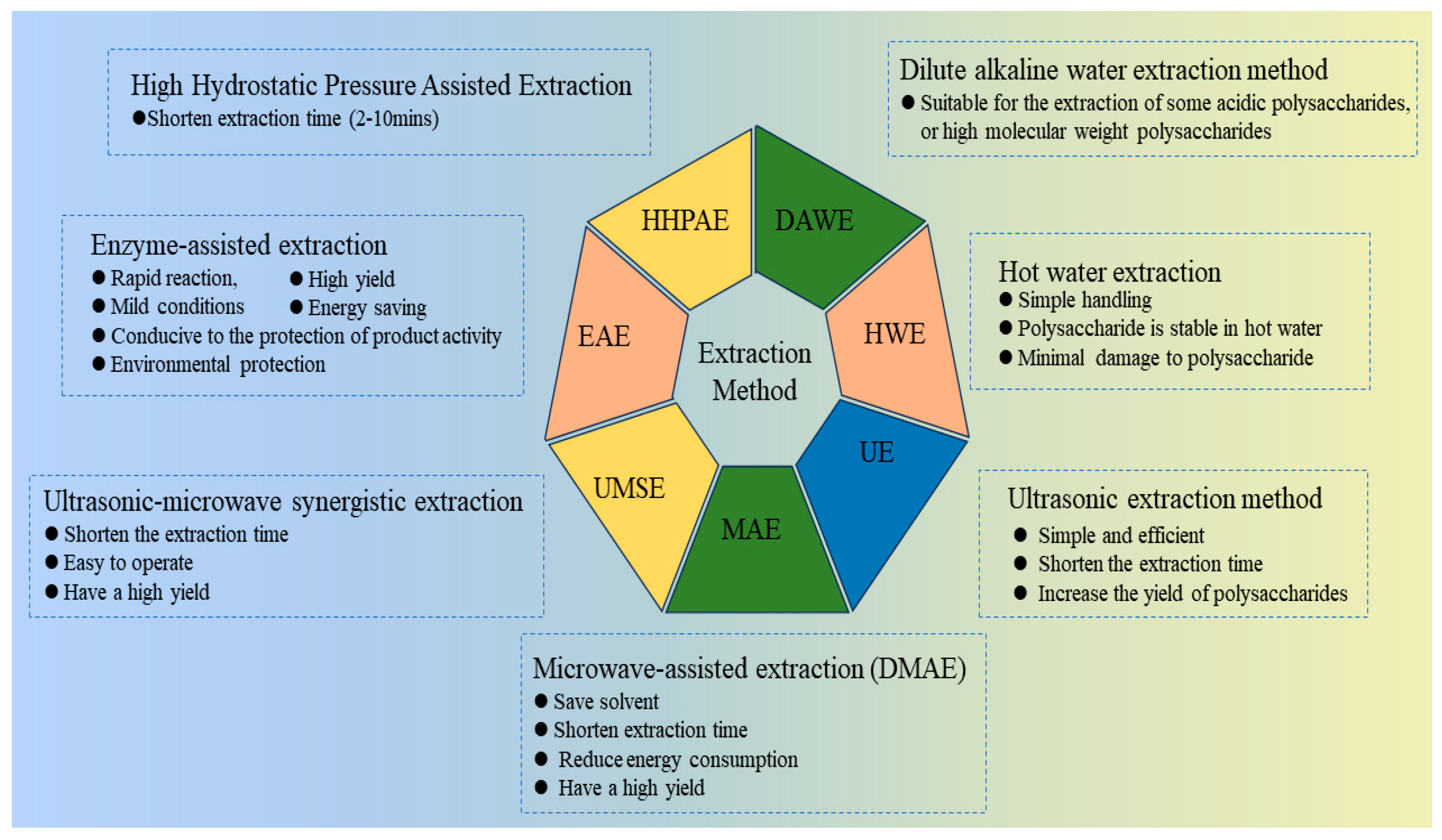

| Number | Extraction Methods | Theory | Key Parameters | Advantages | Disadvantages | References |
|---|---|---|---|---|---|---|
| 1 | Hot water extraction | Polysaccharides are hydrophilic macromolecules, and water enters the cell through the cell wall, causing soluble components such as polysaccharides to dissolve out of the cell. | extraction temperature extraction time material ratio | Simple handling, polysaccharides are stable in hot water, it causes minimal damage to the polysaccharides. | Require high temperature and long extraction time. | [18,19] |
| 2 | Ultrasonic extraction | Under the action of ultrasound, high temperature and pressure appear inside the organism in order to accelerate the internal movement of molecules of substances, thus causing the deformation and rupture of the cell wall of living organisms, so that the effective components can be dissolved more quickly. | material ratio temperature ultrasonic power ultrasonic time | Simple and efficient, shorten the extraction time and increase the yield of polysaccharides | Ultrasound may disrupt the structure of the polysaccharides. | [20] |
| 3 | Microwave-assisted extraction | Through high-frequency microwave radiation, electromagnetic waves advance quickly into the cells, leading to the material internal absorption of the energy and thus rapid warming and instant pressurization, so that the cell wall breaks down, dissolving the required components. | material ratio microwave power temperature microwave time | Saves solvent, shortens extraction time, reduces energy consumption, and has a high yield. | Microwaves may disrupt the structure of the polysaccharides. | [21,22] |
| 4 | Ultrasonic–microwave synergistic extraction | Combine the dual action of ultrasound and microwave to further promote polysaccharides solubilization. | material ratio ultrasonic–microwave temperature power time | Time-saving, easy to operate, high yield | Ultrasound and microwaves may disrupt polysaccharide structure. | [23] |
| 5 | Enzyme-assisted extraction | Pectinase, papain and cellulase can disrupt the cell wall structure, reduce the mass transfer resistance of polysaccharide diffusion from intracellular to solvent, and increase the polysaccharide yield. | material ratio type of enzyme amount of enzyme digestion time digestion temperature pH | Rapid reaction, mild conditions, high yield; it is conducive to the protection of product activity, energy saving and environmental protection | It needs harsh conditions, incurs a high cost, and is rarely used alone, usually combined with other extraction methods. | [24,25] |
| 6 | High hydrostatic pressure assisted extraction | Under the action of ultra-high pressure, the internal and external pressures create a pressure difference inside the cell, promoting the rapid entry of solvents into the cell. When the pressure is released, it causes cell damage and rapid release of polysaccharides from the cell. | HHPE pressure HHPE time material ratio | Short time (2–10 min) | High pressure affects the dissolution of polysaccharides. | [20,26] |
| 7 | Dilute alkaline water extraction | Acidic polysaccharides or high molecular weight polysaccharides are generally more soluble in dilute alkaline solutions than in hot water. | extraction temperature extraction time material ratio | It is suitable for the extraction of some acidic polysaccharides, or high molecular weight polysaccharides | Extraction temperature should be kept below 10 °C, polysaccharides are degraded when the temperature is high. | [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Ma, J.; Li, T.; Li, P.; Yan, D.; Zhu, J.; Zhang, X. Advances in the Preparation, Structure and Bioactivity of Polysaccharides from Lycium ruthenicum Murr.: A Review. Foods 2024, 13, 1995. https://doi.org/10.3390/foods13131995
Liu B, Ma J, Li T, Li P, Yan D, Zhu J, Zhang X. Advances in the Preparation, Structure and Bioactivity of Polysaccharides from Lycium ruthenicum Murr.: A Review. Foods. 2024; 13(13):1995. https://doi.org/10.3390/foods13131995
Chicago/Turabian StyleLiu, Bing, Jingyu Ma, Ting Li, Pei Li, Dehui Yan, Jun Zhu, and Xinguo Zhang. 2024. "Advances in the Preparation, Structure and Bioactivity of Polysaccharides from Lycium ruthenicum Murr.: A Review" Foods 13, no. 13: 1995. https://doi.org/10.3390/foods13131995
APA StyleLiu, B., Ma, J., Li, T., Li, P., Yan, D., Zhu, J., & Zhang, X. (2024). Advances in the Preparation, Structure and Bioactivity of Polysaccharides from Lycium ruthenicum Murr.: A Review. Foods, 13(13), 1995. https://doi.org/10.3390/foods13131995





