Polycyclic Aromatic Hydrocarbons’ Impact on Crops and Occurrence, Sources, and Detection Methods in Food: A Review
Abstract
1. Introduction
| Nation | Types of Food | BaP (μg/kg) | PAH4 (μg/kg) | Ref. |
|---|---|---|---|---|
| European Union | Oil and fats intended for direct human consumption or use as an ingredient in food | 2.0 | 10.0 | [21] |
| Cocoa bean and derived products | 5.0 | 30.0 | ||
| Coconut oil intended for direct human consumption or use as an ingredient in food | 2.0 | 20.0 | ||
| Smoked meat and smoked meat products | 2.0 | 12.0 | ||
| Muscle meat of smoked fish and smoked fishery products | 2.0 | 12.0 | ||
| Smoked sprats and canned smoked sprats; bivalve mollusks (fresh, chilled or frozen) | 5.0 | 30.0 | ||
| Bivalve mollusks (smoked) | 6.0 | 35.0 | ||
| Processed cereal-based food and baby food for infants and young children | 1.0 | 1.0 | ||
| Infant formulae and follow-on formulae, including infant milk and follow-on milk | 1.0 | 1.0 | ||
| Dietary foods for special medical purposes intended specially for infants | 1.0 | 1.0 | ||
| China | Cereals/cereal products | 2.0 | — | [22] |
| Meats/meat products | 5.0 | — | ||
| Aquatic foods/aquatic food products | 5.0 | — | ||
| Milk and dairy products | 10.0 | — | ||
| Oils and fats and their products | 10.0 | — | ||
| Korea | Dried and smoked fish | 10.0 | — | [23] |
| Canada | Olive pomace oils | 3.0 | — | [24] |
| Brazil | Olive pomace oils | 2.0 | — | [25] |
2. Impacts of PAHs on Crops
3. Sources of PAHs in Food
4. Occurrence of PAHs in Food
5. PAH Detection in Food
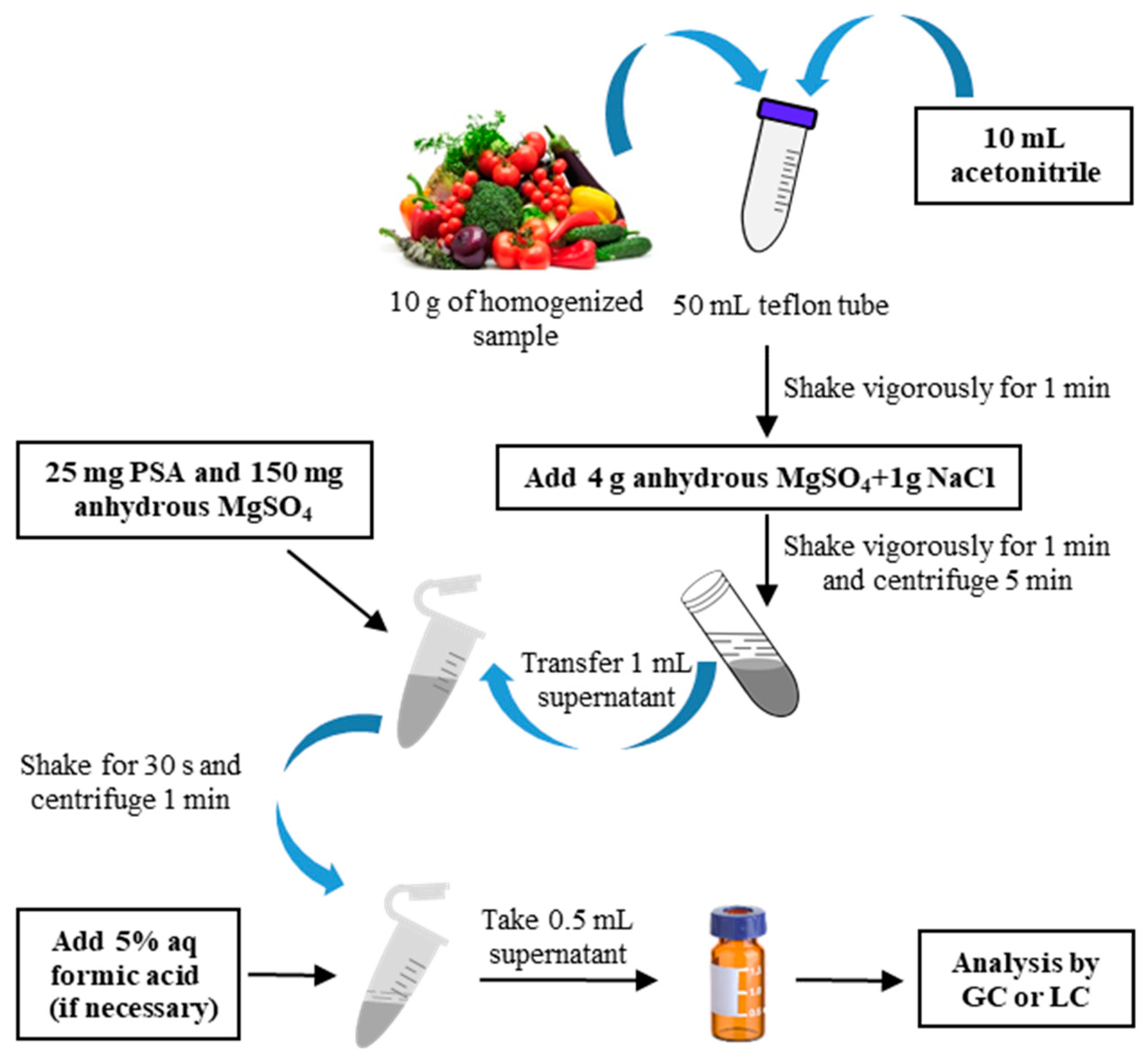
5.1. Sample Preparation
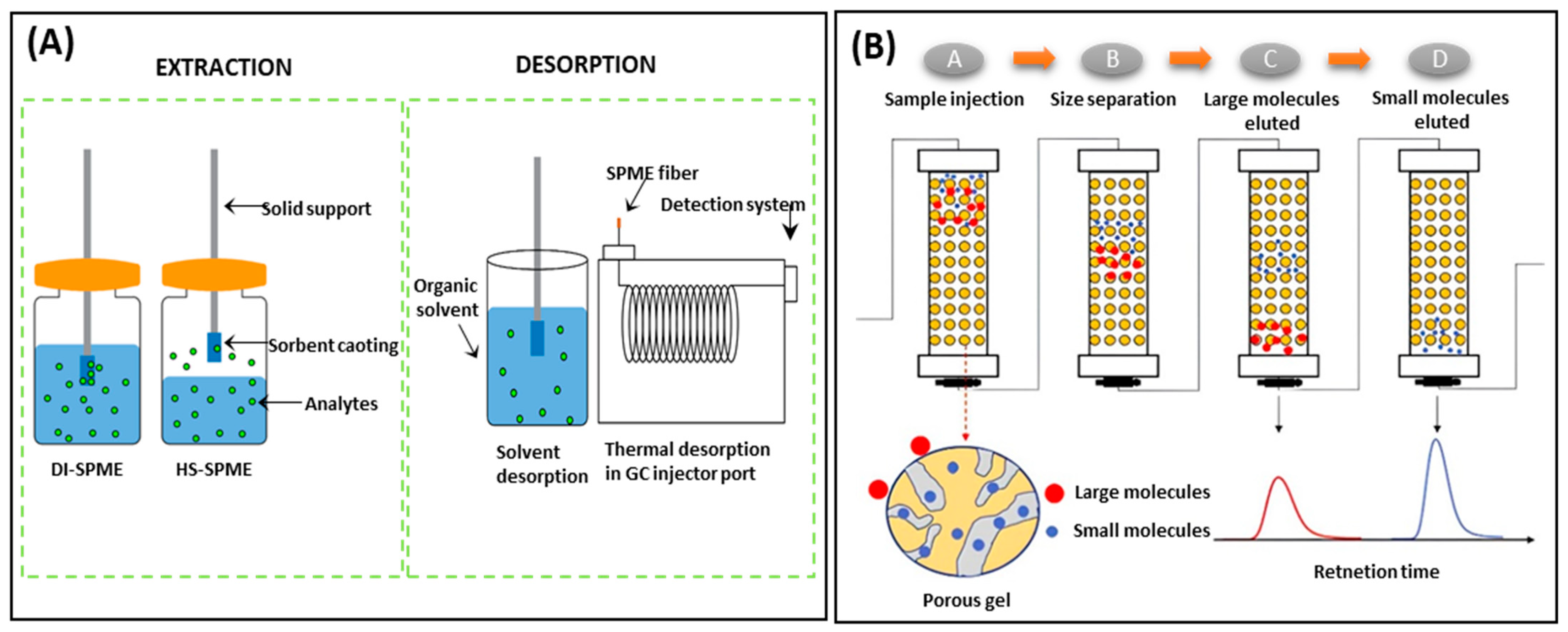
5.1.1. Extraction Methods
5.1.2. Cleanup Procedures
5.2. Detection Strategies
5.2.1. Gas Chromatography
5.2.2. Liquid Chromatography
5.2.3. Other Technologies
| Sample | Analytes | Sample Pretreatment | Instrumental Techniques | Instrumental Details | Performance Characteristics | Ref. |
|---|---|---|---|---|---|---|
| Youtiao | 16 PAHs | UAE with acetonitrile/acetone (3/2, v/v), purification by a C18 SPE cartridge and a Florisil SPE cartridge | GC-MS | DB-5MS capillary column (30 m × 0.25 mm i.d., 0.25 μm); programmed temperature; splitless injection; inlet, transfer line, and ion source temperature at 300 °C, 280 °C, and 230 °C, respectively | Recoveries: 72.2–108.1% LODs: 0.005–0.36 μg/kg | [71] |
| Tea | 22 PAHs | UAE with n-hexane, purification by an SPE cartridge using carboxylated MWCNTs and diatomite as sorbents | HPLC-UV-FLD | C18 column (25 cm × 4.6 mm i.d., 5 μm) at 30 °C with a gradient mobile phase of acetonitrile and water | Recoveries: 82.1–97.4% RSDs: 2.3–5.9% LODs: 0.10–0.75 μg/kg LOQs: 0.33–2.50 μg/kg | [72] |
| Tea | 16 PAHs | UAE with n-hexane, purification by dispersive SPE using PSA and C18 as sorbents | HPLC-UV-FLD | Waters PAH C18 column (25 cm × 4.6 mm i.d., 5 μm) at 27 °C with a gradient mobile phase of acetonitrile and water and UV detection wavelength of 230 nm | Recoveries: 71.5–118% RSDs ˂ 10% LOQs: 0.4–3.0 μg/kg | [73] |
| Dried fish | 16 PAHs | SOX with methylene chloride, purification by GPC with SX-3 Bio Beads as a sorbent and a chromatography column with Al2O3 and silica gel as sorbents | GC-MS/MS | TG-5MS capillary column (30 m × 0.25 mm i.d., 0.25 μm); programmed temperature; splitless injection; inlet and ion source temperature at 270 °C and 280 °C, respectively | Recovery: 50–126% | [104] |
| Aquatic products (grass carp, Macrobrachium rosenbergii, Eriocheir sinensis) | 16 PAHs | SOX with cyclohexane/methylene chloride (1/1, v/v), followed by GPC cleanup | GC-MS | DB-35MS capillary column (30 m × 0.25 mm i.d., 0.25 μm); programmed temperature; splitless injection; both inlet and ion source temperature at 260 °C; transfer line and quadrupole temperature at 280 °C and 150 °C, respectively | Recoveries: 70.3–126.4% RSDs: 0.14–8.91% LODs: 0.017–0.171 μg/kg LOQs: 0.051–0.489 μg/kg | [105] |
| Aquatic products (marine fish, Shrimp, Sea crab) | 16 PAHs | UAE with methylene chloride, purification by an SPE cartridge using C18 and PSA as sorbents | GC-MS | HP-5MS capillary column (30 m × 0.25 mm i.d., 0.25 μm); programmed temperature; splitless injection; inlet, transfer line, and ion source temperature at 250 °C, 280 °C, and 230 °C, respectively | Recoveries: 77.5–107.4% RSDs: 1.8–8.4% LODs: 0.12–0.25 μg/kg LOQs: 0.4–0.82 μg/kg | [109] |
| Herbal tea | 10 PAHs | QuEChERS (acetonitrile, anhydrous MgSO4 and NaCl, PSA and strong anion exchange sorbents) and LLE with hexane | GC-FID | PAH capillary column (30 m × 0.25 mm i.d., 0.36 mm); programmed temperature; split mode at split ratio of 10:1; inlet temperature at 270 °C | Recoveries: 77–85% LODs: 0.08–0.17 μg/kg LOQs: 0.24–0.51 μg/kg | [121] |
| Charcoal-grilled chicken drumsticks | 16 PAHs | QuEChERS (acetone, anhydrous MgSO4 and NaCl, PSA and endcapped octadecylsilane silica gel particle sorbents) | HPLC-FLD | Pinnacle II PAH column (15 cm × 3 mm i.d., 4 μm) at 35 °C with a gradient mobile phase of water and acetonitrile containing 4% tetrahydrofuran and UV detection wavelength of 254 nm | Recoveries: 67–114% RSDs: 1–21% LODs: 0.004–0.25 μg/kg LOQs: 0.01–0.75 μg/kg | [124] |
| Kebabs | 16 PAHs | UAE with acetonitrile and acetonitrile–saturated hexane, cleanup by a PAH-MIP column | HPLC-FLD-DAD | SB C18 column (25 cm × 4.6 mm i.d., 5 μm) at 30 °C with a gradient mobile phase of acetonitrile and water | Recoveries: 81–104% RSDs: 0.95–5.84% LODs: 0.33–3.30 μg/kg LOQs: 1.0–10.0 μg/kg | [145] |
| Oil-tea camellia seed oil | 16 PAHs | Vortex extraction with hexane, cleanup by a PAH-MIP column | GC-MS/MS | HP-5MS capillary column (30 m × 0.25 mm i.d., 0.25 μm); programmed temperature; splitless injection; both inlet and transfer line temperature at 280 °C; ion source and quadrupole temperature at 230 °C and 150 °C, respectively | Recoveries: 71.5–116.3% RSDs: 1.5–13.8% LODs: 0.01–0.20 μg/kg LOQs: 0.04–0.65 μg/kg | [146] |
| Tea infusion | BaA, BbF, and BaP | Sample solution mixed with gelatin aerogel tablet were vortexed and then desorbed by hexane | HPLC-DAD | UPS C18 column (15 cm × 4.6 mm i.d., 5 μm) at 25 °C with an isocratic mobile phase of acetonitrile/water (95/5, v/v) and DAD detection wavelength of 256 nm | Recoveries: 70.1–119.3% RSDs ≤ 6.3% LODs:0.0017–0.0021 μg/L | [151] |
| Fried food | BaP, BbF, and BaA | Saponification and SPE using β-cyclodextrin functionalized graphene oxide-grafted silica as sorbent | HPLC-DAD | C18 column (15 cm × 4.6 mm i.d., 5 μm) at 30 °C with an isocratic mobile phase of methanol/water (80/20, v/v) and DAD detection wavelength of 254 nm | Recoveries: 91.2–109.1% RSDs: 1.6–11.8% LODs: 0.1–0.3 μg/L | [152] |
| Coffee (ground coffee, infusion, and coffee grounds) | Nap, Ant, Aceny, BghiP, BaP, Chr, Fluo, Flu, and Pyr | DI-SPME using a polyacrylate-coated fused silica fiber (85 μm) | GC-MS | SPB-5 fused silica capillary column (30 m × 0.25 μm i.d., 0.25 μm); programmed temperature; splitless injection; both inlet and ion source temperature at 250 °C; transfer line temperature at 300 °C | Recoveries: 59–99% RSDs: 3–11% LODs: 0.3–16.6 μg/kg LOQs: 1.0–55.3 μg/kg | [159] |
| Coffee and tea | 16 EPA PAHs | HS and DI-SPME using a fiber by coating clay–chitosan and dicationic ionic liquid onto a stainless steel wire | GC-MS | TG-5MS capillary column (30 m × 0.25 mm i.d., 0.25 μm); programmed temperature; splitless injection; inlet, ion source, and transfer line temperature at 250 °C, 280 °C, and 250 °C, respectively | Recoveries: 87.5–112% RSDs: 2.5–6.5% LODs: 0.1–10 ng/L | [161] |
| Honey | Acen, Phen, Flu, Ant, and Pyr | HS-SPME using covalent organic framework/Ti3C2Tx composites as coatings | GC-FID | HP-5 capillary fused silica column (30 m × 0.32 mm i.d., 0.25 μm) and programmed temperature | Recoveries: 73.2–112% RSDs ≤ 9.4% LODs: 0.2–0.6 μg/kg LOQs: 0.6–2.0 μg/kg | [162] |
| Grilled meat | 16 PAHs and 36 PCBs | QuEChERS (ethyl acetate/acetone/isooctane (2/2/1, v/v/v), NaCl and ammonium formate, MgSO4, and CH3COONa, PSA and Z-Sep+ made up of C18 and silica coated with zirconium dioxide at a ratio of 2:5 as sorbents) | GC-MS | HP-5MS capillary column (30 m × 0.25 mm i.d., 0.25 μm); programmed temperature; splitless injection; ion source and transfer line temperature at 230 °C and 280 °C, respectively | Recoveries: 72.5–119.5% RSDs: 1.3–16.8% LOQs: 0.5–2 μg/kg | [168] |
| Coffee, tea and water | 15 PAHs | μ-QuEChERS (acetonitrile, anhydrous MgSO4 and NaCl, PSA, and C18 sorbents) followed by enrichment using air-assisted DLLME with diethyl carbonate as the extraction solvent | GC-MS | DB-5MS capillary column (30 m × 0.25 mm i.d., 0.25 μm); programmed temperature; splitless injection; ion source temperature at 230 °C | Recoveries: 90–103% RSDs: 0.2–5.9% LODs: 0.01–2.10 μg/kg LOQs: 0.03–6.36 μg/kg | [169] |
| Bread | 10 PAHs | QuEChERS (acetonitrile, anhydrous MgSO4 and NaCl, PSA sorbent) | GC-MS | DB-5MS capillary column (30 m × 0.25 mm i.d., 0.25 μm); programmed temperature; splitless injection; inlet, quadrupole mass analyzer, transfer line, and ion source temperature at 300 °C, 100 °C, 280 °C, and 230 °C, respectively | Recoveries: 92–106% RSDs: 3–7% LODs: 0.14–0.78 μg/kg LOQs: 0.46–2.60 μg/kg | [170] |
| Pork meat | 16 PAHs | QuEChERS with acetonitrile and Agilent 5982–6650 Extract Pouches, purification by dispersive SPE using PSA as sorbent | GC-MS | SLB-5MS capillary column (30 m × 0.32 mm i.d., 0.25 μm); programmed temperature; splitless injection; both inlet and transfer line temperature at 300 °C; ion source temperature at 220 °C | Recoveries: 71–120% RSDs: 1.7–7.6% LODs: 0.27–5.60 μg/kg LOQs: 0.81–16.8 μg/kg | [171] |
| Bread and potato Tahdig | 16 PAHs | Sample mixed with methanol, refluxed with potassium hydroxide and distilled water, then n-hexane is added, and purified by a Sep-Pak cartridge with methanol/n-hexane (1/1, v/v) as the elution solvent | GC-MS | DB-5MS capillary column (30 m × 0.25 mm i.d., 0.25 μm); programmed temperature; pulsed splitless mode; inlet, transfer line, ion source, and quadrupole temperature at 290, 300, 230, and 150 °C, respectively | Recoveries: 86.2–100.5% | [172] |
| Tea | 16 PAHs | UAE with hexane, purification by dispersive SPE using PSA and C18 as sorbents | GC-MS | DB-5MS capillary column (30 m × 0.25 mm i.d., 0.25 μm); programmed temperature; 20% split injection mode; ion source and transfer line temperature at 230 °C and 280 °C, respectively | Recoveries: 83.2–108.7% RSDs: 0.19–6.9% LODs: 0.10–0.28 μg/kg LOQs: 0.35–1.01 μg/kg | [173] |
| Honey | 33 PAHs | Sample dissolved in 10% methanol/deionized water and extracted using an SPE cartridge and eluting with ethyl acetate | GC-MS/MS | Zebron™ column (20 m × 0.18 mm i.d., 0.18 μm); programmed temperature; pulsed splitless mode; injector port and interface temperature at 290 °C and 250 °C, respectively | Recoveries: 41–115% RSDs: 17–40% LOQs: 0.5 μg/kg | [175] |
| Seafood | 24 PAHs and 18 halogenated PAHs | UAE with acetonitrile/acetone (3/2, v/v), purification by an EMR lipid tube | GC-MS/MS | DB-EUPAH column (20 m × 0.18 mm i.d., 0.14 μm); programmed temperature; splitless injection; inlet, transfer line, ion source, and quadrupole at 280 °C, 300 °C, 230 °C, and 150 °C, respectively | Recoveries: 70.5–116.9% RSDs ≤ 13.1% LODs: 0.01–0.2 μg/kg LOQs: 0.03–0.67 μg/kg | [176] |
| Barbecued meat and meat substitutes | 6 PAHs and 8 oxygenated PAHs | PLE with acetonitrile/ethyl acetate (1/3, v/v), purification by an SPE cartridge using Florisil, zirconia/silica, and C18 as sorbents | GC-HRMS | PAH C18 capillary column (60 m × 0.25 mm i.d., 0.10 μm); programmed temperature; splitless injection; inlet, transfer line, and ion source temperature at 280 °C, 270 °C, and 260 °C, respectively | Recoveries: 72–109% LODs: 0.03–0.17 μg/kg LOQs: 0.10–0.55 μg/kg | [177] |
| Roasted pork meat | BaA, Chr, BaP, BbF, BkF, DiBahA, and BghiP | Homogenization and alkaline hydrolysis of samples with 1 mol/L KOH solution, purification by an SPE cartridge using propyl sulfonic as sorbent | HPLC-FLD | Hypersil Green PAH column (25 cm × 4.6 mm i.d., 5 μm) at 40 °C with an isocratic mobile phase of acetonitrile/water (84/16, v/v) | Recoveries: 61–96% RSDs: 7.8–15.9% LODs: 0.003–0.006 μg/kg LOQs: 0.01–0.02 μg/kg | [196] |
| Tea infusion | BaP, BaA, BbF, and Chr | QuEChERS (acetonitrile containing 1% acetic acid, anhydrous MgSO4 and NaCl, PSA, endcapped octadecyl siloxane, and carbon sorbents) | HPLC-FLD | Pinnacle II PAH column (15 cm × 3.0 mm i.d., 4 μm) with a gradient mobile phase of water and acetonitrile containing 4% tetrahydrofuran | LODs: 0.02–0.11 μg/kg LOQs: 0.08–0.38 μg/kg | [197] |
| Tea and tea infusion | BaA, Chr, BbF, and BaP | Vortex extraction with ethyl acetate, purification by dispersive SPE using silica gel and PSA as sorbents | HPLC-FLD | Vydac 201 TP54 C18 column (25 cm × 4.6mm i.d., 5 μm) at 30 °C with a gradient mobile phase of acetonitrile and water | Recoveries: 54–99% RSDs: 1–21% LODs: 0.03–0.3 μg/kg LOQs: 0.1–0.5 μg/kg | [198] |
| Milk | BaP, BaA, and BbF | Saponification and DLLME using chloroform as the extracting solvent and acetonitrile as the disperser solvent | HPLC-UV | PerfectSil ODS-3 HD column (12.5 cm × 4 mm i.d., 5 μm) at 25 °C with an isocratic mobile phase of acetonitrile/water (90/10, v/v) and UV detection wavelength at 254 nm | Recoveries: 88.4–95.2% RSDs: 3.7–9.7% LODs: 0.06–0.18 ng/mL LOQs: 0.18–0.56 ng/mL | [199] |
| Cooked chicken and roasted coffee | 16 PAHs | QuEChERS (acetonitrile containing 1% acetic acid, MgSO4, CH3COONa, and PSA sorbent) | HPLC-FLD | Spherisorb ODS2 column (25 cm × 4.6 mm i.d., 5 μm) at 30 °C with an isocratic mobile phase of acetonitrile and water (90: 10, v/v) | Recoveries: 52.6–103.9% RSDs < 22% LODs: 0.03–0.6 ng/mL LOQs: 0.09–1.8 ng/mL | [200] |
| Grilled meat | 15 PAHs | Extraction with diatomaceous earth columns and propylsulfonic acid columns using dichloromethane as the elution solvent, and cleanup by a column chromatography containing silica gel and elution with n-hexane/dichloromethane (60/40, v/v) | HPLC-UV-FLD | PAH column (25 cm × 4.6 mm i.d., 5 μm) with a gradient mobile phase of water and acetonitrile and UV detection wavelength at 254 nm | Recoveries: 13.7–132.6% LODs: 0.025–5 μg/kg LOQs: 0.075–15 μg/kg | [201] |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qi, A.A.; Wang, P.C.; Lv, J.H.; Zhao, T.; Huang, Q.; Wang, Y.M.; Zhang, X.F.; Wang, M.; Xiao, Y.; Yang, L.X.; et al. Distributions of PAHs, NPAHs, OPAHs, BrPAHs, and ClPAHs in air, bulk deposition, soil, and water in the Shandong Peninsula, China: Urban-rural gradient, interface exchange, and long-range transport. Ecotoxicol. Environ. Saf. 2023, 265, 115494. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, C.; Villa, S.; Waichman, A.V.; de Souza Nunes, G.S.; de Oliveira, R.; Vighi, M.; Rico, A. Occurrence, sources, and ecological risks of polycyclic aromatic hydrocarbons (PAHs) in the Amazon river. Chemosphere 2023, 336, 139285. [Google Scholar] [CrossRef] [PubMed]
- Paradelo, R.; Celeiro, M.; Herbon, C.; Barral, M.T.; García-Jares, C. Polycyclic aromatic hydrocarbons concentration and spatial distribution in the soils of Santiago de Compostela (northwestern Spain). Geoderma Reg. 2023, 34, e00703. [Google Scholar] [CrossRef]
- Alani, R.; Zhao, S.Z.; Liu, X.; Akinrinade, O.; Agunbiade, F.; Ayejuyo, O.; Zhang, G. Concentrations, profiles and exposure risks of polycyclic aromatic hydrocarbons (PAHs) in passive air samples from Lagos, Nigeria. Atmos. Pollut. Res. 2021, 12, 101162. [Google Scholar] [CrossRef]
- Soursou, V.; Campo, J.; Picó, Y. Revisiting the analytical determination of PAHs in environmental samples: An update on recent advances. Trends Environ. Anal. Chem. 2023, 37, e00195. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Some Non-Heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures. Available online: https://www.ncbi.nlm.nih.gov/books/NBK321712/ (accessed on 13 January 2024).
- Khalili, F.; Shariatifar, N.; Dehghani, M.H.; Yaghmaeian, K.; Nodehi, R.N.; Yaseri, M.; Moazzen, M. Polycyclic aromatic hydrocarbons (PAHs) in meat, poultry, fish and related product samples of Iran: A risk assessment study. J. Environ. Health Sci. Eng. 2023, 21, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.M.; Zhang, Y.X.; Sun, S.D.; Liu, X.J. Concentrations of the 16 US EPA PAHs in 86 vegetable oil samples. Polycycl. Aromat. Compd. 2022, 42, 7336–7353. [Google Scholar] [CrossRef]
- Shoaei, F.; Talebi-Ghane, E.; Amirsadeghi, S.; Mehri, F. The investigation of polycyclic aromatic hydrocarbons (PAHs) in milk and its products: A global systematic review, meta-analysis and health risk assessment. Int. Dairy J. 2023, 142, 105645. [Google Scholar] [CrossRef]
- Singh, L.; Agarwal, T. Polycyclic aromatic hydrocarbons in cooked (tandoori) chicken and associated health risk. Risk Anal. 2023, 43, 2380–2397. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.F.; Xu, X.J.; Huo, X.; Faas, M.M. Effects of polycyclic aromatic hydrocarbons (PAHs) on pregnancy, placenta, and placental trophoblasts. Ecotoxicol. Environ. Saf. 2023, 262, 115314. [Google Scholar] [CrossRef]
- Tartaglione, A.M.; Racca, A.; Ricceri, L. Developmental exposure to polycyclic aromatic hydrocarbons (PAHs): Focus on benzo[a]pyrene neurotoxicity. Reprod. Toxicol. 2023, 119, 108394. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-Y.; Jin, H.; Lu, Q. Effect of polycyclic aromatic hydrocarbons on immunity. J. Transl. Autoimmun. 2022, 5, 100177. [Google Scholar] [CrossRef] [PubMed]
- Mallah, M.A.; Li, C.X.; Mallah, M.A.; Noreen, S.; Liu, Y.; Saeed, M.; Xi, H.; Ahmed, B.; Feng, F.F.; Mirjat, A.A.; et al. Polycyclic aromatic hydrocarbon and its effects on human health: An overeview. Chemosphere 2022, 296, 133948. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhang, Y.; Zhou, H.; Cai, K.; Xu, B. A review of hazards in meat products: Multiple pathways, hazards and mitigation of polycyclic aromatic hydrocarbons. Food Chem. 2024, 445, 138718. [Google Scholar] [CrossRef] [PubMed]
- Agus, B.A.P.; Rajentran, K.; Selamat, J.; Lestari, S.D.; Umar, N.B.; Hussain, N. Determination of 16 EPA PAHs in food using gas and liquid chromatography. J. Food Compos. Anal. 2023, 116, 105038. [Google Scholar] [CrossRef]
- Qiao, B.; Li, Y.S.; Meng, X.Y.; Sun, Y.; Hu, P.; Lu, S.Y.; Ren, H.L.; Liu, Z.S.; Zhou, Y. Development of an indirect competitive ELISA for the detection of acenaphthene and pyrene. Food Agric. Immunol. 2017, 28, 789–800. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, J.; Han, C.Q.; Xie, J.C. A versatile SERS sensor for multiple determinations of polycyclic aromatic hydrocarbons and its application potential in analysis of fried foods. Int. J. Anal. Chem. 2020, 2020, 4248029. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.R.; Hu, L.Q.; Ju, Y.; Zhang, Y.H.; Yin, C.L. Recent advances in pretreatment methods for the detection of polycyclic aromatic hydrocarbons in edible oil. Food Sci. 2022, 43, 373–382. [Google Scholar] [CrossRef]
- Bansal, V.; Kumar, P.; Kwon, E.E.; Kim, K.H. Review of the quantification techniques for polycyclic aromatic hydrocarbons (PAHs) in food products. Crit. Rev. Food Sci. Nutr. 2017, 57, 3297–3312. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) No 1327/2014 of 12 December 2014 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Polycyclic Aromatic Hydrocarbons (PAHs) in Traditionally Smoked Meat and Meat Products and Traditionally Smoked Fish and Fishery Products. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32014R1357 (accessed on 13 January 2024).
- GB 2762-2022; National Health Commission of the PRC, State Administration for Market Regulation. National Food Safety Standard—Maximum Levels of Contaminants in Food. China Standard Press: Beijing, China, 2022.
- Seko, T.; Ishihara, K.; Suzuki, T.; Takagi, S.; Taga, K.; Iida, Y.; Shigematsu, Y.; Itabashi, Y.; Nakamichi, Y.; Fujiwara, Y.; et al. Effects of moisture content of firewood used in the manufacture of Japanese traditional smoked-dried bonito, katsuobushi, on polycyclic aromatic hydrocarbon (PAH) generation. J. Food Compos. Anal. 2022, 111, 104630. [Google Scholar] [CrossRef]
- Government of Canada. Health Canada’s Maximum Levels for Chemical Contaminants in Foods. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/food-safety/chemical-contaminants/maximum-levels-chemical-contaminants-foods.html (accessed on 13 January 2024).
- Zhu, Z.; Xu, Y.; Huang, T.; Yu, Y.; Bassey, A.P.; Huang, M. The contamination, formation, determination and control of polycyclic aromatic hydrocarbons in meat products. Food Control 2022, 141, 109194. [Google Scholar] [CrossRef]
- Zhang, H.M.; Long, M.H.; Qiao, S.Y.; Zhao, T.Y.; Long, B.; Liang, Y.S. Accumulations and physiological performance effects on cucumber after application of polycyclic aromatic hydrocarbons to leaf. Acta Bot. Boreal.-Occident. Sin. 2019, 39, 1064–1074. [Google Scholar] [CrossRef]
- Tang, X.; Long, M.H.; Qiao, S.Y.; Li, P.X.; Zhang, H.M.; Liang, Y.S. Physiological responses of different kinds of vegetable seedings to polycyclic aromatic hydrocarbons stress. Jiangsu Agric. Sci. 2019, 47, 166–170. [Google Scholar] [CrossRef]
- Liang, Y.S.; Huang, X.; Long, M.H.; Wu, G.F.; Zhang, H.M.; Qiao, S.Y. Effects of polycyclic aromatic hydrocarbons stress on growth and physiological characteristics of Brassica parachinensis. J. South. Chin. Agric. Univ. 2018, 39, 54–60. [Google Scholar] [CrossRef]
- Li, Y.; Xie, S.; Chen, Z.L.; Xu, S.Y.; Zhang, L.H. Impacts of zinc, benzo[a]pyrene, and their combination on the growth and antioxidant enzymes activities of wheat (Triticum aestivum L.) seedlings. Chin. J. Ecol. 2013, 32, 358–362. [Google Scholar] [CrossRef]
- Wang, H.C.; Hu, L.L.; Li, M.; Chen, W.F.; Wang, Y.; Zhou, J.J. Growth effects and accumulations of polycyclic aromatic hydrocarbons (PAHs) in rape. Chin. J. Plant Ecol. 2013, 37, 1123–1131. [Google Scholar] [CrossRef]
- Cai, S.X.; He, Y.; Qiu, X.X.; Lan, M.Z. Effects of polycyclic aromatic hydrocarbon pyrene on growth, uptake of pyrene and other elements, nutritious qualities of Chinese cabbage. Chin. J. Soil Sci. 2010, 41, 452–457. [Google Scholar] [CrossRef]
- Qiao, S.Y.; Wu, G.F.; Long, M.H.; Liang, Y.S.; Shen, H.Y.; Tang, X.; Li, P.X.; Zhang, H.M.; Zhao, T.Y. Pathway of PAHs entering Brassica parachinensis and its effects on yield and quality of B. parachinensis. J. South. Agric. 2018, 49, 1202–1207. [Google Scholar] [CrossRef]
- Long, M.H.; Wu, G.F.; Liang, Y.S.; Shen, H.Y.; Long, B.; Tang, X.; Li, P.X.; Zhang, H.N. Influence of PAHs stress on Brassica parachinensis Bailey quality and its detoxification system. J. South. Agric. 2017, 48, 1036–1041. [Google Scholar] [CrossRef]
- Chen, S.J.; Zhu, X.L.; Feng, X.Z.; Huang, L.Q.; Mei, Y.Q. Effects of polycyclic aromatic hydrocarbons on plants. Bull. Biol. 2010, 45, 9–11. [Google Scholar] [CrossRef]
- Bansal, V.; Kim, K.H. Review of PAH contamination in food products and their health hazards. Environ. Int. 2015, 84, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Edna Hee, P.T.; Liang, Z.; Zhang, P.; Fang, Z. Formation mechanisms, detection methods and mitigation strategies of acrylamide, polycyclic aromatic hydrocarbons and heterocyclic amines in food products. Food Control 2024, 158, 110236. [Google Scholar] [CrossRef]
- Gao, Y.Z.; Collins, C.D. Uptake pathways of polycyclic aromatic hydrocarbons in white clover. Environ. Sci. Technol. 2009, 43, 6190–6195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Hou, D.Y.; Xiong, G.N.; Duan, Y.H.; Cai, C.Y.; Wang, X.; Li, J.Y.; Tao, S.; Liu, W.X. Structural equation modeling of PAHs in ambient air, dust fall, soil, and cabbage in vegetable bases of Northern China. Environ. Pollut. 2018, 239, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Li, H.D.; Zhu, D.W.; Lu, X.; Du, H.X.; Guan, S.; Chen, Z.L. Determination and risk assessment of sixteen polycyclic aromatic hydrocarbons in vegetables. J. Environ. Sci. Health Part A 2018, 53, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Desalme, D.; Binet, P.; Chiapusio, G. Challenges in tracing the fate and effects of atmospheric polycyclic aromatic hydrocarbon deposition in vascular plants. Environ. Sci. Technol. 2013, 47, 3967–3981. [Google Scholar] [CrossRef] [PubMed]
- Terzaghi, E.; Zacchello, G.; Scacchi, M.; Raspa, G.; Jones, K.C.; Cerabolini, B.; Di Guardo, A. Towards more ecologically realistic scenarios of plant uptake modelling for chemicals: PAHs in a small forest. Sci. Total Environ. 2015, 505, 329–337. [Google Scholar] [CrossRef]
- Wang, J.F.; Li, J.; Cai, J.; Wu, F.Y. Effects of leaf trichome and stomata on PAHs uptake in the leaf of winter wheat. Chin. J. Eco-Agric. 2023, 31, 31–39. [Google Scholar] [CrossRef]
- Tao, S.; Cui, Y.H.; Xu, F.L.; Li, B.G.; Cao, J.; Liu, W.X.; Schmitt, G.; Wang, X.J.; Shen, W.R.; Qing, B.P.; et al. Polycyclic aromatic hydrocarbons (PAHs) in agricultural soil and vegetables from Tianjin. Sci. Total Environ. 2004, 320, 11–24. [Google Scholar] [CrossRef]
- Wang, L.P.; Xia, Z.H.; Wu, M.M.; Zhang, Q.Q.; Yang, H. Pollution and health hazards of PAHs in vegetables sold in Xuzhou city, China. Asian J. Ecotoxicol. 2017, 12, 526–534. [Google Scholar] [CrossRef]
- Han, M.W.; Liu, F.; Kang, Y.R.; Zhang, R.J.; Yu, K.F.; Wang, Y.H.; Wang, R.X. Occurrence, distribution, sources, and bioaccumulation of polycyclic aromatic hydrocarbons (PAHs) in multi environmental media in estuaries and the coast of the Beibu Gulf, China: A health risk assessment through seafood consumption. Environ. Sci. Pollut. Res. 2022, 29, 52493–52506. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; He, W.; Liu, W.X.; Kong, X.Z.; Xu, F.L.; Giesy, J.P. Tissue distribution, bioaccumulation, and carcinogenic risk of polycyclic aromatic hydrocarbons in aquatic organisms from Lake Chaohu, China. Sci. Total Environ. 2020, 749, 141577. [Google Scholar] [CrossRef] [PubMed]
- Büyükkurt, O.K.; Dincer, E.A.; Cam, I.B.; Candal, C.; Erbas, M. The influence of cooking methods and some marinades on polycyclic aromatic hydrocarbon formation in beef meat. Polycycl. Aromat. Compd. 2020, 40, 195–205. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.K.; Chen, Y.; Xiao, Y.H.; Fu, X.; Feng, T.T. Studied on detection methods and changes of polycyclic aromatic hydrocarbons in instant roasted fish with different processes. Sci. Technol. Food Ind. 2022, 43, 336–343. [Google Scholar] [CrossRef]
- Singh, L.; Varshney, J.G.; Agarwal, T. Polycyclic aromatic hydrocarbons’ formation and occurrence in processed food. Food Chem. 2016, 199, 768–781. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Shi, J.; Duan, X.; Wang, B.; Huang, N.; Zhao, X. Health risk assessment of dietary exposure to polycyclic aromatic hydrocarbons in Taiyuan, China. J. Environ. Sci. 2014, 26, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Du, H.Z.; Tian, X.L.; Liu, J.; Kong, B.H. Formation and control of polycyclic aromatic hydrocarbons in fried foods: A review. Food Sci. 2020, 41, 272–280. [Google Scholar] [CrossRef]
- Hu, G.F.; Cai, K.Z.; Li, Y.Z.; Xie, T.T.; Li, P.J.; Chen, C.G.; Xu, B.C. Influencing factors and control of polycyclic aromatic hydrocarbons formation in food processing. Food Res. Dev. 2020, 41, 196–201. [Google Scholar] [CrossRef]
- Hao, X.; Li, J.; Yao, Z. Changes in PAHs levels in edible oils during deep-frying process. Food Control 2016, 66, 233–240. [Google Scholar] [CrossRef]
- Sharifiarab, G.; Ahmadi, M.; Shariatifar, N.; Ariaii, P. Investigating the effect of type of fish and different cooking methods on the residual amount of polycyclic aromatic hydrocarbons (PAHs) in some Iranian fish: A health risk assessment. Food Chem. X 2023, 19, 100789. [Google Scholar] [CrossRef]
- Karslıoğlu, B.; Kolsarıcı, N. The effects of fat content and cooking procedures on the PAH content of beef Doner Kebabs. Polycycl. Aromat. Compd. 2022, 43, 3291–3304. [Google Scholar] [CrossRef]
- Chung, S.Y.; Yettella, R.R.; Kim, J.S.; Kwon, K.; Kim, M.C.; Min, D.B. Effects of grilling and roasting on the levels of polycyclic aromatic hydrocarbons in beef and pork. Food Chem. 2011, 129, 1420–1426. [Google Scholar] [CrossRef]
- Masuda, M.; Wang, Q.; Tokumura, M.; Miyake, Y.; Amagai, T. Simultaneous determination of polycyclic aromatic hydrocarbons and their chlorinated derivatives in grilled foods. Ecotoxicol. Environ. Saf. 2019, 178, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Kamal, N.H.A.; Selamat, J.; Sanny, M. Simultaneous formation of polycyclic aromatic hydrocarbons (PAHs) and heterocyclic aromatic amines (HCAs) in gas-grilled beef satay at different temperatures. Food Addit. Contam. 2018, 35, 848–869. [Google Scholar] [CrossRef] [PubMed]
- Wongmaneepratip, W.; Vangnai, K. Effects of oil types and pH on carcinogenic polycyclic aromatic hydrocarbons (PAHs) in grilled chicken. Food Control 2017, 79, 119–125. [Google Scholar] [CrossRef]
- Li, S.Q.; Ni, H.G.; Zeng, H. PAHs in polystyrene food contact materials: An unintended consequence. Sci. Total Environ. 2017, 609, 1126–1131. [Google Scholar] [CrossRef] [PubMed]
- Li, S.X.; Qin, J.L.; Zegn, Y.; Chen, J.Q.; Luo, E.L.; Peng, M.J. Determination of the migration of polycyclic aromatic hydrocarbons in four kinds of food contact materials. Sci. Technol. Food Ind. 2018, 39, 194–199. [Google Scholar] [CrossRef]
- Wang, C.Y.; Mai, J.C.; Liao, Q.P.; Ruan, Y.H.; Shen, Y.L.; Xie, T.T. Simultaneous determination of eighteen polycyclic aromatic hydrocarbons in paper packaging materials intended to come into contact with foodstuffs using ultra high performance liquid chromatography combined with microwave assisted extraction. J. Anal. Sci. 2014, 30, 549–553. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, J.; Wang, X.; Li, C.; Peng, T.; Miao, Q.; Du, X.; Lu, X. A facile molecularly imprinted column coupled to GC-MS/MS for sensitive and selective determination of polycyclic aromatic hydrocarbons and study on their migration in takeaway meal boxes. Talanta 2022, 243, 123385. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yu, J.L.; Zhu, Z.W.; Zhang, W.Z. Study on migration law of 16 polycyclic aromatic hydrocarbons in food contact material plastic. Food Ferment. Ind. 2020, 46, 105–109. [Google Scholar] [CrossRef]
- Einolghozati, M.; Talebi-Ghane, E.; Amirsadeghi, S.; Mehri, F. Evaluation of polycyclic aromatic hydrocarbons (PAHs) in processed cereals: A meta-analysis study, systematic review, and health risk assessment. Heliyon 2022, 8, e12168. [Google Scholar] [CrossRef]
- Yousefi, M.; Shemshadi, G.; Khorshidian, N.; Ghasemzadeh-Mohammadi, V.; Fakhri, Y.; Hosseini, H.; Khaneghah, A.M. Polycyclic aromatic hydrocarbons (PAHs) content of edible vegetable oils in Iran: A risk assessment study. Food Chem. Toxicol. 2018, 118, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Roszko, M.; Kaminska, M.; Szymczyk, K.; Jedrzejczak, R. Dietary risk evaluation for 28 polycyclic aromatic hydrocarbons (PAHs) in tea preparations made of teas available on the Polish retail market. J. Environ. Sci. Health Part B 2018, 53, 25–34. [Google Scholar] [CrossRef]
- Thi, L.A.P.; Ngoc, N.T.; Quynh, N.T.; Thanh, N.V.; Kim, T.T.; Anh, D.H.; Viet, P.H. Polycyclic aromatic hydrocarbons (PAHs) in dry tea leaves and tea infusions in Vietnam: Contamination levels and dietary risk assessment. Environ. Geochem. Health 2020, 42, 2853–2863. [Google Scholar] [CrossRef]
- Gao, G.W.; Chen, H.P.; Liu, P.X.; Ma, G.C.; Hao, Z.X.; Wang, C.; Cai, Y.F.; Lu, C.Y. Contaminated levels and source of polycyclic aromatic hydrocarbons in tea. Chin. J. Trop. Crops 2016, 37, 2032–2042. [Google Scholar] [CrossRef]
- Gao, G.W.; Chen, H.P.; Liu, P.X.; Hao, Z.X.; Ma, G.C.; Chai, Y.F.; Wang, C.; Lu, C.Y. Residue pattern of polycyclic aromatic hydrocarbons during green tea manufacturing and their transfer rates during tea brewing. Food Addit. Contam. Part A 2017, 34, 990–999. [Google Scholar] [CrossRef]
- Li, G.; Wu, S.M.; Wang, L.; Akoh, C.C. Concentration, dietary exposure and health risk estimation of polycyclic aromatic hydrocarbons (PAHs) in youtiao, a Chinese traditional fried food. Food Control 2016, 59, 328–336. [Google Scholar] [CrossRef]
- Guo, X.Y.; Chen, F.; Zhang, W.B. Pollution, source and risk assessment of PAHs in Chinese tea. LWT-Food Sci. Technol. 2022, 167, 113851. [Google Scholar] [CrossRef]
- Ma, J.J.; Zhu, Z.; Du, S.J.; Zhang, D.; Li, X.Y.; Zheng, Q.Z.; Shen, J.C.; Xiao, L.H.; Wu, X.L.; Chen, Y.N.; et al. Polycyclic aromatic hydrocarbons in commercial tea from China and implications for human exposure. J. Food Compos. Anal. 2023, 116, 105075. [Google Scholar] [CrossRef]
- Zhang, H.L.; Chen, Y.J.; Li, D.W.; Yang, C.H.; Zhou, Y.D.; Wang, X.Y.; Zhang, Z.C. PAH residue and consumption risk assessment in four commonly consumed wild marine fishes from Zhoushan Archipelago, East China Sea. Mar. Pollut. Bull. 2021, 170, 112670. [Google Scholar] [CrossRef] [PubMed]
- Li, J.F.; Dong, H.; Li, X.G.; Han, B.; Zhu, C.J.; Zhang, D.H. Quantitatively assessing the health risk of exposure to PAHs from intake of smoked meats. Ecotoxicol. Environ. Saf. 2016, 124, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Iwegbue, C.M.A.; Tesi, G.O.; Ogbuta, A.A.; Lari, B.; Igbuku, U.A.; Obi, G.; Martincigh, B.S. Concentrations and risk of polycyclic aromatic hydrocarbons (PAHs) in oil and tomato-based sauces from selected brands of canned fish consumed in Nigeria. Polycycl. Aromat. Compd. 2022, 42, 4621–4634. [Google Scholar] [CrossRef]
- Benson, N.U.; Fred-Ahmadu, O.H.; Olugbuyiro, J.A.O.; Anake, W.U.; Adedapo, A.E.; Olajire, A.A. Concentrations, sources and risk characterisation of polycyclic aromatic hydrocarbons (PAHs) in green, herbal and black tea products in Nigeria. J. Food Compos. Anal. 2018, 66, 13–22. [Google Scholar] [CrossRef]
- Tongo, I.; Ogbeide, O.; Ezemonye, L. Human health risk assessment of polycyclic aromatic hydrocarbons (PAHs) in smoked fish species from markets in Southern Nigeria. Toxicol. Rep. 2017, 4, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Adetunde, O.T.; Mills, G.A.; Oluseyi, T.O.; Oyeyiola, A.O.; Olayinka, K.O.; Alo, B.I. Polycyclic aromatic hydrocarbon in vegetables grown on contaminated soils in a Sub-Saharan tropical environment-Lagos, Nigeria. Polycycl. Aromat. Compd. 2020, 40, 979–989. [Google Scholar] [CrossRef]
- Marcolin, L.C.; Arias, J.L.D.; Kupski, L.; Barbosa, S.C.; Primel, E.G. Polycyclic aromatic hydrocarbons (PAHs) in honey from stingless bees (Meliponinae) in southern Brazil. Food Chem. 2023, 405, 134944. [Google Scholar] [CrossRef] [PubMed]
- Guizellini, G.M.; Sampaio, G.R.; da Silva, S.A.; Soares-Freitas, R.A.M.; de Almeida, A.P.; Da Silva Torres, E.A.F. Concentration and potential health risk of polycyclic aromatic hydrocarbons for consumers of chocolate in Brazil. Food Chem. 2023, 405, 134853. [Google Scholar] [CrossRef] [PubMed]
- Soltani, N.; Moore, F.; Keshavarzi, B.; Sorooshian, A.; Javid, R. Potentially toxic elements (PTEs) and polycyclic aromatic hydrocarbons (PAHs) in fish and prawn in the Persian Gulf, Iran. Ecotoxicol. Environ. Saf. 2019, 173, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, S.F.; Rezaee, R.; Azizi, M.; Giesy, J.P.; Karimi, G. Polycyclic aromatic hydrocarbons, mycotoxins, and pesticides residues in coffee: A probabilistic assessment of risk to health. Int. J. Environ. Anal. Chem. 2022, 104, 1307–1329. [Google Scholar] [CrossRef]
- Sei, K.; Wang, Q.; Tokumura, M.; Suzuki, S.; Miyake, Y.; Amagai, T. Polycyclic aromatic hydrocarbons and their halogenated derivatives in a traditional smoke-dried fish product in Japan: Occurrence and countermeasures. ACS Food Sci. Technol. 2021, 1, 960–966. [Google Scholar] [CrossRef]
- Rozentale, I.; Zacs, D.; Perkons, I.; Bartkevics, V. A comparison of gas chromatography coupled to tandem quadrupole mass spectrometry and high-resolution sector mass spectrometry for sensitive determination of polycyclic aromatic hydrocarbons (PAHs) in cereal products. Food Chem. 2017, 221, 1291–1297. [Google Scholar] [CrossRef]
- Mahugija, J.A.M.; Njale, E. Levels of polycyclic aromatic hydrocarbons (PAHs) in smoked and sun-dried fish samples from areas in Lake Victoria in Mwanza, Tanzania. J. Food Compos. Anal. 2018, 73, 39–46. [Google Scholar] [CrossRef]
- Essumang, D.K.; Dodoo, D.K.; Adjei, J.K. Polycyclic aromatic hydrocarbon (PAH) contamination in smoke-cured fish products. J. Food Compos. Anal. 2012, 27, 128–138. [Google Scholar] [CrossRef]
- Ferrante, M.; Zanghì, G.; Cristaldi, A.; Copat, C.; Grasso, A.; Fiore, M.; Signorelli, S.S.; Zuccarello, P.; Conti, G.O. PAHs in seafood from the Mediterranean Sea: An exposure risk assessment. Food Chem. Toxicol. 2018, 115, 385–390. [Google Scholar] [CrossRef]
- Hasan, G.M.M.A.; Shaikh, M.A.A.; Satter, M.A.; Hossain, M.S. Detection of indicator polychlorinated biphenyls (I-PCBs) and polycyclic aromatic hydrocarbons (PAHs) in cow milk from selected areas of Dhaka, Bangladesh and potential human health risks assessment. Toxicol. Rep. 2022, 9, 1514–1522. [Google Scholar] [CrossRef]
- Waqas, M.; Khan, S.; Chao, C.; Shamshad, I.; Qamar, Z.; Khan, K. Quantification of PAHs and health risk via ingestion of vegetable in Khyber Pakhtunkhwa Province, Pakistan. Sci. Total Environ. 2014, 497, 448–458. [Google Scholar] [CrossRef]
- Al-Farhan, B.S.; Said, T.O.; El-Ghamdi, S.A.; Al-Alamie, A.Y. Distribution and health risk assessment on dietary exposure of PAHs in vegetables and fruits of Asir, Saudi Arabia. Waste Manag. Bull. 2023, 1, 94–102. [Google Scholar] [CrossRef]
- Londoño, V.A.G.; Reynoso, C.M.; Resnik, S.L. Polycyclic aromatic hydrocarbons (PAHs) survey on tea (Camellia sinensis) commercialized in Argentina. Food Control 2015, 50, 31–37. [Google Scholar] [CrossRef]
- Celik-Saglam, I.; Balcik, C.; Cetin, B. Concentrations, sources, and risk assessment of polycyclic aromatic hydrocarbons (PAHs) in black, green and fruit flavored tea in Turkey. J. Food Compos. Anal. 2022, 109, 104504. [Google Scholar] [CrossRef]
- Sari, M.F.; Esen, F. Polycyclic aromatic hydrocarbon (PAH) residues in the honeybee, honey, and pollen and estimation of atmospheric concentrations in Bursa, Turkey. Polycycl. Aromat. Compd. 2024, 44, 457–472. [Google Scholar] [CrossRef]
- Singh, L.; Agarwal, T. PAHs in Indian diet: Assessing the cancer risk. Chemosphere 2018, 202, 366–376. [Google Scholar] [CrossRef]
- Darwish, W.S.; Chiba, H.; El-Ghareeb, W.R.; Elhelaly, A.E.; Hui, S.P. Determination of polycyclic aromatic hydrocarbon content in heat-treated meat retailed in Egypt: Health risk assessment, benzo[a]pyrene induced mutagenicity and oxidative stress in human colon (CaCo-2) cells and protection using rosmarinic and ascorbic acids. Food Chem. 2019, 290, 114–124. [Google Scholar] [CrossRef]
- Racovita, R.C.; Secuianu, C.; Israel-Roming, F. Quantification and risk assessment of carcinogenic polycyclic aromatic hydrocarbons in retail smoked fish and smoked cheeses. Food Control 2021, 121, 107586. [Google Scholar] [CrossRef]
- Ciemniak, A.; Kuźmicz, K.; Rajkowska-Myśliwiec, M.; Cadena, M.F. Assessing the contamination levels of dried teas and their infusions by polycyclic aromatic hydrocarbons (PAHs). J. Consum. Prot. Food Saf. 2019, 14, 263–274. [Google Scholar] [CrossRef]
- Thompson, K.L.; Picard, C.R.; Chan, H.M. Polycyclic aromatic hydrocarbons (PAHs) in traditionally harvested bivalves in northern British Columbia, Canada. Mar. Pollut. Bull. 2017, 121, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jeong, J.H.; Park, S.; Lee, K.G. Monitoring and risk assessment of polycyclic aromatic hydrocarbons (PAHs) in processed foods and their raw materials. Food Control 2018, 92, 286–292. [Google Scholar] [CrossRef]
- Ouro-Sama, K.; Tanouayi, G.; Solitoke, H.D.; Barsan, N.; Mosnegutu, E.; Badassan, T.-E.; Agbere, S.; Adje, K.; Nedeff, V.; Gnandi, K. Polycyclic aromatic hydrocarbons (PAHs) contamination in Chrysichthys nigrodigitatus Lacépède, 1803 from Lake Togo-Lagoon of Aného, Togo: Possible human health risk suitable to their consumption. Int. J. Environ. Res. Public Health 2023, 20, 1666. [Google Scholar] [CrossRef] [PubMed]
- Bandowe, B.A.M.; Bigalke, M.; Boamah, L.; Nyarko, E.; Saalia, F.K.; Wilcke, W. Polycyclic aromatic compounds (PAHs and oxygenated PAHs) and trace metals in fish species from Ghana (West Africa): Bioaccumulation and health risk assessment. Environ. Int. 2014, 65, 135–146. [Google Scholar] [CrossRef]
- Mohammed, S.; Obiri, S.; Ansa-Asare, O.D.; Dartey, G.; Kuddy, R.; Appiah, S. Assessment of concentration of polycyclic aromatic hydrocarbons (PAHs) in vegetables from farms in Accra, Ghana. Environ. Monit. Assess. 2019, 191, 417. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Chen, X.Q.; Zhang, Y. Analytical chemistry, formation, mitigation, and risk assessment of polycyclic aromatic hydrocarbons: From food processing to in vivo metabolic transformation. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1422–1456. [Google Scholar] [CrossRef]
- Xu, Z.H.; Zhu, X.H.; Ge, Y.Q.; Geng, X.B.; Liu, C.W.; Shen, M.F. Determination of 16 polycyclic aromatic hydrocarbons residues in aquaculture environment and aquatic products by automated Soxhlet extraction-gel permeation chromatography-gas chromatography/mass spectrometry mothod. Jiangsu J. Agric. Sci. 2019, 35, 1459–1467. [Google Scholar] [CrossRef]
- Badolato, E.; Martins, M.; Aued-Pimentel, S.; Alaburda, J.; Kumagai, E.; Baptista, G.; Rosenthal, A. Sistematic study of benzo[a]pyrene in coffee samples. J. Braz. Chem. Soc. 2006, 17, 989–993. [Google Scholar] [CrossRef]
- Wandan, E.N.; Elleingand, E.F.; Ndouba, A.M. A screening for benzo[a]pyrène in cocoa beans subjected to different drying methods during on farm processing. Int. J. Eng. Sci. Technol. 2011, 3, 3621–3630. [Google Scholar]
- Pérez, R.A.; Albero, B. Ultrasound-assisted extraction methods for the determination of organic contaminants in solid and liquid samples. TrAC-Trend. Anal. Chem. 2023, 166, 117204. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Y.D.; Lin, W.Q. Determination of the sixteen polycyclic aromatic hydrocarbons in aquatic products by ultrasonic solvent extraction and GC-MS. Chin. J. Anal. Lab. 2019, 38, 805–809. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Yang, B.; Huang, J.; Zhang, S.; Yu, X.W.; Zhu, S.C.; Tang, Y.P. Determination of 16 polycyclic aromatic hydrocarbons(PAHs) in fried squid by ultrasonic extraction-gas chromatography-mass spectrometry. Sci. Technol. Food Ind. 2021, 42, 263–270. [Google Scholar] [CrossRef]
- Yang, S.D.; Tang, T.; Tan, Y.M.; Wang, F.Y.; Zhang, W.B.; Li, T.; Xia, M.Z. Determination of benzo(a)pyrene in fried and baked foods by HPLC combined with vesicular coacervative supramolecular solvent extraction. J. Food Sci. Technol. 2019, 56, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Płotka-Wasylka, J. Green analytical chemistry metrics: A review. Talanta 2022, 238, 123046. [Google Scholar] [CrossRef]
- Perez-Vazquez, A.; Carpena, M.; Barciela, P.; Cassani, L.; Simal-Gandara, J.; Prieto, M.A. Pressurized liquid extraction for the recovery of bioactive compounds from seaweeds for food industry application: A review. Antioxidants 2023, 12, 612. [Google Scholar] [CrossRef]
- Barp, L.; Visnjevec, A.M.; Moret, S. Pressurized liquid extraction: A powerful tool to implement extraction and purification of food contaminants. Foods 2023, 12, 2017. [Google Scholar] [CrossRef]
- Suranová, M.; Semanová, J.; Sklársová, B.; Simko, P. Application of accelerated solvent extraction for simultaneous isolation and pre-cleaning up procedure during determination of polycyclic aromatic hydrocarbons in smoked meat products. Food Anal. Methods 2015, 8, 1014–1020. [Google Scholar] [CrossRef]
- Pissinatti, R.; Nunes, C.M.; de Souza, A.G.; Junqueira, R.G.; de Souza, S.V.C. Simultaneous analysis of 10 polycyclic aromatic hydrocarbons in roasted coffee by isotope dilution gas chromatography-mass spectrometry: Optimization, in-house method validation and application to an exploratory study. Food Control 2015, 51, 140–148. [Google Scholar] [CrossRef]
- Andreu, V.; Picó, Y. Pressurized liquid extraction of organic contaminants in environmental and food samples. Trac-Trends Anal. Chem. 2019, 118, 709–721. [Google Scholar] [CrossRef]
- Prata, R.; López-Ruiz, R.; Nascimento, L.E.S.; Petrarca, M.H.; Godoy, H.T.; Frenich, A.G.; Arrebola, F.J. Method validation for GC-measurable pesticides and PAHs in baby foods using QuEChERS-based extraction procedure. J. Food Compos. Anal. 2024, 129, 106062. [Google Scholar] [CrossRef]
- Oduntan, A.O.; Tavengwa, N.T.; Cukrowska, E.; Mhlanga, S.D.; Chimuka, L. QuEChERS method development for bio-monitoring of low molecular weight polycyclic aromatic hydrocarbons in South African carp fish using HPLC-fluorescence: An initial assessment. S. Afr. J. Chem. 2016, 69, 98–104. [Google Scholar] [CrossRef]
- Dione, C.T.; Delhomme, O.; Diagne, I.; Diebakate, C.; Ndiaye, B.; Cisse, D.; Hane, M.; Dione, M.M.; Diouf, S.; Diop, A.; et al. Application of the QuEChERS method for the determination of pesticides, PAHs and PCBs in fish in Senegal. J. Environ. Sci. Health Part A 2022, 57, 869–879. [Google Scholar] [CrossRef]
- Aziz, A.; Abd Karim, K.; Ahmad, M.A.; Ridhwan, M.J.M. Polycyclic aromatic hydrocarbons (PAHs) levels and toxicity in herbal teas marketed in Malaysia using QuEChERS and GC-FID. Sains Malays. 2022, 51, 3981–3993. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Deng, Y.C.; Zheng, J.F.; Zhang, Y.; Yang, L.H.; Liao, C.J.; Su, L.; Zhou, Y.Y.; Gong, D.X.; Chen, L.; et al. The application of the QuEChERS methodology in the determination of antibiotics in food: A review. TrAC-Trend. Anal. Chem. 2019, 118, 517–537. [Google Scholar] [CrossRef]
- Kim, L.; Lee, D.; Cho, H.K.; Choi, S.D. Review of the QuEChERS method for the analysis of organic pollutants: Persistent organic pollutants, polycyclic aromatic hydrocarbons, and pharmaceuticals. Trends Environ. Anal. Chem. 2019, 22, e00063. [Google Scholar] [CrossRef]
- Chiang, C.F.; Hsu, K.C.; Tsai, T.Y.; Cho, C.Y.; Hsu, C.H.; Yang, D.J. Evaluation of optimal QuEChERS conditions of various food matrices for rapid determination of EU priority polycyclic aromatic hydrocarbons in various foods. Food Chem. 2021, 334, 127471. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.F.; Hsu, K.C.; Cho, C.Y.; Tsai, T.Y.; Hsu, C.H.; Yang, D.J. Comparison and establishment of appropriate methods to determine EU priority PAHs in charcoal-grilled chicken drumsticks with different treatments and their dietary risk assessments. Food Chem. Toxicol. 2020, 142, 111400. [Google Scholar] [CrossRef]
- Zacs, D.; Rozentale, I.; Reinholds, I.; Bartkevics, V. Multi-walled carbon nanotubes as effective sorbents for rapid analysis of polycyclic aromatic hydrocarbons in edible oils using dispersive solid-phase extraction (d-SPE) and gas chromatography-tandem mass spectrometry (GC-MS/MS). Food Anal. Methods 2018, 11, 2508–2517. [Google Scholar] [CrossRef]
- Slámová, T.; Sadowska-Rociek, A.; Franková, A.; Surma, M.; Banout, J. Application of QuEChERS-EMR-Lipid-DLLME method for the determination of polycyclic aromatic hydrocarbons in smoked food of animal origin. J. Food Compos. Anal. 2020, 87, 103420. [Google Scholar] [CrossRef]
- Agus, B.A.P.; Hussain, N.; Selamat, J. Quantification of PAH4 in roasted cocoa beans using QuEChERS and dispersive liquid-liquid micro-extraction (DLLME) coupled with HPLC-FLD. Food Chem. 2020, 303, 125398. [Google Scholar] [CrossRef]
- Onopiuk, A.; Kołodziejczak, K.; Marcinkowska-Lesiak, M.; Poltorak, A. Determination of polycyclic aromatic hydrocarbons using different extraction methods and HPLC-FLD detection in smoked and grilled meat products. Food Chem. 2022, 373, 131506. [Google Scholar] [CrossRef]
- Wang, W.; Guo, R.; Zhao, Y.; Tian, L.; Liu, C.W.; Liang, X.C. Determination of 24 polycyclic aromatic hydrocarbons in infant formula by gas chromatography-triple quadruple mass spectrometry. Phys. Test. Chem. Anal. Part B 2022, 58, 1287–1293. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Guo, Y.M.; Sun, X.M.; Hao, Q.; Cheng, X.; Zhang, L. Determination of 15 polycyclic aromatic hydrocarbons in aquatic products by solid-phase extraction and GC-MS. J. Sep. Sci. 2018, 41, 2188–2196. [Google Scholar] [CrossRef]
- Zachara, A.; Galkowska, D.; Juszczak, L. Method validation and determination of polycyclic aromatic hydrocarbons in vegetable oils by HPLC-FLD. Food Anal. Methods 2017, 10, 1078–1086. [Google Scholar] [CrossRef]
- Zachara, A.; Gałkowska, D.; Juszczak, L. Contamination of smoked meat and fish products from Polish market with polycyclic aromatic hydrocarbons. Food Control 2017, 80, 45–51. [Google Scholar] [CrossRef]
- Bogdanović, T.; Pleadin, J.; Petričević, S.; Listeš, E.; Sokolić, D.; Marković, K.; Ozogul, F.; Šimat, V. The occurrence of polycyclic aromatic hydrocarbons in fish and meat products of Croatia and dietary exposure. J. Food Compos. Anal. 2019, 75, 49–60. [Google Scholar] [CrossRef]
- Mottier, P.; Parisod, V.; Turesky, R.J. Quantitative determination of polycyclic aromatic hydrocarbons in barbecued meat sausages by gas chromatography coupled to mass spectrometry. J. Agric. Food Chem. 2000, 48, 1160–1166. [Google Scholar] [CrossRef]
- Martinez, E.; Gros, M.; Lacorte, S.; Barceló, D. Simplified procedures for the analysis of polycyclic aromatic hydrocarbons in water, sediments and mussels. J. Chromatogr. A 2004, 1047, 181–188. [Google Scholar] [CrossRef]
- Shi, L.K.; Zhang, D.D.; Liu, Y.L. Incidence and survey of polycyclic aromatic hydrocarbons in edible vegetable oils in China. Food Control 2016, 62, 165–170. [Google Scholar] [CrossRef]
- Rascón, A.J.; Azzouz, A.; Ballesteros, E. Multiresidue determination of polycyclic aromatic hydrocarbons in edible oils by liquid-liquid extraction–solid-phase extraction–gas chromatography–mass spectrometry. Food Control 2018, 94, 268–275. [Google Scholar] [CrossRef]
- Girelli, A.M.; Apriceno, A.; Tarola, A.M.; Tortora, F. Determination of polycyclic aromatic hydrocarbons in tea infusions samples by high performance liquid chromatography with fluorimetric detection. J. Food Qual. 2017, 2017, 1076876. [Google Scholar] [CrossRef]
- Hossain, M.A.; Salehuddin, S.M. Polycyclic aromatic hydrocarbons (PAHs) in edible oils by gas chromatography coupled with mass spectroscopy. Arab. J. Chem. 2012, 5, 391–396. [Google Scholar] [CrossRef]
- Guatemala-Morales, G.M.; Beltrán-Medina, E.A.; Murillo-Tovar, M.A.; Ruiz-Palomino, P.; Corona-González, R.I.; Arriola-Guevara, E. Validation of analytical conditions for determination of polycyclic aromatic hydrocarbons in roasted coffee by gas chromatography-mass spectrometry. Food Chem. 2016, 197, 747–753. [Google Scholar] [CrossRef]
- Li, Y.L.; Wang, C.L.; Zou, X.Q.; Feng, Z.Y.; Yao, Y.L.; Wang, T.; Zhang, C.C. Occurrence of polycyclic aromatic hydrocarbons (PAHs) in coral reef fish from the South China Sea. Mar. Pollut. Bull. 2019, 139, 339–345. [Google Scholar] [CrossRef]
- Fang, C.L.; Wang, G.M.; Xiang, L.; Xiao, L.; Ran, X.Z. Determination of 8 polycyclic aromatic hydrocarbons in aquatic products by LC-MS-MS coupled with optimized QuEChERS. Food Ferment. Ind. 2021, 57, 152–156. [Google Scholar] [CrossRef]
- Du, R.; Wan, L.B.; Gao, H.L.; Gao, H.D. Determination of 15 polycyclic aromatic hydrocarbons in vegetable oils by solid phase extraction coupled with high performance liquid chromatography. Cere Oil. 2022, 35, 158–162. [Google Scholar]
- Zhang, Y.S.; Han, J.H.; Tian, Z.; Zhang, D.; Zhang, Z.; Shi, X.X. Determination of 16 polycyclic aromatic hydrocarbons in kebabs by high performance liquid chromatography-fluorescence detector-secondary array tube detector. Food Ferment. Ind. 2022, 48, 274–280. [Google Scholar] [CrossRef]
- Zhong, D.L.; Yu, N.H.; Wang, M.J.; Mo, R.H.; Duan, J.M.; Tang, F.B. Determination of 16 polycyclic aromatic hydrocarbons in oil-tea camellia seed oil using molecularly imprinted solid-phase extraction coupled with GC-MS/MS. China Oil Fats 2022, 47, 118–123. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; El-Nouby, M.A.M.; Kimani, P.K.; Lim, L.W.; Rabea, E.I. A review of the modern principles and applications of solid-phase extraction techniques in chromatographic analysis. Anal. Sci. 2022, 38, 1457–1487. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.J.; Zhang, J.; Ren, Y.; Qi, L.; Yang, W.L.; Huang, Y.R. Quality control on the determination of 16 PAHs in soil using ASE-SPE/GC-MS. J. Instrum. Anal. 2012, 31, 1126–1131. [Google Scholar] [CrossRef]
- Nazir, N.A.M.; Raoov, M.; Mohamad, S. Spent tea leaves as an adsorbent for micro-solid-phase extraction of polycyclic aromatic hydrocarbons (PAHs) from water and food samples prior to GC-FID analysis. Microchem. J. 2020, 159, 105581. [Google Scholar] [CrossRef]
- Asfaram, A.; Dil, E.A.; Arabkhani, P.; Sadeghfar, F.; Ghaedi, M. Magnetic Cu: CuO-GO nanocomposite for efficient dispersive micro-solid phase extraction of polycyclic aromatic hydrocarbons from vegetable, fruit, and environmental water samples by liquid chromatographic determination. Talanta 2020, 218, 121131. [Google Scholar] [CrossRef]
- Zaw, M.M.; Poorahong, S.; Kanatharana, P.; Thavarungkul, P.; Thammakhet-Buranachai, C. A simple gelatin aerogel tablet sorbent for the effective vortex assisted solid phase extraction of polycyclic aromatic hydrocarbons from tea samples. Food Chem. 2022, 383, 132388. [Google Scholar] [CrossRef]
- Wang, N.; Lu, Y.M.; Cui, B. Preparation and application of β-Cyclodextrin functionalised graphene oxide-grafted silica sorbents for solid-phase extraction (SPE) of polycyclic aromatic hydrocarbons from fried food using a box-behnken design. Food Anal. Methods 2021, 14, 1577–1589. [Google Scholar] [CrossRef]
- Villa, C.C.; Sánchez, L.T.; Valencia, G.A.; Ahmed, S.; Gutiérrez, T.J. Molecularly imprinted polymers for food applications: A review. Trends Food Sci. Technol. 2021, 111, 642–669. [Google Scholar] [CrossRef]
- Garballo-Rubio, A.; Soto-Chinchilla, J.; Martín-Pozo, L.; Zafra-Gómez, A. Use of Quick, Easy, Cheap, Effective, Rugged & Safe (QuEChERS) and molecular imprinted polymer followed by gas chromatography with tandem mass spectrometry for the quantitative analysis of polycyclic aromatic hydrocarbons (PAH4) in complex health supplements. J. Food Compos. Anal. 2020, 93, 103588. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Q.; Zhan, C.Q.; Liu, W.Q.; He, M.H.; Qiu, X.Y. Simultaneous determination of 16 kinds of polycyclic aromatic hydrocarbons in tea by gas chromatography-triple quadrupole tandem mass spectrometry. J. Food Saf. Qual. 2021, 12, 7585–7591. [Google Scholar] [CrossRef]
- Jalili, V.; Barkhordari, A.; Ghiasvand, A. Solid-phase microextraction technique for sampling and preconcentration of polycyclic aromatic hydrocarbons: A review. Microchem. J. 2020, 157, 104967. [Google Scholar] [CrossRef]
- Sajid, M.; Nazal, M.K.; Rutkowska, M.; Szczepanska, N.; Namiesnik, J.; Plotka-Wasylka, J. Solid phase microextraction: Apparatus, sorbent materials, and application. Crit. Rev. Anal. Chem. 2019, 49, 271–288. [Google Scholar] [CrossRef]
- Drabinska, N.; Marcinkowska, M.A.; Wieczorek, M.N.; Jelen, H.H. Application of sorbent-based extraction techniques in food analysis. Molecules 2023, 28, 7985. [Google Scholar] [CrossRef]
- Aresta, A.M.; Zambonin, C. Determination of polycyclic aromatic hydrocarbons (PAHs) in coffee samples by DI-SPME-GC/MS. Food Anal. Methods 2023, 16, 1009–1016. [Google Scholar] [CrossRef]
- Fathollahy, I.; Baglari, B.; Pirsa, S. Application of solid-phase microextraction/gas chromatography method for extraction, identification, and comparison of polycyclic aromatic hydrocarbons from industrial and traditional edible oils. Main Group Chem. 2023, 22, 187–200. [Google Scholar] [CrossRef]
- Erdem, P.; Tagaç, A.A.; Bozkurt, S.S.; Merdivan, M. Chitosan and dicationic ionic liquid intercalated clay-coated solid-phase microextraction fiber for determination of sixteen polycyclic aromatic hydrocarbons in coffee and tea samples. Talanta 2021, 235, 122764. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Hu, K.; Liu, X.B.; Li, L.X.; Li, Z.H.; Wang, P.; Zhang, Z.Q.; Zhang, S.S. Covalent organic framework@Ti3C2Tx composite as solid phase microextraction coating for the determination of polycyclic aromatic hydrocarbons in honey samples. Anal. Chim. Acta 2023, 1237, 9. [Google Scholar] [CrossRef]
- Xu, L.; Hu, W.; Luo, X.G.; Zhang, J. Covalent organic framework in situ grown on the metal-organic framework as fiber coating for solid-phase microextraction of polycyclic aromatic hydrocarbons in tea. Microchim. Acta 2023, 190, 344. [Google Scholar] [CrossRef]
- Ma, J.M.; Sun, G.Q.; Sun, D.Q.; Yu, F.; Hu, M.J.; Lu, T. Application of gel permeation chromatography technology in asphalt materials: A review. Constr. Build. Mater. 2021, 278, 122386. [Google Scholar] [CrossRef]
- Shen, X.X.; Zhan, J.L.; Tang, X.Y. Determination of 16 polycyclic aromatic hydrocarbons in roast duck skin by gel permeation chromatography clean-up combined with gas chromatography-mass spectrometry. Meat Res. 2020, 34, 77–82. [Google Scholar] [CrossRef]
- Wang, J.; Jia, L.; Wei, W.; Lang, S.; Shao, P.; Fan, X. Determination of polycyclic aromatic hydrocarbons in edible oil by gel permeation chromatography and ultra-high performance liquid chromatography coupled with diode array detector and fluorescence detector. Acta Chromatogr. 2016, 28, 415–427. [Google Scholar] [CrossRef]
- Cotugno, P.; Massari, F.; Aresta, A.; Zambonin, C.; Ragni, R.; Monks, K.; Avagyan, L.; Böttcher, J. Advanced gel permeation chromatography system with increased loading capacity: Polycyclic aromatic hydrocarbons detection in olive oil as a case of study. J. Chromatogr. A 2021, 1639, 461920. [Google Scholar] [CrossRef] [PubMed]
- Ostadgholami, M.; Zeeb, M.; Amirahmadi, M.; Daraei, B. Multivariate optimization and validation of a modified QuEChERS method for determination of PAHs and PCBs in grilled meat by GC-MS. Foods 2024, 13, 143. [Google Scholar] [CrossRef] [PubMed]
- Kamal El-Deen, A.; Shimizu, K. Modified μ-QuEChERS coupled to diethyl carbonate-based liquid microextraction for PAHs determination in coffee, tea, and water prior to GC–MS analysis: An insight to reducing the impact of caffeine on the GC–MS measurement. J. Chromatogr. B 2021, 1171, 122555. [Google Scholar] [CrossRef] [PubMed]
- Peiravian, F.; Shakoori, A.; Moradi, V.; Salamzadeh, J.; Mahboubi, A. Simultaneous analysis of 10 priority PAHs in Iranian Sangak bread samples by developing a GC-MS method. Iran. J. Pharm. Res. 2021, 20, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Givechev, I.; Tanev, D.; Danalev, D. Development and validation of GC/MS method for simultaneous determination of 16 polycyclic aromatic hydrocarbons (PAHs) in pork meat matrix. Acta Chromatogr. 2021, 33, 57–63. [Google Scholar] [CrossRef]
- Akbari-Adergani, B.; Mahmood-babooi, K.; Salehi, A.; Khaniki, G.J.; Shariatifar, N.; Sadighara, P.; Zeinali, T. GC-MS determination of the content of polycyclic aromatic hydrocarbons in bread and potato Tahdig prepared with the common edible oil. Environ. Monit. Assess. 2021, 193, 540. [Google Scholar] [CrossRef]
- Liao, Z.Y.; Gao, Z.B.; Cao, D.L.; Yang, Q.W. Occurrence and exposure evaluation of polycyclic aromatic hydrocarbons in tea samples consumed in Beijing-Tianjin-Hebei regions of China. J. Food Compos. Anal. 2023, 123, 10. [Google Scholar] [CrossRef]
- Souza Futigami, L.; Barcellos Hoff, R.; Turnes Pasini Deolindo, C.; Kleemann, C.R.; Alves de Oliveira, L.V.; de Francisco de Casas, A.; Burin, V.M. Search for new green natural solid phases for sample preparation for PAHs determination in seafood samples followed by LC and GC–MS/MS analysis. Food Res. Int. 2024, 183, 114240. [Google Scholar] [CrossRef]
- Hungerford, N.L.; Fletcher, M.T.; Tsai, H.H.; Hnatko, D.; Swann, L.J.; Kelly, C.L.; Anuj, S.R.; Tinggi, U.; Webber, D.C.; Were, S.T.; et al. Occurrence of environmental contaminants (pesticides, herbicides, PAHs) in Australian/Queensland Apis mellifera honey. Food Addit. Contam. Part B 2021, 14, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wu, S. Halogenated polycyclic aromatic hydrocarbons and their parent compounds in ready-to-eat seafood rich in salt: Method validation, profiles, correlation, and exposure risks. Food Control 2022, 136, 108864. [Google Scholar] [CrossRef]
- Zastrow, L.; Speer, K.; Schwind, K.H.; Jira, W. A sensitive GC-HRMS method for the simultaneous determination of parent and oxygenated polycyclic aromatic hydrocarbons in barbecued meat and meat substitutes. Food Chem. 2021, 365, 11. [Google Scholar] [CrossRef]
- GB 5009.265-2021; National Health Commission of the PRC, State Administration for Market Regulation. National Food Safety Standard—Detection of PAHs in Food. China Standard Press: Beijing, China, 2022.
- Russo, M.V.; Avino, P.; Notardonato, I. PAH residues in honey by ultrasound-vortex-assisted liquid-liquid micro-extraction followed by GC-FID/IT-MS. Food Anal. Methods 2017, 10, 2132–2142. [Google Scholar] [CrossRef]
- Gómez-Ruiz, J.A.; Cordeiro, F.; López, P.; Wenzl, T. Optimisation and validation of programmed temperature vaporization (PTV) injection in solvent vent mode for the analysis of the 15+1 EU-priority PAHs by GC-MS. Talanta 2009, 80, 643–650. [Google Scholar] [CrossRef]
- Mandal, S.; Poi, R.; Hazra, D.K.; Ansary, I.; Bhattacharyya, S.; Karmakar, R. Review of extraction and detection techniques for the analysis of pesticide residues in fruits to evaluate food safety and make legislative decisions: Challenges and anticipations. J. Chromatogr. B 2023, 1215, 123587. [Google Scholar] [CrossRef]
- Peng, Y.; He, S.Y.; Wang, F.H.; Zheng, H.B.; Meng, Z. Determination of polycyclic aromatic hydrocarbons in edible oil by magnetic solid phase extraction based on a mesoporous molybdenum disulfide/graphite prior to gas chromatography-mass spectrometry. Microchem. J. 2022, 183, 108146. [Google Scholar] [CrossRef]
- Colón, L.P.; Rascon, A.J.; Ballesteros, E. Trace-level determination of polycyclic aromatic hydrocarbons in dairy products available in Spanish supermarkets by semi-automated solid-phase extraction and gas chromatography-mass spectrometry detection. Foods 2022, 11, 713. [Google Scholar] [CrossRef]
- Purcaro, G.; Moret, S.; Conte, L.S. Overview on polycyclic aromatic hydrocarbons: Occurrence, legislation and innovative determination in foods. Talanta 2013, 105, 292–305. [Google Scholar] [CrossRef]
- Analytical Methods Committee AMCTB. Thermal desorption part 1: Introduction and instrumentation. Anal. Methods 2020, 12, 3425–3428. [Google Scholar] [CrossRef]
- Guiffard, I.; Geny, T.; Veyrand, B.; Marchand, P.; Pellouin-Grouhel, A.; Bizec, B.L.; Bichon, E. Quantification of light polycyclic aromatic hydrocarbons in seafood samples using on-line dynamic headspace extraction, thermodesorption, gas chromatography tandem mass spectrometry, based on an isotope dilution approach. J. Chromatogr. A 2020, 1619, 460906. [Google Scholar] [CrossRef] [PubMed]
- Drventić, I.; Šala, M.; Vidović, K.; Kroflič, A. Direct quantification of PAHs and nitro-PAHs in atmospheric PM by thermal desorption gas chromatography with electron ionization mass spectroscopic detection. Talanta 2023, 251, 123761. [Google Scholar] [CrossRef]
- Manzano, C.; Hoh, E.; Simonich, S.L.M. Improved separation of complex polycyclic aromatic hydrocarbon mixtures using novel column combinations in GCxGC/TOF-MS. Environ. Sci. Technol. 2012, 46, 7677–7684. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.M.; Raines, J.M.; Rodriguez, J.M. Determination of polycyclic aromatic hydrocarbons in seafood. Food Anal. Methods 2013, 6, 1330–1336. [Google Scholar] [CrossRef]
- Ekner, H.; Dreij, K.; Sadiktsis, I. Determination of polycyclic aromatic hydrocarbons in commercial olive oils by HPLC/GC/MS-Occurrence, composition and sources. Food Control 2022, 132, 108528. [Google Scholar] [CrossRef]
- Ju, H.; Kim, B.; Kim, J.; Baek, S.Y. Development of candidate reference method for accurate determination of four polycyclic aromatic hydrocarbons in olive oil via gas chromatography/high-resolution mass spectrometry using 13C-labeled internal standards. Food Chem. 2020, 309, 125639. [Google Scholar] [CrossRef] [PubMed]
- Al-Alam, J.; Fajloun, Z.; Chbani, A.; Millet, M. A multiresidue method for the analysis of 90 pesticides, 16 PAHs, and 22 PCBs in honey using QuEChERS-SPME. Anal. Bioanal. Chem. 2017, 409, 5157–5169. [Google Scholar] [CrossRef] [PubMed]
- Duedahl-Olesen, L.; Iversen, N.M.; Kelmo, C.; Jensen, L.K. Validation of QuEChERS for screening of 4 marker polycyclic aromatic hydrocarbons in fish and malt. Food Control 2020, 108, 106434. [Google Scholar] [CrossRef]
- GB 5009.27-2016; National Health and Family Planning Commission of the PRC, State Food and Drug Administration. National Food Safety Standard—Detection of Benzo[a]pyrene in Food. China Standard Press: Beijing, China, 2017.
- Uinted States Environmental Protection Agency. Method 610-Polynuclear Aromatic Hydrocarbons. Available online: https://www.epa.gov/sites/default/files/2015-10/documents/method_610_1984.pdf (accessed on 20 January 2024).
- Bulanda, S.; Janoszka, B. Polycyclic aromatic hydrocarbons (PAHs) in roasted pork meat and the effect of dried fruits on PAH content. Int. J. Environ. Res. Public Health 2023, 20, 4922. [Google Scholar] [CrossRef]
- Harrison, D.M.; Chang, W.C.; Lin, H.T. Dietary exposure and health risk assessment of polycyclic aromatic hydrocarbons in black tea consumed in Taiwan. Toxics 2024, 12, 134. [Google Scholar] [CrossRef]
- Tfouni, S.A.V.; Reis, R.M.; Kamikata, K.; Gomes, F.M.L.; Morgano, M.A.; Furlani, R.P.Z. Polycyclic aromatic hydrocarbons in teas using QuEChERS and HPLC-FLD. Food Addit. Contam. Part B 2018, 11, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudpour, M.; Mohtadinia, J.; Ansarin, M.; Nemati, M. Dispersive liquid-liquid microextraction for HPLC-UV determination of PAHs in milk. J. AOAC Int. 2016, 99, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.; Agarwal, T. Comparative analysis of conventional and greener extraction methods and method validation for analyzing PAHs in cooked chicken and roasted coffee. Food Chem. 2021, 364, 130440. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, E.N.; Hajeb, P.; Selamat, J.; Lee, S.Y.; Razis, A.F.A. Bioaccessibility of polycyclic aromatic hydrocarbons (PAHs) in grilled meat: The effects of meat doneness and fat content. Int. J. Environ. Res. Public Health 2022, 19, 736. [Google Scholar] [CrossRef]
- Koltsakidou, A.; Zacharis, C.K.; Fytianos, K. A validated liquid chromatographic method for the determination of polycyclic aromatic hydrocarbons in honey after homogeneous liquid–liquid extraction using hydrophilic acetonitrile and sodium chloride as mass separating agent. J. Chromatogr. A 2015, 1377, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.F.; Liao, P.L.; Lin, Y.J.; Huang, S.H.; Wu, Y.H.S.; Teng, C.F.; Yang, D.J. Assessment of various conditions for the simultaneous determination of US EPA and EU priority PAHs in coffee samples and their PAHs consumption risk. Food Res. Int. 2023, 169, 112947. [Google Scholar] [CrossRef] [PubMed]
- Jesús Dueñas-Mas, M.; Ballesteros-Gómez, A.; Rubio, S. Characterization of a new sustainable supramolecular solvent and application to the determination of oxy-PAHs in meat, seafood and fish tissues. Food Chem. 2023, 405, 134731. [Google Scholar] [CrossRef]
- Lung, S.C.C.; Liu, C.H. Fast analysis of 29 polycyclic aromatic hydrocarbons (PAHs) and nitro-PAHs with ultra-high performance liquid chromatography-atmospheric pressure photoionization-tandem mass spectrometry. Sci. Rep. 2015, 5, 12992. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, L.; Zhang, X.C.; Chen, Y.J.; Wang, J. Development and identification of monoclonal antibodies against polycyclic aromatic hydrocarbons. Immunol. J. 2015, 31, 712–716. [Google Scholar] [CrossRef]
- Sheryna, J.N.; Azmi, A.A.A.R.; Ahmad, A. Enzyme-linked immunosorbent assay (ELISA)-based-sensor for determination of benzo[a]pyrene in river water using screen-printed gold electrode. Malays. J. Anal. Sci. 2017, 21, 518–526. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Y.S.; Meng, X.Y.; Qiao, B.; Hu, P.; Meng, X.M.; Lu, S.Y.; Ren, H.L.; Liu, Z.S.; Zhou, Y. Fluorescence-linked immunosorbent assay for detection of phenanthrene and its homolog. Anal. Biochem. 2018, 547, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.M.; Li, H.M.; Yin, X.Y.; Si, Y.; Qin, L.N.; Yang, H.F.; Xiao, J.X.; Peng, D.P. Preparation of monoclonal antibody against pyrene and benzo[a]pyrene and development of enzyme-linked immunosorbent assay for fish, shrimp and crab samples. Foods 2022, 11, 3220. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhuang, H.S. A chemical synthesis of benzo[a]pyrene hapten and its application in a streptavidin-horseradish peroxidase-based enzyme-linked immunological analysis. J. Chem. 2018, 2018, 8673476. [Google Scholar] [CrossRef]
- Nilghaz, A.; Mousavi, S.M.; Amiri, A.; Tian, J.F.; Cao, R.; Wang, X.A. Surface-enhanced Raman spectroscopy substrates for food safety and quality analysis. J. Agric. Food Chem. 2022, 70, 5463–5476. [Google Scholar] [CrossRef]
- Su, M.K.; Jiang, Q.; Guo, J.H.; Zhu, Y.X.; Cheng, S.; Yu, T.; Du, S.S.; Jiang, Y.F.; Liu, H.L. Quality alert from direct discrimination of polycyclic aromatic hydrocarbons in edible oil by liquid-interfacial surface-enhanced Raman spectroscopy. LWT-Food Sci. Technol. 2021, 143, 111143. [Google Scholar] [CrossRef]
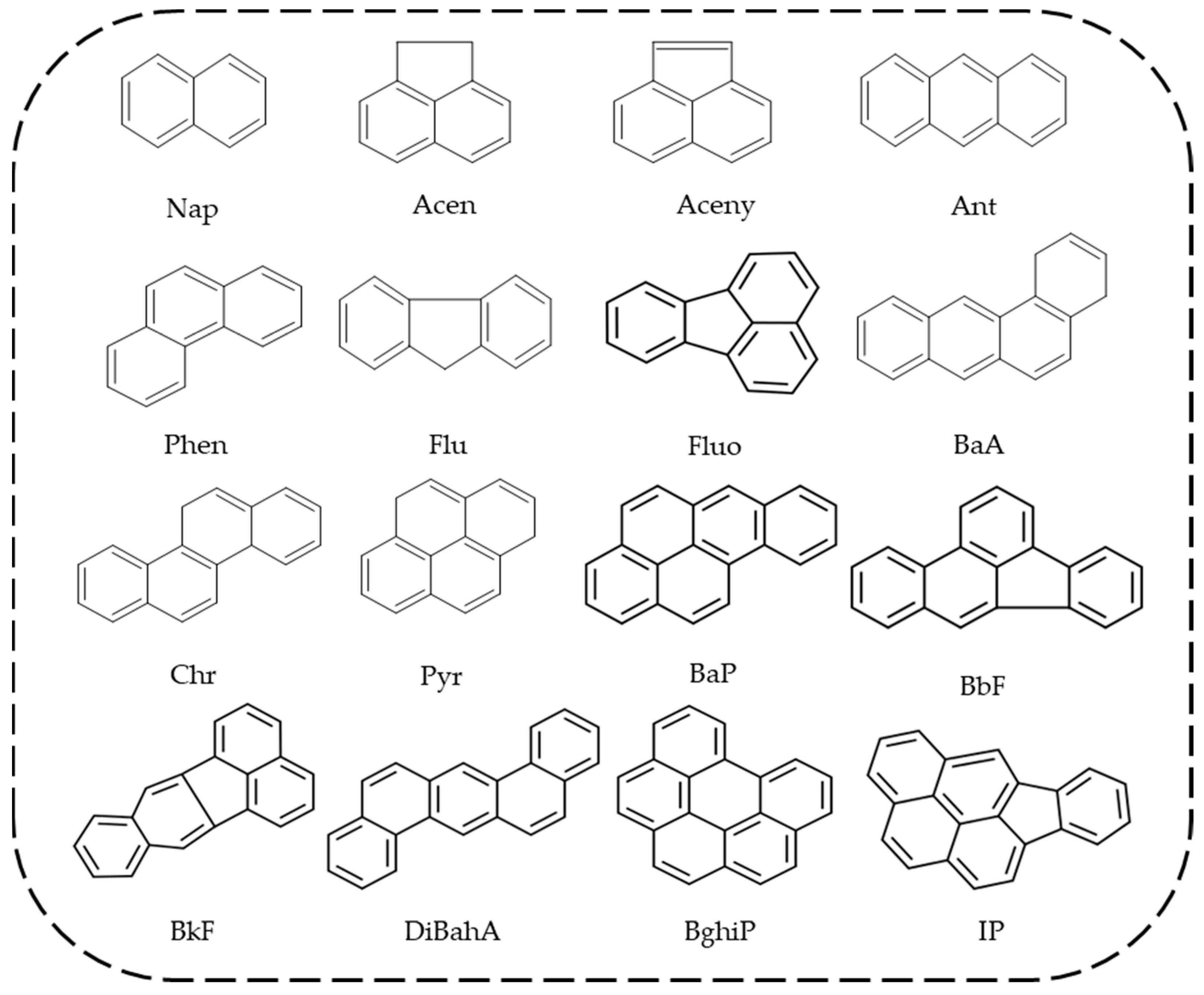
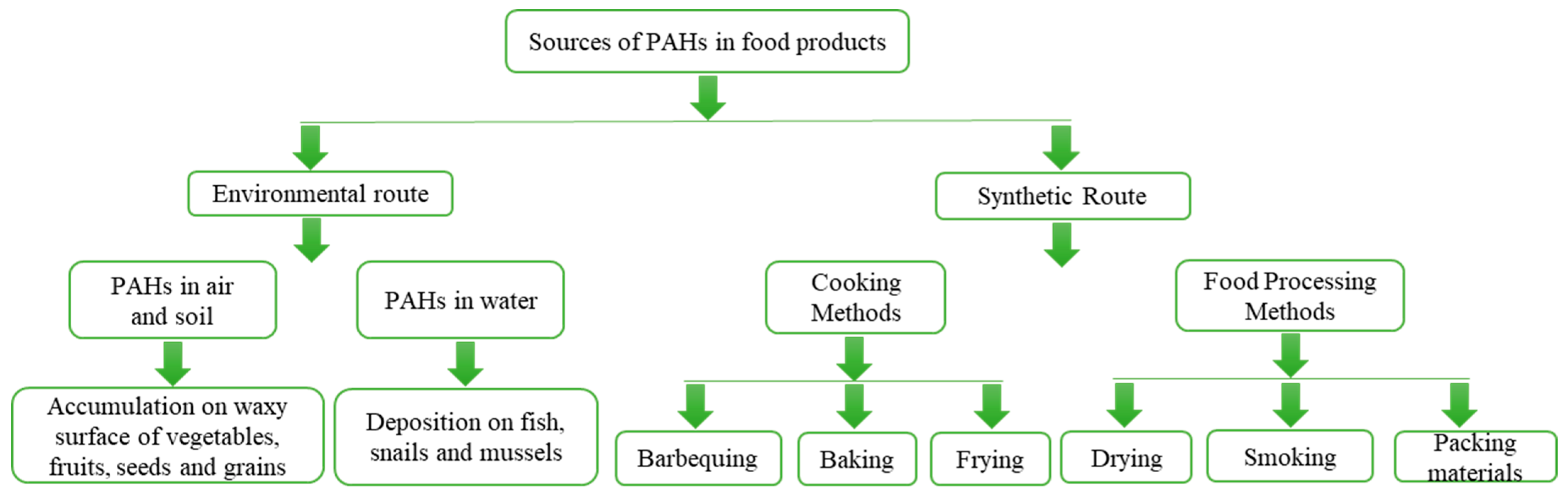
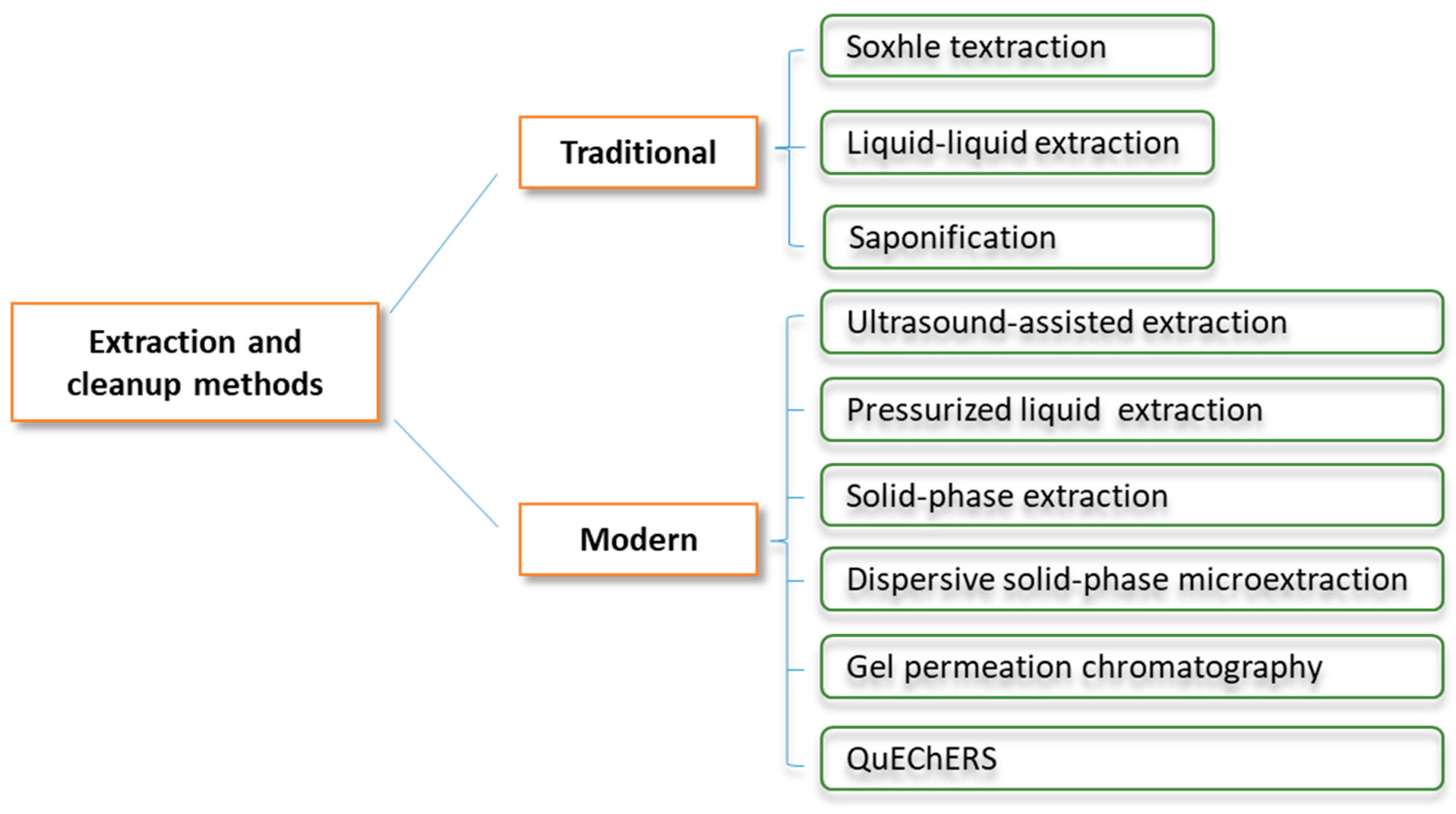
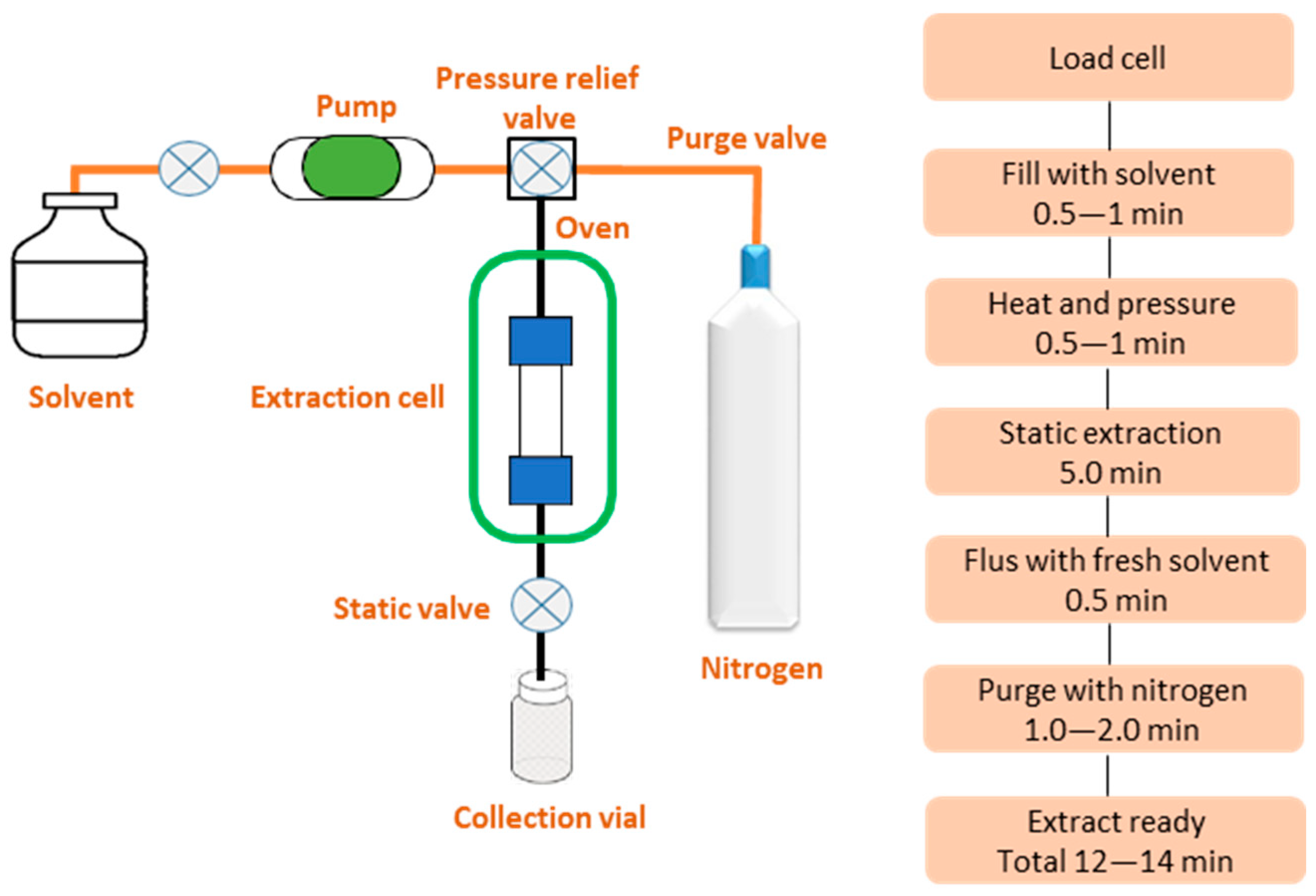

| PAHs | Abbreviation | Formula | Aromatic Ring | Water Solubility (mg/L) | Melting Point (°C) | Boiling Point (°C) | Vapor Pressure (mm Hg) |
|---|---|---|---|---|---|---|---|
| Naphthalene | Nap | C10H8 | 2 | 31 | 80.26 | 218 | 0.087 |
| Acenapthene | Acen | C12H10 | 3 | 3.8 | 95 | 96 | 4.47 × 10−3 |
| Acenaphthylene | Aceny | C14H10 | 3 | 16.1 | 92–93 | 265–275 | 0.029 |
| Anthracene | Ant | C14H10 | 3 | 0.045 | 218 | 340–342 | 1.75 × 10−6 |
| Phenanthrene | Phen | C14H10 | 3 | 1.1 | 100 | 340 | 6.8 × 10−4 |
| Fluorene | Flu | C13H10 | 3 | 1.9 | 116–117 | 295 | 3.2 × 10−4 |
| Fluoranthene | Fluo | C16H10 | 4 | 0.26 | 110.8 | 375 | 5.0 × 10−6 |
| Benzo[a]anthracene | BaA | C20H12 | 4 | 0.011 | 158 | 438 | 2.5 × 10−6 |
| Chrysene | Chr | C18H12 | 4 | 1.5 × 10−3 | 254 | 448 | 6.4 × 10−9 |
| Pyrene | Pyr | C16H10 | 4 | 0.132 | 156 | 393–404 | 2.5 × 10−6 |
| Benzo[a]pyrene | BaP | C20H12 | 5 | 3.8 × 10−3 | 179–179.3 | 495 | 5.6 × 10−9 |
| Benzo[b]fluoranthene | BbF | C20H12 | 5 | 8.0 × 10−4 | 215.7 | 480 | 9.59 × 10−11 |
| Benzo[k]fluoranthene | BkF | C20H12 | 5 | 1.5 × 10−3 | 168.3 | NS | 5.0 × 10−6 |
| Dibenz[a,h]anthracene | DiBahA | C22H14 | 6 | 5.0 × 10−4 | 262 | NS | 1.0 × 10−10 |
| Benzo[g,h,i]perylene | BghiP | C22H12 | 6 | 2.6 × 10−4 | 273 | 550 | 1.03 × 10−10 |
| Indeno[1,2,3-c,d]pyrene | IP | C22H12 | 6 | 0.062 | 163.6 | 530 | 10−16–10−10 |
| Nation | Type of Food | PAHs | Concentration Range (μg/kg) | Ref. |
|---|---|---|---|---|
| China | Youtiao | 16 EPA PAHs | 9.90–89.97 | [71] |
| Tea | 22 PAHs | 136.99–462.51 | [72] | |
| Tea | 16 EPA PAHs | 11.4–1251 | [73] | |
| Wild marine fishes | 16 EPA PAHs | 34.7–108 | [74] | |
| Smoked meat | 16 EPA PAHs | 14.4–56.3 | [75] | |
| Nigeria | Oils and tomato sauces from canned fish | 16 EPA PAHs | 101–698 | [76] |
| Tea | 16 EPA PAHs | 0.76–44.57 | [77] | |
| Smoked fishes | 16 EPA PAHs | 694–3585 | [78] | |
| Vegetables (e.g., white spinach and lettuce) | 16 EPA PAHs | 100–5000 | [79] | |
| Brazil | Honey | 16 EPA PAHs | 1.4–23.3 | [80] |
| Chocolate | PAH4 | 8.38–41.58 | [81] | |
| Iran | Edible vegetable oils | 13 PAHs | 12.63–182.80 | [66] |
| Fish and prawn | 16 EPA PAHs | 2.3–13.81 | [82] | |
| Coffee | 16 EPA PAHs | 13.00–20.78 | [83] | |
| Japan | Grilled foods | 12 PAHs | 0.062–1102 | [57] |
| Smoke-dried bonito | 29 PAHs | 419–1070 | [84] | |
| Latvia | Cereal products | PAH4 | 0.22–1.62 | [85] |
| Tanzania | Smoked and sun-dried fishes | 13 PAHs | 80–33,900 | [86] |
| Ghana | Smoke-cured fish | 16 EPA PAHs | 510.59–1461.79 | [87] |
| Italy | Seafood | 16 EPA PAHs | 20.26–282.2 | [88] |
| Bangladesh | Milk | 16 EPA PAHs | 0.5497–1.077 | [89] |
| Pakistan | Vegetables | 16 EPA PAHs | 51.6–402 | [90] |
| Saudi Arabia | Vegetables and fruits | 16 EPA PAHs | 10.11–798.21 | [91] |
| Argentina | Tea | 16 EPA PAHs | 509.7–2746.5 | [92] |
| Turkey | Tea | 15 PAHs | 212.2–953.9 | [93] |
| Honey | 14 PAHs | 464.32–650.24 | [94] | |
| India | Bread, biscuits, tea, coffee, oils, chocolates, grapes, pepper, and fishes | 16 EPA PAHs | 0.18–61,967 | [95] |
| Egypt | Grilled, pan-fried, and boiled meat | 15 PAHs | 12.21–72.16 | [96] |
| Romania | Smoked fish and smoked cheese | 16 EPA PAHs | 8.54–56.3 | [97] |
| Poland | Tea | 16 EPA PAHs | 41.5–2910.2 | [98] |
| Tea infusion | 52.9–2226.0 ng/L | |||
| Canada | Mussel, clam, and cockle | 17 PAHs | 510–17,550 | [99] |
| Korea | Processed foods and their raw materials (e.g., cereals, nuts, fruit, meat, fish, beverages, and seasonings) | 8 PAHs | 0.08–11.97 | [100] |
| Togo | Fish | 12 PAHs | 5.24–149.0 | [101] |
| Ghana | Fish | 28 PAHs | 71–481 | [102] |
| Vegetables (e.g., Chinese cabbage, lettuce, and garden egg leaves) | 16 EPA PAHs | 3.16–9.29 | [103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Zhang, L.; Pan, L.; Yang, D. Polycyclic Aromatic Hydrocarbons’ Impact on Crops and Occurrence, Sources, and Detection Methods in Food: A Review. Foods 2024, 13, 1977. https://doi.org/10.3390/foods13131977
Liu T, Zhang L, Pan L, Yang D. Polycyclic Aromatic Hydrocarbons’ Impact on Crops and Occurrence, Sources, and Detection Methods in Food: A Review. Foods. 2024; 13(13):1977. https://doi.org/10.3390/foods13131977
Chicago/Turabian StyleLiu, Tengfei, Li Zhang, Leiqing Pan, and Daifeng Yang. 2024. "Polycyclic Aromatic Hydrocarbons’ Impact on Crops and Occurrence, Sources, and Detection Methods in Food: A Review" Foods 13, no. 13: 1977. https://doi.org/10.3390/foods13131977
APA StyleLiu, T., Zhang, L., Pan, L., & Yang, D. (2024). Polycyclic Aromatic Hydrocarbons’ Impact on Crops and Occurrence, Sources, and Detection Methods in Food: A Review. Foods, 13(13), 1977. https://doi.org/10.3390/foods13131977






