Effects of Freeze–Thaw Pretreatment Combined with Hot Air on Snake Gourd (Trichosanthes anguina L.)
Abstract
1. Introduction
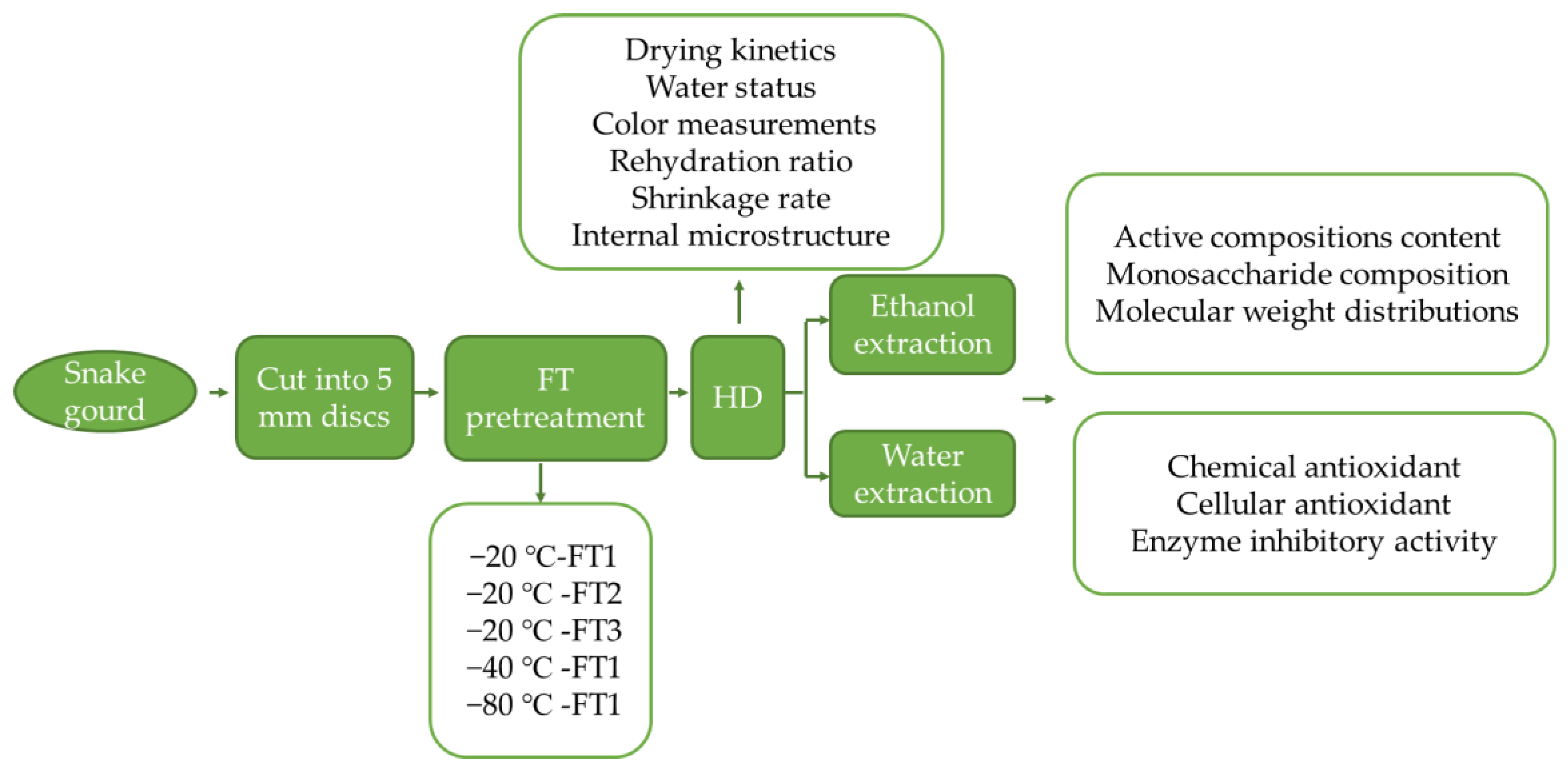
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Drying Experiments
2.2.1. Freeze-Thaw Cycles Pretreatment
2.2.2. Hot Air Drying
2.3. Drying Characteristics Analysis
2.3.1. Drying Kinetics
Determination of Moisture Ratio
Establishment of the Mathematical Model of Drying Kinetics
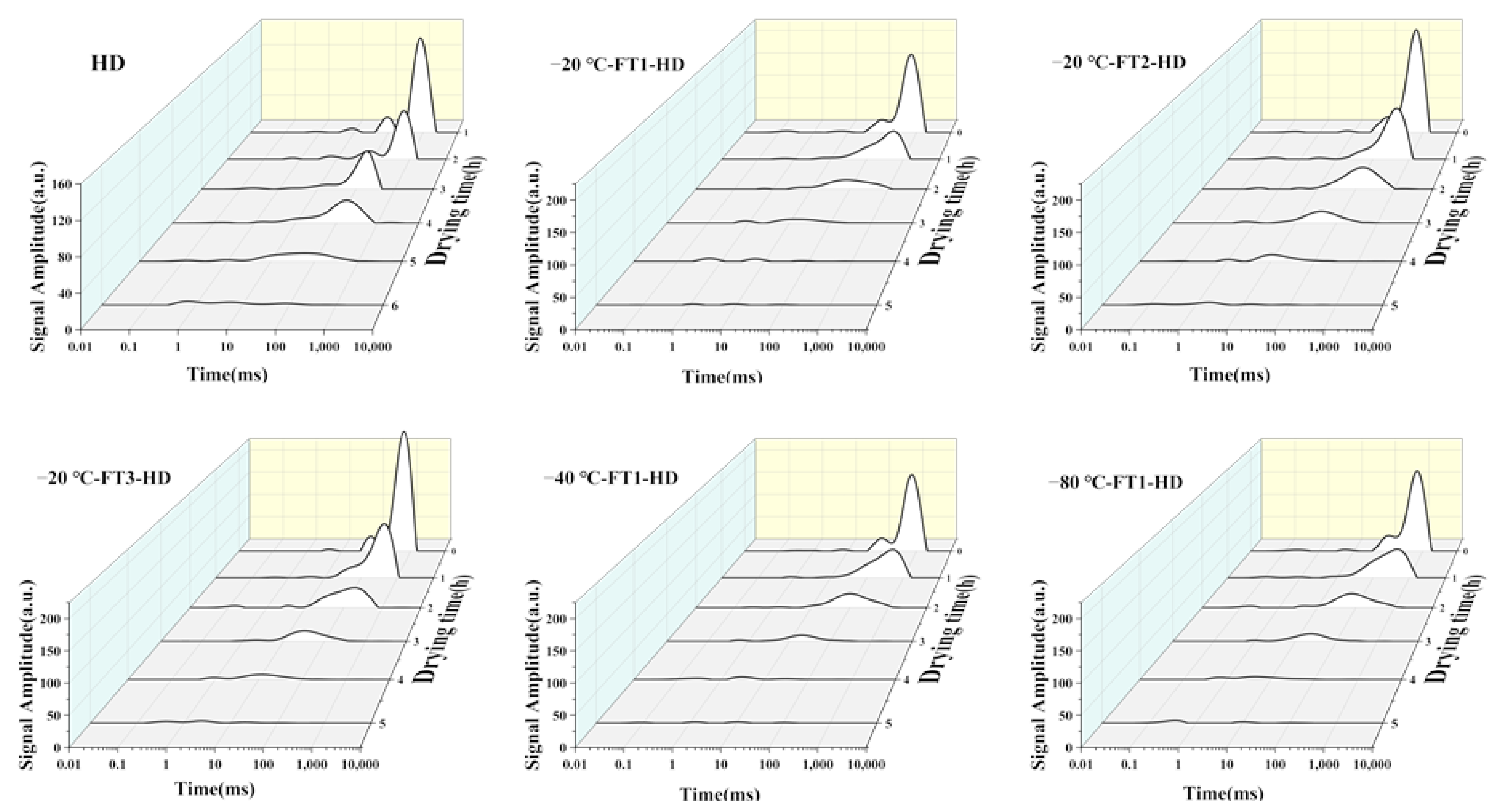
2.3.2. Water Status
2.3.3. Color Measurements
2.3.4. Rehydration Ratio
2.3.5. Shrinkage Rate
2.3.6. Internal Microstructure
2.4. Structure Characterization of Ethanol (TG1) and Water (TG2) Extraction
2.4.1. Preparation of TG1 and TG2
2.4.2. Determination of Active Compositions (Polysaccharide and Total Phenolic) Content
2.4.3. Determination of Monosaccharide Composition
2.4.4. Determination of Molecular Weight Distributions
2.5. Biological Activities of Dried Snake Gourd Extraction
2.5.1. Chemical Antioxidant Evaluation
2.5.2. Cellular Antioxidants Determination
Measurements of ROS Level
Measurements of LDH, SOD, CAT, GSH and MDA
2.5.3. Inhibitory Activity against α-Amylase and α-Glucosidase Assay of TG1 and TG2
2.6. Statistical Analysis
3. Results and Discussion
3.1. Effect of FT Pretreatment on the Drying Process
3.2. Characterization of TG1 and TG2
3.2.1. Active Compositions Content Analysis
3.2.2. Monosaccharide Composition Analysis
3.2.3. Molecular Weight Distributions Analysis
3.3. Biological Activities of TG1 and TG2
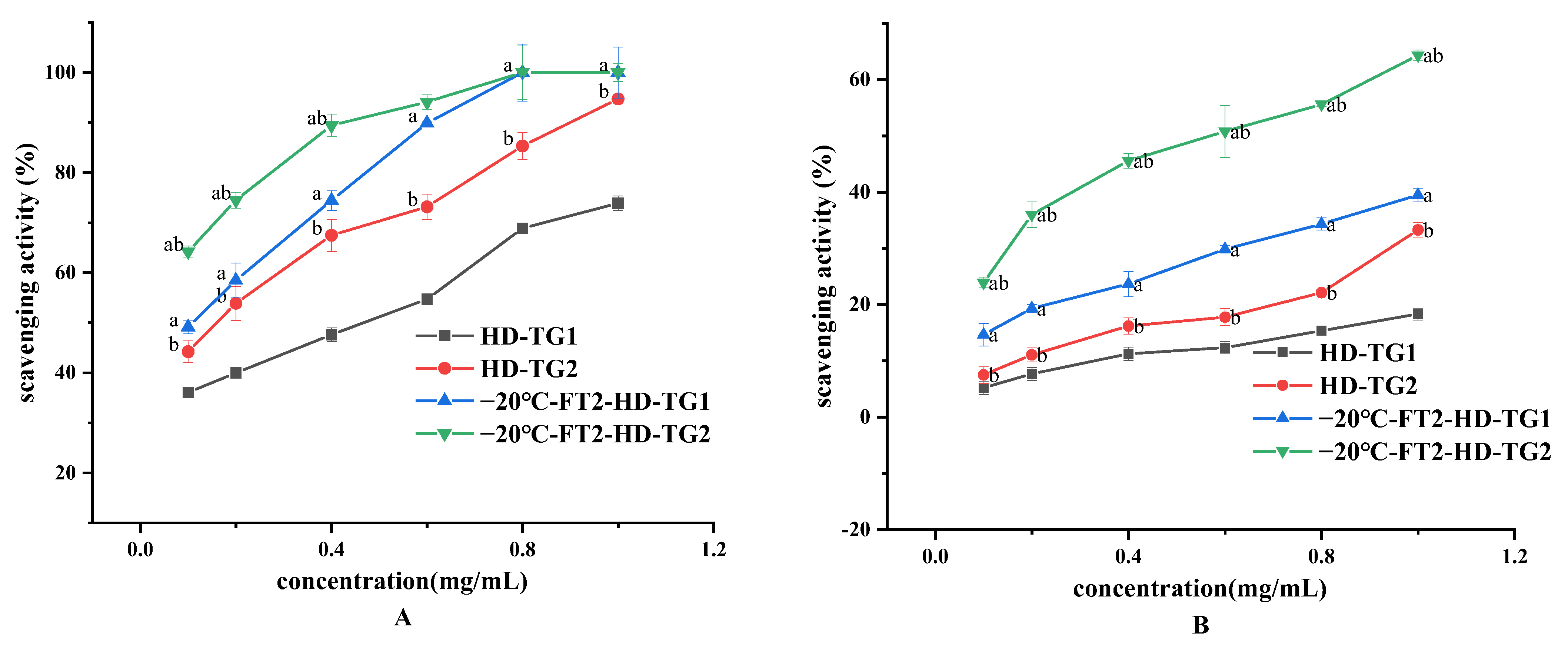
3.3.1. Chemical Antioxidant Activity of TG1 and TG2
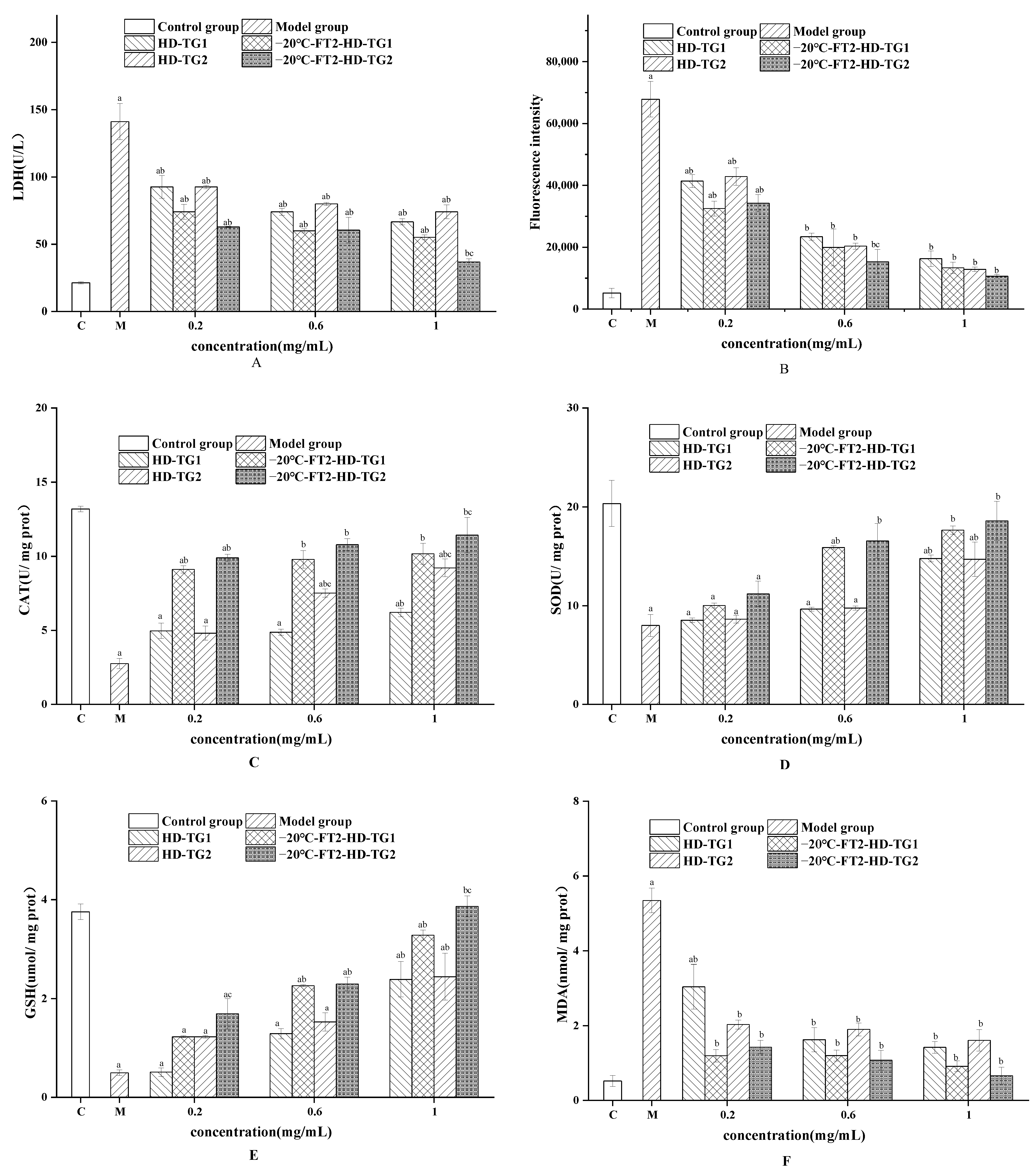
3.3.2. Cellular Antioxidant Activities of TG1 and TG2
Effect of TG1 and TG2 on LDH Levels
Effect of TG1and TG2 on ROS Levels
Effect of TG1 and TG2 on SOD, CAT, GSH and MDA Levels
3.3.3. Inhibitory Activity against α-Amylase and α-Glucosidase of TG1 and TG2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bharathi, L.K.; Behera, T.K.; Sureja, A.K.; John, K.J.; Wehne, T.C. Snake Gourd and Pointed Gourd: Botany and Horticulture. Hortic. Rev. 2013, 41, 457–496. [Google Scholar]
- Devi, N. Medicinal Values of Trichosanthus cucumerina L. (Snake Gourd)—A Review. Br. J. Pharm. Res. 2017, 16, 1–10. [Google Scholar] [CrossRef]
- Cano-Gómez, C.I.; Alonso-Castro, A.J.; Carranza-Alvarez, C.; Wong-Paz, J.E. Advancements in Litchi chinensis Peel Processing: A Scientific Review of Drying, Extraction, and Isolation of Its Bioactive Compounds. Foods 2024, 13, 1461. [Google Scholar] [CrossRef]
- Streule, S.; Freimüller Leischtfeld, S.; Chatelain, K.; Miescher Schwenninger, S. Effect of Pod Storage and Drying Temperature on Fermentation Dynamics and Final Bean Quality of Cacao Nacional in Ecuador. Foods 2024, 13, 1536. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhang, Y.; Li, F.; Zhang, N.; Yin, X.; Zhang, B.; Zhang, B.; Ni, W.; Wang, M.; Fan, J. Effects of traditional and advanced drying techniques on the physicochemical properties of Lycium barbarum L. polysaccharides and the formation of Maillard reaction products in its dried berries. Food Chem. 2023, 409, 135268. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Fan, H.; Li, Y.; Zhang, L. Effect of drying methods on physico-chemical properties and antioxidant activity of Dendrobium officinale. J. Food Meas. Charact. 2017, 12, 1–10. [Google Scholar] [CrossRef]
- Guo, X.; Hao, Q.; Qiao, X.; Li, M.; Qiu, Z.; Zheng, Z.; Zhang, B. An evaluation of different pretreatment methods of hot-air drying of garlic: Drying characteristics, energy consumption and quality properties. LWT-Food Sci. Technol. 2023, 180, 114685. [Google Scholar] [CrossRef]
- Wu, S.; Li, F.; Jia, S.; Ren, H.; Gong, G.; Wang, Y.; Lv, Z.; Liu, Y. Drying effects on the antioxidant properties of polysaccharides obtained from Agaricus blazei Murrill. Carbohydr. Polym. 2014, 103, 414–417. [Google Scholar] [CrossRef]
- Deng, L.-Z.; Mujumdar, A.S.; Zhang, Q.; Yang, X.-H.; Wang, J.; Zheng, Z.-A.; Gao, Z.-J.; Xiao, H.-W. Chemical and physical pretreatments of fruits and vegetables: Effects on drying characteristics and quality attributes—A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1408–1432. [Google Scholar] [CrossRef]
- Dalmau, E.; Araya-Farias, M.; Ratti, C. Cryogenic Pretreatment Enhances Drying Rates in Whole Berries. Foods 2024, 13, 1524. [Google Scholar] [CrossRef]
- Li, D.; Zhu, Z.; Sun, D.-W. Effects of freezing on cell structure of fresh cellular food materials: A review. Trends Food Sci. Technol. 2018, 75, 46–55. [Google Scholar] [CrossRef]
- Piroozi, M.; Noshad, M.; Alizadeh Behbahani, B.; Jooyandeh, H. Preparation of onion powder by freeze-thaw pretreatments: Influence of different thawing methods on drying kinetics and quality properties. Food Chem. Adv. 2023, 2, 100325. [Google Scholar] [CrossRef]
- Zhang, L.H.; Liao, L.; Qiao, Y.; Wang, C.; Shi, D.F.; An, K.J.; Hu, J.Z. Effects of ultrahigh pressure and ultrasound pretreatments on properties of strawberry chips prepared by vacuum-freeze drying. Food Chem. 2020, 303, 125386. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Ping Tan, C.; Zhou, C.; Yagoub, A.E.A.; Xu, B.; Sun, Y.; Ma, H.; Xu, X.; Yu, X. Effect of freeze-thaw cycles pretreatment on the vacuum freeze-drying process and physicochemical properties of the dried garlic slices. Food Chem. 2020, 324, 126883. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, L.; Yagoub, A.E.A.; Yu, X.; Ma, H.; Zhou, C. Effects of ultrasound, freeze-thaw pretreatments and drying methods on structure and functional properties of pectin during the processing of okra. Food Hydrocoll. 2021, 120, 106569. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, L.; Feng, Y.; Zhou, C.; Yagoub, A.E.A.; Wahia, H.; Ma, H.; Zhang, J.; Sun, Y. Ultrasound freeze-thawing style pretreatment to improve the efficiency of the vacuum freeze-drying of okra (Abelmoschus esculentus (L.) Moench) and the quality characteristics of the dried product. Ultrason. Sonochem. 2021, 70, 105300. [Google Scholar] [CrossRef] [PubMed]
- Doymaz, İ.; İsmail, O. Drying characteristics of sweet cherry. Food Bioprod. Process. 2011, 89, 31–38. [Google Scholar] [CrossRef]
- Kaveh, M.; Jahanbakhshi, A.; Abbaspour-Gilandeh, Y.; Taghinezhad, E.; Moghimi, M.B.F. The effect of ultrasound pre-treatment on quality, drying, and thermodynamic attributes of almond kernel under convective dryer using ANNs and ANFIS network. J. Food Process Eng. 2018, 41, 12868. [Google Scholar] [CrossRef]
- Maleki, M.; Shahidi, F.; Varidi, M.J.; Azarpazhooh, E. Hot air drying kinetics of novel functional carrot snack: Impregnated using polyphenolic rich osmotic solution with ultrasound pretreatment. J. Food Process Eng 2019, 43, 13331. [Google Scholar] [CrossRef]
- Abbaspour-Gilandeh, Y.; Kaveh, M.; Jahanbakhshi, A. The effect of microwave and convective dryer with ultrasound pre-treatment on drying and quality properties of walnut kernel. J. Food Process. Preserv. 2019, 43, 14178. [Google Scholar] [CrossRef]
- Kaveh, M.; Abbaspour-Gilandeh, Y.; Chayjan, R.A.; Taghinezhad, E.; Mohammadigol, R. Mass transfer, physical, and mechanical characteristics of terebinth fruit (Pistacia atlantica L.) under convective infrared microwave drying. Heat Mass Transf. 2018, 54, 1879–1899. [Google Scholar] [CrossRef]
- Koua, K.B.; Fassinou, W.F.; Gbaha, P.; Toure, S. Mathematical modelling of the thin layer solar drying of banana, mango and cassava. Energy 2009, 34, 1594–1602. [Google Scholar] [CrossRef]
- Oh, J.-H.; Chung, J.-O.; Lee, C.-Y.; Yun, Y.; Park, M.-Y.; Hong, Y.-D.; Kim, W.-G.; Cha, H.-Y.; Shin, K.-S.; Hong, G.-P.; et al. Characterized Polysaccharides from Green Tea Inhibited Starch Hydrolysis and Glucose Intestinal Uptake by Inducing Microstructural Changes of Wheat Starch. J. Agric. Food. Chem. 2021, 69, 14075–14085. [Google Scholar] [CrossRef] [PubMed]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.-I.; Lee, Y.C. Carbohydrate analysis by a phenol–sulfuric acid method in microplate format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Vasco, C.; Riihinen, K.; Ruales, J.; Kamal-Eldin, A. Phenolic Compounds inRosaceaeFruits from Ecuador. J. Agric. Food. Chem. 2009, 57, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, F.; Li, Q.; Chen, H.; Zhang, W.; Yu, P.; Zhao, T.; Mao, G.; Feng, W.; Yang, L.; et al. Structure characterization of one polysaccharide from Lepidium meyenii Walp., and its antioxidant activity and protective effect against H2O2-induced injury RAW264.7 cells. Int. J. Biol. Macromol. 2018, 118, 816–833. [Google Scholar] [CrossRef]
- Aalim, H.; Belwal, T.; Jiang, L.; Huang, H.; Meng, X.; Luo, Z. Extraction optimization, antidiabetic and antiglycation potentials of aqueous glycerol extract from rice (Oryza sativa L.) bran. LWT-Food Sci. Technol. 2019, 103, 147–154. [Google Scholar] [CrossRef]
- Chung, H.-S.; Kim, D.-S.; Kim, H.-S.; Lee, Y.-G.; Seong, J.-H. Effect of freezing pretreatment on the quality of juice extracted from Prunus mume fruit by osmosis with sucrose. LWT-Food Sci. Technol. 2013, 54, 30–34. [Google Scholar] [CrossRef]
- Noshad, M.; Ghasemi, P. Influence of freezing pretreatments on kinetics of convective air-drying and quality of grapes. Food Biosci. 2020, 38, 100763. [Google Scholar] [CrossRef]
- Li, K.; Zhang, Y.; Wang, Y.F.; El-Kolaly, W.; Gao, M.; Sun, W.; Li, M. Effects of drying variables on the characteristic of the hot air drying for gastrodia elata: Experiments and multi-variable model. Energy 2021, 222, 119982. [Google Scholar] [CrossRef]
- Xu, H.; Wu, M.; Zhang, X.; Wang, B.; Wang, S.; Zheng, Z.; Li, D.; Wang, F. Application of blanching pretreatment in herbaceous peony (Paeonia lactiflora Pall.) flower processing: Improved drying efficiency, enriched volatile profile and increased phytochemical content. Ind. Crops Prod. 2022, 188, 115663. [Google Scholar]
- Nadery Dehsheikh, F.; Taghian Dinani, S. Coating pretreatment of banana slices using carboxymethyl cellulose in an ultrasonic system before convective drying. Ultrason. Sonochem. 2019, 52, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Richter Reis, F.; Marques, C.; Moraes, A.C.S.d.; Masson, M.L. Trends in quality assessment and drying methods used for fruits and vegetables. Food Control. 2022, 142, 109254. [Google Scholar] [CrossRef]
- Zhang, L.H.; Yu, X.J.; Arun S, M.; Zhou, C. Effect of freeze-thaw pretreatment combined with variable temperature on infrared and convection drying of lotus root. LWT-Food Sci. Technol. 2022, 154, 112804. [Google Scholar] [CrossRef]
- Liu, S.; Hu, J.; Zhong, Y.; Hu, X.; Yin, J.; Xiong, T.; Nie, S.; Xie, M. A review: Effects of microbial fermentation on the structure and bioactivity of polysaccharides in plant-based foods. Food Chem. 2024, 440, 137453. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Li, N.; Qiao, X.; Qiu, Z.; Liu, P. Effects of thermal treatment on polysaccharide degradation during black garlic processing. LWT-Food Sci. Technol. 2018, 95, 223–229. [Google Scholar] [CrossRef]
- Liu, L.-N. Protective effects ofRheum tanguticumpolysaccharide against hydrogen peroxide-induced intestinal epithelial cell injury. World J. Gastroenterol. 2005, 11, 1503–1507. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Liu, G.; Wang, M.; Lv, Z.; Du, P. A selenium polysaccharide from Platycodon grandiflorum rescues PC12 cell death caused by H2O2 via inhibiting oxidative stress. Int. J. Biol. Macromol. 2017, 104, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Li, P.; Xue, H.; Li, Q. Cyanidin-3-glucoside prevents hydrogen peroxide (H2O2)-induced oxidative damage in HepG2 cells. Biotechnol. Lett. 2020, 42, 2453–2466. [Google Scholar] [CrossRef]
- Yan, J.-K.; Wu, L.-X.; Qiao, Z.-R.; Cai, W.-D.; Ma, H. Effect of different drying methods on the product quality and bioactive polysaccharides of bitter gourd (Momordica charantia L.) slices. Food Chem. 2019, 271, 588–596. [Google Scholar] [CrossRef]
- Kelishomi, Z.H.; Goliaei, B.; Mahdavi, H.; Nikoofar, A.; Rahimi, M.; Moosavi-Movahedi, A.A.; Mamashli, F.; Bigdeli, B. Antioxidant activity of low molecular weight alginate produced by thermal treatment. Food Chem. 2016, 196, 897–902. [Google Scholar] [CrossRef] [PubMed]





| Temperature (°C) | Times | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Unpretreatment | HD | - | - | - |
| −20 | - | −20 °C-FT1-HD | −20 °C-FT2-HD | −20 °C-FT3-HD |
| −40 | - | −40 °C-FT1-HD | - | - |
| −80 | - | −80 °C-FT1-HD | - | - |
| Model | Equation | References |
|---|---|---|
| Lewis | MR = exp(−kt) | [17] |
| Page | MR = exp(−ktn) | [18] |
| Henderson and Pabis | MR = aexp(−kt) | [19] |
| Logarithmic | MR = aexp(−kt) + c | [20] |
| Two-term exponential | MR = aexp(−kt) + bexp(−k1t) | [21] |
| Wang and Singh | MR = 1 + at + bt2 | [22] |
| Model | Drying Method | Constants | R2 | χ2 | RMSE | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| k | k1 | a | b | c | n | |||||
| Lewis | HD | 0.34386 | / | / | / | / | / | 0.95646 | 0.00521 | 0.07216 |
| −20 °C-FT1-HD | 0.39438 | / | / | / | / | / | 0.96845 | 0.00366 | 0.06054 | |
| −20 °C-FT2-HD | 0.50572 | / | / | / | / | / | 0.98425 | 0.00169 | 0.04113 | |
| −20 °C-FT3-HD | 0.48649 | / | / | / | / | / | 0.98153 | 0.00205 | 0.04529 | |
| −40 °C-FT1-HD | 0.37694 | / | / | / | / | / | 0.95654 | 0.00529 | 0.07274 | |
| −80 °C-FT1-HD | 0.36302 | / | / | / | / | / | 0.94759 | 0.00658 | 0.08112 | |
| Henderson and Pabis | HD | 0.38065 | / | 1.12153 | / | / | / | 0.97059 | 0.06756 | 0.06331 |
| −20 °C-FT1-HD | 0.42937 | / | 1.09775 | / | / | / | 0.97864 | 0.00372 | 0.03722 | |
| −20 °C-FT2-HD | 0.52223 | / | 1.03533 | / | / | / | 0.98581 | 0.00266 | 0.01829 | |
| −20 °C-FT3-HD | 0.51058 | / | 1.05351 | / | / | / | 0.98505 | 0.00166 | 0.01992 | |
| −40 °C-FT1-HD | 0.41628 | / | 1.1183 | / | / | / | 0.97044 | 0.00181 | 0.05759 | |
| −80 °C-FT1-HD | 0.40356 | / | 1.12707 | / | / | / | 0.96353 | 0.00384 | 0.07327 | |
| Page | HD | 0.17561 | / | / | / | / | 1.54124 | 0.99828 | 2.18 × 10−4 | 0.0037 |
| −20 °C-FT1-HD | 0.2476 | / | / | / | / | 1.42445 | 0.99874 | 1.57 × 10−4 | 0.0022 | |
| −20 °C-FT2-HD | 0.41629 | / | / | / | / | 1.21826 | 0.99425 | 6.73 × 10−4 | 0.00741 | |
| −20 °C-FT3-HD | 0.37856 | / | / | / | / | 1.2761 | 0.99675 | 3.94 × 10−4 | 0.00433 | |
| −40 °C-FT1-HD | 0.20221 | / | / | / | / | 1.54557 | 0.99871 | 1.68 × 10−4 | 0.00252 | |
| −80 °C-FT1-HD | 0.17461 | / | / | / | / | 1.62344 | 0.99852 | 1.98 × 10−4 | 0.00298 | |
| Logarithmic | HD | 0.27146 | / | 1.23685 | / | −0.15933 | / | 0.98921 | 0.00145 | 0.02324 |
| −20 °C-FT1-HD | 0.30942 | / | 1.21173 | / | −0.15385 | / | 0.99376 | 0.000836 | 0.01087 | |
| −20 °C-FT2-HD | 0.38083 | / | 1.14078 | / | −0.13992 | / | 0.99841 | 0.000205 | 0.00205 | |
| −20 °C-FT3-HD | 0.36588 | / | 1.17118 | / | −0.15384 | / | 0.99854 | 0.000194 | 0.00194 | |
| −40 °C-FT1-HD | 0.29421 | / | 1.24245 | / | −0.16693 | / | 0.98903 | 0.00153 | 0.02137 | |
| −80 °C-FT1-HD | 0.26773 | / | 1.28796 | / | −0.20953 | / | 0.98718 | 0.00184 | 0.02577 | |
| Two-term exponential | HD | 0.3805 | 0.3805 | 0.56069 | 0.56068 | / | / | 0.97059 | 0.00422 | 0.06331 |
| −20 °C-FT1-HD | 0.42923 | 0.42923 | 0.54878 | 0.54878 | / | / | 0.97864 | 0.0031 | 0.03722 | |
| −20 °C-FT2-HD | 0.52223 | 0.52223 | 0.51766 | 0.51766 | / | / | 0.98581 | 0.00203 | 0.01829 | |
| −20 °C-FT3-HD | 0.51056 | 0.51056 | 0.52675 | 0.52675 | / | / | 0.98505 | 0.00221 | 0.01992 | |
| −40 °C-FT1-HD | 0.41612 | 0.41612 | 0.55915 | 0.55915 | / | / | 0.97044 | 0.00443 | 0.05759 | |
| −80 °C-FT1-HD | 0.40343 | 0.40343 | 0.56346 | 0.56345 | / | / | 0.96353 | 0.00564 | 0.07327 | |
| Wang and Singh | HD | / | / | −0.24637 | 0.015 | / | / | 0.99409 | 7.48 × 10−4 | 0.01271 |
| −20 °C-FT1-HD | / | / | −0.28456 | 0.02027 | / | / | 0.99668 | 4.14 × 10−4 | 0.00579 | |
| −20 °C-FT2-HD | / | / | −0.36091 | 0.03283 | / | / | 0.99744 | 3.00 × 10−4 | 0.0033 | |
| −20 °C-FT3-HD | / | / | −0.34971 | 0.03084 | / | / | 0.99906 | 1.14 × 10−4 | 0.00125 | |
| −40 °C-FT1-HD | / | / | −0.27126 | 0.01823 | / | / | 0.99349 | 8.46 × 10−4 | 0.01268 | |
| −80 °C-FT1-HD | / | / | −0.26057 | 0.01666 | / | / | 0.99108 | 0.0012 | 0.01793 | |
| HD | −20 °C-FT1-HD | −20 °C-FT2-HD | −20 °C-FT3-HD | −40 °C-FT1-HD | −80 °C-FT1-HD | |
|---|---|---|---|---|---|---|
| Bound water (%) | 3.06 ± 0.02 | 2.56 ± 0.08 a | 1.87 ± 0.89 a | 2.71 ± 0.49 a | 2.59 ± 0.49 a | 2.26 ± 0.26 a |
| Immobile water (%) | 14.53 ± 0.41 | 10.99 ± 0.46 a | 7.91 ± 4.43 a | 10.37 ± 0.06 a | 11.67 ± 2.56 a | 9.84 ± 0.48 a |
| Free water (%) | 81.77 ± 0.38 | 86.44 ± 0.51 a | 90.21 ± 5.30 a | 86.91 ± 0.45 a | 85.74 ± 3.05 a | 87.89 ± 0.74 a |
| Treatment | Rehydration Ratio (%) | Shrinkage Rate (%) | Surface Color | |||
|---|---|---|---|---|---|---|
| L* | a* | b* | ΔE | |||
| HD | 7.25 ± 0.17 | 86.67 ± 0.22 | 62.88 ± 0.02 | 1.86 ± 0.04 | 19.00 ± 0.12 | |
| −20 °C-FT1-HD | 4.67 ± 0.17 a | 87.73 ± 0.14 a | 61.14 ± 0.00 a | 0.32 ± 0.04 a | 17.37 ± 0.08 a | 2.84 ± 0.03 |
| −20 °C-FT2-HD | 4.20 ± 0.12 a | 88.79 ± 0.11 a | 62.24 ± 0.00 a | 0.17 ± 0.02 a | 16.84 ± 0.03 a | 2.82 ± 0.03 |
| −20 °C-FT3-HD | 3.34 ± 0.10 a | 88.51 ± 0.41 a | 64.98 ± 0.01 a | 0.23 ± 0.01 a | 17.99 ± 0.03 a | 2.84 ± 0.02 |
| −40 °C-FT1-HD | 4.75 ± 0.13 a | 87.46 ± 0.32 a | 64.71 ± 0.01 a | −1.93 ± 0.02 a | 18.46 ± 0.04 a | 4.25 ± 0.01 a |
| −80 °C-FT1-HD | 5.02 ± 0.08 a | 87.32 ± 0.49 a | 65.27 ± 0.01 a | 0.42 ± 0.04 a | 19.00 ± 0.08 | 2.78 ± 0.02 |
| Active Compositions | HD-TG1(%) | HD-TG2(%) | −20 °C-FT2-HD-TG1 (%) | −20 °C-FT2-HD-TG2 (%) |
|---|---|---|---|---|
| Polysaccharide | 17.52 ± 0.61 | 37.93 ± 0.42 b | 21.70 ± 0.24 ab | 44.34 ± 0.86 ab |
| Total phenol | 1.36 ± 0.06 | 0.66 ± 0.03 b | 1.81 ± 0.09 ab | 0.88 ± 0.04 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, D.; Li, X.; Dong, M.; Ji, W.; Yan, Z.; Zhao, T.; Zhang, M.; Liu, P.; Yue, P.; Mao, G.; et al. Effects of Freeze–Thaw Pretreatment Combined with Hot Air on Snake Gourd (Trichosanthes anguina L.). Foods 2024, 13, 1961. https://doi.org/10.3390/foods13131961
Gu D, Li X, Dong M, Ji W, Yan Z, Zhao T, Zhang M, Liu P, Yue P, Mao G, et al. Effects of Freeze–Thaw Pretreatment Combined with Hot Air on Snake Gourd (Trichosanthes anguina L.). Foods. 2024; 13(13):1961. https://doi.org/10.3390/foods13131961
Chicago/Turabian StyleGu, Dandan, Xiao Li, Mingyue Dong, Wenxuan Ji, Zihao Yan, Ting Zhao, Min Zhang, Peng Liu, Panpan Yue, Guanghua Mao, and et al. 2024. "Effects of Freeze–Thaw Pretreatment Combined with Hot Air on Snake Gourd (Trichosanthes anguina L.)" Foods 13, no. 13: 1961. https://doi.org/10.3390/foods13131961
APA StyleGu, D., Li, X., Dong, M., Ji, W., Yan, Z., Zhao, T., Zhang, M., Liu, P., Yue, P., Mao, G., & Yang, L. (2024). Effects of Freeze–Thaw Pretreatment Combined with Hot Air on Snake Gourd (Trichosanthes anguina L.). Foods, 13(13), 1961. https://doi.org/10.3390/foods13131961






