Unraveling the Physicochemical, Nutritional and Antioxidant Properties of the Honey Produced from the Fallopia japonica Plant
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Sites and Samples
2.2. Chemicals
2.3. Botanical Origin Identification
2.4. Sensory Analysis
2.5. Physicochemical Analysis
2.5.1. Sugar Profile Content
2.5.2. Moisture Content
2.5.3. Electrical Conductivity
2.5.4. Diastase
2.5.5. Hydroxymethylfurfural (HMF)
2.5.6. Acidity and pH Content
2.5.7. Protein Content
2.5.8. Lipid Content
2.6. Total Phenol Content
2.7. Total Flavonoid Content
2.8. Determination Antioxidant Activity of FJH
2.9. Statistical Analysis
3. Results and Discussion
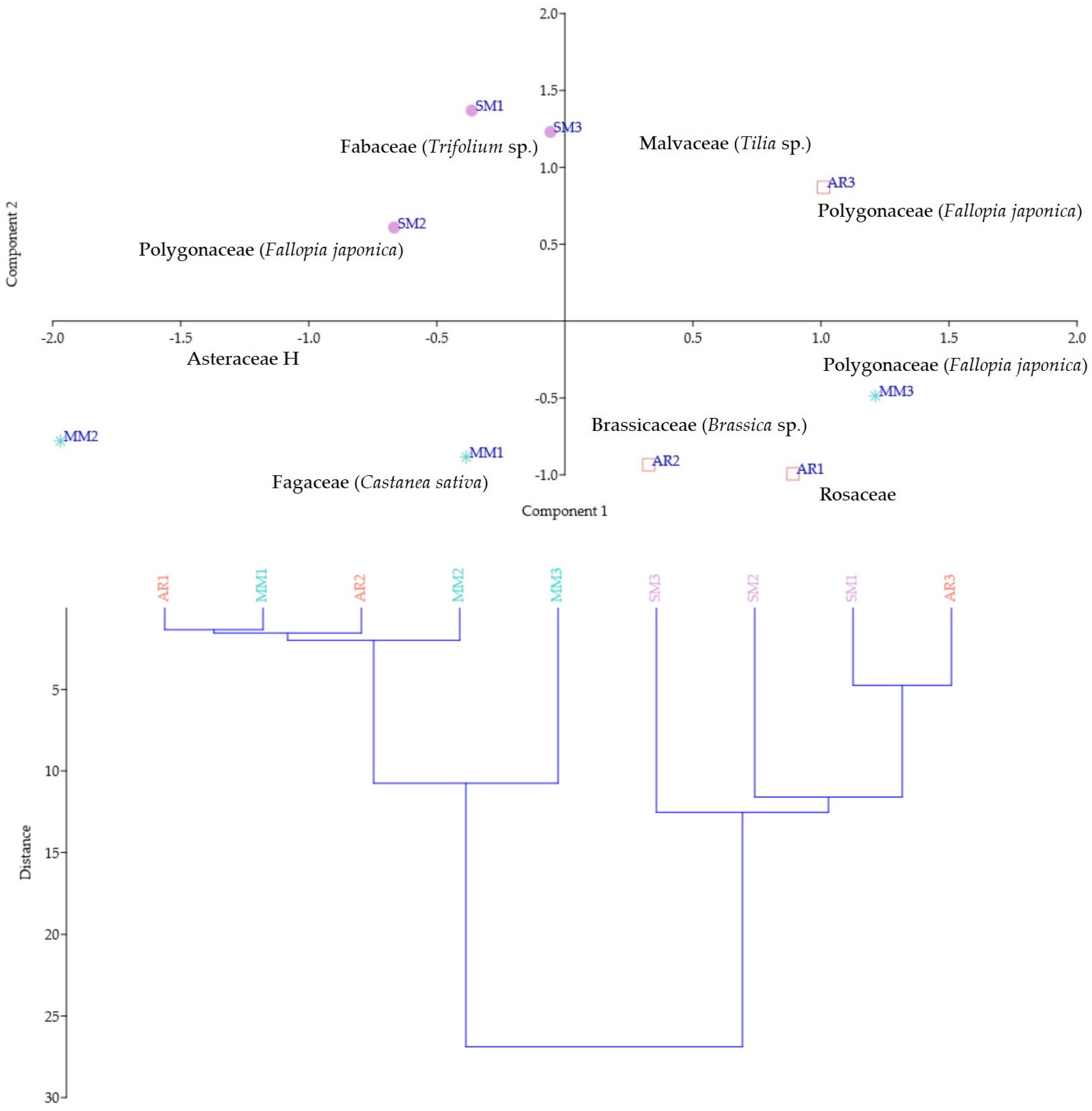
3.1. Botanical Origin Identification of FJH Samples
3.2. Sensory Parameters
3.3. Physicochemical Characteristics of FJH from Different Regions of Romania
3.3.1. Sugar Content
3.3.2. Moisture Content
3.3.3. Electrical Conductivity
3.3.4. pH Content and Acidity
3.3.5. Hydroxymethylfurfural (HMF) Content
3.3.6. Diastase Content
3.4. Nutritional Value of FJH
3.5. Antioxidant Capacity of FJH Samples
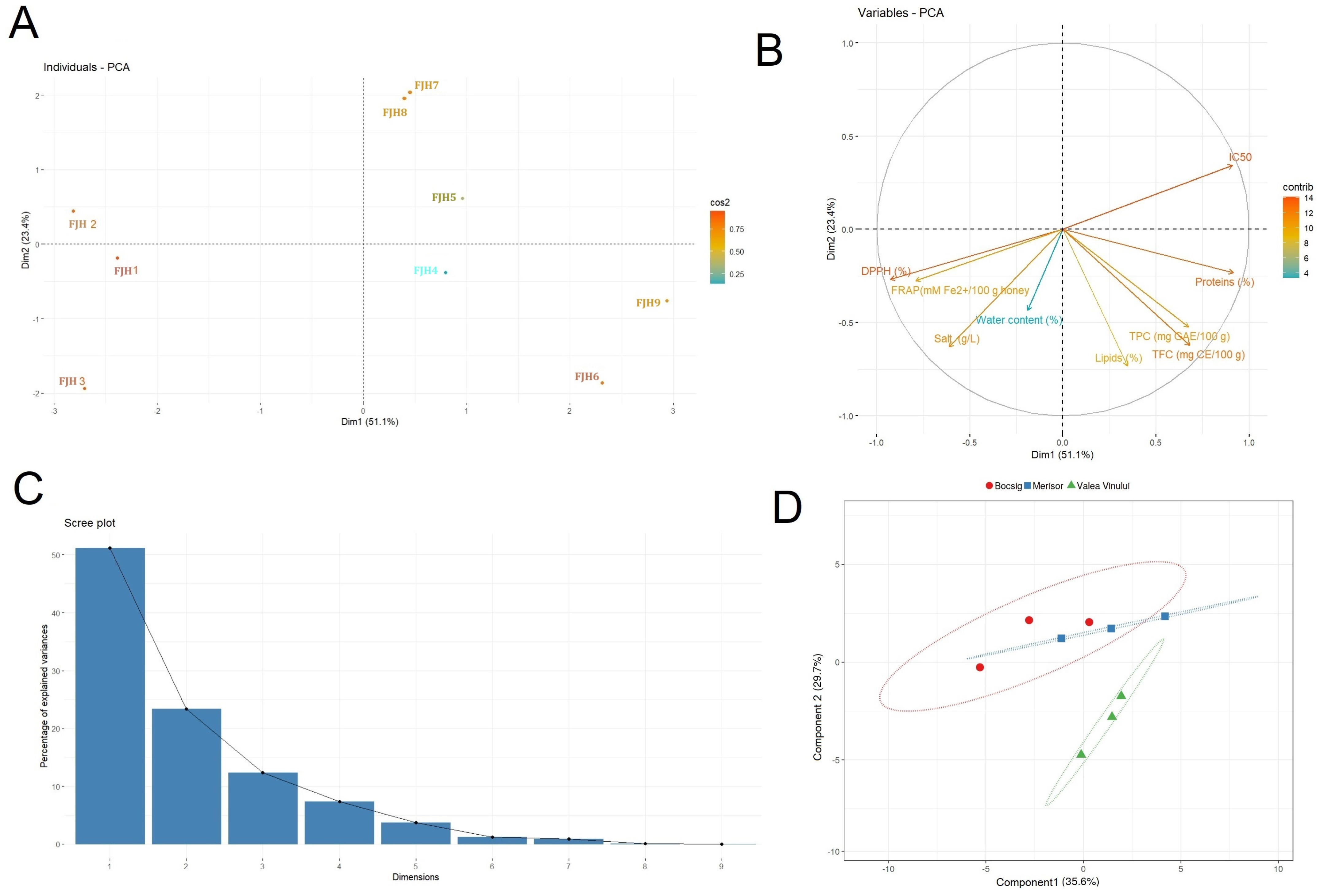
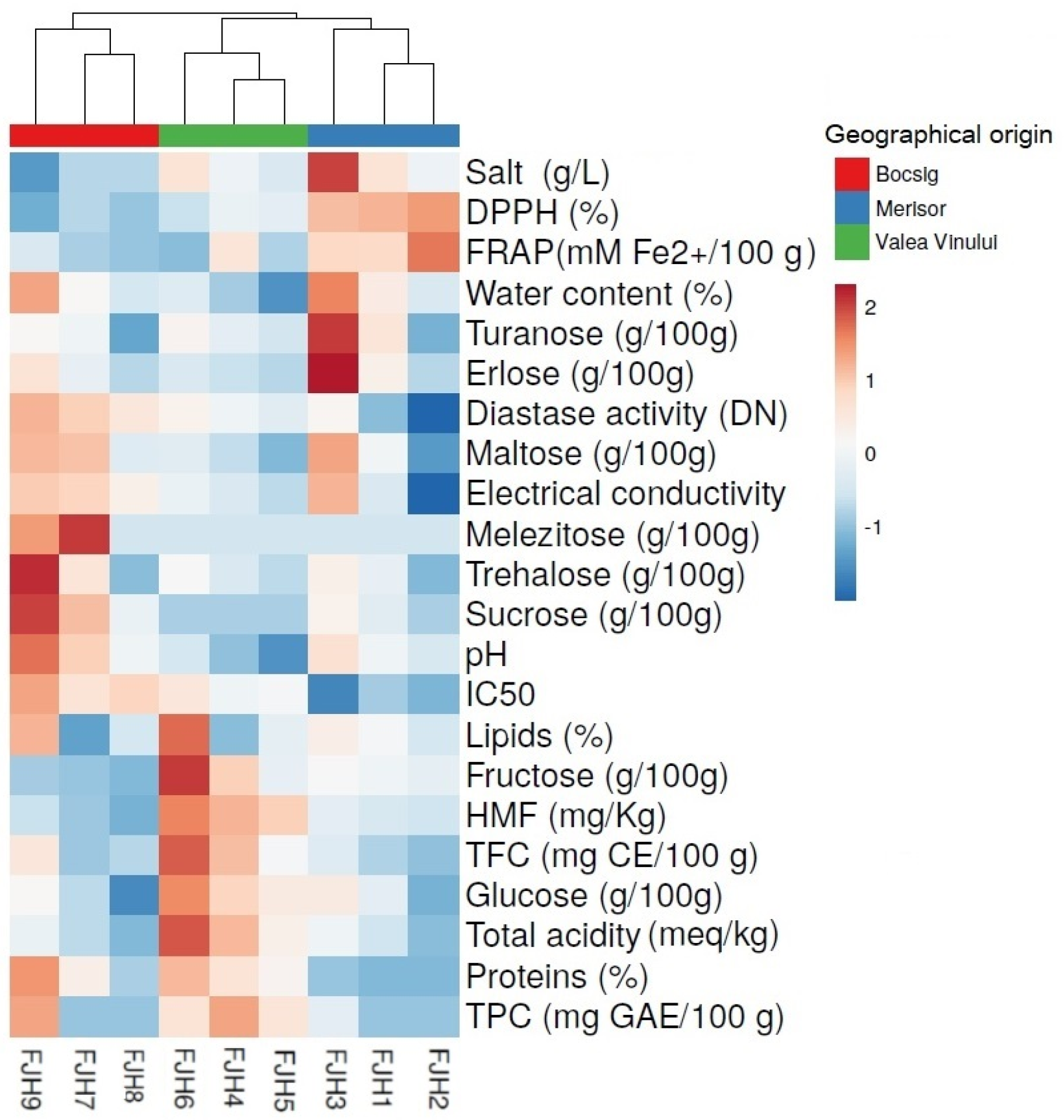
3.6. Geographical Discrimination and Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cucu, A.A.; Baci, G.M.; Moise, A.R.; Dezsi, Ş.; Marc, B.D.; Stângaciu, Ş.; Dezmirean, D.S. Towards a Better Understanding of Nutritional and Therapeutic Effects of Honey and Their Applications in Apitherapy. Appl. Sci. 2021, 11, 4190. [Google Scholar] [CrossRef]
- Puścion-Jakubik, A.; Borawska, M.H.; Socha, K. Modern Methods for Assessing the Quality of Bee Honey and Botanical Origin Identification. Foods 2020, 9, 1028. [Google Scholar] [CrossRef] [PubMed]
- Hernanz, D.; Jara-Palacios, M.J.; Santos, J.L.; Gómez Pajuelo, A.; Heredia, F.J.; Terrab, A. The Profile of Phenolic Compounds by HPLC-MS in Spanish Oak (Quercus) Honeydew Honey and Their Relationships with Color and Antioxidant Activity. LWT 2023, 180, 114724. [Google Scholar] [CrossRef]
- Wang, L.; Ning, F.; Liu, T.; Huang, X.; Zhang, J.; Liu, Y.; Wu, D.; Luo, L. Physicochemical Properties, Chemical Composition, and Antioxidant Activity of Dendropanax Dentiger Honey. LWT 2021, 147, 111693. [Google Scholar] [CrossRef]
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical Composition, Stability and Authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Prđun, S.; Flanjak, I.; Svečnjak, L.; Primorac, L.; Lazarus, M.; Orct, T.; Bubalo, D.; Bilić Rajs, B. Characterization of Rare Himalayan Balsam (Impatiens Glandulifera Royle) Honey from Croatia. Foods 2022, 11, 3025. [Google Scholar] [CrossRef] [PubMed]
- Fröschle, M.; Horn, H.; Spring, O. Characterization of Jatropha Curcas Honeys Originating from the Southern Highlands of Madagascar. LWT 2018, 93, 525–533. [Google Scholar] [CrossRef]
- Bobis, O.; Moise, A.R.; Ballesteros, I.; Reyes, E.S.; Durán, S.S.; Sánchez-Sánchez, J.; Cruz-Quintana, S.; Giampieri, F.; Battino, M.; Alvarez-Suarez, J.M. Eucalyptus Honey: Quality Parameters, Chemical Composition and Health-Promoting Properties. In Food Chemistry; Elsevier Ltd.: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Lamas, L.B.; Flórez, S.M.; Toyos, P.A.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef]
- Persano Oddo, L.; Piro, R. Main European Unifloral Honeys: Descriptive Sheets. Apidologie 2004, 35, 38–81. [Google Scholar] [CrossRef]
- Ferrazzi, P.; Marletto, F. Bee Value of Reynoutria Japonica Houtt. Apic. Mod. 1990, 81, 71–76. [Google Scholar]
- Bartz, R.; Kowarik, I. Assessing the Environmental Impacts of Invasive Alien Plants: A Review of Assessment Approaches. NeoBiota 2019, 43, 69–99. [Google Scholar] [CrossRef]
- Nentwig, W.; Bacher, S.; Kumschick, S.; Pyšek, P.; Vilà, M. More than “100 Worst” Alien Species in Europe. Biol. Invasions 2018, 20, 1611–1621. [Google Scholar] [CrossRef]
- Bailey, J.P.; Bímová, K.; Mandák, B. Asexual Spread versus Sexual Reproduction and Evolution in Japanese Knotweed s.l. Sets the Stage for the “Battle of the Clones”. Biol. Invasions 2009, 11, 1189–1203. [Google Scholar] [CrossRef]
- Holden, C.A.; Morais, C.L.M.; Taylor, J.E.; Martin, F.L.; Beckett, P.; McAinsh, M. Regional Differences in Clonal Japanese Knotweed Revealed by Chemometrics-Linked Attenuated Total Reflection Fourier-Transform Infrared Spectroscopy. BMC Plant Biol. 2021, 21, 522. [Google Scholar] [CrossRef] [PubMed]
- Sołtysiak, J. Heavy Metals Tolerance in an Invasive Weed (Fallopia japonica) under Different Levels of Soils Contamination. J. Ecol. Eng. 2020, 21, 81–91. [Google Scholar] [CrossRef]
- Jones, D.; Bruce, G.; Fowler, M.S.; Law-Cooper, R.; Graham, I.; Abel, A.; Street-Perrott, F.A.; Eastwood, D. Optimising Physiochemical Control of Invasive Japanese Knotweed. Biol. Invasions 2018, 20, 2091–2105. [Google Scholar] [CrossRef]
- Chiocchio, I.; Mandrone, M.; Tomasi, P.; Marincich, L.; Poli, F. Plant Secondary Metabolites: An Opportunity for Circular Economy. Molecules 2021, 26, 495. [Google Scholar] [CrossRef] [PubMed]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of Antioxidant Potential of Plants and Its Relevance to Therapeutic Applications. Int. J. Biol. Sci. 2015, 11, 982. [Google Scholar] [CrossRef] [PubMed]
- Glavnik, V.; Vovk, I. Extraction of Anthraquinones from Japanese Knotweed Rhizomes and Their Analyses by High Performance Thin-Layer Chromatography and Mass Spectrometry. Plants 2020, 9, 1753. [Google Scholar] [CrossRef]
- Hadzik, J.; Choromańska, A.; Karolewicz, B.; Matkowski, A.; Dominiak, M.; Złocińska, A.; Nawrot-Hadzik, I. Oral Wound Healing Potential of Polygoni Cuspidati Rhizoma et Radix Decoction—In Vitro Study. Pharmaceuticals 2023, 16, 267. [Google Scholar] [CrossRef]
- Lachowicz, S.; Oszmianski, J. Profile of Bioactive Compounds in the Morphological Parts of Wild Fallopia japonica (Houtt) and Fallopia sachalinensis (F. Schmidt) and Their Antioxidative Activity. Molecules 2019, 24, 1436. [Google Scholar] [CrossRef] [PubMed]
- Nawrot-Hadzik, I.; Hadzik, J.; Fleischer, M.; Choromańska, A.; Sterczała, B.; Kubasiewicz-Ross, P.; Saczko, J.; Gałczyńska-Rusin, M.; Gedrange, T.; Matkowski, A. Chemical Composition of East Asian Invasive Knotweeds, Their Cytotoxicity and Antimicrobial Efficacy against Cariogenic Pathogens: An In-Vitro Study. Med. Sci. Monit. 2019, 25, 3279–3287. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Hee Park, J.; Park, C.W. Flavonoid Chemistry of Fallopia Section Fallopia (Polygonaceae). Biochem. Syst. Ecol. 2000, 28, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Fu, J.; Yin, X.; Li, X.; Wang, B.; Cao, S.; Zhang, H.; Zhao, Y.; Ni, J. Pharmacological and Other Bioactivities of the Genus Polygonum—A Review. Trop. J. Pharm. Res. 2014, 13, 1749–1759. [Google Scholar]
- Cucu, A.A.; Pașca, C.; Cucu, A.B.; Moise, A.R.; Bobiş, O.; Ștefan, D.; Blaga Petrean, A.; Dezmirean, D.S. Evaluation of the Main Macro-, Micro- and Trace Elements Found in Fallopia japonica Plants and Their Traceability in Its Honey: A Case Study from the Northwestern and Western Part of Romania. Plants 2024, 13, 428. [Google Scholar] [CrossRef] [PubMed]
- Barudanović, S.; Zečić, E.; Macanović, A.; Duraković, B.; Mašić, E. Invasive Alien Plant Species in Global Perspectives with Special References to Bosnia and Herzegovina; Wiley: Hoboken, NJ, USA, 2021. [Google Scholar]
- Davis, E.S.; Kelly, R.; Maggs, C.A.; Stout, J.C. Contrasting Impacts of Highly Invasive Plant Species on Flower-Visiting Insect Communities. Biodivers. Conserv. 2018, 27, 2069–2085. [Google Scholar] [CrossRef]
- Jabłoński, B.; Kołtowski, Z. Nectar Secretion and Honey Potential of Honey-Plants Growing under Poland’s Conditions. Part XII. J. Apic. Sci. 2001, 45, 29–35. [Google Scholar]
- Bobis, O.; Severus Dezmirean, D.; Bonta, V.; Moise, A.; Pașca, C.; Domokos, T.E.; Urcan, A.C. Japanese Knotweed (Fallopia japonica): Landscape Invasive Plant versus High Quality Honey Source. Ser. D. Anim. Sci. Sci. Pap. S. 2019, 62, 231–235. [Google Scholar]
- Cucu, A.A.; Baci, G.M.; Dezsi, Ş.; Nap, M.E.; Beteg, F.I.; Bonta, V.; Bobiş, O.; Caprio, E.; Dezmirean, D.S. New Approaches on Japanese Knotweed (Fallopia japonica) Bioactive Compounds and Their Potential of Pharmacological and Beekeeping Activities: Challenges and Future Directions. Plants 2021, 10, 2621. [Google Scholar] [CrossRef] [PubMed]
- Pătruică, S.; Alexa, E.; Obiștioiu, D.; Cocan, I.; Radulov, I.; Berbecea, A.; Lazăr, R.N.; Simiz, E.; Vicar, N.M.; Hulea, A.; et al. Chemical Composition, Antioxidant and Antimicrobial Activity of Some Types of Honey from Banat Region, Romania. Molecules 2022, 27, 4179. [Google Scholar] [CrossRef]
- Burke, J.M.; Sanchez, A.; Kron, K.; Luckow, M. Placing the Woody Tropical Genera of Polygonaceae: A Hypothesis of Character Evolution and Phylogeny. Am. J. Bot. 2010, 97, 1377–1390. [Google Scholar] [CrossRef] [PubMed]
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of Melissopalynology. Bee World 1978, 59, 139–157. [Google Scholar] [CrossRef]
- Dobrinas, S.; Soceanu, A.; Birghila, S.; Birghila, C.; Matei, N.; Popescu, V.; Constanda, L.M. Chemical Analysis and Quality Assessment of Honey Obtained from Different Sources. Processes 2022, 10, 2554. [Google Scholar] [CrossRef]
- SR 784-1,2,3:2009; Quality Requirements for Honey. Romanian Standards Association: Bucharest, Romania, 2009. Available online: https://e-standard.eu/Search?q=honey (accessed on 14 May 2024).
- Bogdanov, S. Harmonised Methods of the International IHC. Bee Prod. Sci. 2009, 5, 1–62. [Google Scholar]
- Codex Alimentarius Commission. Revised Codex Standard for Honey, Codex Stan 12-1981, Rev. 1, Rev. 2; Food and Agriculture Organization (FAO): Rome, Italy, 2001; pp. 1–7. [Google Scholar]
- EU. Council Directive 2001/110/CE Concerning Honey. Commun. Off. J. Eur. 2002, L10, 47–52. [Google Scholar]
- Pașca, C.; Mărghitaș, L.A.; Matei, I.A.; Bonta, V.; Mărgăoan, R.; Copaciu, F.; Bobiș, O.; Campos, M.G.; Dezmirean, D.S. Screening of Some Romanian Raw Honeys and Their Probiotic Potential Evaluation. Appl. Sci. 2021, 11, 5816. [Google Scholar] [CrossRef]
- Merrill, A.L.; Watt, B.K. USDA Handbook 74; U.S. Government Printing Office: Washington, DC, USA, 1955; No. 74.
- Azeredo, L.D.C.; Azeredo, M.A.A.; De Souza, S.R.; Dutra, V.M.L. Protein Contents and Physicochemical Properties in Honey Samples of Apis Mellifera of Different Floral Origins. Food Chem. 2003, 80, 249–254. [Google Scholar] [CrossRef]
- Almeida-Muradian, L.B.; Pamplona, L.C.; Coimbra, S.; Barth, O.M. Chemical Composition and Botanical Evaluation of Dried Bee Pollen Pellets. J. Food Compos. Anal. 2005, 18, 105–111. [Google Scholar] [CrossRef]
- Duda, S.C.; Mărghitaş, L.A.; Dezmirean, D.; Duda, M.; Mărgăoan, R.; Bobiş, O. Changes in Major Bioactive Compounds with Antioxidant Activity of Agastache Foeniculum, Lavandula Angustifolia, Melissa Officinalis and Nepeta Cataria: Effect of Harvest Time and Plant Species. Ind. Crops Prod. 2015, 77, 499–507. [Google Scholar] [CrossRef]
- Gerginova, D.; Popova, M.; Chimshirova, R.; Trusheva, B.; Shanahan, M.; Guzmán, M.; Solorzano-Gordillo, E.; López-Roblero, E.; Spivak, M.; Simova, S.; et al. The Chemical Composition of Scaptotrigona Mexicana Honey and Propolis Collected in Two Locations: Similarities and Differences. Foods 2023, 12, 3317. [Google Scholar] [CrossRef]
- Mǎrghitaş; Daniel, D.; Moise, A.; Bobis, O.; Laslo, L.; Bogdanov, S. Physico-Chemical and Bioactive Properties of Different Floral Origin Honeys from Romania. Food Chem. 2009, 112, 863–867. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the Total Phenolic, Flavonoid and Proline Contents in Burkina Fasan Honey, as Well as Their Radical Scavenging Activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Aljadi, A.M.; Kamaruddin, M.Y. Evaluation of the Phenolic Contents and Antioxidant Capacities of Two Malaysian Floral Honeys. Food Chem. 2004, 85, 513–518. [Google Scholar] [CrossRef]
- Thrasyvoulou, A.; Tananaki, C.; Goras, G.; Karazafiris, E.; Dimou, M.; Liolios, V.; Kanelis, D.; Gounari, S. Legislación de Criterios y Normas de Miel. J. Apic. Res. 2018, 57, 88–96. [Google Scholar] [CrossRef]
- Panseri, S.; Manzo, A.; Chiesa, L.M.; Giorgi, A. Melissopalynological and Volatile Compounds Analysis of Buckwheat Honey from Different Geographical Origins and Their Role in Botanical Determination. J. Chem. 2013, 2013, 904202. [Google Scholar] [CrossRef]
- Corey, K.; Hamama, A.A.; Li, H.; Siddiqui, R.A.; Kim, C.; Bhardwaj, H.L. Composition of Buckwheat Honey. J. Agric. Sci. 2022, 14, 59. [Google Scholar] [CrossRef]
- Barańska, A.; Jedlińska, A.; Samborska, K. Dehumidified-Air-Assisted Spray Drying of Buckwheat Honey with Maltodextrin and Skim Milk Powder as Carriers. Appl. Sci. 2021, 11, 3150. [Google Scholar] [CrossRef]
- Huda, M.N.; Lu, S.; Jahan, T.; Ding, M.; Jha, R.; Zhang, K.; Zhang, W.; Georgiev, M.I.; Park, S.U.; Zhou, M. Treasure from Garden: Bioactive Compounds of Buckwheat. Food Chem. 2021, 335, 127653. [Google Scholar] [CrossRef] [PubMed]
- Starowicz, M.; Lamparski, G.; Ostaszyk, A.; Szmatowicz, B. Quality Evaluation of Polish Honey: On-Line Survey, Sensory Study, and Consumer Acceptance. J. Sens. Stud. 2021, 36, 12661. [Google Scholar] [CrossRef]
- Dżugan, M.; Grabek-Lejko, D.; Swacha, S.; Tomczyk, M.; Bednarska, S.; Kapusta, I. Physicochemical Quality Parameters, Antibacterial Properties and Cellular Antioxidant Activity of Polish Buckwheat Honey. Food Biosci. 2020, 34, 100538. [Google Scholar] [CrossRef]
- Jiang, L.; Xie, M.; Chen, G.; Qiao, J.; Zhang, H.; Zeng, X. Phenolics and Carbohydrates in Buckwheat Honey Regulate the Human Intestinal Microbiota. Evid. Based Complement. Altern. Med. 2020, 2020, 6432942. [Google Scholar] [CrossRef]
- Pasini, F.; Gardini, S.; Marcazzan, G.L.; Caboni, M.F. Buckwheat Honeys: Screening of Composition and Properties. Food Chem. 2013, 141, 2802–2811. [Google Scholar] [CrossRef]
- Deng, J.; Liu, R.; Lu, Q.; Hao, P.; Xu, A.; Zhang, J.; Tan, J. Biochemical Properties, Antibacterial and Cellular Antioxidant Activities of Buckwheat Honey in Comparison to Manuka Honey. Food Chem. 2018, 252, 243–249. [Google Scholar] [CrossRef]
- Escuredo, O.; Dobre, I.; Fernández-González, M.; Seijo, M.C. Contribution of Botanical Origin and Sugar Composition of Honeys on the Crystallization Phenomenon. Food Chem. 2014, 149, 84–90. [Google Scholar] [CrossRef]
- Pauliuc, D.; Dranca, F.; Oroian, M. Antioxidant Activity, Total Phenolic Content, Individual Phenolics and Physicochemical Parameters Suitability for Romanian Honey Authentication. Foods 2020, 9, 306. [Google Scholar] [CrossRef]
- Živkov Baloš, M.; Popov, N.; Jakšić, S.; Mihaljev, Ž.; Pelić, M.; Ratajac, R.; Ljubojević Pelić, D. Sunflower Honey—Evaluation of Quality and Stability during Storage. Foods 2023, 12, 2585. [Google Scholar] [CrossRef]
- Bogdanov, S.; Jurendic, T.; Sieber, R.; Gallmann, P. Honey for Nutrition and Health: A Review. J. Am. Coll. Nutr. 2008, 27, 677–689. [Google Scholar] [CrossRef] [PubMed]
- European Union. European Council Directive 2001/110 Relating to Honey. Off. J. Eur. Comm. 2001, L10, 47–52. [Google Scholar]
- Machado De-Melo, A.A.; de Almeida-Muradian, L.B.; Sancho, M.T.; Pascual-Maté, A. Composición y Propiedades de La Miel de Apis Mellifera: Una Revisión. J. Apic. Res. 2017, 57, 5–37. [Google Scholar] [CrossRef]
- Nešović, M.; Gašić, U.; Tosti, T.; Horvacki, N.; Šikoparija, B.; Nedić, N.; Blagojević, S.; Ignjatović, L.; Tešić, Ž. Polyphenol Profile of Buckwheat Honey, Nectar and Pollen: Polyphenolics in Buckwheat. R. Soc. Open Sci. 2020, 7, 1–15. [Google Scholar] [CrossRef]
- Boussaid, A.; Chouaibi, M.; Rezig, L.; Hellal, R.; Donsì, F.; Ferrari, G.; Hamdi, S. Physicochemical and Bioactive Properties of Six Honey Samples from Various Floral Origins from Tunisia. Arab. J. Chem. 2018, 11, 265–274. [Google Scholar] [CrossRef]
- Majewska, E.; Drużyńska, B.; Wołosiak, R. Determination of the Botanical Origin of Honeybee Honeys Based on the Analysis of Their Selected Physicochemical Parameters Coupled with Chemometric Assays. Food Sci. Biotechnol. 2019, 28, 1307–1314. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Maia, M.; Karabagias, V.K.; Gatzias, I.; Badeka, A.V. Characterization of Eucalyptus, Chestnut and Heather Honeys from Portugal Using Multi-Parameter Analysis and Chemo-Calculus. Foods 2018, 7, 194. [Google Scholar] [CrossRef]
- Gürbüz, S.; Çakici, N.; Mehmetoǧlu, S.; Atmaca, H.; Demir, T.; Arigül Apan, M.; Atmaca, Ö.F.; Güney, F. Physicochemical Quality Characteristics of Southeastern Anatolia Honey, Turkey. Int. J. Anal. Chem. 2020, 2020, 8810029. [Google Scholar] [CrossRef]
- El Sohaimy, S.A.; Masry, S.H.D.; Shehata, M.G. Physicochemical Characteristics of Honey from Different Origins. Ann. Agric. Sci. 2015, 60, 279–287. [Google Scholar] [CrossRef]
- Prica, N.; Živkov Baloš, M.; Jakšić, S.; Mihaljev, Ž.; Kartalović, B.; Babić, J.; Savić, S. Moisture and Acidity as Indicators of the Quality of Honey Originating from Vojvodina Region. Arch. Vet. Med. 2015, 7, 99–109. [Google Scholar] [CrossRef]
- Shapla, U.M.; Solayman, M.; Alam, N.; Khalil, M.I.; Gan, S.H. 5-Hydroxymethylfurfural (HMF) Levels in Honey and Other Food Products: Effects on Bees and Human Health. Chem. Cent. J. 2018, 12, 35. [Google Scholar] [CrossRef]
- Fallico, B.; Zappalà, M.; Arena, E.; Verzera, A. Effects of Conditioning on HMF Content in Unifloral Honeys. Food Chem. 2004, 85, 305–313. [Google Scholar] [CrossRef]
- Sęk, A.; Porębska, A.; Szczęsna, T. Quality of Commercially Available Manuka Honey Expressed by Pollen Composition, Diastase Activity, and Hydroxymethylfurfural Content. Foods 2023, 12, 2930. [Google Scholar] [CrossRef]
- Pasias, I.N.; Kiriakou, I.K.; Proestos, C. HMF and Diastase Activity in Honeys: A Fully Validated Approach and a Chemometric Analysis for Identification of Honey Freshness and Adulteration. Food Chem. 2017, 229, 425–431. [Google Scholar] [CrossRef]
- Harbane, S.; Escuredo, O.; Saker, Y.; Ghorab, A.; Nakib, R.; Rodríguez-Flores, M.S.; Ouelhadj, A.; Seijo, M.C. The Contribution of Botanical Origin to the Physicochemical and Antioxidant Properties of Algerian Honeys. Foods 2024, 13, 573. [Google Scholar] [CrossRef]
- Soares, S.; Pinto, D.; Rodrigues, F.; Alves, R.C.; Oliveira, M.B.P.P. Portuguese Honeys from Different Geographical and Botanical Origins: A 4-Year Stability Study Regarding Quality Parameters and Antioxidant Activity. Molecules 2017, 22, 1338. [Google Scholar] [CrossRef]
- Miłek, M.; Bocian, A.; Kleczyńska, E.; Sowa, P.; Dżugan, M. The Comparison of Physicochemical Parameters, Antioxidant Activity and Proteins for the Raw Local Polish Honeys and Imported Honey Blends. Molecules 2021, 26, 2423. [Google Scholar] [CrossRef]
- Zhu, M.; Zhao, H.; Wang, Q.; Wu, F.; Cao, W. A Novel Chinese Honey from Amorpha Fruticosa L.: Nutritional Composition and Antioxidant Capacity In Vitro. Molecules 2020, 25, 5211. [Google Scholar] [CrossRef]
- Gupta, U.C.; Gupta, S.C. Sources and Deficiency Diseases of Mineral Nutrients in Human Health and Nutrition: A Review. Pedosphere 2014, 24, 13–38. [Google Scholar] [CrossRef]
- Rossano, R.; Larocca, M.; Polito, T.; Perna, A.M.; Padula, M.C.; Martelli, G.; Riccio, P. What Are the Proteolytic Enzymes of Honey and What They Do Tell Us? A Fingerprint Analysis by 2-D Zymography of Unifloral Honeys. PLoS ONE 2012, 7, e49164. [Google Scholar] [CrossRef]
- Escuredo, O.; Míguez, M.; Fernández-González, M.; Carmen Seijo, M. Nutritional Value and Antioxidant Activity of Honeys Produced in a European Atlantic Area. Food Chem. 2013, 138, 851–856. [Google Scholar] [CrossRef]
- Rivera-Mondragón, A.; Marrone, M.; Bruner-Montero, G.; Gaitán, K.; de Núñez, L.; Otero-Palacio, R.; Añino, Y.; Wcislo, W.T.; Martínez-Luis, S.; Fernández-Marín, H. Assessment of the Quality, Chemometric and Pollen Diversity of Apis Mellifera Honey from Different Seasonal Harvests. Foods 2023, 12, 3656. [Google Scholar] [CrossRef]
- Bogdanov, S. Honey as Nutrient and Functional Food: A Review. Bee Prod. Sci. 2015, 1–47. [Google Scholar]
- Alvarez-Suarez, J.; Giampieri, F.; Battino, M. Honey as a Source of Dietary Antioxidants: Structures, Bioavailability and Evidence of Protective Effects Against Human Chronic Diseases. Curr. Med. Chem. 2013, 20, 621–638. [Google Scholar] [CrossRef]
- Yammine, A.; Namsi, A.; Vervandier-Fasseur, D.; Mackrill, J.J.; Lizard, G.; Latruffe, N. Polyphenols of the Mediterranean Diet and Their Metabolites in the Prevention of Colorectal Cancer. Molecules 2021, 26, 3483. [Google Scholar] [CrossRef]
- Sancho, M.T.; Pascual-Maté, A.; Rodríguez-Morales, E.G.; Osés, S.M.; Escriche, I.; Periche, Á.; Fernández-Muiño, M.A. Critical Assessment of Antioxidant-Related Parameters of Honey. Int. J. Food Sci. Technol. 2016, 51, 30–36. [Google Scholar] [CrossRef]
- Talebi, M.M.; Talebi, M.M.; Farkhondeh, T.; Samarghandian, S. Molecular Mechanism-Based Therapeutic Properties of Hmechanism-Based Therapeutic Properties of Honey. In Biomedicine and Pharmacotherapy; Elsevier Masson SAS: Paris, France, 2020. [Google Scholar] [CrossRef]
- do Nascimento, K.S.; Gasparotto Sattler, J.A.; Lauer Macedo, L.F.; Serna González, C.V.; Pereira de Melo, I.L.; da Silva Araújo, E.; Granato, D.; Sattler, A.; de Almeida-Muradian, L.B. Phenolic Compounds, Antioxidant Capacity and Physicochemical Properties of Brazilian Apis Mellifera Honeys. LWT Food Sci. Technol. 2018, 91, 85–94. [Google Scholar] [CrossRef]
- Kamal, D.A.M.; Ibrahim, S.F.; Kamal, H.; Kashim, M.I.A.M.; Mokhtar, M.H. Physicochemical and Medicinal Properties of Tualang, Gelam and Kelulut Honeys: A Comprehensive Review. Nutrients 2021, 13, 197. [Google Scholar] [CrossRef]
- Kavanagh, S.; Gunnoo, J.; Marques Passos, T.; Stout, J.C.; White, B. Physicochemical Properties and Phenolic Content of Honey from Different Floral Origins and from Rural versus Urban Landscapes. Food Chem. 2019, 272, 66–75. [Google Scholar] [CrossRef]
- Tomczyk, M.; Tarapatskyy, M.; Dżugan, M. The Influence of Geographical Origin on Honey Composition Studied by Polish and Slovak Honeys. Czech J. Food Sci. 2019, 37, 232–238. [Google Scholar] [CrossRef]
- Gheldof, N.; Wang, X.H.; Engeseth, N.J. Buckwheat Honey Increases Serum Antioxidant Capacity in Humans. J. Agric. Food Chem. 2003, 51, 1500–1505. [Google Scholar] [CrossRef]
- Puścion-Jakubik, A.; Karpińska, E.; Moskwa, J.; Socha, K. Content of Phenolic Acids as a Marker of Polish Honey Varieties and Relationship with Selected Honey-Quality-Influencing Variables. Antioxidants 2022, 11, 1312. [Google Scholar] [CrossRef]
- Gośliński, M.; Nowak, D.; Kłębukowska, L. Antioxidant Properties and Antimicrobial Activity of Manuka Honey versus Polish Honeys. J. Food Sci. Technol. 2020, 57, 1269–1277. [Google Scholar] [CrossRef]
- Margaoan, R.; Arădăvoaicei, Ş.; Sisea, C.R.; Cornea-cipcigan, M.; Cordea, M.I. Methods of Microscopic Slides Preparation to Identify the Pollen Grains Derived from Different Bee Products. Sci. Pap. Ser. D. Anim. Sci. 2023, 66, 412–421. [Google Scholar]
- Mărgăoan, R.; Topal, E.; Balkanska, R.; Yücel, B.; Oravecz, T.; Cornea-Cipcigan, M.; Vodnar, D.C. Monofloral Honeys as a Potential Source of Natural Antioxidants, Minerals and Medicine. Antioxidants 2021, 10, 1023. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, Y.K.; Kim, M.S.; Lee, S.H. Antioxidant and Antibacterial Properties of Hovenia (Hovenia dulcis) Monofloral Honey Produced in South Korea. Food Sci. Anim. Resour. 2020, 40, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Mureșan, C.I.; Cornea-Cipcigan, M.; Suharoschi, R.; Erler, S.; Mărgăoan, R. Honey Botanical Origin and Honey-Specific Protein Pattern: Characterization of Some European Honeys. LWT 2022, 154, 112883. [Google Scholar] [CrossRef]


| Sample | Appearance | Consistency | Color | Smell | Taste and Persistency |
|---|---|---|---|---|---|
| FJH 1 | clean, homogeneous, without impurities | fine crystallization | brown-caramel with brown shades | conifer resin, light floral smell | Medium sweet taste and a refreshing aftertaste of fresh mint |
| FJH 2 | clean, homogeneous, without impurities | fine crystallization | brown-caramel with reddish-brown shades | conifer resin, fine floral smell | Medium sweet taste, light metallic and a refreshing aftertaste of fresh mint |
| FJH 3 | clean, homogeneous, without impurities | fine crystallization | brown-caramel with reddish-brown shades | vegetal aroma, conifer resin, fine floral smell | Medium sweet taste, metallic and a refreshing aftertaste of fresh mint |
| FJH4 | clean, homogeneous, without impurities | fine crystallization | brown-caramel | fine floral smell | Medium sweet taste, metallic and a refreshing aftertaste of fresh mint |
| FJH5 | clean, homogeneous, without impurities | fine crystallization | brown-caramel with reddish-brown shades | vegetal aroma, conifer resin | Medium sweet taste, metallic and a refreshing aftertaste of fresh mint |
| FJH6 | clean, homogeneous, without impurities | fine crystallization | brown-caramel | vegetal aroma, conifer resin, fine floral smell | Medium sweet taste, metallic and a refreshing aftertaste of fresh mint |
| FJH7 | clean, homogeneous, without impurities | fine crystallization | brown-caramel | vegetal aroma, conifer resin, fine floral smell | Medium sweet taste, metallic and a refreshing aftertaste of fresh mint |
| FJH8 | clean, homogeneous, without impurities | fluid-viscous | brown-caramel with reddish-brown shades | conifer resin | Medium sweet taste, metallic and a refreshing aftertaste of fresh mint |
| FJH9 | clean, homogeneous, without impurities | fine crystallization | brown-caramel with reddish-brown shades | vegetal aroma, conifer resin | Medium sweet taste, metallic and a refreshing aftertaste of fresh mint |
| Parameter | FJH1 | FJH2 | FJH3 | Merișor Area | FJH4 | FJH5 | FJH6 | Valea Vinului Area | FJH7 | FJH8 | FJH9 | Bocsig Area |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fructose (g/100 g) | 37.05 ± 0.15 bc | 36.72 ± 0.69 c | 37.26 ± 1.01 bc | 37.01 ± 0.27 A | 38.68 ± 1.11 b | 36.79 ± 1.01 c | 40.65 ± 0.91 a | 38.70 ± 1.93 A | 35.35 ± 0.11 d | 35.12 ± 0.13 d | 35.53 ± 0.09 d | 35.33 ± 0.20 B |
| Glucose (g/100 g) | 32.31 ± 1.45 d | 29.39 ± 1.14 de | 34.39 ± 1.11 c | 32.03 ± 2.51 A | 35.79 ± 0.99 b | 34.37 ± 0.87 c | 37.79 ± 0.66 a | 35.98 ± 1.71 A | 30.80 ± 1.55 d | 28.06 ± 2.01 e | 33.45 ± 1.09 cd | 30.77 ± 2.69 A |
| Sucrose (g/100 g) | 0.62 ± 0.35 d | nd | 1.24 ± 0.09 c | 0.62 ± 0.22 B | nd | nd | nd | 0.00 ± 0.00 | 2.12 ± 0.66 b | 0.81 ± 1.21 d | 3.11 ± 1.09 a | 2.01 ± 1.15 A |
| Turanose (g/100 g) | 1.46 ± 0.39 b | 0.72 ± 1.1 d | 2.09 ± 0.39 a | 1.42 ± 0.68 A | 1.12 ± 0.09 c | 0.99 ± 0.05 c | 1.32 ± 0.07 b | 1.14 ± 0.16 A | 1.21 ± 0.19 bc | 0.66 ± 0.95 d | 1.29 ± 0.13 b | 1.05 ± 0.34 A |
| Maltose (g/100 g) | 1.25 ± 0.31 b | 0.68 ± 0.07 c | 1.76 ± 1.1 a | 1.23 ± 0.14 A | 0.98 ± 0.08 c | 0.82 ± 0.06 c | 1.13 ± 0.03 b | 0.97 ± 0.15 A | 1.66 ± 0.18 a | 1.12 ± 0.11 b | 1.68 ± 0.11 a | 1.48 ± 0.31 A |
| Trehalose (g/100 g) | 0.29 ± 0.08 bc | 0.10 ± 0.03 c | 0.39 ± 0.07 b | 0.26 ± 0.14 A | 0.24 ± 0.04 bc | 0.18 ± 0.02 c | 0.34 ± 0.03 b | 0.25 ± 0.08 A | 0.44 ± 0.18 b | 0.11 ± 0.05 c | 0.76 ± 0.04 a | 0.43 ± 0.03 A |
| Erlose (g/100 g) | 0.65 ± 0.52 b | nd | 1.79 ± 0.43 a | 0.81 ± 0.09 A | 0.07 ± 0.06 d | nd | 0.21 ± 0.03 c | 0.09 ± 0.01 A | 0.35 ± 0.23 c | nd | 0.81 ± 0.11 b | 0.38 ± 0.04 A |
| Melezitose (g/100 g) | nd | nd | nd | 0.00 ± 0.00 | nd | nd | nd | 0.00 ± 0.00 | 1.09 ± 0.32 a | nd | 0.81 ± 0.22 a | 0.63 ± 0.05 A |
| F/G | 1.14 ± 0.04 b | 1.24 ± 0.07 a | 1.08 ± 0.05 c | 1.15 ± 0.08 A | 1.08 ± 0.00 c | 1.07 ± 0.03 c | 1.07 ± 0.01 c | 1.07 ± 0.00 A | 1.14 ± 0.05 b | 1.25 ± 0.03 a | 1.06 ± 0.03 c | 1.15 ± 0.09 A |
| Electrical conductivity (µS/cm) | 533 ± 84.58 c | 387 ± 43.78 d | 680 ± 79.34 a | 533 ± 46.5 A | 533 ± 16.16 c | 506 ± 18.8 c | 562 ± 20.19 c | 533 ± 28.00 A | 650 ± 19.21 a | 598 ± 25.66 b | 660 ± 18.39 a | 636.00 ± 33.29 A |
| pH | 3.8 ± 0.05 b | 3.73 ± 0.08 b | 3.92 ± 0.04 a | 3.81 ± 0.09 AB | 3.63 ± 0.05 bc | 3.54 ± 0.09 c | 3.72 ± 0.08 b | 3.63 ± 0.09 B | 3.97 ± 0.08 a | 3.8 ± 0.07 b | 4.1 ± 0.04 a | 3.95 ± 0.15 A |
| Total acidity (meq/kg) | 25.36 ± 2.13 c | 22.13 ± 1.98 d | 29.51 ± 2.11 bc | 25.66 ± 3.69 B | 37.58 ± 3.23 b | 31.7 ± 4.03 b | 42.89 ± 3.33 a | 37.39 ± 5.59 A | 24.56 ± 2.05 c | 21.61 ± 2.01 d | 28.69 ± 2.05 bc | 24.95 ± 3.55 B |
| HMF (mg/Kg) | 1.88 ± 0.15 b | 1.70 ± 0.19 bc | 2.22 ± 0.11 b | 1.93 ± 0.26 B | 4.55 ± 0.30 a | 4.12 ± 0.28 a | 5.16 ± 0.35 a | 4.61 ± 0.52 A | 1.16 ± 0.25 d | 0.72 ± 0.35 d | 1.62 ± 0.33 c | 1.16 ± 0.45 B |
| Diastase activity (DN) | 11.44 ± 1.58 d | 9.11 ± 2.14 d | 14.58 ± 3.00 c | 11.71 ± 2.74 B | 14.04 ± 0.40 c | 13.35 ± 0.28 c | 14.77 ± 0.42 c | 14.05 ± 0.71 B | 16.32 ± 0.40 a | 15.42 ± 0.56 b | 17.01 ± 0.55 a | 16.25 ± 0.79 A |
| Samples/ | Water Content (%) | Proteins (%) | Lipids (%) | Salt (%) | Total Carbohydrates (%) | Energy Value (kcal/100 g) |
|---|---|---|---|---|---|---|
| Parameters | ||||||
| FJH1 | 19.63 ± 1.15 b | 0.19 ± 0.00 b | 0.08 ± 0.01 b | 0.16 ± 0.03 b | 79.91 ± 0.19 bc | 329.38 ± 4.80 b |
| FJH2 | 18.71 ± 1.04 c | 0.19 ± 0.00 b | 0.06 ± 0.00 bc | 0.14 ± 0.02 b | 78.54 ± 0.21 c | 323.42 ± 4.44 c |
| FJH3 | 21.00 ± 1.11 a | 0.20 ± 0.00 b | 0.09 ± 0.03 b | 0.20 ± 0.01 a | 80.93 ± 0.22 b | 332.93 ± 4.76 ab |
| Merișor area | 19.77 ± 1.15 A | 0.19 ± 0.00 A | 0.07 ± 0.01 A | 0.16 ± 0.03 A | 79.79 ± 1.19 A | 328.57 ± 4.80 A |
| FJH4 | 18.17 ± 0.70 c | 0.32 ± 0.03 a | 0.04 ± 0.01 c | 0.14 ± 0.01 b | 81.32 ± 0.74 ab | 335.3 ± 2.80 a |
| FJH5 | 17.40 ± 0.56 c | 0.29 ± 0.01 b | 0.07 ± 0.00 b | 0.13 ± 0.01 b | 80.67 ± 0.82 b | 332.7 ± 3.96 ab |
| FJH6 | 18.80 ± 0.66 c | 0.36 ± 0.04 a | 0.14 ± 0.02 a | 0.16 ± 0.01 b | 82.15 ± 0.77 a | 338.31 ± 3.77 a |
| Valea Vinului area | 18.12 ± 0.70 A | 0.19 ± 0.00 A | 0.08 ± 0.01 A | 0.14 ± 0.01 A | 81.38 ± 0.74 A | 335.43 ± 2.80 A |
| FJH7 | 19.33 ± 1.06 b | 0.3 ± 0.08 a | 0.03 ± 0.04 c | 0.12 ± 0.01 b | 80.2 ± 1.00 b | 330.46 ± 4.40 b |
| FJH8 | 18.60 ± 0.91 c | 0.21 ± 0.02 b | 0.06 ± 0.03 b | 0.12 ± 0.01 b | 78.94 ± 1.03 c | 324.79 ± 6.13 bc |
| FJH9 | 20.70 ± 1.12 a | 0.38 ± 0.07 a | 0.12 ± 0.00 a | 0.10 ± 0.01 b | 80.93 ± 0.96 b | 333.46 ± 5.43 a |
| Bocsig area | 19.54 ± 1.06 A | 0.29 ± 0.008 A | 0.07 ± 0.04 A | 0.11 ± 0.01 B | 80.02 ± 1.00 A | 329.57 ± 4.40 A |
| Parameter | FJH1 | FJH2 | FJH3 | Merișor Area | FJH4 | FJH5 | FJH6 | Valea Vinului Area | FJH7 | FJH8 | FJH9 | Bocsig Area |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TPC (mg GAE/100 g) | 90.12 ± 0.10 d | 90.23 ± 0.95 d | 99.79 ± 1.01 c | 93.38 ± 5.55 A | 120.08 ± 0.62 a | 110.24 ± 0.46 b | 110.71 ± 0.85 bc | 113.67 ± 25.55 A | 89.87 ± 0.95 d | 89.92 ± 0.79 d | 119.88 ± 0.82 a | 99.89 ± 17.31 A |
| TFC (mg CE/100 g) | 19.71 ± 1.23 e | 18.13 ± 0.22 f | 23.13 ± 0.20 d | 20.32 ± 2.55 A | 33.75 ± 0.73 b | 26.25 ± 0.40 c | 39.38 ± 0.49 a | 33.12 ± 6.58 A | 18.75 ± 0.38 ef | 20.17 ± 0.47 e | 29.78 ± 0.98 c | 22.90 ± 6.00 A |
| DPPH (%) | 54.00 ± 2.12 ab | 55.87 ± 0.36 a | 53.40 ± 2.36 b | 54.42 ± 1.28 A | 44.09 ± 0.26 c | 43.16 ± 0.32 c | 40.09 ± 0.42 d | 42.44 ± 2.09 B | 39.31 ± 0.20 d | 37.69 ± 0.80 e | 35.41 ± 0.15 f | 37.46 ± 1.95 C |
| IC50 | 9.9.44 ± 0.35 d | 8.88 ± 0.10 e | 7.83 ± 0.18 f | 8.71 ± 0.81 B | 11.30 ± 0.09 c | 11.49 ± 0.11 c | 12.54 ± 0.13 b | 12.53 ± 0.72 A | 12.74 ± 0.07 b | 13.27 ± 0.28 ab | 14.15 ± 0.06 a | 13.38 ± 0.71 A |
| FRAP (mM Fe2+/100 g honey) | 1.88 ± 0.07 b | 2.32 ± 0.10 a | 1.91 ± 0.08 b | 2.03 ± 0.24 A | 1.78 ± 0.06 c | 1.09 ± 0.09 e | 0.97 ± 0.10 f | 1.28 ± 0.43 B | 1.07 ± 0.05 e | 1.00 ± 0.02 f | 1.28 ± 0.09 d | 1.11 ± 0.14 B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cucu, A.-A.; Bobiș, O.; Bonta, V.; Moise, A.R.; Pașca, C.; Cornea-Cipcigan, M.; Mărgăoan, R.; Dezsi, Ș.; Botezan, S.; Baciu, E.-D.; et al. Unraveling the Physicochemical, Nutritional and Antioxidant Properties of the Honey Produced from the Fallopia japonica Plant. Foods 2024, 13, 1959. https://doi.org/10.3390/foods13131959
Cucu A-A, Bobiș O, Bonta V, Moise AR, Pașca C, Cornea-Cipcigan M, Mărgăoan R, Dezsi Ș, Botezan S, Baciu E-D, et al. Unraveling the Physicochemical, Nutritional and Antioxidant Properties of the Honey Produced from the Fallopia japonica Plant. Foods. 2024; 13(13):1959. https://doi.org/10.3390/foods13131959
Chicago/Turabian StyleCucu, Alexandra-Antonia, Otilia Bobiș, Victorița Bonta, Adela Ramona Moise, Claudia Pașca, Mihaiela Cornea-Cipcigan, Rodica Mărgăoan, Ștefan Dezsi, Sara Botezan, Ecaterina-Daniela Baciu, and et al. 2024. "Unraveling the Physicochemical, Nutritional and Antioxidant Properties of the Honey Produced from the Fallopia japonica Plant" Foods 13, no. 13: 1959. https://doi.org/10.3390/foods13131959
APA StyleCucu, A.-A., Bobiș, O., Bonta, V., Moise, A. R., Pașca, C., Cornea-Cipcigan, M., Mărgăoan, R., Dezsi, Ș., Botezan, S., Baciu, E.-D., Giurgiu, A.-I., Mălinaș, A., & Dezmirean, D. S. (2024). Unraveling the Physicochemical, Nutritional and Antioxidant Properties of the Honey Produced from the Fallopia japonica Plant. Foods, 13(13), 1959. https://doi.org/10.3390/foods13131959

















