Determination of Imidazole Dipeptides and Related Amino Acids in Natural Seafoods by Liquid Chromatography–Tandem Mass Spectrometry Using a Pre-Column Derivatization Reagent
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Reaction of IDPs and Amino Acids with (R)-CIMa-OSu
2.3. LC–MS/MS Analysis
2.4. Sampling of Fish and Shellfish
2.5. Derivatization of IDPs and Amino Acids in Fish and Shellfish Samples
2.6. Validation
3. Results and Discussion
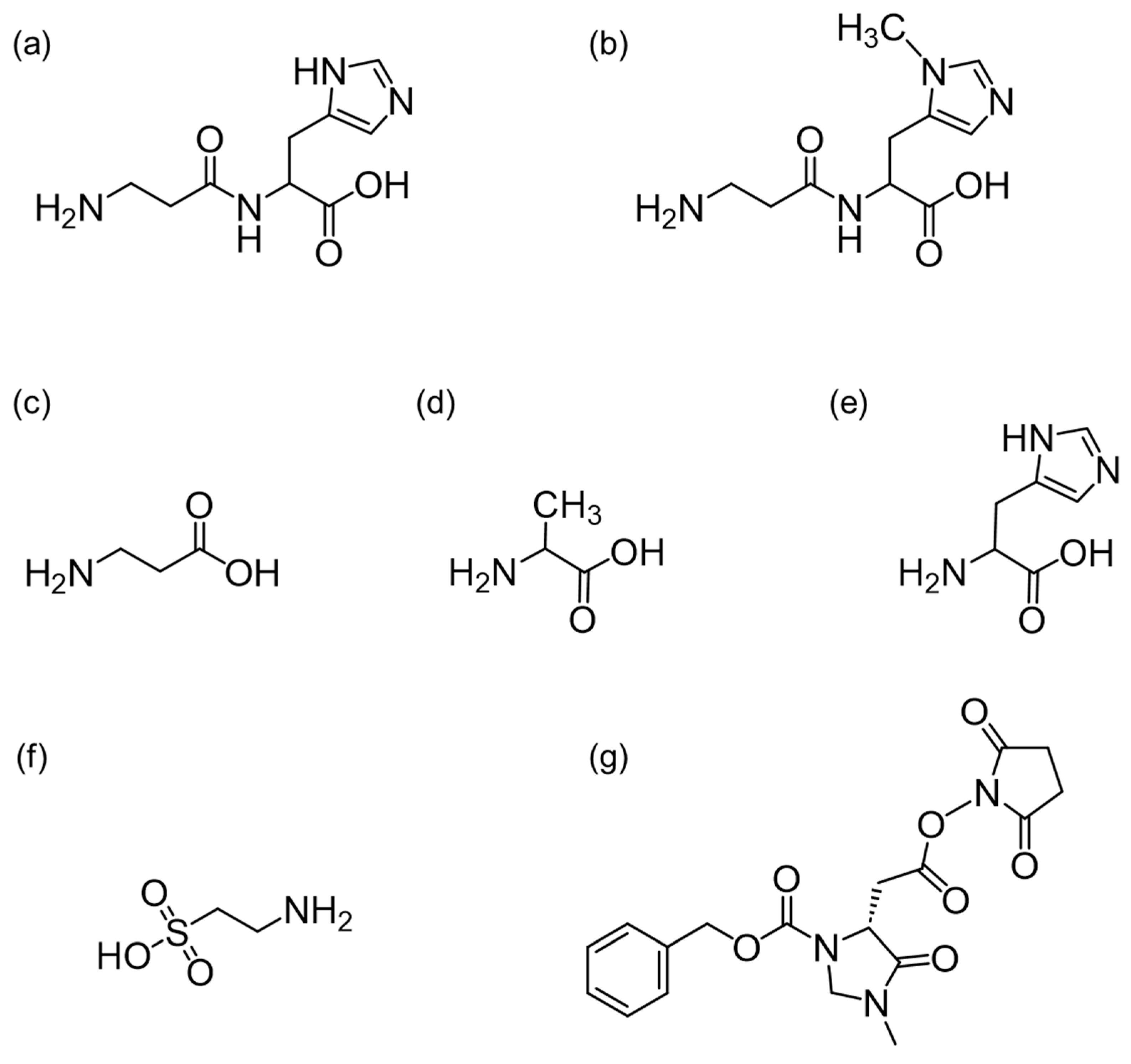
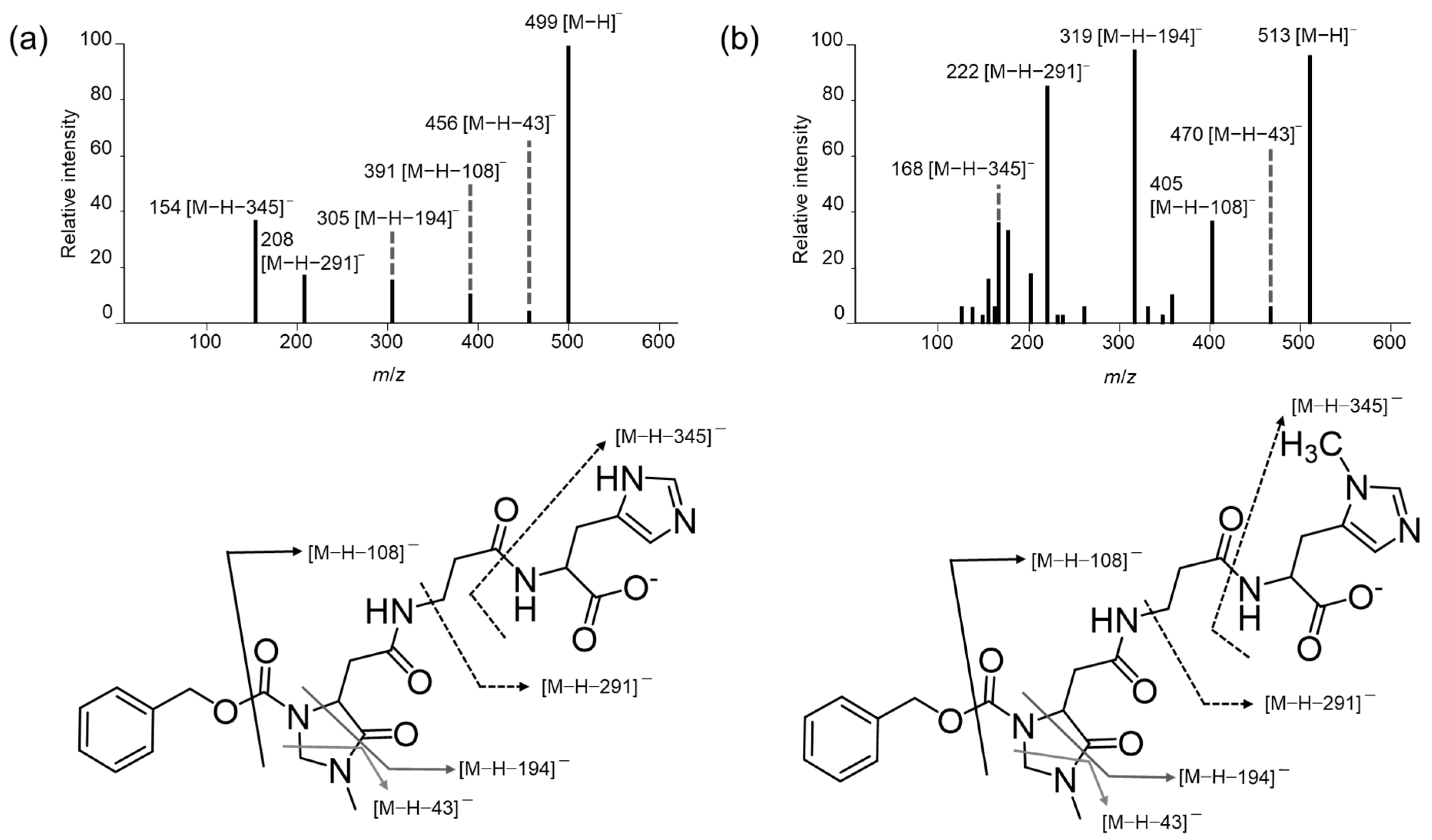
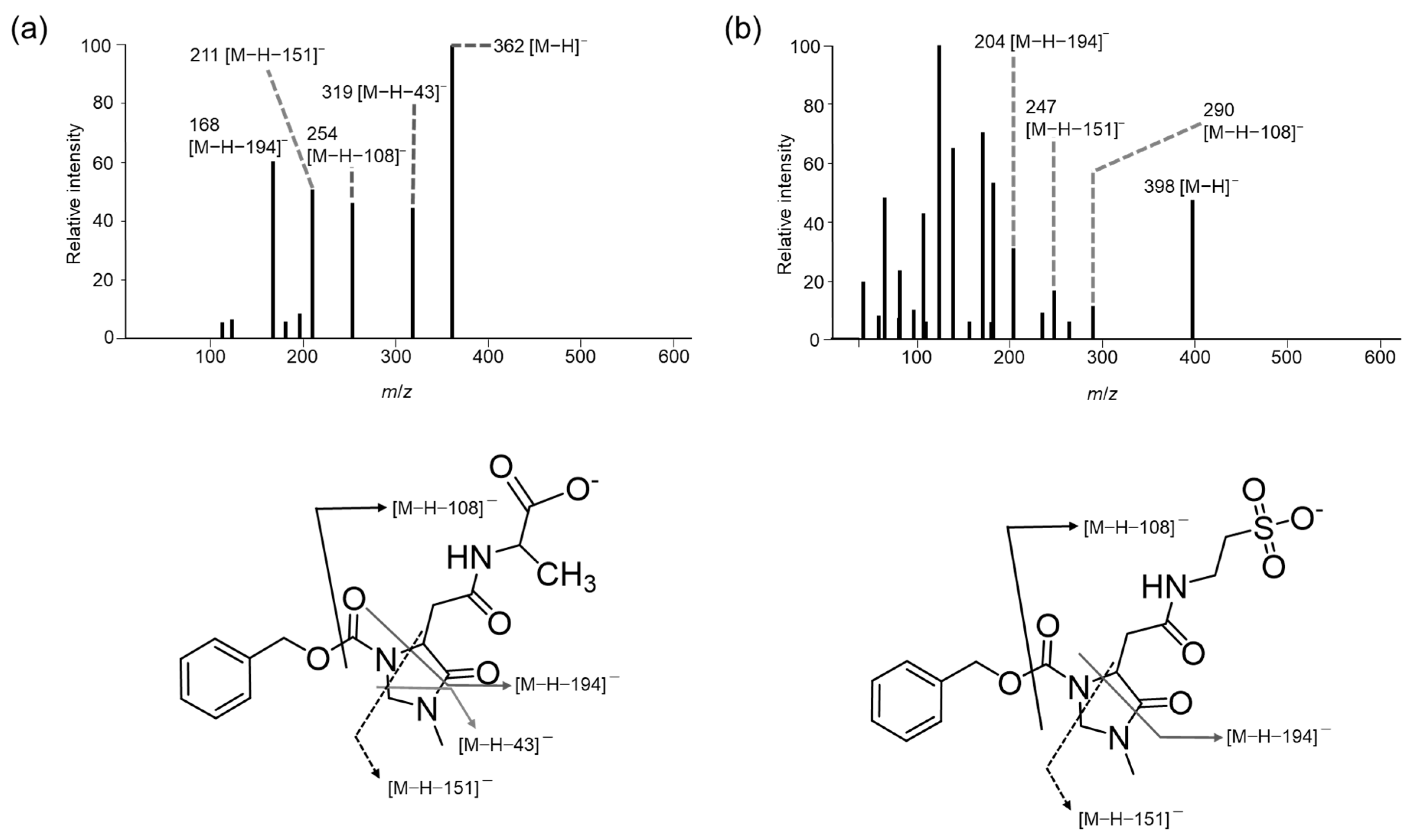
3.1. Mass Spectra of the (R)-CIMa-OSu Derivatives of Car, Ans, His, β-Ala, and Tau
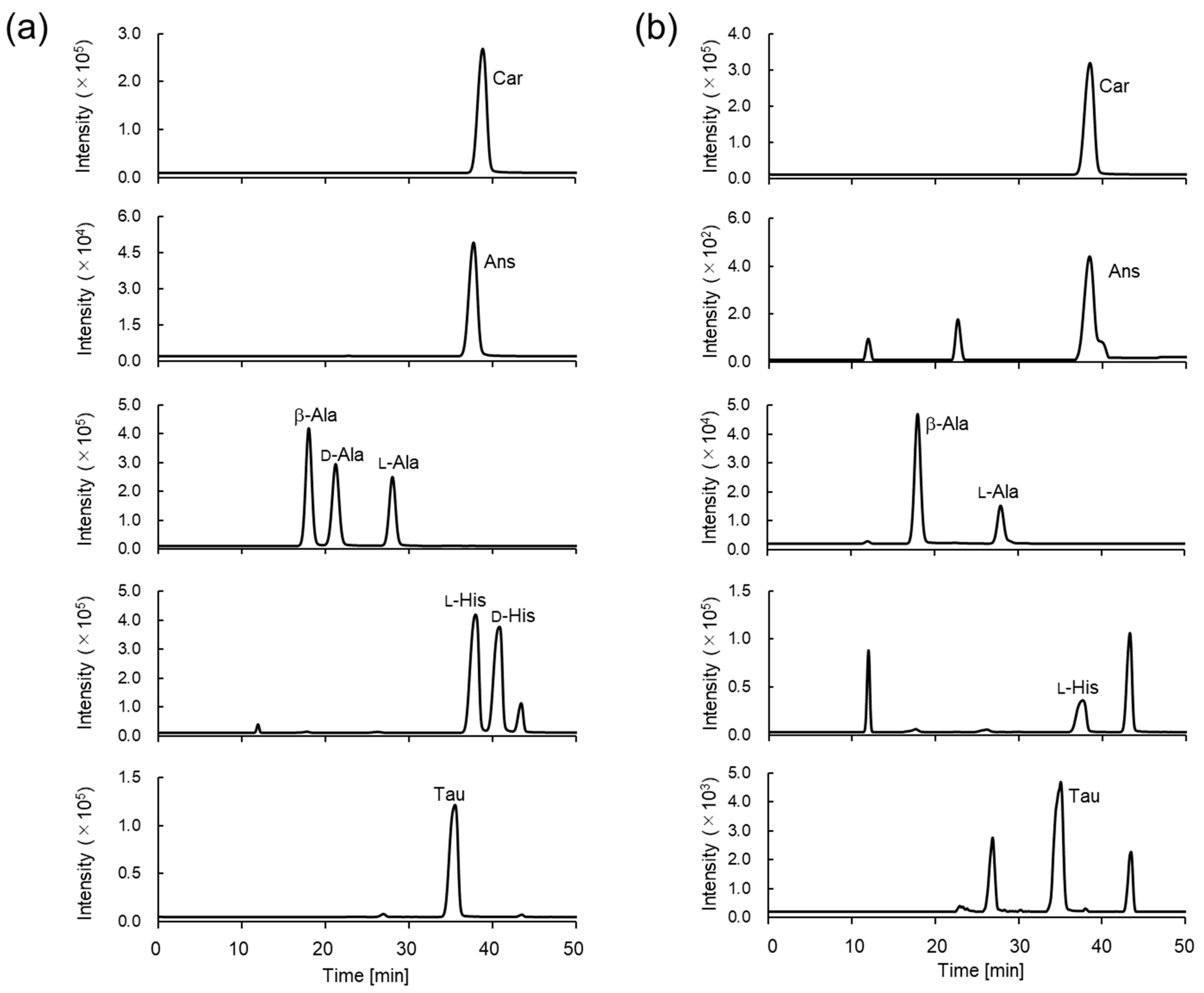
3.2. Separation of Car, Ans, His, β-Ala, and Tau Derivatives
3.3. Validation Data for Car, Ans, His, β-Ala, and Tau Derivatives
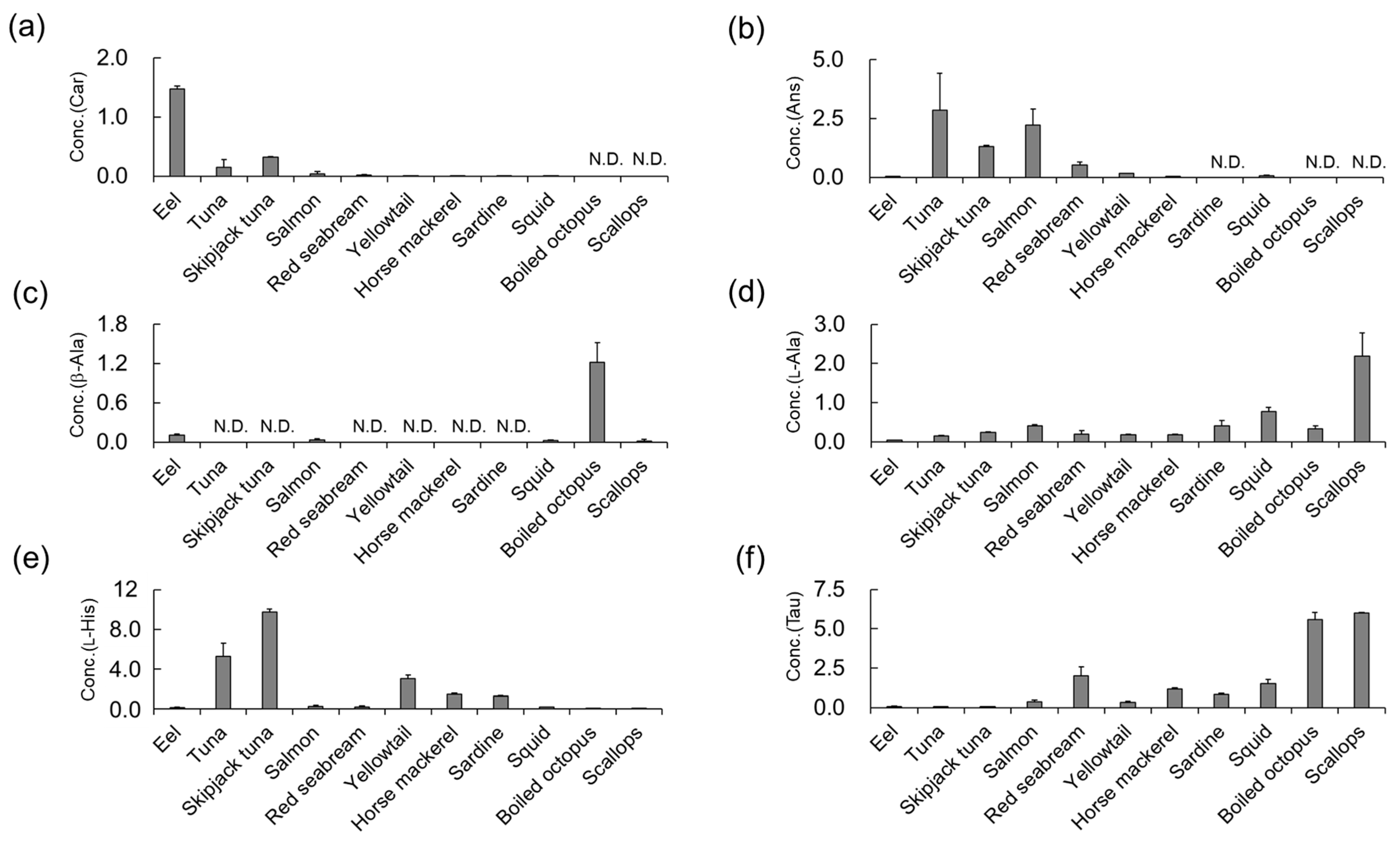
3.4. Determination of Car, Ans, His, β-Ala, and Tau Concentrations in Natural Seafoods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and pathophysiology of carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Chee, M.E.; Zhang, H.; Zhang, W.; Mine, Y. Carnosine—A natural bioactive dipeptide: Bioaccessibility, bioavailability and health benefits. J. Food Bioact. 2019, 5, 8–17. [Google Scholar] [CrossRef]
- Torreggiani, A.; Tamba, M.; Fini, G. Binding of copper(II) to carnosine: Raman and IR spectroscopic study. Biopolymers 2000, 57, 149–159. [Google Scholar] [CrossRef]
- Ghodsi, R.; Kheirouri, S. Carnosine and advanced glycation end products: A systematic review. Amino Acids 2018, 50, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-J.; Kuo, W.-W.; Liu, W.-H.; Yin, M.-C. Antioxidative and anti-inflammatory protection from carnosine in the striatum of MPTP-treated mice. J. Agric. Food Chem. 2010, 58, 11510–11516. [Google Scholar] [CrossRef] [PubMed]
- Hipkiss, A.R. Carnosine, a protective, anti-ageing peptide? Int. J. Biochem. Cell Biol. 1998, 30, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Davey, C.L. The significance of carnosine and anserine in striated skeletal muscle. Arch. Biochem. Biophys. 1960, 89, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Rokicki, J.; Li, L.; Imabayashi, E.; Kaneko, J.; Hisatsune, T.; Matsuda, H. Daily carnosine and anserine supplementation alters verbal episodic memory and resting state network connectivity in healthy elderly adults. Front. Aging Neurosci. 2015, 7, 219. [Google Scholar] [CrossRef] [PubMed]
- Szcześniak, D.; Budzeń, S.; Kopeć, W.; Rymaszewska, J. Anserine and carnosine supplementation in the elderly: Effects on cognitive functioning and physical capacity. Arch. Gerontol. Geriatr. 2014, 59, 485–490. [Google Scholar] [CrossRef]
- Kohen, R.; Yamamoto, Y.; Cundy, K.C.; Ames, B.N. Antioxidant activity of carnosine, homocarnosine, and anserine present in muscle and brain. Proc. Natl. Acad. Sci. USA 1988, 85, 3175–3179. [Google Scholar] [CrossRef]
- Boldyrev, A.A.; Severin, S.E. The histidine-containing dipeptides, carnosine and anserine: Distribution, properties and biological significance. Adv. Enzyme Regul. 1990, 30, 175–194. [Google Scholar] [CrossRef] [PubMed]
- Katakura, Y.; Totsuka, M.; Imabayashi, E.; Matsuda, H.; Hisatsune, T. Anserine/carnosine supplementation suppresses the expression of the inflammatory chemokine CCL24 in peripheral blood mononuclear cells from elderly people. Nutrients 2017, 9, 1199. [Google Scholar] [CrossRef] [PubMed]
- Maemura, H.; Goto, K.; Yoshioka, T.; Sato, M.; Takahata, Y.; Morimatsu, F.; Takamatsu, K. Effects of carnosine and anserine supplementation on relatively high intensity endurance performance. Int. J. Sport Health Sci. 2006, 4, 86–94. [Google Scholar] [CrossRef][Green Version]
- Peters, V.; Calabrese, V.; Forsberg, E.; Volk, N.; Fleming, T.; Baelde, H.; Weigand, T.; Thiel, C.; Trovato, A.; Scuto, M.; et al. Protective actions of anserine under diabetic conditions. Int. J. Mol. Sci. 2018, 19, 2751. [Google Scholar] [CrossRef] [PubMed]
- Kubomura, D.; Yamada, M.; Masui, A. Tuna extract reduces serum uric acid in gout-free subjects with insignificantly high serum uric acid: A randomized controlled trial. Biomed. Rep. 2016, 5, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Sadikali, F.; Darwish, R.; Watson, W.C. Carnosinase activity of human gastrointestinal mucosa. Gut 1975, 16, 585–589. [Google Scholar] [CrossRef][Green Version]
- Chmielewska, K.; Dzierzbicka, K.; Inkielewicz-Stępniak, I.; Przybyłowska, M. Therapeutic potential of carnosine and its derivatives in the treatment of human diseases. Chem. Res. Toxicol. 2020, 33, 1561–1578. [Google Scholar] [CrossRef]
- Van der Stede, T.; Spaas, J.; de Jager, S.; De Brandt, J.; Hansen, C.; Stautemas, J.; Vercammen, B.; De Baere, S.; Croubels, S.; Van Assche, C.H.; et al. Extensive profiling of histidine-containing dipeptides reveals species- and tissue-specific distribution and metabolism in mice, rats, and humans. Acta Physiol. 2023, 239, e14020. [Google Scholar] [CrossRef]
- Hoetker, D.; Chung, W.; Zhang, D.; Zhao, J.; Schmidtke, V.K.; Riggs, D.W.; Derave, W.; Bhatnagar, A.; Bishop, D.; Baba, S.P. Exercise alters and β-alanine combined with exercise augments histidyl dipeptide levels and scavenges lipid peroxidation products in human skeletal muscle. J. Appl. Physiol. 2018, 125, 1767–1778. [Google Scholar] [CrossRef]
- de Souza Gonçalves, L.; Pereira, W.R.; da Silva, R.P.; Yamaguchi, G.C.; Carvalho, V.H.; Vargas, B.S.; Jensen, L.; de Medeiros, M.H.G.; Roschel, H.; Artioli, G.G. Anserine is expressed in human cardiac and skeletal muscles. Physiol. Rep. 2023, 11, e15833. [Google Scholar] [CrossRef]
- O’Flaherty, L.; Stapleton, P.P.; Redmond, H.P.; Bouchier-Hayes, D.J. Intestinal taurine transport: A review. Eur. J. Clin. Investig. 1997, 27, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Spitze, A.R.; Wong, D.L.; Rogers, Q.R.; Fascetti, A.J. Taurine concentrations in animal feed ingredients; cooking influences taurine content. J. Anim. Physiol. Anim. Nutr. 2003, 87, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Oldekop, M.-L.; Herodes, K.; Rebane, R. Comparison of amino acid derivatization reagents for liquid chromatography atmospheric pressure chemical ionization mass spectrometric analysis of seven amino acids in tea extract. Int. J. Mass Spectrom. 2017, 421, 189–195. [Google Scholar] [CrossRef]
- Bernal, J.L.; Nozal, M.J.; Toribio, L.; Diego, J.C.; Ruiz, A. A comparative study of several HPLC methods for determining free amino acid profiles in honey. J. Sep. Sci. 2005, 28, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Onozato, M.; Uekusa, S.; Ichiba, H.; Umino, M.; Shirao, M.; Fukushima, T. Development of derivatization reagents bearing chiral 4-imidazolidinone for distinguishing primary amines from other amino acids and application to the liquid chromatography-tandem mass spectrometric analysis of miso. J. Chromatogr. A 2021, 1652, 462341. [Google Scholar] [CrossRef] [PubMed]
- Onozato, M.; Nakanoue, H.; Sakamoto, T.; Umino, M.; Fukushima, T. Determination of d- and l-amino acids in garlic foodstuffs by liquid chromatography-tandem mass spectrometry. Molecules 2023, 28, 1773. [Google Scholar] [CrossRef] [PubMed]
- Onozato, M.; Shinohara, W.; Osaka, Y.; Sakamoto, T.; Umino, M.; Nishigaki, A.; Okoshi, K.; Fukushima, T. Characterization of polychaetes inhabiting estuaries and inner bays by composition analysis of amino acids and lactate enantiomers. Sci. Rep. 2024, 14, 5494. [Google Scholar] [CrossRef] [PubMed]
- Uenoyama, R.; Miyazaki, M.; Miyazaki, T.; Shigeno, Y.; Tokairin, Y.; Konno, H.; Yamashita, T. LC-ESI-MS/MS quantification of carnosine, anserine, and balenine in meat samples. J. Chromatogr. B 2019, 1132, 121826. [Google Scholar] [CrossRef] [PubMed]
- Pandya, V.K.; Sonwane, B.; Rathore, R.; Unnikrishnan, A.G.; Kumaran, S.; Kulkarni, M.J. Development of multiple reaction monitoring assay for quantification of carnosine in human plasma. RSC Adv. 2020, 10, 763–769. [Google Scholar] [CrossRef]
- Stautemas, J.; Jarzebska, N.; Shan, Z.X.; Blancquaert, L.; Everaert, I.; de Jager, S.; De Baere, S.; Hautekiet, A.; Volkaert, A.; Lefevere, F.B.D.; et al. The role of alanine glyoxylate transaminase-2 (agxt2) in β-alanine and carnosine metabolism of healthy mice and humans. Eur. J. Appl. Physiol. 2020, 120, 2749–2759. [Google Scholar] [CrossRef]
- Uekusa, S.; Onozato, M.; Sakamoto, T.; Umino, M.; Ichiba, H.; Okoshi, K.; Fukushima, T. Development of a derivatization reagent with a 2-nitrophenylsulfonyl moiety for UHPLC-HRMS/MS and its application to detect amino acids including taurine. Molecules 2021, 26, 3498. [Google Scholar] [CrossRef] [PubMed]
- Pucciarini, L.; Gilardoni, E.; Ianni, F.; D’Amato, A.; Marrone, V.; Fumagalli, L.; Regazzoni, L.; Aldini, G.; Carini, M.; Sardella, R. Development and validation of a HPLC method for the direct separation of carnosine enantiomers and analogues in dietary supplements. J. Chromatogr. B 2019, 1126–1127, 121747. [Google Scholar] [CrossRef] [PubMed]
- Everaert, I.; Van der Stede, T.; Stautemas, J.; Hanssens, M.; van Aanhold, C.; Baelde, H.; Vanhaecke, L.; Derave, W. Oral anserine supplementation does not attenuate type-2 diabetes or diabetic nephropathy in BTBR ob/ob mice. Amino Acids 2021, 53, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Peiretti, P.G.; Medana, C.; Visentin, S.; Giancotti, V.; Zunino, V.; Meineri, G. Determination of carnosine, anserine, homocarnosine, pentosidine and thiobarbituric acid reactive substances contents in meat from different animal species. Food Chem. 2011, 126, 1939–1947. [Google Scholar] [CrossRef] [PubMed]
- Schönherr, J. Analysis of products of animal origin in feeds by determination of carnosine and related dipeptides by high-performance liquid chromatography. J. Agric. Food Chem. 2002, 50, 1945–1950. [Google Scholar] [CrossRef]
- Kumagai, M.; Kato, S.; Arakawa, N.; Otsuka, M.; Hamano, T.; Kashiwagi, N.; Yabuki, A.; Yamato, O. Quantification of histidine-containing dipeptides in dolphin serum using a reversed-phase ion-pair high-performance liquid chromatography method. Separations 2021, 8, 128. [Google Scholar] [CrossRef]
- Mora, L.; Sentandreu, M.A.; Toldrá, F. Hydrophilic chromatographic determination of carnosine, anserine, balenine, creatine, and creatinine. J. Agric. Food Chem. 2007, 55, 4664–4669. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.; Hikihara, R.; Ishimaru, M.; Hatate, H.; Tanaka, R. Evaluation of histidine-containing dipeptides in twelve marine organisms and four land animal meats by hydrophilic interaction liquid chromatography with ultraviolet detection. J. Liq. Chromatogr. Relat. Technol. 2018, 41, 849–854. [Google Scholar] [CrossRef]
- Mori, M.; Mizuno, D.; Sadakane, Y.; Konoha-Mizuno, K.; Kawahara, M. Quantitative analysis of carnosine and anserine in foods by performing high performance liquid chromatography. Biomed. Res. Trace Elem. 2015, 26, 147–152. [Google Scholar]
- Hiemori-Kondo, M.; Shinya, D.; Ueta, R. Development of a quantitative method for analyzing three imidazole dipeptides using high-performance liquid chromatography and its application for meat and fish. J. Food Compos. Anal. 2022, 106, 104323. [Google Scholar] [CrossRef]
- Aristoy, M.-C.; Soler, C.; Toldrá, F. A simple, fast and reliable methodology for the analysis of histidine dipeptides as markers of the presence of animal origin proteins in feeds for ruminants. Food Chem. 2004, 84, 485–491. [Google Scholar] [CrossRef]
- Lee, C.J.; Qiu, T.A.; Sweedler, J.V. d-Alanine: Distribution, origin, physiological relevance, and implications in disease. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140482. [Google Scholar] [CrossRef]
- Tsai, G.E.; Yang, P.; Chang, Y.-C.; Chong, M.-Y. d-alanine added to antipsychotics for the treatment of schizophrenia. Biol. Psychiatry 2006, 59, 230–234. [Google Scholar] [CrossRef]
- Marcone, G.L.; Rosini, E.; Crespi, E.; Plooegioni, L. d-amino acids in foods. Appl. Microbiol. Biotechnol. 2020, 104, 555–574. [Google Scholar] [CrossRef]
- Abe, H. Distribution, metabolism and physiological functions of free d-amino acids in aquatic invertebrates. Nippon Suisan Gakkaishi 2002, 68, 515–525. [Google Scholar] [CrossRef][Green Version]
- Sarower, M.G.; Matsui, T.; Abe, H. Distribution and characteristics of d-amino acid and d-aspartate oxidases in fish tissues. J. Exp. Zool. Comp Exp Biol. 2003, 295, 151–159. [Google Scholar] [CrossRef]
- Abe, H. Distribution of free l-histidine and related dipeptides in the muscle of fresh-water fishes. Comp. Biochem. Physiol. B 1983, 76, 35–39. [Google Scholar] [CrossRef]
- Nagano, M.; Omura, Y.; Hayashi, Y.; Akamine, S. Contents of six types of imidazole compounds in five parts of pike conger Muraenesox Cinereus caught in Hyuga-nada and time-course changes in the refrigerated muscle. Nippon Suisan Gakkaishi 2021, 87, 494–503. [Google Scholar] [CrossRef]
- Saito, M.; Kunisaki, N. Determination of anserine and carnosine contents in fish muscles by using high Performance liquid chromatography. Joshi Eiyō Daigaku Kiyou 2004, 35, 57–59. [Google Scholar]
- Shimizu, K.; Fukuda, M.; Yamamoto, H. Effect of repeated intake of imidazole dipeptides-containing drink on healthy people with feeling of fatigue from daily activities. Jpn Pharmacol. Ther. 2009, 37, 255–263. [Google Scholar]
- El Lahamy, A.A.; Khalil, K.I.; El Sherif, S.A.; Abdelazim, S.A.A.; Mahmud, A.A. Effect of frozen storage and cooking method on amino acid composition of mullet fish (Mugil cephalus). MOJ Food Process. Technol. 2018, 6, 458–463. [Google Scholar] [CrossRef]
- Pratama, R.I.; Rostini, I.; Rochima, E. Amino acid profile and volatile flavor compounds of raw and steamed patin catfish (Pangasius hypophthalmus) and narrow-barred spanish mackerel (Scomberomorus commerson). IOP Conf. Ser. Earth Environ. Sci. 2018, 116, 012056. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, Y.; Cao, Y.; Huang, W.; Zhang, S.; Xia, W.; Jiang, Q. Effect of steam cooking on textural properties and taste compounds of shrimp (Metapenaeus ensis). Food Sci. Technol. Res. 2016, 22, 75–81. [Google Scholar] [CrossRef]
- Liu, C.; Ji, W.; Jiang, H.; Shi, Y.; He, L.; Gu, Z.; Zhu, S. Comparison of biochemical composition and non-volatile taste active compounds in raw, high hydrostatic pressure-treated and steamed oysters Crassostrea hongkongensis. Food Chem. 2021, 344, 128632. [Google Scholar] [CrossRef]
- Van Waarde, A. Biochemistry of non-protein nitrogenous compounds in fish including the use of amino acids for anaerobic energy production. Comp. Biochem. Phisiol. B 1988, 91, 207–228. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Li, Y.-R.; Pan, C.; Chen, J.; Jiang, W.; Li, W.-N.; Zhang, X.-L.; Liao, Z.; Yan, X.-J. Quantitative analysis of carnosine, anserine, and homocarnosine in skeletal muscle of aquatic species from East China sea. Biochem. Biophys. Rep. 2021, 25, 100880. [Google Scholar] [CrossRef]
- Abe, H. Metabolism of histidine-related compounds in fish. Kagaku Seibutsu 1985, 23, 809–815. [Google Scholar] [CrossRef][Green Version]
- Suyama, M.; Hirano, T.; Suzuki, T. Buffering capacity of free histidine and its related dipeptides in white and dark muscles of yellowfin tuna. Nippon Suisan Gakkaishi 1986, 52, 2171–2175. [Google Scholar] [CrossRef][Green Version]
- Hiraoka, Y.; Sasaki, Y.; Sonoda, K. Investigation of contents of nitrogenous constituents in the extracts of seafood caught in Ehime (Part 1). Res. Rep. Ehime Inst. Ind. Technol. 2011, 49, 19–22. [Google Scholar]
- Lukton, A.; Olcott, H.S. Content of free imidazole compounds in the muscle tissue of aquatic animals. J. Food Sci. 1958, 23, 611–618. [Google Scholar] [CrossRef]
- Nimura, K.; Ichikawa, R.; Yamazaki, M. The contents of anserine, histidine and taurine in various low-use/underutilized sections of bigeye tuna, albacore and bluefin tuna. J. Fish. Technol. 2022, 15, 39–43. [Google Scholar]
- Zhao, X.; Jia, J.; Lin, Y. Taurine content in Chinese food and daily taurine intake of Chinese men. In Taurine 3: Cellular and Regulatory Mechanisms; Advances in Experimental Medicine and Biology; Schaffer, S., Lombardini, J.B., Huxtable, R.J., Eds.; Springer: Boston, MA, USA, 1998; Volume 442, pp. 501–505. [Google Scholar] [CrossRef]
- Suzuki, T.; Hirano, T.; Shirai, T. Distribution of extractive nitrogenous constituents in white and dark muscles of fresh-water fish. Comp. Biochem. Phys. B 1990, 96, 107–111. [Google Scholar] [CrossRef]

| Derivatized Component | RT 1 (min) | LOD (µg/mL) | Working Range (μM) Linearity (R2) | Spiked Concentration (μM) | Intra-Day (n = 4) | Inter-Day (n = 4) | ||
|---|---|---|---|---|---|---|---|---|
| Accuracy (RME 2, %) | Precision (RSD 3, %) | Accuracy (RME, %) | Precision (RSD, %) | |||||
| Car | 37.9 | 0.00573 | 6.25–500 | 20 | −0.54 | 3.04 | 1.76 | 2.69 |
| 0.99986 | 100 | 2.40 | 1.08 | 1.79 | 1.27 | |||
| 200 | 4.12 | 1.69 | 3.36 | 0.40 | ||||
| Ans | 36.9 | 0.0293 | 6.25–2000 | 50 | −1.91 | 1.52 | 0.88 | 1.52 |
| 0.99965 | 250 | −2.38 | 0.80 | −0.74 | 2.35 | |||
| 500 | −2.52 | 0.95 | 0.09 | 2.86 | ||||
| β-Ala | 17.9 | 0.00122 | 6.25–200 | 10 | −6.18 | 13.2 | −3.31 | 13.9 |
| 0.99961 | 50 | −7.52 | 1.23 | −4.52 | 3.32 | |||
| 100 | −2.99 | 2.09 | −1.94 | 2.12 | ||||
| d-Ala | 21.0 | 0.00194 | 6.25–200 | 10 | −9.64 | 1.89 | −8.58 | 3.83 |
| 0.99962 | 50 | −7.67 | 1.84 | −5.93 | 2.95 | |||
| 100 | −6.69 | 1.31 | −7.04 | 1.48 | ||||
| l-Ala | 27.8 | 0.00201 | 6.25–1000 | 10 | 1.41 | 3.54 | 5.65 | 5.93 |
| 0.99993 | 50 | 0.17 | 1.19 | 1.54 | 0.85 | |||
| 100 | −0.58 | 2.15 | 0.52 | 1.19 | ||||
| l-His | 37.1 | 0.00826 | 6.25–3000 | 50 | −1.78 | 1.51 | −0.19 | 1.45 |
| 0.99977 | 250 | −4.55 | 0.60 | −3.7 | 1.33 | |||
| 500 | −4.09 | 1.74 | −4.53 | 1.32 | ||||
| d-His | 39.9 | 0.00896 | 6.25–200 | 10 | −13.01 | 2.66 | −12.57 | 2.54 |
| 0.99908 | 50 | −11.19 | 1.16 | −11.57 | 0.96 | |||
| 100 | −13.94 | 2.01 | −15.25 | 1.78 | ||||
| Tau | 34.4 | 0.0191 | 6.25–1000 | 20 | −5.17 | 3.07 | −6.56 | 10.8 |
| 0.99994 | 100 | −7.3 | 1.00 | −13.75 | 9.60 | |||
| 200 | −7.75 | 1.07 | −16.52 | 10.6 | ||||
| Derivatization Reagent | Column | Detection | LOD (µg/mL) | Ref. | |
|---|---|---|---|---|---|
| Car | Ans | ||||
| − | Mixed-mode | ESI–MS/MS | 0.0044 | 0.0047 | [28] |
| − | Peptide BEH C18 | ESI–MS/MS with HESI 2 probe | 0.000054 | − | [29] |
| − | C18 | ESI–MS/MS | 0.0013 | 0.0026 | [30] |
| − | C18 | ESI–MS/MS with HESI probe | 0.023 | 0.048 | [33] |
| − | C18 | ESI–MS/MS | 0.011 | 0.011 | [34] |
| Carbazole-9-carbonyl chloride | C18 | UV (254 nm) | 0.54 | − | [35] |
| − | C18 | UV (210 nm) | 0.016 | 0.0096 | [36] |
| − | HILIC | DAD 1 (214 nm) | 5.6 | 8.2 | [37] |
| − | Phosphorylcholine HILIC | UV (214 nm) | 0.060 | 0.10 | [38] |
| − | HypercarbTM | UV (215 nm) | 0.054 | 0.058 | [39] |
| − | Amino | UV (210 nm) | 0.042 | 0.039 | [40] |
| o-Phthalaldehyde | Strong cation exchange | Fluorescence (ex. 340 nm, em. 445 nm) | 0.14 | 0.22 | [41] |
| (R)-CIMa-OSu | Mixed-mode | ESI–MS/MS | 0.0057 | 0.029 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onozato, M.; Horinouchi, M.; Yoshiba, Y.; Sakamoto, T.; Sugasawa, H.; Fukushima, T. Determination of Imidazole Dipeptides and Related Amino Acids in Natural Seafoods by Liquid Chromatography–Tandem Mass Spectrometry Using a Pre-Column Derivatization Reagent. Foods 2024, 13, 1951. https://doi.org/10.3390/foods13121951
Onozato M, Horinouchi M, Yoshiba Y, Sakamoto T, Sugasawa H, Fukushima T. Determination of Imidazole Dipeptides and Related Amino Acids in Natural Seafoods by Liquid Chromatography–Tandem Mass Spectrometry Using a Pre-Column Derivatization Reagent. Foods. 2024; 13(12):1951. https://doi.org/10.3390/foods13121951
Chicago/Turabian StyleOnozato, Mayu, Minori Horinouchi, Yuki Yoshiba, Tatsuya Sakamoto, Hiroshi Sugasawa, and Takeshi Fukushima. 2024. "Determination of Imidazole Dipeptides and Related Amino Acids in Natural Seafoods by Liquid Chromatography–Tandem Mass Spectrometry Using a Pre-Column Derivatization Reagent" Foods 13, no. 12: 1951. https://doi.org/10.3390/foods13121951
APA StyleOnozato, M., Horinouchi, M., Yoshiba, Y., Sakamoto, T., Sugasawa, H., & Fukushima, T. (2024). Determination of Imidazole Dipeptides and Related Amino Acids in Natural Seafoods by Liquid Chromatography–Tandem Mass Spectrometry Using a Pre-Column Derivatization Reagent. Foods, 13(12), 1951. https://doi.org/10.3390/foods13121951







