Elucidating the Interaction of Indole-3-Propionic Acid and Calf Thymus DNA: Multispectroscopic and Computational Modeling Approaches
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. UV–Vis Absorption Spectra
2.3. Fluorescence Spectra
2.4. Potassium Iodide Quenching Measurement
2.5. Effects of Native or Denatured ctDNA on IPA
2.6. Determination of Ionic Strength
2.7. Thermal Melting of ctDNA
2.8. Viscosity Measurements
2.9. Circular Dichroism (CD) Spectra
2.10. DNA Cleavage
2.11. Molecular Docking
2.12. Molecular Dynamics (MD) Simulation
2.13. Statistical Analysis
3. Results and Discussion
3.1. UV–Vis Absorption Spectra
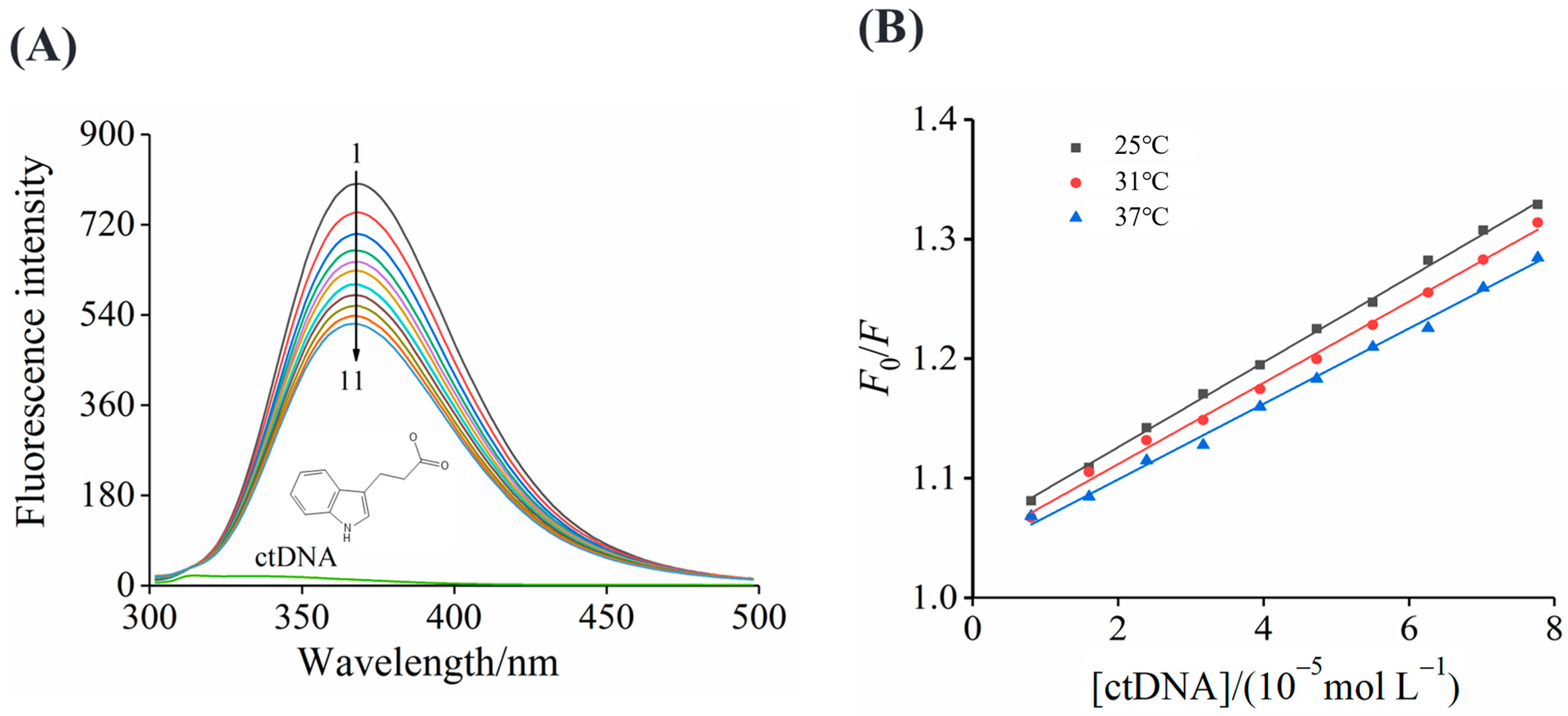
3.2. Fluorescence Spectra
3.3. Binding Constants
3.4. Thermodynamics
3.5. Iodine Ion Quenching
3.6. Quenching Effects of ss ctDNA and ds ctDNA
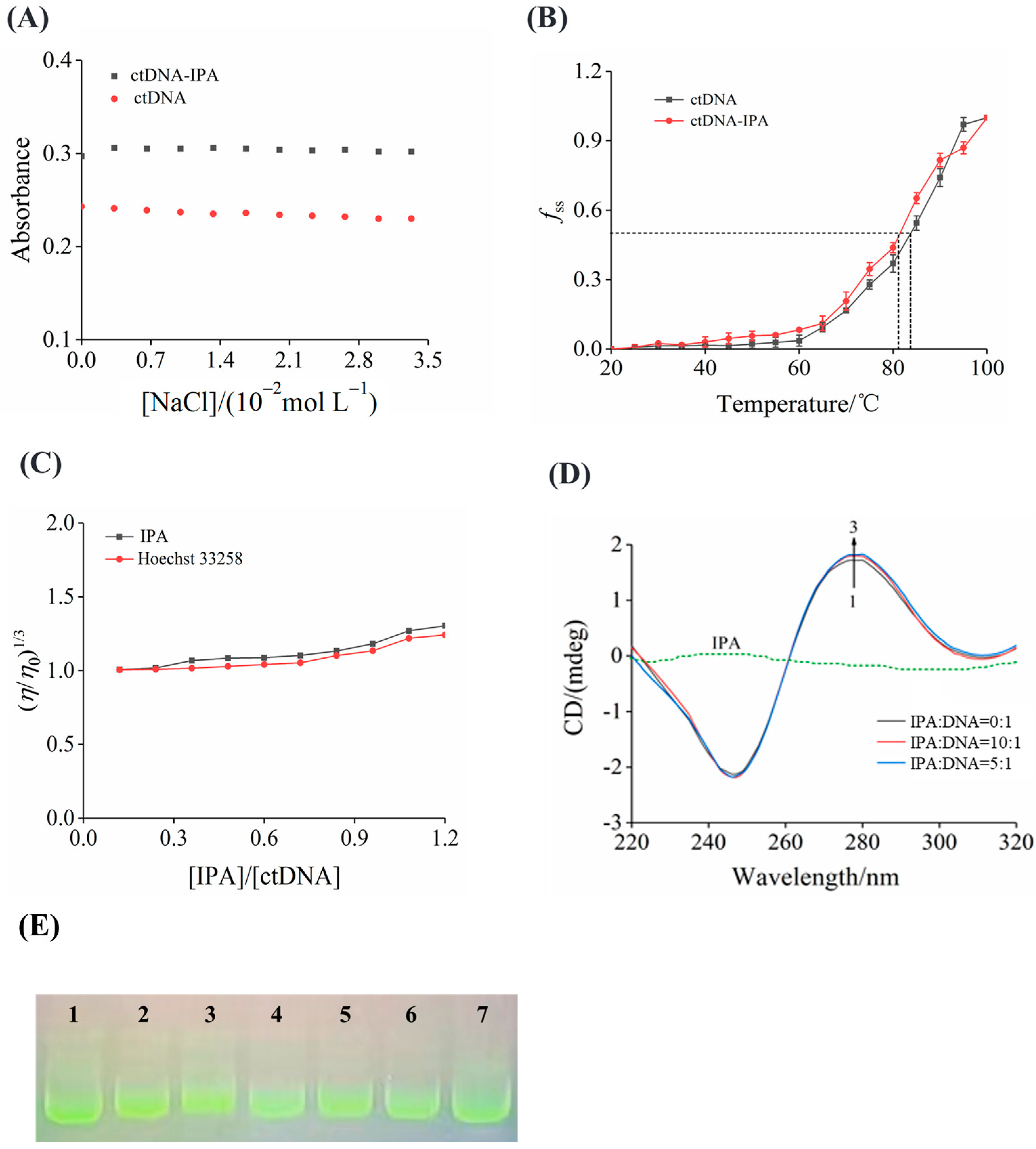
3.7. Ionic Strength
3.8. ctDNA Melting Temperature
3.9. Viscosity
3.10. CD Spectra
3.11. DNA Cleavage Activity of IPA
3.12. Molecular Docking
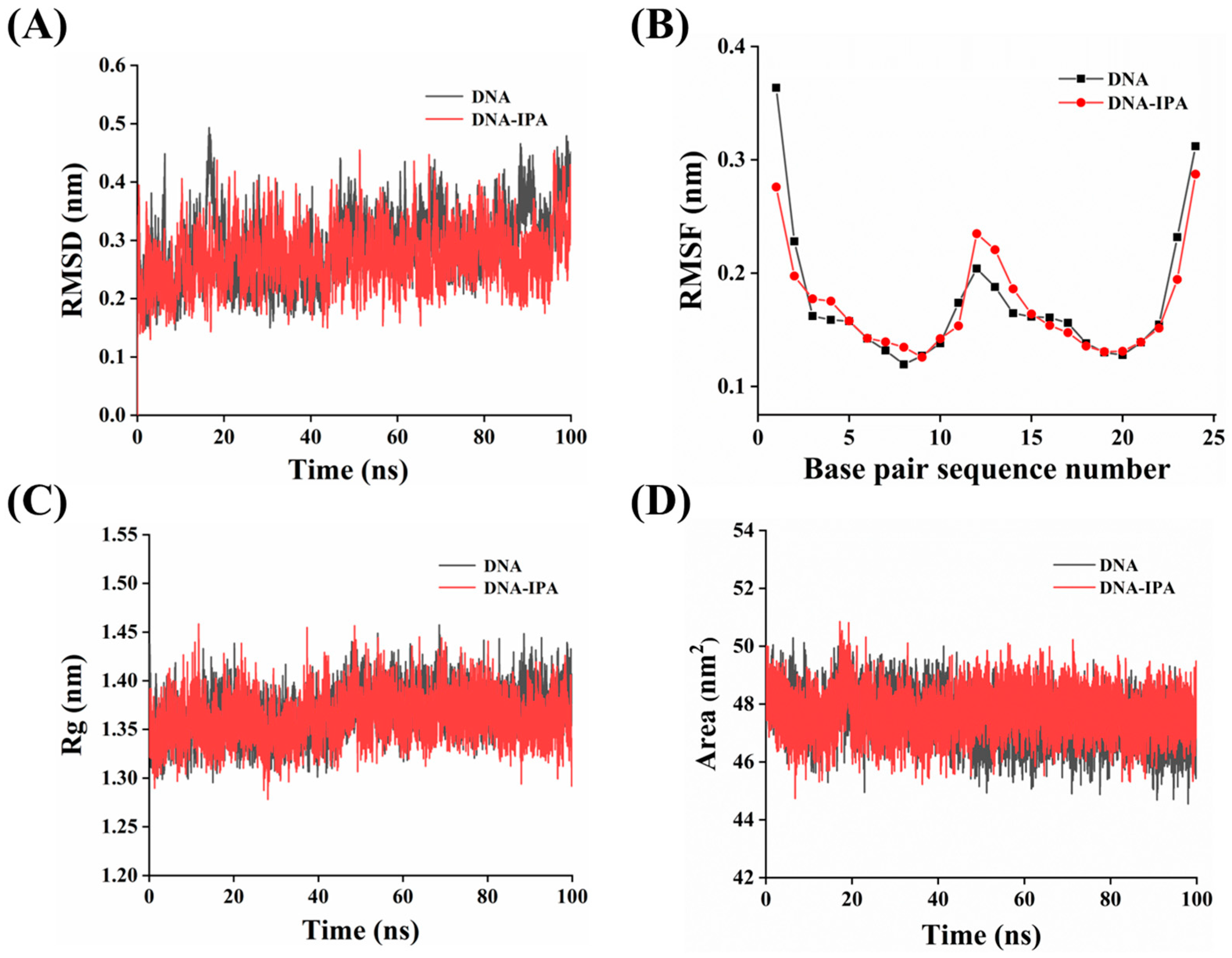
3.13. MD Simulation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giannakoula, A.E.; Ilias, I.F.; Dragišić Maksimović, J.J.; Maksimović, V.M.; Živanović, B.D. The effects of plant growth regulators on growth, yield, and phenolic profile of lentil plants. J. Food Compos. Anal. 2012, 28, 46–53. [Google Scholar] [CrossRef]
- Le, V.N.; Nguyen, Q.T.; Nguyen, T.D.; Nguyen, N.T.; Janda, T.; Szalai, G.; Le, T.G. The potential health risks and environmental pollution associated with the application of plant growth regulators in vegetable production in several suburban areas of hanoi, vietnam. Biol. Futur. 2020, 71, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hao, W. Reproductive and developmental toxicity of plant growth regulators in humans and animals. Pestic. Biochem. Physiol. 2023, 196, 105640. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Z.; Celik, I. Neurotoxic and Immunotoxic effects of indole-3-butyric acid on rats at subacute and subchronic exposure. NeuroToxicology 2009, 30, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Sharma, I.; Kaur, N.; Pati, P.K. Auxin: A master regulator in plant root development. Plant Cell Rep. 2013, 32, 741–757. [Google Scholar] [CrossRef] [PubMed]
- Abildgaard, A.; Elfving, B.; Hokland, M.; Wegener, G.; Lund, S. The microbial metabolite indole-3-propionic acid improves glucose metabolism in rats, but does not affect behaviour. Arch. Physiol. Biochem. 2018, 124, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, W.; Zhang, F.; Zhong, S.; Sun, Y.; Huo, J.; Zhu, J.; Wu, C. The gut microbiota-produced indole-3-propionic acid confers the antihyperlipidemic effect of mulberry-derived 1-deoxynojirimycin. mSystems 2020, 5, e00313-20. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Sun, C.; Chen, Y.; Du, F.; Yang, Y.; Wu, G. Indole-3-propionic acid-aggravated CCl4-induced liver fibrosis via the TGF-Β1/Smads signaling pathway. J. Clin. Transl. Hepatol. 2021, 9, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Konopelski, P.; Chabowski, D.; Aleksandrowicz, M.; Kozniewska, E.; Podsadni, P.; Szczepanska, A.; Ufnal, M. Indole-3-propionic acid, a tryptophan-derived bacterial metabolite, increases blood pressure via cardiac and vascular mechanisms in rats. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2021, 321, R969–R981. [Google Scholar] [CrossRef]
- Dolatabadi, J.E.N.; Kashanian, S. A Review on DNA Interaction with synthetic phenolic food additives. Food Res. Int. 2010, 43, 1223–1230. [Google Scholar] [CrossRef]
- Kashanian, S.; Khodaei, M.M.; Kheirdoosh, F. In vitro DNA binding studies of aspartame, an artificial sweetener. J. Photochem. Photobiol. B Biol. 2013, 120, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Ikhlas, S.; Ahmad, M. Binding studies of guggulsterone-E to calf thymus DNA by multi-Spectroscopic, calorimetric and molecular docking studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 190, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Devaraji, V.; Prasath, R.; Jadhao, M.; Joshi, R.; Bhavana, P.; Ghosh, S.K. Groove binding mediated structural modulation and DNA cleavage by quinoline appended chalcone derivative. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 151, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Wani, T.A.; Bakheit, A.H.; Al-Majed, A.A.; Altwaijry, N.; Baquaysh, A.; Aljuraisy, A.; Zargar, S. Binding and drug displacement study of colchicine and bovine serum albumin in presence of azithromycin using multispectroscopic techniques and molecular dynamic simulation. J. Mol. Liq. 2021, 333, 115934. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Hu, X.; Pan, J.; Zhang, Y.; Gong, D.; Zhang, G. Insights into the mechanism of groove binding between 4–octylphenol and calf thymus DNA. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 238, 118454. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Zhu, M.; Xin, L.; Zhang, G.; Wu, S.; Hu, X.; Gong, D. Change of benzo(a)pyrene during frying and its groove binding to calf thymus DNA. Food Chem. 2021, 350, 129276. [Google Scholar] [CrossRef]
- Zhang, D.; Pan, J.; Gong, D.; Zhang, G. Groove binding of indole-3-butyric acid to calf thymus DNA: Spectroscopic and in silico approaches. J. Mol. Liq. 2022, 347, 118323. [Google Scholar] [CrossRef]
- Lin, J.; Gao, C.; Liu, R. Interaction mechanism of Trp-Arg dipeptide with calf thymus DNA. J. Fluoresc. 2013, 23, 921–927. [Google Scholar] [CrossRef]
- De Carvalho Bertozo, L.; Tutone, M.; Pastrello, B.; da Silva-Filho, L.C.; Culletta, G.; Almerico, A.M.; Farias Ximenes, V. Aminoquinolines: Fluorescent sensors to DNA—A minor groove probe. Experimental and in silico studies. J. Photochem. Photobiol. A Chem. 2023, 444, 114944. [Google Scholar] [CrossRef]
- Zhu, M.; Hu, X.; Zhang, Y.; Pan, J.; Zhang, G. Revealing the groove binding characteristics of plant growth regulator 3-indoleacetic acid with calf thymus DNA. J. Mol. Liq. 2021, 326, 115265. [Google Scholar] [CrossRef]
- Sarwar, T.; Ishqi, H.M.; Rehman, S.U.; Husain, M.A.; Rahman, Y.; Tabish, M. Caffeic acid binds to the minor groove of calf thymus DNA: A multi-spectroscopic, thermodynamics and molecular modelling study. Int. J. Biol. Macromol. 2017, 98, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Venkat Reddy, P.; Rajender Reddy, M.; Avudoddi, S.; Praveen Kumar, Y.; Nagamani, C.; Deepika, N.; Nagasuryaprasad, K.; Satyanarayana Singh, S.; Satyanarayana, S. Design, synthesis, DNA-binding affinity, cytotoxicity, apoptosis, and cell cycle arrest of Ru(II) polypyridyl complexes. Anal. Biochem. 2015, 485, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Pérez, A.; Luque, F.J.; Orozco, M. Frontiers in molecular dynamics simulations of DNA. Acc. Chem. Res. 2012, 45, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Shahabadi, N.; Shiri, F.; Hadidi, S.; Farshadfar, K.; Darbemamieh, M.; Mark Roe, S. The role of both intercalation and groove binding at AT-rich DNA regions in the interaction process of a dinuclear Cu(I) complex probed by spectroscopic and simulation analysis. J. Mol. Liq. 2021, 335, 116290. [Google Scholar] [CrossRef]
- Bodapati, A.T.S.; Reddy, R.S.; Lavanya, K.; Madku, S.R.; Sahoo, B.K. Minor Groove Binding of Antihistamine Drug Bilastine with Calf Thymus DNA: A molecular perspective with thermodynamics using experimental and theoretical methods. J. Mol. Struct. 2024, 1301, 137385. [Google Scholar] [CrossRef]
- Sirajuddin, M.; Ali, S.; Badshah, A. Drug–DNA Interactions and Their Study by UV–Visible, Fluorescence spectroscopies and cyclic voltametry. J. Photochem. Photobiol. B Biol. 2013, 124, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, T.; Husain, M.A.; Rehman, S.U.; Ishqi, H.M.; Tabish, M. Multi-spectroscopic and molecular modelling studies on the interaction of esculetin with calf thymus DNA. Mol. Biosyst. 2015, 11, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Cui, Y.; Liu, Q.; Cui, F.; Zhang, G.; Chi, Y.; Peng, H. Spectroscopic and molecular modeling study on the interaction of ctDNA with 3′-deoxy-3′-azido doxorubicin. J. Lumin. 2013, 141, 144–149. [Google Scholar] [CrossRef]
- Kashanian, S.; Ezzati Nazhad Dolatabadi, J. In vitro studies on calf thymus DNA Interaction and 2-tert-butyl-4-methylphenol food additive. Eur. Food Res. Technol. 2010, 230, 821–825. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Z.; Zhou, Q.; Yan, J.; Zhang, J.; Su, G. Hypouricemic effect in hyperuricemic mice and xanthine oxidase inhibitory mechanism of dietary anthocyanins from purple sweet potato (Ipomoea Batatas L.). J. Funct. Foods 2020, 73, 104151. [Google Scholar] [CrossRef]
- Ponkarpagam, S.; Mahalakshmi, G.; Vennila, K.N.; Elango, K.P. Multi-spectroscopic, voltammetric and molecular docking studies on binding of anti-diabetic drug rosigiltazone with DNA. Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2020, 234, 118268. [Google Scholar] [CrossRef] [PubMed]
- Shahabadi, N.; Fili, S.M.; Kheirdoosh, F. Study on the interaction of the drug mesalamine with calf thymus DNA using molecular docking and spectroscopic techniques. J. Photochem. Photobiol. B Biol. 2013, 128, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, H.; Zhao, L.; Gan, R.; Tang, P.; Sun, Q.; Xiong, X.; Li, H. Capecitabine as a Minor Groove Binder of DNA: Molecular Docking, Molecular Dynamics, and Multi-Spectroscopic Studies. J. Biomol. Struct. Dyn. 2019, 37, 1451–1463. [Google Scholar] [CrossRef] [PubMed]
- Garbett, N.C.; Hammond, N.B.; Graves, D.E. Influence of the amino substituents in the interaction of ethidium bromide with DNA. Biophys. J. 2004, 87, 3974–3981. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.K.; Sukumaran, S.; Sudarsanakumar, C. Photobehaviour and in vitro binding strategy of natural drug, chlorogenic acid with DNA: A case of groove binding. J. Mol. Struct. 2019, 1178, 62–72. [Google Scholar] [CrossRef]
- Tayyab, S.; Sam, S.E.; Kabir, M.Z.; Ridzwan, N.F.W.; Mohamad, S.B. Molecular interaction study of an anticancer drug, ponatinib with human serum albumin using spectroscopic and molecular docking methods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 214, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Farah, M.A.; Al-Lohedan, H.A.; Al-Anazi, K.M. Comprehensive exploration of the anticancer activities of procaine and its binding with calf Thymus DNA: A multi spectroscopic and molecular modelling study. RSC Adv. 2018, 8, 9083–9093. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; He, X. Studies of interaction between safranine T and double helix DNA by spectral methods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1998, 54, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.-H.; Zhou, K.-L.; Lou, Y.-Y.; Pan, D.-Q. Multi-spectroscopic and molecular docking studies on the interaction of darunavir, a HIV protease inhibitor with calf thymus DNA. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 193, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Eskandani, M.; Hamishehkar, H.; Ezzati Nazhad Dolatabadi, J. Cytotoxicity and DNA damage properties of tert-butylhydroquinone (TBHQ) food additive. Food Chem. 2014, 153, 315–320. [Google Scholar] [CrossRef]
- Wani, T.A.; Alsaif, N.; Bakheit, A.H.; Zargar, S.; Al-Mehizia, A.A.; Khan, A.A. Interaction of an abiraterone with calf thymus DNA: Investigation with spectroscopic technique and modelling studies. Bioorganic Chem. 2020, 100, 103957. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.J. Nuclear magnetic resonance studies of drug-nucleic acid interactions at the synthetic DNA level in solution. Am. Chem. Soc. 1979, 12, 118–125. [Google Scholar] [CrossRef]
- Lv, M.; Wang, M.; Lu, K.; Duan, B.; Zhao, Y. Non-covalent interaction between CA–TAT and calf thymus DNA: Deciphering the binding mode by in vitro studies. Int. J. Biol. Macromol. 2018, 114, 1354–1360. [Google Scholar] [CrossRef]
- Husain, M.A.; Ishqi, H.M.; Rehman, S.U.; Sarwar, T.; Afrin, S.; Rahman, Y.; Tabish, M. Elucidating the interaction of sulindac with calf thymus DNA: Biophysical and in silico molecular modelling approach. New J. Chem. 2017, 41, 14924–14935. [Google Scholar] [CrossRef]
- Moradi, S.Z.; Nowroozi, A.; Sadrjavadi, K.; Moradi, S.; Mansouri, K.; Hosseinzadeh, L.; Shahlaei, M. Direct evidences for the groove binding of the clomifene to double stranded DNA. Int. J. Biol. Macromol. 2018, 114, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.U.; Sarwar, T.; Ishqi, H.M.; Husain, M.A.; Hasan, Z.; Tabish, M. Deciphering the interactions between chlorambucil and calf thymus DNA: A Multi-spectroscopic and molecular docking study. Arch. Biochem. Biophys. 2015, 566, 7–14. [Google Scholar] [CrossRef]
- Rehman, S.U.; Sarwar, T.; Husain, M.A.; Ishqi, H.M.; Tabish, M. Studying non-covalent drug-DNA interactions. Arch Biochem. Biophys 2015, 576, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, G.; Wang, L. Probing the binding mode of psoralen to calf thymus DNA. Int. J. Biol. Macromol. 2014, 67, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Tao, M.; Zhang, G.; Pan, J.; Xiong, C. Deciphering the groove binding modes of tau-fluvalinate and flumethrin with calf thymus DNA. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 155, 28–37. [Google Scholar] [CrossRef]
- Haris, P.; Mary, V.; Aparna, P.; Dileep, K.V.; Sudarsanakumar, C. A Comprehensive approach to ascertain the binding mode of curcumin with DNA. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 175, 155–163. [Google Scholar] [CrossRef]
- Shahabadi, N.; Maghsudi, M. Gel electrophoresis and DNA interaction studies of the food colorant quinoline yellow. Dye. Pigment. 2013, 96, 377–382. [Google Scholar] [CrossRef]
- Nair, M.S.; D’Mello, S.; Pant, R.; Poluri, K.M. Binding of resveratrol to the minor groove of DNA sequences with AATT and TTAA segments induces differential stability. J. Photochem. Photobiol. B Biol. 2017, 170, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Vaidergorn, M.M.; Carneiro, Z.A.; Lopes, C.D.; de Albuquerque, S.; Reis, F.C.C.; Nikolaou, S.; Mello, J.F.R.e.; Genesi, G.L.; Trossini, G.H.G.; Ganesan, A.; et al. β-Amino alcohols and their respective 2-phenyl-n-alkyl aziridines as potential DNA minor groove binders. Eur. J. Med. Chem. 2018, 157, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Greener, J.G. Differentiable simulation to develop molecular dynamics force fields for disordered proteins. Chem. Sci. 2024, 15, 4897–4909. [Google Scholar] [CrossRef]
- Pavithra, K.; Priyadharshini, R.D.; Vennila, K.N.; Elango, K.P. Exploring the binding mechanism of pomalidomide drug with ct-DNA: Insights from multi-spectroscopic. Mol. Docking Simul. Studies. J. Mol. Struct. 2024, 1302, 137547. [Google Scholar]
- Farajzadeh-Dehkordi, M.; Darzi, S.; Rahmani, B.; Farhadian, S. A Novel insight into the cytotoxic effects of tphrosin with calf thymus DNA: Experimental and in silico approaches. J. Mol. Liq. 2021, 324, 114728. [Google Scholar] [CrossRef]
- Akter, N.; Bourougaa, L.; Ouassaf, M.; Bhowmic, R.C.; Uddin, K.M.; Bhat, A.R.; Ahmed, S.; Kawsar, S.M.A. Molecular docking, ADME-Tox, DFT and molecular dynamics simulation of butyroyl glucopyranoside derivatives against DNA gyrase inhibitors as antimicrobial agents. J. Mol. Struct. 2024, 1307, 137930. [Google Scholar] [CrossRef]
- Dehkordi, M.F.; Farhadian, S.; Hashemi-Shahraki, F.; Rahmani, B.; Darzi, S.; Dehghan, G. The interaction mechanism of candidone with calf thymus DNA: A multi-spectroscopic and MD simulation study. Int. J. Biol. Macromol. 2023, 235, 123713. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, G.; Hu, M.; Pan, J.; Li, A.; Zhang, Y. Molecular characteristics of gallocatechin gallate affecting protein glycation. Food Hydrocoll. 2020, 105, 105782. [Google Scholar] [CrossRef]



| T (℃) | Ksv (×103 L mol−1) | Ra | Kq (×1011 L mol−1 s−1) | Ka (×104 L mol−1) | Rb | ΔH° (kJ mol−1) | ΔG° (kJ mol−1) | ΔS° (J mol−1 K−1) |
|---|---|---|---|---|---|---|---|---|
| 25 | 3.55 ± 0.06 | 0.9977 | 3.55 ± 0.06 | 1.75 ± 0.10 | 0.9948 | −20.68 | −24.27 | 12.05 |
| 31 | 3.39 ± 0.07 | 0.9961 | 3.39 ± 0.07 | 1.62 ± 0.14 | 0.9958 | −24.34 | ||
| 37 | 3.16 ± 0.08 | 0.9939 | 3.16 ± 0.08 | 1.27 ± 0.08 | 0.9951 | −24.42 |
| Receptor | Ligand | Hydrogen Bonding | Weak Hydrogen Bond | Hydrophobic Interactions |
|---|---|---|---|---|
| 1BNA | IPA | DT7 (2.18Å), DA18 (2.04Å), DT19 (2.66Å) | DT8 (2.80 Å), DC9 (2.71 Å), DT19 (2.71 Å) | DT8 (π-sigma, 2.49 Å) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Zhang, D.; Pan, J.; Gong, D.; Zhang, G. Elucidating the Interaction of Indole-3-Propionic Acid and Calf Thymus DNA: Multispectroscopic and Computational Modeling Approaches. Foods 2024, 13, 1878. https://doi.org/10.3390/foods13121878
Wei Y, Zhang D, Pan J, Gong D, Zhang G. Elucidating the Interaction of Indole-3-Propionic Acid and Calf Thymus DNA: Multispectroscopic and Computational Modeling Approaches. Foods. 2024; 13(12):1878. https://doi.org/10.3390/foods13121878
Chicago/Turabian StyleWei, Yushi, Dan Zhang, Junhui Pan, Deming Gong, and Guowen Zhang. 2024. "Elucidating the Interaction of Indole-3-Propionic Acid and Calf Thymus DNA: Multispectroscopic and Computational Modeling Approaches" Foods 13, no. 12: 1878. https://doi.org/10.3390/foods13121878
APA StyleWei, Y., Zhang, D., Pan, J., Gong, D., & Zhang, G. (2024). Elucidating the Interaction of Indole-3-Propionic Acid and Calf Thymus DNA: Multispectroscopic and Computational Modeling Approaches. Foods, 13(12), 1878. https://doi.org/10.3390/foods13121878








