Improving Muscat Hamburg Wine Quality with Innovative Fermentation Strategies Using Schizosaccharomyces pombe Derived from Fermented Grains of Sauce-Flavor Baijiu
Abstract
1. Introduction
2. Materials and Methods
2.1. Microbial Strains
2.2. Alcohol Fermentation
2.3. Analysis of Metabolites
2.3.1. Determination of Physical and Chemical Indicators Such as Organic Acid, Residual Sugar, Glycerol, and Ethanol in Wine
2.3.2. Analysis of Higher Alcohols and Esters in Wine
2.4. Determination of Wine Color
2.5. Sensory Evaluation of Wine
2.6. Statistical Analysis
3. Results
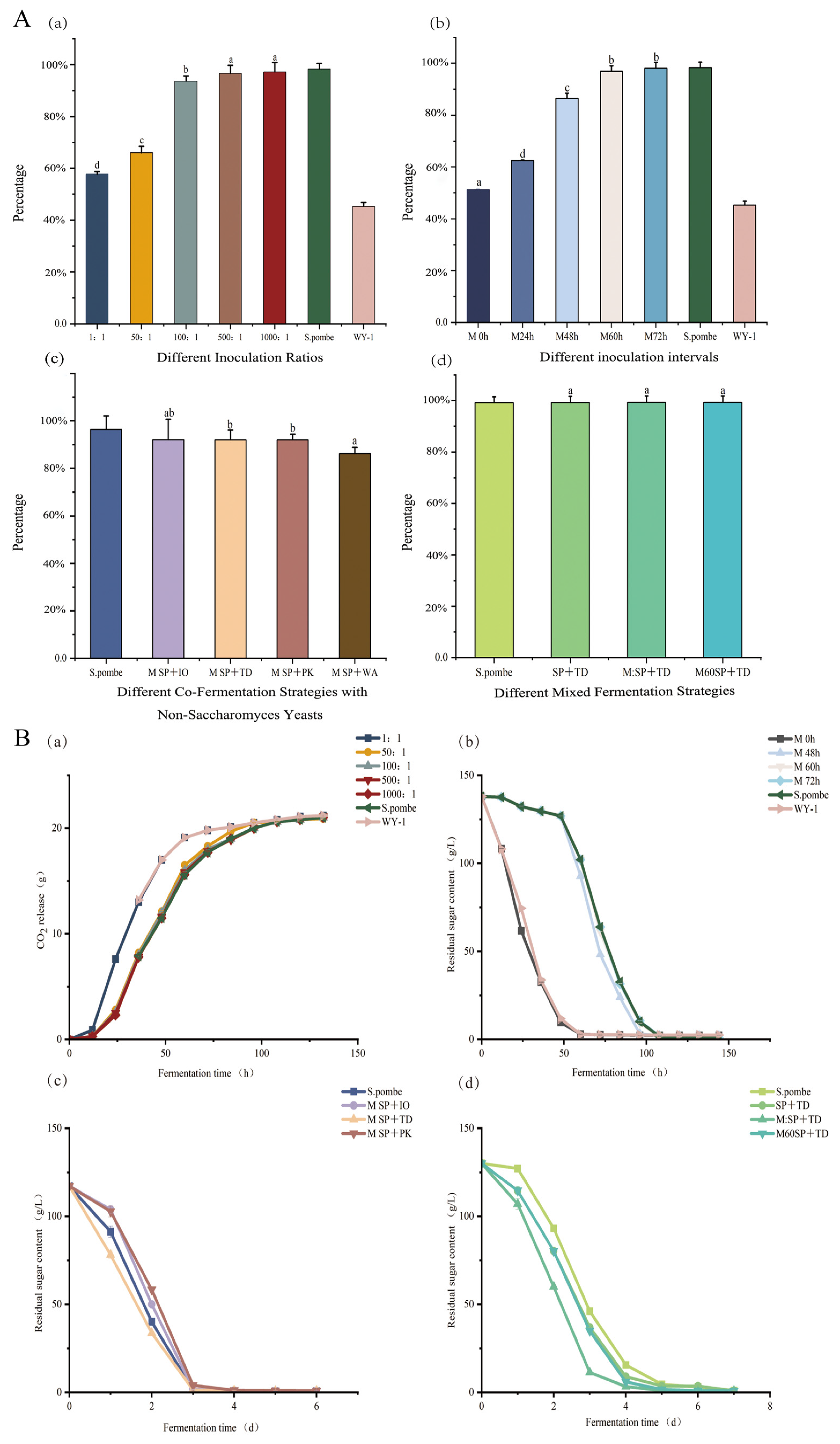
3.1. Wine Fermentation
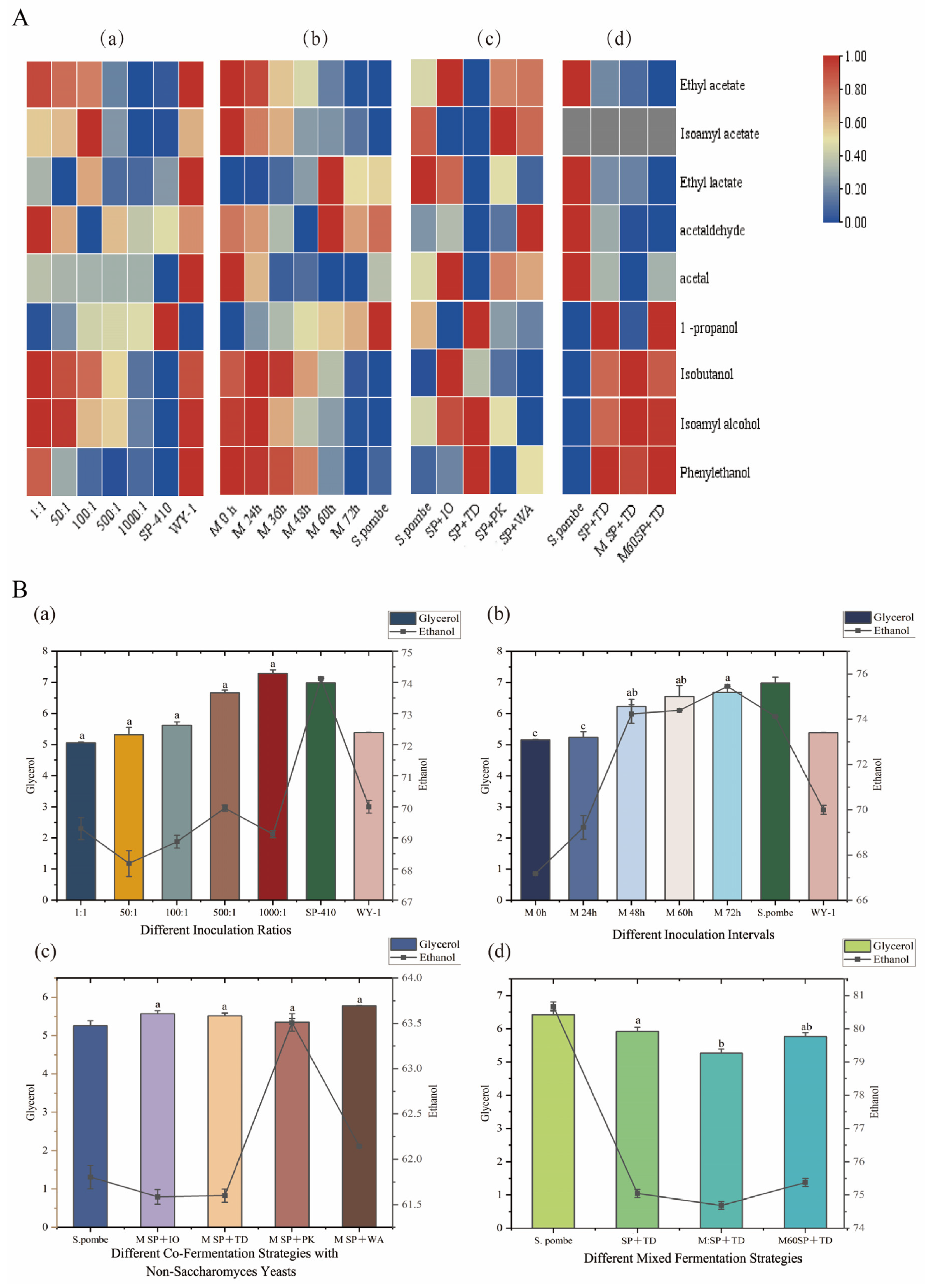
3.2. Volatile Aroma Compounds
3.3. Wine Color
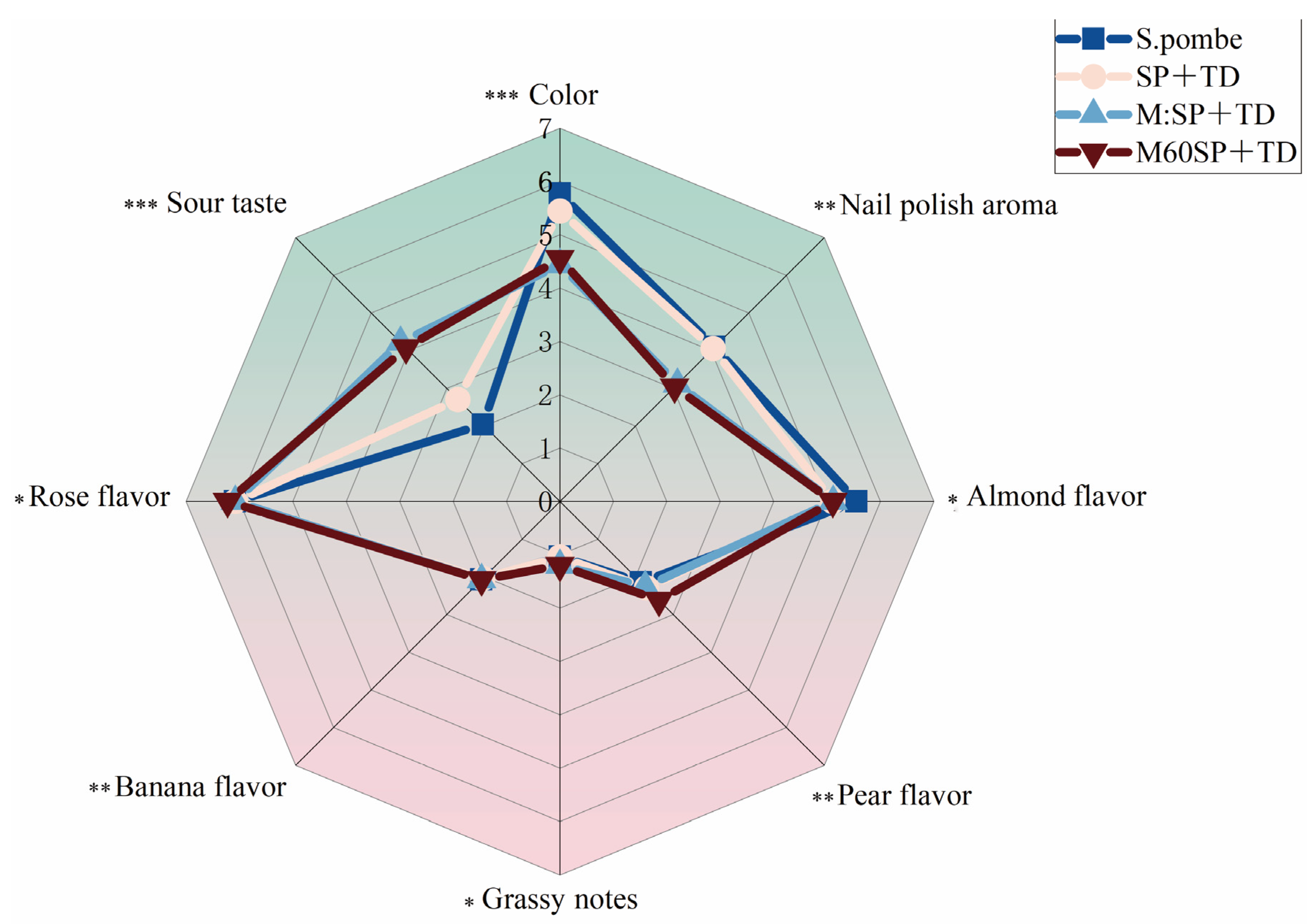
3.4. Sensory Evaluation
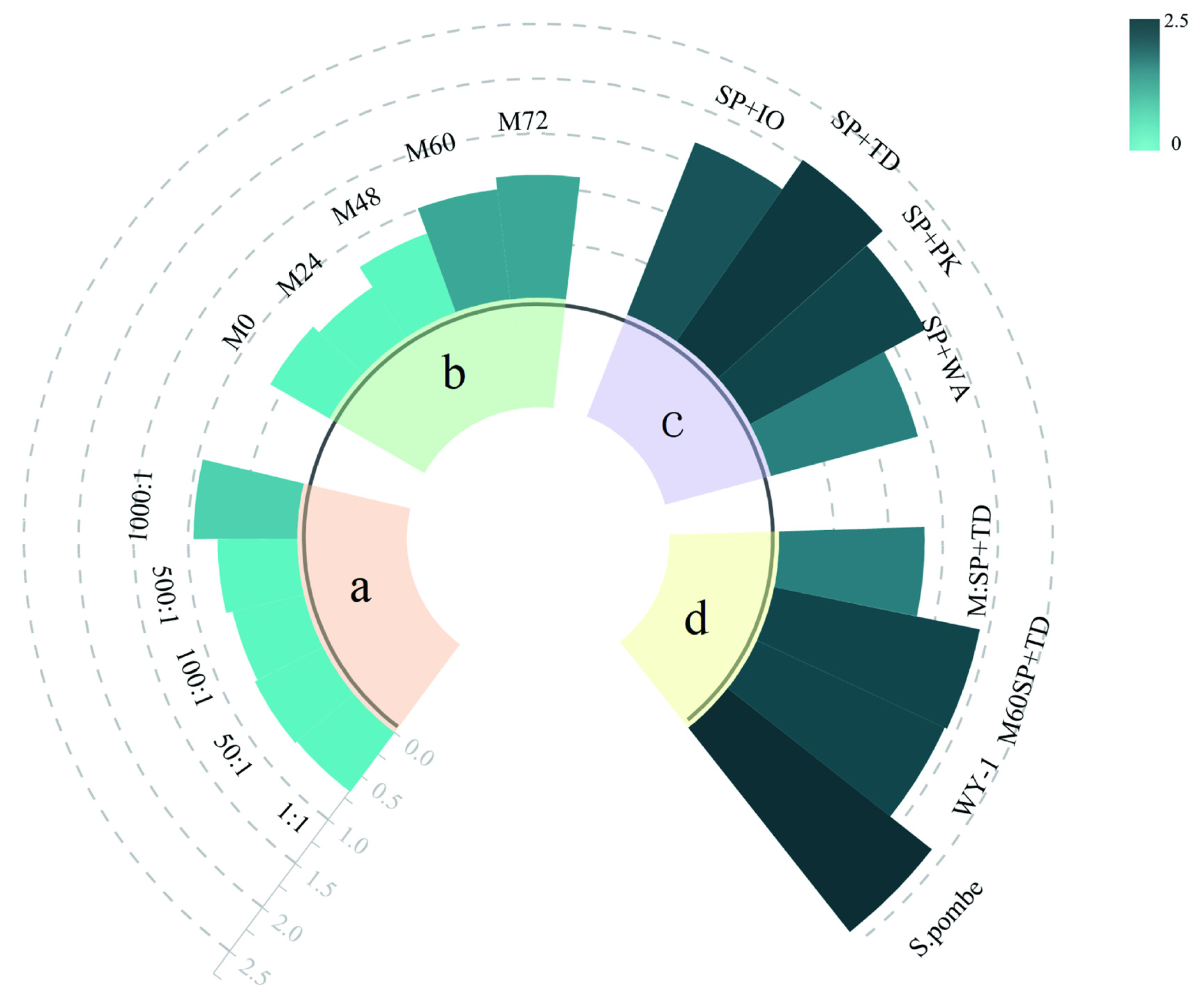
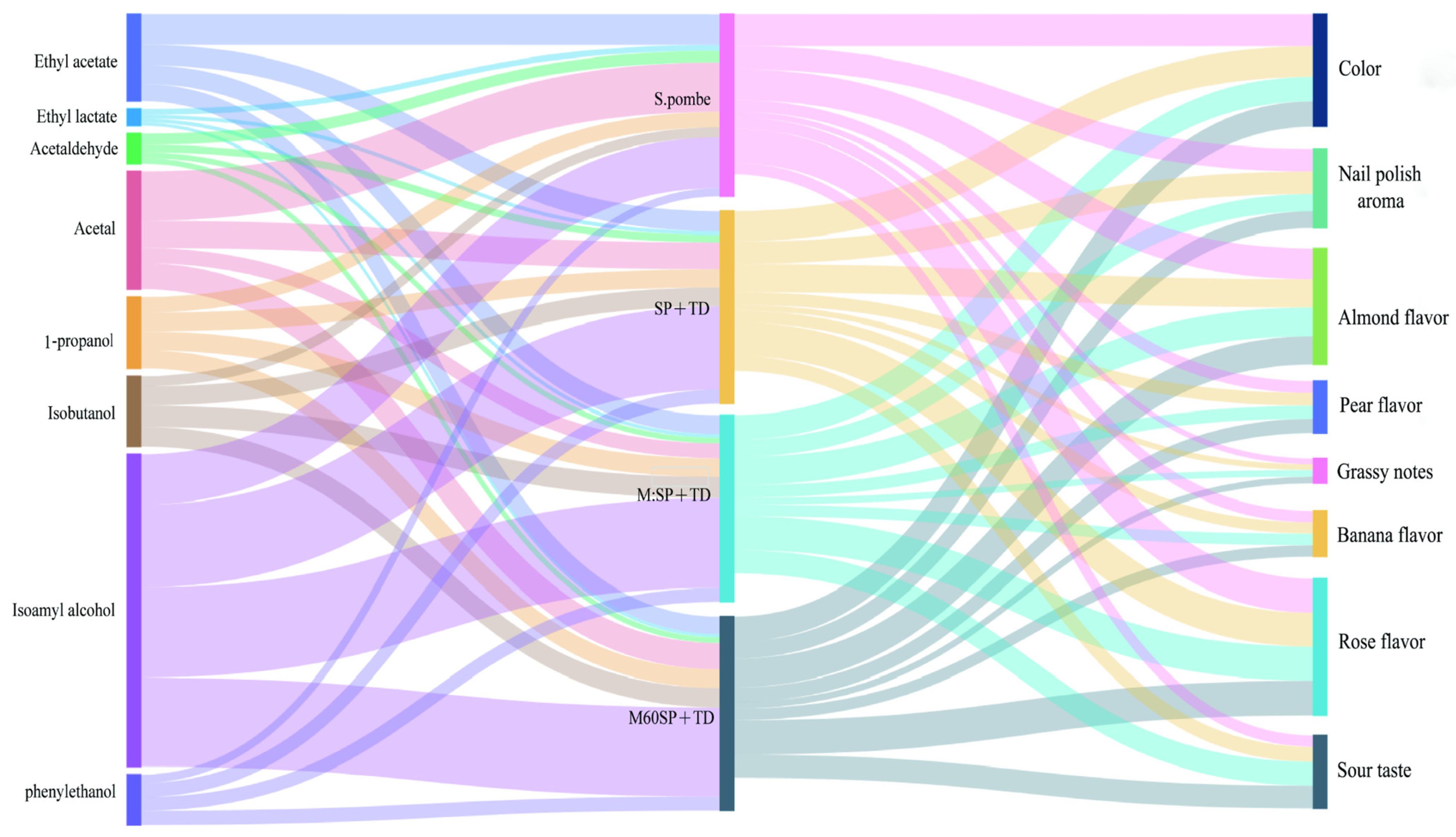
3.5. Relationship between Instrumental Data and Sensory Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Briones-Labarca, V.; Perez-Wom, M.; Habib, G.; Giovagnoli-Vicuña, C.; Cañas-Sarazua, R.; Tabilo-Munizaga, G.; Salazar, F.N. Oenological and Quality Characteristic on Young White Wines (Sauvignon Blanc): Effects of High Hydrostatic Pressure Processing. J. Food Qual. 2017, 2017, 8524073. [Google Scholar] [CrossRef]
- Redzepovic, S.; Orlic, S.; Majdak, A.; Kozina, B.; Volschenk, H.; Viljoen-Bloom, M. Differential malic acid degradation by selected strains of Saccharomyces during alcoholic fermentation. Int. J. Food Microbiol. 2003, 83, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Ruffner, H.P. Metabolism of tartaric and malic acids in Vitis: A review—Part A. Vitis 2016, 21, 247. [Google Scholar]
- Delcourt, F.; Taillandier, P.; Vidal, F.; Strehaiano, P. Influence of pH, malic acid and glucose concentrations on malic acid consumption by Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 1995, 43, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, I.S. Tailoring wine yeast for the new millennium: Novel approaches to the ancient art of winemaking. Yeast 2000, 16, 675–729. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, E.; Reale, A.; Tremonte, P.; Maiuro, L.; Succi, M.; Tipaldi, L.; Di Renzo, T.; Pannella, G.; Coppola, R. Lactobacillus plantarum 29 inhibits Penicillium spp. involved in the spoilage of black truffles (Tuber aestivum). J. Food Sci. 2013, 78, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Tremonte, P.; Reale, A.; Di Renzo, T.; Tipaldi, L.; Di Luccia, A.; Coppola, R.; Sorrentino, E.; Succi, M. Interactions between Lactobacillus sakei and CNC (Staphylococcus xylosus and Kocuria varians) and their influence on proteolytic activity. Lett. Appl. Microbiol. 2010, 51, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Tremonte, P.; Sorrentino, E.; Succi, M.; Tipaldi, L.; Pannella, G.; Ibañez, E.; Mendiola, J.A.; Di Renzo, T.; Reale, A.; Coppola, R. Antimicrobial Effect of Malpighia Punicifolia and Extension of Water Buffalo Steak Shelf-Life. J. Food Sci. 2016, 81, 97–105. [Google Scholar] [CrossRef]
- Tremonte, P.; Succi, M.; Reale, A.; Di Renzo, T.; Sorrentino, E.; Coppola, R. Interactions between strains of Staphylococcus xylosus and Kocuria varians isolated from fermented meats. J. Appl. Microbiol. 2007, 103, 743–751. [Google Scholar] [CrossRef]
- Sumby, K.M.; Bartle, L.; Grbin, P.R.; Jiranek, V. Measures to improve wine malolactic fermentation. Appl. Microbiol. Biotechnol. 2019, 103, 2033–2051. [Google Scholar] [CrossRef]
- Liu, S.Q. A review: Malolactic fermentation in wine—Beyond deacidification. J. Appl. Microbiol. 2002, 92, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Lonvaud-Funel, A. Lactic acid bacteria in the quality improvement and depreciation of wine. Antonie Van Leeuwenhoek 1999, 76, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Tortia, C.; Gerbi, V.; Gandini, A. Impiego di Saccharomyces cerevisiae maloalcolici in vinificazione. Vignevini 1993, 20, 15–20. [Google Scholar]
- Osothsilp, C.; Subden, R.E. Isolation and characterization of Schizosaccharomyces pombe mutants with defective NAD-dependent malic enzyme. Can. J. Microbiol. 1986, 32, 481–486. [Google Scholar] [CrossRef]
- Su, J.; Wang, T.; Wang, Y.; Li, Y.Y.; Li, H. The use of lactic acid-producing, malic acid-producing, or malic acid-degrading yeast strains for acidity adjustment in the wine industry. Appl. Microbiol. Biotechnol. 2014, 98, 2395–2413. [Google Scholar] [CrossRef] [PubMed]
- Barbe, J.C.; De Revel, G.; Bertrand, A. Gluconic acid, its lactones, and SO2 binding phenomena in musts from botrytized grapes. J. Agric. Food Chem. 2002, 50, 6408–6412. [Google Scholar] [CrossRef]
- Morata, A.; Benito, S.; Loira, I.; Palomero, F.; González, M.C.; Suárez-Lepe, J.A. Formation of pyranoanthocyanins by Schizosaccharomyces pombe during the fermentation of red must. Int. J. Food Microbiol. 2012, 159, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Benito, Á.; Calderón, F.; Benito, S. Mixed alcoholic fermentation of Schizosaccharomyces pombe and Lachancea thermotolerans and its influence on mannose-containing polysaccharides wine Composition. AMB Express 2019, 9, 17. [Google Scholar] [CrossRef]
- Benito, Á.; Calderón, F.; Benito, S. The Combined Use of Schizosaccharomyces pombe and Lachancea thermotolerans-Effect on the Anthocyanin Wine Composition. Molecules 2017, 22, 739. [Google Scholar] [CrossRef]
- Petruzzi, L.; Sinigaglia, M.; Corbo, M.R.; Campaniello, D.; Speranza, B.; Bevilacqua, A. Decontamination of ochratoxin A by yeasts: Possible approaches and factors leading to toxin removal in wine. Appl. Microbiol. Biotechnol. 2014, 98, 6555–6567. [Google Scholar] [CrossRef]
- Mylona, A.E.; Del Fresno, J.M.; Palomero, F.; Loira, I.; Bañuelos, M.A.; Morata, A.; Calderón, F.; Benito, S.; Suárez-Lepe, J.A. Use of Schizosaccharomyces strains for wine fermentation—Effect on the wine composition and food safety. Int. J. Food Microbiol. 2016, 232, 63–72. [Google Scholar] [CrossRef]
- Benito, Á.; Calderón, F.; Benito, S. Combined Use of S. pombe and L. thermotolerans in Winemaking. Beneficial Effects Determined Through the Study of Wines’ Analytical Characteristics. Molecules 2016, 21, 1744. [Google Scholar] [CrossRef]
- Benito, Á.; Jeffares, D.; Palomero, F.; Calderón, F.; Bai, F.Y.; Bähler, J.; Benito, S. Selected Schizosaccharomyces pombe Strains Have Characteristics That Are Beneficial for Winemaking. PLoS ONE 2016, 11, e0151102. [Google Scholar] [CrossRef]
- Chen, E.S. Application of the fission yeast Schizosaccharomyces pombe in human nutrition. FEMS Yeast Res. 2023, 23, foac064. [Google Scholar] [CrossRef]
- Takayama, S.; Ozaki, A.; Konishi, R.; Otomo, C.; Kishida, M.; Hirata, Y.; Matsumoto, T.; Tanaka, T.; Kondo, A. Enhancing 3-hydroxypropionic acid production in combination with sugar supply engineering by cell surface-display and metabolic engineering of Schizosaccharomyces pombe. Microb. Cell Fact. 2018, 17, 176. [Google Scholar] [CrossRef] [PubMed]
- Satora, P.; Semik-Szczurak, D.; Tarko, T.; Bułdys, A. Influence of Selected Saccharomyces and Schizosaccharomyces Strains and Their Mixed Cultures on Chemical Composition of Apple Wines. J. Food Sci. 2018, 83, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef]
- Li, C.; Lu, J.; Yan, X.J.; Li, C.W.; Lin, L.C.; Xiao, D.G.; Zhang, C.Y. The eisosomes contribute to acid tolerance of yeast by maintaining cell membrane integrity. Food Microbiol. 2023, 110, 104157. [Google Scholar] [CrossRef] [PubMed]
- Vararu, F.; Moreno-García, J.; Niculaua, M.; Cotea, V.V.; Mayén, M.; Moreno, J. Fermentative volatilome modulation of Muscat Ottonel wines by using yeast starter cultures. LWT 2020, 129, 109575. [Google Scholar] [CrossRef]
- Glories, Y. Le coleur des vins rouges, 2e partie: Mesure, origine et interpretation. OENO ONE 1984, 18, 253–271. [Google Scholar] [CrossRef]
- Lyu, X.T.; Dias Araujo, L.; Quek, S.Y.; Kilmartin, P.A. Effects of antioxidant and elemental sulfur additions at crushing on aroma profiles of Pinot Gris, Chardonnay and Sauvignon Blanc wines. Food Chem. 2021, 346, 128914. [Google Scholar] [CrossRef] [PubMed]
- Mendes, F.A.; Mendes-Faia, A. The Role of Yeasts and Lactic Acid Bacteria on the Metabolism of Organic Acids during Winemaking. Foods 2020, 9, 1231. [Google Scholar] [CrossRef] [PubMed]
- González-Centeno, M.R.; Chira, K.; Teissedre, P.L. Use of oak wood during malolactic fermentation and ageing: Impact on chardonnay wine character. Food Chem. 2019, 278, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Taillandier, P.; Riba, J.P.; Strehaiano, P. Malate utilization by Schizosaccharomyces pombe. Biotechnol. Lett. 1988, 10, 469–472. [Google Scholar] [CrossRef]
- Roca-Domènech, G.; Cordero-Otero, R.; Rozès, N.; Cléroux, M.; Pernet, A.; Mira de Orduña, R. Metabolism of Schizosaccharomyces pombe under reduced osmotic stress conditions afforded by fed-batch alcoholic fermentation of white grape must. Food Res. Int. 2018, 113, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Maicas, S.; Mateo, J.J. The Life of Saccharomyces and Non-Saccharomyces Yeasts in Drinking Wine. Microorganisms 2023, 11, 1178. [Google Scholar] [CrossRef] [PubMed]
- Ivit, N.N.; Loira, I.; Morata, A.; Benito, S.; Palomero, F.; Suárez-Lepe, J.A. Making natural sparkling wines with non-Saccharomyces yeasts. Eur. Food Res. Technol. 2018, 244, 925–935. [Google Scholar] [CrossRef]
- Balmaseda, A.; Rozès, N.; Leal, M.Á.; Bordons, A.; Reguant, C. Impact of changes in wine composition produced by non-Saccharomyces on malolactic fermentation. Int. J. Food Microbiol. 2021, 337, 108954. [Google Scholar] [CrossRef] [PubMed]
- Plata, C.; Millán, C.; Mauricio, J.C.; Ortega, J.M. Formation of ethyl acetate and isoamyl acetate by various species of wine yeasts. Food Microbiol. 2003, 20, 217–224. [Google Scholar] [CrossRef]
- Goold, H.D.; Kroukamp, H.; Williams, T.C.; Paulsen, I.T.; Varela, C.; Pretorius, I.S. Yeast’s balancing act between ethanol and glycerol production in low-alcohol wines. Microb. Biotechnol. 2017, 10, 264–278. [Google Scholar] [CrossRef]
- Wang, J.; Gambetta, J.M.; Jeffery, D.W. Comprehensive Study of Volatile Compounds in Two Australian Rosé Wines: Aroma Extract Dilution Analysis (AEDA) of Extracts Prepared Using Solvent-Assisted Flavor Evaporation (SAFE) or Headspace Solid-Phase Extraction (HS-SPE). J. Agric. Food Chem. 2016, 64, 3838–3848. [Google Scholar] [CrossRef] [PubMed]
- Bovo, B.; Nadai, C.; Vendramini, C.; Fernandes Lemos Junior, W.J.; Carlot, M.; Skelin, A.; Giacomini, A.; Corich, V. Aptitude of Saccharomyces yeasts to ferment unripe grapes harvested during cluster thinning for reducing alcohol content of wine. Int. J. Food Microbiol. 2016, 236, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Porras, P.; Gómez-Plaza, E.; Osete-Alcaraz, A.; Martínez-Pérez, P.; Jurado, R.; Bautista-Ortín, A.B. The effect of ultrasound on Syrah wine composition as affected by the ripening or sanitary status of the grapes. Eur. Food Res. Technol. 2023, 249, 641–651. [Google Scholar] [CrossRef]
- Velenosi, M.; Crupi, P.; Perniola, R.; Marsico, A.D.; Salerno, A.; Alexandre, H.; Archidiacono, N.; Ventura, M.; Cardone, M.F. Color Stabilization of Apulian Red Wines through the Sequential Inoculation of Starmerella bacillaris and Saccharomyces cerevisiae. Molecules 2021, 26, 907. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, Z.; Wei, Z.; Yu, W.; Cui, Y. Effect of tannin addition on chromatic characteristics, sensory qualities and antioxidant activities of red wines. RSC Adv. 2020, 10, 7108–7117. [Google Scholar] [CrossRef] [PubMed]
- García-Estévez, I.; Alcalde-Eon, C.; Puente, V.; Escribano-Bailón, M.T. Enological Tannin Effect on Red Wine Color and Pigment Composition and Relevance of the Yeast Fermentation Products. Molecules 2017, 22, 2046. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.H.; Zhang, C.N.; Zhao, H.; Min, W.H.; Zhu, H.; Wang, H.A.; Lu, H.Y.; Li, X.T.; Xu, Y.Q.; Li, W.W. Effect of Environmental Microorganisms on Fermentation Microbial Community of Sauce-Flavor baijiu. Foods 2022, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.M.; Hu, Y.N.; Jia, L.L.; Zhang, M.; Zhang, Z.X.; Huang, X.N.; Chen, X.X.; Han, B.Z. Response of microbial community assembly and succession pattern to abiotic factors during the second round of light-flavor Baijiu fermentation. Food Res Int. 2022, 162 Pt A, 111915. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.N.; Fan, Y.; Lu, T.; Kang, J.M.; Pang, X.N.; Han, B.Z.; Chen, J.Y. Composition and Metabolic Functions of the Microbiome in Fermented Grain during Light-Flavor Baijiu Fermentation. Microorganisms 2020, 8, 1281. [Google Scholar] [CrossRef]
- Mu, Z.; Yang, Y.; Xia, Y.; Zhang, H.; Ni, B.; Ni, L.; Wang, G.; Xiong, Z.; Zhang, H.; Song, X.; et al. Enhancement of the aromatic alcohols and health properties of Chinese rice wine by using a potentially probiotic Saccharomyces cerevisiae BR14. LWT 2023, 181, 114748. [Google Scholar] [CrossRef]
- Espinosa Vidal, E.; de Morais, M.A., Jr.; François, J.M.; de Billerbeck, G.M. Biosynthesis of higher alcohol flavour compounds by the yeast Saccharomyces cerevisiae: Impact of oxygen availability and responses to glucose pulse in minimal growth medium with leucine as sole nitrogen source. Yeast 2015, 32, 47–56. [Google Scholar] [PubMed]
- Sainz-Mellado, D.C.; Méndez-Hernández, J.E.; López-Miranda, J.; Páez-Lerma, J.B.; Aguilar, C.N.; Soto-Cruz, N.O. Gradually supply of isoamyl alcohol increases the isoamyl acetate production in solid-state fermentation. Lett. Appl. Microbiol. 2023, 76, ovac061. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Yan, W.; Zhang, W.; Dong, W.; Ma, J.; Ochsenreither, K.; Jiang, M.; Xin, F. Current status and perspectives of 2-phenylethanol production through biological processes. Crit. Rev. Biotechnol. 2019, 39, 235–248. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, X.; Zhou, Y.; Li, F.; Zhou, M.; Wei, C.; Lin, L.; Li, X.; Zhang, C. Improving Muscat Hamburg Wine Quality with Innovative Fermentation Strategies Using Schizosaccharomyces pombe Derived from Fermented Grains of Sauce-Flavor Baijiu. Foods 2024, 13, 1648. https://doi.org/10.3390/foods13111648
Lyu X, Zhou Y, Li F, Zhou M, Wei C, Lin L, Li X, Zhang C. Improving Muscat Hamburg Wine Quality with Innovative Fermentation Strategies Using Schizosaccharomyces pombe Derived from Fermented Grains of Sauce-Flavor Baijiu. Foods. 2024; 13(11):1648. https://doi.org/10.3390/foods13111648
Chicago/Turabian StyleLyu, Xiaotong, Yifei Zhou, Furong Li, Meiyi Zhou, Chunhui Wei, Liangcai Lin, Xin Li, and Cuiying Zhang. 2024. "Improving Muscat Hamburg Wine Quality with Innovative Fermentation Strategies Using Schizosaccharomyces pombe Derived from Fermented Grains of Sauce-Flavor Baijiu" Foods 13, no. 11: 1648. https://doi.org/10.3390/foods13111648
APA StyleLyu, X., Zhou, Y., Li, F., Zhou, M., Wei, C., Lin, L., Li, X., & Zhang, C. (2024). Improving Muscat Hamburg Wine Quality with Innovative Fermentation Strategies Using Schizosaccharomyces pombe Derived from Fermented Grains of Sauce-Flavor Baijiu. Foods, 13(11), 1648. https://doi.org/10.3390/foods13111648







