NMR Metabolomics of Arctium lappa L., Taraxacum officinale and Melissa officinalis: A Comparison of Spontaneous and Organic Ecotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Chemicals
2.3. Extraction Procedure for NMR Analysis
2.4. NMR Analysis
2.5. Statistical Analysis
3. Results and Discussions
3.1. NMR Assignment of Hydroalcoholic and Organic Fractions
NMR Identification of Polyphenols
3.2. Characterisation and Comparison among the Three Ecotypes of the Same Species
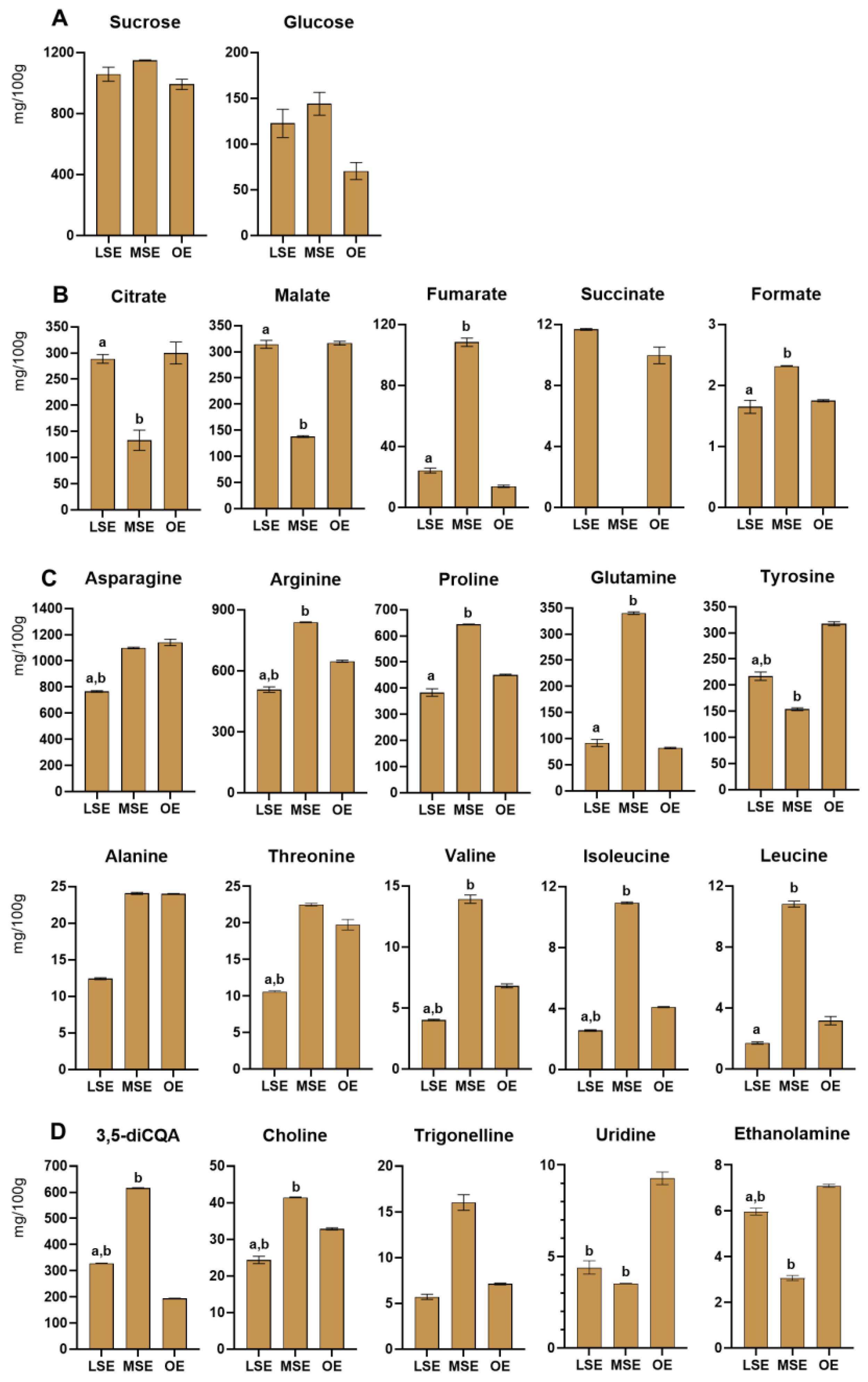
3.2.1. Burdock—Arctium lappa L.
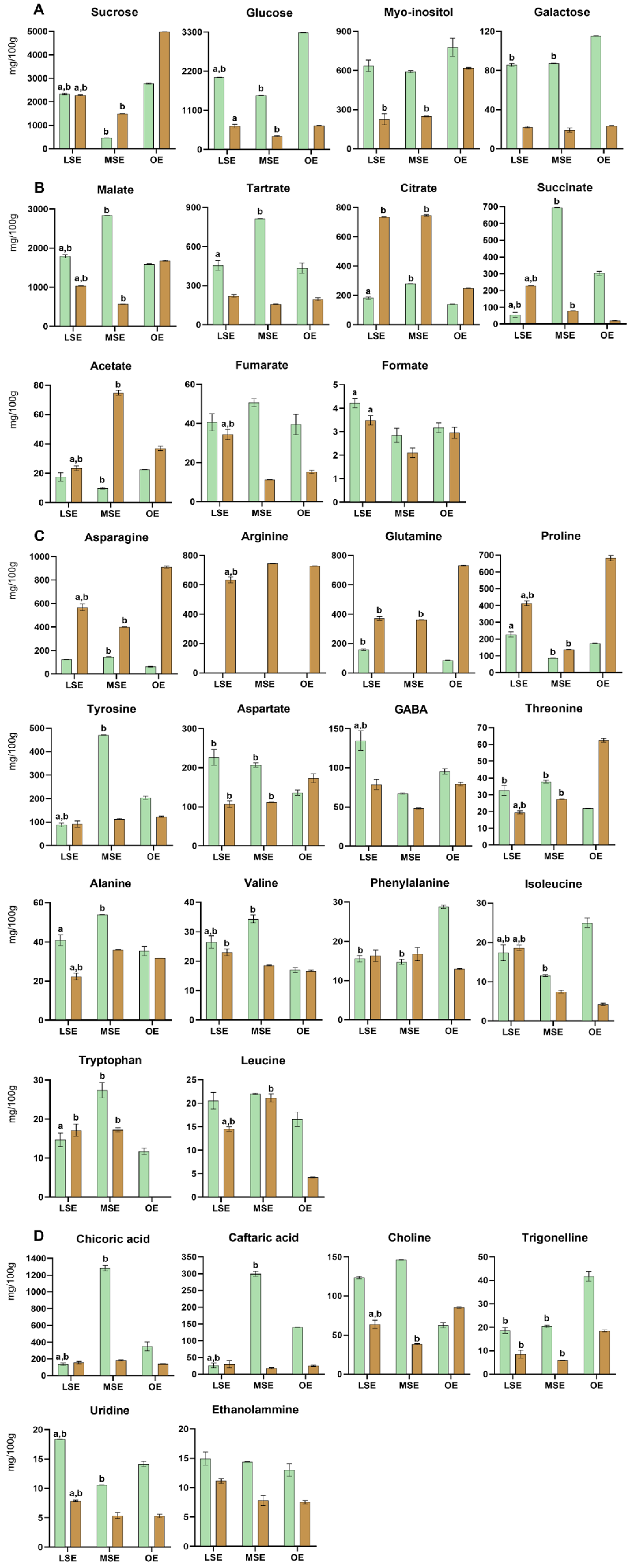
3.2.2. Dandelion—Taraxacum officinale
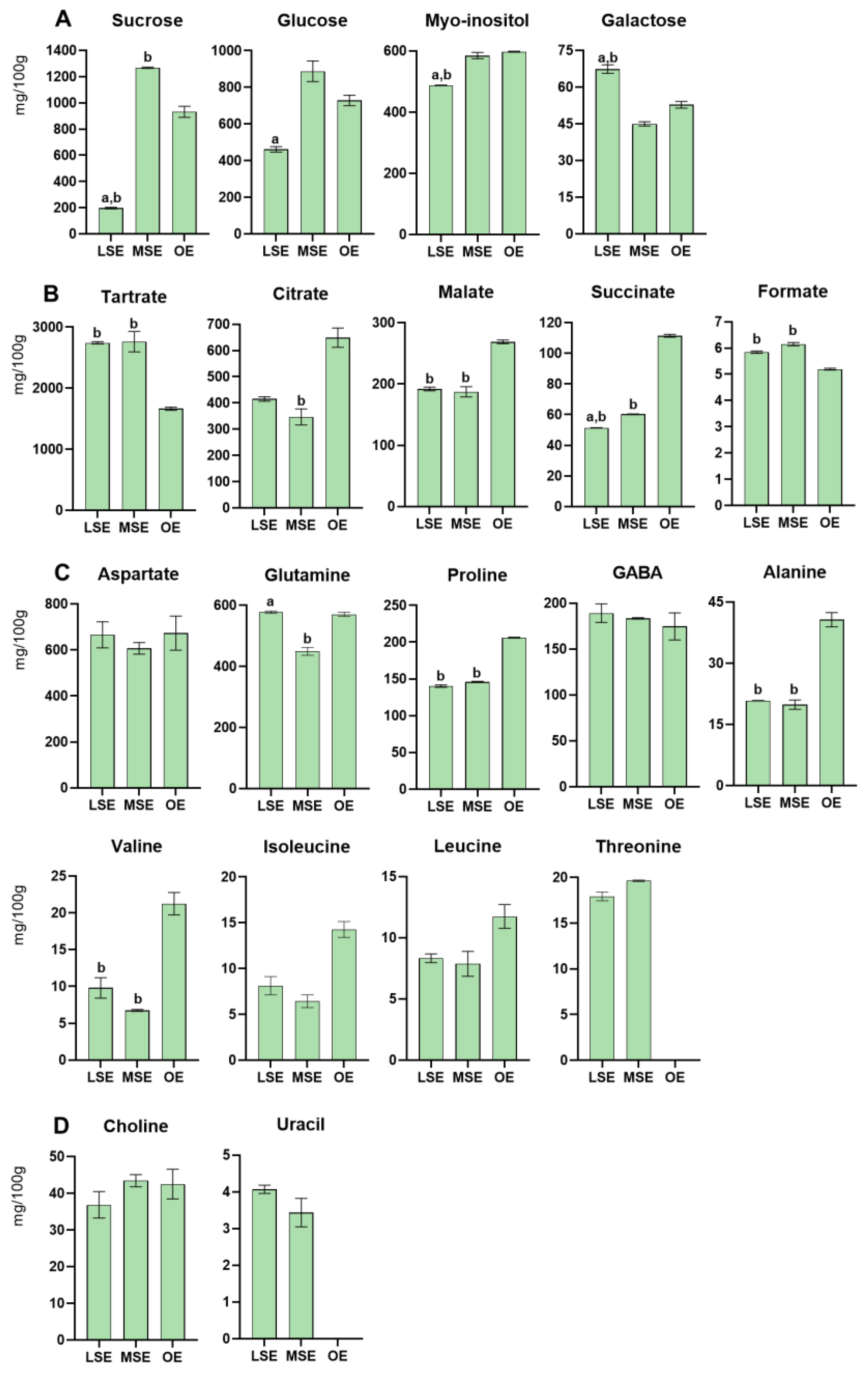
3.2.3. Lemon Balm—Melissa officinalis
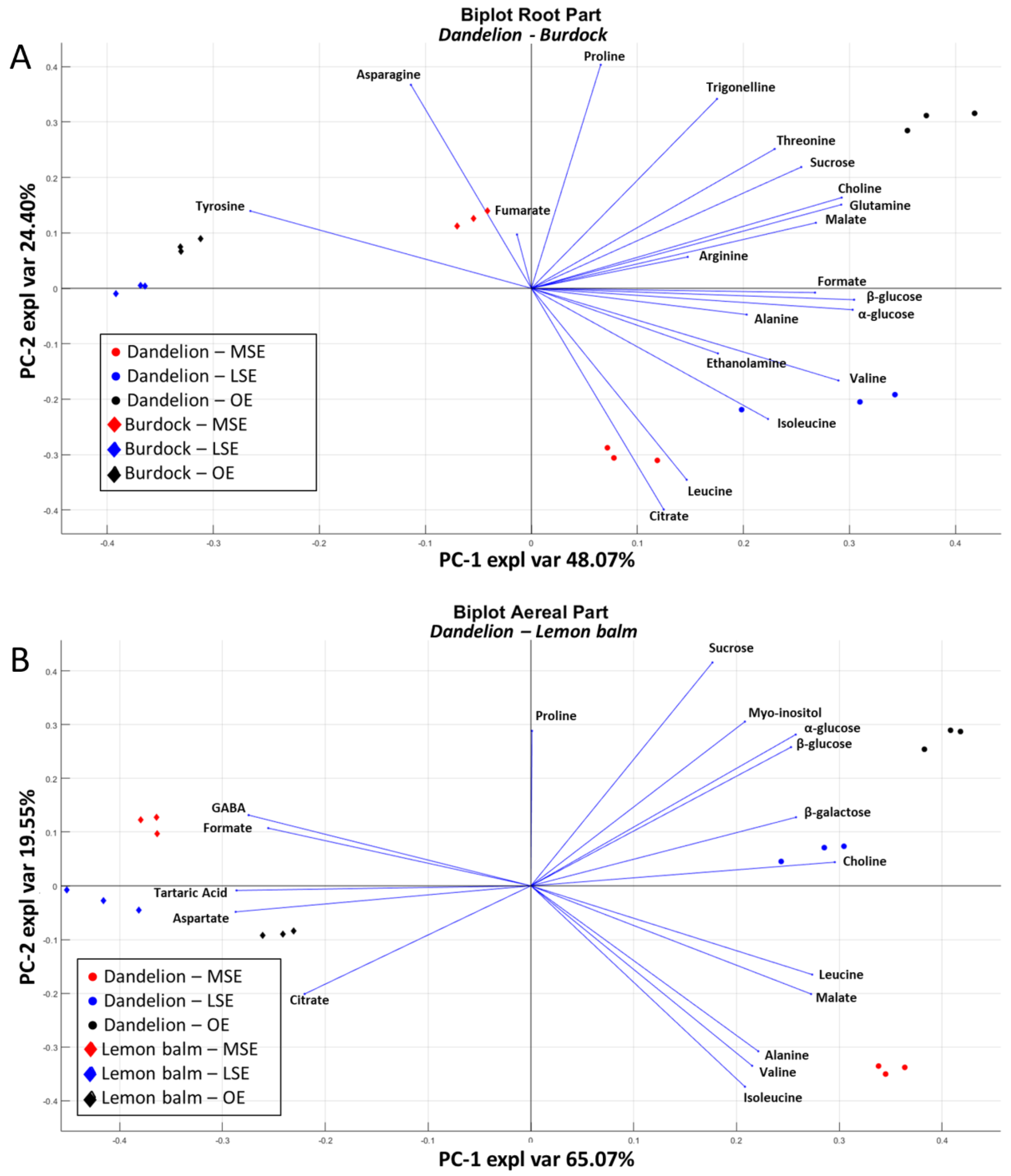
3.3. Comparison of Burdock, Dandelion and Lemon Balm
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| DGG | Digalactosyldiacylglycerol |
| DUFA | Di-unsaturated fatty acids |
| GABA | γ-amino-butyric acid |
| GC-MS | Gas Chromatography–Mass Spectroscopy |
| 1H-13C | HSQC Heteronuclear single quantum coherence Spectroscopy |
| 1H-13C | HMBC Heteronuclear Multiple Bond Correlation Spectroscopy |
| 1H-1H | TOCSY Total Correlation Spectroscopy |
| HPLC-DAD | High-Performance Liquid Chromatography–Diode Array Detector |
| HPLC-MS | High-Performance Liquid Chromatography–Mass Spectroscopy |
| LSE | Land Spontaneous Ecotype |
| MUFA | Mono-unsaturated fatty acids |
| MSE | Mountain Spontaneous Ecotype |
| NMR | Nuclear Magnetic Resonance |
| OE | Organic Ecotype |
| PCA | Principal Component Analysis |
| QTRAP-MS | Mass Spectroscopy |
| UFA | Unsaturated Fatty Acids |
| UHPLC-MS | Ultra-High-Performance Liquid Chromatography-Mass Spectroscopy |
| UPLC | Ultra-Pressure Liquid Chromatography |
| TBZ | 3,4,5-Trimethoxybenzaldehyde |
| TSP | 3-(trimethylsilyl)-propionic-2,2,3,3-d4 acid sodium salt |
| TUFA | Tri-unsaturated fatty acids |
References
- Aye, M.M.; Aung, H.T.; Sein, M.M.; Armijos, C. A Review on the Phytochemistry, Medicinal Properties and Pharmacological Activities of 15 Selected Myanmar Medicinal Plants. Molecules 2019, 24, 293. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.; Hamid, A.; Khalil, A.; Ghaffar, A.; Tayyaba, N.; Saeed, A.; Ali, M.; Naveed, A. Review on Medicinal Uses, Pharmacological, Phytochemistry and Immunomodulatory Activity of Plants. Int. J. Immunopathol. Pharmacol. 2014, 27, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Bommakanti, V.; Puthenparambil Ajikumar, A.; Sivi, C.M.; Prakash, G.; Mundanat, A.S.; Ahmad, F.; Haque, S.; Prieto, M.A.; Rana, S.S. An Overview of Herbal Nutraceuticals, Their Extraction, Formulation, Therapeutic Effects and Potential Toxicity. Separations 2023, 10, 4187. [Google Scholar] [CrossRef]
- European Commission EU Novel Food Catalogue. Available online: https://food.ec.europa.eu/safety/novel-food/novel-food-status-catalogue_en (accessed on 14 May 2024).
- González-Castejón, M.; Visioli, F.; Rodriguez-Casado, A. Diverse Biological Activities of Dandelion. Nutr. Rev. 2012, 70, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Sheng, W.; Gao, R.; Feng, J.; Huang, W.; Cui, L.; Liu, J.; Li, Y. Ethanolic Extract of Root from Arctium lappa L. Ameliorates Obesity and Hepatic Steatosis in Rats by Regulating the AMPK/ACC/CPT-1 Pathway. J. Food Biochem. 2022, 46, 14455. [Google Scholar] [CrossRef] [PubMed]
- Moro, T.M.; Clerici, M.T. Burdock (Arctium lappa L.) Roots as a Source of Inulin-Type Fructans and Other Bioactive Compounds: Current Knowledge and Future Perspectives for Food and Non-Food Applications. Food Res. Int. 2021, 141, 109889. [Google Scholar] [CrossRef] [PubMed]
- Munarin, E.E.O.; Herediazárate, N.A.; Vieira, M.C.; Rosa, Y.B.C.J.; Rodrigues, E.T. Espaçamentos Entre Plantas e Cobertura Do Solo Com Cama-de-Frango Na Produção Da Bardana (Arctium lappa L.). Rev. Bras. Plantas Med. 2010, 12, 141–148. [Google Scholar] [CrossRef]
- Petrisor, G.; Motelica, L.; Craciun, L.N.; Oprea, O.C.; Ficai, D.; Ficai, A. Melissa officinalis: Composition, Pharmacological Effects and Derived Release Systems—A Review. Int. J. Mol. Sci. 2022, 23, 3591. [Google Scholar] [CrossRef]
- Azizov, U.M.; Khadzhieva, U.A.; Rakhimov, D.A.; Mezhlumyan, L.G.; Salikhov, S.A. Chemical Composition of Dry Extract of Arctium lappa Roots. Chem. Nat. Compd. 2012, 47, 834. [Google Scholar] [CrossRef]
- Carlotto, J.; de Souza, L.M.; Baggio, C.H.; Werner, M.F.d.P.; Maria-Ferreira, D.; Sassaki, G.L.; Iacomini, M.; Cipriani, T.R. Polysaccharides from Arctium lappa L.: Chemical Structure and Biological Activity. Int. J. Biol. Macromol. 2016, 91, 954–960. [Google Scholar] [CrossRef]
- Han, Z.; Wang, M.; Wang, L.; Qu, H.; Li, P.; Wang, C. Chemical Analysis of Burdock Root Constituents. Asian J. Chem. 2013, 25, 2573–2576. [Google Scholar] [CrossRef]
- Lee, D.; Kim, C.Y. Influence of Roasting Treatment on the Antioxidant Activities and Color of Burdock Root Tea. Prev. Nutr. Food Sci. 2017, 22, 6793. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Herrera-Balandrano, D.D.; Huang, W.; Chai, Z.; Beta, T.; Wang, J.; Feng, J.; Li, Y. Comparison of Nutritional and Nutraceutical Properties of Burdock Roots Cultivated in Fengxian and Peixian of China. Foods 2021, 10, 2095. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Bădărau, A.S.; Swamy, M.K.; Shaw, S.; Maggi, F.; da Silva, L.E.; López, V.; Yeung, A.W.K.; Mocan, A.; Atanasov, A.G. Arctium Species Secondary Metabolites Chemodiversity and Bioactivities. Front. Plant Sci. 2019, 10, 834. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Filho, J.M.; Costa, M.; Gomes, C.; Trolin, G.G. Isolation of Onopordopicrin, the Toxic Constituent of Arctium lappa L. J. Braz. Chem. Soc. 1993, 3. [Google Scholar] [CrossRef]
- Lis, B.; Olas, B. Pro-Health Activity of Dandelion (Taraxacum officinale L.) and Its Food Products—History and Present. J. Funct. Foods 2019, 59, 40–48. [Google Scholar] [CrossRef]
- Türkmen, B.M.; Teyin, G.; Lokman, U.; Memis Kocaman, E. Functional Effects of Dandelion (Taraxacum officinale) and Its Use in the Traditional Cuisines. J. Culin. Sci. Technol. 2023. [Google Scholar] [CrossRef]
- Mingarro, D.M.; Plaza, A.; Galán, A.; Vicente, J.A.; Martínez, M.P.; Acero, N. The Effect of Five Taraxacum Species on in Vitro and in Vivo Antioxidant and Antiproliferative Activity. Food Funct. 2015, 6, 6477. [Google Scholar] [CrossRef]
- Choi, U.K.; Lee, O.H.; Yim, J.H.; Cho, C.W.; Rhee, Y.K.; Lim, S.I.; Kim, Y.C. Hypolipidemic and Antioxidant Effects of Dandelion (Taraxacum officinale) Root and Leaf on Cholesterol-Fed Rabbits. Int. J. Mol. Sci. 2010, 11, 67–78. [Google Scholar] [CrossRef]
- Hu, C.; Kitts, D.D. Dandelion (Taraxacum officinale) Flower Extract Suppresses Both Reactive Oxygen Species and Nitric Oxide and Prevents Lipid Oxidation in Vitro. Phytomedicine 2005, 12, 588–597. [Google Scholar] [CrossRef]
- Duan, L.; Zhang, C.; Zhao, Y.; Chang, Y.; Guo, L. Comparison of Bioactive Phenolic Compounds and Antioxidant Activities of Different Parts of Taraxacum mongolicum. Molecules 2020, 25, 3260. [Google Scholar] [CrossRef] [PubMed]
- NIH, National Center for Complementary and Integrative Health, Dandelion. Available online: https://www.nccih.nih.gov/health/dandelion (accessed on 14 May 2024).
- Shakeri, A.; Sahebkar, A.; Javadi, B. Melissa officinalis L.—A Review of Its Traditional Uses, Phytochemistry and Pharmacology. J. Ethnopharmacol. 2016, 188, 204–228. [Google Scholar] [PubMed]
- Ghiulai, R.; Avram, S.; Stoian, D.; Pavel, I.Z.; Coricovac, D.; Oprean, C.; Vlase, L.; Farcas, C.; Mioc, M.; Minda, D.; et al. Lemon Balm Extracts Prevent Breast Cancer Progression in Vitro and in Ovo on Chorioallantoic Membrane Assay. Evid.-Based Complement. Altern. Med. 2020, 2020, 6489159. [Google Scholar] [CrossRef] [PubMed]
- Zam, W.; Quispe, C.; Sharifi-Rad, J.; Lopez, M.D.; Shoebitz, M.; Martorell, M.; Sharopov, F.; Fokou, P.V.T.; Mishra, A.P.; Chandran, D.; et al. An Updated Review on the Properties of Melissa officinalis L.: Not Exclusively Anti-Anxiety. Front. Biosci.-Sch. 2022, 14, 16. [Google Scholar] [CrossRef]
- Lobach, A.R.; Schmidt, F.; Fedrizzi, D.; Müller, S. Toxicological Safety Evaluation of an Aqueous Lemon Balm (Melissa officinalis) Extract. Food Chem. Toxicol. 2024, 187, 114565. [Google Scholar] [CrossRef] [PubMed]
- Chizzola, R.; Lohwasser, U.; Franz, C. Biodiversity within Melissa officinalis: Variability of Bioactive Compounds in a Cultivated Collection. Molecules 2018, 23, 294. [Google Scholar] [CrossRef] [PubMed]
- Ghazizadeh, J.; Hamedeyazdan, S.; Torbati, M.; Farajdokht, F.; Fakhari, A.; Mahmoudi, J.; Araj-khodaei, M.; Sadigh-Eteghad, S. Melissa officinalis L. Hydro-Alcoholic Extract Inhibits Anxiety and Depression through Prevention of Central Oxidative Stress and Apoptosis. Exp Physiol 2020, 105, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Eivani, M.; Khosronezhad, N. Melissa officinalis: A Memory Enhancer Remedy. Physiol. Pharmacol. 2020, 24, 159–164. [Google Scholar] [CrossRef]
- Carocho, M.; Barros, L.; Calhelha, R.C.; Ćirić, A.; Soković, M.; Santos-Buelga, C.; Morales, P.; Ferreira, I.C.F.R. Melissa officinalis L. Decoctions as Functional Beverages: A Bioactive Approach and Chemical Characterization. Food Funct. 2015, 6, 2240–2248. [Google Scholar] [CrossRef]
- Younes, M.; Aquilina, G.; Castle, L.; Engel, K.-H.; Fowler, P.; Fernandez, M.J.F.; Fürst, P.; Gundert-Remy, U.; Gürtler, R.; et al.; EFSA Panel on Food Additives and Flavourings (FAF) Scientific Opinion on Flavouring Group Evaluation 72, Revision 2 (FGE.72Rev2): Consideration of aliphatic, branched-chain saturated and unsaturated alcohols, aldehydes, acids and related esters evaluated by JECFA (61st, 68th and 69th meetings) and structurally related to flavouring substances in FGE.05Rev3. EFSA J. 2020, 18, e06029. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the use of oregano and lemon balm extracts as a food additive. EFSA J. 2010, 8, 1514. Available online: https://efsa.onlinelibrary.wiley.com/doi/abs/10.2903/j.efsa.2010.1514 (accessed on 14 May 2024).
- Committee on Herbal Medicinal Products (HMPC). Assessment Report on Melissa officinalis L., Folium; European Medicines Agency: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Cornara, L.; Malaspina, P.; Betuzzi, F.; Di Gristina, E.; D’Arrigo, M.; Ingegneri, M.; Trombetta, D.; Smeriglio, A. The Influence of Pedo-Climatic Conditions on the Micromorphological, Phytochemical Features, and Biological Properties of Leaves of Saponaria Sicula Raf. Int. J. Mol. Sci. 2023, 24, 11693. [Google Scholar] [CrossRef] [PubMed]
- Hussaan, M.; Javed, M.T.; Akram, M.S.; Saleem, M.H.; Chaudhary, H.J. Abiotic Stress in Plants and Metabolic Responses. In Improvement of Plant Production in the Era of Climate Change, 1st ed.; CRC Press: Boca Raton, FL, USA, 2022; Chapter 7. [Google Scholar]
- Sama, H.; Traoré, D.K.; Guenné, S.; Hilou, A.; Dicko, M.H. Effect of Pedo-Climatic Conditions on Physicochemical Characteristics and Agro-Industrial Potential of Three Native Oilseeds Fruits from Burkina Faso. BMC Plant Biol. 2022, 22, 321. [Google Scholar] [CrossRef] [PubMed]
- Iorizzo, M.; Sicilia, A.; Nicolosi, E.; Forino, M.; Picariello, L.; Lo Piero, A.R.; Vitale, A.; Monaco, E.; Ferlito, F.; Succi, M.; et al. Investigating the Impact of Pedoclimatic Conditions on the Oenological Performance of Two Red Cultivars Grown throughout Southern Italy. Front. Plant Sci. 2023, 14, 1250208. [Google Scholar] [CrossRef] [PubMed]
- Cornara, L.; Sgrò, F.; Raimondo, F.M.; Ingegneri, M.; Mastracci, L.; D’Angelo, V.; Germanò, M.P.; Trombetta, D.; Smeriglio, A. Pedoclimatic Conditions Influence the Morphological, Phytochemical and Biological Features of Mentha pulegium L. Plants 2023, 12, 11693. [Google Scholar] [CrossRef] [PubMed]
- Noleto-Dias, C.; Picoli, E.A.d.T.; Porzel, A.; Wessjohann, L.A.; Tavares, J.F.; Farag, M.A. Metabolomics Characterizes Early Metabolic Changes and Markers of Tolerant Eucalyptus ssp. Clones against Drought Stress. Phytochemistry 2023, 212, 113715. [Google Scholar] [CrossRef] [PubMed]
- Grauso, L.; Emrick, S.; Bonanomi, G.; Lanzotti, V. Metabolomics of the Alimurgic Plants Officinale, Papaver Rhoeas and Urtica Dioica by Combined NMR and GC–MS Analysis. Phytochem. Anal. 2019, 30, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Ha, M.; Lee, J.; Ahn, Y.G.; Kwak, J.H.; Ryu, D.H.; Hwang, G.S. Metabolite Profiling of the Response of Burdock Roots to Copper Stress. J Agric Food Chem 2015, 63, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Schütz, K.; Kammerer, D.R.; Carle, R.; Schieber, A. Characterization of Phenolic Acids and Flavonoids in Dandelion (Taraxacum officinale WEB. Ex WIGG.) Root and Herb by High-Performance Liquid Chromatography/Electrospray Ionization Mass Spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 179–186. [Google Scholar] [CrossRef]
- Abdellatif, F.; Begaa, S.; Messaoudi, M.; Benarfa, A.; Ouakouak, H.; Hassani, A.; Sawicka, B.; Simal Gandara, J. HPLC–DAD Analysis, Antimicrobial and Antioxidant Properties of Aromatic Herb Melissa officinalis L., Aerial Parts Extracts. Food Anal. Methods 2023, 16, 45–54. [Google Scholar] [CrossRef]
- Ingallina, C.; Sobolev, A.P.; Circi, S.; Spano, M.; Fraschetti, C.; Filippi, A.; Di Sotto, A.; Di Giacomo, S.; Mazzoccanti, G.; Gasparrini, F.; et al. Cannabis sativa L. Inflorescences from Monoecious Cultivars Grown in Central Italy: An Untargeted Chemical Characterization from Early Flowering to Ripening. Molecules 2020, 25, 1908. [Google Scholar] [CrossRef] [PubMed]
- Spano, M.; Di Matteo, G.; Ingallina, C.; Botta, B.; Quaglio, D.; Ghirga, F.; Balducci, S.; Cammarone, S.; Campiglia, E.; Giusti, A.M.; et al. A Multimethodological Characterization of Cannabis sativa L. Inflorescences from Seven Dioecious Cultivars Grown in Italy: The Effect of Different Harvesting Stages. Molecules 2021, 26, 2912. [Google Scholar] [CrossRef] [PubMed]
- Chiavaroli, A.; Masciulli, F.; Ingallina, C.; Mannina, L.; Maria Loreta, L.; Di Simone, S.C.; Acquaviva, A.; Nilofar; Recinella, L.; Leone, S.; et al. Comprehensive Metabolite and Biological Profile of “Sulmona Red Garlic” Ecotype’s Aerial Bulbils. Food Res. Int. 2024, 175, 113654. [Google Scholar] [CrossRef] [PubMed]
- Mannina, L.; Sobolev, A.P.; Capitani, D. Applications of NMR Metabolomics to the Study of Foodstuffs: Truffle, Kiwifruit, Lettuce, and Sea Bass. Electrophoresis 2012, 33, 2290–2313. [Google Scholar] [CrossRef]
- Spano, M.; Maccelli, A.; Di Matteo, G.; Ingallina, C.; Biava, M.; Crestoni, M.E.; Bardaud, J.X.; Giusti, A.M.; Mariano, A.; D’abusco, A.S.; et al. Metabolomic Profiling of Fresh Goji (Lycium barbarum L.) Berries from Two Cultivars Grown in Central Italy: A Multi-Methodological Approach. Molecules 2021, 26, 5412. [Google Scholar] [CrossRef] [PubMed]
- Spano, M.; Di Matteo, G.; Fernandez Retamozo, C.A.; Lasalvia, A.; Ruggeri, M.; Sandri, G.; Cordeiro, C.; Sousa Silva, M.; Totaro Fila, C.; Garzoli, S.; et al. A Multimethodological Approach for the Chemical Characterization of Edible Insects: The Case Study of Acheta Domesticus. Foods 2023, 12, 2331. [Google Scholar] [CrossRef] [PubMed]
- Nemzer, B.; Edwards, J.; Kalita, D. Matrix-Specific Effects on Caffeine and Chlorogenic Acid Complexation in a Novel Extract of Whole Coffea Arabica Coffee Cherry by NMR Spectroscopy. Molecules 2022, 27, 7803. [Google Scholar] [CrossRef] [PubMed]
- Exarchou, V.; Troganis, A.; Gerothanassis, I.P.; Tsimidou, M.; Boskou, D. Identification and Quantification of Caffeic and Rosmarinic Acid in Complex Plant Extracts by the Use of Variable-Temperature Two-Dimensional Nuclear Magnetic Resonance Spectroscopy. J. Agric. Food Chem. 2001, 49, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Cusack, L.K.; Fernandez, M.L.; Volek, J.S. The Food Matrix and Sterol Characteristics Affect the Plasma Cholesterol Lowering of Phytosterol/Phytostanol. Adv. Nutr. 2013, 4, 633–643. [Google Scholar] [CrossRef]
- Gomez, L.; Vercambre, G.; Jordan, M.O. Spatial-Temporal Management of Nitrogen and Carbon on the Peach Tree (Prunus persicae L. Batsch.). Sci. Hortic. 2020, 273, 109613. [Google Scholar] [CrossRef]
- Kim, S.; Shin, M.H.; Hossain, M.A.; Yun, E.J.; Lee, H.; Kim, K.H. Metabolite Profiling of Sucrose Effect on the Metabolism of Melissa officinalis by Gas Chromatography-Mass Spectrometry. Anal. Bioanal. Chem. 2011, 399, 3519–3528. [Google Scholar] [CrossRef]
- Marchetti, L.; Rossi, M.C.; Pellati, F.; Benvenuti, S.; Bertelli, D. HR-1H NMR Spectroscopy and Multivariate Statistical Analysis to Determine the Composition of Herbal Mixtures for Infusions. Phytochem. Anal. 2021, 32, 544–553. [Google Scholar] [CrossRef]
- Souihi, M.; Amri, I.; Souissi, A.; Hosni, K.; Ben Brahim, N.; Annabi, M. Essential Oil and Fatty Acid Composition of Melissa officinalis L. Prog. Nutr. 2020, 22, 3591. [Google Scholar]

| Plant | Harvesting Time | Ecotype | Altitude | Treatment |
|---|---|---|---|---|
| Burdock (root) | October | Land Spontaneous (LSE) | 150 m | No treat, wild grown |
| Mountain Spontaneous (MSE) | 800 m | No treat, wild grown | ||
| Organic (OE) | 150 m | Remotion of other vegetable species from the soil | ||
| Dandelion (aerial part and root) | October | Land Spontaneous (LSE) | 150 m | No treat, wild grown |
| Mountain Spontaneous (MSE) | 800 m | No treat, wild grown | ||
| Organic (OE) | 150 m | Remotion of other vegetable species from the soil | ||
| Lemon Balm (aerial part) | May | Land Spontaneous (LSE) | 150 m | No treat, wild grown |
| Mountain Spontaneous (MSE) | 800 m | No treat, wild grown | ||
| Organic (OE) | 150 m | Remotion of other vegetable species from the soil |
| Compound | Assignment | 1H (ppm) | Multiplicity [J(Hz)] | 13C (ppm) | Burdock | Dandelion | Lemon Balm |
|---|---|---|---|---|---|---|---|
| Sugars | |||||||
| α-D-Fructofuranose | C-2 | 105.9 | ● | ● | ● | ||
| CH-3 | 4.11 | 82.3 | |||||
| β-D-Fructofuranose | C-2 | 102.6 | ● | ● | ● | ||
| CH-4 | 4.12 | 75.6 | |||||
| β-D-Fructopyranose | C-2 | 99.3 | ● | ● | ● | ||
| CH-3 | 3.80 | 68.6 | |||||
| β-Galactose | CH-1 | 4.60 D,L | d [7.9] | 97.3 | ● | ● | |
| CH-2 | 3.51 | ||||||
| CH-3 | 3.67 | ||||||
| CH-4 | 3.95 | ||||||
| CH-5 | 4.05 | ||||||
| CH-6 | 3.78 | ||||||
| α-Glucose | CH-1 | 5.23 B,D,L | d [3.8] | 93.3 | ● | ● | ● |
| CH-2 | 3.54 | 72.6 | |||||
| CH-3 | 3.72 | 73.3 | |||||
| CH-4 | 3.41 | 70.8 | |||||
| CH-5 | 3.84 | 72.5 | |||||
| β-Glucose | CH-1 | 4.66 B,D,L | d [7.9] | 97.0 | ● | ● | ● |
| CH-2 | 3.25 | 75.3 | |||||
| Inulin | CH-1 (Glc) | 5.44 | 93.9 | ● | ● | ||
| CH-2 | 3.57 | 72.1 | |||||
| CH-3 | 3.78 | 73.6 | |||||
| CH-4 | 3.48 | 70.2 | |||||
| CH-5 | 3.85 | 73.4 | |||||
| CH2-6 | 3.83 | 61.1 | |||||
| CH2-1′ (Fru) | 3.75; 3.89 | ||||||
| CH-3′a | 4.21 | 77.5 | |||||
| CH-3′b | 4.26 | ||||||
| CH-4′a | 4.05 | 74.9 | |||||
| CH-5′ | 3.89 | 82.2 | |||||
| Myo-inositol | CH-2,5 | 3.56 | ● | ● | |||
| CH-3,6 | 3.65 | ||||||
| CH-4 | 3.28 D,L | 75.0 | |||||
| Sucrose | CH-1 (Glc) | 5.42 B,D,L | d [3.9] | 93.1 | ● | ● | ● |
| CH-2 | 3.53 | 71.8 | |||||
| CH–3 | 3.72 | 73.6 | |||||
| CH–4 | 3.42 | 70.2 | |||||
| CH–5 | 3.84 | 73.5 | |||||
| C2′ (Fru) | 104.8 | ||||||
| CH–3′ | 4.23 | 77.4 | |||||
| CH–5′ | 3.92 | 82.4 | |||||
| CH–4′ | 3.83 | 61.2 | |||||
| Organic acids | |||||||
| Acetate | CH3 | 1.92 B,D | s | 24.4 | ● | ● | ● |
| COO- | 184.4 | ||||||
| Citrate | α, γ-CH | 2.56 B,D,L | d [16.0] | 46.0 | ● | ● | ● |
| α′, γ′-CH | 2.68 | 46.0 | |||||
| β-C | 73.2 | ||||||
| 1,5-COO- | 180.2 | ||||||
| 6-COO- | 183.0 | ||||||
| Formate | HCOO- | 8.46 B,D,L | s | ● | ● | ● | |
| Fumarate | α, β-CH=CH | 6.53 B,D | s | 136.7 | ● | ● | |
| Lactate | β -CH3 | 1.34 B | d [7.1] | 21.4 | ● | ||
| Malate | α-CH | 4.31 B,D,L | dd [9.9; 3.1] | 71.0 | ● | ● | ● |
| β-CH | 2.68 | dd [15.5; 3.1] | 43.5 | ||||
| β′-CH | 2.41 | dd [15.5; 9.9] | 43.5 | ||||
| Succinate | α, β-CH2 | 2.42 B,D,L | s | 35.2 | ● | ● | ● |
| Tartrate | CH(OH)COO- | 4.34 D,L | s | 75.3 | ● | ● | |
| Amino acids | |||||||
| Alanine | α-CH | 3.80 | 51.0 | ● | ● | ● | |
| β-CH3 | 1.49 B,D,L | d [7.2] | 17.3 | ||||
| COO- | 178.6 | ||||||
| Arginine | α-CH | 3.77 | 55.4 | ● | ● * | ||
| β-CH2 | 1.91 | m | 28.0 | ||||
| γ-CH | 1.67 B,D | m | 25.3 | ||||
| γ′-CH | 1.74 | m | 25.3 | ||||
| δ-CH3 | 3.24 | 41.5 | |||||
| Asparagine | α-CH | 4.00 | 52.8 | ● | ● | ||
| β, β′-CH2 | 2.88 B; 2.96 D | dd [7.7; 16.8] dd [4.3; 16.8] | 35.6 | ||||
| COO- | 176.5 | ||||||
| Aspartate | β, β′-CH2 | 2.70 L; 2.81 D | dd [3.7; 17.5] | 37.0 | ● | ● | |
| α-CH | 3.92 | 54.0 | |||||
| γ-COO- | 177.3 | ||||||
| GABA | α-CH2 | 2.30 D,L | t [7.4] | 35.4 | ● | ● | |
| β-CH2 | 1.90 | 24.9 | |||||
| γ-CH2 | 3.01 | t [7.5] | 40.4 | ||||
| Glutamine | α-CH | 3.76 | 55.3 | ● | ● | ● | |
| β, β′-CH2 | 2.14 | m | 27.5 | ||||
| γ-CH | 2.45 B,D,L | m | 31.9 | ||||
| Glycine | α-CH2 | 3.58 | s | 42.5 | ● | ||
| Isoleucine | α-CH | 1.97 | 38.0 | ● | ● | ● | |
| β-CH | 1.27 | 29.4 | |||||
| γ-CH3 | 1.01 B,D,L | d [7.1] | 15.7 | ||||
| δ-CH3 | 0.89 | d [7.4] | |||||
| Leucine | β-CH2 | 1.74 | 41.0 | ● | ● | ● | |
| γ-CH | 1.71 | ||||||
| δ-CH3 | 0.97 B,D,L | d [6.2] | 23.1 | ||||
| δ′-CH3 | 0.96 | 22.1 | |||||
| Phenylalanine | CH-2,6 | 7.33 | m | 130.7 | ● | ||
| CH-4 | 7.38 | ||||||
| CH-3,5 | 7.43 D | m | 130.6 | ||||
| Proline | α-CH | 4.13 L | 62.5 | ● | ● | ● | |
| β, β′-CH2 | 2.07, 2.33 | 30.0 | |||||
| γ-CH3 | 2.01 B,D | m | 25.0 | ||||
| δ, δ′-CH3 | 3.33, 3.41 | 47.4 | |||||
| Threonine | α-CH | 3.59 | 62.3 | ● | ● | ● | |
| γ-CH3 | 1.33 B,D,L | d [6.7] | 20.7 | ||||
| Tyrosine | CH-2, 6 ring | 7.22 | d [8.2] | 132.0 | ● | ● | |
| CH-3, 5 ring | 6.96 B,D | d [8.5] | 117.0 | ||||
| Tryptophan | CH-4 ring | 7.74 | ● | ● | |||
| CH-5 ring | 7.20 | ||||||
| CH-6 ring | 7.29 | ||||||
| CH-7 ring | 7.53 B,D | d [8.1] | |||||
| Valine | α-CH | 3.60 | ● | ● | ● | ||
| β-CH | 2.27 | ||||||
| γ-CH3 | 1.10 | d [7.1] | 17.7 | ||||
| γ′-CH3 | 1.04 B,D,L | d [7.1] | 19.0 | ||||
| Other compounds | |||||||
| Choline | +N(CH3)3 | 3.20 B,D,L | s | 55.1 | ● | ● | ● |
| Ethanolamine | α, β-CH2 | 3.14 B,D | t [5.2] | 42.1 | ● | ● | |
| Trigonelline | CH-1 | 9.13 B,D | s | ● | ● | ||
| CH-3 | 4.42 | s | |||||
| CH-4 | 8.09 | ||||||
| Caftaric acid | CH-10 | 5.31 D | d [2.2] | ● | |||
| CH-11 | 4.57 | d [2.2] | |||||
| CH-2 | 6.49 | d [16.0] | 115.4 | ||||
| CH-3 | 7.72 | d [16.0] | 148.0 | ||||
| CH-8 ring | 6.96 | d [8.2] | 117.6 | ||||
| CH-9 ring | 7.17 | dd [2.0; 8.2] | 124.2 | ||||
| CH-5 ring | 7.25 | d [2.0] | 117.5 | ||||
| COO− | 171.4 | ||||||
| Chicoric acid | CH-10,10a | 5.54 D | s | 75.9 | ● | ||
| CH-2, 2a | 6.49 | d [16.0] | 115.4 | ||||
| CH-3, 3a | 7.72 | d [16.0] | 148.0 | ||||
| CH-8, 8a ring | 6.96 | d [8.2] | 117.6 | ||||
| CH-9, 9a ring | 7.17 | dd [2.0; 8.2] | 124.2 | ||||
| CH-5, 5a ring | 7.25 | d [2.0] | 117.5 | ||||
| COO− | 171.4 | ||||||
| Chlorogenic acid | CH-2 | 6.22 | d [16.0] | 115.8 | ● | ||
| CH-3 | 7.37 | d [16.0] | 146.8 | ||||
| CH2-2′ | 1.89; 2.10 | m | 41.7 | ||||
| CH-4′ | 3.90 | m | 70.8 | ||||
| CH2-6′ | 1.99; 2.06 | m | 38.6 | ||||
| 3,5-Di-caffeoylquinic acid | CH-2, 2a | 6.13, 6.25 B | d [16.0] | 115.7, 116.5 | ● | ||
| CH-3, 3a | 7.40, 7.32 | d [16.0] | 148.0, 147.8 | ||||
| CH-3′, 5′ | 5.335.37 | m m | 72.3 72.9 | ||||
| CH2-2′, 6′ | 1.99, 2.02 | m | 40.0 | ||||
| CH-8, 8a ring | 6.58, 6.66 | d [8.3] | 116,9, 117.0 | ||||
| CH-9, 9a ring | 6.70, 6.74 | dd [1.7; 8.3] | 123.9, 124.0 | ||||
| CH-5, 5a ring | 7.21, 7.24 | d [1.7] | 116.2, 117.0 | ||||
| Rosmarinic acid | CH-2 | 6.22 | d [16.0] | 115.8 | ● | ||
| CH-3 | 7.37 | d [16.0] | 146.8 | ||||
| CH-1a | 5.01 | m | 77.5 | ||||
| CH2-2a | 2.85; 2.93 | m | 37.9 | ||||
| Uracil | CH | 7.84 L | d | ● | |||
| CH | 5.80 | d | |||||
| Uridine | CH-6 | 5.90 B | d [8.1] | ● | ● | ||
| CH-5 | 7.87 D | d [8.1] | |||||
| CH-1′ | 5.89 | d [4.8] | |||||
| ppm | Group | Compounds | Burdock | Dandelion | Lemon Balm | ||
|---|---|---|---|---|---|---|---|
| Root | Leaves | ||||||
| Iβ-Sit | 0.65 | CH3-18 | β-Sitosterol | ● | ● | ● | ● |
| IStig | 0.67 | CH3-18 | Stigmasterol | ● | ● | ● | ● |
| IFA | 2.30 | CH2-11 | Totally fatty acids | ● | ● | ● | ● |
| IDUFA | 2.73 | CH2-11 | Linoleic acid | ● | ● | ● | ● |
| ITUFA | 2.77 | CH2-11,14 | Linolenic acid | ● | ● | ● | ● |
| IPCG | 3.23 | N(CH3)3 | Glyceroylphosphatidylcholine | ● | ● | ● | ● |
| IDGG | 4.87 | CH-1 | Glyceroyldigalactose | ● | ● | ||
| IPHEO | 11.15 | CH-5 | Pheophytin | ● | ● | ||
| ICHL | 11.13 | CH-5 | Chlorophyll | ● | ● | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambroselli, D.; Masciulli, F.; Romano, E.; Guerrini, R.; Ingallina, C.; Spano, M.; Mannina, L. NMR Metabolomics of Arctium lappa L., Taraxacum officinale and Melissa officinalis: A Comparison of Spontaneous and Organic Ecotypes. Foods 2024, 13, 1642. https://doi.org/10.3390/foods13111642
Ambroselli D, Masciulli F, Romano E, Guerrini R, Ingallina C, Spano M, Mannina L. NMR Metabolomics of Arctium lappa L., Taraxacum officinale and Melissa officinalis: A Comparison of Spontaneous and Organic Ecotypes. Foods. 2024; 13(11):1642. https://doi.org/10.3390/foods13111642
Chicago/Turabian StyleAmbroselli, Donatella, Fabrizio Masciulli, Enrico Romano, Ruggero Guerrini, Cinzia Ingallina, Mattia Spano, and Luisa Mannina. 2024. "NMR Metabolomics of Arctium lappa L., Taraxacum officinale and Melissa officinalis: A Comparison of Spontaneous and Organic Ecotypes" Foods 13, no. 11: 1642. https://doi.org/10.3390/foods13111642
APA StyleAmbroselli, D., Masciulli, F., Romano, E., Guerrini, R., Ingallina, C., Spano, M., & Mannina, L. (2024). NMR Metabolomics of Arctium lappa L., Taraxacum officinale and Melissa officinalis: A Comparison of Spontaneous and Organic Ecotypes. Foods, 13(11), 1642. https://doi.org/10.3390/foods13111642











