Influence of Harvesting Stages on Phytonutrients and Antioxidant Properties of Leaves of Five Purple-Fleshed Sweet Potato (Ipomoea batatas) Genotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Site
2.3. Cultivation
2.4. Harvesting of Leaves and Preparation of Samples
2.5. Analysis
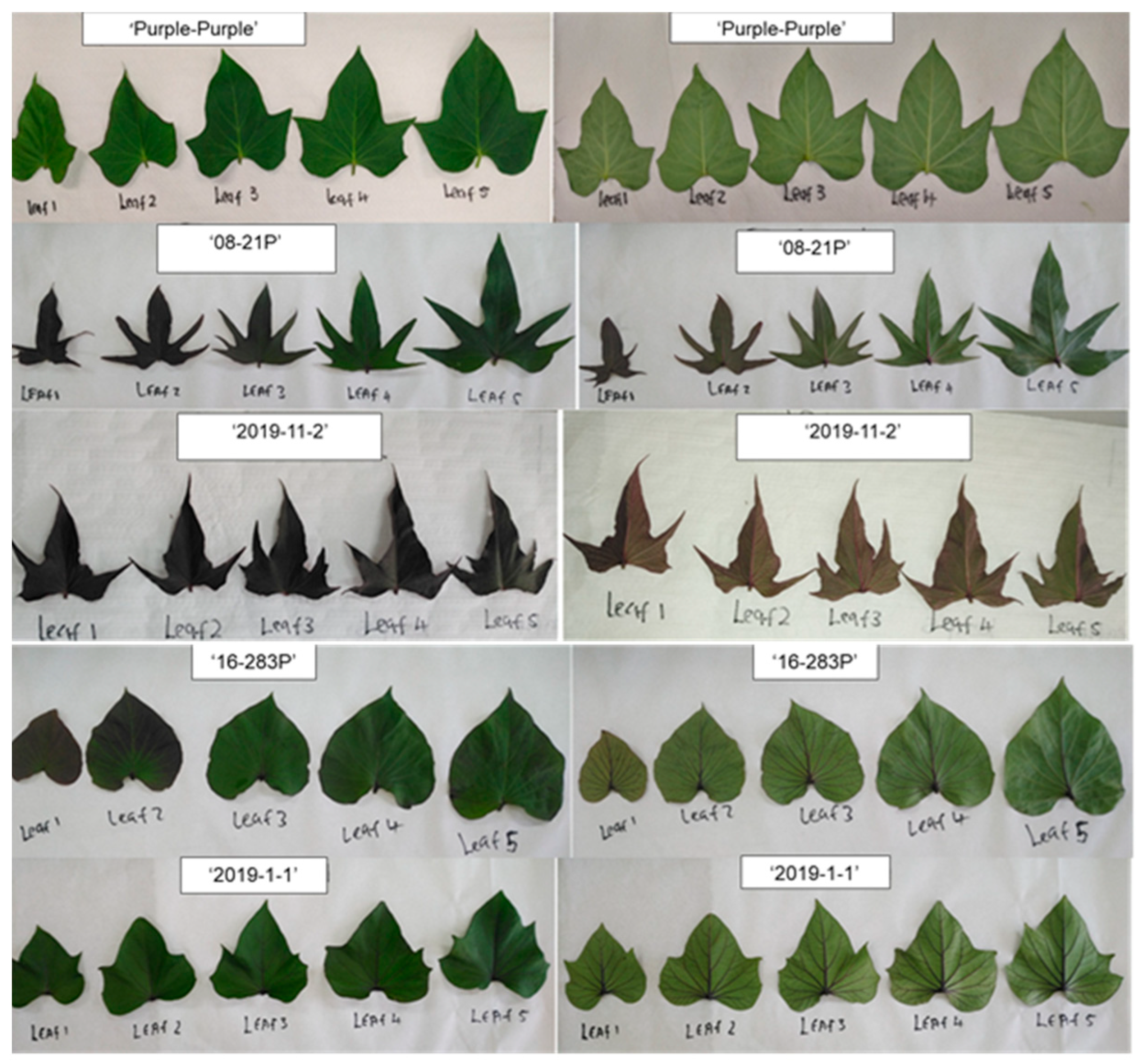
2.5.1. Morphological Analysis
2.5.2. Colour and Chlorophyll
2.5.3. Leaf Area Index (LAI)
2.5.4. Mineral Analysis
2.5.5. Extraction for Total Phenol Content (TPC) and Antioxidant Activities Assays
2.5.6. TPC
2.5.7. Ferric Reducing Antioxidant Power (FRAP)
2.5.8. 2,2-Azino-bis-3-ethylbenzothiazoline-6-sulfonic (ABTS) Scavenging Activity
2.5.9. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Activity
2.5.10. Total Carotenoids
2.5.11. Quantification of Phenolic and Anthocyanin Compounds Using UPLC-QTOF/MS
2.5.12. Quantification of Carotenoid Components Using HPLC-PDA
2.6. Statistical Analysis
3. Results and Discussion
3.1. Morphological Characterisation
3.2. Leaf Area Index (LAI)
3.3. Chlorophyll
3.4. Leaf Colour
3.5. TPC
3.6. UHPLC-QTOF-MS Identification and Characterisation of Phenolic Compounds
3.6.1. Anthocyanins
3.6.2. Phenolic Acids and Flavonoids
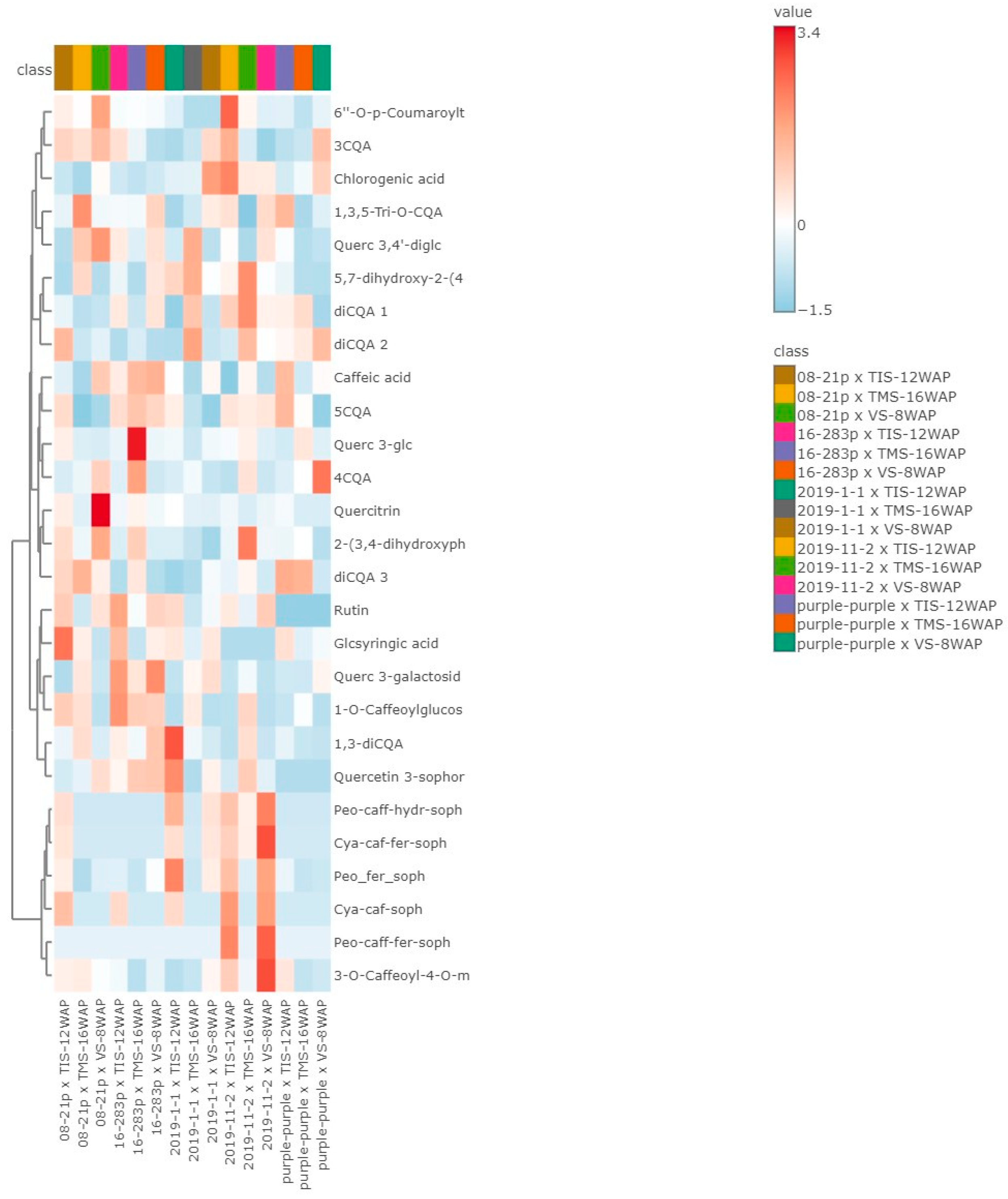
3.6.3. Heat Map
3.7. Total Carotenoids (TC) and Carotenoid Components
3.8. Antioxidant Activities
3.9. Mineral Contents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dinu, M.; Soare, R.; Hoza, G.; Becherescu, A.D.; Băbeanu, C. Bioactive compounds content and antioxidant activity in the leaves of some sweet potato cultivars (Ipomoea batatas L.). Sci. Pap. Ser. B Hortic. 2021, LXV, 415–422. [Google Scholar]
- Nguyen, H.C.; Chen, C.C.; Lin, K.H.; Chao, P.Y.; Lin, H.H.; Huang, M.Y. Bioactive Compounds, Antioxidants, and Health Benefits of Sweet Potato Leaves. Molecules 2021, 26, 1820. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.K.; Gowder, S.J.T. Members of Antioxidant Machinery and Their Functions. In Basic Principles and Clinical Significance of Oxidative Stress; Gowder, S.J.T., Ed.; InTech: London, UK, 2015. [Google Scholar] [CrossRef]
- Motsa, N.M.; Modi, A.T.; Mabhaudhi, T. Influence of agro-ecological production areas on antioxidant activity, reducing sugar content, and selected phytonutrients of orange-fleshed sweet potato cultivars. Food Sci. Technol. 2015, 35, 32–37. [Google Scholar] [CrossRef]
- Lee, S.-L.; Chin, T.-Y.; Tu, S.-C.; Wang, Y.-J.; Hsu, Y.-T.; Kao, M.-C.; Wu, Y.-C. Purple sweet potato leaf extract induces apoptosis and reduces inflammatory adipokine expression in 3t3-l1 differentiated adipocytes. Evid. Based Complement. Altern. Med. 2015, 2015, 126302. [Google Scholar] [CrossRef] [PubMed]
- Vorster, H.I.; van Rensburg Willem, J.; Van Zijl, J.; Sonja, L.V. The importance of traditional leafy vegetables in South Africa. Afr. J. Food Agric. Nutr. Dev. 2007, 7, 1–13. [Google Scholar] [CrossRef]
- Ahmed, M.; Akter, M.S.; Lee, J.-C.; Eun, J.-B. Encapsulation by spray drying of bioactive components, physicochemical and morphological properties from purple sweet potato. LWT Food Sci. Technol. 2010, 43, 1307–1312. [Google Scholar] [CrossRef]
- Makori, S.I.; Mu, T.-H.; Sun, H.-N. Total polyphenol content, antioxidant activity, and individual phenolic composition of different edible parts of 4 sweet potato cultivars. Nat. Prod. Commun. 2020, 15, 1–12. [Google Scholar] [CrossRef]
- Su, X.; Griffin, J.; Xu, J.; Ouyang, P.; Zhao, Z.; Wang, W. Identification and quantification of anthocyanins in purple-fleshed sweet potato leaves. Heliyon 2019, 5, e01964. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C.; Giusti, M.M. Anthocyanins. Adv. Nutr. 2015, 6, 620–622. [Google Scholar] [CrossRef]
- Zhang, L.; Tu, Z.; Wang, H.; Fu, Z.; Wen, Q.; Chang, H.; Huang, X. Comparison of different methods for extracting polyphenols from Ipomoea batatas leaves, and identification of antioxidant constituents by HPLC-QTOF-MS2. Food Res. Int. 2015, 70, 101–109. [Google Scholar] [CrossRef]
- Chirwa-Moonga, T.; Muzungaile, T.; Siyumbano, N.; Moonga, H.B.; Nyau, V. Nutrient composition of raw and steamed, green and purple sweet potato leaf varieties (Ipomoea batatas). J. Med. Act. Plants 2020, 9, 253–261. [Google Scholar] [CrossRef]
- Islam, S. Sweetpotato (Ipomoea batatas L.) Leaf: Its potential effect on human health and nutrition. J. Food Sci. 2006, 71, R13–R121. [Google Scholar] [CrossRef]
- Liao, M.; Zou, B.; Chen, J.; Yao, Z.; Huang, L.; Luo, Z.; Wang, Z. Effect of domestic cooking methods on the anthocyanins and antioxidant activity of deeply purple-fleshed sweetpotato GZ9. Heliyon 2019, 5, e01515. [Google Scholar] [CrossRef]
- Howe, L. Sweet potato leaves for family nutrition. In Proceedings of Trellis Fund Project by a Cow Ethiopia 2018. Available online: https://horticulture.ucdavis.edu/sites/g/files/dgvnsk1816/files/extension_material_files/Sweetpota-to-leaves-family-nutrition-August-2018.pdf (accessed on 15 November 2023).
- Gakige, J.K.; Gachuri, C.; Butterbach-bahl, K.; Goopy, J.P. Early vine harvesting of dual-purpose sweet potato (Ipomoea batatas) increases feeding quality and total biomass without comprising tuber production. Afr. J. Food Agric. Nutr. Dev. 2020, 20, 15538–15548. [Google Scholar] [CrossRef]
- Nkongho, G.O.; Achidi, A.U.; Ntonifor, N.N.; Numfor, F.A.; Dingha, B.N.; Jackai, L.E.; Bonsi, C.K. Sweet potatoes in Cameroon: Nutritional profile of leaves and their potential new use in local foods. Afr. J. Agric. Res. 2014, 9, 1371–1377. [Google Scholar] [CrossRef]
- Jia, R.; Tang, C.; Chen, J.; Zhang, X.; Wang, Z. Total phenolics and anthocyanins contents and antioxidant activity in four different aerial parts of leafy sweet potato (Ipomoea batatas L.). Molecules 2022, 27, 3117. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, S.I.M.; Laurie, S.M.; Shimelis, H.; Laing, M.D. Morpho-agronomical characterisation of local and international sweetpotato germplasm from the South African collection. S. Afr. J. Plant Soil 2020, 37, 308–320. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Niederwieser, J.G. Guide to Sweet Potato Production in South Africa; ARC–Roodeplaat: Pretoria, South Africa, 2004. [Google Scholar]
- Huamán, Z. Descriptors for Sweet Potato; International Board for Plant Genetic Resources 1991. Available online: https://cipotato.org/genebankcip/wp-content/uploads/sites/3/2017/05/Descriptors_for_sweet_potato_Descripteurs_pour_la_patate_douce_Descriptores_de_la_batata_263.pdf (accessed on 10 June 2023).
- Vargas, L.; Kapoor, R.; Nemzer, B.; Feng, H. Application of different drying methods for evaluation of phytochemical content and physical properties of broccoli, kale, and spinach. LWT Food Sci. Technol. 2022, 155, 112892. [Google Scholar] [CrossRef]
- Fan, G.; Han, Y.; Gu, Z.; Gu, F. Composition and colour stability of anthocyanins extracted from fermented purple sweet potato culture. LWT Food Sci. Technol. 2008, 41, 1412–1416. [Google Scholar] [CrossRef]
- Phahlane, C.J.; Laurie, S.M.; Shoko, T.; Manhivi, V.E.; Sivakumar, D. An evaluation of phenolic compounds, carotenoids, and antioxidant properties in leaves of South African cultivars, Peruvian 199062.1 and USA’s Beauregard. Front. Nutr. 2021, 8, 773550. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Bolanos, J. Antioxidant Properties and Anti-Cancer Effects of Polyphenols in Sweetpotato Leaves. Master’s Thesis, University of Arkansas, Fayetteville, AR, USA, December 2020. Available online: https://scholarworks.uark.edu/etd/3934 (accessed on 2 February 2024).
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O. SWATH-MS/MS and DIA-MS: MS-DIAL: Data independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.; Tsugawa, H.; Wohlgemuth, G.; Mehta, S.; Mueller, M.; Zheng, Y.; Ogiwara, A.; Meissen, J.; Showalter, M.; Takeuchi, K.; et al. Identifying metabolites by integrating metabolome databases with mass spectrometry cheminformatics. Nat. Methods 2018, 15, 53–56. [Google Scholar] [CrossRef]
- Deng, J.; Wu, D.; Shi, J.; Balfour, K.; Wang, H.; Zhu, G.; Liu, Y.; Wang, J.; Zhu, Z. Multiple MYB activators and repressors collaboratively regulate the juvenile red fading in leaves of sweetpotato. Front. Plant Sci. 2020, 11, 941. [Google Scholar] [CrossRef]
- Mbayaki, C.W.; Karuku, G.N. Growth and yield of sweet potato (Ipomoea batatas L.) monocrops versus intercrops in the semi-arid Katumani, Kenya. Trop. Subtrop. Agroecosyst. 2021, 24, 16. [Google Scholar] [CrossRef]
- Widaryanto, E.; Saitama, A. Analysis of plant growth of ten varieties of sweet potato (Ipomoea batatas L.) cultivated in rainy season. Asian J. Plant Sci. 2017, 16, 193–199. [Google Scholar] [CrossRef]
- Koshy, A.M.; Joseph, V.; Ravi, V.; Byju, G. Rapid method for estimation of total chlorophyll, chlorophyll a and b and carotene content in leaves of cassava and sweet potato using SPAD meter. J. Root Crop. 2018, 44, 37–40. [Google Scholar]
- Jackson, D.M.; Harrison, H.F.; Jarret, R.L.; Wadl, P.A. Phenotypic variation in leaf morphology of the usda, ars sweetpotato (Ipomoea batatas) germplasm collection. HortScience 2020, 55, 465–475. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Li, K.-T. Leaf color as a morpho-physiological index for screening heat tolerance and improved water use efficiency in rabbiteye blueberry (Vaccinium virgatum Aiton). Sci. Hortic. 2021, 278, 109864. [Google Scholar] [CrossRef]
- Carvalho, I.S.; Cavaco, T.; Carvalho, L.M.; Duque, P. Effect of photoperiod on flavonoid pathway activity in sweet potato (Ipomoea batatas (L.) Lam.) leaves. Food Chem. 2010, 118, 384–390. [Google Scholar] [CrossRef]
- Fukano, Y.; Yamori, W.; Misu, H.; Sato, M.P.; Shirasawa, K.; Tachiki, Y.; Uchida, K. From green to red: Urban heat stress drives leaf color evolution. Sci. Adv. 2023, 9, eabq3542. [Google Scholar] [CrossRef]
- Tan, W.; Guo, X.; Wang, Z.; Zhang, R.; Tang, C.; Jiang, B.; Jia, R.; Deng, Y.; Yang, S.; Chen, J. Metabolic profile and morphological characteristics of leaf tips among different sweet potato (Ipomoea batatas Lam.) varieties. J. Integr. Agric. 2024, 23, 494–510. [Google Scholar] [CrossRef]
- Takács-Hájos, M.; Vargas-Rubóczki, T. Evaluation of bioactive compounds in leaf and root of five beetroot varieties. J. Agric. Food Res. 2022, 7, 100280. [Google Scholar] [CrossRef]
- Suárez, S.; Mu, T.; Sun, H.; Añón, M.C. Antioxidant activity, nutritional, and phenolic composition of sweet potato leaves as affected by harvesting period. Int. J. Food Prop. 2020, 23, 178–188. [Google Scholar] [CrossRef]
- Krochmal-Marczak, B.; Cebulak, T.; Kapusta, I.; Oszmianski, J.; Kaszuba, J.; Zurek, N. The content of phenolic acids and flavonols in the leaves of nine varieties of sweet potatoes (Ipomoea batatas L.) depending on their development, grown in Central Europe. Molecules 2020, 25, 3473. [Google Scholar] [CrossRef]
- Sun, H.; Mu, T.; Xi, L.; Zhang, M.; Chen, J. Sweet potato (Ipomoea batatas L.) leaves as nutritional and functional foods. Food Chem. 2014, 156, 380–389. [Google Scholar] [CrossRef]
- Islam, M.S.; Jalaluddin, M.; Garner, J.O.; Yoshimoto, M.; Yamakawa, O. Artificial shading and temperature influence on anthocyanin compositions in sweetpotato leaves. HortScience 2005, 40, 176–180. [Google Scholar] [CrossRef]
- Sasaki, K.; Oki, T.; Kai, Y.; Nishiba, Y.; Okuno, S. Effect of repeated harvesting on the content of caffeic acid and seven species of caffeoylquinic acids in sweet potato leaves. Biosci. Biotechnol. Biochem. 2015, 79, 1308–1314. [Google Scholar] [CrossRef]
- Vishnu, V.R.; Renjith, R.S.; Mukherjee, A.; Anil, S.R.; Sreekumar, J.; Jyothi, A.N. Comparative study on the chemical structure and in vitro antiproliferative activity of anthocyanins in purple root tubers and leaves of sweet potato (Ipomoea batatas). J. Agric. Food Chem. 2019, 67, 2467–2475. [Google Scholar] [CrossRef]
- Li, G.; Lin, Z.; Zhang, H.; Liu, Z.; Xu, Y.; Xu, G.; Li, H.; Ji, R.; Luo, W.; Qiu, Y.; et al. Anthocyanin accumulation in the leaves of the purple sweet potato (Ipomoea batatas L.) cultivars. Molecules 2019, 24, 3743. [Google Scholar] [CrossRef]
- Zhang, D.; Tan, Y.; Dong, F.; Zhang, Y.; Huang, Y.; Zhou, Y.; Zhao, Z.; Yin, Q.; Xie, X.; Gao, X.; et al. The expression of IbMYB1 is essential to maintain the purple color of leaf and storage root in sweet potato [Ipomoea batatas (L.) Lam]. Front. Plant Sci. 2021, 12, 688707. [Google Scholar] [CrossRef]
- Chen, X.; Li, M.; Ni, J.; Hou, J.; Shu, X.; Zhao, W.; Su, P.; Wang, D.; Shah, F.A.; Huang, S. The R2R3-MYB transcription factor SsMYB1 positively regulates anthocyanin biosynthesis and determines leaf color in Chinese tallow (Sapium sebiferum Roxb.). Ind. Crop. Prod. 2021, 164, 113335. [Google Scholar] [CrossRef]
- Zhang, K.-M.; Yu, H.-J.; Shi, K.; Zhou, Y.-H.; Yu, J.-Q.; Xia, X.-J. Photoprotective roles of anthocyanins in Begonia semperflorens. Plant Sci. 2010, 179, 202–208. [Google Scholar] [CrossRef]
- Jung, J.-K.; Lee, S.-U.; Kozukue, N.; Levin, C.E.; Friedman, M. Distribution of phenolic compounds and antioxidative activities in parts of sweet potato (Ipomoea batata L.) plants and in home processed roots. J. Food Compos. Anal. 2011, 24, 29–37. [Google Scholar] [CrossRef]
- Luo, C.; Wang, X.; Gao, G.; Wang, L.; Li, Y.; Sun, C. Identification and quantification of free, conjugate and total phenolic compounds in leaves of 20 sweetpotato cultivars by HPLC–DAD and HPLC–ESI–MS/MS. Food Chem. 2013, 141, 2697–2706. [Google Scholar] [CrossRef]
- Hossain, M.A.S.; Islam, A.; Miah, M.N.H.; Khan, M.M.H. Leaf chlorophylls and carotenoids status and their correlation with storage root weight of some local and exotic sweetpotato genotypes. Asian J. Plant Sci. 2019, 2, 1–11. [Google Scholar]
- Czeczuga, B. Carotenoid contents in leaves grown under various light intensities. Biochem. Syst. Ecol. 1987, 15, 523–527. [Google Scholar] [CrossRef]
- Mibei, E.K.; Ambuko, J.; Giovannoni, J.J.; Onyango, A.N.; Owino, W.O. Carotenoid profiling of the leaves of selected African eggplant accessions subjected to drought stress. Food Sci. Nutr. 2017, 5, 113–122. [Google Scholar] [CrossRef]
- Ooko Abong, G.; Muzhingi, T.; Wandayi Okoth, M.; Ng’ang’a, F.; Ochieng, P.E.; Mahuga Mbogo, D.; Malavi, D.; Akhwale, M.; Ghimire, S. Phytochemicals in leaves and roots of selected Kenyan orange fleshed sweet potato (OFSP) varieties. Int. J. Food Sci. 2020, 2020, 3567972. [Google Scholar] [CrossRef]
- Drapal, M.; Rossel, G.; Heider, B.; Fraser, P.D. Metabolic diversity in sweet potato (Ipomoea batatas, Lam.) leaves and storage roots. Hortic. Res. 2019, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Dhami, N.; Tissue, D.T.; Cazzonelli, C.I. Leaf-age dependent response of carotenoid accumulation to elevated CO2 in Arabidopsis. Arch. Biochem. Biophys. 2018, 647, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Ofori, G.; Oduro, I.; Ellis, W.; Dapaah, K.H. Assessment of vitamin A content and sensory attributes of new sweet potato (Ipomoea batatas) genotypes in Ghana. Afr. J. Food Sci. 2009, 3, 184–192. [Google Scholar]
- Hughes, N.M.; Neufeld, H.S.; Burkey, K.O. Functional role of anthocyanins in high-light winter leaves of the evergreen herb Galax urceolata. New Phytol. 2005, 168, 575–587. [Google Scholar] [CrossRef]
- Rahman, K. Studies on free radicals, antioxidants, and co-factors. Clin. Interv. Aging 2007, 2, 219–236. [Google Scholar] [PubMed]
- Kim, M.-J.; Hyun, J.-N.; Kim, J.-A.; Park, J.-C.; Kim, M.-Y.; Kim, J.-G.; Lee, S.-J.; Chun, S.-C.; Chung, I.-M. Relationship between phenolic compounds, anthocyanins content and antioxidant activity in colored barley germplasm. J. Agric. Food Chem. 2007, 55, 4802–4809. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, H.; Morita, Y.; Ukeda, H.; Sawamura, M.; Terahara, N. Superoxide Anion-Scavenging Activity of Anthocyanin Pigments. J. Jpn. Soc. Food Sci. 2003, 50, 499–505. [Google Scholar] [CrossRef]
- Luo, X.; Wang, R.; Wang, J.; Li, Y.; Luo, H.; Chen, S.; Zeng, X.; Han, Z. Acylation of anthocyanins and their applications in the food industry: Mechanisms and recent research advances. Foods 2022, 11, 2166. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, K.; Modi, H. The importance of potassium in plant growth–a review. Indian J. Plant Sci. 2012, 1, 177–186. [Google Scholar]
- Kanwar, P.; Baby, D.; Bauer, P. Interconnection of iron and osmotic stress signalling in plants: Is FIT a regulatory hub to cross-connect abscisic acid responses? Plant Biol. 2021, 23, 31–38. [Google Scholar] [CrossRef]
- Hamzah Saleem, M.; Usman, K.; Rizwan, M.; Al Jabri, H.; Alsafran, M. Functions and strategies for enhancing zinc availability in plants for sustainable agriculture. Front. Plant Sci. 2022, 13, 1033092. [Google Scholar] [CrossRef] [PubMed]
- Verlinden, M.S.; AbdElgawad, H.; Ven, A.; Verryckt, L.T.; Wieneke, S.; Janssens, I.A.; Vicca, S. Phosphorus stress strongly reduced plant physiological activity, but only temporarily, in a mesocosm experiment with Zea mays colonized by arbuscular mycorrhizal fungi. Biogeosci. Discuss. 2021, 2021, 1–14. [Google Scholar] [CrossRef]
- Umar, O.B.; Ranti, L.A.; Abdulbaki, A.S.; Bola, A.; Abdulhamid, A.; Biola, M.; Victor, K. Stresses in plants: Biotic and abiotic. Curr. Trends Wheat Res. 2021, 2021, 1–8. [Google Scholar]
- Kumari, V.V.; Banerjee, P.; Verma, V.C.; Sukumaran, S.; Chandran, M.A.S.; Gopinath, K.A.; Venkatesh, G.; Yadav, S.K.; Singh, V.K.; Awasthi, N.K. Plant nutrition: An effective way to alleviate abiotic stress in agricultural crops. Int. J. Mol. Sci. 2022, 23, 8519. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, S.; Gaur, S.; Singh, S.; Yadav, V.; Liu, S.; Singh, V.P.; Sharma, S.; Srivastava, P.; Prasad, S.M. Acquisition and homeostasis of iron in higher plants and their probable role in abiotic stress tolerance. Front. Env. Sci. 2018, 5, 86. [Google Scholar] [CrossRef]
| Genotype X Harvesting Stage | TPC CAE mg/g dw | Total Carotenoids mg/g dw | ||||
|---|---|---|---|---|---|---|
| S1 | S2 | Means | S1 | S2 | Means | |
| Purple-purple X VS-8WAP | 169.08 ± 1.81 j | 125.5 ± 1.33 h | 147.3 ± 23.89 j | 5.08 ± 0.12 c | 7.85 ± 0.05 c | 6.465 ± 1.52 d |
| Purple-purple X TIS-12WAP | 224.76 ± 2.35 g | 150.4 ± 2.61 f | 190.1 ± 38.06 f | 4.53 ± 0.08 d | 4.03 ± 0.03 ij | 4.278 ± 0.28 g |
| Purple-purple X TMS-16WAP | 133.39 ± 1.89 m | 115.7 ± 3.52 i | 124.5 ± 10.02 m | 4.14 ± 0.03 e | 4.43 ± 0.18 h | 4.283 ± 0.19 g |
| 08-21P X VS-8WAP | 256.14 ± 0.90 e | 104.4 ± 1.33 k | 180.3 ± 83.13 h | 6.18 ± 0.62 b | 8.02 ± 0.0 b | 7.100 ± 1.08 b |
| 08-21P X TIS-12WAP | 285.75 ± 1.80 c | 183.8 ± 1.33 c | 234.8 ± 55.86 c | 6.35 ± 0.19 b | 7.03 ± 0.04 e | 6.863 ± 0.58 c |
| 08-21P X TMS-16WAP | 125.16 ± 3.24 n | 94.5 ± 1.74 l | 109.8 ± 19.64 n | 3.22 ± 0.13 f | 7.37 ± 0.124 d | 5.125 ± 2.09 e |
| 2019-11-2 X VS-8WAP | 301.24 ± 0.59 a | 177.7 ± 1.33 d | 239.4 ± 67.63 b | 3.48 ± 0.03 f | 5.92 ± 0.04 f | 4.700 ± 1.34 f |
| 2019-11-2 X TIS-12WAP | 282.41 ± 2.56 d | 242.1 ± 2.5 a | 262.2 ± 22.22 a | 4.04 ± 0.13 e | 3.38 ± 0.05 l | 3.970 ± 0.12 h |
| 2019-11-2 X TMS-16WAP | 202.23 ± 2.01 h | 170.5 ± 1.81 e | 186.3 ± 17.49 g | 2.12 ± 0.10 h | 3.90 ± 0.06 jk | 2.752 ± 0.70 k |
| 16-283P X VS-8WAP | 193.16 ± 0.63 i | 131.3 ± 0.50 g | 162.2 ± 33.87 i | 6.75 ± 0.07 a | 8.64 ± 0.18 a | 7.693 ± 1.04 a |
| 16-283P X TIS-12WAP | 252.70 ± 2.82 f | 192.8 ± 0.87 b | 222.7 ± 32.87 e | 4.37 ± 0.38 de | 3.85 ± 0.12 k | 4.190 ± 0.31 gh |
| 16-283P X TMS-16WAP | 164.06 ± 0.99 k | 108.7 ± 3.92 j | 136.4 ± 30.41 k | 2.63 ± 0.18 g | 4.01 ± 0.06 ijk | 3.237 ± 0.68 j |
| 2019-1-1 X VS-8WAP | 138.72 ± 0.87 l | 125.8 ± 1.51 h | 132.3 ± 7.15 l | 3.11 ± 0.03 f | 7.25 ± 0.07 d | 5.182 ± 2.27 e |
| 2019-1-1 X TIS-12WAP | 295.10 ± 0.33 b | 168.1 ± 1.32 e | 231.9 ± 69.79 d | 2.75 ± 0.17 g | 4.09 ± 0.10 i | 3.892 ± 1.27 ij |
| 2019-1-1 X TMS-16WAP | 107.87 ± 0.87 o | 62.1 ± 1.33 m | 85.0 ± 25.11 o | 2.11 ± 0.03 h | 5.04 ± 0.12 g | 3.100 ± 1.09 i |
| LSD | 2.87 | 3.29 | 0.38 | 0.17 | ||
| Molecular Formula | Compound | Concentrations in mg/kg in the Solid versus Neochlorogenic Acid (3CQA) Calibration Curve | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 16-283p x VS-8WAP | 16-283p x TIS-12WAP | 16-283p x TMS-16WAP | 08-21p x VS-8WAP | 08-21p x TIS-12WAP | 08-21p x TMS-16WAP | 2019-1-1 x VS-8WAP | 2019-1-1 x TIS-12WAP | 2019-1-1 x TMS-18WAP | 2019-11-2 x VS-8WAP | 2019-11-2 x TIS-12WAP | 2019-11-2 x TMS-16WAP | Purple-purple x VS-8WAP | Purple-purple x TIS-12WAP | Purple-purple x TMS-16WAP | ||
| C42H45O24 | Cyanidin-caffeoyl-sophoroside-glucoside | nd | 9.86 ± 1.72 c | nd | nd | 13.46 ± 0.41 b | nd | nd | 14.56 ± 0.43 b | nd | 17.64 ± 0.87 a | 18.13 ± 0.74 a | nd | nd | nd | nd |
| C44H49O24 | Peonidin feruloyl-sophoroside-glucoside | 66.30 ± 0.47 h | 66.48 ± 0.66 g | 28.28 ± 1.34 m | 64.72 ± 1.35 i | 87.35 ± 1.99 f | 14.52 ± 0.44 o | 88.76 ± 1.25 e | 267.10 ± 3.13 b | 44.15 ± 3.51 j | 161.74 ± 4.63 c | 134.67 ± 1.80 d | 43.74 ± 2.09 k | 32.01 ± 3.19 l | 298.52 ± 444.01 a | 27.29 ± 0.76 n |
| C52H53O27 | Cyanidin-caffeoyl-feruloyl-sophoroside-glucoside | nd | nd | nd | nd | 13.68 ± 0.30 d | nd | 13.32 ± 0.53 d | 21.56 ± 1.28 c | nd | 41.51 ± 3.33 a | 27.51 ± 0.61 b | 11.17 ± 0.89 d | nd | nd | nd |
| C50H51O26 | Peonidin-caffeoyl-hydroxybenzoyl-sophoriside-glucoside | nd | nd | nd | nd | 13.99 ± 1.08 d | nd | 13.43 ± 0.54 d | 22.83 ± 2.15 c | nd | 45.24 ± 1.61 a | 29.13 ± 0.40 b | 10.43 ± 0.29 e | nd | nd | nd |
| C53H55O27 | Peonidin caffeoyl-feruloyl-sophoroside-glucoside | nd | nd | nd | nd | nd | nd | nd | nd | nd | 24.47 ± 1.67 a | 21.16 ± 0.14 b | nd | nd | nd | nd |
| RT/ [M-H]- | Molecular Formula | Compound | Concentrations in mg/Kg in the Solid versus Neochlorogenic Acid (3CQA) Calibration Curve | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 16-283p x VS-8WAP | 16-283p x TIS-12WAP | 16-283p x TMS-16WAP | 08-21p x VS-8WAP | 08-21p x TIS-12WAP | 08-21p x TMS-16WAP | 2019-1-1 x VS-8WAP | 2019-1-1 x TIS-12WAP | 2019-1-1 x TMS-18WAP | 2019-11-2 x VS-8WAP | 2019-11-2 x TIS-12WAP | 2019-11-2 x TMS-16WAP | Purple-purple x VS-8WAP | Purple-purple x TIS-12WAP | Purple-purple x TMS-16WAP | |||
| 3.77/ 359.09 | C15H20O10 | Glucosyringic acid; Syringin 4-O-beta-glucoside | 48.62 ± 1.23 d | 49.82 ± 0.82 c | 24.03 ± 0.58 i | 15.57 ± 1.51 m | 68.81 ± 1.83 b | 34.21 ± 2.49 h | 36.39 ± 0.62 g | 36.90 ± 0.74 f | 21.92 ± 0.25 j | 11.66 ± 0.27 n | 11.65 ± 0.83 o | 16.52 ± 0.59 l | 38.86 ± 1.55 e | 113.57 ± 189.43 a | 21.91 ± 1.21 k |
| 4.44/ 353.08 | C16H18O9 | Chlorogenic acid (3CQA) | 1023.68 ± 23.95 d | 994.74 ± 28.43 e | 300.43 ± 7.57 k | 722.20 ± 43.38 g | 612.18 ± 10.83 h | 54.72 ± 2.16 o | 580.94 ± 3.08 i | 1225.95 ± 11.91 c | 121.48 ± 5.05 n | 1337.75 ± 26.14 b | 816.14 ± 13.65 f | 161.24 ± 5.29 l | 502.71 ± 7.06 j | 3436.47 ± 5705.74 a | 122.17 ± 1.46 m |
| 5.08/ 341.09 | C15H18O9 | 1-O-Caffeoylglucose | 84.71 ± 2.17 j | 86.20 ± 2.17 i | 60.32 ± 1.58 l | 116.61 ± 6.07 d | 86.60 ± 0.95 h | 50.93 ± 1.62 n | 102.47 ± 1.09 g | 137.02 ± 4.05 b | 64.59 ± 1.63 k | 106.21 ± 2.48 e | 119.32 ± 2.35 c | 55.91 ± 1.61 m | 102.61 ± 0.72 f | 396.52 ± 657.99 a | 47.82 ± 0.99 o |
| 5.11/ 359.08 | C18H16O8 | 2-(3,4-dihydroxyphenyl)-5-hydroxy-3,6,7-trimethoxy-4H-chromen-4-one | 207.34 ± 8.11 c | 205.17 ± 27.17 d | 45.21 ± 1.41 j | 79.95 ± 5.30 h | 406.69 ± 8.72 b | 25.17 ± 1.98 l | 123.66 ± 4.47 g | 203.51 ± 5.99 e | 15.28 ±1.33 n | 24.95 ± 1.27 m | 70.97 ± 3.81 i | 10.20 ± 1.00 o | 128.03 ± 5.66 f | 1091.55 ± 1826.59 a | 29.25 ± 2.19 k |
| 5.29/ 707.18 | C16H18O9 | Chlorogenic acid | 1366.14 ± 98.61 c | 1290.55 ± 206.01 d | 13.22 ± 0.68 k | 85.91 ± 3.44 i | 1109.61 ± 75.64 e | 0.27 ± 0.47 o | 78.60 ± 2.86 j | 2064.07 ± 103.18 b | 2.08 ± 2.22 n | 578.08 ± 46.32 g | 881.00 ± 59.74 f | 11.60 ± 1.84 l | 88.03 ± 27.37 h | 6688.82 ± 11,201.25 a | 3.13 ± 1.29 m |
| 5.29/ 353.09 | C16H18O9 | Chlorogenic acid (5CQA) | 3496.49 ± 153.12 c | 3314.37 ± 244.52 e | 545.67 ± 15.12 k | 1284.58 ± 67.53 h | 3341.79 ± 64.01 d | 128.76 ± 9.86 o | 1035.82 ± 16.67 j | 4243.55 ± 75.56 b | 234.45 ± 10.73 n | 3137.42 ± 86.85 g | 3141.00 ± 89.10 f | 419.77 ± 18.94 l | 1041.20 ± 25.21 i | 10,916.61 ± 18,124.68 a | 263.62 ± 17.55 m |
| 5.62/ 179.03 | C9H8O4 | Caffeic acid | 663.70 ± 8.14 e | 655.66 ± 56.66 f | 629.45 ± 21.20 g | 802.95 ± 61.90 d | 436.15 ± 9.07 j | 163.39 ± 2.43 o | 619.37 ± 3.48 h | 555.08 ± 23.90 i | 179.06 ± 3.35 n | 2023.06 ± 106.85 a | 1067.31 ± 16.11 c | 416.25 ± 19.33 k | 398.78 ± 25.62 l | 1668.72 ± 2769.16 b | 265.09 ± 6.40 m |
| 5.73/ 353.09 | C16H18O9 | Chlorogenic acid (4CQA) | 213.92 ± 3.80 d | 203.40 ± 5.13 e | 76.25 ± 2.81 k | 76.95 ± 4.15 j | 253.11 ± 4.71 c | 25.43 ± 0.86 o | 119.73 ± 0.81 h | 266.47 ± 7.14 b | 27.32 ± 1.06 n | 195.83 ± 4.58 f | 123.96 ± 0.64 g | 45.82 ± 1.30 l | 91.28 ± 1.87 i | 557.67 ± 927.91 a | 45.28 ± 0.93 m |
| 6.02/ 771.20 | C33H40O21 | Quercetin 3-sophoroside-7-rhamnoside | 4.65 ± 0.45 de | 3.80 ± 0.33 e | 4.48 ± 0.17 de | 9.81 ± 0.47 cd | 14.38 ± 1.81 c | 1.76 ± 0.88 e | 4.55 ± 0.93 de | 9.85 ± 0.26 cd | 0 | 139.93 ± 8.30 a | 132.78 ± 3.37 a | 44.35 ± 3.57 b | 0 | 0 | 0 |
| 6.28/ 625.14 | C27H30O17 | Quercetin 3,4′-diglucoside | 43.93 ± 2.49 c | 41.24 ± 1.23 c | 40.21 ± 2.60 c | 960.00 ± 46.96 abc | 1843.07 ± 36.94 abc | 516.53 ± 14.18 bc | 196.69 ± 4.92 bc | 349.98 ± 6.68 bc | 84.90 ± 2.55 c | 4387.54 ± 86.93 a | 3558.71 ± 22.77 ab | 1679.59 ± 50.85 abc | 211.30 ± 2.42 bc | 2672.41 ± 4434.14 abc | 266.33 ± 5.68 bc |
| 6.48/ 367.10 | C17H20O9 | 3-O-Caffeoyl-4-O-methylquinic acid | 21.28 ± 17.67 k | 27.56 ± 0.42 h | 12.16 ± 0.79 n | 29.80 ± 2.18 g | 36.64 ± 1.55 e | 5.60 ± 0.58 o | 34.42 ± 1.72 f | 116.20 ± 5.65 b | 22.45 ± 0.26 j | 88.22 ± 2.26 c | 70.84 ± 1.25 d | 26.26 ± 0.88 i | 17.62 ± 0.25 l | 144.79 ± 240.55 a | 14.46 ± 0.17 m |
| 6.94/ 609.15 | C27H30O16 | Rutin | 523.04 ± 5.86 a | 499.00 ± 22.46 ab | 336.23 ± 33.62 c | 31.91 ± 1.21 e | 38.74 ± 16.65 e | 16.66 ± 0.51 e | 201.12 ± 68.26 d | 346.79 ± 110.04 c | 110.66 ± 1.11 de | 387.42 ± 6.33 bc | 442.93 ± 55.85 abc | 168.54 ± 3.77 d | 0 | 0 | 0 |
| 7.12/ 463.09 | C21H20O12 | Quercetin 3-galactoside | 829.10 ± 23.77 i | 778.36 ± 26.89 j | 455.13 ± 10.78 m | 362.90 ± 23.20 o | 1066.87 ± 12.09 g | 442.59 ± 7.74 n | 695.64 ± 11.68 k | 1303.50 ± 31.28 d | 532.94 ± 3.88 l | 1114.58 ± 24.86 f | 1280.30 ± 37.36 e | 856.03 ± 281.72 h | 1457.26 ± 596.22 c | 4440.52 ± 7988.66 a | 1631.68 ± 37.63 b |
| 7.19/ 463.09 | C21H20O12 | Quercetin 3-glucoside | 1841.13 ± 60.71 g | 1755.14 ± 84.36 h | 977.69 ± 13.20 o | 1205.94 ± 58.73 l | 3148.21 ± 31.66 b | 1338.57 ± 8.01 j | 1989.18 ± 4.83 f | 2828.49 ± 58.88 c | 1192.43 ± 14.03 m | 2044.06 ± 36.91 e | 2182.17 ± 44.90 d | 1016.90 ± 285.66 n | 1451.40 ± 585.40 i | 9064.57 ± 15,266.96 a | 1318.79 ± 507.70 k |
| 7.41/ 515.12 | C25H24O12 | 1,3-Dicaffeoylquinic acid | 5628.98 ± 148.03 b | 5406.35 ± 217.99 c | 2581.02 ± 35.47 e | 1784.22 ± 92.81 g | 3371.92 ± 74.59 d | 465.04 ± 4.23 l | 156.95 ± 2.08 o | 971.08 ± 21.29 k | 252.23 ± 6.65 n | 1194.78 ± 30.87 j | 2153.33 ± 84.09 f | 459.68 ± 10.96 m | 1562.58 ± 26.21 h | 16,752.67 ± 28,022.12 a | 1213.85 ± 21.59 i |
| 7.57/ 447.09 | C21H20O11 | Quercitrin | 186.44 ± 3.56 d | 180.64 ± 8.33 f | 94.52 ±14.08 n | 89.35 ± 5.12 o | 294.47 ± 8.73 b | 138.55 ± 1.51 j | 138.98 ± 3.10 i | 222.93 ±0.64 c | 146.41 ± 4.84 h | 167.32 ± 6.83 g | 182.41 ± 2.60 e | 123.02 ± 2.87 k | 121.62 ± 1.56 m | 591.06 ± 986.92 a | 122.19 ± 0.87 l |
| 7.60/ 515.12 | C25H24O12 | Dicaffeoylquinic acid isomer 1 (diCQA 1) | 5807.08 ± 195.76 d | 5530.04 ± 270.98 e | 1051.02 ± 333.66 k | 2555.37 ± 137.16 h | 3724.60 ± 26.86 g | 217.49 ± 10.74 o | 2487.34 ± 28.98 i | 11217.07 ± 215.35 b | 720.16 ± 15.32 m | 5141.49 ± 80.57 f | 6709.48 ± 98.04 c | 947.11 ± 7.73 l | 1579.57 ± 9.63 j | 16,302.09 ± 27,303.72 a | 615.24 ± 15.97 n |
| 7.86/ 477.10 | C22H22O12 | 5,7-dihydroxy-2-(4-hydroxy-3-methoxyphenyl)-3-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-4H-chromen-4-one | 51.04 ± 2.42 ab | 48.36 ± 1.47 ab | 22.90 ± 0.20 ab | 17.03 ± 0.48 b | 151.68 ± 3.89 a | 81.95 ± 1.53 ab | 39.52 ± 1.33 ab | 83.09 ± 1.15 ab | 74.73 ± 7.34 ab | 39.02 ± 1.10 ab | 64.57 ± 0.75 ab | 86.18 ± 0.33 ab | 17.26 ± 0.90 b | 100.64 ± 168.14 ab | 17.56 ± 0.56 b |
| 8.13/ 515.12 | C25H24O12 | Dicaffeoylquinic acid isomer 2 (diCQA 2) | 1183.62 ± 50.88 bc | 1111.71 ± 40.60 bc | 191.32 ± 3.51 c | 311.99 ± 17.26 bc | 506.51 ± 12.19 bc | 22.63 ± 2.36 c | 158.85 ± 4.47 c | 1099.61 ± 37.26 bc | 57.30 ± 5.06 c | 2848.34 ± 1150.57 a | 1842.83 ± 64.43 ab | 497.90 ± 15.91 bc | 83.23 ± 25.71 c | 977.54 ± 1637.79 bc | 35.21 ± 10.06 c |
| 8.67/ 515.12 | C25H24O12 | Dicaffeoylquinic acid isomer 3 (diCQA 3) | 115.49 ± 1.30 ab | 116.96 ± 9.02 ab | 37.17 ± 1.28 ab | 47.88 ± 3.52 ab | 63.68 ± 1.79 ab | 5.38 ± 0.94 b | 26.93 ± 0.36 b | 108.30 ± 3.05 ab | 10.94 ± 1.24 b | 261.64 ± 10.50 a | 234.66 ± 2.62 ab | 46.01 ± 0.96 ab | 17.27 ± 0.65 b | 176.80 ± 296.17 ab | 8.85 ± 1.30 b |
| 9.40/ 677.15 | C34H30O15 | 1,3,5-Tri-O-caffeoylquinic acid | 49.05 ± 0.13 d | 45.68 ± 2.67 e | 45.53 ± 1.02 f | 45.39 ± 1.66 g | 41.69 ± 1.63 i | 6.88 ± 0.50 o | 41.26 ± 1.57 j | 143.50 ± 5.75 b | 23.54 l± 0.80 l | 69.31 ± 1.13 c | 44.65 ± 1.42 h | 12.11 ± 0.68 n | 27.10 ± 1.25 k | 239.29 ± 405.51 a | 15.31 ± 0.15 m |
| 9.53/ 593.13 | C30H26O13 | 6″-O-p-Coumaroyltrifolin | 33.04 ± 0.61 h | 35.38 ± 3.34 f | 35.66 ± 2.32 e | 101.15 ± 3.83 d | 33.89 ± 1.19 g | 26.73 ± 1.24 i | 0.31 ± 0.53 n | 1.49 ± 1.09 m | nd | 14.90 ± 0.93 j | 9.12 ± 0.65 k | 3.03 ± 0.81 l | 249.14 ± 12.78 b | 717.03 ± 1201.93 a | 104.39 ± 8.11 c |
| Genotypes x Harvesting Stages | Lutein mg/100 g dw | All Trans-β-carotene mg/100 g dw | Zeaxanthin mg/100 g dw | Cis-β-carotene mg/100 g dw |
|---|---|---|---|---|
| Purple-purple X VS-8WAP | 100.22 ± 0.38 b | 56.94 ± 0.26 b | 33.60 ± 0.16 c | 7.11 ± 0.00 b |
| Purple-purple X TIS-12WAP | 76.15 ± 0.09 f | 22.83 ± 0.07 i | 9.85 ± 0.01 j | 5.68 ± 0.00 f |
| Purple-purple X TMS-16WAP | 63.59 ± 0.00 k | 14.02 ± 0.05 l | 11.85 ± 0.01 h | 4.04 ± 0.01 j |
| 08-21P X VS-8WAP | 128.35 ± 0.23 a | 60.20 ± 0.01 a | 46.83 ± 0.04 a | 9.20 ± 0.03 a |
| 08-21P X TIS-12WAP | 70.64 ± 0.04 j | 53.52 ± 0.20 c | 11.76 ± 0.04 h | 6.16 ± 0.03 c |
| 08-21P X TMS-16WAP | 56.94 ± 0.23 l | 13.41 ± 0.23 m | 9.62 ± 0.06 k | 3.92 ± 0.02 k |
| 2019-11-2 X VS-8WAP | 75.17 ± 0.19 g | 32.44 ± 0.10 g | 26.46 ± 0.27 d | 5.61 ± 0.04 f |
| 2019-11-2 X TIS-12WAP | 71.14 ± 0.18 i | 30.76 ± 0.20 h | 7.98 ± 0.05 l | 4.99 ± 0.06 g |
| 2019-11-2 X TMS-16WAP | 100.40 ± 0.08 b | 9.11 ± 0.00 n | 14.57 ± 0.30 g | 3.07 ± 0.08 l |
| 16-283P X VS-8WAP | 82.66 ± 0.04 e | 41.42 ± 0.13 e | 40.50 ± 0.13 b | 6.00 ± 0.09 e |
| 16-283P X TIS-12WAP | 71.89 ± 0.04 h | 17.19 ± 0.02 k | 10.88 ± 0.09 i | 4.99 ± 0.05 g |
| 16-283P X TMS-16WAP | 86.53 ± 0.12 d | 33.16 ± 0.21 f | 18.44 ± 0.10 f | 4.70 ± 0.06 h |
| 2019-1-1 X VS-8WAP | 88.12 ± 0.00 c | 22.21 ± 0.04 d | 22.21 ± 0.04 e | 6.07 ± 0.03 d |
| 2019-1-1 X TIS-12WAP | 87.94 ± 0.23 c | 18.80 ± 24.42 j | 5.37 ± 0.01 n | 4.52 ± 0.00 i |
| 2019-1-1 X TMS-16WAP | 52.43 ± 0.17 m | 9.11 ± 0.77 n | 18.80 ± 24.42 m | 3.07 ± 0.00 l |
| LSD | 2.88 | 2.43 | 2.14 | 0.71 |
| Main and Interaction effects | ||||
| Genotype | 10,075.27 *** | 47,652.27 *** | 23,659.72 *** | 555.56 a*** |
| Harvest | 228,192.57 *** | 389,611.10 *** | 276,844.88 *** | 3467.32 *** |
| Genotype X harvesting stage | 131,531.47 *** | 44,032.25 *** | 8265.98 *** | 173.15 *** |
| Genotypes X Harvesting Stages | FRAP TEAC mM/g | ABTS IC50 mg/mL | DPPH IC50 mg/mL | ||||||
|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | Mean | S1 | S2 | Mean | S1 | S2 | Mean | |
| Purple-purple X VS-8WAP | 31.25±1.32 e | 48.75±0.87 bc | 40.00±9.64 d | 0.83±0.03 g | 0.42±0.03 de | 0.63±0.23 h | 2.22±0.09f | 1.43±0.08 h | 1.83±0.44 h |
| Purple-purple X TIS-12WAP | 38.75±1.32 b | 49.92±0.76 b | 44.33±6.19 b | 0.55±0.02 cde | 0.36±0.01 bcd | 0.46±0.10 de | 1.46±0.06 d | 1.24±0.15 g | 1.35±0.16 f |
| Purple-purple XTMS-16WAP | 9.42±1.04 h | 39.42±1.04 f | 24.42±16.46 i | 1.31±0.11 h | 0.53±0.07 g | 0.92±0.43 i | 2.99±0.19 g | 1.85±0.06 i | 2.42±0.64 i |
| 08-21P X VS-8WAP | 34.25±1.00 d | 46.58±1.16 d | 40.42±6.82 cd | 0.62±0.04 def | 0.36±0.00 bc | 0.49±0.14 ef | 1.54±0.01 de | 1.30±0.02 fg | 1.42±0.13 fg |
| 08-21P X TIS-12WAP | 36.42±1.04 c | 46.92±2.75 cd | 41.67±6.05 c | 0.42±0.10 ab | 0.21±0.02 a | 0.32±0.13 b | 1.14±0.04 c | 0.68±0.04 c | 0.91±0.25 c |
| 08-21P X TMS-16WAP | 7.75±0.01 h | 44.92±1.04 d | 26.33±20.37 h | 1.52±0.14 i | 0.62±0.05 h | 1.07±0.50 j | 4.33±0.18 h | 1.35±0.04 gh | 2.54±1.63 j |
| 2019-11-2 X VS-8WAP | 40.08±1.44 b | 50.42±1.61 b | 45.25±5.82 b | 0.18±0.01 a | 0.22±0.01 a | 0.20±0.02 a | 0.73±0.061 a | 0.58±0.04 b | 0.66±0.10 a |
| 2019-11-2 X TIS-12WAP | 50.08±1.61 a | 57.75±1.73 a | 53.92±4.46 a | 0.21±0.02 a | 0.23±0.01 a | 0.22±0.02 ab | 0.79±0.06 ab | 0.37±0.03 a | 0.58±0.23 a |
| 2019-11-2 X TMS-16WAP | 29.58±0.29 e | 42.08±0.76 e | 35.83±6.87 e | 0.49±0.02 bc | 0.27±0.04 a | 0.38±0.12 c | 1.6±0.06 de | 0.71±0.07 c | 1.17±0.51 e |
| 16-283P X VS-8WAP | 26.42±0.58 f | 35.75±0.50 g | 31.08±5.14 f | 0.64±0.09 ef | 0.41±0.02 cd | 0.52±0.14 fg | 2.17±0.11 f | 0.56±0.04 b | 1.37±0.88 f |
| 16-283P X TIS-12WAP | 34.08±0.58 d | 40.08±2.02 ef | 37.08±3.55 e | 0.36±0.03 b | 0.34±0.02 b | 0.35±0.02 c | 1.17±0.01 c | 0.43±0.05 a | 0.80±0.41 b |
| 16-283P X TMS-16WAP | 24.42±0.29 g | 33.92±0.76 g | 29.17±5.23 g | 0.54±0.02 cd | 0.47±0.04 ef | 0.50±0.05 gh | 1.54±0.14 de | 0.58±0.02 b | 1.06±0.53 d |
| 2019-1-1 X VS-8WAP | 23.42±1.53 g | 23.25±0.50 i | 23.33±1.02 i | 1.63±0.03 j | 0.38±0.01 bcd | 1.01±0.69 i | 1.19±0.02 c | 1.06±0.07 e | 1.12±0.08 de |
| 2019-1-1 X TIS-12WAP | 34.58±0.29 d | 28.92±0.29 h | 31.75±3.11 f | 0.67±0.06 f | 0.26±0.02 a | 0.47±0.23 cd | 0.93±0.04 b | 0.94±0.01 d | 0.94±0.02 c |
| 2019-1-1 X TMS-16WAP | 9.25±0.50 h | 21.08±0.29 i | 15.17±6.49 j | 2.49±0.04 k | 0.52±0.06 fg | 1.31±1.08 k | 1.65±0.19 e | 1.28±0.02 fg | 1.47±0.23 g |
| LSD | 1.76 | 2.12 | 1.31 | 0.09 | 0.05 | 0.50 | 0.17 | 0.09 | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tshilongo, L.; Mianda, S.M.; Seke, F.; Laurie, S.M.; Sivakumar, D. Influence of Harvesting Stages on Phytonutrients and Antioxidant Properties of Leaves of Five Purple-Fleshed Sweet Potato (Ipomoea batatas) Genotypes. Foods 2024, 13, 1640. https://doi.org/10.3390/foods13111640
Tshilongo L, Mianda SM, Seke F, Laurie SM, Sivakumar D. Influence of Harvesting Stages on Phytonutrients and Antioxidant Properties of Leaves of Five Purple-Fleshed Sweet Potato (Ipomoea batatas) Genotypes. Foods. 2024; 13(11):1640. https://doi.org/10.3390/foods13111640
Chicago/Turabian StyleTshilongo, Lavhelani, Sephora Mutombo Mianda, Faith Seke, Sunette M. Laurie, and Dharini Sivakumar. 2024. "Influence of Harvesting Stages on Phytonutrients and Antioxidant Properties of Leaves of Five Purple-Fleshed Sweet Potato (Ipomoea batatas) Genotypes" Foods 13, no. 11: 1640. https://doi.org/10.3390/foods13111640
APA StyleTshilongo, L., Mianda, S. M., Seke, F., Laurie, S. M., & Sivakumar, D. (2024). Influence of Harvesting Stages on Phytonutrients and Antioxidant Properties of Leaves of Five Purple-Fleshed Sweet Potato (Ipomoea batatas) Genotypes. Foods, 13(11), 1640. https://doi.org/10.3390/foods13111640








