Characterization of Polyphenol Composition and Starch and Protein Structure in Brown Rice Flour, Black Rice Flour and Their Mixtures
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Reagents
2.2. Characterization of Rice Flours
2.2.1. Proximate Analysis
2.2.2. Total Amylose Analysis
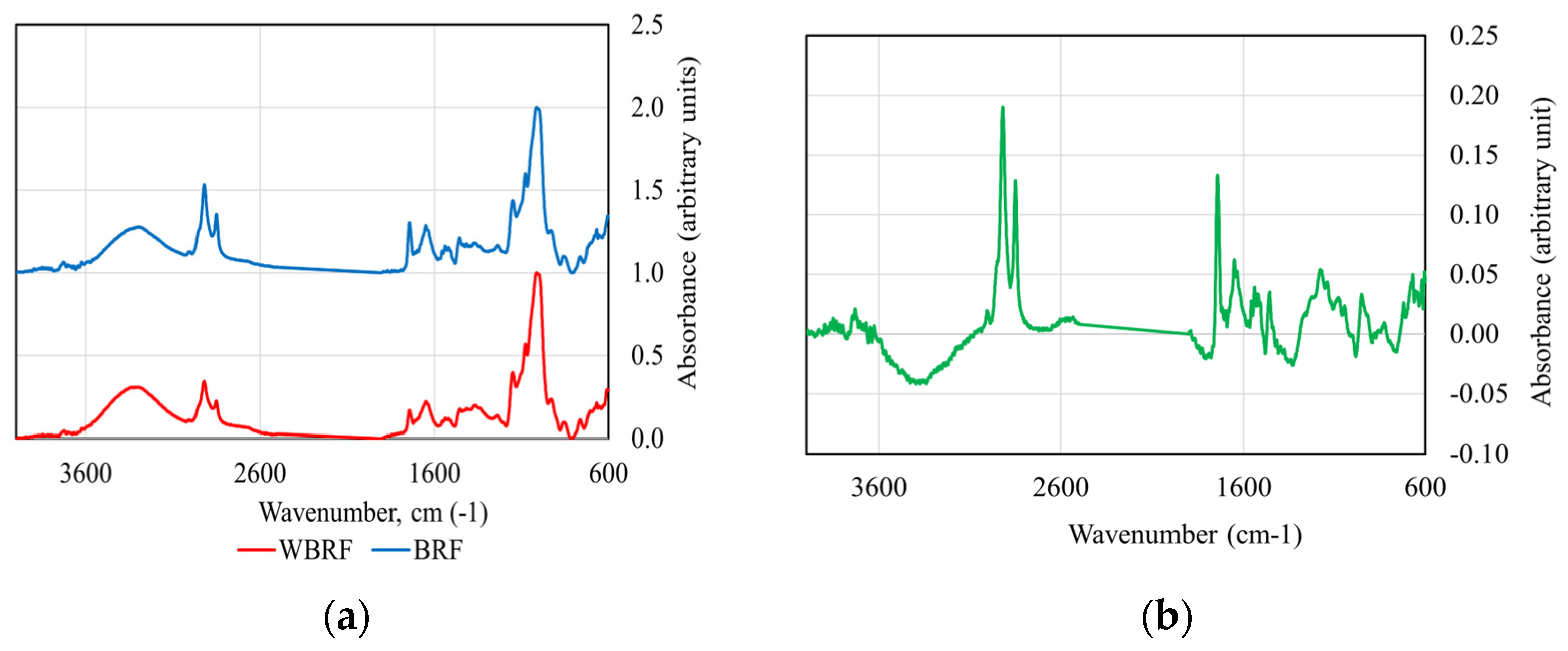
2.2.3. FTIR Analysis
2.2.4. Extraction and Quantification of Total Polyphenols
2.2.5. Extraction and Quantification of Total Flavonoids and Total Proanthocyanidins
2.2.6. Quantification of Individual Polyphenols
2.2.7. Color Analysis
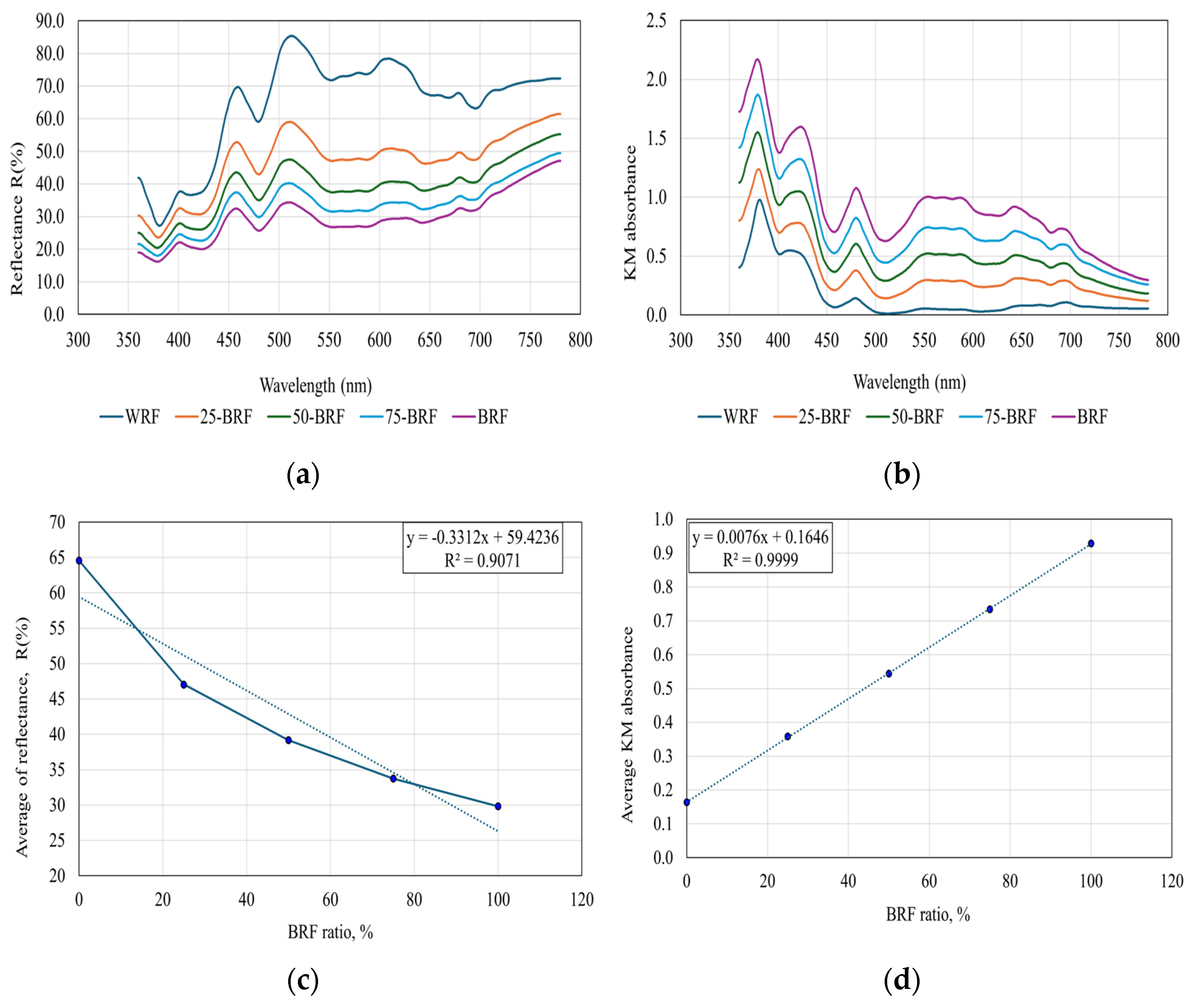
2.2.8. Reflectance Spectra
2.3. Statistical Analysis
3. Results
3.1. Proximate Composition of Rice Flours
3.2. FTIR Spectroscopic Analysis of Rice Flours
3.3. The Contents of Total Polyphenols, Total Flavonoids and Total Proanthocyanidins
3.4. Quantification of Individual Polyphenols
3.5. Color Analysis of Flours
3.6. Reflectance Spectra
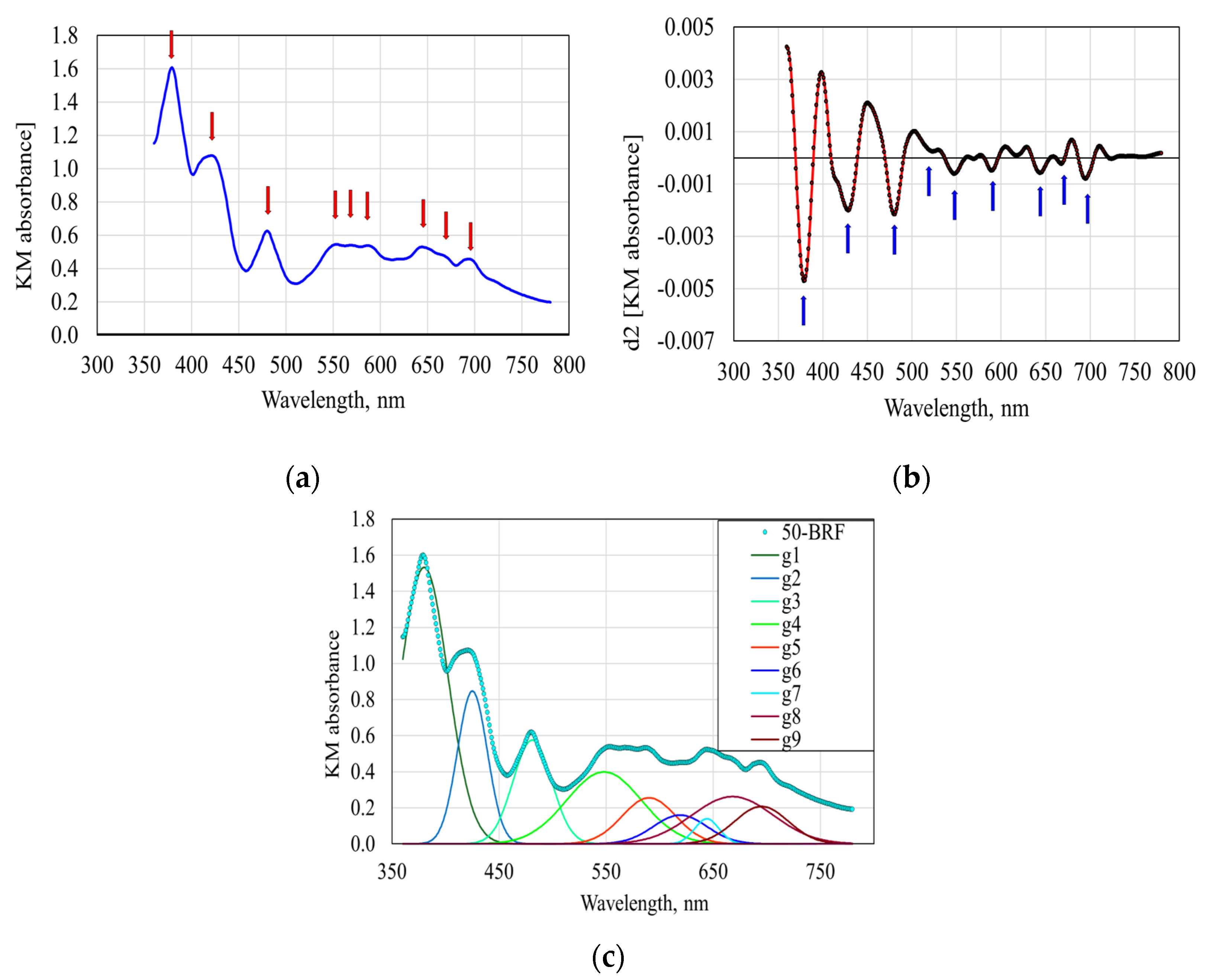
- (i)
- With the exception of area A# 6, assigned to the wavelength at 619 nm, the concentrations of four proanthocyanins correlate significantly (p < 0.05) with the areas resulting from the deconvolutions. The best correlation is registered in the case of area A# 9 (695 nm), for which the values of R2 were in the range of 0.9905 to 0.9977, the values of ER(%) were in the range of 3.0616–5.1745% and the predictive power of the model ranged from 5.37% to 8.31%.
- (ii)
- (iii)
- The concentration of protocatechuic acid significantly correlates (p < 0.00007) with the area A#2 assigned to the wavelength 429 nm, with values of 0.9970, 5.2081% and 5.5689% for the R2, ER (%) and predictive power, respectively.
- (iv)
- The concentration of vanillic acid significantly correlates (p < 0.00004) with area A#9 (695 nm), with values of R2, ER (%) and predictive power of 0.9979, 2.7378% and 4.3299%.
- (v)
- The syringic acid concentration is statistically correlated (p < 0.0219) with the area A#4 assigned to the wavelength at 548 nm, with an R2 of 0.8651, ER (%) of 14.6277% and predictive power of 24.7955%.
- (vi)
- The concentrations of p-coumaric acid and ferulic acid are not significantly correlated to any of the nine areas resulting from the deconvolution process. The minimum values of the statistical probability level were 0.2138 and 0.3704, respectively. The positioning of the absorption maximum values for coumaric acid at 211, 225 and 310 nm and for ferulic acid at 275 and 326 nm [5] outside the analyzed spectral range explains the lack of correlations.
- (vii)
- The concentrations of sinapic acid and ellagic acid both correlate significantly (p < 0.0116 and p < 0.0002) with area A#1 assigned to the wavelength of 380 nm with values of 0.9111 and 0.9950 for the R2, 3.8234% and 3.8730% for the ER (%) and a predictive power of 6.9287% and 7.5309%, respectively.
| Areas Associated with the Gaussian Distributions (g1–g9) in Figure 4c | Wavelengths Associated with the Gaussian Distributions, nm | Wavelength-Assigned Compounds from the Application of Multiple Linear Regression (SLR) to Deconvoluted Reflectance Spectra | Wavelengths of Maximum Absorption of Compounds Reported in the Literature | The Values of the Areas Associated with the Gaussian Distributions (g1–g9) in Figure 4c | ||||
|---|---|---|---|---|---|---|---|---|
| WRF | 25-BRF | 50-BRF | 75-BRF | BRF | ||||
| A#1 associated with g1 | 380 | Proanthocyanins | Peonidin 3-O-glucoside, 330 nm [5] Malvidin 3-O-glucoside, 348 nm [5] | 38.45 ± 0.61 e | 59.45 ± 0.41 d | 84.44 ± 1.34 c | 105.29 ± 0.43 b | 128.12 ± 2.78 a |
| Sinapic acid | 238 nm, 318 nm [5] | |||||||
| Ellagic acid | 256 nm, 360–368 nm [5] | |||||||
| A#2 associated with g2 | 429 | Proanthocyanins | Malvidin 3-O-glucoside, 421 nm [5] | 16.42 ± 0.27 e | 22.99 ± 0.27 d | 28.70 ± 0.92 c | 33.83 ± 0.17 b | 39.82 ± 1.48 a |
| Protocatechuic acid | 297 nm, 258 nm [5] | |||||||
| A#3 associated with g3 | 481 | Proanthocyanins | Cyanidin-3-(6-O-coumaroyl) glucoside, 441 nm [5] | 3.72 ± 0.13 e | 13.22 ± 0.16 d | 24.92 ± 0.72 c | 35.29 ± 0.28 b | 53.65 ± 1.29 a |
| A#4 associated with g4 | 548 | Proanthocyanins | Cyanidin-3-O-glucoside, 516 nm [5] | 2.28 ± 0.10 e | 12.34 ± 0.08 d | 26.05 ± 0.77 c | 29.11 ± 0.09 b | 47.59 ± 1.28 a |
| Peonidin 3-O-glucoside, 517 nm [5] | ||||||||
| Petunidin 3-O-glucoside, 526 nm [5] | ||||||||
| Malvidin 3-O-glucoside, 528 nm [5] | ||||||||
| Syringic acid | Co-pigments based on syringic acid, 550 nm [40] | |||||||
| A#5 associated with g5 | 590 | Proanthocyanins | Peonidin-3-(6-O-coumaroyl)glucoside, 522 nm Petunidin-3-(6-O-coumaroyl)glucoside, 534 nm Malvidin-3-(6-O-coumaroyl)glucoside, 534 nm Malvidin-3-(6-O-ca_eoyl)glucoside, 532 nm Malvidin-3-O-rutinoside, 526 nm [5] | 0.47 ± 0.03 e | 6.71 ± 0.06 d | 10.64 ± 0.19 c | 19.10 ± 0.24 b | 26.54 ± 0.66 a |
| A#6 associated with g6 | 619 | - | Anthocyanins, 627 nm [39] | 0 | 0 | 4.10 ± 0.22 b | 0 | 7.84 ± 0.31 a |
| A#7 associated with g7 | 644 | Proanthocyanins | Anthocyanins, 679 nm [41] Protein-tannin complexes, 679 nm [41] Chlorophyll a [42] | 0.15 ± 0.01 e | 1.81 ± 0.03 d | 3.75 ± 0.10 b | 7.30 ± 0.06 b | 8.49 ± 0.19 a |
| A#8 associated with g8 | 668 | Proanthocyanins | 0.63 ± 0.04 e | 12.95 ± 0.22 d | 14.48 ± 0.24 c | 24.08 ± 0.45 b | 36.25 ± 0.95 a | |
| A#9 associated with g9 | 695 | Proanthocyanins | Methylated anthocyanins, 717 nm [43] Acylated anthocyanins, 717 nm [43] Protein-tannin complexes, 717 nm [43] | 13.10 ± 0.70 e | 38.41 ± 0.41 d | 57.39 ± 2.50 c | 83.68 ± 1.36 b | 94.76 ± 3.84 a |
| Vanillic acid | Co-pigments based on vanillic acid, 540 nm [44] | |||||||
| Sum of areas g1–g9 | 75.22 | 167.88 | 254.47 | 337.68 | 443.06 | |||
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Juliano, B. Rice: Overview. In Encyclopedia of Food Grains, 2nd ed.; Wrigley, C., Corke, H., Seetharaman, K., Faubion, J., Eds.; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Lehmann, U.; Robin, F. Slowly digestible starch e its structure and health implications: A review. Trends Food Sci. Technol. 2007, 18, 346–355. [Google Scholar] [CrossRef]
- Ito, V.C.; Lacerda, L.G. Black rice (Oryza sativa L.): A review of its historical aspects, chemical composition, nutritional and functional properties, and applications and processing technologies. Food Chem. 2019, 301, 125304. [Google Scholar] [CrossRef]
- Pang, Y.; Ahmed, S.; Xu, Y.; Beta, T.; Zhu, T.; Shao, Y.; Bao, J. Bound phenolic compounds and antioxidant properties of whole grain and bran of white, red and black rice. Food Chem. 2018, 240, 212–221. [Google Scholar] [CrossRef]
- Goufo, P.; Trindade, H. Rice antioxidants: Phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, γ-oryzanol, and phytic acid. Food Sci. Nutr. 2014, 2, 75–104. [Google Scholar] [CrossRef]
- Das, M.; Dash, U.; Mahanand, S.S.; Nayak, P.K.; Kesavan, R.K. Black rice: A comprehensive review on its bioactive compounds, potential health benefits and food applications. Food Chem. Adv. 2023, 3, 100462. [Google Scholar] [CrossRef]
- Hou, Z.; Qin, P.; Zhang, Y.; Cui, S.; Ren, G. Identification of anthocyanins isolated from black rice (Oryza sativa L.) and their degradation kinetics. Food Res. Int. 2013, 50, 691–697. [Google Scholar] [CrossRef]
- Rahim, M.A.; Umar, M.; Habib, A.; Imran, M.; Khalid, W.; Gonçalves Lima, C.M.; Shoukat, A.; Itrat, N.; Nazir, A.; Ejaz, A.; et al. Photochemistry, functional properties, food applications, and health prospective of black rice. J. Chem. 2022, 2022, 2755084. [Google Scholar] [CrossRef]
- Solaesa, A.G.; Villanueva, M.; Vela, A.J.; Ronda, F. Impact of microwave radiation on in vitro starch digestibility, structural and thermal properties of rice flour. From dry to wet treatments. Int. J. Biol. Macromol. 2022, 222, 1768–1777. [Google Scholar] [CrossRef]
- Heras-Roger, J.; Alonso-Alonso, O.; Gallo-Montesdeoca, A.; Díaz-Romero, C.; Darias-Martín, J. Influence of copigmentation and phenolic composition on wine color. J. Food Sci. Technol. 2016, 53, 2540–2547. [Google Scholar] [CrossRef]
- Xie, L.H.; Tang, S.Q.; Chen, N.; Luo, J.; Jiao, G.A.; Shao, G.N.; Wei, X.J.; Hu, P.S. Optimisation of near-infrared reflectance model in measuring protein and amylose content of rice flour. Food Chem. 2014, 142, 92–100. [Google Scholar] [CrossRef]
- AOAC 945.18-B; Kjeldahl’s Method for Protein Determination in Cereals and Feed. AOAC: Rockville, MD, USA, 1995.
- AOAC 920.39.B; Kjeldahl’s Method for Fat Extraction. UDK 127 Operating Manual. AOAC: Rockville, MD, USA, 1995.
- AOAC 942.05; Determination of Ash of Animal Feed. AOAC: Rockville, MD, USA, 1995. Available online: http://files.foodmate.com/2013/files_2718.html (accessed on 5 September 2023).
- Commission Regulation (EC) No. 152/2009 Laying Down the Methods of Sampling and Analysis for the Official Control of Feed. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32009R0152 (accessed on 5 September 2023).
- AACC 76-13; Total Starch Assay Procedure. AOAC: Rockville, MD, USA, 1997. Available online: https://prod-media.megazyme.com/media/pdf/d2/7b/20/collaborative-evaluation-of-a-simplified-assay-for-total-starch-in-cereal-products.pdf (accessed on 5 September 2023).
- Juliano, B.O. A simplified assay for milled rice amylose. Cereal Sci. Today. 1971, 16, 334–338. [Google Scholar]
- Vela, A.J.; Villanueva, M.; Solaesa, A.G.; Ronda, F. Impact of high-intensity ultrasound waves on structural, functional,thermal and rheological properties of rice flour and its biopolymers structural features. Food Hydrocoll. 2021, 113, 106480. [Google Scholar] [CrossRef]
- Zhang, G.; Xuan, Y.; Lyu, F.; Ding, Y. Microstructural, physicochemical properties and starch digestibility of brown rice flour treated with extrusion and heat moisture. Int. J. Biol. Macromol. 2023, 242, 124594. [Google Scholar] [CrossRef] [PubMed]
- Shamanin, V.P.; Tekin-Cakmak, Z.H.; Gordeeva, E.I.; Karasu, S.; Pototskaya, I.; Chursin, A.S.; Pozherukova, V.E.; Ozulku, G.; Morgounov, A.I.; Sagdic, O.; et al. Antioxidant capacity and profiles of phenolic acids in various genotypes of purple wheat. Foods 2022, 11, 2515. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Xu, Z. Lipophilic and hydrophilic antioxidants and their antioxidant activities in purple rice bran. J. Agric. Food Chem. 2009, 57, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.J.; Ng, L.T. Antioxidant and free radical scavenging activities of wild bitter melon (Momordica charantia Linn. var. abbreviata Ser.) in Taiwan. LWT-Food Sci. Technol. 2008, 41, 323–330. [Google Scholar] [CrossRef]
- Marinova, D.; Ribarova, F.; Atanassova, M. Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J. Univ. Chem. Technol. Metall. 2005, 40, 255–260. [Google Scholar]
- Skerget, M.; Kotnik, P.; Hadolin, M.; Hraš, A.R.; Simoniš, M.; Knez, Z. Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005, 89, 191–198. [Google Scholar] [CrossRef]
- Martinović, J.; Lukinac, J.; Jukić, M.; Ambrus, R.; Planinić, M.; Šelo, G.; Klarić, A.-M.; Perković, G.; Bucić-Kojić, A. In vitro bioaccessibility assessment of phenolic compounds from encapsulated grape pomace extract by ionic gelation. Molecules 2023, 28, 5285. [Google Scholar] [CrossRef]
- Šelo, G.; Planinić, M.; Tišma, M.; Grgić, J.; Perković, G.; Koceva Komlenić, D.; Bucić-Kojić, A.A. Comparative study of the influence of various fungal-based pretreatments of grape pomace on phenolic compounds recovery. Foods 2022, 11, 1665. [Google Scholar] [CrossRef]
- Banu, I.; Aprodu, I. Investigation on functional, thermo-mechanical and bread-making properties of some white and black rice flours. Appl. Sci. 2022, 12, 4544. [Google Scholar] [CrossRef]
- Farooq, M.A.; Murtaza, M.A.; Aadil, R.M.; Arshad, R.; Rahaman, A.; Siddique, R.; Hassan, S.; Akhtar, H.M.S.; Manzoor, M.F.; Karrar, E.; et al. Investigating the structural properties and in vitro digestion of rice flours. Food Sci. Nutr. 2021, 9, 2668–2675. [Google Scholar] [CrossRef]
- Goufo, P.; Trindade, H. Factors influencing antioxidant compounds in rice. Crit. Rev. Food Sci. Nutr. 2017, 57, 893–922. [Google Scholar]
- Brunet-Loredo, A.; López-Belchí, M.D.; Cordero-Lara, K.; Noriega, F.; Cabeza, R.A.; Fischer, S.; Careaga, P.; Garriga, M. Assessing grain quality changes in white and black rice under water deficit. Plants 2023, 12, 4091. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, X.; Yang, Z.; Gu, C.; Tao, Y.; Guo, Q.; Guo, D.; Zhang, H.; Xu, P.; Liao, Y.; et al. Analysis of quality involving in minerals, amylose, protein, polyphenols and antioxidant capacity in different coloured rice varieties. Food Sci. Technol. Res. 2019, 25, 141–148. [Google Scholar] [CrossRef]
- Paiva, C.L.; Viera, V.A.; Ferreira, M.L.; Schaffert, R.E.; de Oliveira, A.C.; da Silva, C.S. Mineral content of sorghum genotypes and the influence of water stress. Food Chem. 2017, 214, 400–405. [Google Scholar] [CrossRef]
- Goufo, P.; Pereira, J.; Figueiredo, N.; Beatriz, M.; Oliveira, P.P.; Carranca, C.; Rosa, E.A.S.; Trindade, H. Effect of elevated carbon dioxide (CO2) on phenolic acids, flavonoids, tocopherols, tocotrienols, γ-oryzanol and antioxidant capacities of rice (Oryza sativa L.). J. Cereal Sci. 2014, 59, 15–24. [Google Scholar] [CrossRef]
- Biduski, B.; Silva, Y.M.F.; Colussi, R.; El Halal, S.L.M.; Lim, L.T.; Dias, A.R.G.; Elessandra da Rosa Zavareze, E.R. Starch hydrogels: The influence of the amylose content and gelatinization method. Int. J. Biol. Macromol. 2018, 113, 443–449. [Google Scholar] [CrossRef]
- Huang, S.-H.; Ng, L.-T. Quantification of polyphenolic content and bioactive constituents of some commercial rice varieties in Taiwan. J. Food Compos. Anal. 2012, 26, 122–127. [Google Scholar] [CrossRef]
- Kong, S.; Lee, J. Antioxidants in milling fractions of black rice cultivars. Food Chem. 2010, 120, 278–281. [Google Scholar] [CrossRef]
- Liyana-Pathirana, C.M.; Shahidi, F. Importance of insoluble-bound phenolics to antioxidant properties of wheat. J. Agric. Food Chem. 2006, 54, 1256–1264. [Google Scholar] [CrossRef]
- Shao, Y.; Bao, J. Rice. In Chapter 8—Rice Phenolics and Other Natural Products; Chemistry and Technology; Bao, J., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 221–271. [Google Scholar]
- Chowdhury, M.; Ngo, V.-D.; Islam, M.N.; Ali, M.; Islam, S.; Rasool, K.; Park, S.-U.; Chung, S.-O. Estimation of glucosinolates and anthocyanins in kale leaves grown in a plant factory using spectral reflectance. Horticulturae 2021, 7, 56. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, H.; Lou, L.; Chen, Y.; Ye, X.; Chen, J. Copigmentation effect of three phenolic acids on color and thermal stability of Chinese bayberry anthocyanins. Food Sci. Nutr. 2020, 8, 3234–3242. [Google Scholar] [CrossRef]
- Aleixandre-Tudo, J.L.; du Toit, W. Frontiers and new trends in the science of fermented food and beverages. In The Role of UV-Visible Spectroscopy for Phenolic Compounds Quantification in Winemaking; Solís-Oviedo, R.L., de la Cruz Pech-Canul, A., Eds.; Intech Open: London, UK, 2018; pp. 1–21. [Google Scholar]
- Merzlyak, M.N.; Solovchenko, A.E.; Gitelson, A.A. Reflectance spectral features and non-destructive estimation of chlorophyll, carotenoid and anthocyanin content in apple fruit. Postharvest Biol. Technol. 2023, 27, 197–211. [Google Scholar] [CrossRef]
- Saha, S.; Singh, J.; Paul, A.; Sarkar, R. Anthocyanin profiling using UV-Vis spectroscopy and liquid chromatography mass spectrometry. J. AOAC Int. 2020, 103, 23–39. [Google Scholar] [CrossRef]
- Chen, X.; Gao, Q.; Liao, S.; Zou, Y.; Yan, J.; Li, Q. Co-Pigmentation mechanism and thermal reaction kinetics of mulberry anthocyanins with different phenolic acids. Foods 2022, 11, 3806. [Google Scholar] [CrossRef]
- Brodkorb, A.; Balance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; Clemente, A.; Corredig, M.; Dupont, D.; Dufour, C.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nature Protocols 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Yusufoğlu, B.; Karakuş, E.; Yaman, M. In vitro glycemic response determination based on digestion enzymes in some snacks via hydrolysis index procedure. INJIR 2021, 5, 38–43. [Google Scholar]
- Klunklin, W.; Savage, G. Physicochemical, antioxidant properties and in vitro digestibility of wheat–purple rice flour mixtures. Int. J. Food Sci. Tech. 2018, 53, 1962–1971. [Google Scholar] [CrossRef]
- Goni, I.; Garcia-Alonso, A.; Saura-Calixto, F. A starch hydrolysis procedure to estimate glycemic index. Nutr. Res. 1997, 17, 427–437. [Google Scholar] [CrossRef]
- Xiang, G.; Li, J.; Lin, Q.; Zhang, Y.; Ding, Y.; Guo, X.; Pan, Q.; Liu, Q.; Fu, X.; Yang, Y. The effect of heat-moisture treatment changed the binding of starch, protein and lipid in rice flour to affect its hierarchical structure and physicochemical properties. Food Chem. X 2023, 19, 100785. [Google Scholar] [CrossRef] [PubMed]
- Ying, D.Y.; Hlaing, M.M.; Lerissona, J.; Pitts, K.; Cheng, L.; Sanguansri, L.; Augustin, M.A. Physical properties and FTIR analysis of rice-oat flour and maize-oat flour based extruded food products containing olive pomace. Food Res. Int. 2017, 100, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Copikova, J.; Synytsya, A.; Cerna, M.; Kaasova, J.; Novotna, M. Application of FT-IR spectroscopy in detection of food hydrocolloids in confectionery jellies and food supplements. Czech J. Food Sci. 2001, 19, 51–56. [Google Scholar] [CrossRef]

| Parameter | Black Rice Flour Substitution, % | |||||
|---|---|---|---|---|---|---|
| 0 (Brown Rice Flour) WRF | 25 25-BRF | 50 50-BRF | 75 75-BRF | 100 (Black Rice Flour) BRF | ||
| Moisture, g/100 g | 12.79 ± 0.32 a | 12.58 ± 0.41 a | 12.42 ± 0.11 a | 12.28 ± 0.20 a | 12.14 ± 0.29 a | |
| Protein, g/100 g | 8.73 ± 0.18 d | 9.32 ± 0.11 c | 9.78 ± 0.12 c | 10.33 ± 0.15 b | 10.98 ± 0.28 a | |
| Total lipids, g/100 g | 2.11 ± 0.02 e | 2.49 ± 0.02 d | 3.11 ± 0.03 c | 3.50 ± 0.04 b | 4.01 ± 0.11 a | |
| Ash, g/100 g | 0.72 ± 0.01 e | 0.83 ± 0.01 d | 1.01 ± 0.01 c | 1.18 ± 0.01 b | 1.36 ± 0.02 a | |
| Crude fiber, g/100 g | 2.54 ± 0.08 e | 3.11 ± 0.06 d | 3.82 ± 0.09 c | 4.39 ± 0.14 b | 5.11 ± 0.18 a | |
| Total starch, g/100 g | 71.08 ± 3.38 a | 70.03 ± 2.39 a | 68.98 ± 2.84 a | 67.93 ± 2.08 a | 66.87 ± 2.25 a | |
| Carbohydrates, g/100 g | 85.90 ± 4.3a | 84.25 ± 2.90 a | 82.28 ± 3.92 a | 80.60 ± 3.29 a | 78.54 ± 1.78a | |
| Energy, Kcal/100 g | 402.59 ± 16.71 a | 402.91 ± 9.55 a | 403.87 ± 5.78 a | 404.00 ± 8.56 a | 404.39 ± 12.58 a | |
| Amylose content, % | 19.16 ± 0.70 c | 20.11 ± 0.45 bc | 20.92 ± 0.24 ab | 21.46 ± 0.20 ab | 22.30 ± 0.99 a | |
| Polyphenols, mg GAE/100 g | Free polyphenols | 93.13 ± 3.41 e | 105.34 ± 4.43 d | 117. 23 ± 2.78 c | 131.13 ± 1.60 b | 142.42 ± 3.46 a |
| Bound polyphenols | 54.63 ± 2.47 e | 93.68 ± 3.89 d | 132.80 ± 2.88 c | 169.65 ± 5.19 b | 209.79 ± 7.34 a | |
| Total polyphenols | 147.76 ± 4.09 e | 199.02 ± 8.14 d | 250.03 ± 8.33 c | 300.78 ± 9.35 b | 352.21 ± 9.72 a | |
| Total flavonoids, mgQE/g | 0.41 ± 0.01 e | 0.90 ± 0.02 d | 1.46 ± 0.02 c | 2.33 ± 0.06 b | 3.47 ± 0.09 a | |
| Total proanthocyanins, mgC/g | 0.01 ± 0.00 e | 0.38 ± 0.00 d | 0.74 ± 0.00 c | 1.16 ± 0.01 b | 1.49 ± 0.04 a | |
| CIEL*a*b* coordinates | L* | 89.31 ± 0.27 a | 76.15 ± 0.09 b | 69.60 ± 0.33 c | 66.02 ±0.05 d | 60.72 ± 0.34 e |
| a* | −7.77 ± 0.06 e | −6.31 ± 0.03 d | −5.23 ± 0.02 c | −4.80 ± 0.02 b | −3.96 ±0.03 a | |
| b* | 15.05 ± 0.17 a | 6.74 ± 0.03 b | 4.77 ± 0.05 c | 3.78 ± 0.3 d | 2.72 ± 0.05 e | |
| RGB coordinates | R | 11.19 ± 0.11 a | 7.09 ± 0.019 b | 5.67 ±0.07 c | 4.96 ± 0.01 d | 4.07 ±0.06 e |
| G | 10.93 ± 0.07 a | 8.35 ±0.027 b | 6.91 ± 0.08 c | 6.19 ± 0.01 d | 5.16 ±0.064 e | |
| B | 13.72 ± 0.10 a | 9.27 ±0.03 b | 7.43 ±0.09 c | 6.54 ±0.01 d | 5.35 ± 0.07 e | |
| Images of flour samples |  |  |  |  |  | |
| Flour Type | Protein Secondary Structure Analysis (%) | Starch Bands | ||||
|---|---|---|---|---|---|---|
| α-Helix | β-Sheet | β-Turns | Random Coil | R1047/1022 | R995/1022 | |
| WRF | 14.22 ± 0.72 e | 30.52 ± 1.26 a | 18.80 ± 0.41 a | 36.46 ± 0.89 a | 1.12 ± 0.03 d | 1.32 ± 0.05 c |
| 25-BRF | 15.72 ± 0.65 d | 31.01 ± 1.45 a | 16.66 ± 0.41 b | 36.61 ± 1.15 a | 1.17 ± 0.06 cd | 1.38 ± 0.06 bc |
| 50-BRF | 17.38 ± 0.37 c | 31.44 ± 0.98 a | 14.42 ± 0.28 c | 36.76 ± 1.62 a | 1.22 ± 0.03 bc | 1.45 ± 0.04 ab |
| 75-BRF | 18.92 ± 0.57 b | 31.89 ± 1.30 a | 12.30 ± 0.37 d | 36.89 ± 1.80 a | 1.27 ± 0.03 ab | 1.51 ± 0.05 a |
| BRF | 20.47 ± 0.23 a | 32.40 ± 0.93 a | 10.12 ± 0.27 e | 37.01 ± 1.88 a | 1.32 ± 0.02 a | 1.57 ± 0.03 a |
| (a) | ||||||||
| Sample | Proanthocyanins, µg/g | |||||||
| Cyanidin-3-O-glucoside | Peonidin-3-O-glucoside | Malvidin 3-O-glucoside | Petunidin Chloride | Total Amount of Proanthocyanins | ||||
| WRF | Nd | nd | nd | nd | - | |||
| 25-BRF | 130.23 ± 5.25 d | 68.07 ± 7.12 d | 1.60 ± 0.18 d | 0.35 ± 0.03 d | 200.25 | |||
| 50-BRF | 265.47 ± 7.81 c | 14.34 ± 2.37 c | 2.82 ± 0.08 c | 0.59 ± 0.02 c | 283.22 | |||
| 75-BRF | 441.19 ± 1.56 b | 203.74 ± 5.66 b | 4.62 ± 0.38 b | 0.88 ± 0.01 b | 650.43 | |||
| BRF | 481.68 ± 7.63 a | 226.33 ± 0.06 a | 5.60 ± 0.04 a | 1.07 ± 0.05 a | 714.68 | |||
| (b) | ||||||||
| Sample | Phenolic Acids, µg/g | |||||||
| Protocatechuic Acid | Vanillic Acid | Syringic Acid | p-Coumaric Acid | Ferulic Acid | Sinapic Acid | Ellagic Acid | Total Amount of Phenolic Acids | |
| WRF | nd | 0.27 ± 0.00 e | 1.13 ± 1.13 c | 1.03 ± 0.01 ab | 4.84 ± 0.03 a | 0.34 ± 0.01 c | 0.25 ± 0.03 e | 7.86 |
| 25-BRF | 54.92 ± 4.40 d | 7.36 ± 0.34 d | 3.48 ± 0.00 b | 1.02 ± 0.01 b | 3.13 ± 0.00 c | 0.32 ± 0.01 c | 1.75 ± 0.04 d | 71.98 |
| 50-BRF | 105.54 ± 3.11 c | 14.43 ± 0.34 c | 4.81 ± 0.28 ab | 1.09 ± 0.02 ab | 3.29 ± 0.05 c | 0.42 ± 0.01 b | 3.69 ± 0.08 c | 133.27 |
| 75-BRF | 162.18 ± 4.62 b | 22.22 ± 0.28 b | 3.84 ± 0.07 b | 1.13 ± 0.03 a | 3.60 ± 0.02 b | 0.46 ± 0.04 ab | 5.06 ± 0.09 b | 198.49 |
| BRF | 205.80 ± 1.71 a | 25.07 ± 1.06 a | 6.03 ± 0.14 a | 0.89 ± 0.03 c | 3.59 ± 0.04 b | 0.51 ± 0.05 a | 7.37 ± 0.19 a | 249.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uivarasan, A.; Lukinac, J.; Jukić, M.; Šelo, G.; Peter, A.; Nicula, C.; Mihaly Cozmuta, A.; Mihaly Cozmuta, L. Characterization of Polyphenol Composition and Starch and Protein Structure in Brown Rice Flour, Black Rice Flour and Their Mixtures. Foods 2024, 13, 1592. https://doi.org/10.3390/foods13111592
Uivarasan A, Lukinac J, Jukić M, Šelo G, Peter A, Nicula C, Mihaly Cozmuta A, Mihaly Cozmuta L. Characterization of Polyphenol Composition and Starch and Protein Structure in Brown Rice Flour, Black Rice Flour and Their Mixtures. Foods. 2024; 13(11):1592. https://doi.org/10.3390/foods13111592
Chicago/Turabian StyleUivarasan, Alexandra, Jasmina Lukinac, Marko Jukić, Gordana Šelo, Anca Peter, Camelia Nicula, Anca Mihaly Cozmuta, and Leonard Mihaly Cozmuta. 2024. "Characterization of Polyphenol Composition and Starch and Protein Structure in Brown Rice Flour, Black Rice Flour and Their Mixtures" Foods 13, no. 11: 1592. https://doi.org/10.3390/foods13111592
APA StyleUivarasan, A., Lukinac, J., Jukić, M., Šelo, G., Peter, A., Nicula, C., Mihaly Cozmuta, A., & Mihaly Cozmuta, L. (2024). Characterization of Polyphenol Composition and Starch and Protein Structure in Brown Rice Flour, Black Rice Flour and Their Mixtures. Foods, 13(11), 1592. https://doi.org/10.3390/foods13111592








