Unveiling the Potent Antiviral and Antioxidant Activities of an Aqueous Extract from Caesalpinia mimosoides Lamk: Cheminformatics and Molecular Docking Approaches
Abstract
:1. Introduction
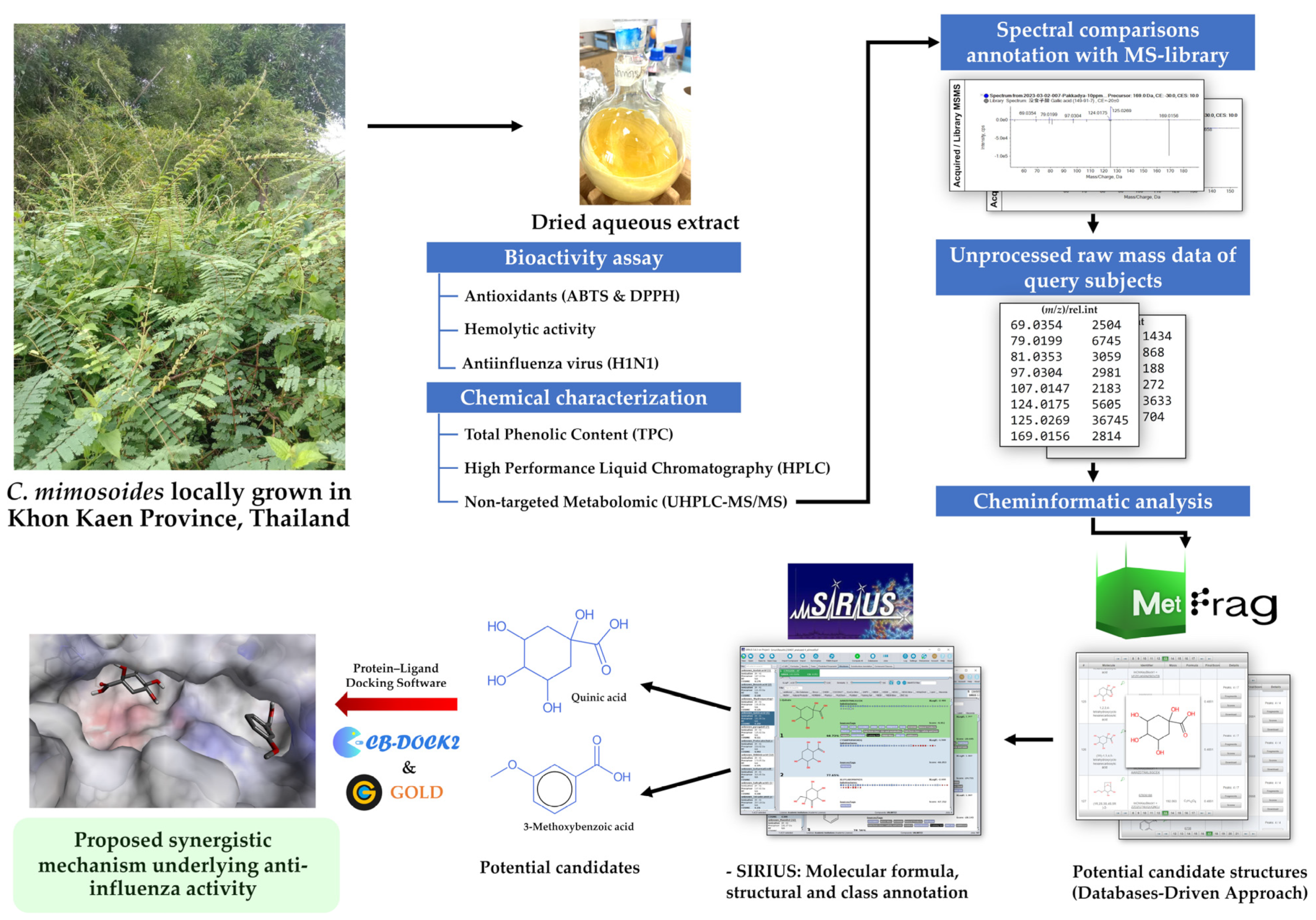
2. Materials and Methods
2.1. Plant Collection and Preparation of C. mimosoides Aqueous Extract
2.2. Total Phenolic Content (TPC)
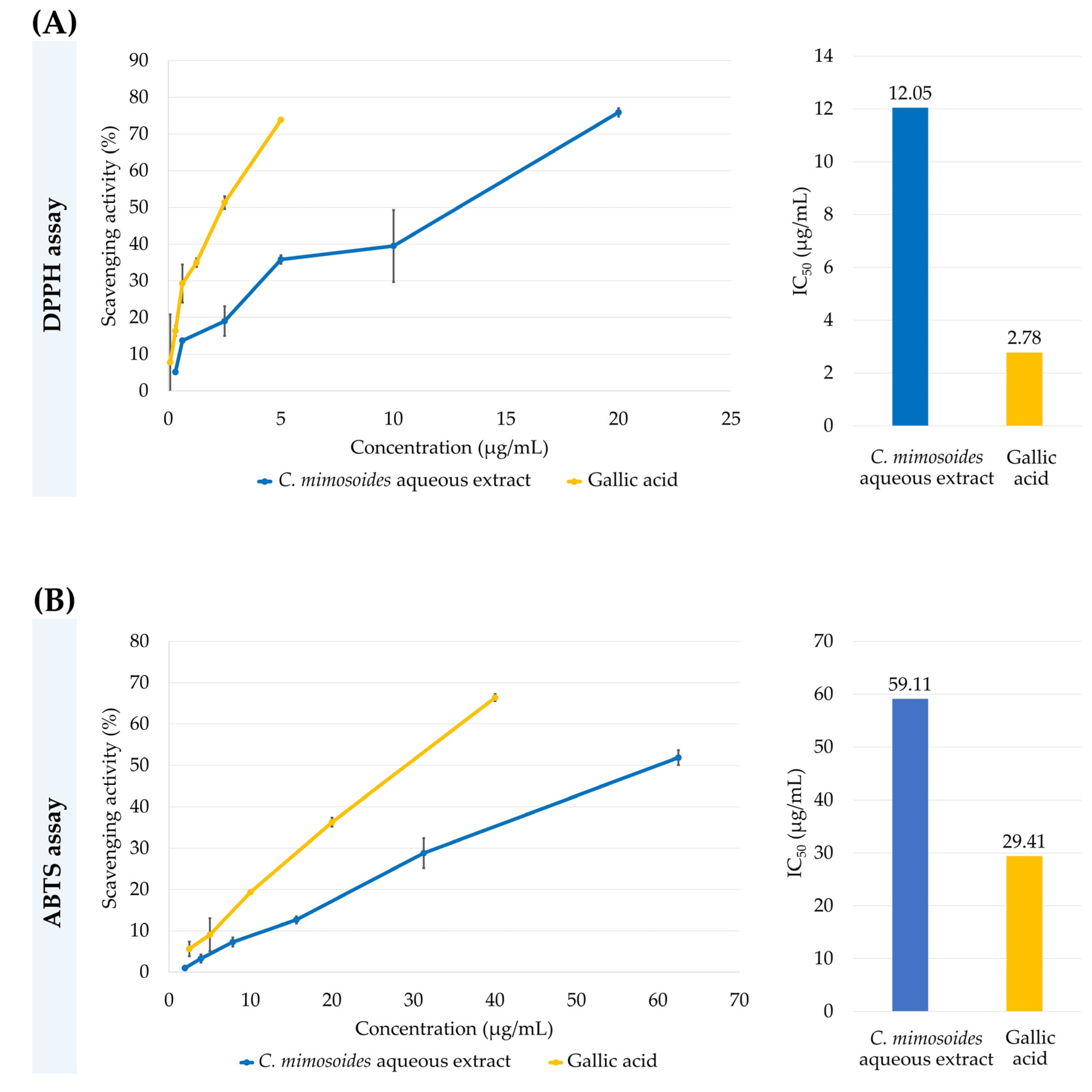
2.3. Antioxidant Assays
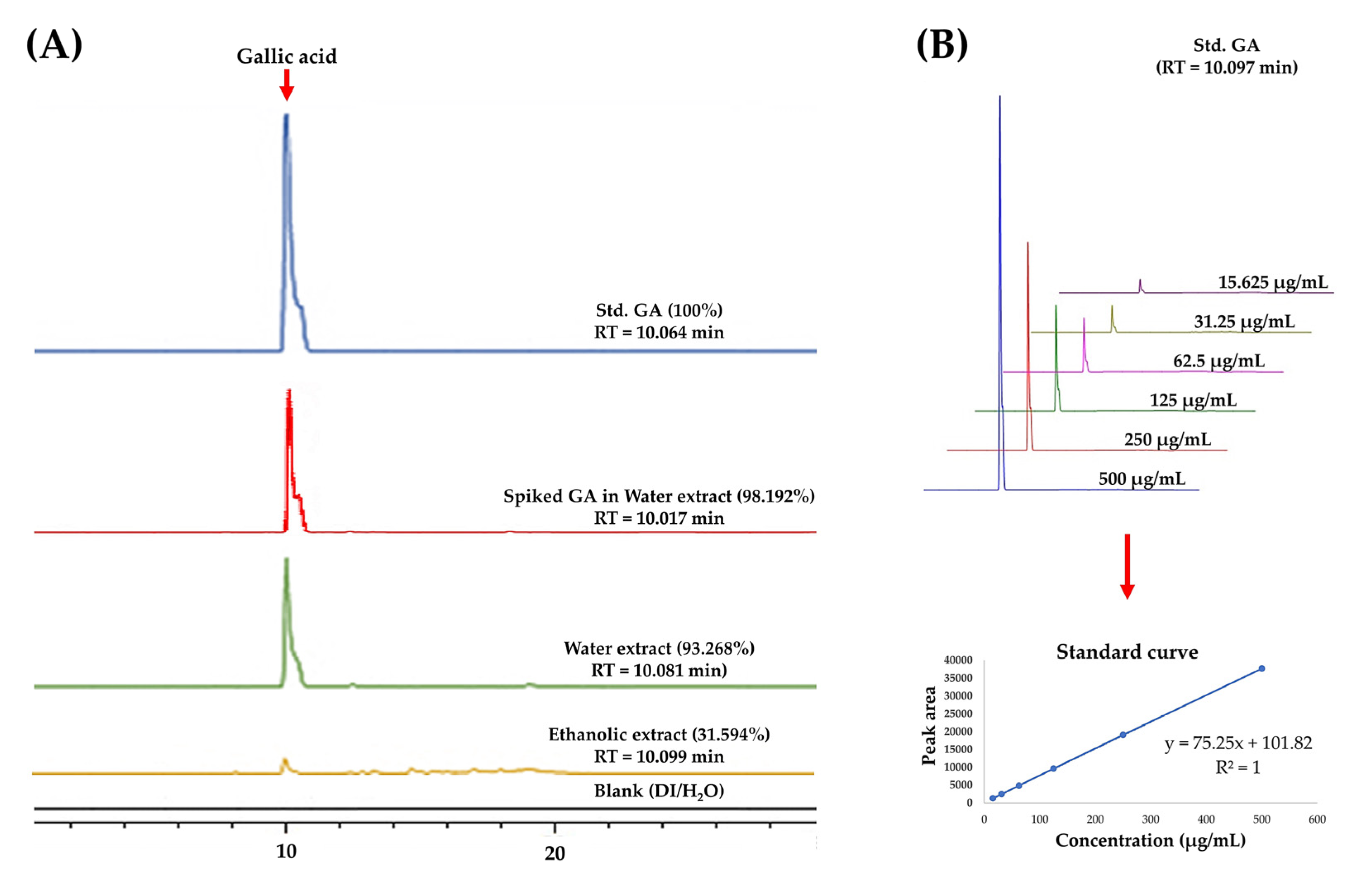
2.4. HPLC Analysis of GA (1) in the C. mimosoides Aqueous Extract
2.5. Detection of Metabolites Using LC-MS/MS
2.6. Structural Annotation Using MetFrag Webservice
2.7. Structural Elucidation Using SIRIUS
2.8. Anti-Influenza Virus Screening Assay
2.9. Hemolytic Activity Assay
2.10. Molecular Docking
3. Results
3.1. Radical Scavenging Activities of the C. mimosoides Aqueous Extract
3.2. Determination of GA (1) Using High-Performance Liquid Chromatography
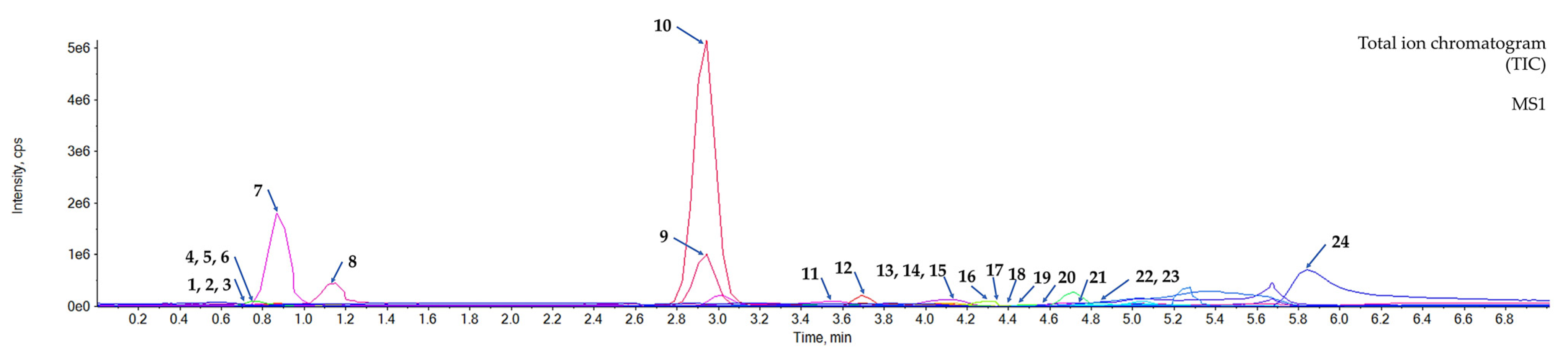
3.3. Metabolic Profiling of the Aqueous Extract of the C. mimosoides Using UPLC-ESI(-)-QTOF-MS/MS
3.4. Structural Annotation Using MetFrag
3.5. Structural Annotation Using SIRIUS
3.5.1. Simple Phenolic Substances
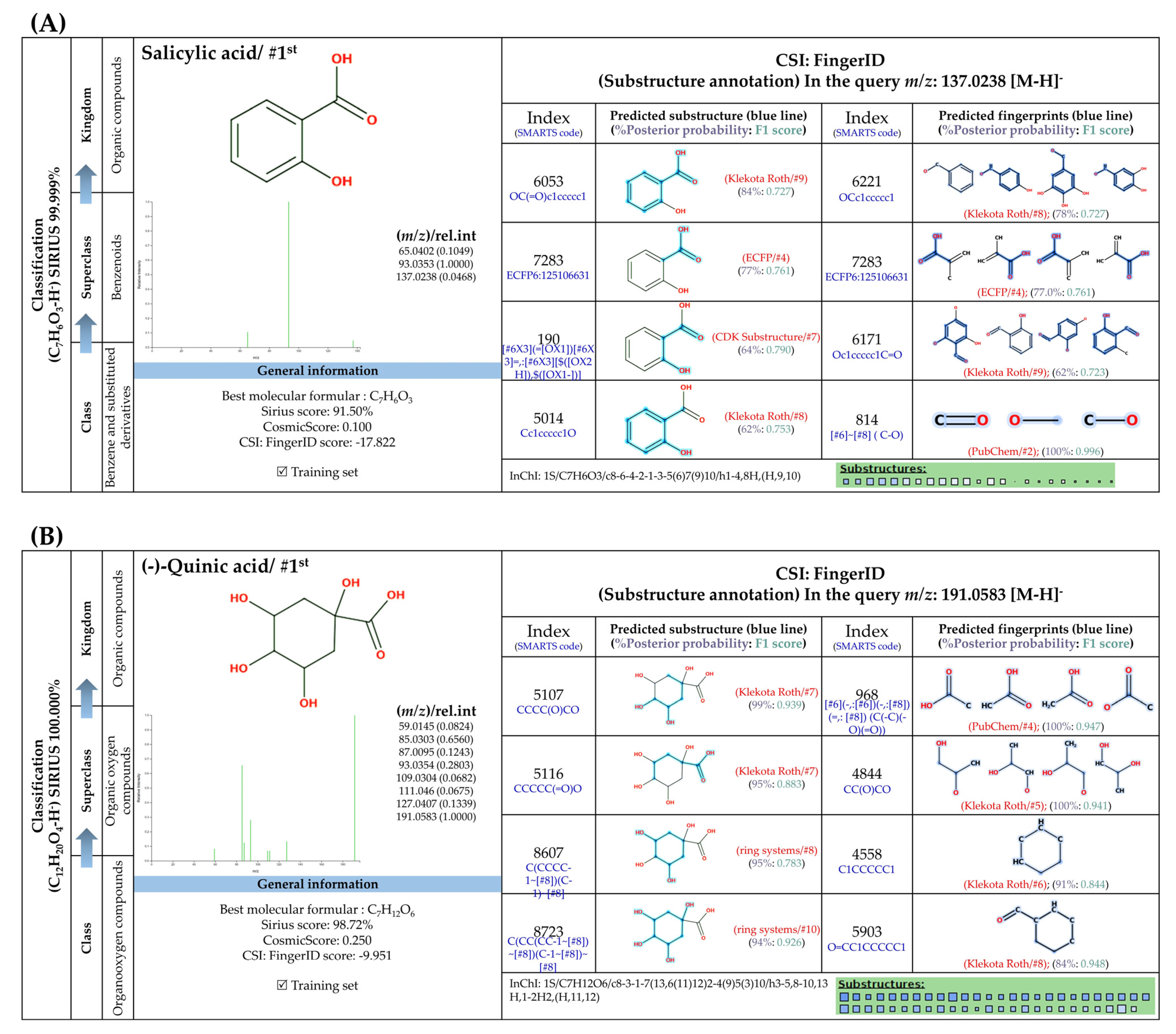
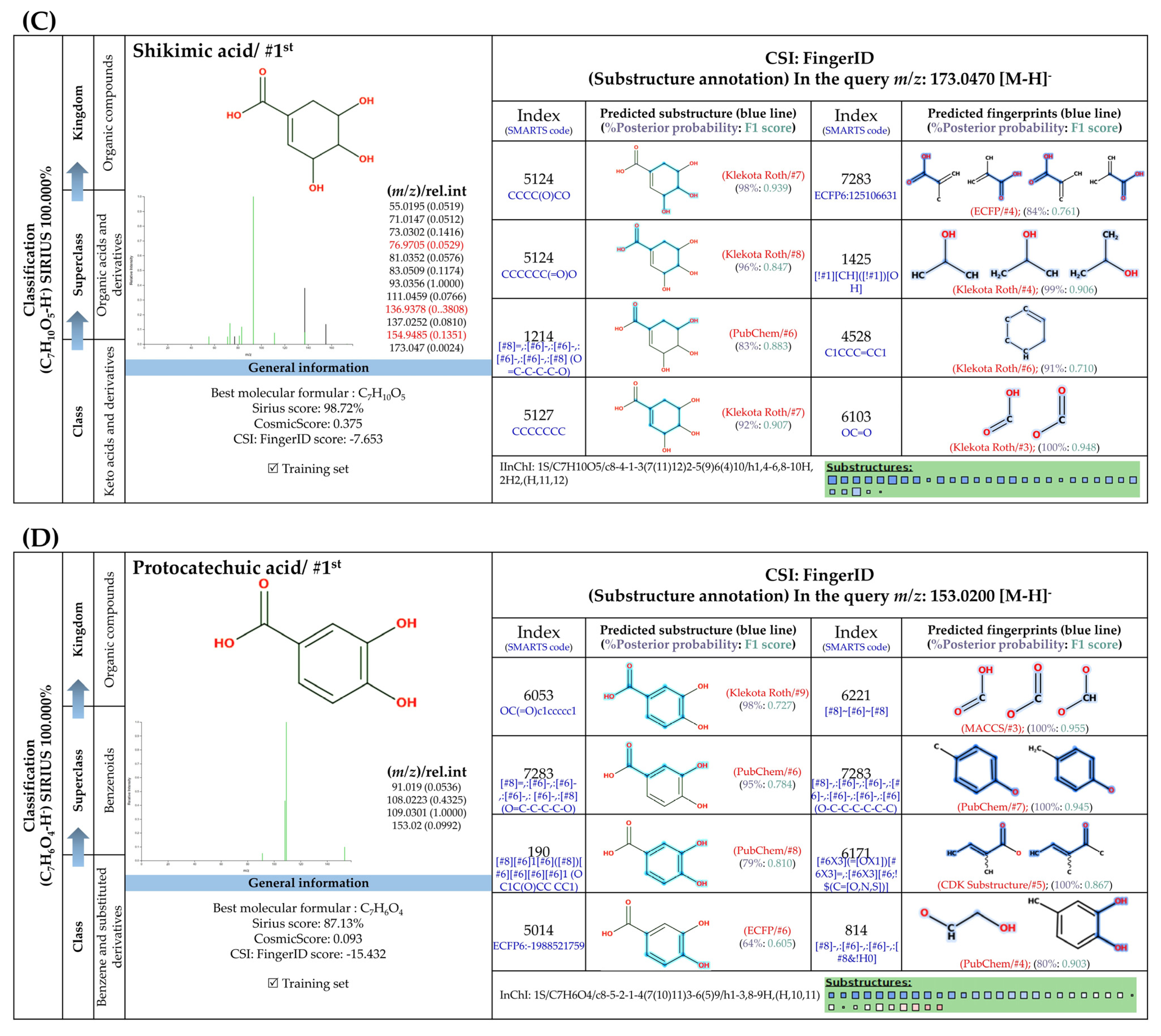
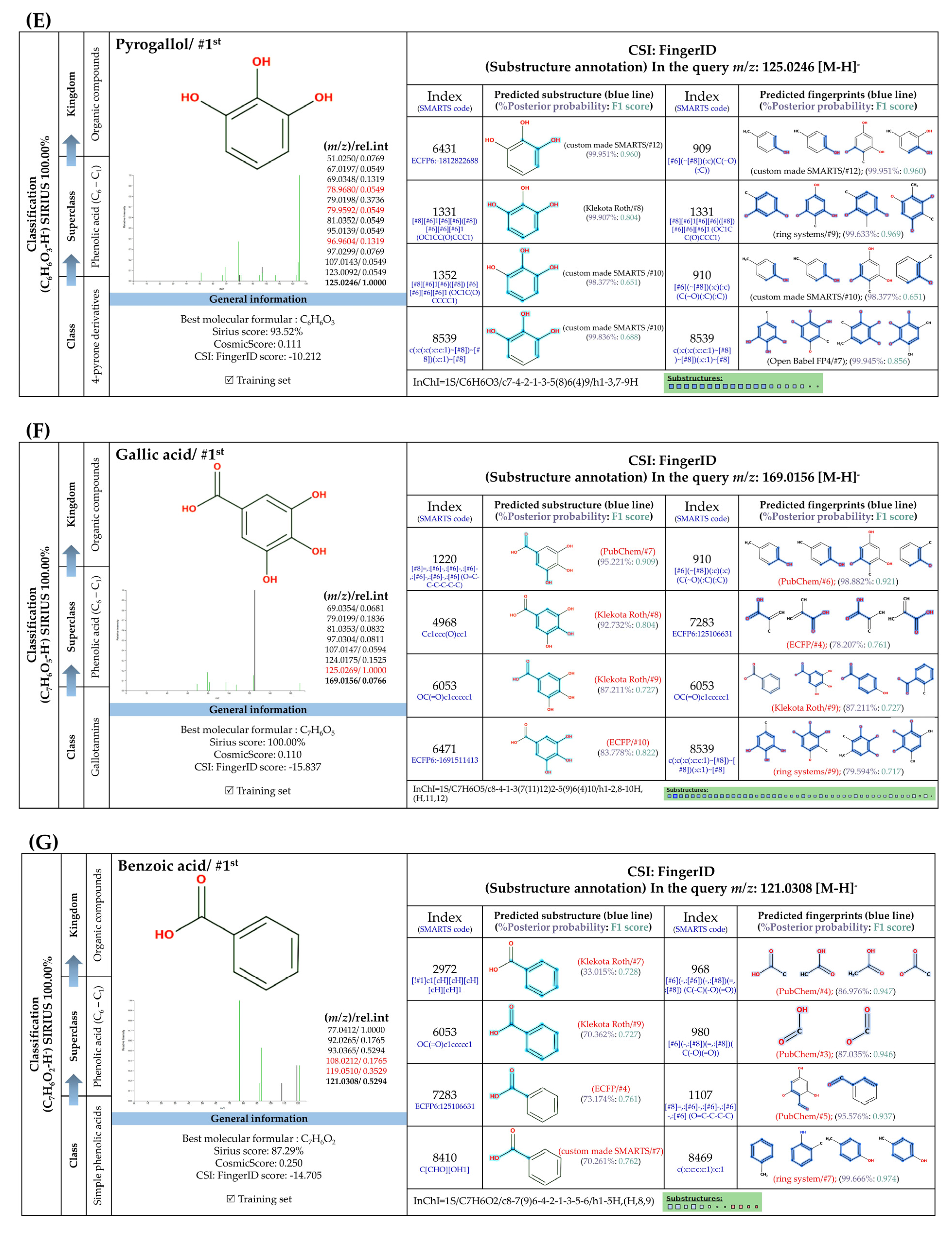
3.5.2. Methylated Analogs
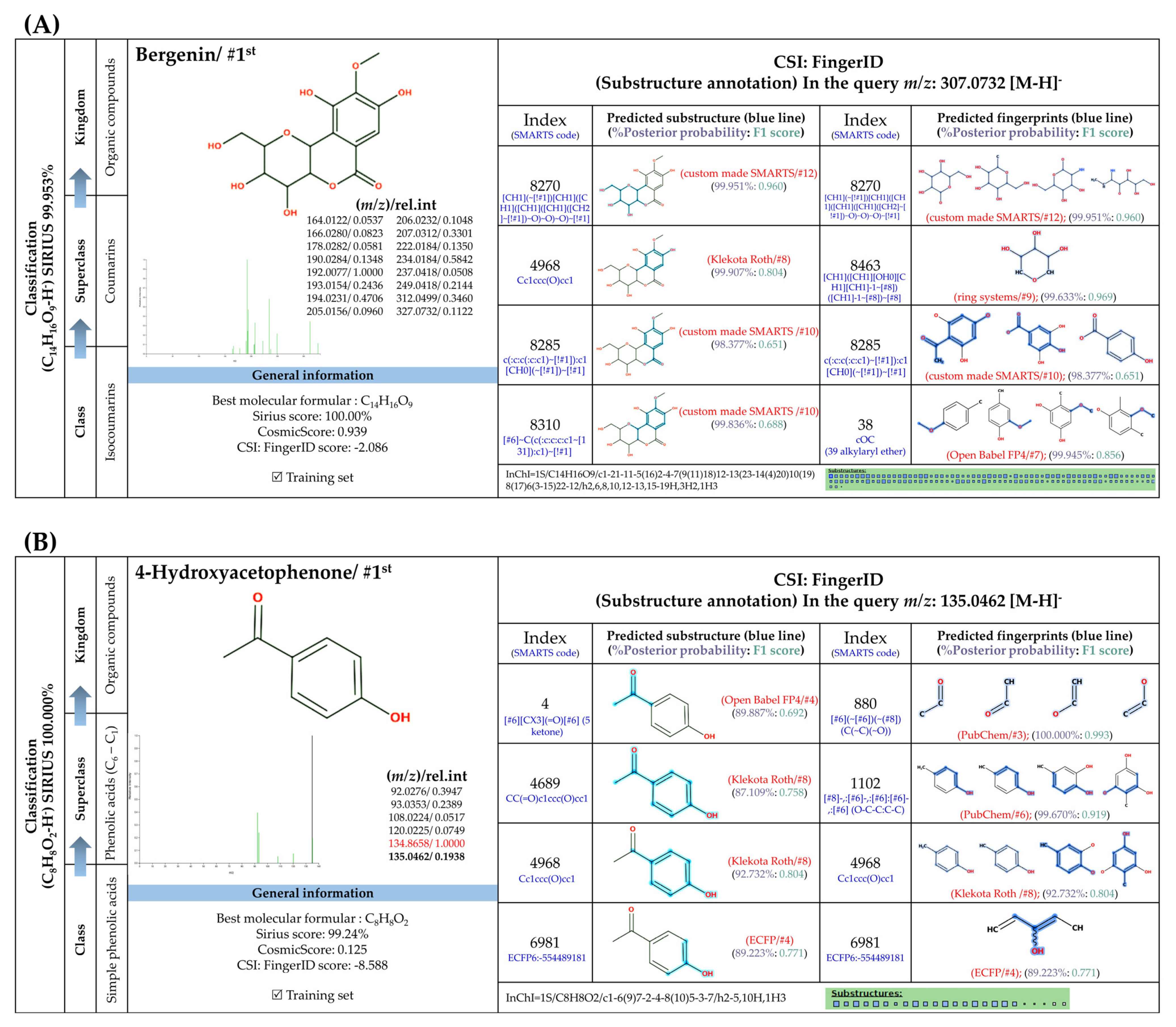
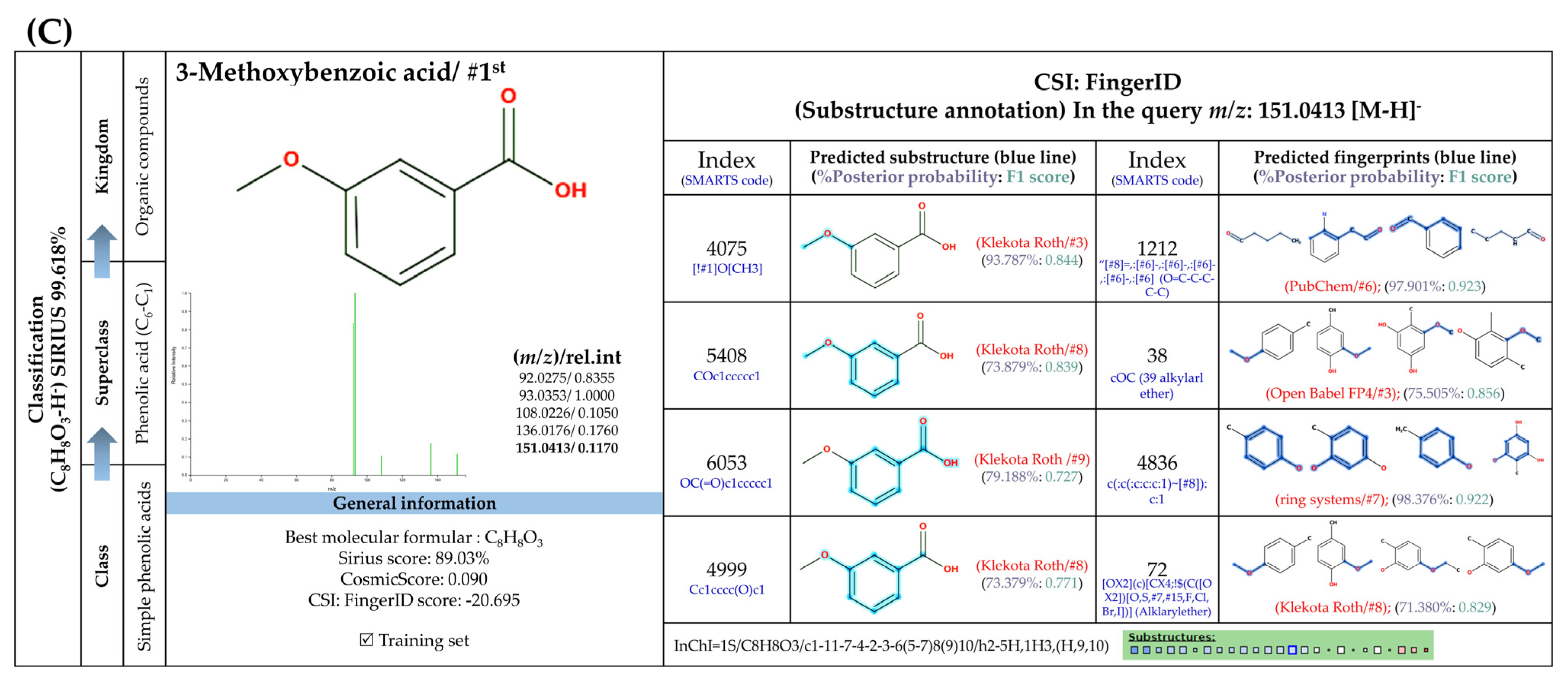
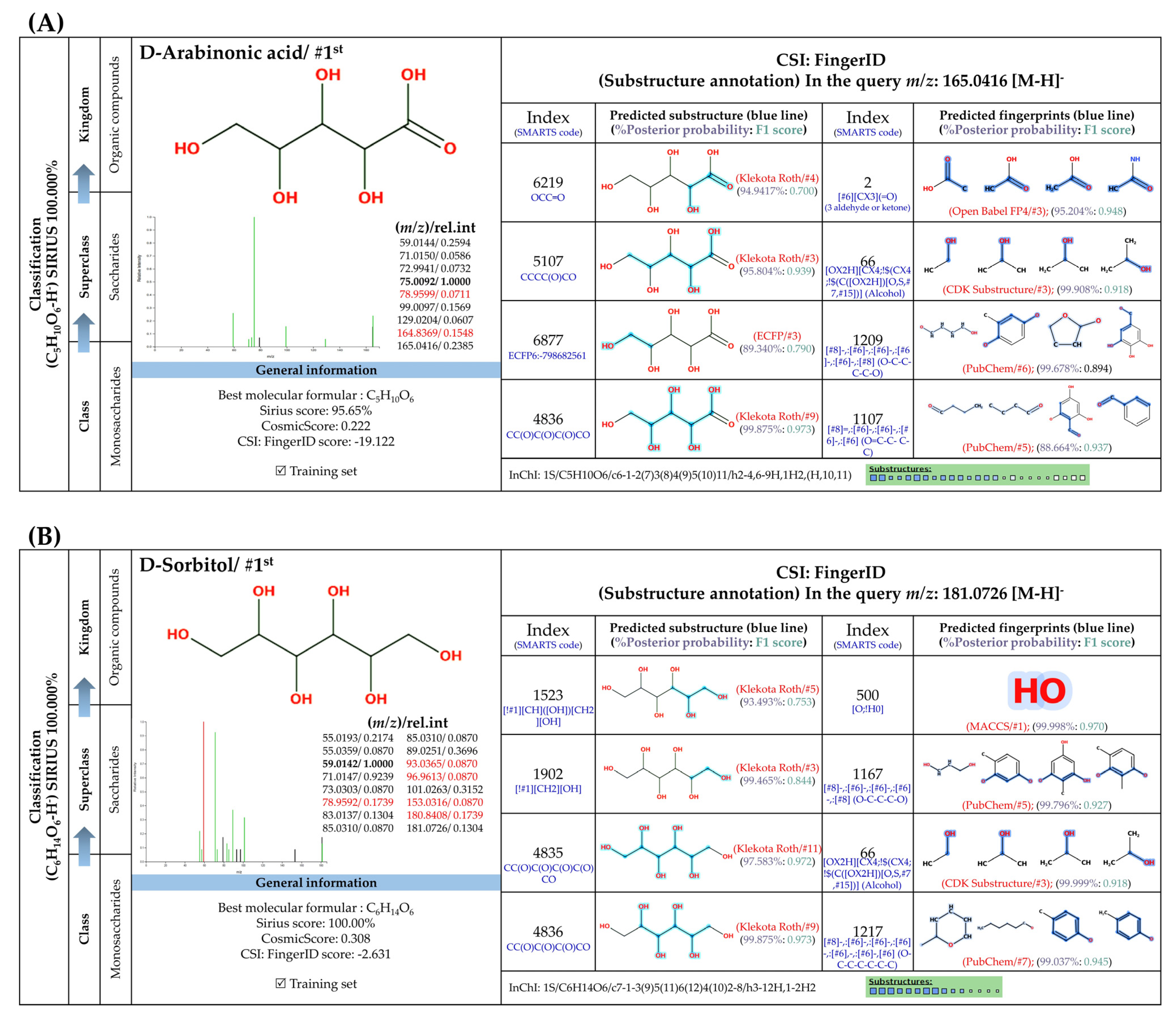
3.5.3. Sugar Derivatives
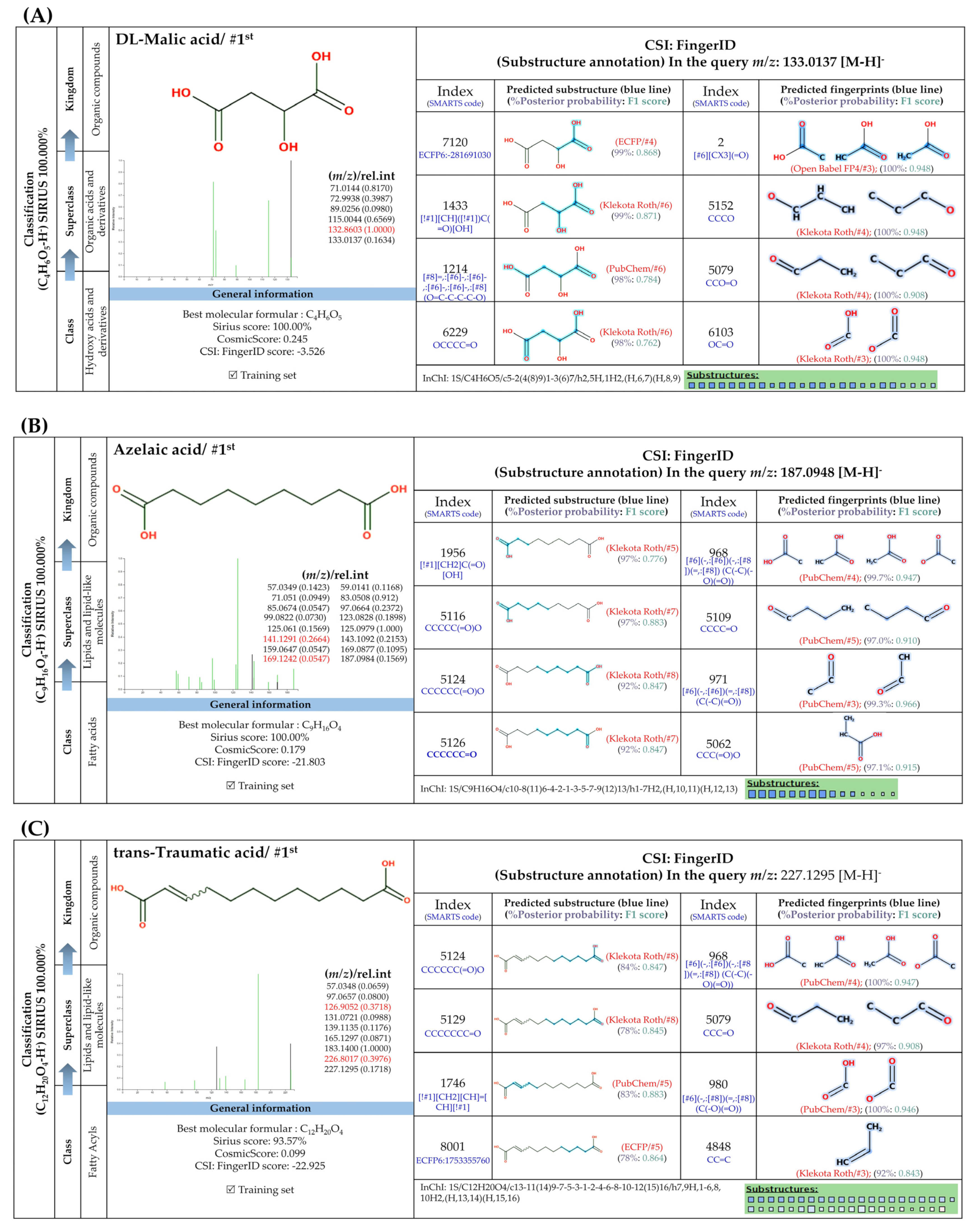
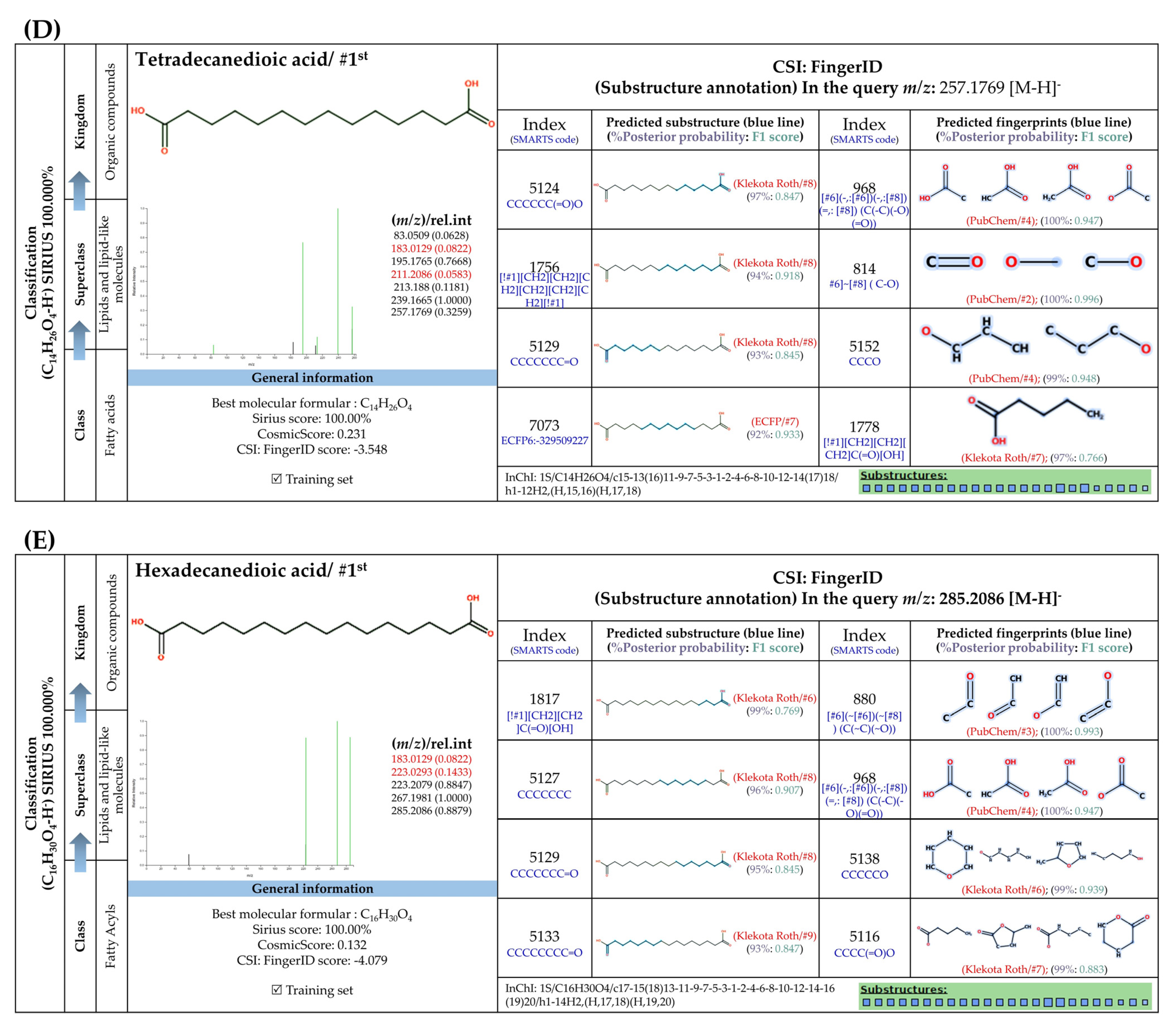
3.5.4. Dicarboxylic Acids
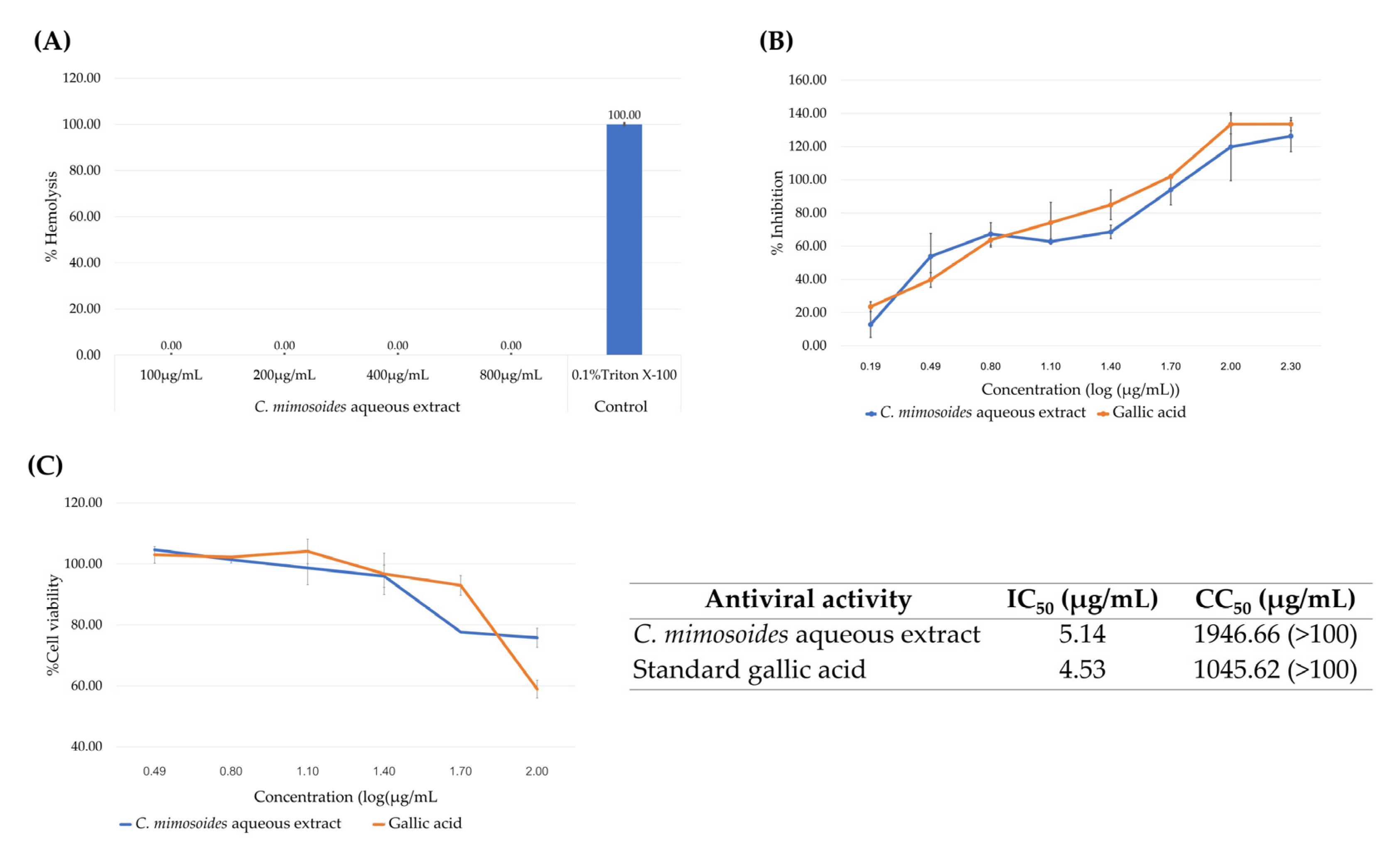
3.6. Antiviral and Hemolytic Activity of the C. mimosoides Aqueous Extract
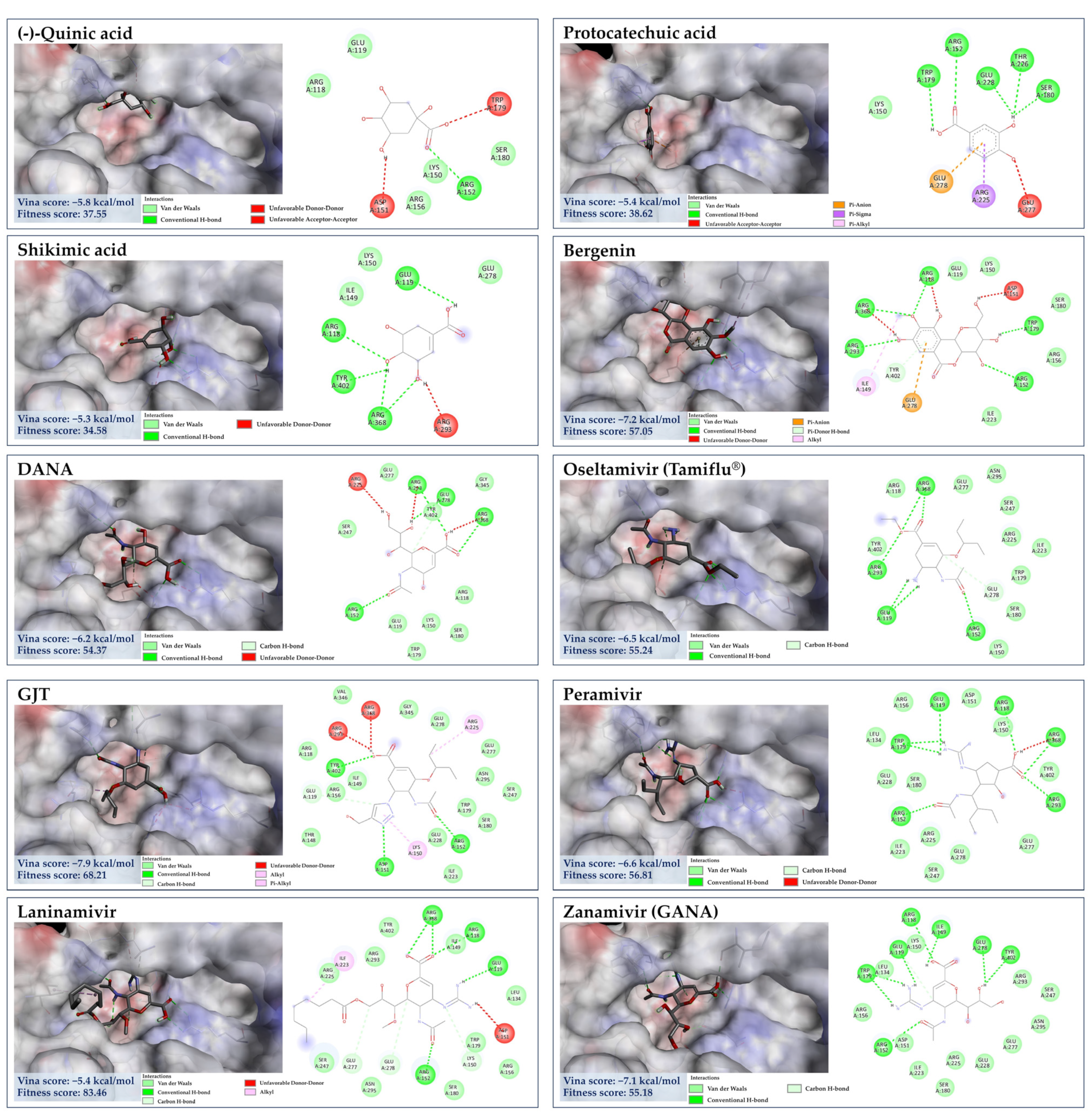
3.7. Molecular Docking for Anti-Influenza Activity
3.7.1. Target Specificity towards Viral Surface Proteins
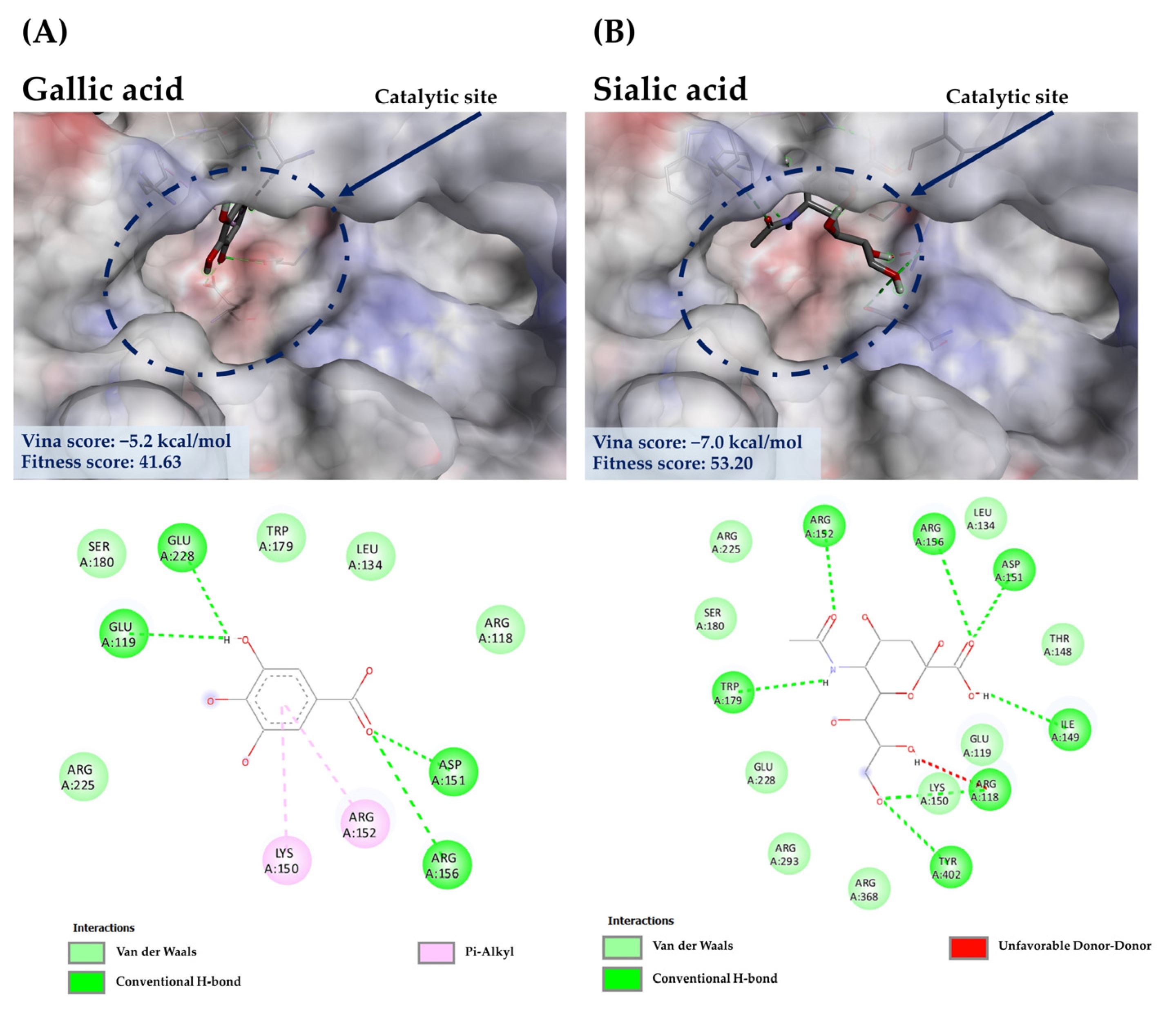
3.7.2. Molecular Docking of GA against NA
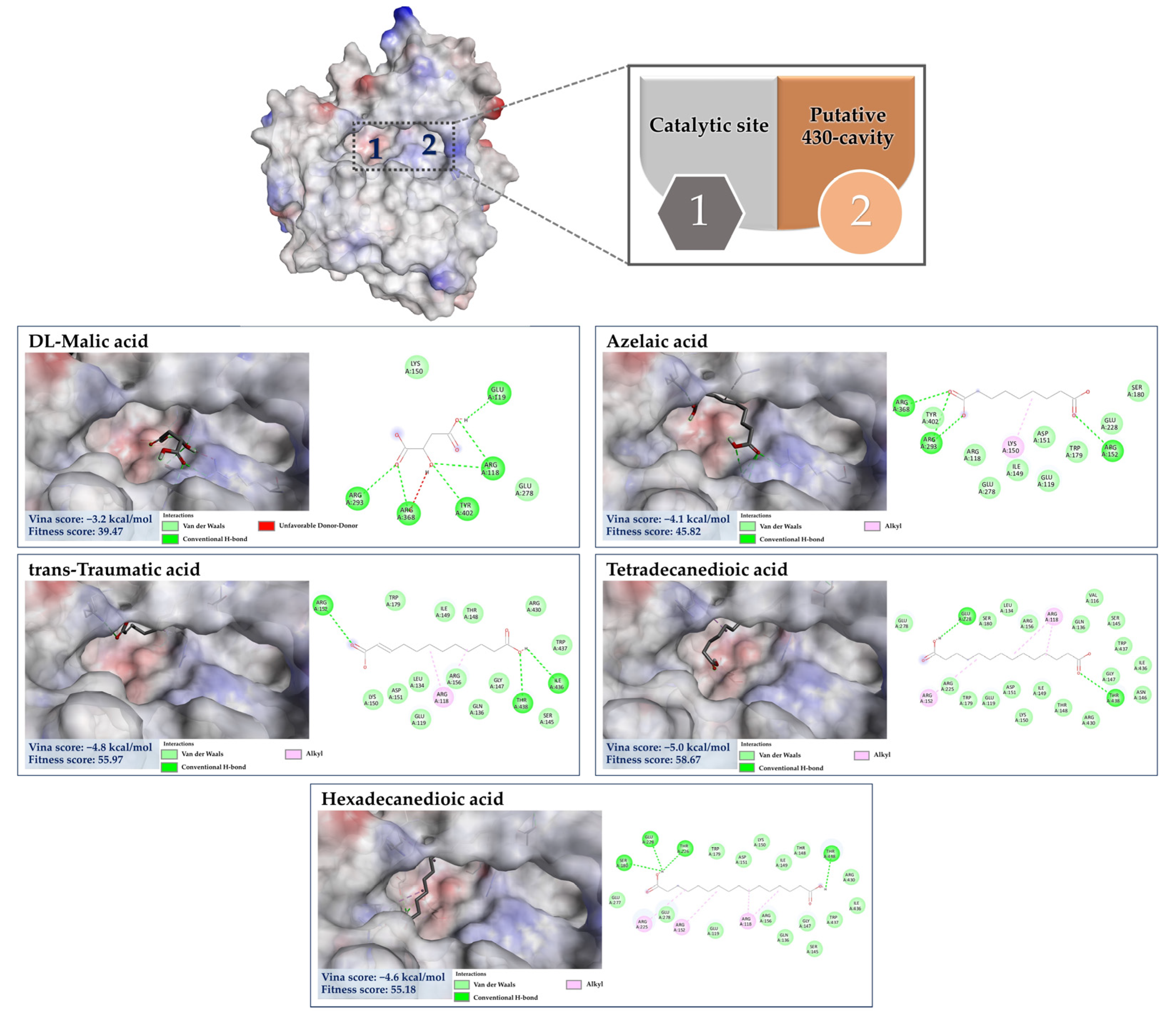
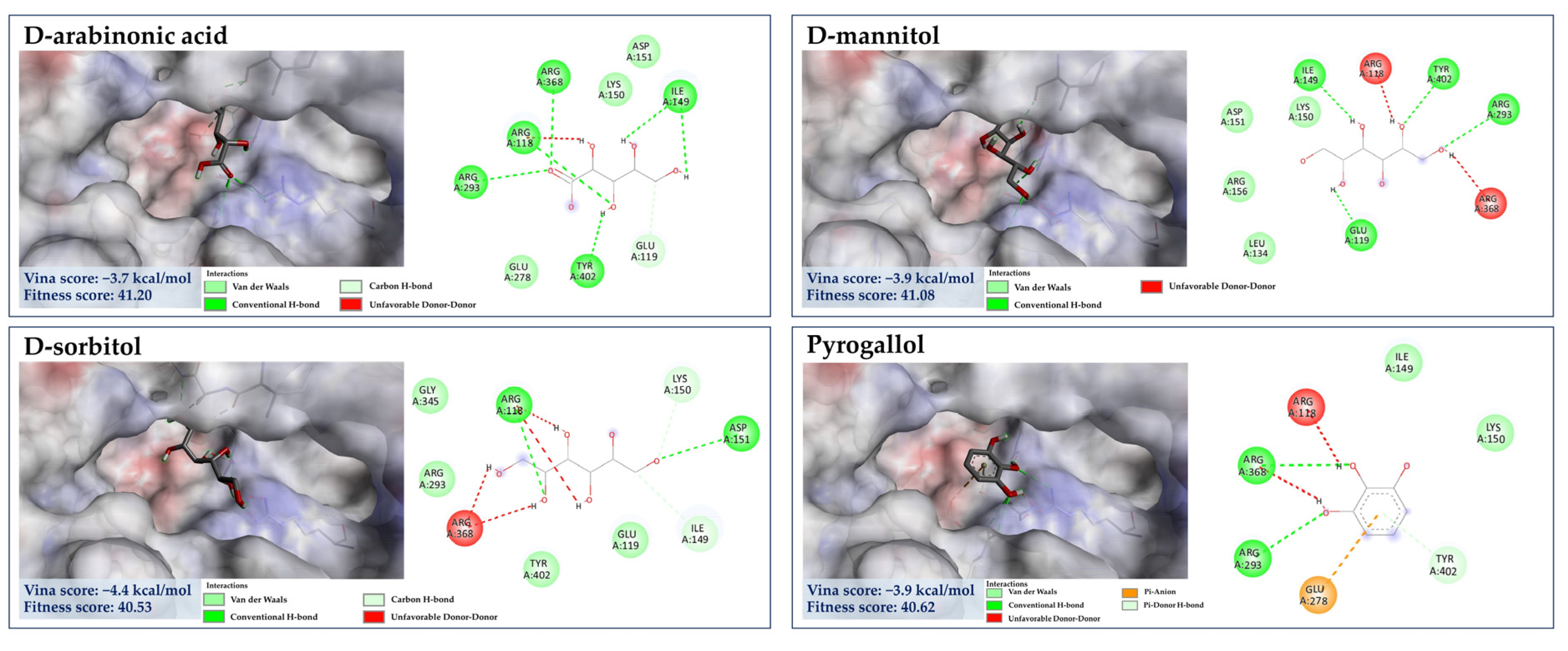
3.7.3. Molecular Docking of the Minor Constituents against NA Catalytic Region
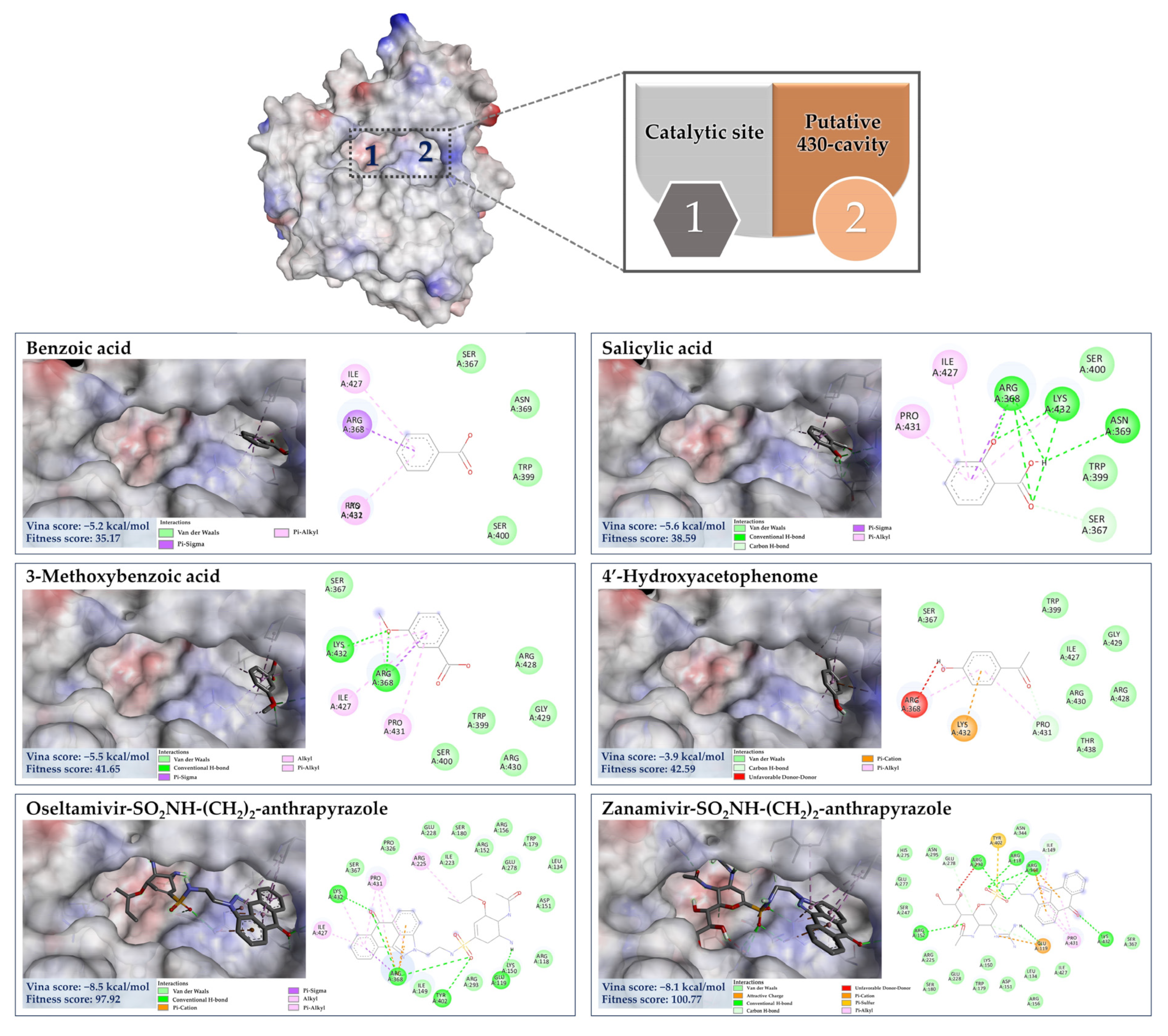
3.7.4. Molecular Docking of the Minor Constituents against the 430-Cavity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mukhtar, M.; Arshad, M.; Ahmad, M.; Pomerantz, R.J.; Wigdahl, B.; Parveen, Z. Antiviral Potentials of Medicinal Plants. Virus Res. 2008, 131, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.H.; Alamri, S.A.; Al-Whaibi, M.H.; Hussain, Z.; Ali, H.M.; El-Zaidy, M.E. A Mini-Review of Anti-Hepatitis B Virus Activity of Medicinal Plants. Biotechnol. Biotechnol. Equip. 2017, 31, 9–15. [Google Scholar] [CrossRef]
- Ganjhu, R.K.; Mudgal, P.P.; Maity, H.; Dowarha, D.; Devadiga, S.; Nag, S.; Arunkumar, G. Herbal Plants and Plant Preparations as Remedial Approach for Viral Diseases. Virusdisease 2015, 26, 225–236. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Denaro, M.; Smeriglio, A.; Barreca, D.; De Francesco, C.; Occhiuto, C.; Milano, G.; Trombetta, D. Antiviral Activity of Plants and Their Isolated Bioactive Compounds: An Update. Phytother. Res. 2020, 34, 742–768. [Google Scholar] [CrossRef]
- Naseer, S.; Hussain, S.; Naeem, N.; Pervaiz, M.; Rahman, M. The Phytochemistry and Medicinal Value of Psidium guajava (Guava). Clin. Phytosci. 2018, 4, 32. [Google Scholar] [CrossRef]
- Chattopadhyay, D.; Sarkar, M.C.; Chatterjee, T.; Sharma Dey, R.; Bag, P.; Chakraborti, S.; Khan, M.T.H. Recent Advancements for the Evaluation of Antiviral Activities of Natural Products. New Biotechnol. 2009, 25, 347–368. [Google Scholar] [CrossRef]
- Dhama, K.; Karthik, K.; Khandia, R.; Munjal, A.; Tiwari, R.; Rana, R.; Khurana, S.K.; Ullah, S.; Khan, R.U.; Alagawany, M.; et al. Medicinal and Therapeutic Potential of Herbs and Plant Metabolites/Extracts Countering Viral Pathogens—Current Knowledge and Future Prospects. Curr. Drug Metab. 2018, 19, 236–263. [Google Scholar] [CrossRef]
- Chojnacka, K.; Skrzypczak, D.; Izydorczyk, G.; Witek-Krowiak, A.; Mikula, K.; Szopa, D. Antiviral Properties of Polyphenols from Plants. Foods 2021, 10, 2277. [Google Scholar] [CrossRef]
- Yang, Z.F.; Bai, L.P.; Huang, W.B.; Li, X.Z.; Zhao, S.S.; Zhong, N.S.; Jiang, Z.H. Comparison of in Vitro Antiviral Activity of Tea Polyphenols against Influenza A and B Viruses and Structure–Activity Relationship Analysis. Fitoterapia 2014, 93, 47–53. [Google Scholar] [CrossRef]
- Kamboj, A.; Solutions, J. Antiviral Activity of Plant Polyphenols. J. Pharm. Res. 2012, 5, 2402–2412. [Google Scholar]
- You, H.L.; Chen, C.J.; Eng, H.L.; Liao, P.L.; Huang, S.T. The Effectiveness and Mechanism of Toona sinensis Extract Inhibit Attachment of Pandemic Influenza A (H1N1) Virus. Evid.-Based Complement. Altern. Med. 2013, 2013, 479718. [Google Scholar] [CrossRef] [PubMed]
- You, H.L.; Huang, C.C.; Chen, C.J.; Chang, C.C.; Liao, P.L.; Huang, S.T. Anti-Pandemic Influenza A (H1N1) Virus Potential of Catechin and Gallic Acid. J. Chin. Med. Assoc. 2018, 81, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Kwon, M.J.; Yoo, J.Y.; Choi, H.J.; Ahn, Y.J. Antiviral Activity and Possible Mode of Action of Ellagic Acid Identified in Lagerstroemia speciosa Leaves toward Human Rhinoviruses. BMC Complement. Altern. Med. 2014, 14, 171. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, J.I.; Lee, I.; Lee, S.; Hwang, M.W.; Bae, J.Y.; Heo, J.; Kim, D.; Han, S.Z.; Park, M.S. Aronia melanocarpa and Its Components Demonstrate Antiviral Activity against Influenza Viruses. Biochem. Biophys. Res. Commun. 2013, 440, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Gramza-Michałowska, A.; Sidor, A.; Kulczyński, B. Berries as a Potential Anti-Influenza Factor—A Review. J. Funct. Foods 2017, 37, 116–137. [Google Scholar] [CrossRef]
- Devi, A.B.; Sarala, R. Substantial Effect of Phytochemical Constituents against the Pandemic Disease Influenza—A Review. Futur. J. Pharm. Sci. 2021, 7, 120. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, P.; Lante, A. Environmentally Friendly Techniques for the Recovery of Polyphenols from Food By-Products and Their Impact on Polyphenol Oxidase: A Critical Review. Appl. Sci. 2022, 12, 1923. [Google Scholar] [CrossRef]
- Pagano, I.; Campone, L.; Celano, R.; Piccinelli, A.L.; Rastrelli, L. Green Non-Conventional Techniques for the Extraction of Polyphenols from Agricultural Food by-Products: A Review. J. Chromatogr. A 2021, 1651, 462295. [Google Scholar] [CrossRef]
- Panja, P. Green Extraction Methods of Food Polyphenols from Vege Materials. Curr. Opin. Food Sci. 2018, 23, 173–182. [Google Scholar] [CrossRef]
- Smitinand, T.; Larsen, K. Flora of Thailand; Applied Scientific Research Corporation of Thailand: Bangkok, Thailand, 1970; ISBN 9786163162885. [Google Scholar]
- Jacquat, C.; Bertossa, G. Plants from the Markets of Thailand, 1st ed.; Editions Duang Kamol: Bangkok, Thailand, 1990; ISBN 9742105065. [Google Scholar]
- Pullaiah, T. Bioactives and Pharmacology of Legumes, 1st ed.; Apple Academic Press: New York, NY, USA, 2023; ISBN 9781000613261. [Google Scholar]
- Tangsaengvit, N.; Kitphati, W.; Tadtong, S.; Bunyapraphatsara, N.; Nukoolkarn, V. Neurite Outgrowth and Neuroprotective Effects of Quercetin from Caesalpinia mimosoides Lamk. on Cultured P19-Derived Neurons. Evid.-Based Complement. Altern. Med. 2013, 2013, 838051. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, B.O.; David, J.M. Chemical Composition, Biological Activities and Traditional Uses of Plants from the Segregated Genus Caesalpinia sensu Lato. Phytochem. Rev. 2023, in press. [Google Scholar] [CrossRef]
- Bhat, P.; Upadhya, V.; Hegde, G.R.; Hegde, H.V.; Roy, S. Attenuation of Dermal Wounds through Topical Application of Ointment Containing Phenol Enriched Fraction of Caesalpinia mimosoides Lam. Front. Pharmacol. 2022, 13, 1025848. [Google Scholar] [CrossRef] [PubMed]
- Palasap, A.; Limpaiboon, T.; Boonsiri, P.; Thapphasaraphong, S.; Daduang, S.; Suwannalert, P.; Daduang, J. Cytotoxic Effects of Phytophenolics from Caesalpinia mimosoides Lamk on Cervical Carcinoma Cell Lines through an Apoptotic Pathway. Asian Pac. J. Cancer Prev. 2014, 15, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Bhat, P.; Patil, V.S.; Anand, A.; Bijjaragi, S.; Hegde, G.R.; Hegde, H.V.; Roy, S. Ethyl Gallate Isolated from Phenol-Enriched Fraction of Caesalpinia mimosoides Lam. Promotes Cutaneous Wound Healing: A Scientific Validation through Bioassay-Guided Fractionation. Front. Pharmacol. 2023, 14, 1214220. [Google Scholar] [CrossRef] [PubMed]
- Rangsinth, P.; Prasansuklab, A.; Duangjan, C.; Gu, X.; Meemon, K.; Wink, M.; Tencomnao, T. Leaf Extract of Caesalpinia mimosoides Enhances Oxidative Stress Resistance and Prolongs Lifespan in Caenorhabditis elegans. BMC Complement. Altern. Med. 2019, 19, 164. [Google Scholar] [CrossRef] [PubMed]
- Baldim Zanin, J.L.; De Carvalho, B.A.; Martineli, P.S.; Dos Santos, M.H.; Lago, J.H.G.; Sartorelli, P.; Viegas, C.; Soares, M.G. The Genus Caesalpinia L. (Caesalpiniaceae): Phytochemical and Pharmacological Characteristics. Molecules 2012, 17, 7887–7902. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.; Park, S.J.; Song, J.H.; Ko, H.J.; Kim, S.H. Chemical Components from the Twigs of Caesalpinia latisiliqua and Their Antiviral Activity. J. Nat. Med. 2020, 74, 26–33. [Google Scholar] [CrossRef]
- Rattanata, N.; Klaynongsruang, S.; Daduang, S.; Tavichakorntrakool, R.; Limpaiboon, T.; Lekphrom, R.; Boonsiri, P.; Daduang, J. Inhibitory Effects of Gallic Acid Isolated from Caesalpinia mimosoides Lamk on Cholangiocarcinoma Cell Lines and Foodborne Pathogenic Bacteria. Asian Pac. J. Cancer Prev. 2016, 17, 1341–1345. [Google Scholar] [CrossRef]
- Kiselova-Kaneva, Y.; Galunska, B.; Nikolova, M.; Dincheva, I.; Badjakov, I. High Resolution LC-MS/MS Characterization of Polyphenolic Composition and Evaluation of Antioxidant Activity of Sambucus ebulus Fruit Tea Traditionally Used in Bulgaria as a Functional Food. Food Chem. 2022, 367, 130759. [Google Scholar] [CrossRef]
- Wu, A.H.B.; Gerona, R.; Armenian, P.; French, D.; Petrie, M.; Lynch, K.L. Role of Liquid Chromatography–High-Resolution Mass Spectrometry (LC-HR/MS) in Clinical Toxicology. Clin. Toxicol. 2012, 50, 733–742. [Google Scholar] [CrossRef]
- Lebel, P.; Waldron, K.; Furtos, A. Rapid Determination of 24 Synthetic and Natural Cannabinoids for LC–MS-MS Screening in Natural Products and Drug Inspection Applications. LCGC Suppl. 2015, 13, 8–14. [Google Scholar]
- Hoffmann, T.; Krug, D.; Hüttel, S.; Müller, R. Improving Natural Products Identification through Targeted LC-MS/MS in an Untargeted Secondary Metabolomics Workflow. Anal. Chem. 2014, 86, 10780–10788. [Google Scholar] [CrossRef] [PubMed]
- Dührkop, K.; Shen, H.; Meusel, M.; Rousu, J.; Böcker, S. Searching Molecular Structure Databases with Tandem Mass Spectra Using CSI:FingerID. Proc. Natl. Acad. Sci. USA 2015, 112, 12580–12585. [Google Scholar] [CrossRef] [PubMed]
- Dührkop, K.; Fleischauer, M.; Ludwig, M.; Aksenov, A.A.; Melnik, A.V.; Meusel, M.; Dorrestein, P.C.; Rousu, J.; Böcker, S. SIRIUS 4: A Rapid Tool for Turning Tandem Mass Spectra into Metabolite Structure Information. Nat. Methods 2019, 16, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Dührkop, K.; Nothias, L.F.; Fleischauer, M.; Reher, R.; Ludwig, M.; Hoffmann, M.A.; Petras, D.; Gerwick, W.H.; Rousu, J.; Dorrestein, P.C.; et al. Systematic Classification of Unknown Metabolites Using High-Resolution Fragmentation Mass Spectra. Nat. Biotechnol. 2021, 39, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Ruttkies, C.; Schymanski, E.L.; Wolf, S.; Hollender, J.; Neumann, S. MetFrag Relaunched: Incorporating Strategies beyond in Silico Fragmentation. J. Cheminform. 2016, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ren, X.; Wu, J.; Li, H.; Yang, L.; Zhang, Y.; Wang, X.; Li, Z. Protocatechuic Acid Protects Mice from Influenza A Virus Infection. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Su, Y.; Li, J.; Lu, Y.; Mei, X.; Wang, J. Integration of LC/MS-Based Molecular Networking and Molecular Docking Allows in-Depth Annotation and Prediction of the Metabolome: A Study of Salvia miltiorrhiza Bunge. Ind. Crops Prod. 2022, 186, 115298. [Google Scholar] [CrossRef]
- Bhattarai, K.; Paudel, B.; Dahal, S.; Yadav, P.; Aryal, N.; Baral, B.; Bhattarai, H.D. Bioprospecting the Metabolome of Plant Urtica dioica, L.: A Fast Dereplication and Annotation Workflow in Plant Metabolomics. Evid.-Based Complement. Altern. Med. 2022, 2022, 3710791. [Google Scholar] [CrossRef]
- Folin, O.; Ciocalteu, V. On Tyrosine and Tryptophane Determinations in Proteins. J. Biol. Chem. 1927, 73, 627–650. [Google Scholar] [CrossRef]
- Lamuela-Raventós, R.M. Folin–Ciocalteu Method for the Measurement of Total Phenolic Content and Antioxidant Capacity. Meas. Antioxid. Act. Capacit. Recent. Trends Appl. 2017, 107–115. [Google Scholar] [CrossRef]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for Antioxidant Assays for Food Components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 2018, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Erviana, R.; Saengkun, Y.; Rungsa, P.; Jangpromma, N.; Tippayawat, P.; Klaynongsruang, S.; Daduang, J.; Daduang, S. Novel Antimicrobial Peptides from a Cecropin-like Region of Heteroscorpine-1 from Heterometrus laoticus Venom with Membrane Disruption Activity. Molecules 2021, 26, 5872. [Google Scholar] [CrossRef] [PubMed]
- Zohra, M.; Fawzia, A. Hemolytic Activity of Different Herbal Extracts Used in Algeria. Int. J. Pharma Sci. Res. 2014, 5, 495–500. [Google Scholar]
- GOLD User Guide. Available online: https://www.ccdc.cam.ac.uk/media/Documentation/0C5D99BC-7CC3-49B6-8319-06BEA8CA342D/GOLD_User_Guide_2020_1.pdf (accessed on 12 September 2023).
- Elswaifi, S.F.; Palmieri, J.R.; Hockey, K.S.; Rzigalinski, B.A. Antioxidant Nanoparticles for Control of Infectious Disease. Infect. Disord. Drug Targets 2012, 9, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Choe, U.; Li, Y.; Liu, Z.; Yao, Y.; He, X.; Sun, J.; Wu, X.; Pehrsson, P.; Zhang, Y.; Gao, B.; et al. Chemical Compositions of Dill (Anethum graveolens L.) Water and Ethanol Extracts and Their Potential in Blocking the SARS-CoV-2 Entry into Cells and Free Radical Scavenging Capacities. ACS Food Sci. Technol. 2023, 3, 1654–1662. [Google Scholar] [CrossRef]
- Nováková, L.; Vildová, A.; Mateus, J.P.; Gonalves, T.; Solich, P. Development and Application of UHPLC–MS/MS Method for the Determination of Phenolic Compounds in Chamomile Flowers and Chamomile Tea Extracts. Talanta 2010, 82, 1271–1280. [Google Scholar] [CrossRef]
- Bataglion, G.A.; Da Silva, F.M.A.; Eberlin, M.N.; Koolen, H.H.F. Determination of the Phenolic Composition from Brazilian Tropical Fruits by UHPLC–MS/MS. Food Chem. 2015, 180, 280–287. [Google Scholar] [CrossRef]
- Singh, A.P.; Wilson, T.; Luthria, D.; Freeman, M.R.; Scott, R.M.; Bilenker, D.; Shah, S.; Somasundaram, S.; Vorsa, N. LC-MS–MS Characterisation of Curry Leaf Flavonols and Antioxidant Activity. Food Chem. 2011, 127, 80–85. [Google Scholar] [CrossRef]
- Wu, S.; Chen, W.; Lu, S.; Zhang, H.; Yin, L. Metabolic Engineering of Shikimic Acid Biosynthesis Pathway for the Production of Shikimic Acid and Its Branched Products in Microorganisms: Advances and Prospects. Molecules 2022, 27, 4779. [Google Scholar] [CrossRef] [PubMed]
- Soto-Hernández, M.; García-Mateos, R.; Palma-Tenango, M.; Soto-Hernández, M.; García-Mateos, R.; Palma-Tenango, M. Plant Physiological Aspects of Phenolic Compounds; IntechOpen: London, UK, 2019; ISBN 978-1-78984-034-6. [Google Scholar]
- Koirala, N.; Thuan, N.H.; Ghimire, G.P.; Van Thang, D.; Sohng, J.K. Methylation of Flavonoids: Chemical Structures, Bioactivities, Progress and Perspectives for Biotechnological Production. Enzym. Microb. Technol. 2016, 86, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Meena, M.; Prasad, V.; Zehra, A.; Gupta, V.K.; Upadhyay, R.S. Mannitol Metabolism during Pathogenic Fungal-Host Interactions under Stressed Conditions. Front. Microbiol. 2015, 6, 1019. [Google Scholar] [CrossRef] [PubMed]
- Kausar, S.; Said Khan, F.; Ishaq Mujeeb Ur Rehman, M.; Akram, M.; Riaz, M.; Rasool, G.; Hamid Khan, A.; Saleem, I.; Shamim, S.; Malik, A. A Review: Mechanism of Action of Antiviral Drugs. Int. J. Immunopathol. Pharmacol. 2021, 35, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Evteev, S.; Nilov, D.; Polenova, A.; Švedas, V. Bifunctional Inhibitors of Influenza Virus Neuraminidase: Molecular Design of a Sulfonamide Linker. Int. J. Mol. Sci. 2021, 22, 13112. [Google Scholar] [CrossRef] [PubMed]
- McAuley, J.L.; Gilbertson, B.P.; Trifkovic, S.; Brown, L.E.; McKimm-Breschkin, J.L. Influenza Virus Neuraminidase Structure and Functions. Front. Microbiol. 2019, 10, 432609. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Ulrich-Merzenich, G. Synergy Research: Approaching a New Generation of Phytopharmaceuticals. Phytomedicine 2009, 16, 97–110. [Google Scholar] [CrossRef]
- Caesar, L.K.; Cech, N.B.; Kubanek, J.; Linington, R.; Luesch, H. Synergy and Antagonism in Natural Product Extracts: When 1 + 1 Does Not Equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Z.; Li, S.; Ye, X.; Li, X.; He, K. Synergy Effects of Herb Extracts: Pharmacokinetics and Pharmacodynamic Basis. Fitoterapia 2014, 92, 133–147. [Google Scholar] [CrossRef]
- Rhee, C.H.; Kang, Y.E.; Han, B.; Kim, Y.W.; Her, M.; Jeong, W.; Kim, S. Virucidal Efficacy of Seven Active Substances in Commercial Disinfectants Used against H9N2 Low Pathogenic Avian Influenza Virus. J. Appl. Poult. Res. 2021, 30, 100198. [Google Scholar] [CrossRef]
- Tesch, M.L.; Westover, J.B.; Sanchez-Gonzalez, M.A.; Rahaghi, F.F. Exploring Chemotherapeutic Agents as Countermeasures against Respiratory Viruses: Antiviral Potential of Sugar Alcohols. Med. Res. Arch. 2023, 11, 1–13. [Google Scholar] [CrossRef]
- Mandai, T.; Oshitari, T. Efficient Asymmetric Synthesis of Oseltamivir from D-Mannitol. Synlett 2009, 2009, 783–786. [Google Scholar] [CrossRef]
- Singh, P.; Gupta, E.; Mishra, N.; Mishra, P. Shikimic Acid as Intermediary Model for the Production of Drugs Effective against Influenza Virus. Phytochem. Lead. Compd. New Drug Discov. 2020, 245–256. [Google Scholar] [CrossRef]
- Mahal, A.; Duan, M.; Zinad, D.S.; Mohapatra, R.K.; Obaidullah, A.J.; Wei, X.; Pradhan, M.K.; Das, D.; Kandi, V.; Zinad, H.S.; et al. Recent Progress in Chemical Approaches for the Development of Novel Neuraminidase Inhibitors. RSC Adv. 2021, 11, 1804–1840. [Google Scholar] [CrossRef] [PubMed]
- Chintakrindi, A.S.; Gohil, D.J.; Chowdhary, A.S.; Kanyalkar, M.A. Design, Synthesis and Biological Evaluation of Substituted Flavones and Aurones as Potential Anti-Influenza Agents. Bioorg. Med. Chem. 2020, 28, 115191. [Google Scholar] [CrossRef] [PubMed]
- Atigadda, V.R.; Brouillette, W.J.; Duarte, F.; Babu, Y.S.; Bantia, S.; Chand, P.; Chu, N.; Montgomery, J.A.; Walsh, D.A.; Sudbeck, E.; et al. Hydrophobic Benzoic Acids as Inhibitors of Influenza Neuraminidase. Bioorg. Med. Chem. 1999, 7, 2487–2497. [Google Scholar] [CrossRef]
- Guo, M.; Ni, J.; Yu, J.; Jin, J.; Ma, L.; Zhang, H.; Wang, D.; Zhang, X.; Dou, J.; Zhou, C. Antiviral Activity of Benzoic Acid Derivative NC-5 Against Influenza A Virus and Its Neuraminidase Inhibition. Int. J. Mol. Sci. 2019, 20, 6261. [Google Scholar] [CrossRef]
- Singh, M.; Jha, A.; Kumar, A.; Hettiarachchy, N.; Rai, A.K.; Sharma, D. Influence of the Solvents on the Extraction of Major Phenolic Compounds (Punicalagin, Ellagic Acid and Gallic Acid) and Their Antioxidant Activities in Pomegranate aril. J. Food Sci. Technol. 2014, 51, 2070–2077. [Google Scholar] [CrossRef]
- Dewick, P.M. The Shikimate Pathway: Aromatic Amino Acids and Phenylpropanoids. In Medicinal Natural Products: A Biosynthetic Approach; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; pp. 137–186. [Google Scholar]
- Candeias, N.R.; Assoah, B.; Simeonov, S.P. Production and Synthetic Modifications of Shikimic Acid. Chem. Rev. 2018, 118, 10458–10550. [Google Scholar] [CrossRef]
- Estevez, M.A.; Estevez, J.R. A Short Overview on the Medicinal Chemistry of (—)-Shikimic Acid. Mini-Rev. Med. Chem. 2012, 12, 1443–1454. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.M.; Sainsbury, M.; Searle, P.A. The Biosynthesis and Synthesis of Shikimic Acid, Chorismic Acid, and Related Compounds. Synthesis 1993, 1993, 179–193. [Google Scholar] [CrossRef]
- Blaženović, I.; Kind, T.; Ji, J.; Fiehn, O. Software Tools and Approaches for Compound Identification of LC-MS/MS Data in Metabolomics. Metabolites 2018, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, D.L.; Lima, E.T.G.; Silva, J.C.; Medeiros, M.A.; Pinheiro, E.B.F. Rhamnetin: A Review of Its Pharmacology and Toxicity. J. Pharm. Pharmacol. 2022, 74, 793–799. [Google Scholar] [CrossRef]
- Lemoui, R.; Benyahia, S.; Noman, L.; Bencherchar, I.; Oke-Altuntas, F.; Rebbas, K.; Benayache, S.; Benayache, F.; Demirtas, I. Isolation of Phytoconstituents and Evaluation of Biological Potentials of Berberis hispanica from Algeria. Bangladesh J. Pharmacol. 2018, 13, 179–186. [Google Scholar] [CrossRef]
- Bate-Smith, E.C.; Harborne, J.B.; Davenport, S.M. Occurrence of Azaleatin and Caryatin in Eucryphia. Nature 1966, 212, 1065–1066. [Google Scholar] [CrossRef]
- Klamrak, A.; Nabnueangsap, J.; Nualkaew, N. Biotransformation of Benzoate to 2,4,6-Trihydroxybenzophenone by Engineered Escherichia coli. Molecules 2021, 26, 2779. [Google Scholar] [CrossRef] [PubMed]
- Klamrak, A.; Nabnueangsap, J.; Puthongking, P.; Nualkaew, N. Synthesis of Ferulenol by Engineered Escherichia coli: Structural Elucidation by Using the In Silico Tools. Molecules 2021, 26, 6264. [Google Scholar] [CrossRef]
- Zhang, T.; Lo, C.Y.; Xiao, M.; Cheng, L.; Pun Mok, C.K.; Shaw, P.C. Anti-Influenza Virus Phytochemicals from Radix Paeoniae Alba and Characterization of Their Neuraminidase Inhibitory Activities. J. Ethnopharmacol. 2020, 253, 112671. [Google Scholar] [CrossRef]
- Zima, V.; Albiñana, C.B.; Rojíková, K.; Pokorná, J.; Pachl, P.; Řezáčová, P.; Hudlicky, J.; Navrátil, V.; Majer, P.; Konvalinka, J.; et al. Investigation of Flexibility of Neuraminidase 150-Loop Using Tamiflu Derivatives in Influenza A Viruses H1N1 and H5N1. Bioorg. Med. Chem. 2019, 27, 2935–2947. [Google Scholar] [CrossRef]
- Uhlendorff, J.; Matrosovich, T.; Klenk, H.D.; Matrosovich, M. Functional Significance of the Hemadsorption Activity of Influenza Virus Neuraminidase and Its Alteration in Pandemic Viruses. Arch. Virol. 2009, 154, 945–957. [Google Scholar] [CrossRef]
- Dai, M.; McBride, R.; Dortmans, J.C.F.M.; Peng, W.; Bakkers, M.J.G.; de Groot, R.J.; van Kuppeveld, F.J.M.; Paulson, J.C.; de Vries, E.; de Haan, C.A.M. Mutation of the Second Sialic Acid-Binding Site, Resulting in Reduced Neuraminidase Activity, Preceded the Emergence of H7N9 Influenza A Virus. J. Virol. 2017, 91, e00049-17. [Google Scholar] [CrossRef]
| Fresh Aerial C. mimosoides Parts (g) | Dried Material (g) | Total Phenolic Content (g) |
|---|---|---|
| 15.00 | 3.11 | 1.71 |
| 551 mg (GAE)/g. dried weight |
| No. | Exp.RT/RT (Minute) | Predicted Metabolite | Neutral Formula | Neutral Mass (m/z) | Precursor Mass (m/z) [M-H]− | Exp. Mass (m/z) [M-H]− | Mass Error (ppm) | Library Score (%) | Relative Peak Area | Relative Peak Area 1 (%) (All Metabolites)/Rank | Relative Peak Area 2 (%) (24 Metabolites Detected)/Rank | Relative Peak Area 3 (%) (18 Metabolites Selected)/Rank |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.71/0.80 | DL-Malic acid | C4H6O5 | 133.87470 | 132.868 | 132.8682 | 1.505253 | 100 | 6.026 × 105 | 0.58/21 | 0.82/10 | 0.87/7 |
| 2 | 0.71/0.88 | Salicylic acid | C7H6O3 | 137.86958 | 136.863 | 136.8630 | 0 | 100 | 1.966 × 105 | 0.19/43 | 0.27/16 | 0.28/11 |
| 3 | 0.71/0.75 | Digalactouronic acid | C12H18O13 | 370.07154 | 369.068 | 369.0684 | 1.083811 | 95.2 | 1.049 × 105 | 0.10/54 | 0.14/23 | - |
| 4 | 0.75/0.77 | D-arabinonic acid | C5H10O6 | 166.04819 | 165.041 | 165.0414 | 2.423640 | 96.8 | 1.690 × 105 | 0.16/47 | 0.23/19 | 0.24/14 |
| 5 | 0.75/0.75 | D-sorbitol | C6H14O6 | 182.07596 | 181.073 | 181.073 | 0 | 95.7 | 2.466 × 105 | 0.24/39 | 0.34/14 | 0.35/10 |
| 6 | 0.75/0.76 | 5-keto-D-gluconic acid | C6H10O7 | 194.04362 | 193.037 | 193.0371 | 0.518000 | 95.5 | 8.989 × 105 | 0.87/14 | 1.22/7 | - |
| 7 | 0.87/0.88 | (-)-Quinic acid | C7H12O6 | 192.06664 | 191.060 | 191.0607 | 3.663771 | 98.7 | 1.301 × 107 | 12.53/2 | 17.73/2 | 18.72/2 |
| 8 | 1.13/1.14 | Shikimic acid | C7H10O5 | 174.05405 | 173.047 | 173.0472 | 1.155755 | 99.4 | 3.766 × 106 | 3.63/5 | 5.13/4 | 5.42/4 |
| 9 | 2.92/2.93 | Pyrogallol | C6H6O3 | 126.03248 | 125.026 | 125.0258 | −1.599667 | 98.9 | 7.780 × 106 | 7.49/4 | 10.60/3 | 11.20/3 |
| 10 | 2.92/2.93 | Gallic acid | C7H6O5 | 170.02842 | 169.022 | 169.0241 | 12.424418 | 96.3 | 3.967 × 107 | 38.21/1 | 54.06/1 | 57.09/1 |
| 11 | 3.53/3.51 | Protocatechuic acid | C7H6O4 | 154.02666 | 153.020 | 153.020 | 0 | 97.6 | 1.146 × 105 | 0.11/50 | 0.16/21 | 0.16/16 |
| 12 | 3.69/3.70 | Bergenin | C14H16O9 | 328.08096 | 327.074 | 327.0743 | 0.917224 | 96.7 | 1.410 × 106 | 1.36/9 | 1.92/5 | 2.03/5 |
| 13 | 4.13/4.09 | 4′-hydroxyacetophenone | C8H8O2 | 136.08886 | 135.082 | 135.0822 | 1.480582 | 83.3 | 7.230 × 105 | 0.70/17 | 0.99/9 | 1.04/6 |
| 14 | 4.13/4.06 | 1,2-Benzenedicarboxylic acid | C13H16O4 | 166.09928 | 165.093 | 165.0926 | −2.422877 | 99.2 | 5.482 × 105 | 0.53/25 | 0.75/13 | - |
| 15 | 4.13/4.08 | Benzoic acid | C7H6O2 | 122.07921 | 121.066 | 121.0663 | 0.247920 | 62.4 | 5.680 × 105 | 0.55/23 | 0.77/11 | 0.82/8 |
| 16 | 4.29/4.30 | Azelaic acid | C9H16O4 | 188.10488 | 187.098 | 187.0982 | 1.068959 | 85.2 | 1.737 × 105 | 0.17/45 | 0.24/17 | 0.25/12 |
| 17 | 4.33/4.31 | Betulinuc acid | C30H48O3 | 456.25676 | 455.250 | 455.2501 | 0.219660 | 100 | 8.733 × 105 | 0.84/15 | 1.19/8 | - |
| 18 | 4.40/4.39 | 3-Methoxybenzoic acid | C8H8O3 | 152.04700 | 151.040 | 151.0402 | 1.324153 | 93.2 | 6.116 × 104 | 0.06/58 | 0.08/24 | 0.09/18 |
| 19 | 4.45/4.58 | trans-Traumatic acid | C12H20O4 | 228.20954 | 227.203 | 227.2021 | −3.961215 | 88.5 | 1.643 × 105 | 0.16/49 | 0.22/20 | 0.24/15 |
| 20 | 4.56/4.64 | 3-Hydroxy-4-methoxycinnamic acid | C10H10O4 | 194.09463 | 193.088 | 193.0878 | −1.035797 | 72.3 | 1.051 × 105 | 0.10/53 | 0.14/22 | 0.15/17 |
| 21 | 4.76/4.73 | Hexadecanedioic acid | C16H30O4 | 286.21477 | 285.208 | 285.2081 | 0.350621 | 99.7 | 1.708 × 105 | 0.16/46 | 0.23/18 | 0.25/13 |
| 22 | 4.84/4.84 | Isorhamatin | C16H12O7 | 316.26016 | 315.253 | 315.2536 | 1.903233 | 88.1 | 1.233 × 106 | 1.19/10 | 1.68/6 | - |
| 23 | 4.84/4.85 | Tetradecanedioic acid | C14H26O4 | 258.18427 | 257.178 | 257.1776 | −1.555343 | 95.9 | 5.536 × 105 | 0.53/24 | 0.75/12 | 0.80/9 |
| 24 | 5.84/5.89 | 3-hydroxy-4-methoxybenzoic acid | C8H8O4 | 167.84063 | 166.834 | 166.8340 | 0 | 84.7 | 2.432 × 105 | 0.23/40 | 0.33/15 | - |
| No. | Retention Time (Minute) | Putative Peak * (m/z; [M-H]−) | Neutral Formula | Database (Rank/Entire Candidate; F1 Score) Monitoring Based on In-House Library | (Number of Peaks) Matched Peaks ● Matched ● Not matched ● Excluded | Candidate Structure ** (InChIKeyBlock1) | |||
|---|---|---|---|---|---|---|---|---|---|
 |  |  |  | ||||||
| 1 | 0.71 | DL-Malic acid (133.0137) | C4H6O5 | (1/4: 1.0) | N.D. | (42/153: 0.8173) | N.D. | (4/4) 71.0144 72.9938 89.0256 115.0044 132.8683 133.0137 | 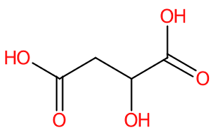 BJEPYKJPYRNKOW |
| 2 | 0.71 | Salicylic acid (136.8630) | C7H6O3 | N.D. | (1/13: 0.8173) | (31/308: 0.6744) | N.A. | (2/2) 65.0402 93.0353 136.8630 | 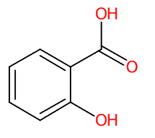 YGSDEFSMJLZEOE |
| 3 | 0.71 | Digalactouronic (369.0684) | N.D. | N.D. | N.D. | N.D. | N.A. | N.D. | N.D. |
| 4 | 0.75 | D-arabinonic acid (165.0414) | C5H10O6 | (1/6: 1.0) | (1/1: 1.0) | (11/112: 0.7562) | N.A. | (5/7) 59.0144 71.0150 72.9941 75.0092 78.9599 99.0097 129.0204 164.8369 165.0416 | 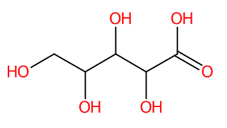 QXKAIJAYHKCRRA |
| 5 | 0.75 | D-sorbitol (181.073) | C6H14O6 | (1/6: 1) | (1/1: 1.0) | (26/185: 0.7835) | N.A. | (4/13) 55.0193 57.0359 59.0142 71.0147 73.0303 78.9592 83.0137 85.0310 89.0251 93.0365 96.9613 101.0263 153.0316 180.8408 181.0726 | 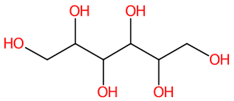 FBPFZTCFMRRESA |
| 6 | 0.75 | 5-keto-D-gluconic acid (193.0371) | N.D. | N.D. | N.D. | N.D. | N.A. | N.D. | N.D. |
| 7 | 0.87 | (-)-Quinic acid (191.0607) | C7H12O6 | (1/5: 1) | (2/4: 0.8985) | (126/702: 1.0) | N.A. | (4/7) 59.0145 85.0303 87.0095 93.0354 109.0304 111.0460 127.0407 191.0583 | 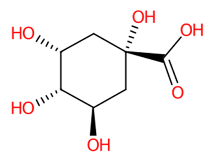 AAWZDTNXLSGCEK |
| 8 | 1.13 | Shikimic acid (173.0472) | C7H10O5 | (5/5: 0.6902) | (4/10: 0.6902) | (193/170: 0.4491) | N.A. | (5/11) 55.0195 71.0147 73.0302 76.9705 81.0352 83.0509 93.0356 111.0459 136.9378 137.0252 154.9485 173.047 | 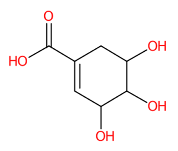 JXOHGGNKMLTUBP |
| 9 | 2.92 | Pyrogallol (125.0258) | C6H6O3 | (5/7: 0.7328) | (7/12: 0.7328) | (216/365: 0.6497) | N.A. | (8/11) 51.0250 67.0197 69.0348 78.9680 79.0198 79.9592 81.0352 95.0139 96.9604 97.0299 107.0143 123.0092 124.0173 125.0246 | 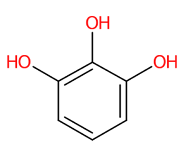 WQGWDDDVZFFDIG |
| 10 | 2.92 | Gallic acid (169.0241) | C7H6O5 | (1/1: 1.0) | (2/2: 1.0) | (89/146: 0.6436) | N.A. | (4/7) 69.0354 79.0199 81.0353 97.0304 107.0147 124.0175 125.0269 169.0156 | 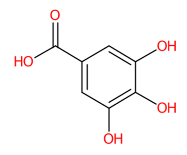 LNTHITQWFMADLM |
| 11 | 3.53 | Protocatechuic acid (153.0200) | C7H6O4 | (2/4: 1.0) | (4/13: 1.0) | (16/325: 0.9806) | N.A. | (3/3) 91.01903 108.0222 109.0300 153.020 | 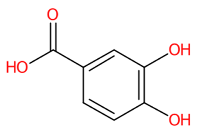 YQUVCSBJEUQKSH |
| 12 | 3.69 | Bergenin (327.0743) | C14H16O9 | (1/2: 1.0) | N.D. | (2/89: 0.9733) | N.A. | (15/15) 164.0122 166.0280 178.0282 190.0284 192.0077 193.0154 194.0231 205.0156 206.0232 207.0312 222.0184 234.0184 237.0418 249.0418 312.0499 327.0732 | 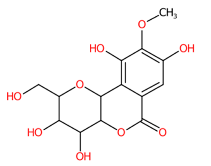 YWJXCIXBAKGUKZ |
| 13 | 4.13 | 4-hydroxyacetophenone (135.0822) | C8H8O2 | (1/15: 1.0) | (2/27: 1.0) | (21/833: 0.7430) | N.A. | (4/4) 92.0276 93.0353 108.0224 120.0225 134.8658 135.0462 | 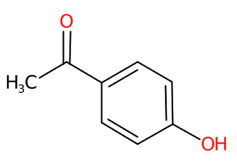 TXFPEBPIARQUIG |
| 14 | 4.13 | 1,2-Benzenedicarboxylic acid (165.0926) | N.D. | N.D. | N.D. | N.D. | N.A. | N.D. | N.D. |
| 15 | 4.13 | Benzoic acid (121.0663) | C7H6O2 | (5/6: 0.7078) | (9/9: 0.6598) | (216/298: 0.5669) | N.D. | (2/4) 77.0412 92.0265 93.0365 108.0212 119.0510 121.0308 121.0653 | 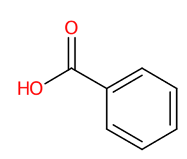 WPYMKLBDIGXBTP WPYMKLBDIGXBTP |
| 16 | 4.29 | Azelaic acid (187.0982) | C9H16O4 | (1/2: 1) | (4/33: 0.8571) | (531/3149: 0.6948) | (1/1: 1.0) | (10/17) 57.03487 59.0141 71.0510 83.0507 85.0673 97.0664 99.0822 123.0828 125.0610 125.0979 141.1291 143.0517 143.1091 159.0646 168.8642 169.0876 169.1241 187.0983 187.1354 | 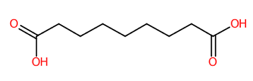 BDJRBEYXGGNYIS |
| 17 | 4.33 | Betulinuc acid (455.2502) | N.D. | N.D. | N.D. | N.D. | N.A. | N.D. | N.D. |
| 18 | 4.40 | 3-Methoxybenzoic acid (151.0402) | C8H8O3 | N.D. | (20/40: 0.7570) | (168/1077: 0.5668) | N.A. | (4/4) 92.0275 93.0353 108.0226 136.0176 151.0061 151.0413 | 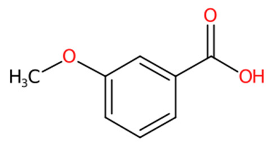 XHQZJYCNDZAGLW |
| 19 | 4.45 | trans-Traumatic acid (227.2021) | C12H20O4 | (1/1: 1.0) | (1/15: 1.0) | (187/3696: 0.8825) | (2/5: 1.0) | (5/7) 57.03475 97.06573 126.9052 131.0720 139.1134 165.12972 183.13998 226.8016 227.0368 227.1295 227.1669 227.2030 | 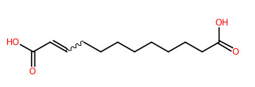 MAZWDMBCPDUFDJ |
| 20 | 4.56 | 3-Hydroxy-4-methoxycinnamic acid (193.0878) | C10H10O4 | (6/9: 1.0) | (15/32: 1.0) | (837/2148: 0.6964) | N.A. | (2/2) 177.0566 178.0643 193.0500 | 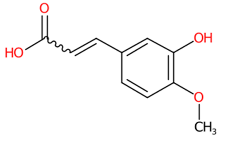 QURCVMIEKCOAJU |
| 21 | 4.76 | Hexadecanedioic acid (285.2081) | C16H30O4 | (2/2: 1.0) | (2/15: 1.0) | (82/1425: 0.8668) | (2/3: 1.0) | (3/4) 59.0142 223.0293 223.2079 267.1981 285.1173 285.2086 | 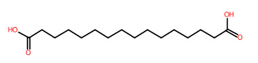 QQHJDPROMQRDLA |
| 22 | 4.83 | Isorhamatin (315.2536) | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 23 | 4.83 | Tetradecanedioic acid (257.1776) | C14H26O4 | N.D. | (1/21: 1.0) | (91/2047: 0.7704) | (1/4: 1.0) | (4/6) 83.0509 183.0129 195.1765 211.2086 213.1880 239.1665 257.0506 257.1769 257.2139 | 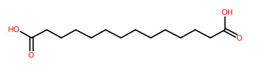 HQHCYKULIHKCEB |
| 24 | 5.84 | 3-hydroxy-4-methoxybenzoic acid (166.8340) | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| No. | Compounds | PubChem CID | HA (PDB: 1RU7) | NA (PDB: 6HP0) | ||
|---|---|---|---|---|---|---|
| Fitness Score (GOLD) | Vina Score (CB Dock) (kcal/mol) | Fitness Score (GOLD) | Vina Score (CB Dock) (kcal/mol) | |||
| C. mimosoides’s metabolites | ||||||
| 1 | Hexadecanedioic acid | 10459 | 53.78 | −3.9 | 55.18 | −4.6 |
| 2 | Tetradecanedioic acid | 13185 | 56.72 | −4.4 | 58.67 | −5.0 |
| 3 | Bergenin | 66065 | 41.19 | −5.7 | 57.05 | −7.2 |
| 4 | trans-Traumatic acid | 5283028 | 50.48 | −4.7 | 55.97 | −4.8 |
| 5 | Azelaic acid | 2266 | 43.83 | −4.2 | 45.82 | −4.1 |
| 6 | Gallic acid | 370 | 36.34 | −4.1 | 41.63 | −5.2 |
| 7 | D-arabinonic acid | 122045 | 34.48 | −3.7 | 41.20 | −3.7 |
| 8 | Pyrogallol | 1057 | 41.38 | −4.2 | 40.62 | −3.9 |
| 9 | D-sorbitol | 5780 | 35.64 | −3.3 | 40.53 | −4.4 |
| 10 | D-mannitol | 6251 | 36.56 | −3.8 | 41.08 | −3.9 |
| 11 | DL-Malic acid | 525 | 31.69 | −3.1 | 39.47 | −3.2 |
| 12 | Protocatechuic acid | 72 | 37.05 | −4.7 | 38.62 | −5.4 |
| 13 | (-)-Quinic acid | 6508 | 28.74 | −4.3 | 37.55 | −5.8 |
| 14 | Shikimic acid | 8742 | 32.23 | −4.2 | 34.58 | −5.3 |
| 15 | 3-Methoxybenzoic acid | 11461 | 43.83 | −4.7 | 41.65 | −5.5 |
| 16 | 4′-Hydroxyacetophenone | 7469 | 36.64 | −2.5 | 42.59 | −3.9 |
| 17 | Salicylic acid | 338 | 43.55 | −4.2 | 38.59 | −5.6 |
| 18 | Benzoic acid | 243 | 36.16 | −5.0 | 35.17 | −5.2 |
| Control ligand: Natural substrate | ||||||
| 1 | Sialic acid | 445063 | 42.37 | −4.6 | 53.20 | −7.0 |
| Control ligand: anti-influenza agents | ||||||
| 1 | GJT | 139030257 | 42.59 | −6.3 | 68.21 | −7.9 |
| 2 | Laninamivir | 9847629 | 55.47 | −5.1 | 83.46 | −5.4 |
| 3 | Peramivir | 154234 | 40.49 | −4.6 | 56.81 | −6.6 |
| 4 | Oseltamivir (Tamiflu®) | 65028 | 48.14 | −4.7 | 55.24 | −6.5 |
| 5 | DANA | 65309 | 39.99 | −4.9 | 54.37 | −6.2 |
| 6 | Zanamivir (GANA) | 60855 | 44.35 | −4.2 | 53.18 | −7.1 |
| 7 | Oseltamivir-SO2NH-(CH2)2-anthrapyrazole | Evteev et al. [61] | 70.42 | −5.8 | 97.92 | −8.5 |
| 8 | Zanamivir-SO2NH-(CH2)2-anthrapyrazole | Evteev et al. [61] | 71.26 | −6.3 | 100.77 | −8.1 |
| No. | Compound Name | Chemical Bond Interaction | ||||
|---|---|---|---|---|---|---|
| H-Bond ● Conventional H-Bond ● Carbon H-Bond ● π-Donor H-Bond | Charge ● π-cation ● π-anion ● Attractive Charges | Hydrophobic ● Alkyl ● π-sigma ● π-alkyl ● π-sulfur | Van der Waals (VdW) | Unfavorable ● Acceptor-Acceptor Clashes ● Donor-Donor Clashes | ||
| C. mimosoides’s metabolites | ||||||
| 1 | Hexadecanedioic acid | ● Conventional H-Bond Ser180 (4.83 Å) Thr226 (4.19 Å) Glu228 (4.82 Å) Thr438 (4.31 Å) | N.D. | ● Alkyl Arg118 (5.45, 5.48 Å) Arg152 (5.77 Å) Arg225 (6.94 Å) | ● VdW Glu119, Gln136, Ser145, Gly147, Thr148, Ile149, Lys150, Asp151, Arg156, Trp179, Glu277, Glu278, Arg430, Ile436, Trp437 | N.D. |
| 2 | Tetradecanedioic acid | ● Conventional H-Bond Glu228 (4.95 Å) Thr438 (4.00 Å) | N.D. | ● Alkyl Arg118 (4.47, 4.88 Å) Agr152 (6.32 Å) | ● VdW Val116, Glu119, Leu134, Gln136, Ser145, Asn146, Gly147, Thr148, Lys150, Asp151, Arg156, Trp179, Ser180, Arg225, Glu278, Arg430, Ile436, Trp437 | N.D. |
| 3 | Bergenin | ● Conventional H-Bond Arg118 (6.48 Å) Arg152 (3.82 Å) Trp179 (4.62 Å) Arg293 (6.82 Å) Arg368 (6.32 Å) ● π-donor H-Bond Tyr402 (7.14 Å) | ● π-anion Glu278 (7.81 Å) | ● Alkyl Ile149 (5.04 Å) | ● VdW Glu119, Lys150, Arg156, Ser180, Ile223 | ● Donor-Donor clashes Arg118 (4.67 Å) Asp151 (4.04 Å) Arg368 (5.92 Å) |
| 4 | trans-Traumatic acid | ● Conventional H-Bond Arg152 (4.15 Å) Ile436 (6.00 Å) Thr438 (4.11 Å) | N.D. | ● Alkyl Arg118 (4.28, 4.33 Å) | ● VdW Glu119, Leu134, Gln136, Ser145, Gly147, Thr148, Ile149, Lys150, Asp151, Arg156, Trp179, Arg430, Trp437 | N.D. |
| 5 | Azelaic acid | ● Conventional H-Bond Arg152 (4.64 Å) Arg368 (6.71 Å) Arg293 (6.24, 6.38 Å) | N.D. | ● Alkyl Lys150 (4.80 Å) | ● VdW Arg118, Glu119, Ile149, Asp151, Trp179, Ser180, Glu228, Glu278, Tyr402 | N.D. |
| 6 | Gallic acid | ● Conventional H-Bond Glu119 (5.37 Å) Asp151 (3.10 Å) Arg156 (3.33 Å) Glu228 (2.56 Å) | N.D. | ● π-alkyl Lys150 (5.17 Å) Arg152 (5.23 Å) | ● VdW Arg118, Leu134, Trp179, Ser180, Arg225 | N.D. |
| 7 | D-arabinonic acid | ● Conventional H-Bond Arg118 (4.98 Å) Arg293 (6.54 Å) Arg368 (7.05 Å) Tyr402 (5.12 Å) Ile149 (5.82, 5.90 Å) ● Carbon H-Bond Glu119 (5.48 Å) | ● π-anion Arg118 (5.41 Å) | N.D. | ● VdW Lys150, Asp151, Glu278 | N.D. |
| 8 | Pyrogallol | ● Conventional H-Bond Arg293 (6.37 Å) Arg368 (6.42 Å) ● π-donor H-Bond Tyr402 (6.29 Å) | ● π-anion Glu278 (8.43 Å) | N.D. | ● VdW Ile149, Lys150 | ● Donor-Donor clashes Arg118 (5.18 Å) Arg368 (6.01 Å) |
| 9 | D-sorbitol | ● Conventional H-Bond Arg118 (6.40 Å) Asp151 (4.99 Å) ● Carbon H-Bond Ile149 (6.79 Å) Lys150 (3.89 Å) | N.D. | N.D. | ● VdW Glu119, Arg293, Gly345, Tyr402 | ● Donor-Donor clashes Arg118 (4.13, 4.98 Å) Arg368 (5.59, 5.73 Å) |
| 10 | D-mannitol | ● Conventional H-Bond Glu119 (5.14 Å) Ile149 (5.01 Å) Arg293 (6.50 Å) Tyr402 (5.48 Å) | N.D. | N.D. | ● VdW Leu134, Lys150, Asp151, Arg156 | ● Donor-Donor clashes Arg118 (5.44 Å) Arg368 (6.19 Å) |
| 11 | DL-Malic acid | ● Conventional H-Bond Arg118 (4.77, 6.02 Å) Glu119 (4.70 Å) Arg293 (6.36 Å) Arg368 (6.78 Å) Tyr402 (5.42 Å) | N.D. | N.D. | ● VdW Lys150, Glu278 | ● Donor-Donor clashes Arg368 (5.64 Å) |
| 12 | Protocatechuic acid | ● Conventional H-Bond Arg152 (3.00 Å) Trp179 (2.87 Å) Ser180 (2.70 Å) Thr226 (2.71 Å) Glu228 (2.23 Å) | ● π-anion Glu278 (4.02 Å) | ● π-sigma Arg225 (3.68 Å) ● π-alkyl Arg225 (4.30 Å) | ● VdW Lys150 | ● Acceptor-Acceptor clashes Glu227 (2.82 Å) |
| 13 | (-)-Quinic acid | ● Conventional H-Bond Arg152 (4.04 Å) | N.D. | N.D. | ● VdW Arg118, Glu119, Lys150, Arg156, Ser180 | ● Acceptor-Acceptor clashes Trp179 (4.75 Å) ● Donor-Donor clashes Asp151 (3.96 Å) |
| 14 | Shikimic acid | ● Conventional H-Bond Arg118 (6.36 Å) Glu119 (5.80 Å) Arg368 (6.13, 6.90 Å) Tyr402 (4.98 Å) | N.D. | N.D. | ● VdW Ile149, Lys150, Glu278 | ● Donor-Donor clashes Arg293 (6.33 Å) |
| 15 | 3-Methoxybenzoic acid | ● Conventional H-Bond Arg368 (3.55 Å) Lys432 (4.91 Å) | N.D. | ● Alkyl Arg368 (3.47 Å) ● π-sigma Arg368 (4.42 Å) ● π-alkyl Ile427 (4.59 Å) Pro431 (5.82 Å) Lys432 (4.51 Å) | ● VdW Ser367, Trp399, Ser400, Arg428, Gly429, Arg430 | N.D. |
| 16 | 4′-hydroxyacetophenone | ● Carbon H-Bond Pro431 (4.82 Å) | ● π-cation Lys432 (4.14 Å) | ● π-alkyl Arg368 (4.73 Å) Pro431 (4.99 Å) | ● VdW Ser367, Trp399, Ile427, Arg428, Gly429, Arg430, Thr438 | ● Donor-Donor clashes Arg368 (3.69 Å) |
| 17 | Salicylic acid | ● Conventional H-Bond Arg368 (3.55, 4.98, 5.60 Å) Asn369 (4.21 Å) Lys432 (4.03, 4.77 Å) ● Carbon H-Bond Ser367 (4.14 Å) | N.D. | ● π-sigma Arg368 (3.87 Å) ● π-alkyl Ile427 (5.46 Å) Pro431 (5.96 Å) Lys432 (4.30 Å) | ● VdW Trp399, Ser400 | N.D. |
| 18 | Benzoic acid | N.D. | N.D. | ● π-sigma Arg368 (3.56 Å) ● π-alkyl Arg368 (4.26 Å) Ile427 (4.89 Å) Lys432 (4.89, 4.96 Å) | ● VdW Ser367, Asn369, Trp399, Ser400 | N.D. |
| Control ligand: Natural substrate | ||||||
| 1 | Sialic acid | ● Conventional H-Bond Arg118 (6.40 Å) Ile149 (5.50 Å) Asp151 (4.36 Å) Arg152 (4.04 Å) Arg156 (5.86 Å) Trp179 (5.67 Å) Tyr402 (5.78 Å) | N.D. | N.D. | ● VdW Glu119, Leu134, Thr148, Lys150, Ser180, Arg225, Glu228, Arg293, Arg368 | ● Donor-Donor clashes Arg118 (4.42 Å) |
| Control ligand: anti-influenza agents | ||||||
| 1 | GJT | ● Conventional H-Bond Asp151 (4.80 Å) Arg152 (4.40 Å) Tyr402 (5.92 Å) ● Carbon H-Bond Glu119 (6.21 Å) | N.D. | ● Alkyl Arg225 (463 Å) ● π-alkyl Lys150 (4.69 Å) | ● VdW Arg118, Thr148, Ile149, Arg156, Trp179, Ser180, Ile223, Glu228, Ser247, Glu277, Glu278, Asn295, Gly345, Val346 | ● Donor-Donor clashes Arg293 (5.64 Å) Arg368 (6.41 Å) |
| 2 | Laninamivir | ● Conventional H-Bond Arg118 (6.53 Å) Glu119 (4.96 Å) Arg152 (4.12 Å) Arg368 (6.42, 6.46 Å) ● Carbon H-Bond Glu277 (5.44 Å) Glu278 (6.83 Å) Lys150 (4.19 Å) | N.D. | ● Alkyl Ile223 (6.21 Å) | ● VdW Leu134, Ile149, Arg156, Trp179, Ser180, Arg225, Ser247, Arg293,Asn295, Tyr402 | ● Donor-Donor clashes Asp151 (4.31 Å) |
| 3 | Peramivir | ● Conventional H-Bond Arg118 (6.44 Å) Glu119 (5.60 Å) Arg152 (4.49 Å) Trp179 (5.52, 6.07 Å) Arg293 (6.17 Å) Arg368 (7.03 Å) ● Carbon H-Bond Arg152 (3.60 Å) | N.D. | N.D. | ● VdW Leu134, Lys150, Asp151, Arg156, Ser180, Ile223, Arg225, Glu228, Ser247, Glu277, Glu278, Tyr402 | ● Donor-Donor clashes Arg368 (5.47 Å) |
| 4 | Oseltamivir (Tamifilu®) | ● Conventional H-Bond Glu119 (5.58, 6.27 Å) Arg152 (4.08 Å) Arg293 (6.89 Å) Arg368 (6.23, 6.93 Å) ● Carbon H-Bond Glu278 (6.60 Å) | N.D. | N.D. | ● VdW Arg118, Lys150, Trp179, Ser180, Ile223, Arg225, Ser246, Glu277, Asn295, Tyr402 | N.D. |
| 5 | DANA | ● Conventional H-Bond Arg152 (4.06 Å) Glu278 (6.32 Å) Arg293 (5.27 Å) Arg368 (6.30 Å) ● Carbon H-Bond Glu278 (6.74 Å) | N.D. | N.D. | ● VdW Arg118, Glu119, Lys150, Ser180, Glu277, Ser247, Gly345, Tyr402 | ● Donor-Donor clashes Arg225 (4.40 Å) Arg293 (4.63 Å) Arg368 (6.12 Å) |
| 6 | Zanamivir (GANA) | ● Conventional H-Bond Arg118 (5.26 Å) Glu119 (4.83 Å) Ile149 (4.65 Å) Arg152 (4.34 Å) Trp179 (4.84, 5.85 Å) Glu278 (5.03 Å) Tyr402 (6.58 Å) ● Carbon H-Bond Glu119 (6.73 Å) | N.D. | N.D. | ● VdW Leu134, Lys150, Asp151, Arg156, Ser180, Ile223, Arg225, Glu228, Ser247, Glu277, Arg293, Asn295 | N.D. |
| Control ligands: anti-influenza drug derivatives | ||||||
| 1 | Oseltamivir-SO2NH-(CH2)2-anthrapyrazole | ● Conventional H-bond Glu119 (4.34 Å), Lys432 (5.15 Å), Arg368 (3.92, 6.73 Å), Tyr402 (6.24 Å) | ● π-cation Arg368 (4.11, 4.14, 5.46 Å) | ● Alkyl Arg225 (4.81 Å) ● π-alkyl Ile427 (6.45 Å) Lys432 (4.74 Å) Pro431 (5.56, 5.73, 6.52 Å) ● π-sigma Arg368 (3.37 Å) | ● VdW Arg118, Leu134, Ile149, Lys150, Asp151, Arg152, Arg156, Trp179, Ser180, Ile223, Glu228, Glu278, Arg293, Pro326, Ser367 | N.D. |
| 2 | Zanamivir-SO2NH-(CH2)2-anthrapyrazole | ● Conventional H-bond Arg118 (6.07 Å) Glu119 (4.54 Å) Arg152 (4.07 Å) Arg293 (6.34 Å) Tyr402 (6.20 Å) Lys432 (5.47 Å) Arg 368 (6.14, 6.74 Å) ● Carbon H-bond Glu119 (6.46 Å) Glu278 (6.50 Å) Ile149 (4.60 Å) | ● π-cation Arg368 (4.79, 5.78, 5.72 Å) ● Attractive Charges Glu119 (6.60 Å) | ● π-alkyl Ile149 (5.11 Å) Pro431 (5.40, 5.91, 6.08 Å) ● π-sulfur Tyr402 (6.20 Å) | ● VdW Leu134, Lys150, Asp151, Arg156, Trp179, Ser180, Arg225, Glu228, Ser247, His275, Glu277, Asn295, Asn344, Ser367, Ile427 | ● Donor-Donor clashes Arg293 (5.01 Å) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klamrak, A.; Nabnueangsap, J.; Narkpuk, J.; Saengkun, Y.; Janpan, P.; Nopkuesuk, N.; Chaveerach, A.; Teeravechyan, S.; Rahman, S.S.; Dobutr, T.; et al. Unveiling the Potent Antiviral and Antioxidant Activities of an Aqueous Extract from Caesalpinia mimosoides Lamk: Cheminformatics and Molecular Docking Approaches. Foods 2024, 13, 81. https://doi.org/10.3390/foods13010081
Klamrak A, Nabnueangsap J, Narkpuk J, Saengkun Y, Janpan P, Nopkuesuk N, Chaveerach A, Teeravechyan S, Rahman SS, Dobutr T, et al. Unveiling the Potent Antiviral and Antioxidant Activities of an Aqueous Extract from Caesalpinia mimosoides Lamk: Cheminformatics and Molecular Docking Approaches. Foods. 2024; 13(1):81. https://doi.org/10.3390/foods13010081
Chicago/Turabian StyleKlamrak, Anuwatchakij, Jaran Nabnueangsap, Jaraspim Narkpuk, Yutthakan Saengkun, Piyapon Janpan, Napapuch Nopkuesuk, Arunrat Chaveerach, Samaporn Teeravechyan, Shaikh Shahinur Rahman, Theerawat Dobutr, and et al. 2024. "Unveiling the Potent Antiviral and Antioxidant Activities of an Aqueous Extract from Caesalpinia mimosoides Lamk: Cheminformatics and Molecular Docking Approaches" Foods 13, no. 1: 81. https://doi.org/10.3390/foods13010081
APA StyleKlamrak, A., Nabnueangsap, J., Narkpuk, J., Saengkun, Y., Janpan, P., Nopkuesuk, N., Chaveerach, A., Teeravechyan, S., Rahman, S. S., Dobutr, T., Sitthiwong, P., Maraming, P., Nualkaew, N., Jangpromma, N., Patramanon, R., Daduang, S., & Daduang, J. (2024). Unveiling the Potent Antiviral and Antioxidant Activities of an Aqueous Extract from Caesalpinia mimosoides Lamk: Cheminformatics and Molecular Docking Approaches. Foods, 13(1), 81. https://doi.org/10.3390/foods13010081






