Enological Suitability of Indigenous Yeast Strains for ‘Verdejo’ Wine Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strains
2.2. Grape Must
2.3. Characterization of Yeasts Fermentative Performances
2.4. Pilot Scale Vinifications with the Selected Yeast Strains
2.5. Oenological Parameters Analysis
2.6. Determination of Wine Aroma Compounds
2.7. Organoleptic Evaluation
2.8. Statistical Analysis
3. Results
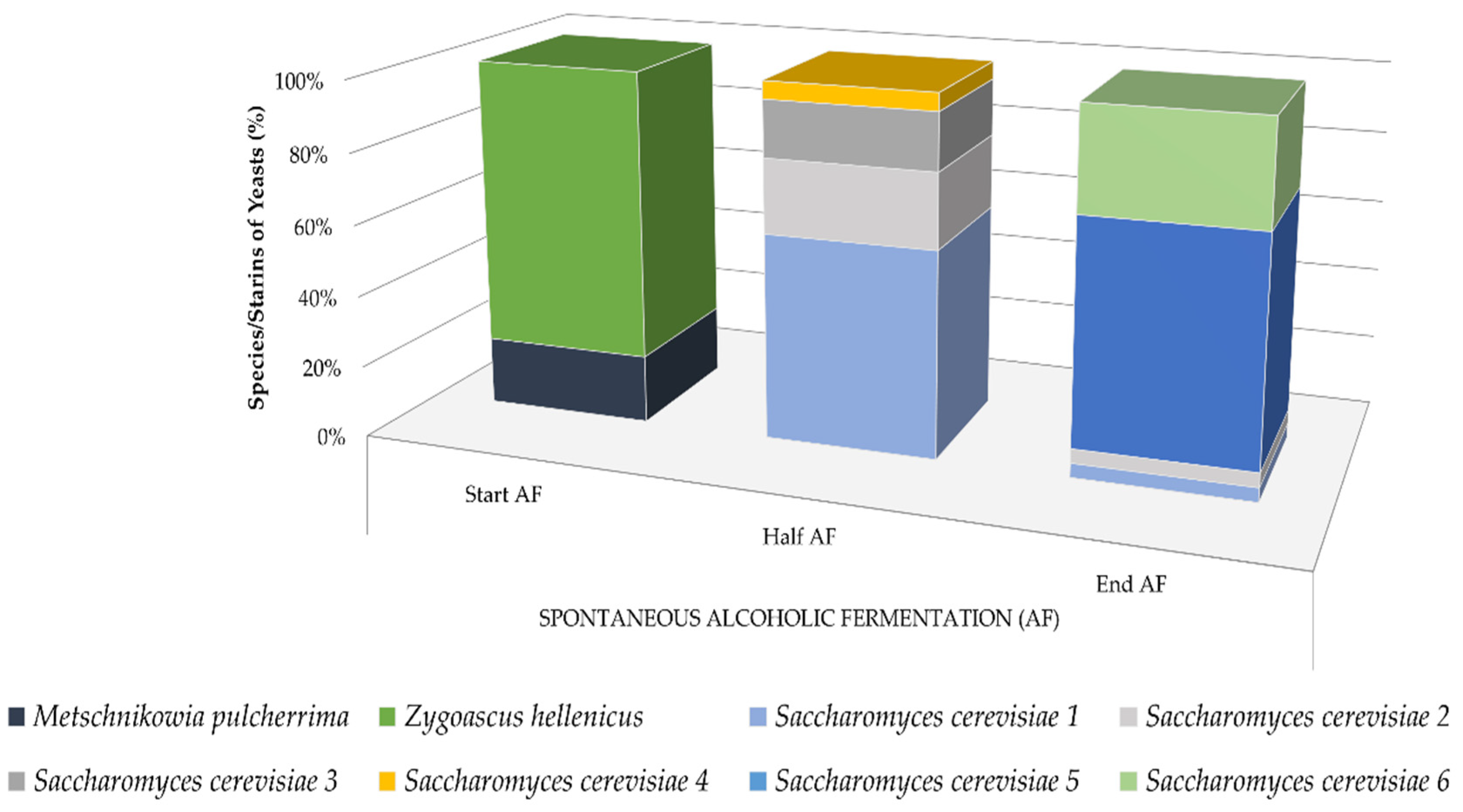
3.1. Fermentative Potential of Yeast Isolates
3.2. Evolution of Density and Yeast Population at Pilot Scale Fermentations
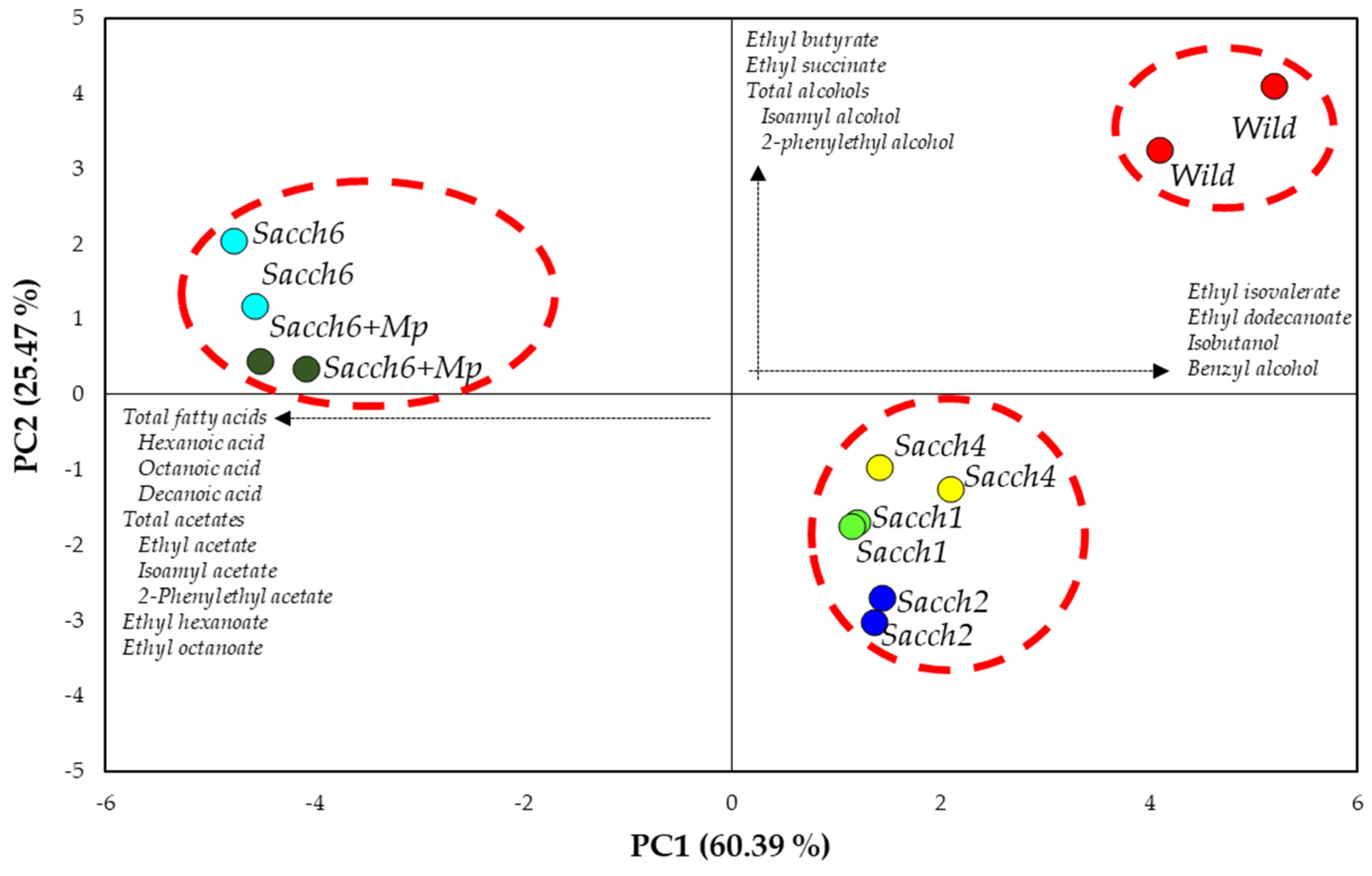
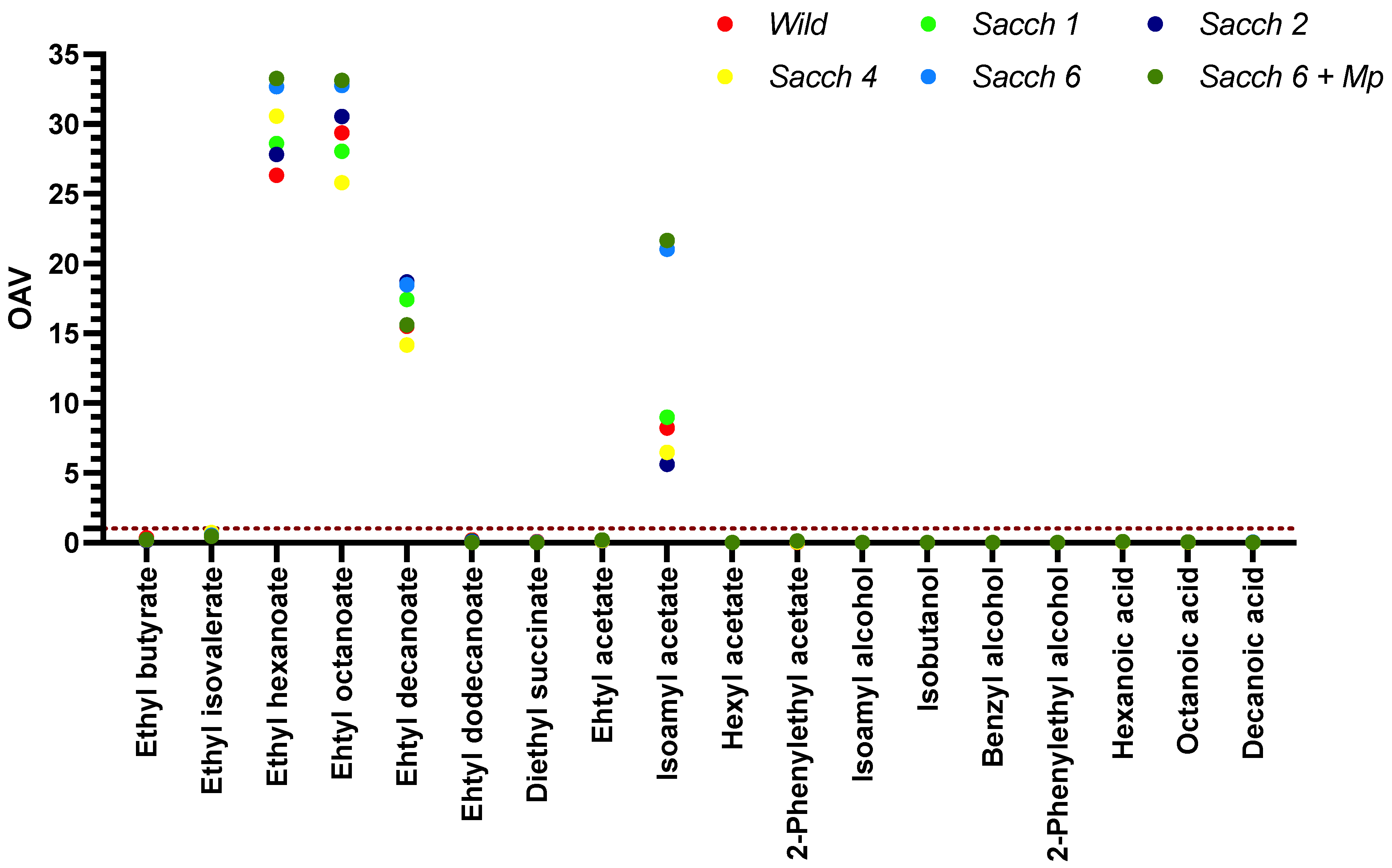
3.3. Basic Parameters and Volatile Composition of Wines
3.4. Sensory Profiles of Final Wines
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
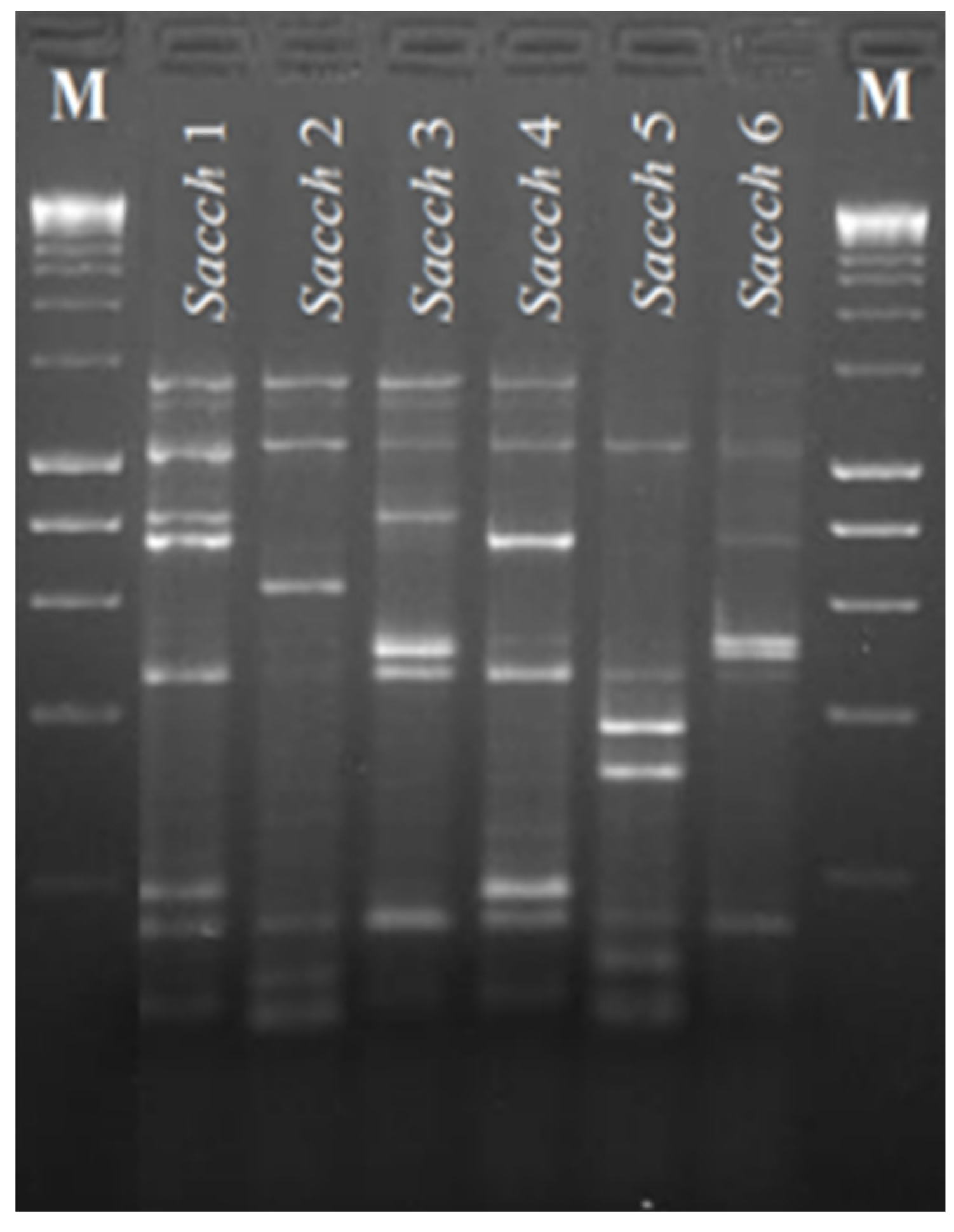
Appendix B
| Families & Compounds | Descriptor | Odor Threshold (µg/L) |
|---|---|---|
| Esters | ||
| Ethyl butyrate | Pineapple, strawberry | 400 (d) |
| Ethyl hexanoate | Fruit, green apple, anise | 80 (d) |
| Ethyl octanoate | Pineapple, pear, floral, sweet | 580 (a) |
| Ethyl decanoate | Fruit, grape, nice | 500(a) |
| Ethyl dodecanoate | Floral, fruit, sweet, cream | 1500 (c) |
| Ethyl isovaleriate | Fruit, apple | 3 (d;e) |
| Diethyl succinate | Fruit, melon | 1200 (a) |
| Alcohols | ||
| Isoamyl alcohol | Flowers, solvent | 60,000 (a) |
| Isobutanol | Flowers, nail polish | 75,000 (a) |
| Benzyl alcohol | Citrus, sweet | 200,000 (b) |
| 2-phenylethyl alcohol | Roses, pollen, perfume | 200,000 (a) |
| Acetates | ||
| Ethyl acetate | Sweet fruit, pinapple, solvent, balsamic | 7500 (f) |
| Isoamyl acetate | Banana, sweet | 160 (a) |
| Hexyl acetate | Pear, apple, cherry | 670 (a) |
| 2-phenylethyl acetate | Green tea, fruit, flowery | 1800 (a) |
| Fatty Acids | ||
| Hexanoic acid | Soapy, cheese | 3000 (a) |
| Octanoic acid | Soapy, rancid | 10000 (a) |
| Decanoic acid | Soapy, rancid | 6000 (a) |
References
- Romano, P.; Caruso, M.; Capece, A.; Lipani, G.; Paraggio, M.; Fiore, C. Metabolic Diversity of Saccharomyces Cerevisiae Strains from Spontaneously Fermented Grape Musts. World J. Microbiol. Biotechnol. 2003, 19, 311–315. [Google Scholar] [CrossRef]
- Gammacurta, M.; Marchand, S.; Albertin, W.; Moine, V.; de Revel, G. Impact of Yeast Strain on Ester Levels and Fruity Aroma Persistence during Aging of Bordeaux Red Wines. J. Agric. Food Chem. 2014, 62, 5378–5389. [Google Scholar] [CrossRef] [PubMed]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not Your Ordinary Yeast: Non-Saccharomyces Yeasts in Wine Production Uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Comitini, F.; Ciani, M. Influence of Fungicide Treatments on the Occurrence of Yeast Flora Associated with Wine Grapes. Ann. Microbiol. 2008, 58, 489–493. [Google Scholar] [CrossRef]
- Castrillo, D.; Rabuñal, E.; Neira, N.; Blanco, P. Yeast Diversity on Grapes from Galicia, NW Spain: Biogeographical Patterns and the Influence of the Farming System. Oeno One 2019, 53, 573–587. [Google Scholar] [CrossRef]
- Fleet, G. The Commercial and Community Significance of Yeasts in Food and Beverage Production. In Yeasts in Food and Beverages; Querol, A., Fleet, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar] [CrossRef]
- Barrio, E.; González, S.S.; Arias, A.; Belloch, C.; Querol, A. Molecular Mechanisms Involved in the Adaptive Evolution of Industrial Yeasts. In Yeasts in Food and Beverages; Querol, A., Fleet, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar] [CrossRef]
- González, S.S.; Barrio, E.; Querol, A. Molecular Identification and Characterization of Wine Yeasts Isolated from Tenerife (Canary Island, Spain). J. Appl. Microbiol. 2007, 102, 1018–1025. [Google Scholar] [CrossRef]
- Cray, J.A.; Bell, A.N.W.; Bhaganna, P.; Mswaka, A.Y.; Timson, D.J.; Hallsworth, J.E. The Biology of Habitat Dominance; Can Microbes Behave as Weeds? Microb. Biotechnol. 2013, 6, 453–492. [Google Scholar] [CrossRef]
- Beltran, G.; Torija, M.J.; Novo, M.; Ferrer, N.; Poblet, M.; Guillamón, J.M.; Rozès, N.; Mas, A. Analysis of yeast populations during alcoholic fermentation: A six year follow-up study. Syst. Appl. Microbiol. 2002, 25, 287–293. [Google Scholar] [CrossRef]
- Padilla, B.; García-Fernández, D.; González, B.; Izidoro, I.; Esteve-Zarzoso, B.; Beltran, G.; Mas, A. Yeast Biodiversity from DOQ Priorat Uninoculated Fermentations. Front. Microbiol. 2016, 7, 930. [Google Scholar] [CrossRef]
- Mas, A.; Guillamon, J.M.; Torija, M.J.; Beltran, G.; Cerezo, A.B.; Troncoso, A.M.; Garcia-Parrilla, M.C. Bioactive Compounds Derived from the Yeast Metabolism of Aromatic Amino Acids during Alcoholic Fermentation. Biomed Res. Int. 2014, 2014, 898045. [Google Scholar] [CrossRef]
- Nisiotou, A.A.; Dourou, D.; Filippousi, M.E.; Diamantea, E.; Fragkoulis, P.; Tassou, C.; Banilas, G. Genetic and Technological Characterisation of Vineyard- and Winery-Associated Lactic Acid Bacteria. Biomed Res. Int. 2015, 2015, 508254. [Google Scholar] [CrossRef] [PubMed]
- Andorrà, I.; Miró, G.; Espligares, N.; Mislata, A.M.; Puxeu, M.; Ferrer-Gallego, R. Wild Yeast and Lactic Acid Bacteria of Wine. In Yeasts Biotechnology; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Suárez-Lepe, J.A.; Morata, A. New Trends in Yeast Selection for Winemaking. Trends Food Sci. Technol. 2012, 23, 39–50. [Google Scholar] [CrossRef]
- Kontogiannatos, D.; Troianou, V.; Dimopoulou, M.; Hatzopoulos, P.; Kotseridis, Y. Oenological Potential of Autochthonous Saccharomyces Cerevisiae Yeast Strains from the Greek Varieties of Agiorgitiko and Moschofilero. Beverages 2021, 7, 27. [Google Scholar] [CrossRef]
- Barbosa, C.; Lage, P.; Esteves, M.; Chambel, L.; Mendes-Faia, A.; Mendes-Ferreira, A. Molecular and Phenotypic Characterization of Metschnikowia Pulcherrima Strains from Douro Wine Region. Fermentation 2018, 4, 8. [Google Scholar] [CrossRef]
- Andorrà, I.; Berradre, M.; Rozès, N.; Mas, A.; Guillamón, J.M.; Esteve-Zarzoso, B.; Guillamón, J.M. Effect of Pure and Mixed Cultures of the Main Wine Yeast Species on Grape Must Fermentations. Eur. Food Res. Technol. 2010, 231, 215–224. [Google Scholar] [CrossRef]
- Vicente, J.; Ruiz, J.; Belda, I.; Benito-Vázquez, I.; Marquina, D.; Calderón, F.; Santos, A.; Benito, S. The Genus Metschnikowia in Enology. Microorganisms 2020, 8, 1038. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; Tesfaye, W.; Bañuelos, M.A.; González, C.; Suárez Lepe, J.A. Lachancea Thermotolerans Applications in Wine Technology. Fermentation 2018, 4, 53. [Google Scholar] [CrossRef]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected Non-Saccharomyces Wine Yeasts in Controlled Multistarter Fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef]
- Ciani, M.; Capece, A.; Comitini, F.; Canonico, L.; Siesto, G.; Romano, P. Yeast Interactions in Inoculated Wine Fermentation. Front. Microbiol. 2016, 7, 555. [Google Scholar] [CrossRef]
- Esteve-Zarzoso, B.; Belloch, C.; Uruburul, F.; Queroi2, A. Identification of Yeasts by RFLP Analysis of the 5.85 RRNA Gene and the Two Ribosomal Internal Transcribed Spacers. Int. J. Syst. Bacteriol. 1999, 49, 329–337. [Google Scholar] [CrossRef]
- Legras, J.L.; Karst, F. Optimisation of Interdelta Analysis for Saccharomyces cerevisiae Strain Characterisation. FEMS Microbiol. Lett. 2003, 221, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Kurtzman, C.P.; Robnett, C.J. Identification and Phylogeny of Ascomycetous Yeasts from Analysis of Nuclear Large Subunit (26S) Ribosomal DNA Partial Sequences. Antonie Van Leeuwenhoek 1998, 73, 331–371. [Google Scholar] [CrossRef] [PubMed]
- OIV. International Organisation of Vine and Wine. Compendium of International Methods of Wine and Must Analysis. In Paris, France. Volumes 1 and 2. 2020. Available online: http://www.oiv.int (accessed on 7 May 2020).
- Mislata, A.M.; Puxeu, M.; Tomás, E.; Nart, E.; Ferrer-Gallego, R. Influence of the Oxidation in the Aromatic Composition and Sensory Profile of Rioja Red Aged Wines. Eur. Food Res. Technol. 2020, 246, 1167–1181. [Google Scholar] [CrossRef]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Yong-Sheng, T.; Li, H. Active volatiles of cabernet Sauvignon wine from Changli County. Health 2009, 1, 176–182. [Google Scholar] [CrossRef]
- Francis, I.L.; Newton, J.L. Determining wine aroma from compositional data. Aust. J. Grape Wine Res. 2005, 11, 114–126. [Google Scholar] [CrossRef]
- Peinado, R.A.; Moreno, J.; Bueno, J.E.; Moreno, J.A.; Mauricio, J.C. Comparative study of aromatic compounds in tao Young white wines subjected to pre-fermentative cryomaceration. Food Chem. 2004, 84, 585–590. [Google Scholar] [CrossRef]
- Guth, H. Quantitation and Sensory Studies of Character Impact Odorants of Different White Wine Varieties. J. Agric. Food Chem 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Ivit, N.N.; Kemp, B. The Impact of Non-Saccharomyces Yeast on Traditional Method Sparkling Wine. Fermentation 2018, 4, 73. [Google Scholar] [CrossRef]
- Lambrechts, M.; Pretorius, I. Yeast and its importance to wine aroma—A review. S. Afr. J. Enol. Vitic. 2000, 21, 97–129. [Google Scholar] [CrossRef]
- Gómez-Míguez, M.J.; Cacho, J.F.; Ferreira, V.; Vicario, I.M.; Heredia, F.J. Volatile components of Zalema white wines. Food Chem. 2007, 100, 1464–1473. [Google Scholar] [CrossRef]
- Vilanova, M.; Martínez, C. First Study of Determination of Aromatic Compounds of Red Wine from Vitis Vinifera Cv. Castañal Grown in Galicia (NW Spain). Eur. Food Res. Technol. 2007, 224, 431–436. [Google Scholar] [CrossRef]
- Vilanova, M.; Genisheva, Z.; Graña, M.; Oliveira, J.M. Determination of Odorants in Varietal Wines from International Grape Cultivars (Vitis Vinífera) Grown in NW Spain. S. Afr. J. Enol. Vitic. 2013, 34, 212–222. [Google Scholar] [CrossRef]
- Pretorius, I.S. Tailoring Wine Yeast for the New Millennium: Novel Approaches to the Ancient Art of Winemaking. Yeast 2000, 16, 675–729. [Google Scholar] [CrossRef] [PubMed]
- Combina, M.; Elía, A.; Mercado, L.; Catania, C.; Ganga, A.; Martinez, C. Dynamics of Indigenous Yeast Populations during Spontaneous Fermentation of Wines from Mendoza, Argentina. Int. J. Food. Microbiol. 2005, 99, 237–243. [Google Scholar] [CrossRef]
- Fleet, G.H. Wine Yeasts for the Future. FEMS Yeast Res. 2008, 8, 979–995. [Google Scholar] [CrossRef]
- Jolly, N.P.; Augustyn, O.P.H.; Pretorius, I.S. The Role and Use of Non-Saccharomyces Yeasts in Wine Production. S. Afr. J. Enol. Vitic. 2006, 27, 15–38. [Google Scholar] [CrossRef]
- Torija, M.J.; Rozès, N.; Poblet, M.; Guillamón, J.M.; Mas, A. Yeast Population Dynamics in Spontaneous Fermentations: Comparison between Two Different Wine-Producing Areas over a Period of Three Years. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2001, 79, 345–352. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; Escott, C.; del Fresno, J.M.; Bañuelos, M.A.; Suárez-Lepe, J.A. Applications of Metschnikowia Pulcherrima in Wine Biotechnology. Fermentation 2019, 5, 63. [Google Scholar] [CrossRef]
- Varela, C.; Borneman, A.R. Yeasts Found in Vineyards and Wineries. Yeast 2017, 34, 111–128. [Google Scholar] [CrossRef]
- Albergaria, H.; Arneborg, N. Dominance of Saccharomyces cerevisiae in Alcoholic Fermentation Processes: Role of Physiological Fitness and Microbial Interactions. App. Microbiol. Biotechnol. 2016, 100, 2035–2046. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.V.; Panon, G. The yeast Metschnikowia pulcherrima has an inhibitory effect against various yeast species. Sci. Aliments 1998, 18, 515–526. [Google Scholar]
- Pallmann, C.; Brown, J.; Olineka, T.; Cocolin, L.; Mills, D.; Bisson, L. Use of WL Medium to Profile Native Flora Fermentations. Am. J. Enol. Vitic. 2001, 52, 198–203. [Google Scholar] [CrossRef]
- Fleet, G.H. Yeast Interactions and Wine Flavour. Int. J. Food Microbiol. 2003, 86, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Swiegers, J.; Bartowsky, E.; Henschke, P.A.; Pretorius, I. Yeast and bacterial modulation of wine aroma and flavour. Aus. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- King, E.S.; Swiegers, J.H.; Travis, B.; Francis, I.L.; Bastian, S.E.P.; Pretorius, I.S. Coinoculated Fermentations Using Saccharomyces Yeasts Affect the Volatile Composition and Sensory Properties of Vitis Vinifera L. Cv. Sauvignon Blanc Wines. J. Agric. Food Chem. 2008, 56, 10829–10837. [Google Scholar] [CrossRef]
- Santos, M.C.; Nunes, C.; Saraiva, J.A.; Coimbra, M.A. Chemical and Physical Methodologies for the Replacement/Reduction of Sulfur Dioxide Use during Winemaking: Review of Their Potentialities and Limitations. Eur. Food Res. Technol. 2012, 234, 1–12. [Google Scholar] [CrossRef]
- Monro, T.M.; Moore, R.L.; Nguyen, M.C.; Ebendorff-Heidepriem, H.; Skouroumounis, G.K.; Elsey, G.M.; Taylor, D.K. Sensing Free Sulfur Dioxide in Wine. Sensors 2012, 12, 10759–10773. [Google Scholar] [CrossRef]
- Howe, P.A.; Worobo, R.; Sacks, G.L. Conventional Measurements of Sulfur Dioxide (SO2) in Red Wine Overestimate SO2 Antimicrobial Activity. Am. J. Enol. Vitic. 2018, 69, 210–220. [Google Scholar] [CrossRef]
- Benito, S.; Hofmann, T.; Laier, M.; Lochbühler, B.; Schüttler, A.; Ebert, K.; Fritsch, S.; Röcker, J.; Rauhut, D. Effect on Quality and Composition of Riesling Wines Fermented by Sequential Inoculation with Non-Saccharomyces and Saccharomyces Cerevisiae. Eur. Food Res. Technol. 2015, 241, 707–717. [Google Scholar] [CrossRef]
- Hranilovic, A.; Gambetta, J.M.; Jeffery, D.W.; Grbin, P.R.; Jiranek, V. Lower-Alcohol Wines Produced by Metschnikowia Pulcherrima and Saccharomyces Cerevisiae Co-Fermentations: The Effect of Sequential Inoculation Timing. Int. J. Food Microbiol. 2020, 329, 108651. [Google Scholar] [CrossRef] [PubMed]
- Garciá, M.; Esteve-Zarzoso, B.; Cabellos, J.M.; Arroyo, T. Sequential Non-Saccharomyces and Saccharomyces Cerevisiae Fermentations to Reduce the Alcohol Content in Wine. Fermentation 2020, 6, 60. [Google Scholar] [CrossRef]
- Vilela, A. Modulating Wine Pleasantness throughout Wine-Yeast Co-Inoculation or Sequential Inoculation. Fermentation 2020, 6, 22. [Google Scholar] [CrossRef]
- Prior, B.A.; Toh, T.H.; Jolly, N.; Baccari, C.; Mortimer, R.K. Impact of yeast breeding for elevated glycerol production on fermentative activity and metabolite formation in Chardonnay wine. S. Afr. J. Enol. Vitic. 2000, 21, 92–99. [Google Scholar] [CrossRef]
- Ruiz, J.; Belda, I.; Beisert, B.; Navascués, E.; Marquina, D.; Calderón, F.; Rauhut, D.; Santos, A.; Benito, S. Analytical Impact of Metschnikowia Pulcherrima in the Volatile Profile of Verdejo White Wines. App. Microbiol. Biotechnol. 2018, 102, 8501–8509. [Google Scholar] [CrossRef] [PubMed]
- Cioch-Skoneczny, M.; Satora, P.; Skoneczny, S.; Klimczak, K. Physicochemical Characterization of Wines Produced Using Indigenous Yeasts from Cold Climate Grapes. Eur. Food Res. Technol. 2021, 247, 201–209. [Google Scholar] [CrossRef]
- Contreras, A.; Curtin, C.; Varela, C. Yeast Population Dynamics Reveal a Potential ‘Collaboration’ between Metschnikowia pulcherrima and Saccharomyces uvarum for the Production of Reduced Alcohol Wines during Shiraz Fermentation. Appl. Microbiol. Biotechnol. 2015, 99, 1885–1895. [Google Scholar] [CrossRef]
- Cabredo-Pinillos, S.; Cedrón-Fernández, T.; González-Briongos, M.; Puente-Pascual, L.; Sáenz-Barrio, C. Ultrasound-Assisted Extraction of Volatile Compounds from Wine Samples: Optimisation of the Method. Talanta 2006, 69, 1123–1129. [Google Scholar] [CrossRef]
- Massera, A.; Assof, M.; Sturm, M.E.; Sari, S.; Jofré, V.; Cordero-Otero, R.; Combina, M. Selection of Indigenous Saccharomyces Cerevisiae Strains to Ferment Red Musts at Low Temperature. Ann. Microbiol. 2012, 62, 367–380. [Google Scholar] [CrossRef]
- Ferreira, V.; Fernández, P.; Peña, C.; Escudero, A.; Cacho, J.F. Investigation on the role played by fermentation esters in the aroma of young Spanish wines by multivariate analysis. J. Sci. Food Agric. 1995, 67, 381–392. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Traité d’Oenologie: 2. Chimie du vin, Stabilitation et Traitements; Dunod: Bordas, Paris, 1998; Volume 2, p. 1184. [Google Scholar]
- Dutraive, O.; Benito, S.; Fritsch, S.; Beisert, B.; Patz, C.D.; Rauhut, D. Effect of Sequential Inoculation with Non-Saccharomyces and Saccharomyces Yeasts on Riesling Wine Chemical Composition. Fermentation 2019, 5, 79. [Google Scholar] [CrossRef]
- Saerens, S.M.G.; Delvaux, F.R.; Verstrepen, K.J.; Thevelein, J.M. Production and Biological Function of Volatile Esters in Saccharomyces Cerevisiae. Microb. Biotechnol. 2010, 3, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Rapp, A.; Giuseppe, V. Influence of Nitrogen Compounds in Grapes on Aroma Compounds of Wine. In Proceedings of the International Symposium on Nitrogen in Grapes and Wine, Seattle, WA, USA, 18–19 June 1991; Rantz, J., Ed.; American Society for Enology and Viticulture: Davis, CA, USA, 1991; p. 15664. [Google Scholar]
- Robinson, A.L.; Ebeler, S.E.; Heymann, H.; Boss, P.K.; Solomon, P.S.; Trengove, R.D. Interactions between Wine Volatile Compounds and Grape and Wine Matrix Components Influence Aroma Compound Headspace Partitioning. J. Agric. Food Chem. 2009, 57, 10313–10322. [Google Scholar] [CrossRef] [PubMed]
- Peynaud, E. Connaissance et Travail du Vin; Dunod: Bordas, Paris, 1984; pp. 133–135. [Google Scholar]



| Yeast Strain | Total SO2 (mg/L) | Alcoholic Strength (vol.%) | Residual Sugar (g/L) | Acetic Acid (g/L) | Glycerol (g/L) |
|---|---|---|---|---|---|
| Sacch 1 | 5.0 ± 2.0 d | 11.80 ± 0.06 bc | 1.5 ± 0.6 bc | 0.32 ± 0.02 c | 4.6 ± 0.2 a |
| Sacch 2 | 9.0 ± 1.0 c | 11.93 ± 0.04 ab | 1.9 ± 0.0 b | 0.38 ± 0.01 b | 4.5 ± 0.3 a |
| Sacch 3 | 24.0 ± 1.0 a | 11.02 ± 0.07 e | 5.1 ± 0.1 a | 0.56 ± 0.01 a | 4.3 ± 0.2 a |
| Sacch 4 | 11.0 ± 1.0 bc | 11.75 ± 0.04 cd | 1.6 ± 0.1 b | 0.55 ± 0.03 a | 4.4 ± 0.1 a |
| Sacch 5 | 13.0 ± 1.0 b | 11.66 ± 0.07 d | 5.0 ± 0.2 a | 0.53 ± 0.02 a | 4.6 ± 0.2 a |
| Sacch 6 | 5.0 ± 1.0 d | 12.04 ± 0.02 a | 0.8 ± 0.7 c | 0.20 ± 0.00 d | 4.6 ± 0.1 a |
| Compounds | Wild | Sacch 1 | Sacch 2 | Sacch 4 | Sacch 6 | Sacch 6 + Mp |
|---|---|---|---|---|---|---|
| Basic parameters | ||||||
| Total SO2 (mg/L) | 65 ± 2 b | 87 ± 2 a | 80 ± 2 a | 66 ± 5 b | 85 ± 3 a | 80 ± 3 a |
| Free SO2 (mg/L) | 20 ± 1 c | 34 ± 3 ab | 39 ± 1 a | 27 ± 2 bc | 31 ± 2 ab | 32 ± 2 ab |
| Free SO2/Total SO2 | 0.31 ± 0.01 a | 0.39 ± 0.03 b | 0.48 ± 0.03 c | 0.41 ± 0.00 b | 0.36 ± 0.02 d | 0.40 ± 0.01 b |
| Alcoholic grade (vol.%) | 13.55 ± 0.03 a | 13.30 ± 0.07 bc | 13.21 ± 0.06 c | 13.20 ± 0.01 c | 13.42 ± 0.02 ab | 13.44 ± 0.02 ab |
| Residual sugar (g/L) | 0.4 ± 0.0 bc | 1.3 ± 0.7 ab | 1.8 ± 0.0 a | 1.6 ± 0.1 a | 0.9 ± 0.0 abc | 0.1 ± 0.0 c |
| pH | 3.44 ± 0.09 a | 3.52 ± 0.10 a | 3.63 ± 0.10 a | 3.56 ± 0.10 a | 3.6 ± 0.10 a | 3.6 ± 0.10 a |
| ATT (g/L) | 5.9 ± 0.1 a | 5.1 ± 0.1 ab | 4.7 ± 0.4 b | 4.6 ± 0.3 b | 5 ± 0.0 b | 4.9 ± 0.0 b |
| Acetic acid (g/L) | 0.18 ± 0.00 d | 0.48 ± 0.00 a | 0.41 ± 0.00 c | 0.44 ± 0.00 b | 0.12 ± 0.00 e | 0.07 ± 0.00 f |
| Malic acid (g/L) | 3.20 ± 0.00 a | 2.60 ± 0.00 b | 2.60 ± 0.00 b | 1.50 ± 0.00 e | 2.40 ± 0.00 c | 2.10 ± 0.00 d |
| Lactic acid (g/L) | 0.1 ± 0.0 b | 0 ± 0.0 bc | 0.1 ± 0.0 b | 0.7 ± 0.0 a | 0 ± 0.0 c | 0 ± 0.0 c |
| Glycerol (g/L) | 5.7 ± 0.0 a | 4.2 ± 0.0 e | 3.8 ± 0.0 f | 4.4 ± 0.0 d | 5.1 ± 0.0 c | 5.3 ± 0.0 b |
| Total esters (µg/L) | 27,415.7 ± 423.7 a | 27,543.1 ± 33.2 a | 29,490.4 ± 3127.5 a | 24,676.1 ± 2968.2 a | 31,042.8 ± 1151.9 a | 29,810.4 ± 1284.3 a |
| Ethyl butyrate | 142.4 ± 7.1 a | 58.0 ± 0.6 c | 53.9 ± 6.7 c | 76.8 ± 8.3 bc | 89.1 ± 7.8 b | 85.7 ± 1.8 b |
| Ethyl isovalerate | 2.0 ± 0.2 a | 1.8 ± 0.1 ab | 1.5 ± 0.2 b | 2.2 ± 0.1 a | 1.6 ± 0.2 b | 1.4 ± 0.1 b |
| Ehyl hexanoate | 2105.6 ± 52.2 e | 2288.4 ± 48.5 cd | 2224.9 ± 38.8 de | 2444.5 ± 63.6 bc | 2604.6 ± 21.1 ab | 2659.9 ± 21.8 a |
| Ehtyl octanoate | 17,033.3 ± 22.5 ab | 16,266.2 ± 254.2 ab | 17,707.9 ± 1652.64 ab | 14,954.4 ± 1780.7 b | 19,001.9 ± 415.2 ab | 19,214.6 ± 696.5 a |
| Ethyl decanoate | 7759.3 ± 512.8 a | 8715.7 ± 184.1 a | 9335.5 ± 1501.8 a | 7082.9 ± 1248.1 a | 9236.5 ± 740.6 a | 7796.7 ± 561.6 a |
| Ethyl dodecanoate | 285.6 ± 11.2 a | 190.7 ± 10.6 b | 146.3 ± 18.5 b | 92.7 ± 10.6 c | 71.3 ± 9.2 cd | 29.8 ± 2.2 d |
| Diethyl succinate | 87.2 ± 18.3 a | 22.2 ± 0.5 b | 20.2 ± 0.3 b | 22.5 ± 0.9 b | 27.7 ± 0.1 b | 22.4 ± 0.5 b |
| Total acetates (µg/L) | 2255.2 ± 467.0 b | 2434.1 ± 26.1 b | 1829.1 ± 143.5 b | 2132.5 ± 241.4 b | 4950.6 ± 358.5 a | 5068.8 ± 38.2 a |
| Ethyl acetate | 884.9 ± 127.5 ab | 917.8 ± 13.3 ab | 878.3 ± 84.6 b | 1045.6 ± 186.9 ab | 1358.3 ± 164.2 a | 1359.2 ± 34.8 a |
| Isoamyl acetate | 1316.4 ± 329.6 b | 1436.8 ± 38.4 b | 896.4 ± 59.4 b | 1033.6 ± 55.8 b | 3364.3 ± 193.1 a | 3465.1 ± 6.8 a |
| Hexyl acetate | 13.5 ± 2.2 a | 11.4 ± 0.1 a | 12.1 ± 1.0 a | 11.8 ± 1.9 a | 12.0 ± 0.3 a | 10.9 ± 1.1 a |
| 2-Phenylethyl acetate | 40.5 ± 7.9 d | 68.1 ± 1.1 c | 42.3 ± 1.5 d | 41.6 ± 3.2 d | 216.1 ± 1.0 b | 233.6 ± 4.4 a |
| Total alcohols (µg/L) | 1762.1 ± 49.2 a | 1125.9 ± 8.6 cd | 1001.9 ± 57.4 d | 1227.4 ± 34.5 bc | 1376.8 ± 56.0 b | 1233.7 ± 2.8 bc |
| Isoamyl alcohol | 1275.4 ± 33.9 a | 749.1 ± 8.4 bc | 661.2 ± 49.7 c | 863.9 ± 73.3 b | 893.2 ± 54.5 b | 786.9 ± 8.6 bc |
| Isobutanol | 58.7 ± 2.7 a | 35.9 ± 0.1 c | 41.2 ± 4.6 bc | 48.8 ± 4.9 ab | 18.9 ± 1.3 d | 16.1 ± 0.9 d |
| Benzyl alcohol | 3.7 ± 0.2 a | 3.1 ± 0.1 ab | 3.0 ± 0.2 ab | 3.4 ± 0.5 ab | 3.0 ± 0.1 ab | 2.6 ± 0.1 b |
| 2-Phenylethyl alcohol | 424.2 ± 12.3 a | 337.8 ± 0.4 b | 296.6 ± 3.3 b | 311.2 ± 43.2 b | 461.7 ± 0.3 a | 428.1 ± 6.7 a |
| Total fatty acids (µg/L) | 591.0 ± 9.1 c | 686.7 ± 6.8 c | 657.9 ± 0.9 c | 697.2 ± 70.5 c | 987.6 ± 3.5 a | 843.6 ± 19.3 b |
| Hexanoic acid | 143.6 ± 1.0 c | 167.1 ± 0.1 b | 155.9 ± 7.8 bc | 176.5 ± 11.6 b | 227.6 ± 0.5 a | 217.6 ± 1.4 a |
| Octanoic acid | 367.7 ± 6.7 c | 410.5 ± 6.9 c | 400.8 ± 3.1 c | 416.9 ± 41.3 c | 605.0 ± 0.1 a | 521.3 ± 9.3 b |
| Decanoic acid | 79.8 ± 3.3 b | 109.1 ± 0.1 b | 101.2 ± 3.8 b | 103.8 ± 17.6 b | 154.9 ± 3.0 a | 104.6 ± 8.5 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez, J.; Mislata, A.M.; Vendrell, V.; Moro, C.; de Lamo, S.; Ferrer-Gallego, R.; Andorrà, I. Enological Suitability of Indigenous Yeast Strains for ‘Verdejo’ Wine Production. Foods 2023, 12, 1888. https://doi.org/10.3390/foods12091888
Vázquez J, Mislata AM, Vendrell V, Moro C, de Lamo S, Ferrer-Gallego R, Andorrà I. Enological Suitability of Indigenous Yeast Strains for ‘Verdejo’ Wine Production. Foods. 2023; 12(9):1888. https://doi.org/10.3390/foods12091888
Chicago/Turabian StyleVázquez, Jennifer, Ana Maria Mislata, Victor Vendrell, Carlos Moro, Sergi de Lamo, Raúl Ferrer-Gallego, and Imma Andorrà. 2023. "Enological Suitability of Indigenous Yeast Strains for ‘Verdejo’ Wine Production" Foods 12, no. 9: 1888. https://doi.org/10.3390/foods12091888
APA StyleVázquez, J., Mislata, A. M., Vendrell, V., Moro, C., de Lamo, S., Ferrer-Gallego, R., & Andorrà, I. (2023). Enological Suitability of Indigenous Yeast Strains for ‘Verdejo’ Wine Production. Foods, 12(9), 1888. https://doi.org/10.3390/foods12091888