Emulsion-Templated Liquid Oil Structuring with Egg White Protein Microgel- Xanthan Gum
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Aqueous Dispersions
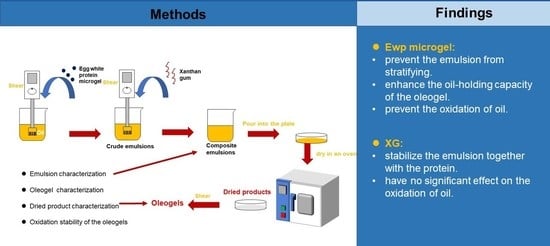
2.3. Preparation of Emulsion-Templated Oleogels
2.4. Emulsion Characterization
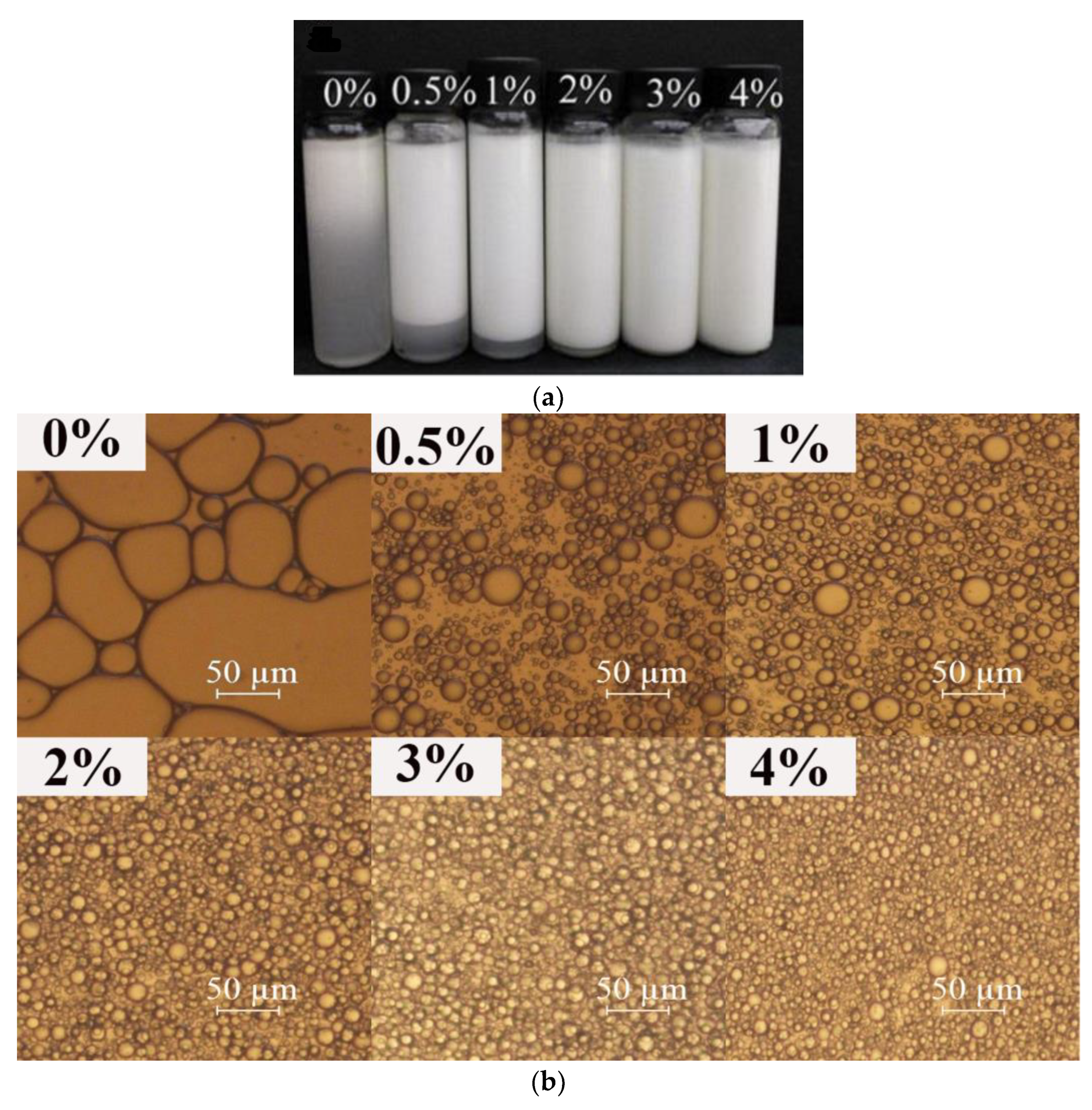
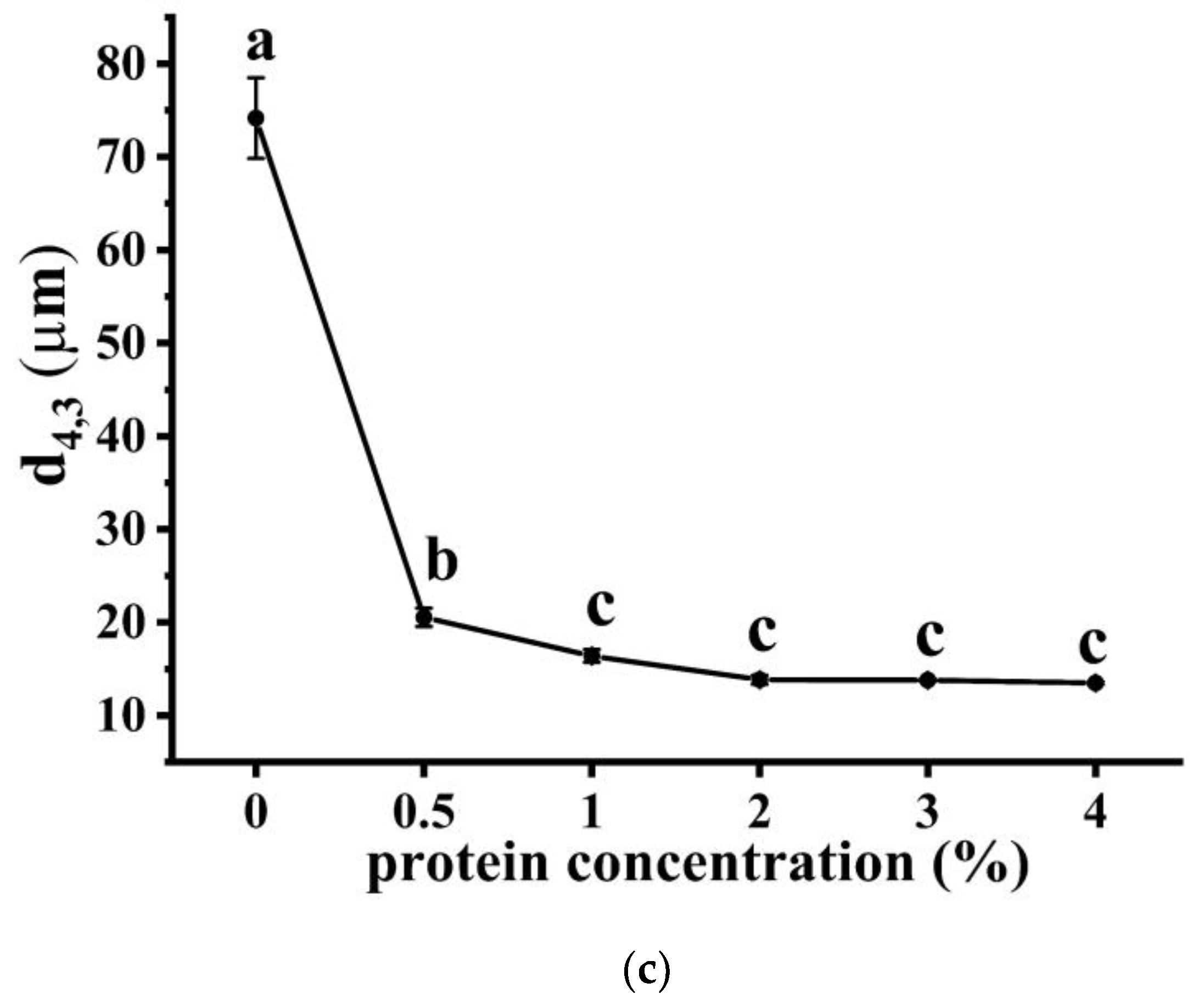
2.4.1. Determination of Physical Stability and Droplet Size
2.4.2. Microstructure of the Emulsions
2.4.3. Rheological characterization of the emulsions
2.5. Characterization of Oleogels and Dried Products
2.5.1. Microstructure of the Dried Products
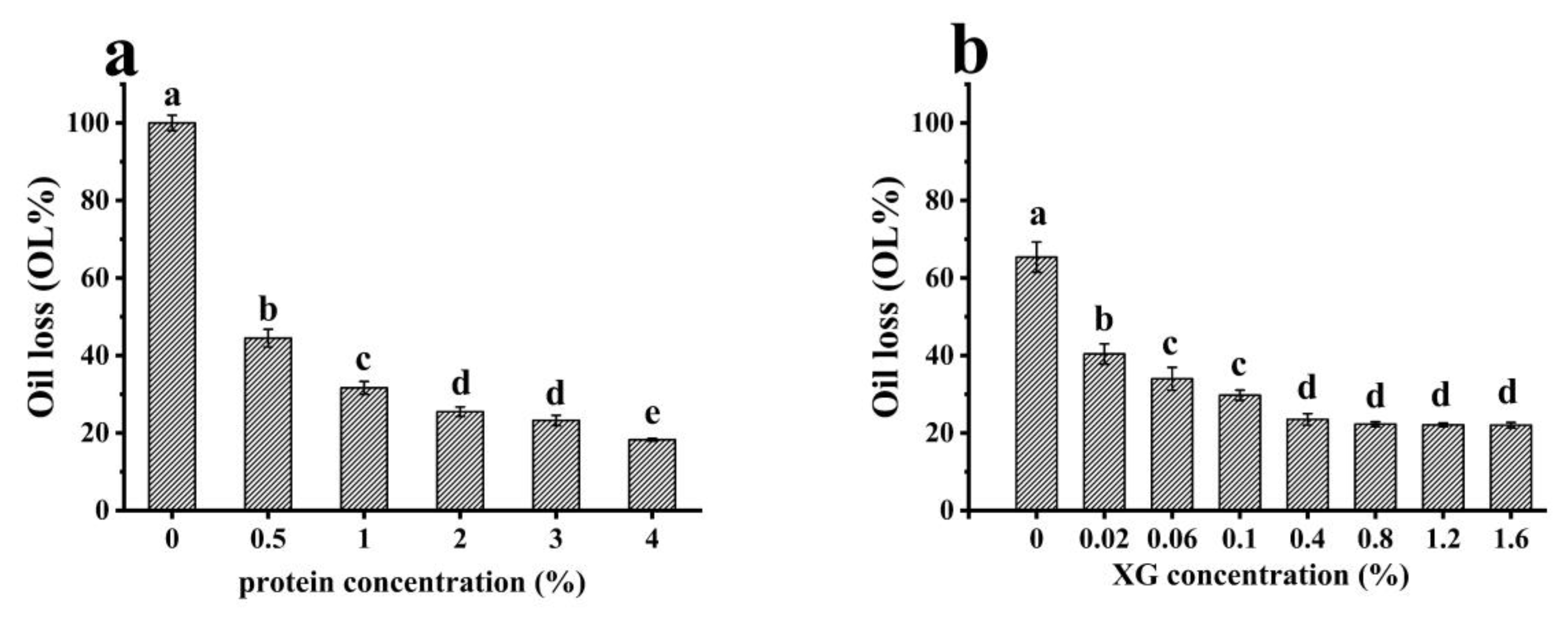
2.5.2. Determination of Oil Loss Rate of the Oleogels
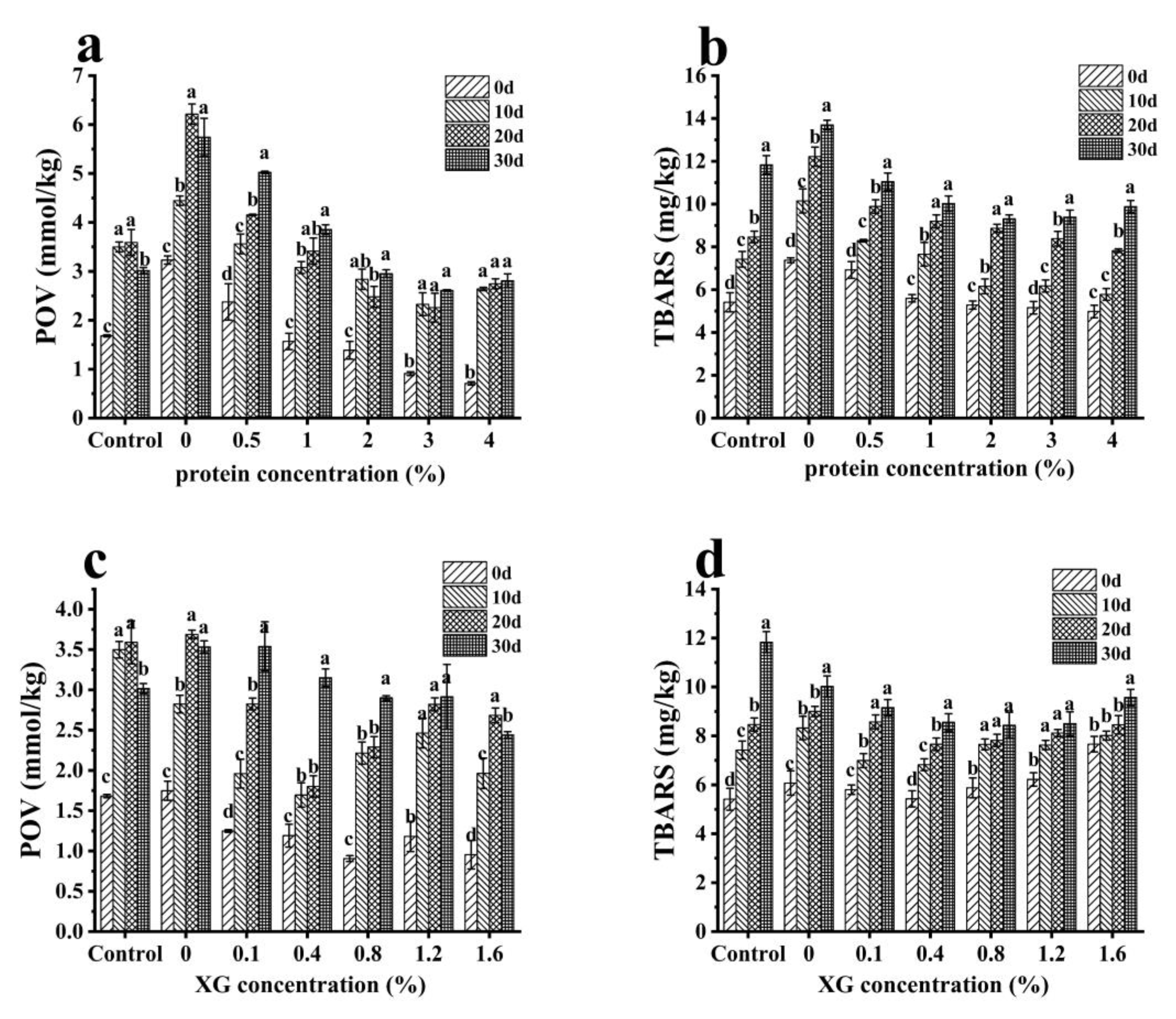
2.5.3. Determination of Oxidation Stability of the Oleogels
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Emulsions
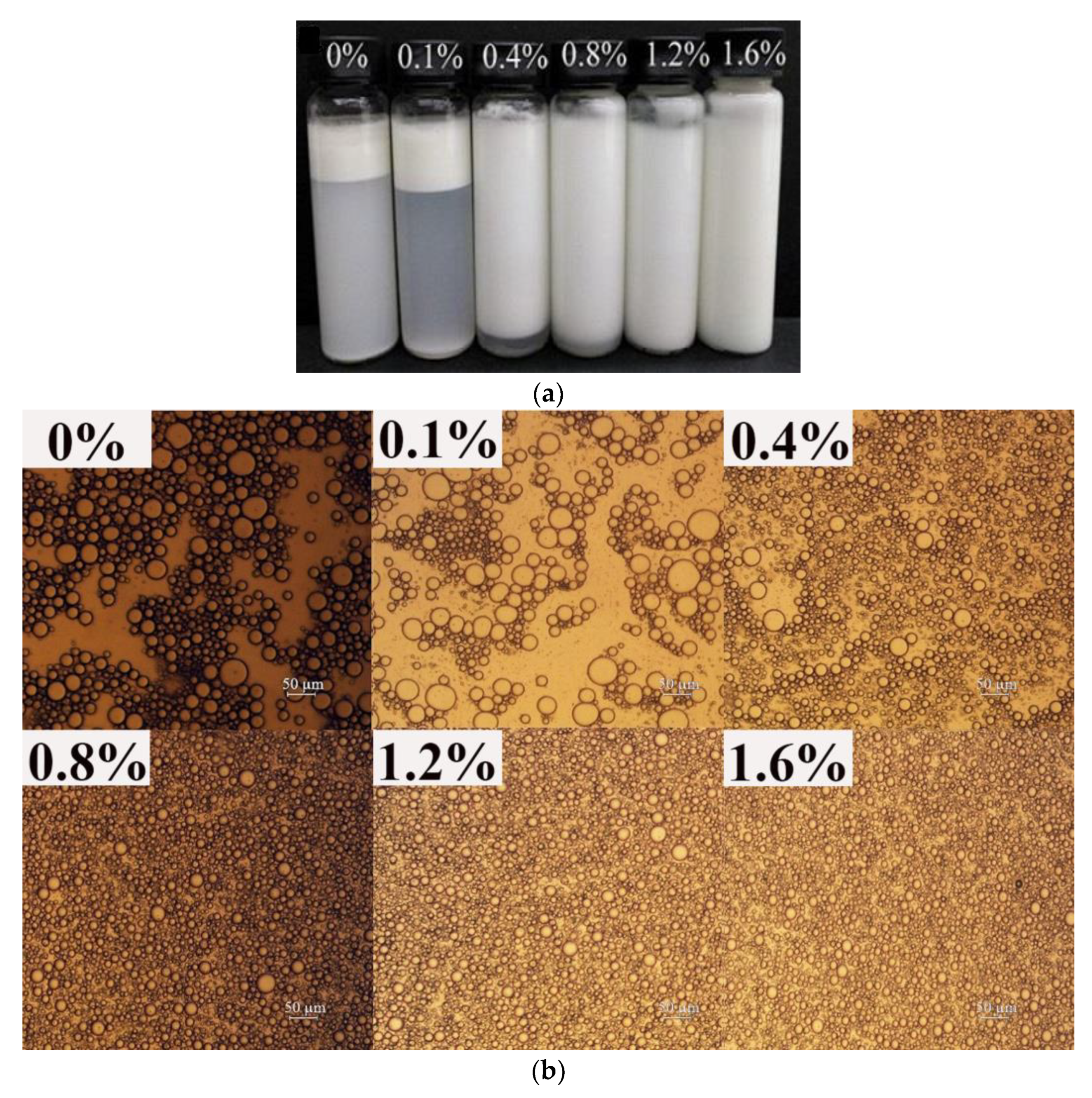
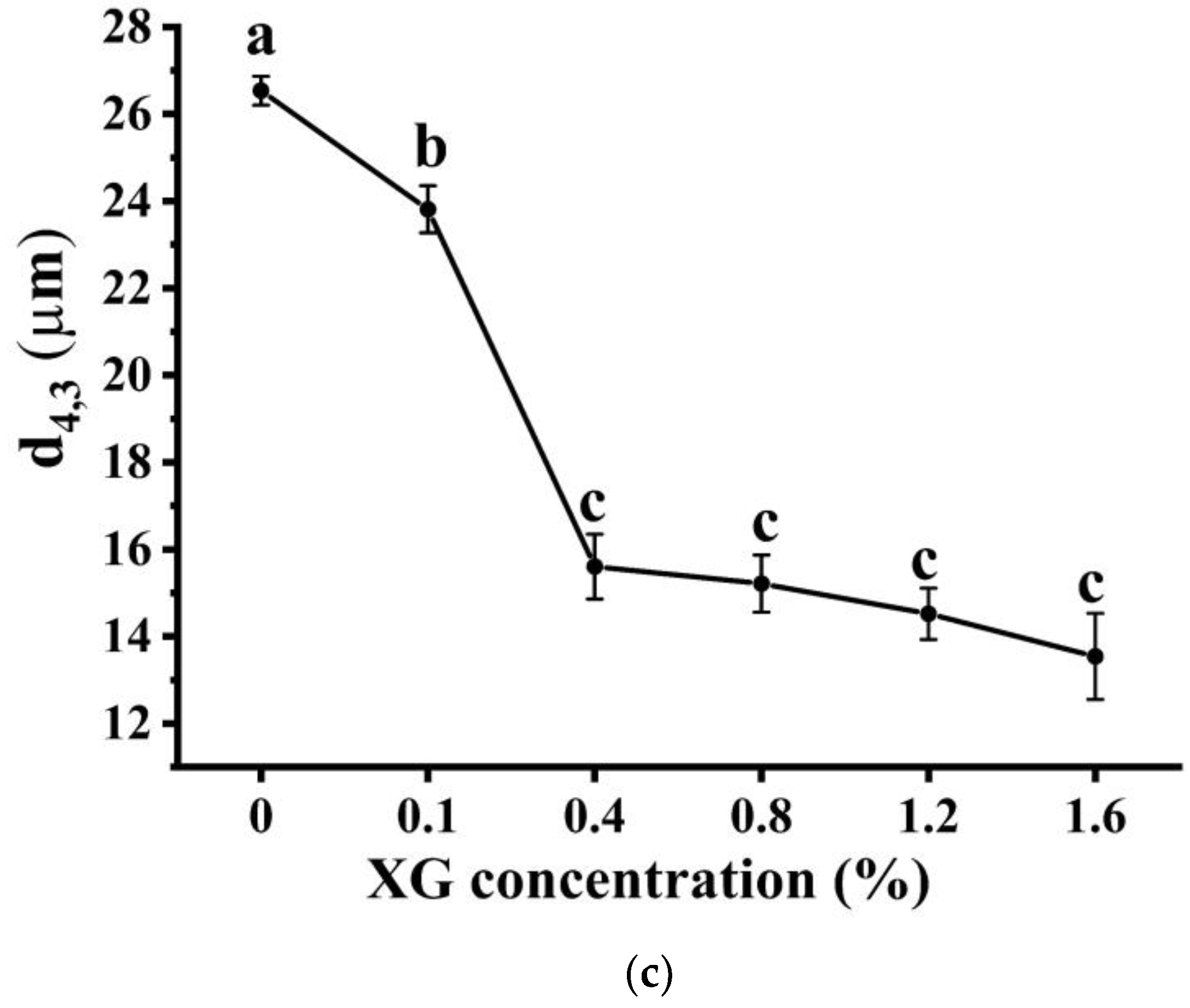
3.1.1. Macro-, Micro-Structure, and Droplet Size of Emulsions
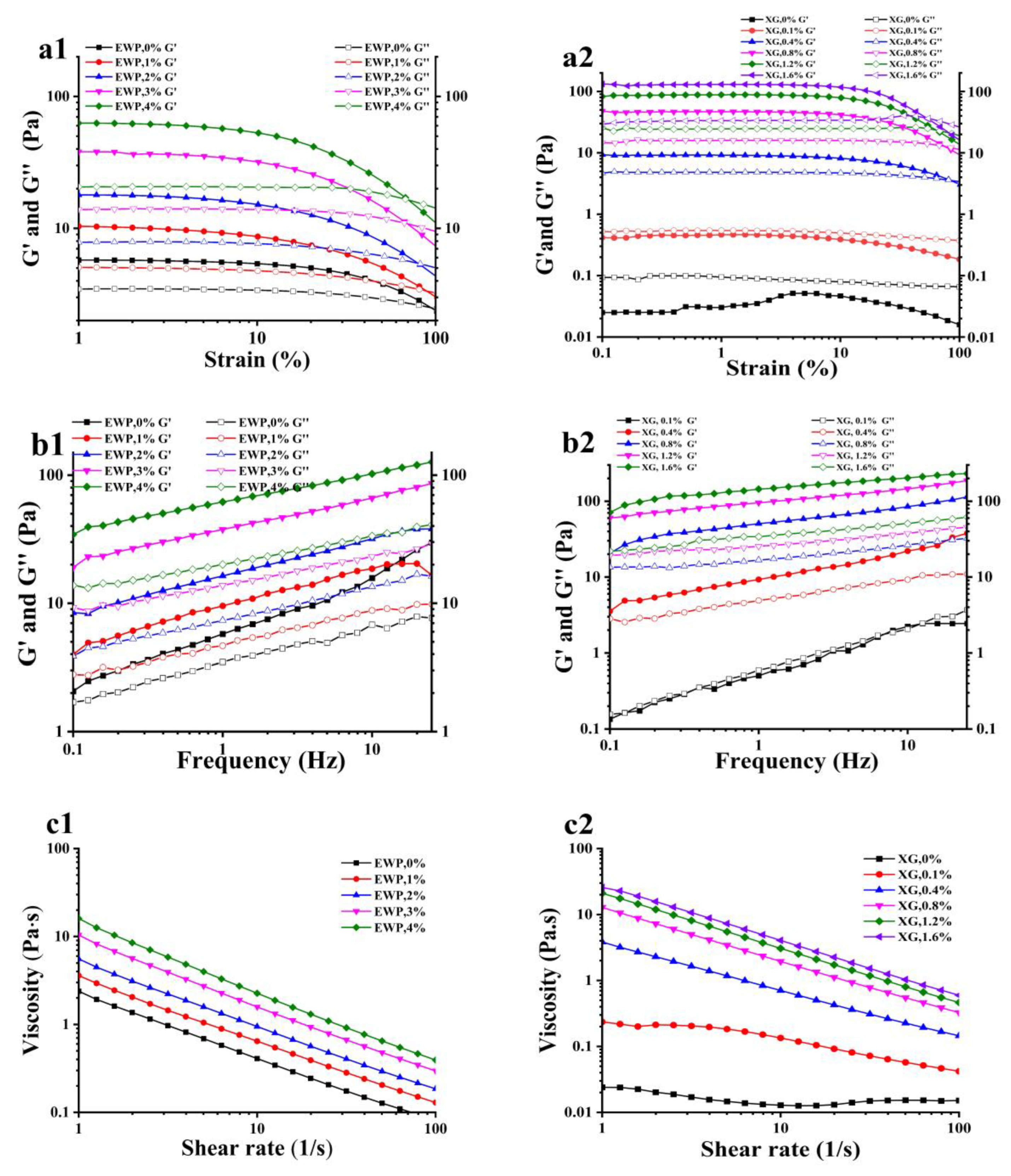
3.1.2. Rheology of Emulsions
3.2. Characterization of Oleogels and Dried Products
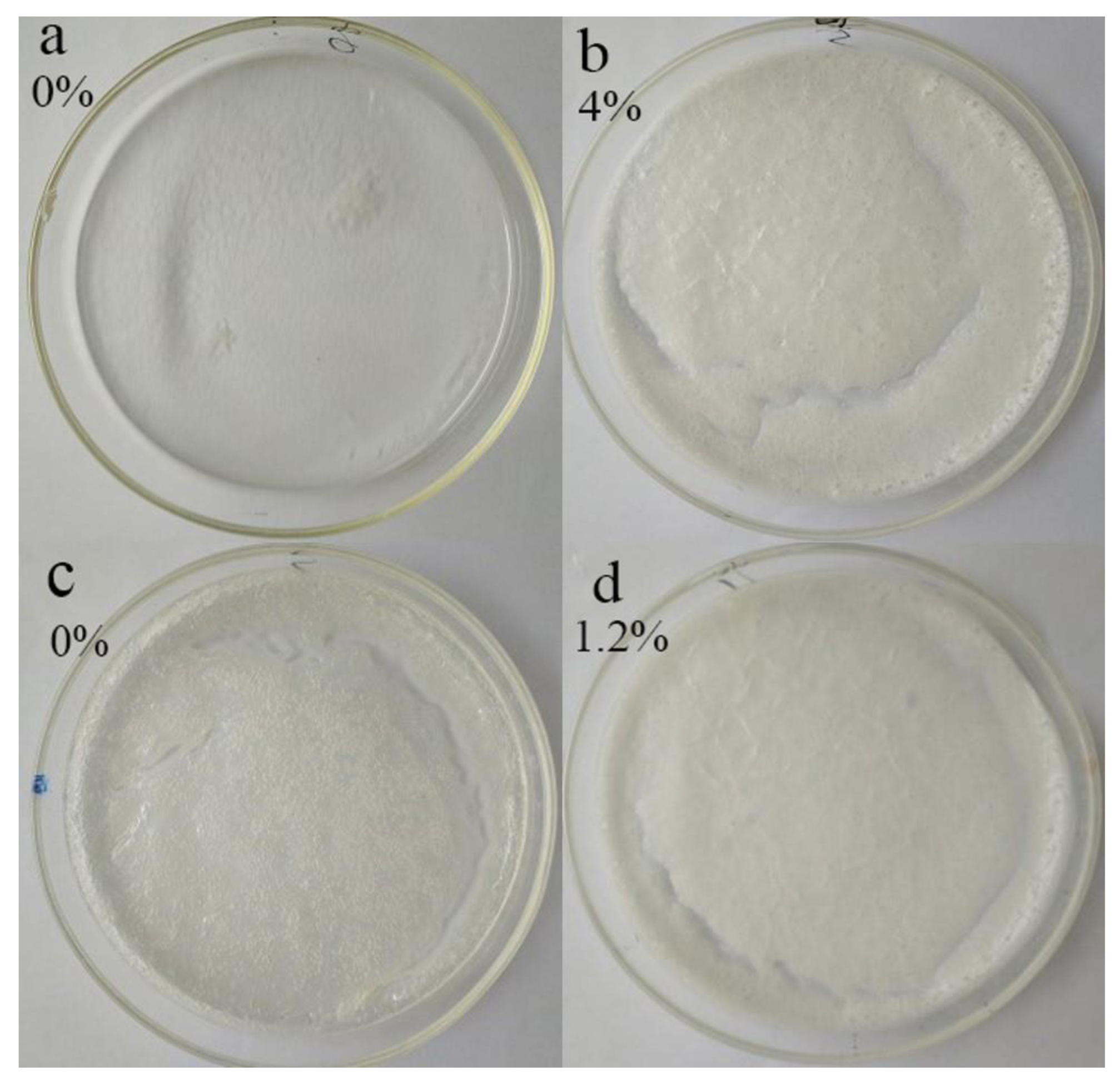
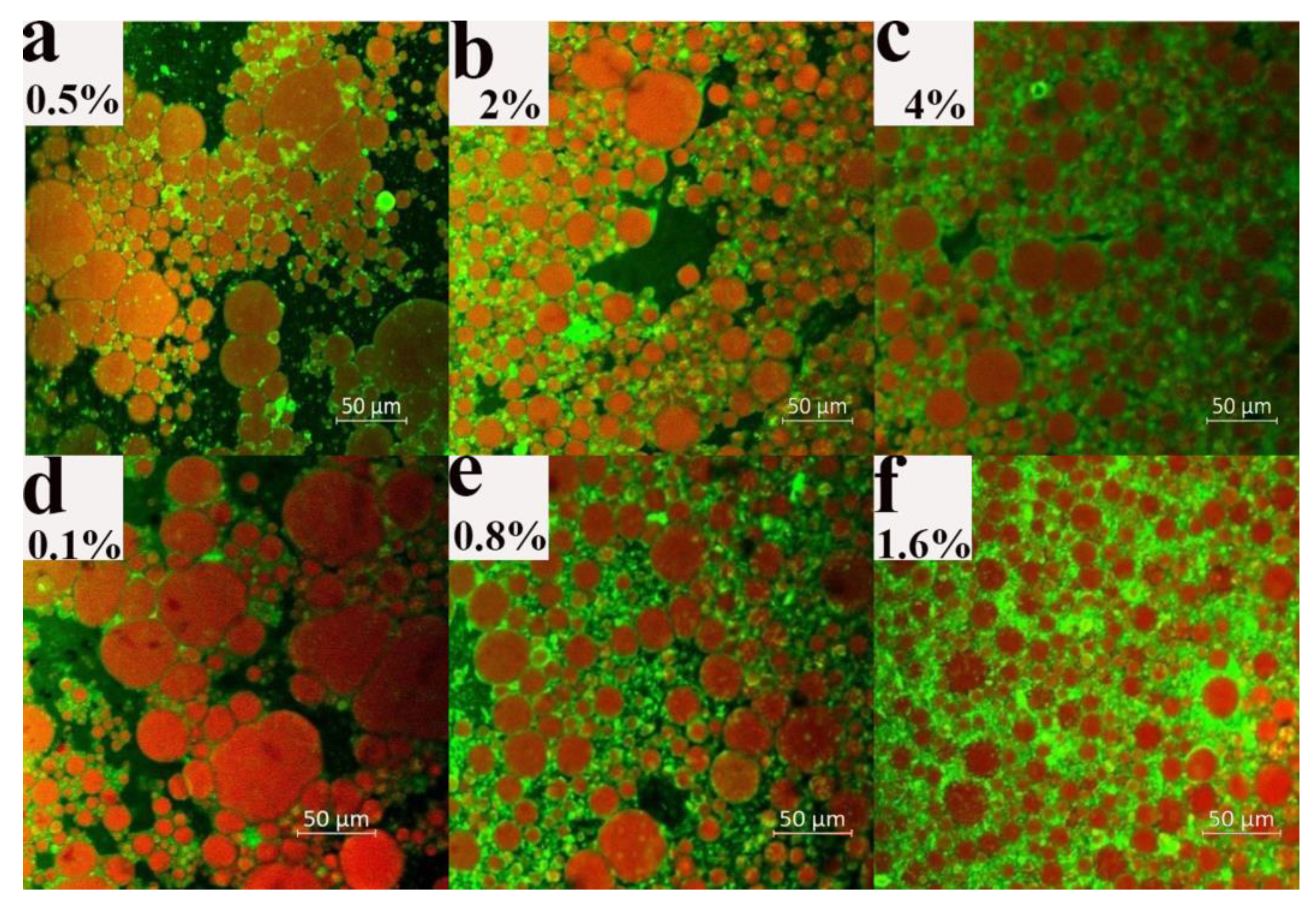
3.2.1. Microstructure of Dried Products
3.2.2. Stability of Oleogels
3.3. Oxidation Stability of Oleogels
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Kris-Etherton, P.M.; Petersen, K.; Van Horn, L. Convincing evidence supports reducing saturated fat to decrease cardiovascular disease risk. BMJ Nutr. Prev. Health 2018, 1, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Zahringer, J.; Beyerbach, J.; Werner, S.W.; Heseker, H.; Koletzko, B.; Meerpohl, J.J. Total Dietary Fat Intake, Fat Quality, and Health Outcomes: A Scoping Review of Systematic Reviews of Prospective Studies. Ann. Nut. Meta. 2021, 77, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Samateh, M.; Sagiri, S.S.; Sanni, R.; Chee, C.A.; Satapathy, S.; John, G. Tuning Aesthetic and Mechanical Properties of Oleogels via Formulation of Enzyme-Enabled Stereoisomeric Molecular Gelators. J. Agric. Food Chem. 2020, 68, 13282–13290. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R.; Dewettinck, K. Edible oil structuring: An overview and recent updates. Food Funct. 2016, 7, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.C.; Gravelle, A.J.; Blake, A.I.; Marangoni, A.G. Novel trans fat replacement strategies. Curr. Opin. Food Sci. 2016, 7, 27–34. [Google Scholar] [CrossRef]
- Ashok, R.P. Alternative Routes to Oil Structuring; Springer International Publishing: New York, NY, USA, 2015; pp. 4–8. [Google Scholar]
- Scholten, E. Edible oleogels: How suitable are proteins as a structurant? Curr. Opin. Food Sci. 2019, 27, 36–42. [Google Scholar] [CrossRef]
- Feichtinger, A.; Scholten, E. Preparation of Protein Oleogels: Effect on Structure and Functionality. Foods 2020, 9, 1745. [Google Scholar] [CrossRef]
- Patel, A.R.; Schatteman, D.; De Vos, W.H.; Lesaffer, A.; Dewettinck, K. Preparation and rheological characterization of shellac oleogels and oleogel-based emulsions. J. Colloid Interface Sci. 2013, 411, 114–121. [Google Scholar] [CrossRef]
- Patel, A.R.; Cludts, N.; Bin Sintang, M.D.; Lesaffer, A.; Dewettinck, K. Edible oleogels based on water soluble food polymers: Preparation, characterization and potential application. Food Funct. 2014, 5, 2833–2841. [Google Scholar] [CrossRef]
- Patel, A.R.; Rajarethinem, P.S.; Cludts, N.; Lewille, B.; De Vos, W.H.; Lesaffer, A.; Dewettinck, K. Biopolymer-Based Structuring of Liquid Oil into Soft Solids and Oleogels Using Water-Continuous Emulsions as Templates. Langmuir 2015, 31, 2065–2073. [Google Scholar] [CrossRef]
- Pan, H.; Xu, X.; Qian, Z.; Cheng, H.; Shen, X.; Chen, S.; Ye, X. Xanthan gum-assisted fabrication of stable emulsion-based oleogel structured with gelatin and proanthocyanidins. Food Hydrocoll. 2021, 115, 106596. [Google Scholar] [CrossRef]
- Romoscanu, A.I.; Mezzenga, R. Emulsion-templated fully reversible protein-in-oil gels. Langmuir 2006, 22, 7812–7818. [Google Scholar] [CrossRef]
- Khadije, A.; Leyla, A.; Kooshan, N.; Marzieh Hosseini, S.; Reyhane, S. Oleogel production based on binary and ternary mixtures of sodium caseinate, xanthan gum, and guar gum: Optimization of hydrocolloids concentration and drying method. J. Texture Stud. 2020, 51, 290–299. [Google Scholar] [CrossRef]
- Tavernier, I.; Patel, A.R.; Van der Meeren, P.; Dewettinck, K. Emulsion-templated liquid oil structuring with soy protein and soy protein: Kappa-carrageenan complexes. Food Hydrocoll. 2017, 65, 107–120. [Google Scholar] [CrossRef]
- Zou, Y.; Xi, Y.; Pan, J.; Ijaz Ahmad, M.; Zhang, A.; Zhang, C.; Li, Y.; Zhang, H. Soy oil and SPI based-oleogels structuring with glycerol monolaurate by emulsion-templated approach: Preparation, characterization and potential application. Food Chem. 2022, 397, 133767. [Google Scholar] [CrossRef]
- Espert, M.; Hernandez, M.J.; Sanz, T.; Salvador, A. Rheological properties of emulsion templated oleogels based on xanthan gum and different structuring agents. Curr. Res. Food Sci. 2022, 5, 564–570. [Google Scholar] [CrossRef]
- Silva-Avellaneda, E.; Bauer-Estrada, K.; Prieto-Correa, R.E.; Quintanilla-Carvajal, M.X. The effect of composition, microfluidization and process parameters on formation of oleogels for ice cream applications. Sci. Rep. 2021, 11, 7161. [Google Scholar] [CrossRef]
- Wei, G.; Xiao-lu, G.; Hai-bo, H.; Xiang, L.; Yu, X.; Jiang-Ning, H. Structural characterization of modified whey protein isolates using cold plasma treatment and its applications in emulsion oleogels. Food Chem. 2021, 356, 129703. [Google Scholar] [CrossRef]
- Mine, Y. Recent advances in egg protein functionality in the food system. World Poult. Sci J. 2002, 58, 31–39. [Google Scholar] [CrossRef]
- Yao, X.; McClements, D.J.; Su, Y.; Li, J.; Chang, C.; Wang, J.; Yang, Y.; Gu, L. Fabrication, Structural and Emulsifying Properties of Egg White Protein-Dextran Conjugates through Maillard Reaction. Food Biophys. 2022, 17, 650–661. [Google Scholar] [CrossRef]
- Chang, C.; Fuge, N.; Luping, G.; Xin, L.; Hao, Y.; Bei, Z.; Junwei, W.; Yujie, S.; Yanjun, Y. Formation of fibrous or granular egg white protein microparticles and properties of the integrated emulsions. Food Hydrocoll. 2016, 61, 477–486. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Murray, B.S.; Sarkar, A. Combination of egg white protein and microgels to stabilize foams: Impact of processing treatments. J. Food Eng. 2020, 275, 109860. [Google Scholar] [CrossRef]
- Li, X.; Murray, B.S.; Yang, Y.; Sarkar, A. Egg white protein microgels as aqueous Pickering foam stabilizers: Bubble stability and interfacial properties. Food Hydrocoll. 2020, 98, 105292. [Google Scholar] [CrossRef]
- Velez-Erazo, E.M.; Okuro, P.K.; Gallegos-Soto, A.; da Cunha, R.L.; Hubinger, M.D. Protein-based strategies for fat replacement: Approaching different protein colloidal types, structured systems and food applications. Food Res. Int. 2022, 156, 111346. [Google Scholar] [CrossRef] [PubMed]
- Bascuas, S.; Morell, P.; Hernando, I.; Quiles, A. Recent trends in oil structuring using hydrocolloids. Food Hydrocoll. 2021, 118, 106612. [Google Scholar] [CrossRef]
- Jaberi, R.; Pedram Nia, A.; Naji-Tabasi, S.; Elhamirad, A.H.; Shafafi Zenoozian, M. Rheological and structural properties of oleogel base on soluble complex of egg white protein and xanthan gum. J. Texture Stud. 2020, 51, 925–936. [Google Scholar] [CrossRef]
- Meng, Z.; Qi, K.; Guo, Y.; Wang, Y.; Liu, Y. Effects of thickening agents on the formation and properties of edible oleogels based on hydroxypropyl methyl cellulose. Food Chem. 2018, 246, 137–149. [Google Scholar] [CrossRef]
- Bascuas, S.; Hernando, I.; Moraga, G.; Quiles, A. Structure and stability of edible oleogels prepared with different unsaturated oils and hydrocolloids. Int. J. Food Sci. Technol. 2020, 55, 1458–1467. [Google Scholar] [CrossRef]
- Bouyer, E.; Mekhloufi, G.; Rosilio, V.; Grossiord, J.-L.; Agnely, F. Proteins, polysaccharides, and their complexes used as stabilizers for emulsions: Alternatives to synthetic surfactants in the pharmaceutical field? Int. J. Pharm. 2012, 436, 359–378. [Google Scholar] [CrossRef]
- Pocan, P.; Ilhan, E.; Oztop, M.H. Characterization of Emulsion Stabilization Properties of Gum Tragacanth, Xanthan Gum and Sucrose Monopalmitate: A Comparative Study. J. Food Sci. 2019, 84, 1087–1093. [Google Scholar] [CrossRef]
- Schmitt, C.; Moitzi, C.; Bovay, C.; Rouvet, M.; Bovetto, L.; Donato, L.; Leser, M.; Schurtenberger, P.; Stradner, A. Internal structure and colloidal behaviour of covalent whey protein microgels obtained by heat treatment. Soft Matter. 2010, 6, 4876–4884. [Google Scholar] [CrossRef]
- Kanagaratnam, S.; Hoque, M.E.; Sahri, M.M.; Spowage, A. Investigating the effect of deforming temperature on the oil-binding capacity of palm oil based shortening. J. Food Eng. 2013, 118, 90–99. [Google Scholar] [CrossRef]
- Chaiyasit, W.; Silvestre, M.P.C.; McClements, D.J.; Decker, E.A. Ability of surfactant hydrophobic tail group size to alter lipid oxidation in oil-in-water emulsions. J. Agric. Food Chem. 2000, 48, 3077–3080. [Google Scholar] [CrossRef]
- Zhao, Q.; Selomulya, C.; Wang, S.; Xiong, H.; Chen, X.D.; Li, W.; Peng, H.; Xie, J.; Sun, W.; Zhou, Q. Enhancing the oxidative stability of food emulsions with rice dreg protein hydrolysate. Food Res. Int. 2012, 48, 876–884. [Google Scholar] [CrossRef]
- Wijaya, W.; Van der Meeren, P.; Wijaya, C.H.; Patel, A.R. High internal phase emulsions stabilized solely by whey protein isolate-low methoxyl pectin complexes: Effect of pH and polymer concentration. Food Funct. 2017, 8, 584–594. [Google Scholar] [CrossRef]
- Chang, C.; Niu, F.; Su, Y.; Qiu, Y.; Gu, L.; Yang, Y. Characteristics and emulsifying properties of acid and acid-heat induced egg white protein. Food Hydrocoll. 2016, 54, 342–350. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Li, X.; Chang, C.; Zhang, M.; Gu, L.; Su, Y.; Yang, Y. Emulsifying properties of glycation or glycation-heat modified egg white protein. Food Res. Int. 2019, 119, 227–235. [Google Scholar] [CrossRef]
- Sun, C.; Gunasekaran, S.; Richards, M.P. Effect of xanthan gum on physicochemical properties of whey protein isolate stabilized oil-in-water emulsions. Food Hydrocoll. 2007, 21, 555–564. [Google Scholar] [CrossRef]
- Chityala, P.K.; Khouryieh, H.; Williams, K.; Conte, E. Effect of xanthan/enzyme-modified guar gum mixtures on the stability of whey protein isolate stabilized fish oil-in-water emulsions. Food Chem. 2016, 212, 332–340. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions: Principles, Practices, and Techniques; CRC Press: Boca Raton, FL, USA, 2015; p. 609. [Google Scholar]
- Velez-Erazo, E.M.; Bosqui, K.; Rabelo, R.S.; Kurozawa, L.E.; Hubinger, M.D. High internal phase emulsions (HIPE) using pea protein and different polysaccharides as stabilizers. Food Hydrocoll. 2020, 105, 105775. [Google Scholar] [CrossRef]
- Liu, F.; Tang, C.-H. Cold, gel-like whey protein emulsions by microfluidisation emulsification: Rheological properties and microstructures. Food Chem. 2011, 127, 1641–1647. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, L.; Wang, B.; Sui, X.; Zhong, Y.; Zhang, L.; Mao, Z.; Xu, H. Cellulose-rich oleogels prepared with an emulsion-templated approach. Food Hydrocoll. 2018, 77, 460–464. [Google Scholar] [CrossRef]
- Meng, Z.; Qi, K.; Guo, Y.; Wang, Y.; Liu, Y. Macro-micro structure characterization and molecular properties of emulsion-templated polysaccharide oleogels. Food Hydrocoll. 2018, 77, 17–29. [Google Scholar] [CrossRef]
- Tang, Y.R.; Ghosh, S. Canola protein thermal denaturation improved emulsion-templated oleogelation and its cake-baking application. RSC Adv. 2021, 11, 25141–25157. [Google Scholar] [CrossRef]
- Baraki, S.Y.; Jiang, Y.; Li, X.; Debeli, D.K.; Wang, B.; Feng, X.; Mao, Z.; Sui, X. Stable sunflower oil oleogel from oil/water pickering emulsion with regenerated chitin. Lwt-Food Sci. Technol. 2021, 146, 111483. [Google Scholar] [CrossRef]
- Wijaya, W.; Sun, Q.-Q.; Vermeir, L.; Dewettinck, K.; Patel, A.R.; Van der Meeren, P. pH and protein to polysaccharide ratio control the structural properties and viscoelastic network of HIPE-templated biopolymeric oleogels. Food Struct. 2019, 21, 100112. [Google Scholar] [CrossRef]
- Pan, J.; Tang, L.; Dong, Q.; Li, Y.; Zhang, H. Effect of oleogelation on physical properties and oxidative stability of camellia oil-based oleogels and oleogel emulsions. Food Res. Int. 2021, 140, 110057. [Google Scholar] [CrossRef]
- Coupland, J.N.; McClements, D.J. Lipid oxidation in food emulsions. Trends Food Sci. Technol. 1996, 7, 83–91. [Google Scholar] [CrossRef]
- Hu, M.; McClements, D.J.; Decker, E.A. Lipid oxidation in corn oil-in-water emulsions stabilized by casein, whey protein isolate, and soy protein isolate. J. Agric. Food Chem. 2003, 51, 1696–1700. [Google Scholar] [CrossRef]
- Fernanadez, L.C.; Romero, C.M.; Rolhaiser, F.; Fogar, A.R.; Doval, M.M. Fat substitutes based on bovine blood plasma and flaxseed oil as functional ingredients. Int. J. Gastron. Food Sci. 2021, 25, 100365. [Google Scholar] [CrossRef]
- Maryam, M.; Hossein Goli, S.A.; Goly, F. Effect of biopolymers concentration and drying methods on physicochemical properties of emulsion-templated oleogel. J. Food Sci. Technol. 2022, 59, 1994–2003. [Google Scholar] [CrossRef]
| Amount/g in a 100 g Basis of Emulsion | 0% | 0.5% | 1% | 2% | 3% | 4% |
|---|---|---|---|---|---|---|
| EWP microgels | 0 | 0.275 | 0.55 | 1.1 | 1.65 | 2.2 |
| XG | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 |
| Oil | 30 | 30 | 30 | 30 | 30 | 30 |
| Water | 69.82 | 69.545 | 69.27 | 68.72 | 68.17 | 67.62 |
| Amount/g in a 100 g Basis of Emulsion | 0% | 0.1% | 0.4% | 0.8% | 1.2% | 1.6% |
|---|---|---|---|---|---|---|
| EWP microgels | 1.1 | 1.1 | 1.1 | 1.1 | 1.1 | 1.1 |
| XG | 0 | 0.0425 | 0.17 | 0.34 | 0.51 | 0.68 |
| Oil | 30 | 30 | 30 | 30 | 30 | 30 |
| Water | 68.9 | 68.8575 | 68.73 | 68.56 | 68.39 | 68.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Y.; Zhang, W.; Liu, R.; Chang, C.; Li, J.; Xiong, W.; Yang, Y.; Gu, L. Emulsion-Templated Liquid Oil Structuring with Egg White Protein Microgel- Xanthan Gum. Foods 2023, 12, 1884. https://doi.org/10.3390/foods12091884
Su Y, Zhang W, Liu R, Chang C, Li J, Xiong W, Yang Y, Gu L. Emulsion-Templated Liquid Oil Structuring with Egg White Protein Microgel- Xanthan Gum. Foods. 2023; 12(9):1884. https://doi.org/10.3390/foods12091884
Chicago/Turabian StyleSu, Yujie, Wanqiu Zhang, Ruidan Liu, Cuihua Chang, Junhua Li, Wen Xiong, Yanjun Yang, and Luping Gu. 2023. "Emulsion-Templated Liquid Oil Structuring with Egg White Protein Microgel- Xanthan Gum" Foods 12, no. 9: 1884. https://doi.org/10.3390/foods12091884
APA StyleSu, Y., Zhang, W., Liu, R., Chang, C., Li, J., Xiong, W., Yang, Y., & Gu, L. (2023). Emulsion-Templated Liquid Oil Structuring with Egg White Protein Microgel- Xanthan Gum. Foods, 12(9), 1884. https://doi.org/10.3390/foods12091884