In Vitro Antimicrobial and Antibiofilm Properties and Bioaccessibility after Oral Digestion of Chemically Characterized Extracts Obtained from Cistus × incanus L., Scutellaria lateriflora L., and Their Combination
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Chemical Characterization of C. incanus and S. lateriflora Extracts Using Reversed-Phase, Ultra-High-Performance Liquid Chromatography (RP-UHPLC) Coupled with a Q-Exactive Hybrid Quadrupole Orbitrap Mass Spectrometer
2.3. In Vitro Bioaccessibility of C. incanus and S. lateriflora Extracts Using Simulated Oral Digestion Processes and RP-UHPLC-Photodiode Array Detector (PDA) Analysis
2.4. Antimicrobial Activity of C. incanus and S. lateriflora Extracts against P. gingivalis
2.5. In Vitro Cell Model Systems
2.5.1. Human Keratinocyte Epithelial Cells (HaCaT)
2.5.2. Cytotoxic Activity of C. incanus and S. lateriflora Extracts on HaCaT Cells
2.5.3. In Vitro Effects of C. incanus and S. lateriflora Extracts on Invasive Capacity of P. gingivalis Targeting HaCaT Cells
2.5.4. Effects of C. incanus and S. lateriflora Extracts on Pre-Formed Biofilm Mass Reduction
2.6. Statistical Analysis
3. Results
3.1. Metabolic Profile of C. incanus and S. lateriflora Extracts
3.2. Bioaccessibility of C. incanus and S. lateriflora Extracts after In Vitro Simulated Oral Digestion
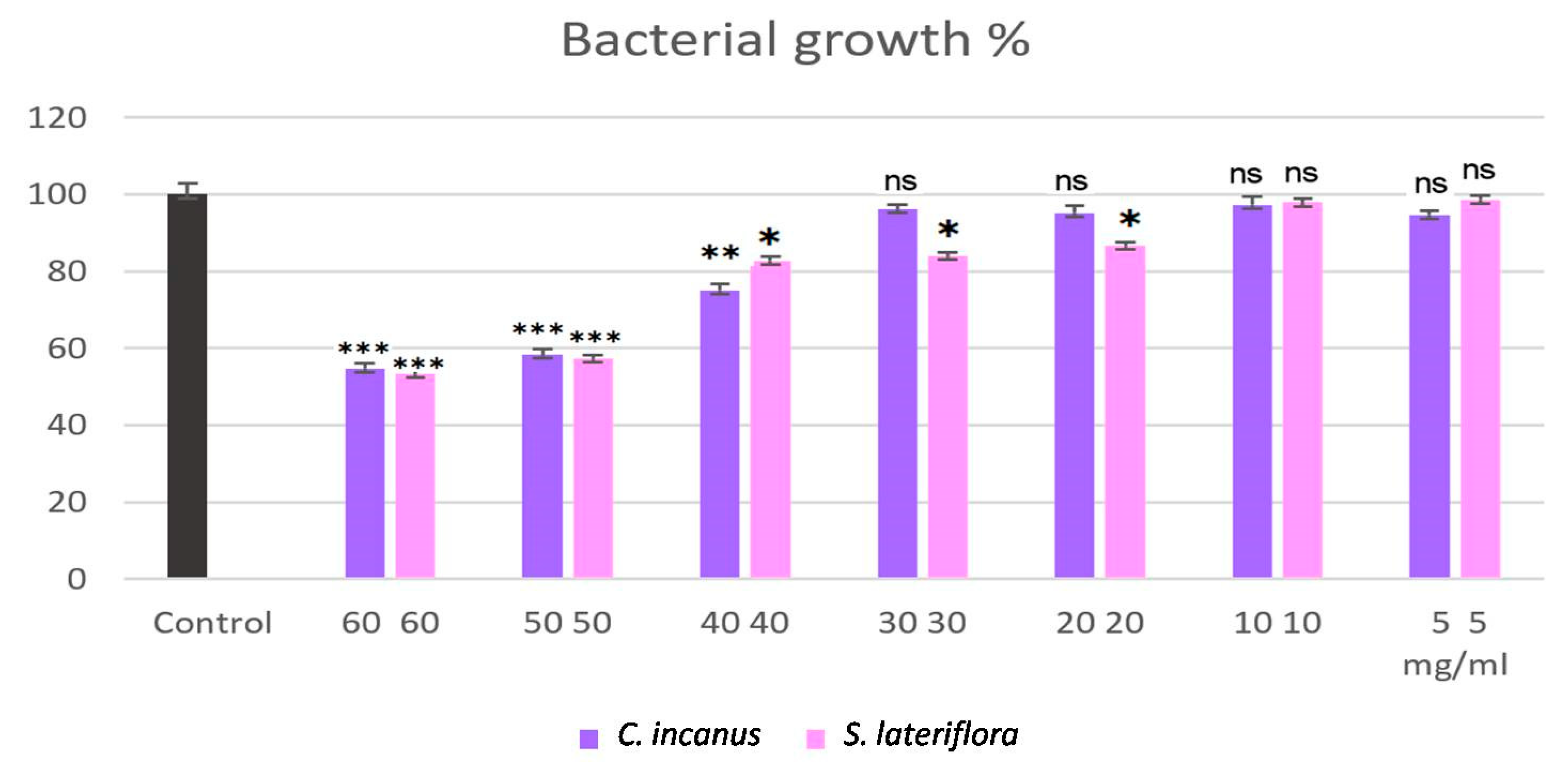
3.3. Antibacterial Activity of C. incanus and S. lateriflora Extracts against P. gingivalis
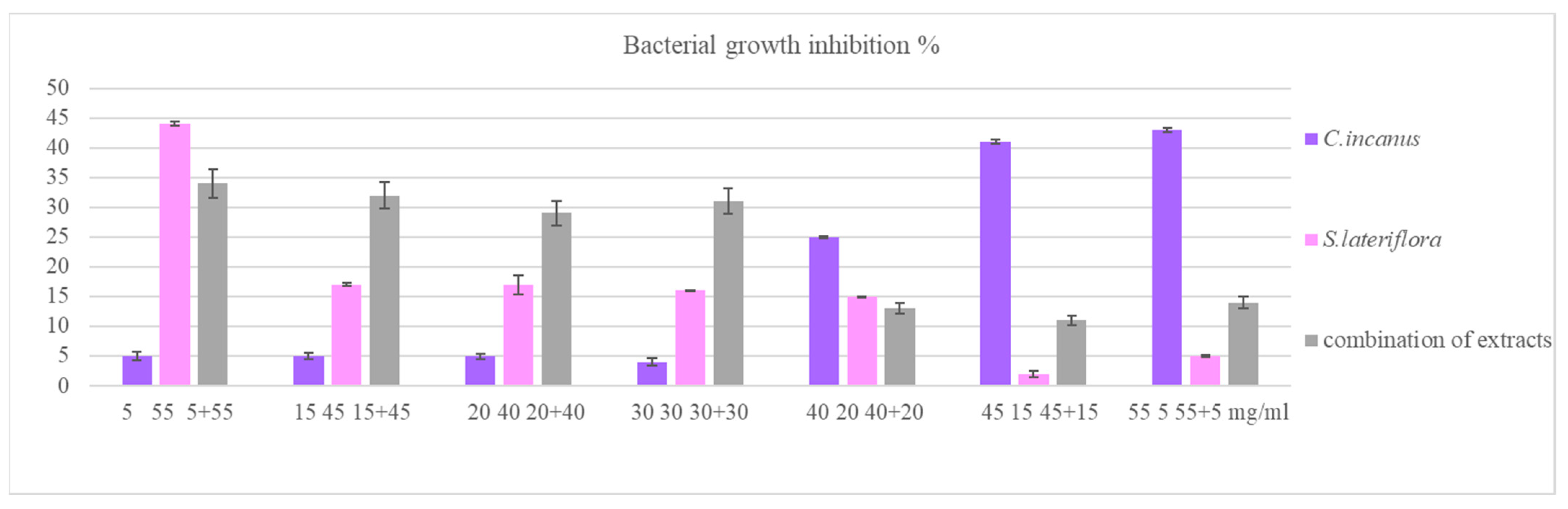
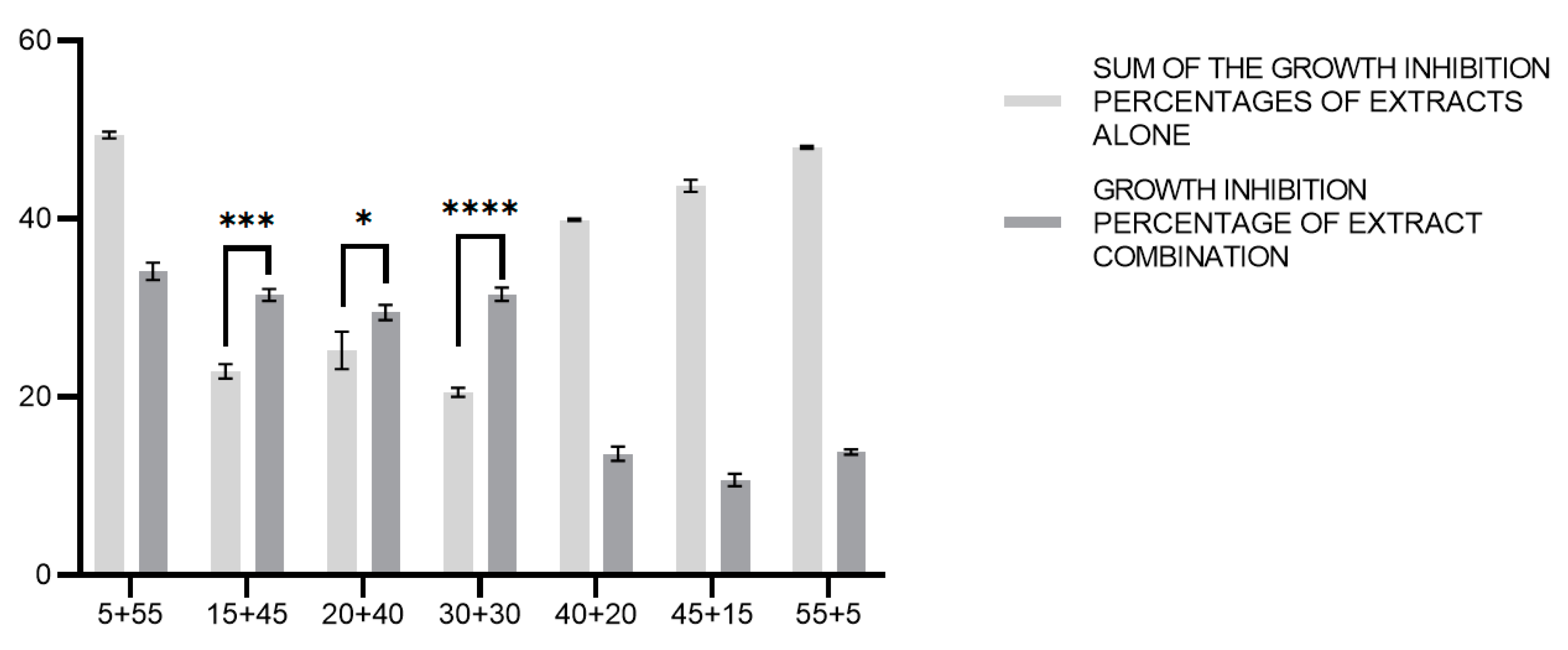
3.4. Modulating Effects of C. incanus and S. lateriflora Extracts and Their Combinations on P. gingivalis Cell Invasive Capacity
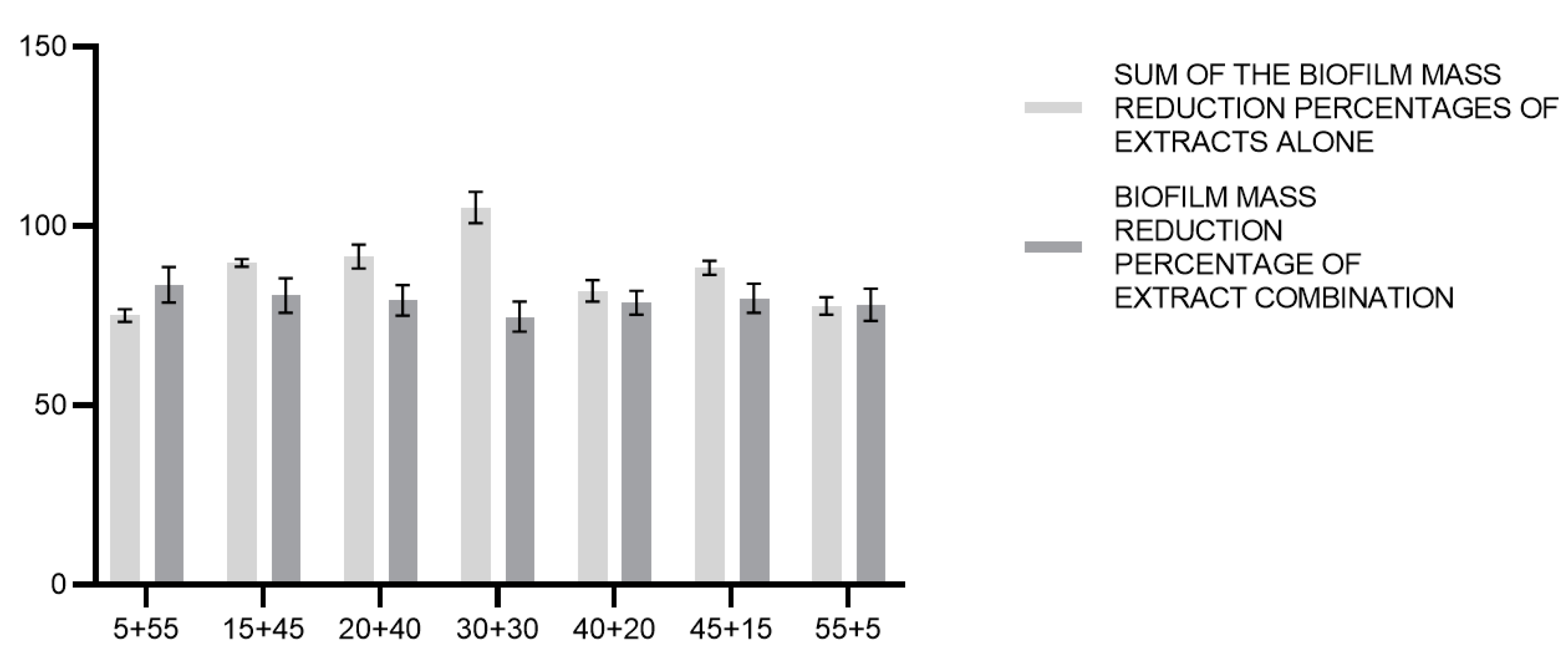
3.5. Effects of C. incanus and S. lateriflora Extracts and Their Combinations on the Degradation of Pre-Formed P. gingivalis Biofilm
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeffcoat, M.K.; Hauth, J.C.; Geurs, N.C.; Reddy, M.S.; Cliver, S.P.; Hodgkins, P.M.; Goldenberg, R.L. Periodontal disease and preterm birth: Results of a pilot intervention study. J. Periodontol. 2003, 74, 1214–1218. [Google Scholar] [CrossRef] [PubMed]
- Gotsman, I.; Lotan, C.; Soskolne, W.A.; Rassovsky, S.; Pugatsch, T.; Lapidus, L.; Novikov, Y.; Masrawa, S.; Stabholz, A. Periodontal destruction is associated with coronary artery disease and periodontal infection with acute coronary syndrome. J. Periodontol. 2007, 78, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Chavakis, T.; Lambris, J.D. Current understanding of periodontal disease pathogenesis and targets for host-modulation therapy. Periodontology 2000 2020, 84, 14–34. [Google Scholar] [CrossRef] [PubMed]
- Armitage, G.C. Periodontal diagnoses and classification of periodontal diseases. Periodontology 2000 2004, 34, 9–21. [Google Scholar] [CrossRef]
- Rosier, B.T.; Marsh, P.D.; Mira, A. Resilience of the oral microbiota in health: Mechanisms that prevent dysbiosis. J. Dent. Res. 2018, 97, 371–380. [Google Scholar] [CrossRef]
- Herrero, E.R.; Fernandes, S.; Verspecht, T.; Ugarte-Berzal, E.; Boon, N.; Proost, P.; Bernaerts, K.; Quirynen, M.; Teughels, W. Dysbiotic biofilms deregulate the periodontal inflammatory response. J. Dent. Res. 2018, 97, 547–555. [Google Scholar] [CrossRef]
- Hajishengallis, G. The inflammophilic character of the periodontitis-associated microbiota. Mol. Oral Microbiol. 2014, 29, 248–257. [Google Scholar] [CrossRef]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Prim. 2017, 3, 17038. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention, Periodontal Disease. 2013. Available online: https://www.cdc.gov/oralhealth/conditions/periodontal-disease.html (accessed on 2 November 2022).
- Qasim, S.S.B.; Al-Otaibi, D.; Al-Jasser, R.; Gul, S.S.; Zafar, M.S. An evidence-based update on the molecular mechanisms underlying periodontal diseases. Int. J. Mol. Sci. 2020, 21, 3829. [Google Scholar] [CrossRef]
- Loos, B.G. Systemic markers of inflammation in periodontitis. J. Periodontol. 2005, 76, 2106–2115. [Google Scholar] [CrossRef]
- Martínez-García, M.; Hernández-Lemus, E. Periodontal inflammation and systemic diseases: An overview. Front. Physiol. 2021, 12, 709438. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. 2017, 11, 72–80. [Google Scholar]
- Bonez, P.C.; dos Santos Alves, C.F.; Dalmolin, T.V.; Agertt, V.A.; Mizdal, C.R.; da Costa Flores, V.; Marques, J.B.; Santos, R.C.V.; de Campos, M.M.A. Chlorhexidine activity against bacterial biofilms. Am. J. Infect. Control 2013, 41, e119–e122. [Google Scholar] [CrossRef] [PubMed]
- Buxser, S. Has resistance to chlorhexidine increased among clinically-relevant bacteria? A systematic review of time course and subpopulation data. PLoS ONE 2021, 16, e0256336. [Google Scholar] [CrossRef]
- Cruz Martínez, C.; Diaz Gómez, M.; Oh, M.S. Use of traditional herbal medicine as an alternative in dental treatment in Mexican dentistry: A review. Pharm. Biol. 2017, 55, 1992–1998. [Google Scholar] [CrossRef]
- Kumar, G.; Jalaluddin, M.D.; Rout, P.; Mohanty, R.; Dileep, C.L. Emerging trends of herbal care in dentistry. J. Clin. Diagn. Res. 2013, 7, 1827–1829. [Google Scholar]
- Irani, S. Herbal medicine and oral health: A review. J. Int. Oral Health 2016, 8, 989–994. [Google Scholar]
- Hannig, C.; Spitzmüller, B.; Al-Ahmad, A.; Hannig, M. Effects of Cistus-tea on bacterial colonization and enzyme activities of the in situ pellicle. J. Dent. 2008, 36, 540–545. [Google Scholar] [CrossRef]
- Hickl, J.; Argyropoulou, A.; Sakavitsi, M.E.; Halabalaki, M.; Al-Ahmad, A.; Hellwig, E.; Aligiannis, N.; Skaltsounis, A.L.; Wittmer, A.; Vach, K.; et al. Mediterranean herb extracts inhibit microbial growth of representative oral microorganisms and biofilm formation of Streptococcus mutans. PLoS ONE 2018, 13, e0207574. [Google Scholar] [CrossRef]
- Sherman, S.H.; Joshee, N. Current status of research on medicinal plant Scutellaria lateriflora: A review. J. Med. Act. Plants 2022, 11, 22–38. [Google Scholar]
- Leung, K.C.F.; Seneviratne, C.J.; Li, X.; Leung, P.C.; Lau, C.B.S.; Wong, C.H.; Pang, K.Y.; Wong, C.W.; Wat, E.; Jin, L. Synergistic antibacterial effects of nanoparticles encapsulated with Scutellaria baicalensis and pure chlorhexidine on oral bacterial biofilms. Nanomaterials 2016, 6, 61. [Google Scholar] [CrossRef] [PubMed]
- Sommella, E.; Pagano, F.; Salviati, E.; Chieppa, M.; Bertamino, A.; Manfra, M.; Sala, M.; Novellino, E.; Campiglia, P. Chemical profiling of bioactive constituents in hop cones and pellets extracts by online comprehensive two-dimensional liquid chromatography with tandem mass spectrometry and direct infusion Fourier transform ion cyclotron resonance mass spectrometry. J. Sep. Sci. 2018, 41, 1548–1557. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardized static in vitro digestion method suitable for food–an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Schilling, D.; Pittelkow, M.R.; Kumar, R. IEX-1, an immediate early gene, increases the rate of apoptosis in keratinocytes. Oncogene 2001, 20, 7992–7997. [Google Scholar] [CrossRef]
- Misba, L.; Zaidi, S.; Khan, A.U. A comparison of antibacterial and antibiofilm efficacy of phenothiazinium dyes between Gram positive and Gram negative bacterial biofilm. Photodiagn. Photodyn. Ther. 2017, 18, 24–33. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis: Chemical analysis working group (CAWG) metabolomics standards initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Sao, P.; Vats, S.; Singh, S. Porphyromonas gingivalis resistance and virulence: An integrated functional network analysis. Gene 2022, 839, 146734. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Zhu, X.; Cao, P.; Wei, S.; Lu, Y. Antibacterial and antibiofilm activities of eugenol from essential oil of Syzygium aromaticum (L.) Merr. & LM Perry (clove) leaf against periodontal pathogen Porphyromonas gingivalis. Microb. Pathog. 2017, 113, 396–402. [Google Scholar]
- Müller-Heupt, L.K.; Vierengel, N.; Groß, J.; Opatz, T.; Deschner, J.; von Loewenich, F.D. Antimicrobial activity of Eucalyptus globulus, Azadirachta indica, Glycyrrhiza glabra, Rheum palmatum extracts and rhein against Porphyromonas gingivalis. Antibiotics 2022, 11, 186. [Google Scholar] [CrossRef]
- Awad, R.; Arnason, J.T.; Trudeau, V.; Bergeron, C.; Budzinski, J.W.; Foster, B.C.; Merali, Z. Phytochemical and biological analysis of skullcap (Scutellaria lateriflora L.): A medicinal plant with anxiolytic properties. Phytomedicine 2003, 10, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Bernacka, K.; Bednarska, K.; Starzec, A.; Mazurek, S.; Fecka, I. Antioxidant and antiglycation effects of Cistus × incanus water infusion, its phenolic components, and respective metabolites. Molecules 2022, 27, 2432. [Google Scholar] [CrossRef] [PubMed]
- Wittpahl, G.; Kölling-Speer, I.; Basche, S.; Herrmann, E.; Hannig, M.; Speer, K.; Hannig, C. The polyphenolic composition of Cistus incanus herbal tea and its antibacterial and anti-adherent activity against Streptococcus mutans. Planta Med. 2015, 81, 1727–1735. [Google Scholar] [CrossRef] [PubMed]
- Jeszka-Skowron, M.; Zgoła-Grześkowiak, A.; Frankowski, R. Cistus incanus a promising herbal tea rich in bioactive compounds: LC–MS/MS determination of catechins, flavonols, phenolic acids and alkaloids—A comparison with Camellia sinensis, Rooibos and Hoan Ngoc herbal tea. J. Food Compos. Anal. 2018, 74, 71–81. [Google Scholar] [CrossRef]
- Riehle, P.; Vollmer, M.; Rohn, S. Phenolic compounds in Cistus incanus herbal infusions—Antioxidant capacity and thermal stability during the brewing process. Food Res. Int. 2013, 53, 891–899. [Google Scholar] [CrossRef]
- Fecka, I.; Włodarczyk, M.; Starzec, A. Isolation and structure elucidation of cistusin: A new ellagitannin from Cistus × incanus L. leaves. Ind. Crops Prod. 2020, 158, 112971. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.H.; Smillie, T.J.; Khan, I.A. Identification of phenolic compounds from Scutellaria lateriflora by liquid chromatography with ultraviolet photodiode array and electrospray ionization tandem mass spectrometry. J. Pharm. Biomed. Anal. 2012, 63, 120–127. [Google Scholar] [CrossRef]
- Kuroda, M.; Iwabuchi, K.; Mimaki, Y. Chemical constituents of the aerial parts of Scutellaria lateriflora and their α-glucosidase inhibitory activities. Nat. Prod. Commun. 2012, 7, 1934578X1200700413. [Google Scholar] [CrossRef]
- Kozłowska, M.; Ścibisz, I.; Przybył, J.L.; Laudy, A.E.; Majewska, E.; Tarnowska, K.; Małajowicz, J.; Ziarno, M. Antioxidant and antibacterial activity of extracts from selected plant material. Appl. Sci. 2022, 12, 9871. [Google Scholar] [CrossRef]
- Duffy, C.F.; Power, R.F. Antioxidant and antimicrobial properties of some Chinese plant extracts. Int. J. Antimicrob. Agents 2001, 17, 527–529. [Google Scholar] [CrossRef]
- Hunstad, D.A.; Justice, S.S. Intracellular lifestyles and immune evasion strategies of uropathogenic Escherichia coli. Annu. Rev. Microbiol. 2010, 64, 203–221. [Google Scholar] [CrossRef] [PubMed]
- Mah, T.F. Biofilm-specific antibiotic resistance. Future Microbiol. 2012, 7, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Martínez, F.J.; Borrás-Rocher, F.; Micol, V.; Barrajón-Catalán, E. Artificial intelligence applied to improve scientific reviews: The antibacterial activity of Cistus plants as proof of concept. Antibiotics 2023, 12, 327. [Google Scholar] [CrossRef] [PubMed]
- Nur Onal, F.; Ozturk, I.; Aydin Kose, F.; Der, G.; Kilinc, E.; Baykan, S. Comparative evaluation of polyphenol contents and biological activities of five Cistus L. species native to Turkey. Chem. Biodivers. 2023, 20, e202200915. [Google Scholar] [CrossRef]
- Chen, W.; Li, B.; Li, S.; Ou, Y.W.; Ou, Q. Effects of Scutellaria baicalensis on activity and biofilm formation of Klebsiella pneumoniae. Chin. Med. Sci. J. 2016, 31, 180–184. [Google Scholar] [CrossRef]
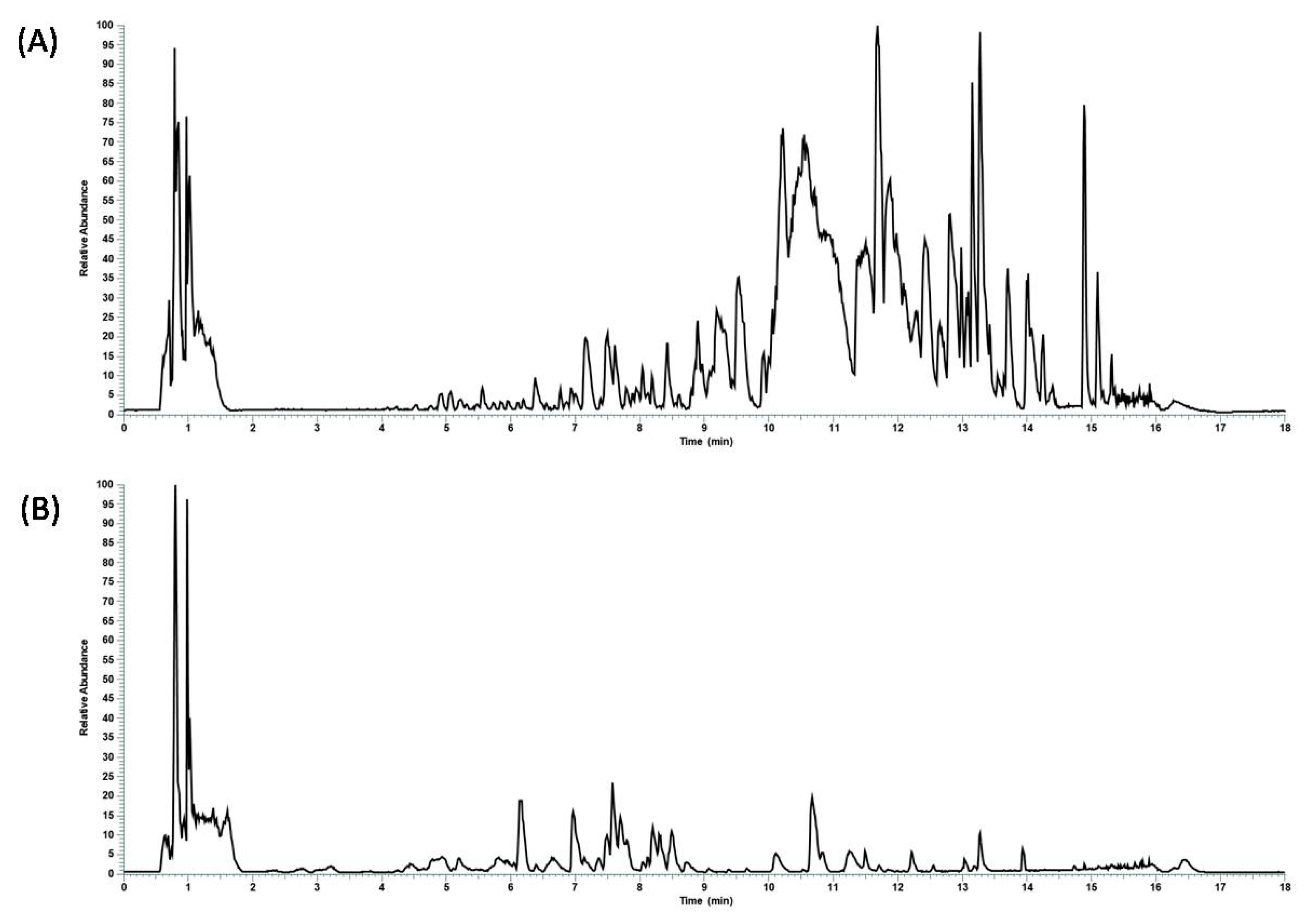

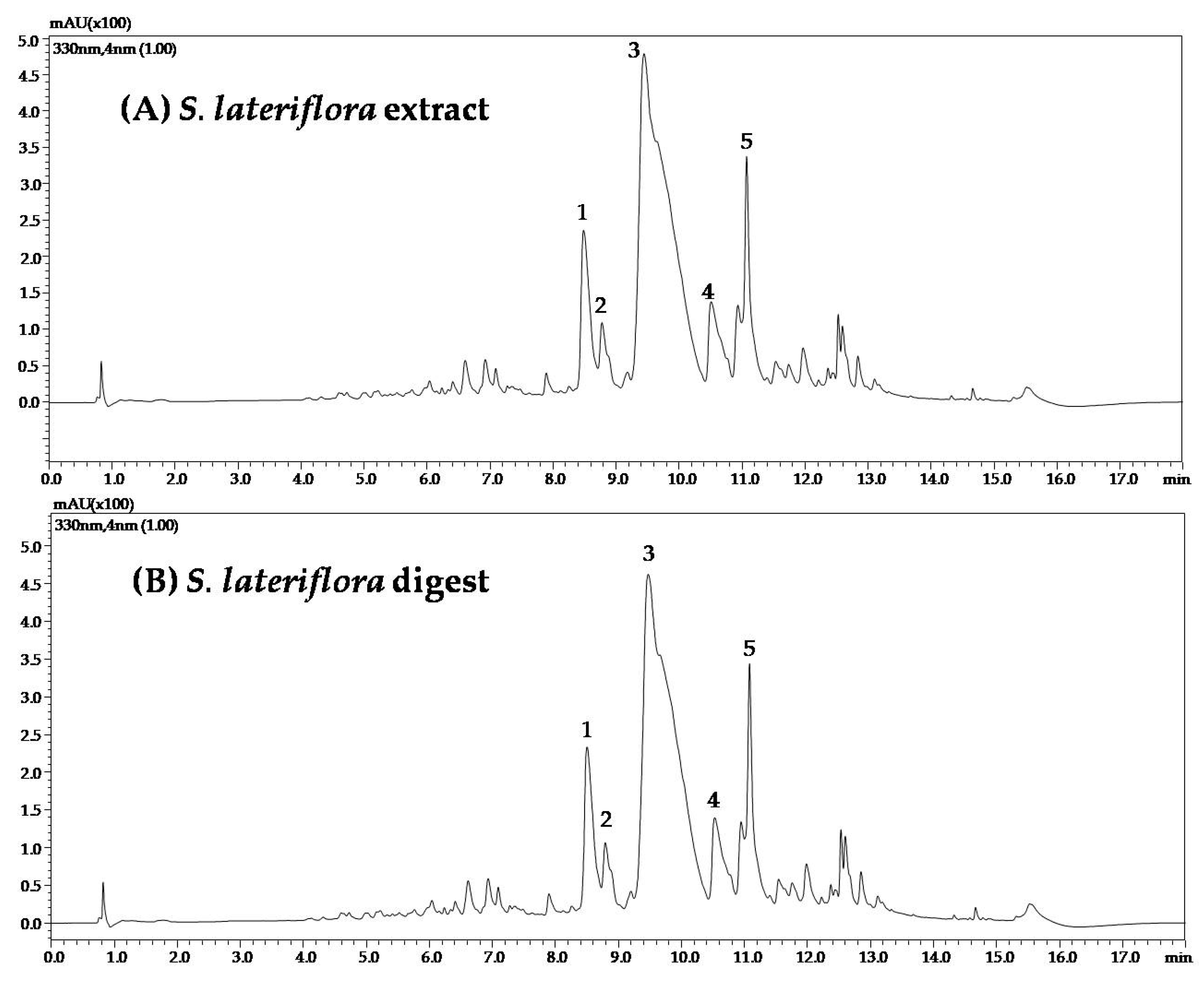

| C. incanus Compound | RT (min) | Mean Area before Digestion | Mean Area after Digestion | Area Reduction Percentage (%) |
|---|---|---|---|---|
| Myricetin 3-hexoside | 6.45 | 3.66 × 105 | 3.42× 105 | 6.5 |
| Myricetin 3 alpha L-arabinofuranoisde | 6.95 | 1.05 × 105 | 9.25 × 104 | 11.6 |
| Quercetin-3-O-glucopyranoside | 7.06 | 3.93 × 105 | 3.76 × 105 | 4.3 |
| Quercetin-3-O-glucopyranoside isomer | 7.17 | 4.13 × 105 | 3.75 × 105 | 9.1 |
| Gujaverin | 7.69 | 1.54 × 105 | 1.45 × 105 | 6.4 |
| Gujaverin isomer | 7.79 | 7.40 × 104 | 6.61 × 104 | 10.6 |
| Kaempferol 3-(3″-p-coumaoroylhexoside) | 10.06 | 4.20 × 105 | 2.27 × 105 | 46.0 |
| S. lateriflora Compounds | RT (min) | Mean Area Phytcomplex | Mean Area Digest | Area Reduction Percentage (%) |
|---|---|---|---|---|
| Scutellarin | 8.48 | 1.15 × 106 | 1.05 × 106 | 8.9 |
| Isoscutellarin | 8.78 | 4.66 × 105 | 4.67 × 105 | 0.0 |
| Baicalein-6-glucuronide | 9.44 | 3.01 × 107 | 2.99 × 107 | 1.0 |
| Quercitrin | 10.51 | 1.00 × 106 | 9.32 × 105 | 7.1 |
| Oroxylin A-glucuronide | 11.07 | 3.39 × 106 | 3.39 × 106 | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, H.; Minno, A.D.; Filippis, A.D.; Sommella, E.; Buccato, D.G.; Lellis, L.F.D.; El-Seedi, H.R.; Khalifa, S.A.M.; Piccinocchi, R.; Galdiero, M.; et al. In Vitro Antimicrobial and Antibiofilm Properties and Bioaccessibility after Oral Digestion of Chemically Characterized Extracts Obtained from Cistus × incanus L., Scutellaria lateriflora L., and Their Combination. Foods 2023, 12, 1826. https://doi.org/10.3390/foods12091826
Ullah H, Minno AD, Filippis AD, Sommella E, Buccato DG, Lellis LFD, El-Seedi HR, Khalifa SAM, Piccinocchi R, Galdiero M, et al. In Vitro Antimicrobial and Antibiofilm Properties and Bioaccessibility after Oral Digestion of Chemically Characterized Extracts Obtained from Cistus × incanus L., Scutellaria lateriflora L., and Their Combination. Foods. 2023; 12(9):1826. https://doi.org/10.3390/foods12091826
Chicago/Turabian StyleUllah, Hammad, Alessandro Di Minno, Anna De Filippis, Eduardo Sommella, Daniele Giuseppe Buccato, Lorenza Francesca De Lellis, Hesham R. El-Seedi, Shaden A. M. Khalifa, Roberto Piccinocchi, Massimiliano Galdiero, and et al. 2023. "In Vitro Antimicrobial and Antibiofilm Properties and Bioaccessibility after Oral Digestion of Chemically Characterized Extracts Obtained from Cistus × incanus L., Scutellaria lateriflora L., and Their Combination" Foods 12, no. 9: 1826. https://doi.org/10.3390/foods12091826
APA StyleUllah, H., Minno, A. D., Filippis, A. D., Sommella, E., Buccato, D. G., Lellis, L. F. D., El-Seedi, H. R., Khalifa, S. A. M., Piccinocchi, R., Galdiero, M., Campiglia, P., & Daglia, M. (2023). In Vitro Antimicrobial and Antibiofilm Properties and Bioaccessibility after Oral Digestion of Chemically Characterized Extracts Obtained from Cistus × incanus L., Scutellaria lateriflora L., and Their Combination. Foods, 12(9), 1826. https://doi.org/10.3390/foods12091826












