Kombucha Beverages Produced from Fruits, Vegetables, and Plants: A Review on Their Pharmacological Activities and Health Benefits
Abstract
1. Introduction
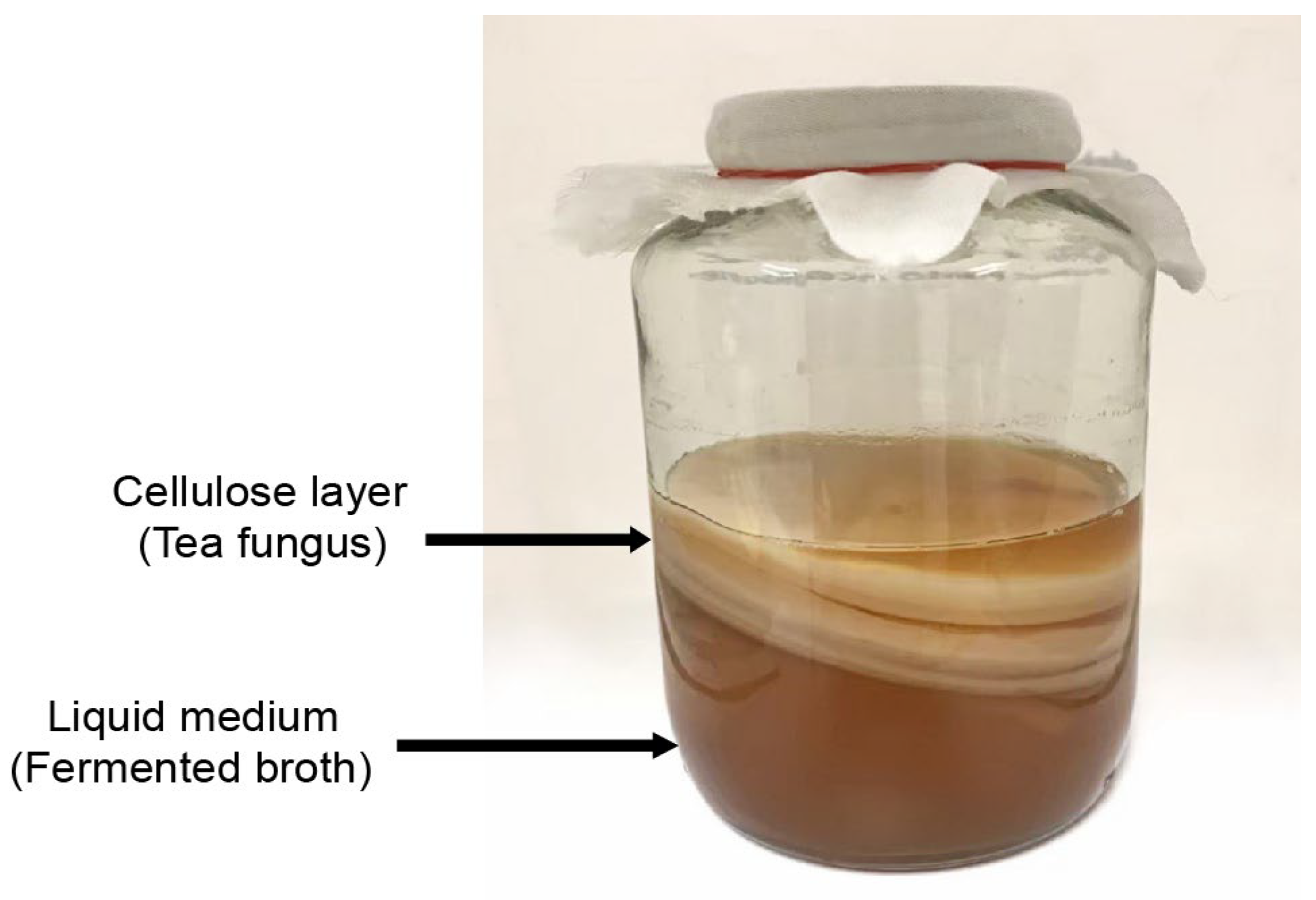
2. Kombucha
2.1. Symbiotic Culture of Bacteria and Yeast (SCOBY)
2.1.1. Bacteria
2.1.2. Yeast
2.2. Fermentation Process
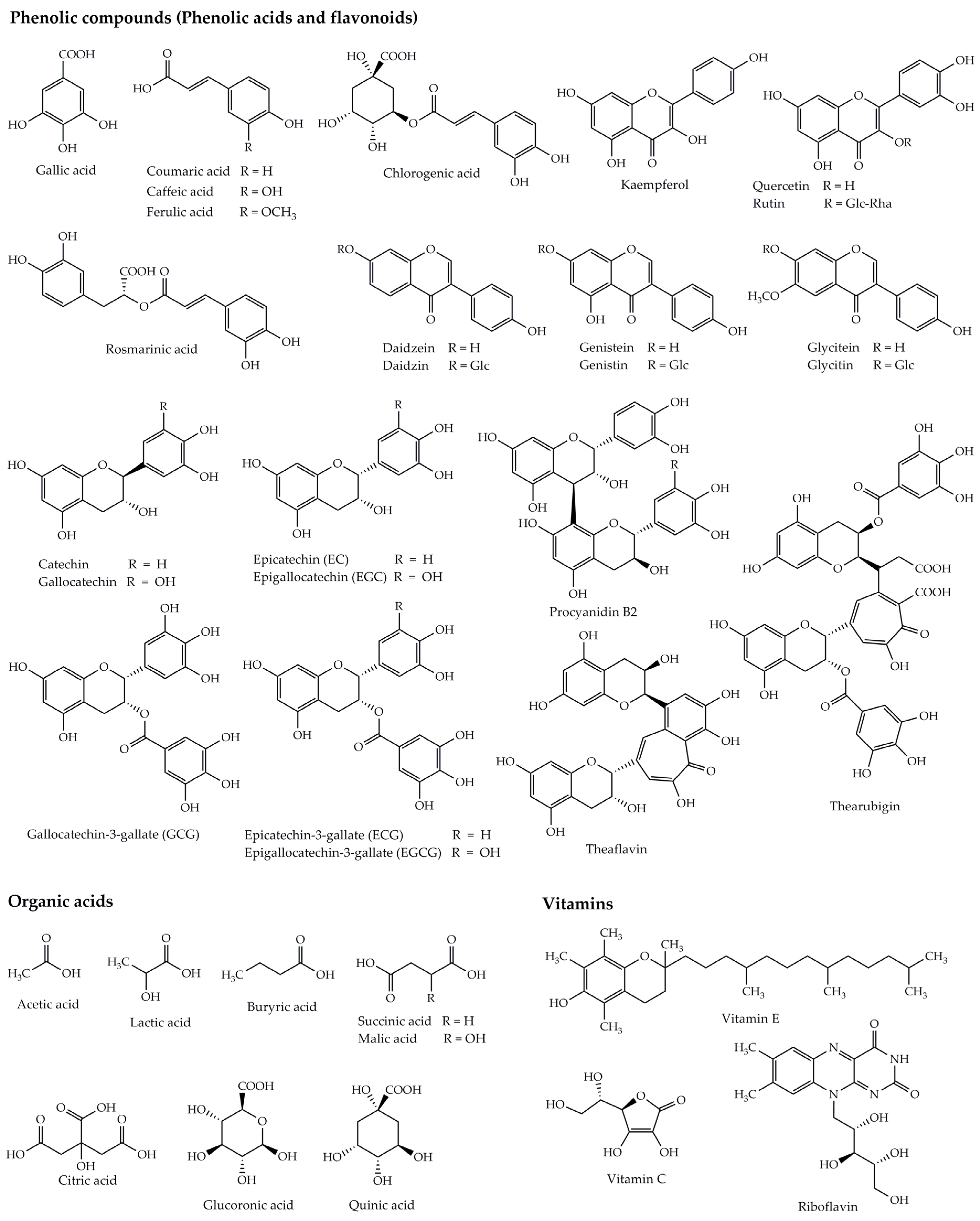
2.3. Substances and Biological Components Found in Kombucha Beverages
2.4. Adverse Effects and Toxicities of Kombucha Beverages
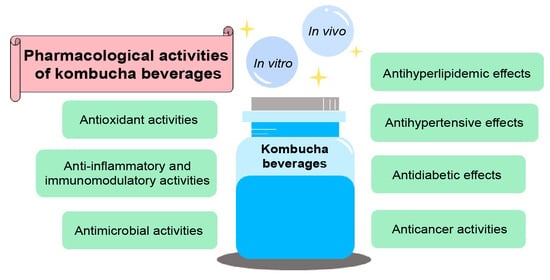
3. Pharmacological Activities of Kombucha Beverages
3.1. Antioxidant Activities
| Name of Substrates | Active Ingredients | Biological Assays | Findings | Ref. |
|---|---|---|---|---|
| In vitro studies | ||||
| Soy milk |
|
|
| [14] |
| Soy whey |
|
|
| [15] |
| Coffee |
|
|
| [24] |
| Apples, pears, carrots |
|
|
| [48] |
| Black and green teas and rooibos leaves (Aspalathus linearis) |
|
|
| [54] |
| Yerba-maté (Ilex paraguariensis) |
|
|
| [58] |
| Arabica green coffee |
|
|
| [59] |
| Black tea and Javanese turmeric |
|
|
| [60] |
| Black tea and lemon balm |
|
|
| [61] |
| Black tea and purple basil (Ocimum basilicum) |
|
|
| [62] |
| Oolong tea, kitchen mint (Mentha cordifolia) |
|
|
| [63] |
| Black tea, green tea, winter savory (Satureja montana), peppermint (Mentha×piperita), stinging nettle (Urtica dioica), wild thyme (Thymus serpyllum), elderberry (Sambucus nigra), and quince (Cydonia oblonga) |
|
|
| [64] |
| Gum arabic tree Acacia arabica (AA), Aegle marmelos root bark (AM-RB), Aerva lanata (Ala), Asteracan-tha longifolia (Alo), Cassia auriculata (CA), Hemidesmus indicus (HI), Hordeum vulgare (HV), Phyllanthus emblica (PE), Tinospora cordifolia (TC) |
|
|
| [65] |
| Black tea, white oak leaves (Quercus resinosa, Q. arizonica, Q. convallata) |
|
|
| [66] |
| Oak leaves (Q. arizonica, Q. convallata) |
|
|
| [67] |
| Oolong tea, royal lotus pollen (Nelumbo nucifera), butterfly pea flower (Clitoria ternatea) |
|
|
| [68] |
| Yarrow (Achillea millefolium) |
|
|
| [69] |
| Black tea and garlic |
|
|
| [70] |
| Broccoli (Brassica oleracea) and spinach (Amaranthus spp.) |
|
|
| [71] |
| African mustard (Brassica tournefortii) |
|
|
| [72] |
| Black carrot (obtained from Konya and Hatay varieties) and green tea |
|
|
| [73] |
| Black and green teas and laver (Porphyra dentata) |
|
|
| [74] |
| Apple varieties (Anna, Fuji, Granny Smith, Manalagi, Red Delicious, Rome Beauty, Royal Gala) |
|
|
| [75] |
| Snake fruit (Salacca zalacca) |
|
|
| [76,77] |
| Cactus pear |
|
|
| [78] |
| Red grape |
|
|
| [79] |
| Date palm (Phoenix dactylifera) fruit and black tea |
|
|
| [80] |
| King coconut (Cocos nucifera var. aurantiaca) |
|
|
| [81] |
| Red goji berry (Lycium barbarum), black goji berry (Lycium ruthenicum), and black tea |
|
|
| [82] |
| Acerola |
|
|
| [83] |
| Green tea, pitanga (Eugenia uniflora), and umbu-caja’ (Spondia tuberosa) fruit |
|
|
| [84] |
| In vivo studies | ||||
| Black tea |
|
|
| [46] |
| Green tea |
|
|
| [51] |
| Black tea |
|
|
| [55] |
| Black tea |
|
|
| [56] |
| Oak leaves (Q. arizonica and Q. convallata) |
|
|
| [67] |
| Snake fruit (S. zalacca) and black tea |
|
|
| [77] |
3.2. Anti-Inflammatory and Immunomodulatory Activities
3.3. Antimicrobial Activities
| Name of Substrates | Active Ingredients | Biological Assays | Findings | Ref. |
|---|---|---|---|---|
| In vitro studies of antifungal activities | ||||
| Black and green teas |
|
|
| [23] |
| Yarrow (Achillea millefolium) |
|
|
| [69] |
| Black tea |
|
|
| [106] |
| Black and green teas |
|
|
| [107] |
| Black tea |
|
|
| [109] |
| Lemon balm (Melissa officinalis) |
|
|
| [112] |
| Thyme (Thymus vulgaris), lemon verbena (Lippia citriodora), rosemary (Rosmarinus officinalis), fennel (Foeniculum vulgare), and peppermint (Mentha piperita) |
|
|
| [113] |
| Black tea |
|
|
| [115] |
| In vivo studies of antiviral activities | ||||
| Licorice (Glycyrrhizae Radix), Grosvenor Momordica (Momordica Grosvenori), Chry-santhemum (Dendranthema morifolium), and green tea (CamelliaSinensis) |
|
|
| [116] |
3.4. Anticancer Activities
3.5. Antidiabetic Activities
3.6. Antihypertensive Effects
3.7. Antihyperlipidemic Effects
4. Conclusions
5. Limitation
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Jayabalan, R.; Waisundara, V.Y. Kombucha as a functional beverage. In Functional and Medicinal Beverages, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 413–446. [Google Scholar]
- Laavanya, D.; Shirkole, S.; Balasubramanian, P. Current challenges, applications and future perspectives of SCOBY cellulose of kombucha fermentation. J. Clean. Prod. 2021, 295, 126454. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.P.; Taillandier, P. Understanding kombucha tea fermentation: A review. J. Food Sci. 2018, 83, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Jayabalan, R.; Malbaša, R.V.; Lončar, E.S.; Vitas, J.S.; Sathishkumar, M. A review on kombucha tea-microbiology, composition, fermentation, beneficial effects, toxicity, and tea fungus. Compr. Rev. Food Sci. Food Saf. 2014, 13, 538–550. [Google Scholar] [CrossRef]
- Bortolomedi, B.M.; Paglarin, C.S.; Brod, F.C.A. Bioactive compounds in kombucha: A review of substrate effect and fermentation conditions. Food Chem. 2022, 385, 132719. [Google Scholar] [CrossRef] [PubMed]
- Antolak, H.; Piechota, D.; Kucharska, A. Kombucha tea-A double power of bioactive compounds from tea and symbiotic culture of bacteria and yeasts (SCOBY). Antioxidants 2021, 10, 1541. [Google Scholar] [CrossRef] [PubMed]
- Diez-Ozaeta, I.; Astiazaran, O.J. Recent advances in Kombucha tea: Microbial consortium, chemical parameters, health implications and biocellulose production. Int. J. Food Microbiol. 2022, 377, 109783. [Google Scholar] [CrossRef]
- de Miranda, J.F.; Ruiz, L.F.; Silva, C.B.; Uekane, T.M.; Silva, K.A.; Gonzalez, A.G.M.; Fernandes, F.F.; Lima, A.R. Kombucha: A review of substrates, regulations, composition, and biological properties. J. Food Sci. 2022, 87, 503–527. [Google Scholar] [CrossRef]
- Leal, J.M.; Suárez, L.V.; Jayabalan, R.; Oros, J.H.; Escalantr-Aburto, A. A review on health benefits of kombucha nutritional compounds and metabolites. CYTA J. Food 2018, 16, 390–399. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; González-Paramás, A.M.; Oludemi, T.; Ayuda-Durán, B.; González-Manzano, S. Plant phenolics as functional food ingredients. Adv. Food Nutr. Res. 2019, 90, 183–257. [Google Scholar]
- Dutta, H.; Paul, S.K. Kombucha drink: Production, quality, and safety aspects. In Production and Management of Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 259–288. [Google Scholar]
- Sinir, G.Ö.; Tamer, C.E.; Suna, S. Kombucha tea: A promising fermented functional beverage. In Fermented Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 401–432. [Google Scholar]
- Kapp, J.M.; Sumner, W. Kombucha: A systematic review of the empirical evidence of human health benefit. Ann. Epidemiol. 2019, 30, 66–70. [Google Scholar] [CrossRef]
- Xia, X.; Dai, Y.; Wu, H.; Liu, X.; Wang, Y.; Yin, L.; Wang, Z.; Li, X.; Zhou, J. Kombucha fermentation enhances the health-promoting properties of soymilk beverage. J. Funct. Foods 2019, 62, 103549. [Google Scholar] [CrossRef]
- Tu, C.; Tang, S.; Azi, F.; Hu, W.; Dong, M. Use of kombucha consortium to transform soy whey into a novel functional beverage. J. Funct. Foods 2019, 52, 81–89. [Google Scholar] [CrossRef]
- Vīna, I.; Semjonovs, P.; Linde, R.; Deniņa, I. Current evidence on physiological activity and expected health effects of kombucha fermented beverage. J. Med. Food 2014, 17, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Vargas, B.K.; Fabricio, M.F.; Ayub, M.A.Z. Health effects and probiotic potential of kombucha: A bibliometric and systematic review. Food Biosci. 2021, 44, 101332. [Google Scholar] [CrossRef]
- Chakravorty, S.; Bhattacharya, S.; Chatzinotas, A.; Chakraborty, W.; Bhattacharya, D.; Gachhui, R. Kombucha tea fermentation: Microbial and biochemical dynamics. Int. J. Food Microbiol. 2016, 220, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Marsh, A.J.; O’Sullivan, O.; Hill, C.; Ross, R.P.; Cotter, P.D. Sequence-based analysis of the bacterial and fungal compositions of multiple kombucha (tea fungus) samples. Food Microbiol. 2014, 38, 171–178. [Google Scholar] [CrossRef]
- Dufrense, C.; Farnworth, E. Tea, Kombucha, and health: A review. Food Res. Int. 2000, 33, 409–421. [Google Scholar]
- Sievers, M.; Lanini, C.; Weber, A.; Schuler-Schmid, U.; Teuber, M. Microbiology and fermentation balance in a kombucha beverage obtained from a tea fungus fermentation. Syst. Appl. Microbiol. 1995, 18, 590–594. [Google Scholar] [CrossRef]
- Nguyen, N.K.; Nguyen, P.B.; Nguyen, H.T.; Le, P.H. Screening the optimal ratio of symbiosis between isolated yeast and acetic acid bacteria strain from traditional kombucha for high-level production of glucuronic acid. LWT Food Sci. Technol. 2015, 64, 1149–1155. [Google Scholar] [CrossRef]
- Greenwalt, C.J.; Ledford, R.A.; Steinkraus, K.H. Determination and characterization of the antimicrobial activity of the fermented tea kombucha. LWT Food Sci. Technol. 1998, 31, 291–296. [Google Scholar] [CrossRef]
- Watawana, M.I.; Jayawardena, N.; Waisundara, V.Y. Enhancement of the functional properties of coffee through fermentation by “tea fungus” (kombucha). J. Food Process. Preserv. 2015, 39, 2596–2603. [Google Scholar] [CrossRef]
- Vijayaraghavan, R.; Singh, M.; Rao, P.; Bhattacharya, R.; Kumar, P.; Sugendran, K.; Kumar, O.; Pant, S.; Singh, R. Subacute (90 days) oral toxicity studies of Kombucha tea. Biomed. Environ. Sci. 2000, 13, 293–299. [Google Scholar] [PubMed]
- Pauline, T.; Dipti, P.; Anju, B.; Kavimani, S.; Sharma, S.; Kain, A.; Sarada, S.; Sairam, M.; Ilavazhagan, G.; Devendra, K. Studies on toxicity, anti-stress and hepato-protective properties of kombucha tea. Biomed. Environ. Sci. 2001, 14, 207–213. [Google Scholar] [PubMed]
- Centers for Disease Control and Prevention (CDC). Unexplained severe illness possibly associated with consumption of kombucha tea—Iowa, 1995. Morb. Mortal. Wkly. Rep. 1995, 44, 892–900. [Google Scholar]
- Srinivasan, R.; Smolinske, S.; Greenbaum, D. Probable gastrointestinal toxicity of kombucha tea: Is this beverage healthy or harmful? J. Gen. Intern. Med. 1997, 12, 643–645. [Google Scholar] [CrossRef] [PubMed]
- BC Center for Disease Control, Food Safety Assessment of Kombucha Tea Recipe and Food Safety Plan. 2020. Available online: http://www.bccdc.ca/resourcegallery/Documents/Educational%20Materials/EH/FPS/Food/kombucha1.pdf (accessed on 22 April 2022).
- SungHee Kole, A.; Jones, H.D.; Christensen, R.; Gladstein, J. A case of Kombucha tea toxicity. J. Intensive Care Med. 2009, 24, 205–207. [Google Scholar] [CrossRef]
- Gedela, M.; Potu, K.C.; Gali, V.L.; Alyamany, K.; Jha, L.K. A case of hepatotoxicity related to kombucha tea consumption. SD Med. 2016, 69, 26–28. [Google Scholar]
- Holbourn, A.; Hurdman, J. Kombucha: Is a cup of tea good for you? Case Rep. 2017, 2017, bcr-2017-221702. [Google Scholar] [CrossRef]
- Jayabalan, R.; Malini, K.; Sathishkumar, M.; Swaminathan, K.; Yun, S.E. Biochemical characteristics of tea fungus produced during kombucha fermentation. Food Sci. Biotechnol. 2010, 19, 843–847. [Google Scholar] [CrossRef]
- Phan, T.G.; Duggin, G.; Estell, J.; Beer, I.; Smith, D.; Ferson, M.J. Lead poisoning from drinking kombucha tea brewed in a ceramic pot. Med. J. Aust. 1998, 169, 644–646. [Google Scholar] [CrossRef]
- Nummer, B.A. Special report: Kombucha brewing under the Food and Drug Administration Model Food Code: Risk analysis and processing guidance. J. Environ. Health 2013, 76, 8–11. [Google Scholar] [PubMed]
- Skou, S.T.; Mair, F.S.; Fortin, M.; Guthrie, B.; Nunes, B.P.; Miranda, J.J.; Boyd, C.M.; Pati, S.; Mtenga, S.; Smith, S.M. Multimorbidity. Nat. Rev. Dis. Prim. 2022, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.P.; Vissoci, J.R.N.; França, D.G.; Caruzzo, N.M.; Batista, S.R.R.; de Oliveira, C.; Nunes, B.P.; Silveira, E.A. Multimorbidity patterns and hospitalisation occurrence in adults and older adults aged 50 years or over. Sci. Rep. 2022, 12, 11643. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H.B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Ighodaro, O.; Akinloye, O. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Kałduńska, J.; Kochman, J.; Janda, K. Chemical profile and antioxidant activity of the kombucha beverage derived from white, green, black and red tea. Antioxidants 2020, 9, 447. [Google Scholar] [CrossRef]
- Aboulwafa, M.M.; Youssef, F.S.; Gad, H.A.; Altyar, A.E.; Al-Azizi, M.M.; Ashour, M.L. A comprehensive insight on the health benefits and phytoconstituents of Camellia sinensis and recent approaches for its quality control. Antioxidants 2019, 8, 455. [Google Scholar] [CrossRef]
- Naveed, M.; Bibi, J.; Kamboh, A.A.; Suheryani, I.; Kakar, I.; Fazlani, S.A.; FangFang, X.; Kalhoro, S.A.; Yunjuan, L.; Kakar, M.U.; et al. Pharmacological values and therapeutic properties of black tea (Camellia sinensis): A comprehensive overview. Biomed. Pharmacother. 2018, 100, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Gachhui, R.; Sil, P.C. Effect of kombucha, a fermented black tea in attenuating oxidative stress mediated tissue damage in alloxan induced diabetic rats. Food Chem. Toxicol. 2013, 60, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.P.; Renard, T.; Rollan, S.; Taillandier, P. Impact of fermentation conditions on the production of bioactive compounds with anticancer, anti-inflammatory and antioxidant properties in kombucha tea extracts. Process. Biochem. 2019, 83, 44–54. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, J.; Fan, L.; Qin, Z.; Chen, Q.; Zhao, L. Antioxidant properties of a vegetable-fruit beverage fermented with two Lactobacillus plantarum strains. Food Sci. Biotechnol. 2018, 27, 1719–1726. [Google Scholar] [CrossRef]
- Cardoso, R.R.; Neto, R.O.; dos Santos D’Almeida, C.T.; do Nasci-mento, T.P.; Pressete, C.G.; Azevedo, L.; Martino, H.S.D.L.; Cameron, C.; Ferreira, M.S.L.; de Barros, F.A.R. Kombuchas from green and black teas have different phenolic profile, which impacts their antioxidant capacities, antibacterial and antiproliferative activities. Food Res. Int. 2020, 128, 108782. [Google Scholar] [CrossRef]
- Fu, C.; Yan, F.; Cao, Z.; Xie, F.; Lin, J. Antioxidant activities of kombucha prepared from three different substrates and changes in content of probiotics during storage. Food Sci. Technol. 2014, 34, 123–126. [Google Scholar] [CrossRef]
- Bellassoued, K.; Ghrab, F.; Makni-Ayadi, F.; Van Pelt, J.; Elfeki, A.; Ammar, E. Protective effect of kombucha on rats fed a hypercholesterolemic diet is mediated by its antioxidant activity. Pharm. Biol. 2015, 53, 1699–1709. [Google Scholar] [CrossRef]
- Kaewkod, T.; Bovonsombut, S.; Tragoolpua, Y. Efficacy of kombucha obtained from green, oolong, and black teas on inhibition of pathogenic bacteria, antioxidation, and toxicity on colorectal cancer cell line. Microorganisms 2019, 7, 700. [Google Scholar] [CrossRef]
- Watawana, M.I.; Jayawardena, N.; Waisundara, V.Y. Value-added tea (Camellia sinesis) as a functional food using the kombucha ‘tea fungus’. Chiang Mai J. Sci. 2018, 45, 136–146. [Google Scholar]
- Gaggìa, F.; Baffoni, L.; Galiano, M.; Nielsen, D.S.; Jakobsen, R.R.; Castro-Mejía, J.L.; Bosi, S.; Truzzi, F.; Musumeci, F.; Dinelli, G.; et al. Kombucha beverage from green, black and rooibos teas: A comparative study looking at microbiology, chemistry and antioxidant activity. Nutrients 2019, 11, 1. [Google Scholar] [CrossRef]
- Yang, Z.W.; Ji, B.P.; Zhou, F.; Li, B.; Luo, Y.; Yang, L.; Li, T. Hypocholesterolaemic and antioxidant effects of kombucha tea in high-cholesterol fed mice. J. Sci. Food Agric. 2009, 89, 150–156. [Google Scholar] [CrossRef]
- Sai Ram, M.; Anju, B.; Pauline, T.; Dipti, P.; Kain, A.K.; Mongia, S.S.; Sharma, S.K.; Singh, B.; Singh, R.; Ilavazhagan, G.; et al. Effect of kombucha tea on chromate (VI)-induced oxidative stress in albino rats. J. Ethnopharmacol. 2000, 71, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Barakat, N.; Beaufort, S.; Rizk, Z.; Bouajila, J.; Taillandier, P.; El Rayess, Y. Kombucha analogues around the world: A review. Crit. Rev. Food Sci. Nutr. 2022, 1–25. [Google Scholar] [CrossRef]
- Lopes, D.R.; Santos, L.O.; Prentice-Hernández, C. Antioxidant and antibacterial activity of a beverage obtained by fermentation of yerba-maté (Ilex paraguariensis) with symbiotic kombucha culture. J. Food Process. Preserv. 2021, 45, e15101. [Google Scholar] [CrossRef]
- Nizioł-Łukaszewska, Z.; Ziemlewska, A.; Bujak, T.; Zagórska-Dziok, M.; Zarebska, M.; Hordyjewicz-Baran, Z.; Wasilewski, T. Effect of fermentation time on antioxidant and anti-ageing properties of green coffee kombucha ferments. Molecules 2020, 25, 5394. [Google Scholar]
- Zubaidah, E.; Dea, E.C.; Sujuti, H. Physicochemical and microbiological characteristics of kombucha based on various concentration of Javanese turmeric (Curcuma xanthorrhiza). Biocatal. Agric. Biotechnol. 2022, 44, 102467. [Google Scholar] [CrossRef]
- Velićanski, A.S.; Cvetković, D.D.; Markov, S.L.; Šaponjac, V.T.; Vulić, J.J. Antioxidant and antibacterial activity of the beverage obtained by fermentation of sweetened lemon balm (Melissa officinalis L.) tea with symbiotic consortium of bacteria and yeasts. Food Technol. Biotechnol. 2014, 52, 420–429. [Google Scholar] [CrossRef]
- Yıkmış, S.; Tuğgum, S. Evaluation of microbiological, physicochemical and sensorial properties of purple basil kombucha beverage. Turk. J. Agric. Food Sci. Technol. 2019, 7, 1321–1327. [Google Scholar] [CrossRef]
- Tanticharakunsiri, W.; Mangmool, S.; Wongsariya, K.; Ochaikul, D. Characteristics and upregulation of antioxidant enzymes of kitchen mint and oolong tea kombucha beverages. J. Food Biochem. 2021, 45, e13574. [Google Scholar] [CrossRef]
- Vitas, J.; Vukmanović, S.; Čakarević, J.; Popović, L.; Malbaša, R. Kombucha fermentation of six medicinal herbs: Chemical profile and biological activity. Chem. Ind. Chem. Eng. Q. 2020, 26, 157–170. [Google Scholar] [CrossRef]
- Waisundara, V.Y. Usage of Kombucha ‘Tea Fungus’ for enhancement of functional properties of herbal beverages. In Frontiers and New Trends in the Science of Fermented Food and Beverages; IntechOpen: London, UK, 2018; pp. 1–14. [Google Scholar]
- Vázquez-Cabral, B.D.; Larrosa-Pérez, M.; Gallegos-Infante, J.A.; Moreno-Jiménez, M.R.; González-Laredo, R.F.; Rutiaga-Quiñones, J.G.; Gamboa-Gómez, C.I.; Rocha-Guzmán, N.E. Oak kombucha protects against oxidative stress and inflammatory processes. Chem.-Biol. Interact. 2017, 272, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gamboa-Gómez, C.I.; Simental-Mendía, L.E.; González-Laredo, R.F.; Alcantar-Orozco, E.J.; Monserrat-Juarez, V.H.; Ramírez-España, J.C.; Gallegos-Infante, J.A.; Moreno-Jiménez, M.R.; Rocha-Guzmán, N.E. In vitro and in vivo assessment of anti-hyperglycemic and antioxidant effects of oak leaves (Quercus convallata and Quercus arizonica) infusions and fermented beverages. Food Res. Int. 2017, 102, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Wongthai, N.; Tanticharakunsiri, W.; Mangmool, S.; Ochaikul, D. Characteristics and antioxidant activity of royal lotus pollen, butterfly pea flower, and oolong tea kombucha beverages. Asia Pac. J. Sci. Technol. 2021, 26, APST-26-04-17. [Google Scholar]
- Vitas, J.S.; Cvetanović, A.D.; Mašković, P.Z.; Švarc-Gajić, J.V.; Malbaša, R.V. Chemical composition and biological activity of novel types of kombucha beverages with yarrow. J. Funct. Foods 2018, 44, 95–102. [Google Scholar] [CrossRef]
- Pure, A.E.; Pure, M.E. Antioxidant, antibacterial and color analysis of garlic fermented in kombucha and red grape vinegar. Appl. Food Biotechnol. 2016, 3, 246–252. [Google Scholar]
- Artanti, N.; Susilowati, A.; Aspiyanto; Lotulung, P.D.N.; Maryati, Y. Antioxidant activity of fermented broccoli and spinach by kombucha culture. AIP Conf. Proc. 2017, 1904, 020069. [Google Scholar]
- Rahmani, R.; Beaufort, S.; Villarreal-Soto, S.A.; Taillandier, P.; Bouajila, J.; Debouba, M. Kombucha fermentation of African mustard (Brassica tournefortii) leaves: Chemical composition and bioactivity. Food Biosci. 2019, 30, 100414. [Google Scholar] [CrossRef]
- Yildiz, E.; Guldas, M.; Gurbuz, O. Determination of in-vitro phenolics, antioxidant capacity and bioaccessibility of Kombucha tea produced from black carrot varieties grown in Turkey. Food Sci. Technol. 2021, 41, 180–187. [Google Scholar] [CrossRef]
- Aung, T.; Eun, J.B. Impact of time and temperature on the physicochemical, microbiological, and nutraceutical properties of laver kombucha (Porphyra dentata) during fermentation. LWT Food Sci. Technol. 2022, 154, 112643. [Google Scholar] [CrossRef]
- Zubaidah, E.; Yurista, S.; Rahmadani, N.R. Characteristic of physical, chemical, and microbiological kombucha from various varieties of apples. IOP Conf. Ser. Earth Environ. Sci. 2018, 131, 012040. [Google Scholar] [CrossRef]
- Zubaidah, E.; Dewantari, F.J.; Novitasari, F.R.; Srianta, I.; Blanc, P.J. Potential of snake fruit (Salacca zalacca (Gaerth.) Voss) for the development of a beverage through fermentation with the kombucha consortium. Biocatal. Agric. Biotechnol. 2018, 13, 198–203. [Google Scholar] [CrossRef]
- Zubaidah, E.; Afgani, C.A.; Kalsum, U.; Srianta, I.; Blanc, P.J. Comparison of in vivo antidiabetes activity of snake fruit kombucha, black tea kombucha and metformin. Biocatal. Agric. Biotechnol. 2019, 17, 465–469. [Google Scholar] [CrossRef]
- Ayed, L.; Hamdi, M. Manufacture of a beverage from cactus pear juice using “tea fungus” fermentation. Ann. Microbiol. 2015, 65, 2293–2299. [Google Scholar] [CrossRef]
- Ayed, L.; Ben Abid, S.; Hamdi, M. Development of a beverage from red grape juice fermented with the kombucha consortium. Ann. Microbiol. 2017, 67, 111–121. [Google Scholar] [CrossRef]
- Khosravi, S.; Safari, M.; Emam-Djomeh, Z.; Golmakani, M.T. Development of fermented date syrup using kombucha starter culture. J. Food Process. Preserv. 2019, 43, e13872. [Google Scholar] [CrossRef]
- Watawana, M.I.; Jayawardena, N.; Gunawardhana, C.B.; Waisundara, V.Y. Enhancement of the antioxidant and starch hydrolase inhibitory activities of king coconut water (Cocos nucifera var. Aurantiaca) by fermentation with kombucha ‘tea fungus’. Int. J. Food Sci. Technol. 2016, 51, 490–498. [Google Scholar] [CrossRef]
- Abuduaibifu, A.; Tamer, C.E. Evaluation of physicochemical and bioaccessibility properties of goji berry kombucha. J. Food Process. Preserv. 2019, 43, e14077. [Google Scholar] [CrossRef]
- Leonarski, E.; Cesca, K.; Zanella, E.; Stambuk, B.U.; Oliveira, D.; Poletto, P. Production of kombucha-like beverage and bacterial cellulose by acerola byproduct as raw material. LWT Food Sci. Technol. 2021, 135, 110075. [Google Scholar] [CrossRef]
- da Silva Júnior, J.C.; Magnani, M.; Almeida da Costa, W.K.; Madruga, M.S.; Olegário, L.S.; da Silva Campelo Borges, G.; Dantas, A.M.; dos Santos Lima, M.; de Lima, L.C.; de Lima Brito, I.; et al. Traditional and flavored kombuchas with pitanga and umbu-cajá pulps: Chemical properties, antioxidants, and bioactive compounds. Food Biosci. 2021, 44, 101380. [Google Scholar] [CrossRef]
- Sutthiphatkul, T.; Mangmool, S.; Rungjindamai, N.; Ochaikul, D. Characteristics and antioxidant activities of kombucha from black tea and roselle by a mixed starter culture. Curr. Appl. Sci. Technol. 2023, 23, 4. [Google Scholar] [CrossRef]
- Pure, A.E.; Mofidi, S.M.G.; Keyghobadi, F.; Pure, M.E. Chemical composition of garlic fermented in red grape vinegar and kombucha. J. Funct. Foods 2017, 34, 347–355. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Morales, D. Biological activities of kombucha beverages: The need of clinical evidence. Trends Food Sci. Technol. 2020, 105, 323–333. [Google Scholar] [CrossRef]
- Haghmorad, D.; Sanaz Yazdanpanah, E.; Sadighimoghaddam, B.; Yousefi, B.; Sahafi, P.; Ghorbani, N.; Rashidy-Pour, A.; Kokhaei, P. Kombucha ameliorates experimental autoimmune encephalomyelitis through activation of Treg and Th2 cells. Acta Neurol. Belg. 2021, 121, 1685–1692. [Google Scholar] [CrossRef]
- Joseph, S.V.; Edirisinghe, I.; Burton-Freeman, B.M. Fruit polyphenols: A review of anti-inflammatory effects in humans. Crit. Rev. Food Sci. Nutr. 2016, 56, 419–444. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Rubió, L.; Motilva, M.J.; Romero, M.P. Recent advances in biologically active compounds in herbs and spices: A review of the most effective antioxidant and anti-inflammatory active principles. Crit. Rev. Food Sci. Nutr. 2013, 53, 943–953. [Google Scholar] [CrossRef]
- Serafini, M.; Peluso, I. Functional foods for health: The interrelated antioxidant and anti-inflammatory role of fruits, vegetables, herbs, spices and cocoa in humans. Curr. Pharm. Des. 2016, 22, 6701–6715. [Google Scholar] [CrossRef]
- Shakoor, H.; Feehan, J.; Apostolopoulos, V.; Platat, C.; Al Dhaheri, A.S.; Ali, H.I.; Ismail, L.C.; Bosevski, M.; Stojanovska, L. Immunomodulatory effects of dietary polyphenols. Nutrients 2021, 13, 728. [Google Scholar] [CrossRef]
- Sknepnek, A.; Tomić, S.; Miletić, D.; Lević, S.; Čolić, M.; Nedović, V.; Nikšić, M. Fermentation characteristics of novel Coriolus versicolor and Lentinus edodes kombucha beverages and immunomodulatory potential of their polysaccharide extracts. Food Chem. 2021, 342, 128344. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, J.Y.; Yoo, D.G.; Jeon, Y.B.; Yoon, H.S.; Kim, C.H. Functional characteristics of kombucha fermented with lactic acid bacteria, yeast, and acetic acid bacteria derived from Korea traditional foods. J. Dairy Sci. Biotechnol. 2022, 40, 23–34. [Google Scholar] [CrossRef]
- Wang, P.; Feng, Z.; Sang, X.; Chen, W.; Zhang, X.; Xiao, J.; Chen, Y.; Chen, Q.; Yang, M.; Su, J. Kombucha ameliorates LPS-induced sepsis in a mouse model. Food Funct. 2021, 12, 10263–10280. [Google Scholar] [CrossRef]
- Wisastra, R.; Dekker, F.J. Inflammation, cancer and oxidative lipoxygenase activity are intimately linked. Cancers 2014, 6, 1500–1521. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.; Hassarajani, S.A.; Maity, B.; Narayan, G.; Bandyopadhyaya, S.K.; Chattopadhyay, S. Comparative healing property of kombucha tea and black tea against indomethacin-induced gastric ulceration in mice: Possible mechanism of action. Food Funct. 2010, 1, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Marzban, F.; Azizi, G.; Afraei, S.; Sedaghat, R.; Seyedzadeh, M.H.; Razavi, A. Kombucha tea ameliorates experimental autoimmune encephalomyelitis in mouse model of multiple sclerosis. Food Agric. Immunol. 2015, 26, 782–793. [Google Scholar] [CrossRef]
- Zubaidah, E.; Iastika, A.R.; Widyaningsih, T.D.; Febrianto, K. Immunomodulatory activity of black tea kombucha (Camellia sinensis) and arabica coffee leaves tea kombucha (coffee arabica) for Salmonella typhi-infected mice. IOP Conf. Ser. Earth Environ. Sci. 2021, 733, 012128. [Google Scholar] [CrossRef]
- Zubaidah, E.; Nisak, Y.K.; Susanti, I.; Widyaningsih, T.D.; Srianta, I.; Tewfik, I. Turmeric Kombucha as effective immunomodulator in Salmonella typhi-infected experimental animals. Biocatal. Agric. Biotechnol. 2021, 37, 102181. [Google Scholar] [CrossRef]
- Yang, C.L.; Chik, S.C.; Lau, A.S.; Chan, G.C. Coriolus versicolor and its bioactive molecule are potential immunomodulators against cancer cell metastasis via inactivation of MAPK pathway. J. Ethnopharmacol. 2023, 301, 115790. [Google Scholar] [CrossRef]
- Gaitán-Hernández, R.; López-Peña, D.; Esqueda, M.; Gutiérrez, A. Review of bioactive molecules production, biomass, and basidiomata of shiitake culinary-medicinal mushrooms, Lentinus edodes (Agaricomycetes). Int. J. Med. Mushrooms 2019, 21, 841–850. [Google Scholar] [CrossRef]
- Fan, X.; Ngo, H.; Wu, C. Natural and Bio-based Antimicrobials: A Review. In Natural and Bio-Based Antimicrobials for Food Applications; American Chemical Society: Washington, DC, USA, 2018; pp. 1–24. [Google Scholar]
- Al-Mohammadi, A.R.; Ismaiel, A.A.; Ibrahim, R.A.; Moustafa, A.H.; Abou Zeid, A.; Enan, G. Chemical constitution and antimicrobial activity of kombucha fermented beverage. Molecules 2021, 26, 5026. [Google Scholar] [CrossRef]
- Battikh, H.; Chaieb, K.; Bakhrouf, A.; Ammar, E. Antibacterial and antifungal activities of black and green kombucha teas. J. Food Biochem. 2013, 37, 231–236. [Google Scholar] [CrossRef]
- Valiyan, F.; Koohsari, H.; Fadavi, A. Use of response surface methodology to investigate the effect of several fermentation conditions on the antibacterial activity of several kombucha beverages. J. Food Sci. Technol. 2021, 58, 1877–1891. [Google Scholar] [CrossRef] [PubMed]
- Ivanišová, E.; Meňhartová, K.; Terentjeva, M.; Harangozo, L.; Kántor, A.; Kačániová, M. The evaluation of chemical, antioxidant, antimicrobial and sensory properties of kombucha tea beverage. J. Food Sci. Technol. 2020, 57, 1840–1846. [Google Scholar] [CrossRef] [PubMed]
- Talawat, S.; Ahantharik, P.; Laohawiwattanakul, S.; Premsuk, A.; Ratanapo, S. Efficacy of fermented teas in antibacterial activ-ity. Kasetsart J. Nat. Sci. 2006, 40, 925–933. [Google Scholar]
- Shahbazi, H.; Hashemi Gahruie, H.; Golmakani, M.T.; Eskandari, M.H.; Movahedi, M. Effect of medicinal plant type and concentration on physicochemical, antioxidant, antimicrobial, and sensorial properties of kombucha. Food Sci. Nutr. 2018, 6, 2568–2577. [Google Scholar] [CrossRef]
- Četojević-Simin, D.D.; Velićanski, A.S.; Cvetković, D.D.; Markov, S.L.; Mrđanović, J.Ž.; Bogdanović, V.V.; Šolajić, S.V. Bioactivity of lemon balm kombucha. Food Bioprocess Technol. 2012, 5, 1756–1765. [Google Scholar] [CrossRef]
- Battikh, H.; Bakhrouf, A.; Ammar, E. Antimicrobial effect of kombucha analogues. LWT Food Sci. Technol. 2012, 47, 71–77. [Google Scholar] [CrossRef]
- Mulyani, H.; Maryati, Y.; Filailla, E.; Susilowati, A.; Lotulung, P.D.N.; Aspiyanto, A. Benefits of fermented beet (Beta vulgaris L.) against digestive infection Escherichia coli and free radicals prevention. AIP Conf. Proc. 2019, 2175, 020025. [Google Scholar]
- Yuniarto, A.; Anggadiredja, K.; Nur Aqidah, R.A. Antifungal activity of kombucha tea against human pathogenic fungi. Asian J. Pharm. Clin. Res. 2016, 9, 253–255. [Google Scholar] [CrossRef]
- Fu, N.; Wu, J.; Lv, L.; He, J.; Jiang, S. Anti-foot-and-mouth disease virus effects of Chinese herbal kombucha in vivo. Braz. J. Microbiol. 2015, 46, 1245–1255. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Jayabalan, R.; Chen, P.-N.; Hsieh, Y.-S.; Prabhakaran, K.; Pitchai, P.; Marimuthu, S.; Thangaraj, P.; Swaminathan, K.; Yun, S.E. Effect of solvent fractions of kombucha tea on viability and invasiveness of cancer cells-characterization of dimethyl 2-(2-hydroxy-2-methoxypropylidine) malonate and vitexin. Indian J. Biotechnol. 2011, 10, 75–82. [Google Scholar]
- Srihari, T.; Arunkumar, R.; Arunakaran, J.; Satyanarayana, U. Downregulation of signalling molecules involved in angiogenesis of prostate cancer cell line (PC-3) by kombucha (lyophilized). Biomed. Prev. Nutr. 2013, 3, 53–58. [Google Scholar] [CrossRef]
- Deghrigue, M.; Chriaa, J.; Battikh, H.; Abid, K.; Bakhrouf, A. Antiproliferative and antimicrobial activities of kombucha tea. Afr. J. Microbiol. Res. 2013, 7, 3466–3470. [Google Scholar]
- Villarreal-Soto, S.A.; Bouajila, J.; Pace, M.; Leech, J.; Cotter, P.D.; Souchard, J.-P.; Taillandier, P.; Beaufort, S. Metabolome-microbiome signatures in the fermented beverage, Kombucha. Int. J. Food Microbiol. 2020, 333, 108778. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- American Diabetes Association (ADA). Diagnosis and classification of diabetes mellitus. Diabetes Care 2014, 37, S81–S90. [Google Scholar] [CrossRef]
- Harding, J.L.; Pavkov, M.E.; Magliano, D.J.; Shaw, J.E.; Gregg, E.W. Global trends in diabetes complications: A review of current evidence. Diabetologia 2019, 62, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Padhi, S.; Nayak, A.K.; Behera, A. Type II diabetes mellitus: A review on recent drug based therapeutics. Biomed. Pharm. 2020, 131, 110708. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.A.; Gorjian, M.; Rasouli, L.; Shirali, S. A comparison between the effect of green tea and kombucha prepared from green tea on the weight of diabetic rats. Biosci. Biotechnol. Res. Asia 2015, 20, 141–145. [Google Scholar] [CrossRef]
- Shenoy, C. Hypoglycemic activity of bio-tea in mice. Blood 2000, 38, 278–279. [Google Scholar]
- Srihari, T.; Karthikesan, K.; Ashokkumar, N.; Satyanarayana, U. Antihyperglycaemic efficacy of kombucha in streptozotocin-induced rats. J. Funct. Foods 2013, 5, 1794–1802. [Google Scholar] [CrossRef]
- Aloulou, A.; Hamden, K.; Elloumi, D.; Ali, M.B.; Hargafi, K.; Jaouadi, B.; Ayadi, F.; Elfeki, A.; Ammar, E. Hypoglycemic and antilipidemic properties of kombucha tea in alloxan-induced diabetic rats. BMC Complement Altern. Med. 2012, 12, 63. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Y.; Wang, J.; Geng, W. Kombucha reduces hyperglycemia in type 2 diabetes of mice by regulating gut microbiota and its metabolites. Foods 2022, 11, 754. [Google Scholar] [CrossRef] [PubMed]
- Dias, F.; Shenoy, C. Protective effect of kombucha on diabetic nephropathy in streptozotocin-induced diabetic rats. Int. J. Sci. Res. 2016, 5, 945–948. [Google Scholar]
- Kallel, L.; Desseaux, V.; Hamdi, M.; Stocker, P.; Ajandouz, E.H. Insights into the fermentation biochemistry of Kombucha teas and potential impacts of Kombucha drinking on starch digestion. Int. Food Res. J. 2012, 49, 226–232. [Google Scholar] [CrossRef]
- Coskun, O.; Kanter, M.; Korkmaz, A.; Oter, S. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and β-cell damage in rat pancreas. Pharmacol. Res. 2005, 51, 117–123. [Google Scholar] [CrossRef]
- Forrester, S.J.; Booz, G.W.; Sigmund, C.D.; Coffman, T.M.; Kawai, T.; Rizzo, V.; Scalia, R.; Eguchi, S. Angiotensin II signal transduction: An update on mechanisms of physiology and pathophysiology. Physiol. Rev. 2018, 98, 1627–1738. [Google Scholar] [CrossRef]
- Lauren, B.; Arendse, A.H.; Jan Danser, A.H.; Poglitsch, M.; Touyz, R.M.; Burnett, J.C.; Llorens-Cortes, C.; Ehlers, M.R.; Sturrock, E.D.; Barker, E.L. Novel therapeutic approaches targeting the renin-angiotensin system and associated peptides in hypertension and heart failure. Pharmacol. Rev. 2019, 71, 539–570. [Google Scholar]
- James, P.A.; Oparil, S.; Carter, B.; Cushman, W.; Dennison-Himmelfarb, C.; Handler, J.; Lackland, D.T.; LeFevre, M.L.; MacKenzie, T.D.; Ogedegbe, O.; et al. Evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014, 311, 507–520. [Google Scholar] [CrossRef]
- Hrnjez, D.; Vaštag, Ž.; Milanović, S.; Vukić, V.; Iličić, M.; Popović, L.; Kanurić, K. The biological activity of fermented dairy products obtained by kombucha and conventional starter cultures during storage. J. Funct. Foods 2014, 10, 336–345. [Google Scholar] [CrossRef]
- Elkhtab, E.; El-Alfy, M.; Shenana, M.; Mohamed, A.; Yousef, A.E. New potentially antihypertensive peptides liberated in milk during fermentation with selected lactic acid bacteria and kombucha cultures. J. Dairy Sci. 2017, 100, 9508–9520. [Google Scholar] [CrossRef] [PubMed]
- López-Fandiño, R.; Otte, J.; Van Camp, J. Physiological, chemical and technological aspects of milk-protein-derived peptides with antihypertensive and ACE-inhibitory activity. Int. Dairy J. 2006, 16, 1277–1293. [Google Scholar] [CrossRef]
- Gamboa-Gómez, C.I.; González-Laredo, R.F.; Gallegos-Infante, J.A.; Pérez, M.d.M.L.; Moreno-Jiménez, M.R.; Flores-Rueda, A.G.; Rocha-Guzmán, N.E. Antioxidant and angiotensin-converting enzyme inhibitory activity of Eucalyptus camaldulensis and Litsea glaucescens infusions fermented with kombucha consortium. Food Technol. Biotechnol. 2016, 54, 367. [Google Scholar] [CrossRef] [PubMed]
- Parichatikanond, W.; Pinthong, D.; Mangmool, S. Blockade of the renin-angiotensin system with delphinidin, cyanin and quercetin. Planta Med. 2012, 78, 1626–1632. [Google Scholar] [CrossRef]
- Lobo, R.O.; Sagar, B.C.; Shenoy, C.K. Bio-tea prevents membrane destabilization during isoproterenol-induced myocardial injury. J. Microsc. Ultrastruct. 2017, 5, 146–154. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ ASPC/NLA/PCNA Guideline on the management of blood cholesterol: Executive summary: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar]
- Shattat, G.F. A review article on hyperlipidemia: Types, treatments and new drug targets. Biomed. Pharmacol. J. 2015, 7, 399–409. [Google Scholar] [CrossRef]
- Adriani, L.; Mayasari, N.; Kartasudjana, R. The effect of feeding fermented kombucha tea on HLD, LDL and total cholesterol levels in the duck bloods. Biotechnol. Anim. Husb. 2011, 27, 1749–1755. [Google Scholar] [CrossRef]
- Al-dulaimi, F.K.; Abd-alwahab, W.I.; Hasan, A.S. Bioactivity study of kombucha black tea and kombucha with skim milk on some of physiological and biochemical parameters in male albino rats. Int. J. Pharm. Res. 2018, 10, 301. [Google Scholar]
- Doudi, M.; Hooshmandi, Z.; Saedi, S.; Setorki, M. Effects of kombucha tea on side effects of high cholesterol diet in rabbits. Pharm. Biomed. Res. 2020, 6, 123–132. [Google Scholar] [CrossRef]
- Alaei, Z.; Doudi, M.; Setorki, M. The protective role of kombucha extract on the normal intestinal microflora, high-cholesterol diet caused hypercholesterolemia, and histological structures changes in New Zealand white rabbits. Avicenna J. Phytomedicine 2020, 10, 604. [Google Scholar]
- Nofer, J.-R.; Kehrel, B.; Fobker, M.; Levkau, B.; Assmann, G.; von Eckardstein, A. HDL and arteriosclerosis: Beyond reverse cholesterol transport. Atherosclerosis 2002, 161, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Vergès, B. Pathophysiology of diabetic dyslipidaemia: Where are we? Diabetologia 2015, 58, 886–899. [Google Scholar] [CrossRef] [PubMed]
- Nakai, M.; Fukui, Y.; Asami, S.; Toyoda-Ono, Y.; Iwashita, T.; Shibata, H.; Mitsunaga, T.; Hashimoto, F.; Kiso, Y. Inhibitory effects of oolong tea polyphenols on pancreatic lipase in vitro. J. Agric. Food Chem. 2005, 53, 4593–4598. [Google Scholar] [CrossRef] [PubMed]
| Name of Substrates | Active Ingredients | Biological Assays | Findings | Ref. |
|---|---|---|---|---|
| In vitro studies | ||||
| Black tea |
|
|
| [47] |
| Black tea, white oak leaves (Quercus resinosa, Q. arizonica, Q. convallata) |
|
|
| [66] |
| Mushrooms (Coriolus versicolor and Lentinus edodes) |
|
|
| [95] |
| Green tea |
|
|
| [96] |
| In vivo studies | ||||
| Black tea |
|
|
| [89] |
| Green tea |
|
|
| [97] |
| Black tea |
|
|
| [99] |
| Black tea |
|
|
| [100] |
| Black tea and arabica coffee |
|
|
| [101] |
| Black tea and turmeric |
|
|
| [102] |
| Name of Substrates | Active Ingredients | Biological Assays | Findings | Ref. |
|---|---|---|---|---|
| In vitro studies | ||||
| Soy whey |
|
|
| [15] |
| Black and green teas |
|
|
| [49] |
| Black, green, and oolong teas |
|
|
| [52] |
| Yerba-maté (Ilex paraguariensis) |
|
|
| [58] |
| Lemon balm (Melissa officinalis) |
|
|
| [61] |
| Yarrow (Achillea millefolium) |
|
|
| [69] |
| Black tea and garlic |
|
|
| [70] |
| Apple varieties (Anna, Fuji, Granny Smith, Manalagi, Red Delicious, Rome Beauty, Royal Gala) |
|
|
| [75] |
| Snake fruit (Salacca zalacca) |
|
|
| [76] |
| Cactus pear |
|
|
| [78] |
| Red grape |
|
|
| [79] |
| Green tea |
|
|
| [96] |
| Black tea |
|
|
| [106] |
| Black and green teas |
|
|
| [107] |
| Black and green tea, lemon verbena (Lippia citriodora), and peppermint (Mentha piperita) |
|
|
| [108] |
| Black tea |
|
|
| [109] |
| Black, green, oolong, and mulberry teas |
|
|
| [110] |
| Green tea and spices (cinnamon, cardamom, and Shirazi thyme) |
|
|
| [111] |
| Lemon balm (Melissa officinalis) |
|
|
| [112] |
| Thyme (Thymus vulgaris), lemon verbena (Lippia citriodora), rosemary (Rosmarinus officinalis), fennel (Foeniculum vulgare), and peppermint (Mentha piperita) |
|
|
| [113] |
| Beet (Beta Vulgaris) |
|
|
| [114] |
| Name of Substrates | Active Ingredients | Biological Assays | Findings | Ref. |
|---|---|---|---|---|
| In vitro studies | ||||
| Black tea |
|
|
| [47] |
| Yarrow (Achillea millefolium L.) |
|
|
| [69] |
| African mustard (Brassica tournefortii) leaves |
|
|
| [72] |
| Lemon balm (Melissa officinalis L.) |
|
|
| [112] |
| Black tea |
|
|
| [118] |
| Black tea |
|
|
| [119] |
| Green tea and black tea |
|
|
| [120] |
| Name of Substrates | Active Ingredients | Biological Assays | Findings | Ref. |
|---|---|---|---|---|
| In vitro studies | ||||
| Soymilk |
|
|
| [14] |
| Chinese black tea, oolong tea, green tea and Sri Lankan black tea |
|
|
| [53] |
| Oak leaves [Quercus convallata (QC) and Quercus arizonica (QA)] |
|
|
| [67] |
| In vivo studies | ||||
| Oak leaves [Quercus convallata (QC) and Quercus arizonica (QA)] |
|
|
| [67] |
| Snake fruit (Salak Suwaru cultiva) and black tea |
|
|
| [77] |
| Black tea |
|
|
| [129] |
| Name of Substrates | Active Ingredients | Biological Assays | Findings | Ref. |
|---|---|---|---|---|
| In vitro studies | ||||
| Black tea, green tea, winter savory (Satureja montana), peppermint (Mentha × piperita), stinging nettle (Urtica dioica), wild thyme (Thymus serpyllum), elderberry (Sambucus nigra), and quince (Cydonia oblonga) |
|
|
| [64] |
| Milk |
|
|
| [137] |
| Ultra-high temperature-treated milk |
|
|
| [138] |
| Eucalyptus camaldulensis and Litsea glaucescens |
|
|
| [140] |
| Name of Substrates | Active Ingredients | Biological Assays | Findings | Ref. |
|---|---|---|---|---|
| In vivo studies | ||||
| Green tea (Camellia sinensis) |
|
|
| [51] |
| Traditional kombucha tea (TKT) and modified kombucha tea (MKT; tea broth fermented by a single Gluconacetobacter sp. A4) |
|
|
| [55] |
| Snake fruit (Salak Suwaru cultiva) and black tea |
|
|
| [77] |
| Black tea (Camellia sinensis) |
|
|
| [129] |
| Black tea (Camellia sinensis) and skim milk |
|
|
| [146] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anantachoke, N.; Duangrat, R.; Sutthiphatkul, T.; Ochaikul, D.; Mangmool, S. Kombucha Beverages Produced from Fruits, Vegetables, and Plants: A Review on Their Pharmacological Activities and Health Benefits. Foods 2023, 12, 1818. https://doi.org/10.3390/foods12091818
Anantachoke N, Duangrat R, Sutthiphatkul T, Ochaikul D, Mangmool S. Kombucha Beverages Produced from Fruits, Vegetables, and Plants: A Review on Their Pharmacological Activities and Health Benefits. Foods. 2023; 12(9):1818. https://doi.org/10.3390/foods12091818
Chicago/Turabian StyleAnantachoke, Natthinee, Ratchanee Duangrat, Tanyarat Sutthiphatkul, Duangjai Ochaikul, and Supachoke Mangmool. 2023. "Kombucha Beverages Produced from Fruits, Vegetables, and Plants: A Review on Their Pharmacological Activities and Health Benefits" Foods 12, no. 9: 1818. https://doi.org/10.3390/foods12091818
APA StyleAnantachoke, N., Duangrat, R., Sutthiphatkul, T., Ochaikul, D., & Mangmool, S. (2023). Kombucha Beverages Produced from Fruits, Vegetables, and Plants: A Review on Their Pharmacological Activities and Health Benefits. Foods, 12(9), 1818. https://doi.org/10.3390/foods12091818