Insight into the Effects of Drying Methods on Lanzhou Lily Rehydration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Drying Methods
2.3. Rehydration Performance Evaluation
2.4. Low Field Nuclear Magnetic Resonance (LF-NMR)
2.5. Morphology Observation by Scanning Electron Microscopy
2.6. Micro X-ray Computer Tomography (CT) Imaging
2.7. Lily Starch Isolation
2.8. X-ray Diffraction
2.9. Pasting Profiles Determination
2.10. Solubility and Swelling Power
2.11. Statistical Analysis
3. Results
3.1. Rehydration Performance
3.2. Water Migration and Distribution within Lily Scales during Rehydration
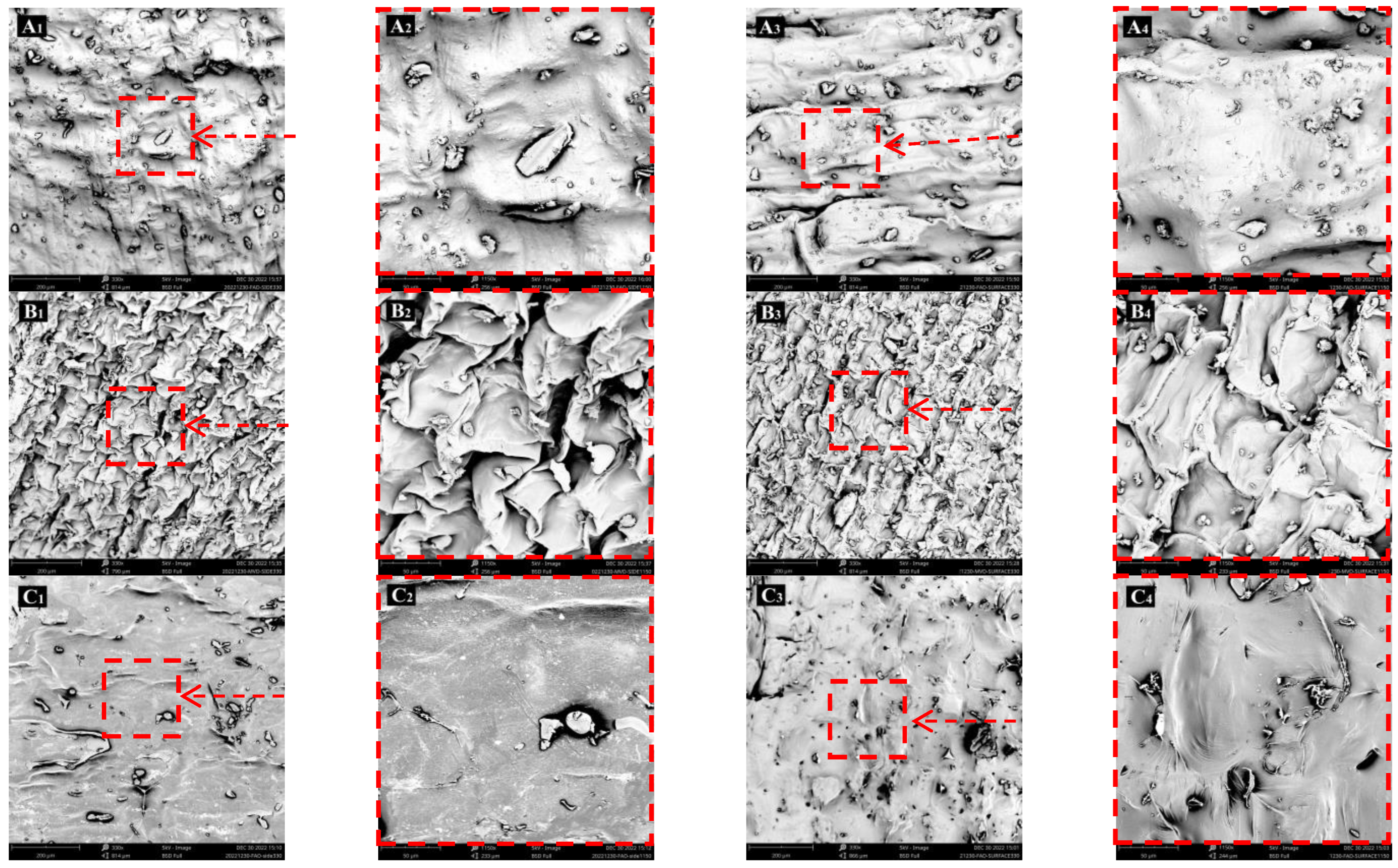
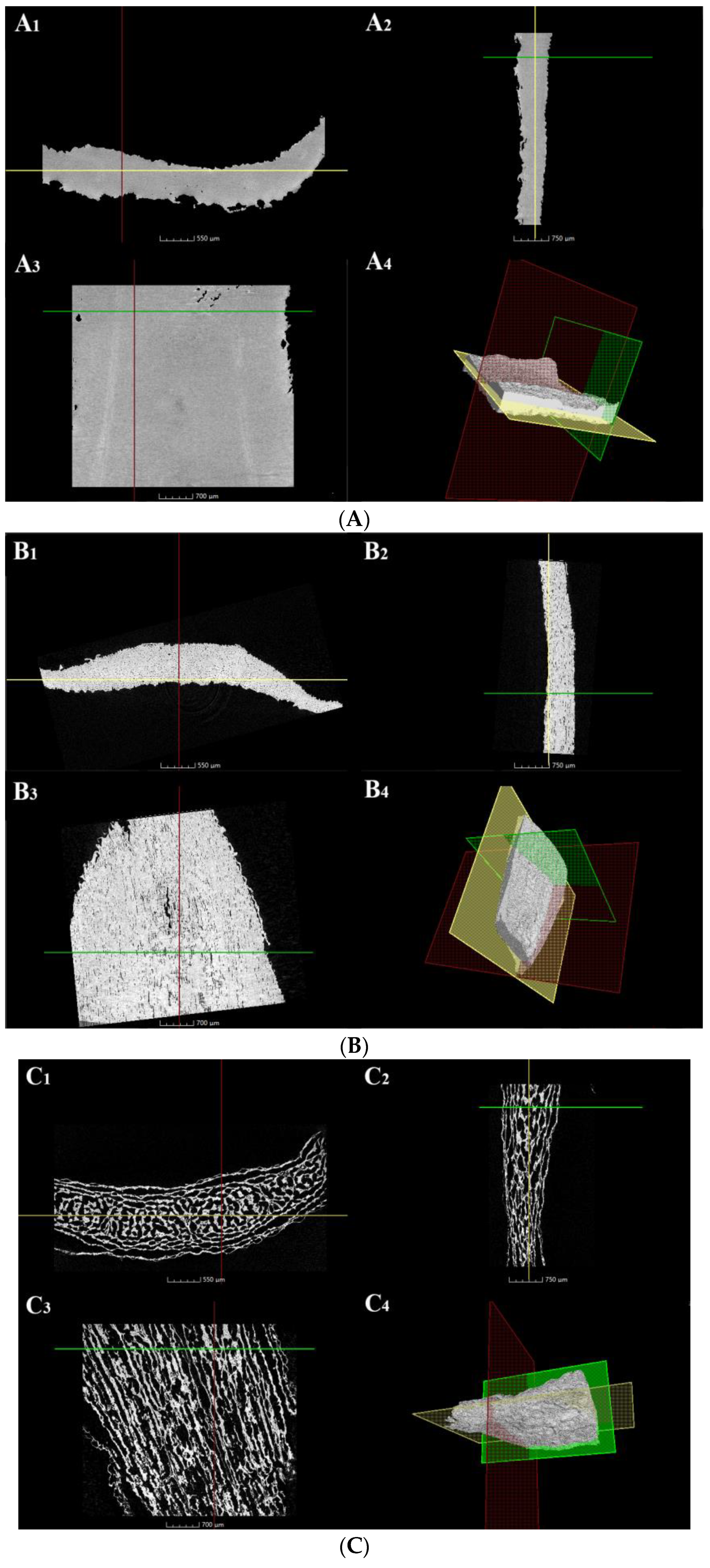
3.3. Morphology and Microstructure of Dried Lily Scale
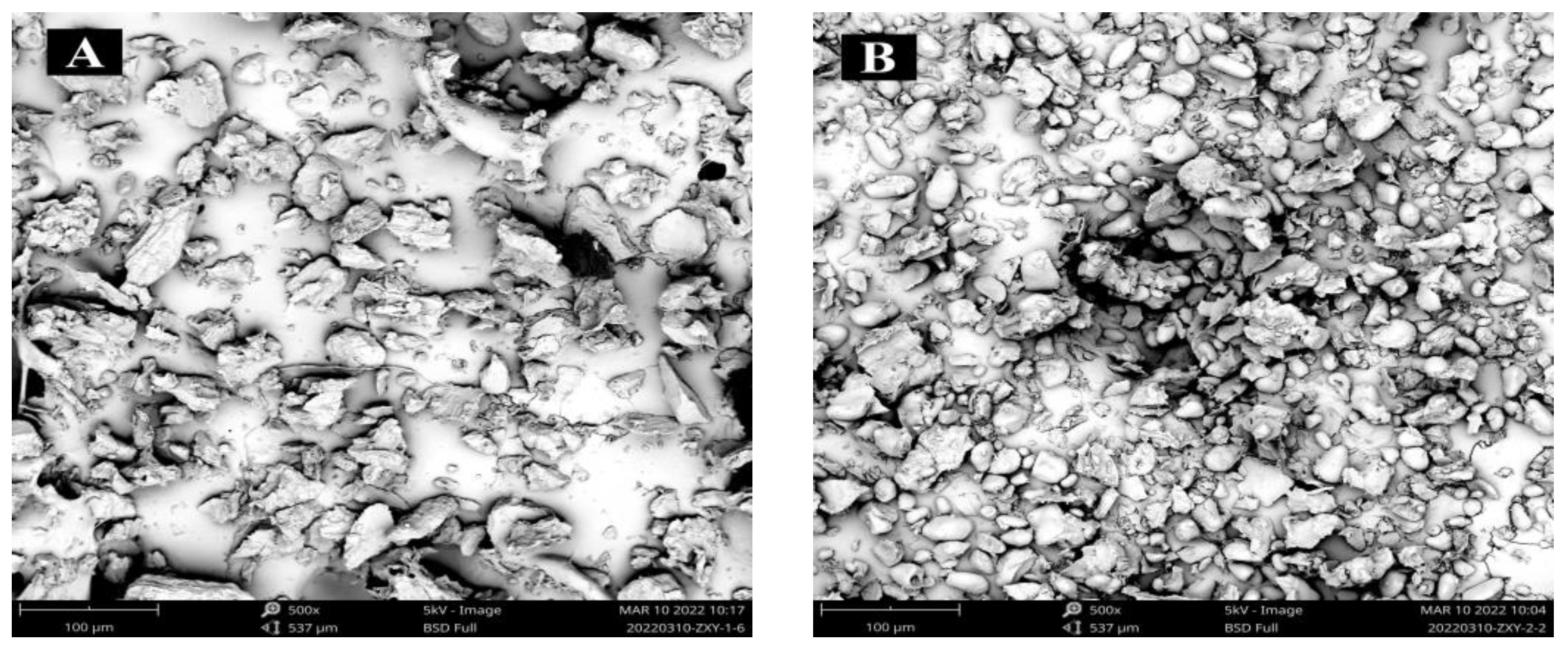
3.4. Starch Morphology by SEM
3.5. X-ray Diffraction Patterns of Lily Starch
3.6. Pasting Profile
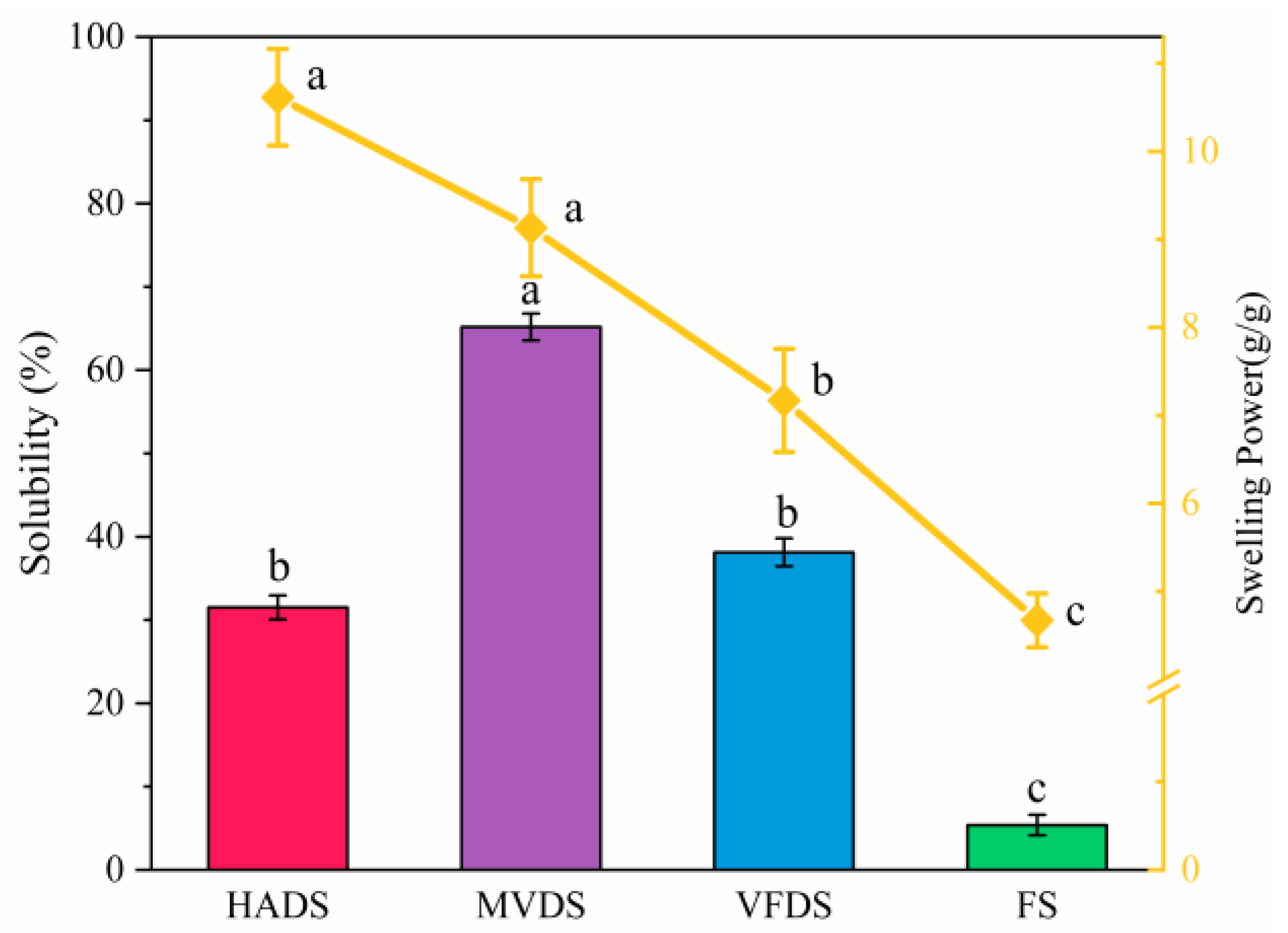
3.7. Solubility and Swelling Power
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xie, M.; Tan, H.; Zhao, G. A clean and sustainable strategy to produce bio-lubricant with high-bearing and good anti-oxidation ability from Lanzhou lily. J. Clean. Prod. 2022, 371, 133333. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Zhang, W.; Wu, H.; Wang, Z. Evaluation of nutrition components in Lanzhou lily bulb by confocal Raman microscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 244, 118837. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xia, Y.-P.; Zhang, J.-P.; Du, F.; Zhang, L.; Ma, Y.-D.; Zhou, H. Low humic acids promote in vitro lily bulblet enlargement by enhancing roots growth and carbohydrate metabolism. J. Zhejiang Univ. B 2016, 17, 892–904. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Y.; Wei, H.; Zhang, Y.; Guo, Z.; Qiu, Y.; Wen, L.; Xie, Z. Structural characterization of Lanzhou lily (Lilium davidii var. unicolor) polysaccharides and determination of their associated antioxidant activity. J. Sci. Food Agric. 2020, 100, 5603–5616. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, Y.; Li, W.; Qiu, Y.; Hua, C.; Zhang, Y.; Guo, Z.; Xie, Z. Immunomodulatory activity and active mechanisms of a low molecular polysaccharide isolated from Lanzhou lily bulbs in RAW264.7 macrophages. J. Funct. Foods 2022, 92, 105071. [Google Scholar] [CrossRef]
- Zhang, H.; Jin, L.; Zhang, J.-B.; Niu, T.; Guo, T.; Chang, J. Chemical constituents from the bulbs of Lilium davidii var. unicolor and anti-insomnia effect. Fitoterapia 2022, 161, 105071. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, J.; Li, A.; Wang, Z.; Xiong, F. Morphology and Physicochemical Properties of 3 Lilium Bulb Starches. J. Food Sci. 2015, 80, C1661–C1669. [Google Scholar] [CrossRef]
- Li, H.; Wang, R.; Liu, J.; Zhang, Q.; Li, G.; Shan, Y.; Ding, S. Effects of heat-moisture and acid treatments on the structural, physicochemical, and in vitro digestibility properties of lily starch. Int. J. Biol. Macromol. 2020, 148, 956–968. [Google Scholar] [CrossRef]
- Huang, D.; Li, W.; Dawuda, M.M.; Huo, J.; Li, C.; Wang, C.; Liao, W. Hydrogen sulfide reduced colour change in Lanzhou lily-bulb scales. Postharvest Biol. Technol. 2021, 176, 111520. [Google Scholar] [CrossRef]
- Yang, P.; Huang, D.; Lv, S.; Wang, R.; Sherif, S.A.; Li, W. Sun Drying and Far-Infrared Drying Characteristics of Lily. In Proceedings of the ASME 2021 Heat Transfer Summer Conference collocated with the ASME 2021 15th International Conference on Energy Sustainability, Virtual, 16–18 June 2021. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Wei, X.; Wu, D.; Dai, J.; Liu, S.; Qin, W. Effect of radio frequency-assisted hot-air drying on drying kinetics and quality of Sichuan pepper (Zanthoxylum bungeanum maxim.). LWT 2021, 147, 111572. [Google Scholar] [CrossRef]
- Marques, L.G.; Silveira, A.M.; Freire, J.T. Freeze-Drying Characteristics of Tropical Fruits. Dry. Technol. 2006, 24, 457–463. [Google Scholar] [CrossRef]
- Haizhu, Z.; Zheng, L.; Zhang, X.; Cui, X.; Wang, C.; Qu, Y. A study of the freeze-drying process and quality evaluation of Angelica sinensis. Int. J. Food Eng. 2020, 17, 411–422. [Google Scholar] [CrossRef]
- Jiang, N.; Liu, C.; Li, D.; Zhang, Z.; Liu, C.; Wang, D.; Niu, L.; Zhang, M. Evaluation of freeze drying combined with microwave vacuum drying for functional okra snacks: Antioxidant properties, sensory quality, and energy consumption. Food Sci. Technol. 2017, 82, 216–226. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, H.; Xu, J.; Zhuang, W.; Zheng, B.; Lo, Y.M.; Tian, Y. Microwave vacuum drying of lotus (Nelumbo nucifera Gaertn.) seeds: Effects of ultrasonic pretreatment on color, antioxidant activity, and rehydration capacity. LWT 2021, 149, 111603. [Google Scholar] [CrossRef]
- Aravindakshan, S.; Nguyen, T.H.A.; Kyomugasho, C.; Van Loey, A.; Hendrickx, M.E. The rehydration attributes and quality characteristics of ‘Quick-cooking’ dehydrated beans: Implications of glass transition on storage stability. Food Res. Int. 2022, 157, 111377. [Google Scholar] [CrossRef]
- Wang, R.; Chen, C.; Guo, S. Effects of drying methods on starch crystallinity of gelatinized foxtail millet (α-millet) and its eating quality. J. Food Eng. 2017, 207, 81–89. [Google Scholar] [CrossRef]
- Lopez-Quiroga, E.; Prosapio, V.; Fryer, P.J.; Norton, I.T.; Bakalis, S. Model discrimination for drying and rehydration kinetics of freeze-dried tomatoes. J. Food Process. Eng. 2019, 43, e13192. [Google Scholar] [CrossRef]
- Chitrakar, B.; Zhang, M.; Zhang, X. The determination of drying end-point for asparagus by-products with the use of LF-NMR spectra. Dry. Technol. 2020, 39, 1158–1164. [Google Scholar] [CrossRef]
- Yang, D.; Wu, G.; Lu, Y.; Li, P.; Qi, X.; Zhang, H.; Wang, X.; Jin, Q. Comparative analysis of the effects of novel electric field frying and conventional frying on the quality of frying oil and oil absorption of fried shrimps. Food Control. 2021, 128, 108195. [Google Scholar] [CrossRef]
- Mugodo, K.; Workneh, T.S. The kinetics of thin-layer drying and modelling for mango slices and the influence of differing hot-air drying methods on quality. Heliyon 2021, 7, e07182. [Google Scholar] [CrossRef]
- Ramachandran, R.P.; Erkinbaev, C.; Thakur, S.; Paliwal, J. Three dimensional characterization of micronized soybean seeds using X-ray microtomography. Food Bioprod. Process. 2021, 127, 388–397. [Google Scholar] [CrossRef]
- Pozo, C.; Rodríguez-Llamazares, S.; Bouza, R.; Barral, L.; Castaño, J.; Müller, N.; Restrepo, I. Study of the structural order of native starch granules using combined FTIR and XRD analysis. J. Polym. Res. 2018, 25, 266. [Google Scholar] [CrossRef]
- Ziegler, V.; Ferreira, C.D.; Goebel, J.T.S.; El Halal, S.L.M.; Santetti, G.S.; Gutkoski, L.C.; Zavareze, E.D.R.; Elias, M.C. Changes in properties of starch isolated from whole rice grains with brown, black, and red pericarp after storage at different temperatures. Food Chem. 2017, 216, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Waterschoot, J.; Gomand, S.V.; Delcour, J.A. Impact of swelling power and granule size on pasting of blends of potato, waxy rice and maize starches. Food Hydrocoll. 2016, 52, 69–77. [Google Scholar] [CrossRef]
- Kocabay, G.; Ismail, O. Investigation of rehydration kinetics of open-sun dried okra samples. Heat Mass Transf. 2017, 53, 2155–2163. [Google Scholar] [CrossRef]
- Kumar, A.; Begum, A.; Hoque, M.; Hussain, S.; Srivastava, B. Textural degradation, drying and rehydration behaviour of ohmically treated pineapple cubes. LWT 2021, 142, 110988. [Google Scholar] [CrossRef]
- Peng, J.; Lu, L.; Zhu, K.-X.; Guo, X.-N.; Chen, Y.; Zhou, H.-M. Effect of rehydration on textural properties, oral behavior, kinetics and water state of textured wheat gluten. Food Chem. 2022, 376, 131934. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, M.; Mujumdar, A.S. Evaluation of potential application of artificial intelligent control aided by LF-NMR in drying of carrot as model material. Dry. Technol. 2020, 39, 1149–1157. [Google Scholar] [CrossRef]
- Song, Y.; Huang, F.; Li, X.; Han, D.; Zhao, L.; Liang, H.; Rui, M.; Wang, J.; Zhang, C. Water status evolution of pork blocks at different cooking procedures: A two-dimensional LF-NMR T1-T2 relaxation study. Food Res. Int. 2021, 148, 110614. [Google Scholar] [CrossRef]
- Zhou, C.; Feng, Y.; Zhang, L.; Yagoub, A.E.A.; Wahia, H.; Ma, H.; Sun, Y.; Yu, X. Rehydration characteristics of vacuum freeze- and hot air-dried garlic slices. LWT 2021, 143, 111158. [Google Scholar] [CrossRef]
- Van Dyck, T.; Verboven, P.; Herremans, E.; Defraeye, T.; Van Campenhout, L.; Wevers, M.; Claes, J.; Nicolaï, B. Characterisation of structural patterns in bread as evaluated by X-ray computer tomography. J. Food Eng. 2014, 123, 67–77. [Google Scholar] [CrossRef]
- Aravindakshan, S.; Nguyen, T.H.A.; Kyomugasho, C.; Buvé, C.; Dewettinck, K.; Van Loey, A.; Hendrickx, M.E. The Impact of Drying and Rehydration on the Structural Properties and Quality Attributes of Pre-Cooked Dried Beans. Foods 2021, 10, 1665. [Google Scholar] [CrossRef]
- Li, H.; Wang, R.; Zhang, Q.; Li, G.; Shan, Y.; Ding, S. Morphological, structural, and physicochemical properties of starch isolated from different lily cultivars grown in China. Int. J. Food Prop. 2019, 22, 737–757. [Google Scholar] [CrossRef]
- Monteiro, R.L.; Link, J.V.; Tribuzi, G.; Carciofi, B.A.; Laurindo, J.B. Microwave vacuum drying and multi-flash drying of pumpkin slices. J. Food Eng. 2018, 232, 1–10. [Google Scholar] [CrossRef]
- Süfer, Ö.; Palazoğlu, T.K. A study on hot-air drying of pomegranate. J. Therm. Anal. Calorim. 2019, 137, 1981–1990. [Google Scholar] [CrossRef]
- Chen, L.; Tian, Y.; Sun, B.; Cai, C.; Ma, R.; Jin, Z. Measurement and characterization of external oil in the fried waxy maize starch granules using ATR-FTIR and XRD. Food Chem. 2018, 242, 131–138. [Google Scholar] [CrossRef]
- Zavareze, E.D.R.; Dias, A.R.G. Impact of heat-moisture treatment and annealing in starches: A review. Carbohydr. Polym. 2011, 83, 317–328. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, H.; Jin, Y.; Xu, D.; Xu, X. Impact of superheated steam on the moisture transfer, structural characteristics and rheological properties of wheat starch. Food Hydrocoll. 2021, 122, 107089. [Google Scholar] [CrossRef]
- Liu, X.; Huang, S.; Chao, C.; Yu, J.; Copeland, L.; Wang, S. Changes of starch during thermal processing of foods: Current status and future directions. Trends Food Sci. Technol. 2021, 119, 320–337. [Google Scholar] [CrossRef]
- Liu, Y.; Chao, C.; Yu, J.; Wang, S.; Wang, S.; Copeland, L. New insights into starch gelatinization by high pressure: Comparison with heat-gelatinization. Food Chem. 2020, 318, 126493. [Google Scholar] [CrossRef]
- Teobaldi, A.G.; Barrera, G.N.; Sciarini, L.S.; Ribotta, P.D. Pasting, gelatinization, and rheological properties of wheat starch in the presence of sucrose and gluten. Eur. Food Res. Technol. 2021, 247, 1083–1093. [Google Scholar] [CrossRef]
- Xu, F.; Liu, W.; Zhang, L.; Liu, Q.; Wang, F.; Zhang, H.; Hu, H.; Blecker, C. Thermal, structural, rheological and morphological properties of potato starch-gluten model dough systems: Effect of degree of starch pre-gelatinization. Food Chem. 2022, 396, 133628. [Google Scholar] [CrossRef] [PubMed]
- Bento, J.A.C.; Ferreira, K.C.; de Oliveira, A.L.M.; Lião, L.M.; Caliari, M.; Júnior, M.S.S. Extraction, characterization and technological properties of white garland-lily starch. Int. J. Biol. Macromol. 2019, 135, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Reddy, C.K.; Xu, B. Morphological, physico-chemical and functional properties of underutilized starches in China. Int. J. Biol. Macromol. 2020, 158, 648–655. [Google Scholar] [CrossRef]
- Liu, X.; Chao, C.; Yu, J.; Copeland, L.; Wang, S. Mechanistic studies of starch retrogradation and its effects on starch gel properties. Food Hydrocoll. 2021, 120, 106914. [Google Scholar] [CrossRef]





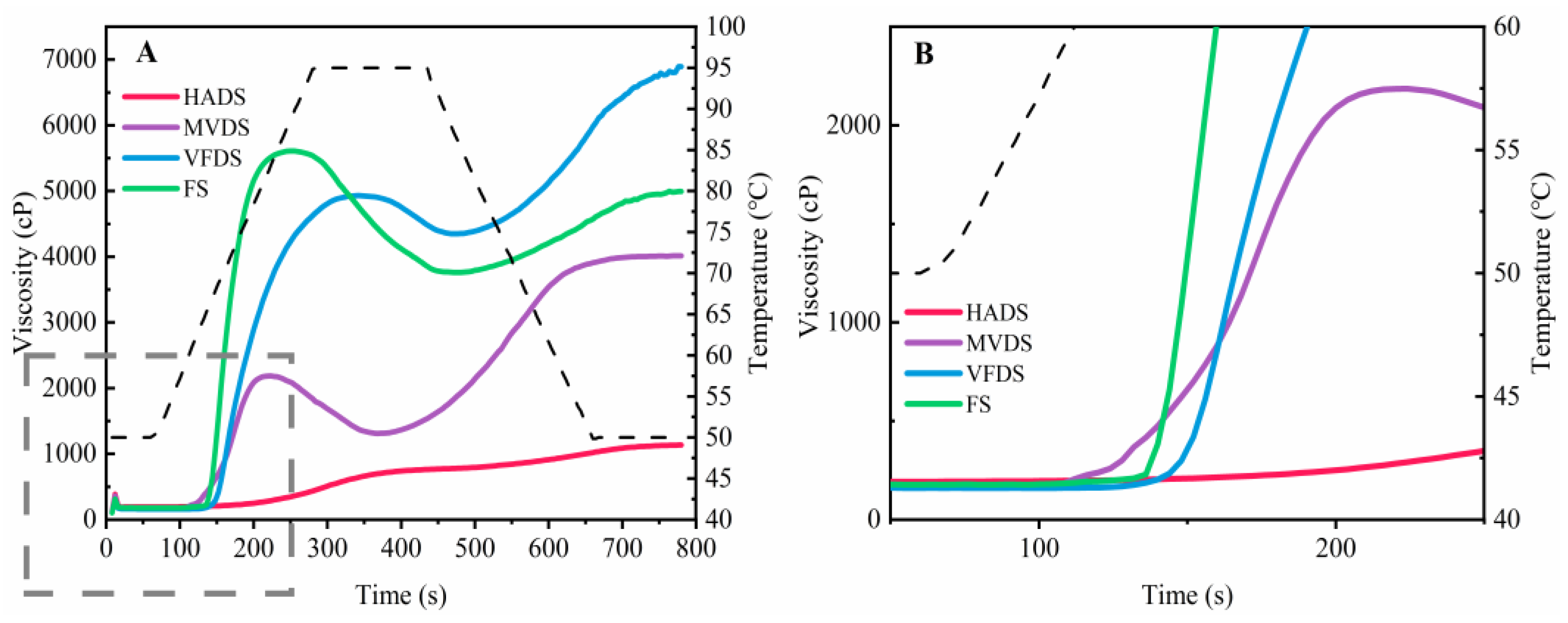
| Parameters | HADS | MVDS | VFDS | FS |
|---|---|---|---|---|
| PV(cp) | 624.67 ± 27.47 d | 2186.60 ± 7.77 c | 5097.33 ± 63.07 b | 5973.00 ± 88.84 a |
| TV(cp) | 536.00 ± 30.33 d | 1313.00 ± 36.51 c | 4279.00 ± 74.20 a | 3685.00 ± 38.69 b |
| BD(cp) | 84.67 ± 10.50 c | 856.32 ± 18.74 b | 788.00 ± 52.61 b | 2318.67 ± 60.00 a |
| FV(cp) | 1120.00 ± 63.79 d | 3997.68 ± 30.99 c | 6932.33 ± 59.43 a | 4981.67 ± 78.56 b |
| SB(cp) | 461.33 ± 6.86 c | 2680.87 ± 12.11 a | 2568.33 ± 15.50 ab | 1298.00 ± 10.33 b |
| PT(°C) | ND | 62.03 ± 4.30 b | 65.02 ± 7.23 ab | 65.97 ± 6.66 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Xue, L.; Wu, Z.; Zhang, W.; Zhang, H.; Zhao, C.; Liu, D. Insight into the Effects of Drying Methods on Lanzhou Lily Rehydration. Foods 2023, 12, 1817. https://doi.org/10.3390/foods12091817
Zhang X, Xue L, Wu Z, Zhang W, Zhang H, Zhao C, Liu D. Insight into the Effects of Drying Methods on Lanzhou Lily Rehydration. Foods. 2023; 12(9):1817. https://doi.org/10.3390/foods12091817
Chicago/Turabian StyleZhang, Xinyu, Lu Xue, Zijian Wu, Wen Zhang, Han Zhang, Cuiyu Zhao, and Dandan Liu. 2023. "Insight into the Effects of Drying Methods on Lanzhou Lily Rehydration" Foods 12, no. 9: 1817. https://doi.org/10.3390/foods12091817
APA StyleZhang, X., Xue, L., Wu, Z., Zhang, W., Zhang, H., Zhao, C., & Liu, D. (2023). Insight into the Effects of Drying Methods on Lanzhou Lily Rehydration. Foods, 12(9), 1817. https://doi.org/10.3390/foods12091817