Identification and Quantification of Selected Benzoxazinoids and Phenolics in Germinated Spelt (Triticum spelta)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Germination Method
2.3. Extraction of Free Phenolic Compounds
2.4. Extraction of Bound Phenolic Compounds
2.5. Total Phenolic Content (TPC)
2.6. DPPH Radical Scavenging Activity
2.7. ABTS Radical Cation Scavenging Activity
2.8. Purification of Extracts
2.9. Liquid Chromatography–Mass Spectrometry Analysis
2.10. NMR Spectroscopy
2.11. Statistical Analysis
3. Results and Discussion
3.1. Effect of Germination on Total Phenolic Content and Antioxidant Activity
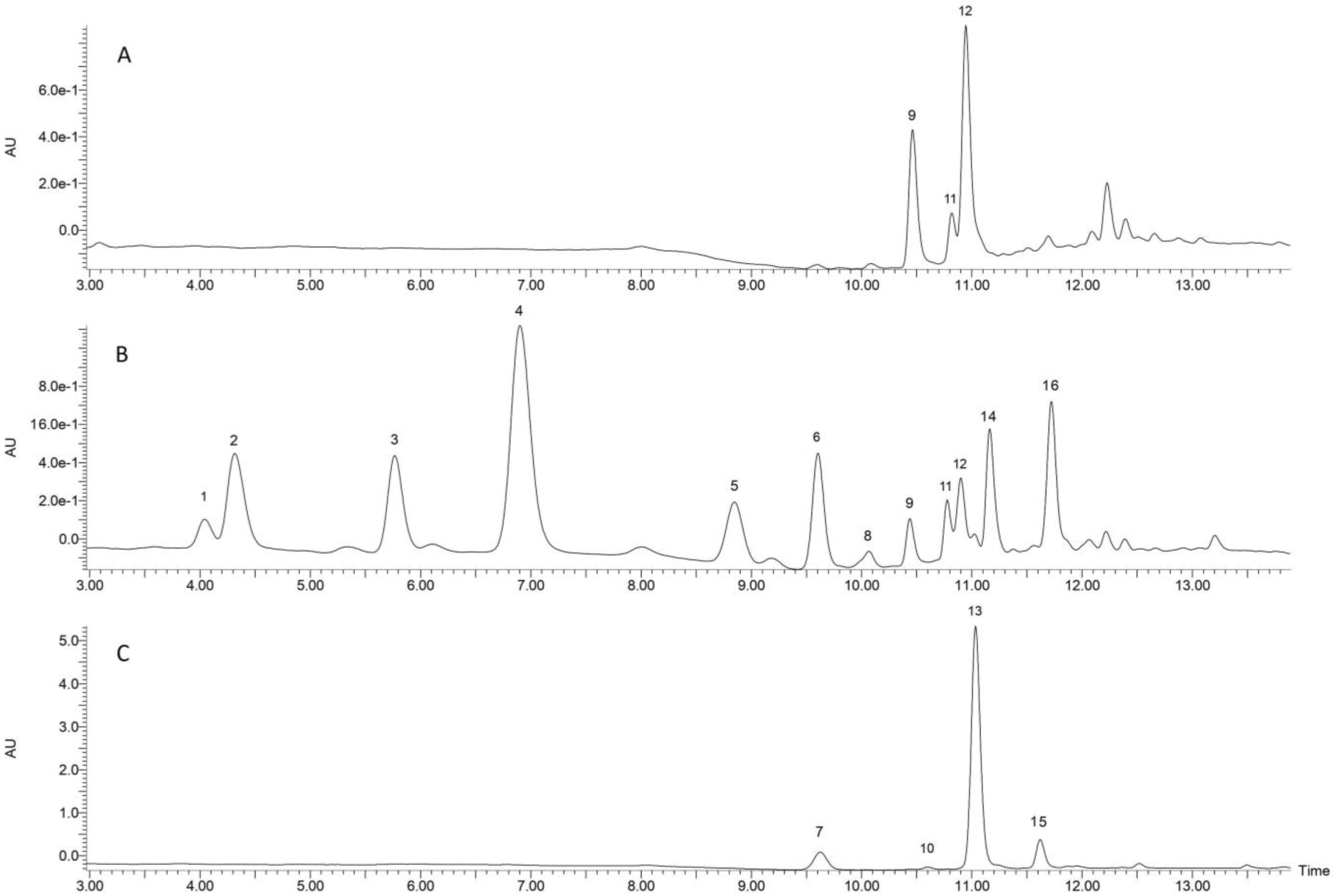
3.2. Phenolic Characterization by Liquid Chromatography–Mass Spectrometry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Molberg, Ø.; Uhlen, A.K.; Jensen, T.; Flæte, N.S.; Fleckenstein, B.; Arentz-Hansen, H.; Raki, M.; Lundin, K.E.A.; Sollid, L.M. Mapping of gluten T-cell epitopes in the bread wheat ancestors: Implications for celiac disease. Gastroenterology 2005, 128, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Șerban, L.R.; Păucean, A.; Man, S.M.; Chiş, M.S.; Mureşan, V. Ancient wheat species: Biochemical profile and impact on sourdough bread characteristics—A review. Processes 2021, 9, 2008. [Google Scholar] [CrossRef]
- Gan, R.Y.; Lui, W.Y.; Wu, K.; Chan, C.L.; Dai, S.H.; Sui, Z.Q.; Corke, H. Bioactive compounds and bioactivities of germinated edible seeds and sprouts: An updated review. Trends Food Sci. Technol. 2017, 59, 1–14. [Google Scholar] [CrossRef]
- Muñoz-Insa, A.; Selciano, H.; Zarnkow, M.; Becker, T.; Gastl, M. Malting process optimization of spelt (Triticum spelta L.) for the brewing process. LWT-Food Sci. Technol. 2013, 50, 99–109. [Google Scholar] [CrossRef]
- Hübner, F.; Arendt, E.K. Germination of Cereal Grains as a Way to Improve the Nutritional Value: A Review. Crit. Rev. Food Sci. Nutr. 2013, 53, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Herchi, W.; Bahashwan, S.; Sebei, K.; Ben Saleh, H.; Kallel, H.; Boukhchina, S. Effects of germination on chemical composition and antioxidant activity of flaxseed (Linum usitatissimum L.) oil. Grasas Aceites 2015, 66, e057. [Google Scholar] [CrossRef]
- Živković, A.; Polak, T.; Cigić, B.; Požrl, T. Germinated Buckwheat: Effects of Dehulling on Phenolics Profile and Antioxidant Activity of Buckwheat Seeds. Foods 2021, 10, 740. [Google Scholar] [CrossRef]
- Li, L.; Shewry, P.R.; Ward, J.L. Phenolic acids in wheat varieties in the healthgrain diversity screen. J. Agric. Food Chem. 2008, 56, 9732–9739. [Google Scholar] [CrossRef]
- Bei, Q.; Liu, Y.; Wang, L.; Chen, G.; Wu, Z. Improving free, conjugated, and bound phenolic fractions in fermented oats (Avena sativa L.) with Monascus anka and their antioxidant activity. J. Funct. Foods 2017, 32, 185–194. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Li, H.; Deng, Z.; Tsao, R. A review on insoluble-bound phenolics in plant-based food matrix and their contribution to human health with future perspectives. Trends Food Sci. Technol. 2020, 105, 347–362. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Schulz, M.; Marocco, A.; Tabaglio, V.; Macias, F.A.; Molinillo, J.M.G. Benzoxazinoids in Rye Allelopathy–From Discovery to Application in Sustainable Weed Control and Organic Farming. J. Chem. Ecol. 2013, 39, 154–174. [Google Scholar] [CrossRef] [PubMed]
- Pihlava, J.M.; Kurtelius, T. Determination of benzoxazinoids in wheat and rye beers by HPLC-DAD and UPLC-QTOF MS. Food Chem. 2016, 204, 400–408. [Google Scholar] [CrossRef]
- Prinz, S.; Schauberger, D.; Bauer, I.M.; Knasmueller, S.; Kopp, B. Aneugenic 2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one (DIMBOA) and 2,4-dihydroxy-1,4-benzoxazin-3-one (DIBOA) in sprouts of Triticum aestivum cultivars—A “safety health food”? Food Chem. 2010, 121, 973–979. [Google Scholar] [CrossRef]
- Adhikari, K.B.; Tanwir, F.; Gregersen, P.L.; Steffensen, S.K.; Jensen, B.M.; Poulsen, L.K.; Nielsen, C.H.; Høyer, S.; Borre, M.; Fomsgaard, I.S. Benzoxazinoids: Cereal phytochemicals with putative therapeutic and health-protecting properties. Mol. Nutr. Food Res. 2015, 59, 1324–1338. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, M.; Lloyd, A.J.; Haldar, S.; Seal, C.; Brandt, K.; Draper, J. Hydroxylated phenylacetamides derived from bioactive benzoxazinoids are bioavailable in humans after habitual consumption of whole grain sourdough rye bread. Mol. Nutr. Food Res. 2013, 57, 1859–1873. [Google Scholar] [CrossRef]
- Adhikari, K.B.; Lærke, H.N.; Mortensen, A.G.; Fomsgaard, I.S. Plasma and urine concentrations of bioactive dietary benzoxazinoids and their glucuronidated conjugates in rats fed a rye bread-based diet. J. Agric. Food Chem. 2012, 60, 11518–11524. [Google Scholar] [CrossRef]
- Jensen, B.M.; Adhikari, K.B.; Schnoor, H.J.; Juel-Berg, N.; Fomsgaard, I.S.; Poulsen, L.K. Quantitative analysis of absorption, metabolism, and excretion of benzoxazinoids in humans after the consumption of high- and low-benzoxazinoid diets with similar contents of cereal dietary fibres: A crossover study. Eur. J. Nutr. 2017, 56, 387–397. [Google Scholar] [CrossRef]
- Dang, J.; Paudel, Y.N.; Yang, X.; Ren, Q.; Zhang, S.; Ji, X.; Liu, K.; Jin, M. Schaftoside Suppresses Pentylenetetrazol-Induced Seizures in Zebrafish via Suppressing Apoptosis, Modulating Inflammation, and Oxidative Stress. ACS Chem. Neurosci. 2021, 12, 2542–2552. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Wu, J.; Chen, J.; Zhou, Y.; Chen, X.; Wu, Q.; Xu, Y.; Tu, W.; Lou, X.; Yang, G.; et al. Schaftoside ameliorates oxygen glucose deprivation-induced inflammation associated with the TLR4/Myd88/Drp1-related mitochondrial fission in BV2 microglia cells. J. Pharmacol. Sci. 2019, 139, 15–22. [Google Scholar] [CrossRef]
- Polonskiy, V.; Loskutov, I.; Sumina, A. Biological role and health benefits of antioxidant compounds in cereals. Biol. Commun. 2020, 65, 53–67. [Google Scholar] [CrossRef]
- Watts, J.E.; De Villiers, O.T.; Watts, L. Sterilization of wheat seeds for tissue culture purposes. S. Afr. J. Bot. 1993, 59, 641–642. [Google Scholar] [CrossRef]
- Abramovič, H.; Grobin, B.; Ulrih, N.P.; Cigić, B. Relevance and standardization of in vitro antioxidant assays: ABTS, DPPH, and Folin–Ciocalteu. J. Chem. 2018, 2018, 4608405. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Sayed, E.; Abdel-Aal, M.; Rabalski, I. Bioactive Compounds and their Antioxidant Capacity in Selected Primitive and Modern Wheat Species. Open Agric. J. 2008, 2, 7–14. [Google Scholar] [CrossRef]
- Yilmaz, V.A.; Brandolini, A.; Hidalgo, A. Phenolic acids and antioxidant activity of wild, feral and domesticated diploid wheats. J. Cereal Sci. 2015, 64, 168–175. [Google Scholar] [CrossRef]
- Mathew, S.; Emilia, T.; Zainul, A.; Zakaria, A. Reactivity of phenolic compounds towards free radicals under in vitro conditions. J. Food Sci. Technol. 2015, 52, 5790–5798. [Google Scholar] [CrossRef]
- Cho, D.H.; Lim, S.T. Changes in phenolic acid composition and associated enzyme activity in shoot and kernel fractions of brown rice during germination. Food Chem. 2018, 256, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Cevallos-Casals, B.A.; Cisneros-Zevallos, L. Impact of germination on phenolic content and antioxidant activity of 13 edible seed species. Food Chem. 2010, 119, 1485–1490. [Google Scholar] [CrossRef]
- Dixon, D.P.; Sellars, J.D.; Kenwright, A.M.; Steel, P.G. The maize benzoxazinone DIMBOA reacts with glutathione and other thiols to form spirocyclic adducts. Phytochemistry 2012, 77, 171–178. [Google Scholar] [CrossRef]
- Ma, X.; Lin, H.; Yong, Y.; Ju, X.; Li, Y.; Liu, X.; Yu, Z.; Wujin, C.; She, Y.; Zhang, J.; et al. Molecularly imprinted polymer-specific solid-phase extraction for the determination of 4-hydroxy-2(3H)benzoxazolone isolated from Acanthus ilicifolius Linnaeus using high-performance liquid chromatography-tandem mass spectrometry. Front. Nutr. 2022, 9, 2581. [Google Scholar] [CrossRef]
- Otaka, J.; Subbarao, G.V.; Ono, H.; Yoshihashi, T. Biological nitrification inhibition in maize—Isolation and identification of hydrophobic inhibitors from root exudates. Biol. Fertil. Soils 2022, 58, 251–264. [Google Scholar] [CrossRef]
- Tanwir, F.; Dionisio, G.; Adhikari, K.B.; Fomsgaard, I.S.; Gregersen, P.L. Biosynthesis and chemical transformation of benzoxazinoids in rye during seed germination and the identification of a rye Bx6-like gene. Phytochemistry 2017, 140, 95–107. [Google Scholar] [CrossRef]
- Zou, Y.; Yang, M.; Zhang, G.; He, H.; Yang, T. Antioxidant Activities and Phenolic Compositions of Wheat Germ as Affected by the Roasting Process. JAOCS J. Am. Oil Chem. Soc. 2015, 92, 1303–1312. [Google Scholar] [CrossRef]
- Siipola, S.M.; Kotilainen, T.; Sipari, N.; Morales, L.O.; Lindfors, A.V.; Robson, T.M.; Aphalo, P.J. Epidermal UV-A absorbance and whole-leaf flavonoid composition in pea respond more to solar blue light than to solar UV radiation. Plant Cell Environ. 2015, 38, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Casal, J.J. Photoreceptor signaling networks in plant responses to shade. Annu. Rev. Plant Biol. 2013, 64, 403–427. [Google Scholar] [CrossRef]
- Mencin, M.; Mikulic-Petkovsek, M.; Veberič, R.; Terpinc, P. Development and Optimisation of Solid-Phase Extraction of Extractable and Bound Phenolic Acids in Spelt (Triticum spelta L.) Seeds. Antioxidants 2021, 10, 1085. [Google Scholar] [CrossRef] [PubMed]
- Mencin, M.; Abramovič, H.; Jamnik, P.; Mikulič Petkovšek, M.; Veberič, R.; Terpinc, P. Abiotic stress combinations improve the phenolics profiles and activities of extractable and bound antioxidants from germinated spelt (Triticum spelta L.) seeds. Food Chem. 2020, 344, 128704. [Google Scholar] [CrossRef] [PubMed]
- Žilić, S.; Basić, Z.; Hadži-Tašković Šukalović, V.; Maksimović, V.; Janković, M.; Filipović, M. Can the sprouting process applied to wheat improve the contents of vitamins and phenolic compounds and antioxidant capacity of the flour? Int. J. Food Sci. Technol. 2014, 49, 1040–1047. [Google Scholar] [CrossRef]
- Hosseinian, F.S.; Mazza, G. Triticale bran and straw: Potential new sources of phenolic acids, proanthocyanidins, and lignans. J. Funct. Foods 2009, 1, 57–64. [Google Scholar] [CrossRef]
- Istasse, T.; Jacquet, N.; Berchem, T.; Haubruge, E.; Nguyen, B.K.; Richel, A. Extraction of honey polyphenols: Method development and evidence of cis isomerization. Anal. Chem. Insights 2016, 2016, 49–57. [Google Scholar] [CrossRef]
- Tang, Y.; Hao, J.; Fan, C.; Cao, X. Preparative separation of high-purity trans- and cis-ferulic acid from wheat bran by pH-zone-refining counter-current chromatography. J. Chromatogr. A 2021, 1636, 461772. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Wang, F.; Xu, K.; Zhang, Z.; Yan, J.; Yan, J.; Tian, Y.; Liu, J.; Zhang, Y.; Zhang, Y.; et al. Accumulation of Wheat Phenolic Acids under Different Nitrogen Rates and Growing Environments. Plants 2022, 11, 2237. [Google Scholar] [CrossRef] [PubMed]
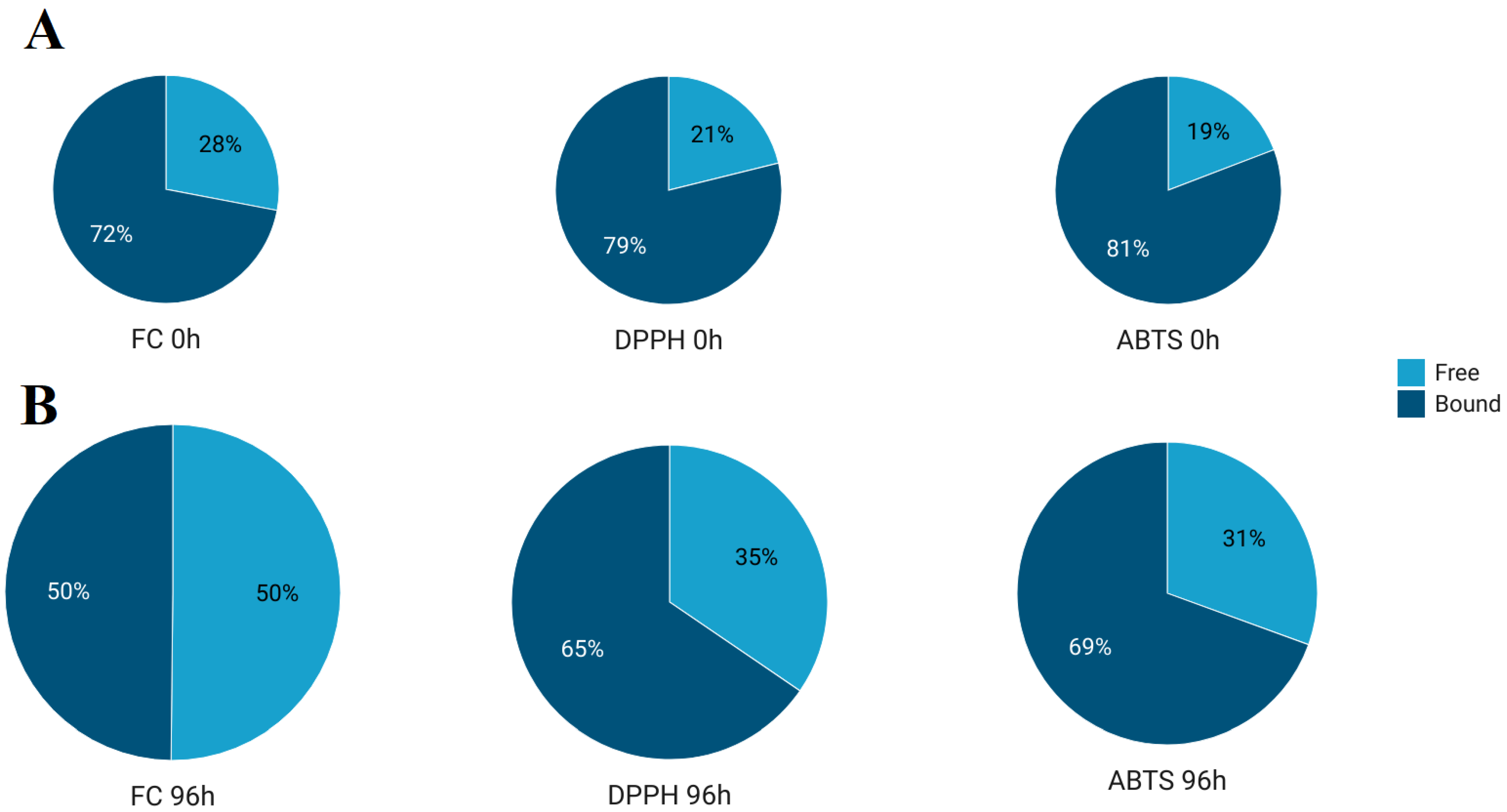
| Analysis | Measure | Germination Time (h) | ||||||
|---|---|---|---|---|---|---|---|---|
| Nongerminated | 12 | 24 | 36 | 48 | 72 | 96 | ||
| Total phenolic content (mg TE/g dry weight) | ||||||||
| FC | Free | 1.17 ± 0.08 a | 1.39 ± 0.06 ab | 1.47 ± 0.07 ab | 1.76 ± 0.11 b | 2.40 ± 0.15 c | 3.12 ± 0.28 d | 4.57 ± 0.57 e |
| Bound | 3.01 ± 0.16 a | 3.12 ± 0.24 ab | 3.35 ± 0.28 ab | 3.45 ± 0.57 ab | 3.69 ± 0.29 b | 4.34 ± 0.20 c | 4.54 ± 0.40 c | |
| Total | 4.18 ± 0.18 a | 4.51 ± 0.29 a | 4.82 ± 0.30 a | 5.21 ± 0.59 ab | 6.09 ± 0.41 b | 7.46 ± 0.46 c | 9.11 ± 0.96 d | |
| Antioxidant activity (mgTE/g dry weight) | ||||||||
| DPPH | Free | 0.36 ± 0.03 a | 0.43 ± 0.05 ab | 0.50 ± 0.05 b | 0.61 ± 0.04 c | 0.72 ± 0.06 d | 0.92 ± 0.07 e | 1.13 ± 0.09 f |
| Bound | 1.34 ± 0.07 a | 1.31 ± 0.05 a | 1.42 ± 0.04 a | 1.67 ± 0.02 b | 1.80 ± 0.06 bc | 1.96 ± 0.08 c | 2.14 ± 0.19 d | |
| Total | 1.70 ± 0.07 a | 1.74 ± 0.09 a | 1.92 ± 0.09 a | 2.28 ± 0.04 b | 2.52 ± 0.11 b | 2.88 ± 0.14 c | 3.27 ± 0.28 d | |
| ABTS | Free | 2.22 ± 0.12 a | 2.72 ± 0.06 b | 2.89 ± 0.12 b | 3.34 ± 0.16 c | 3.95 ± 0.13 d | 4.80 ± 0.06 e | 6.14 ± 0.56 f |
| Bound | 9.33 ± 0.19 a | 9.41 ± 0.34 a | 9.69 ± 0.32 a | 10.43 ± 0.48 a | 11.66 ± 0.96 b | 13.09 ± 0.67 c | 13.98 ± 1.05 c | |
| Total | 11.55 ± 0.22 a | 12.13 ± 0.39 a | 12.58 ± 0.40 ab | 13.77 ± 0.45 b | 15.61 ± 0.98 c | 17.89 ± 0.69 d | 20.12 ± 1.61 e | |
| Compound | Content of Benzoxazinoids and Phenolics (µg/g DW) during Germination (h) for the Nongerminated and Germinated Spelt Seeds | ||||||
|---|---|---|---|---|---|---|---|
| Nongerminated | 12 | 24 | 36 | 48 | 72 | 96 | |
| Free fraction | |||||||
| Schaftoside | 13.58 ± 1.54 b | 9.62 ± 1.22 a | 12.3 ± 0.52 b | 9.21 ± 0.55 a | 12.89 ± 1.40 b | 18.53 ± 0.58 c | 53.69 ± 2.65 d |
| Schaftoside isomer 1 | 46.78 ± 5.41 bc | 39.8 ± 5.20 ab | 47.94 ± 2.04 c | 35.08 ± 1.75 a | 46.82 ± 7.44 bc | 45.51 ± 1.71 bc | 47.48 ± 1.56 c |
| Schaftoside isomer 2 | 91.28 ± 15.26 b | 83.38 ± 15.77 ab | 92.97 ± 3.97 b | 71.30 ± 3.00 a | 89.71 ± 9.43 b | 85.20 ± 5.56 ab | 90.42 ± 3.38 b |
| BOA | ND | ND | 4.13 ± 1.32 a | 11.88 ± 1.33 b | 34.67 ± 3.00 d | 19.77 ± 4.41 c | 20.49 ± 0.86 c |
| MBOA | ND | ND | 6.98 ± 2.55 a | 38.78 ± 5.97 b | 111.52 ± 10.38 c | 181.05 ± 29.94 d | 277.61 ± 15.29 e |
| HBOA | ND | ND | ND | 14.97 ± 6.37 a | 26.05 ± 5.72 a | 130.08 ± 30.88 b | 219.65 ± 46.01 c |
| trans-ferulic acid | 1.90 ± 0.19 a | 1.90 ± 0.39 a | 1.63 ± 0.36 a | 2.06 ± 0.46 a | 2.05 ± 0.37 a | 1.90 ± 0.18 a | 3.00 ± 0.70 b |
| cis-ferulic acid | 0.96 ± 0.40 ab | 1.27 ± 0.33 b | 0.95 ± 0.33 ab | 1.14 ± 0.47 ab | 1.32 ± 0.32 b | 0.52 ± 0.13 a | 0.52 ± 0.11 a |
| trans-p-Coumaric acid | ND | ND | 0.97 ± 0.19 a | 1.34 ± 0.14 ab | 1.13 ± 0.09 a | 1.12 ± 0.14 a | 1.59 ± 0.20 b |
| Bound fraction | |||||||
| trans-ferulic acid | 359.92 ± 9.21 a | 406.61 ± 12.03 a | 419.48 ± 11.44 ab | 439.96 ± 32.99 ab | 489.16 ± 42.94 b | 580.89 ± 38.23 c | 753.27 ± 95.87 d |
| cis-ferulic acid | 76.69 ± 7.51 a | 97.29 ± 14.07 a | 93.46 ± 22.90 a | 110.44 ± 27.16 ab | 140.38 ± 13.47 bc | 150.84 ± 17.75 c | 203.66 ± 29.30 d |
| trans-p-Coumaric acid | 16.87 ± 1.75 a | 17.21 ± 0.58 a | 18.44 ± 0.81 a | 20.18 ± 2.41 a | 25.36 ± 1.07 a | 50.05 ± 3.69 b | 119.77 ± 15.42 c |
| cis-p-Coumaric acid | 1.59 ± 0.36 a | 1.68 ± 0.23 a | 1.73 ± 0.22 a | 1.69 ± 0.28 a | 2.33 ± 0.29 ab | 3.41 ± 0.56 b | 9.18 ± 1.56 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Živković, A.; Gođevac, D.; Cigić, B.; Polak, T.; Požrl, T. Identification and Quantification of Selected Benzoxazinoids and Phenolics in Germinated Spelt (Triticum spelta). Foods 2023, 12, 1769. https://doi.org/10.3390/foods12091769
Živković A, Gođevac D, Cigić B, Polak T, Požrl T. Identification and Quantification of Selected Benzoxazinoids and Phenolics in Germinated Spelt (Triticum spelta). Foods. 2023; 12(9):1769. https://doi.org/10.3390/foods12091769
Chicago/Turabian StyleŽivković, Andrej, Dejan Gođevac, Blaž Cigić, Tomaž Polak, and Tomaž Požrl. 2023. "Identification and Quantification of Selected Benzoxazinoids and Phenolics in Germinated Spelt (Triticum spelta)" Foods 12, no. 9: 1769. https://doi.org/10.3390/foods12091769
APA StyleŽivković, A., Gođevac, D., Cigić, B., Polak, T., & Požrl, T. (2023). Identification and Quantification of Selected Benzoxazinoids and Phenolics in Germinated Spelt (Triticum spelta). Foods, 12(9), 1769. https://doi.org/10.3390/foods12091769









