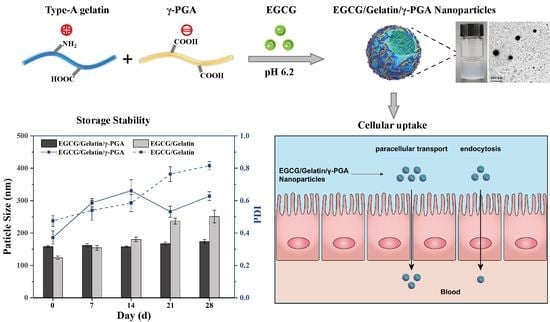
Preparation of Type-A Gelatin/Poly-γ-Glutamic Acid Nanoparticles for Enhancing the Stability and Bioavailability of (-)-Epigallocatechin Gallate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Complex Nanoparticles
2.3. Characterization of the Produced Nanoparticles
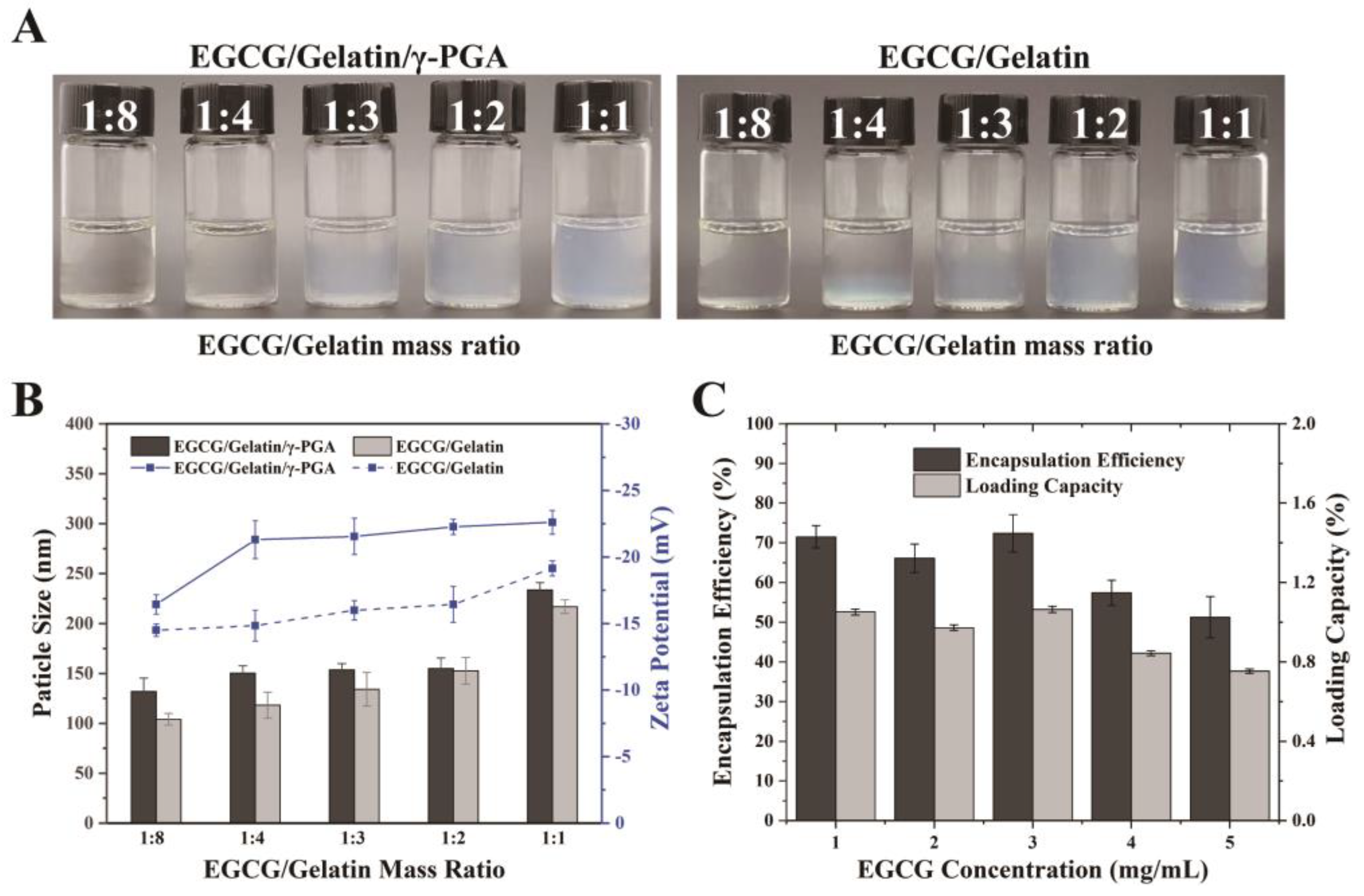
2.4. Assessments of Encapsulation Efficiency of EGCG in Nanoparticles
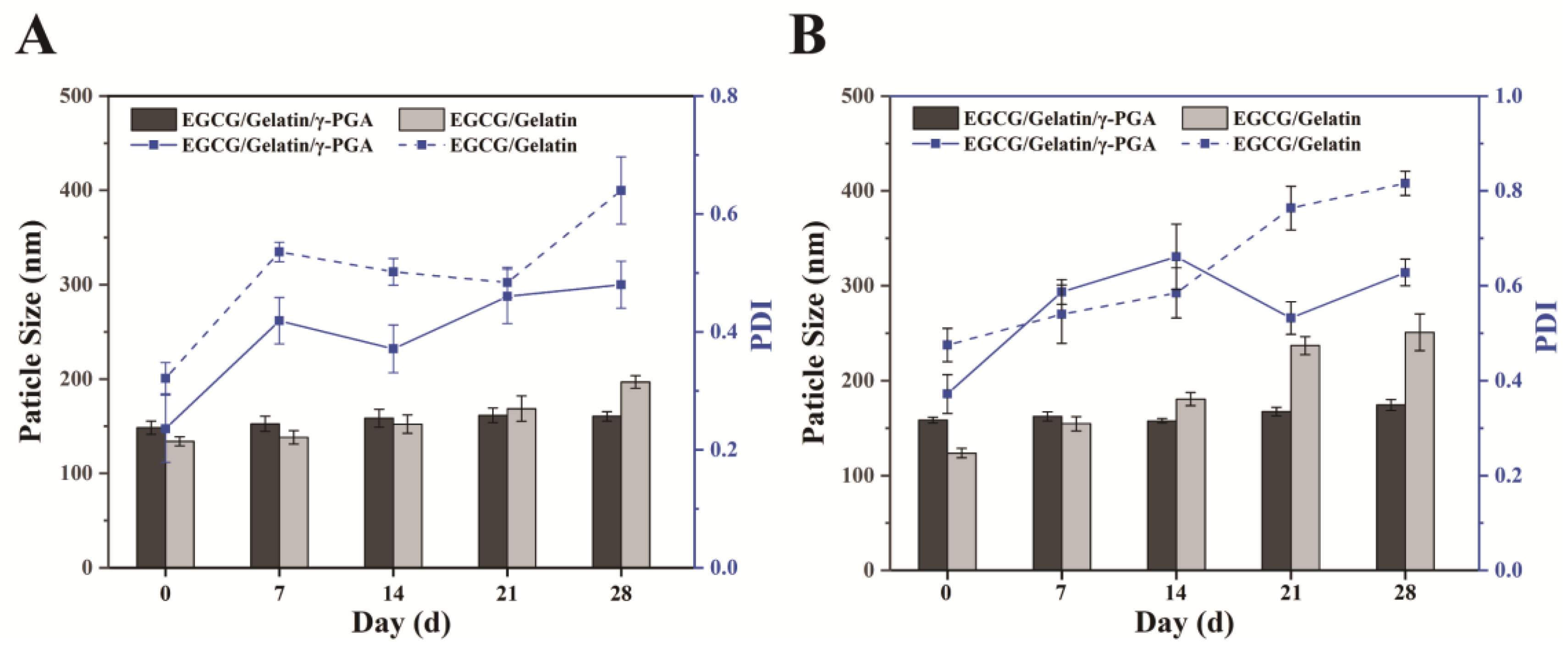
2.5. Storage Stability of EGCG/Gelatin/γ-PGA Nanoparticles
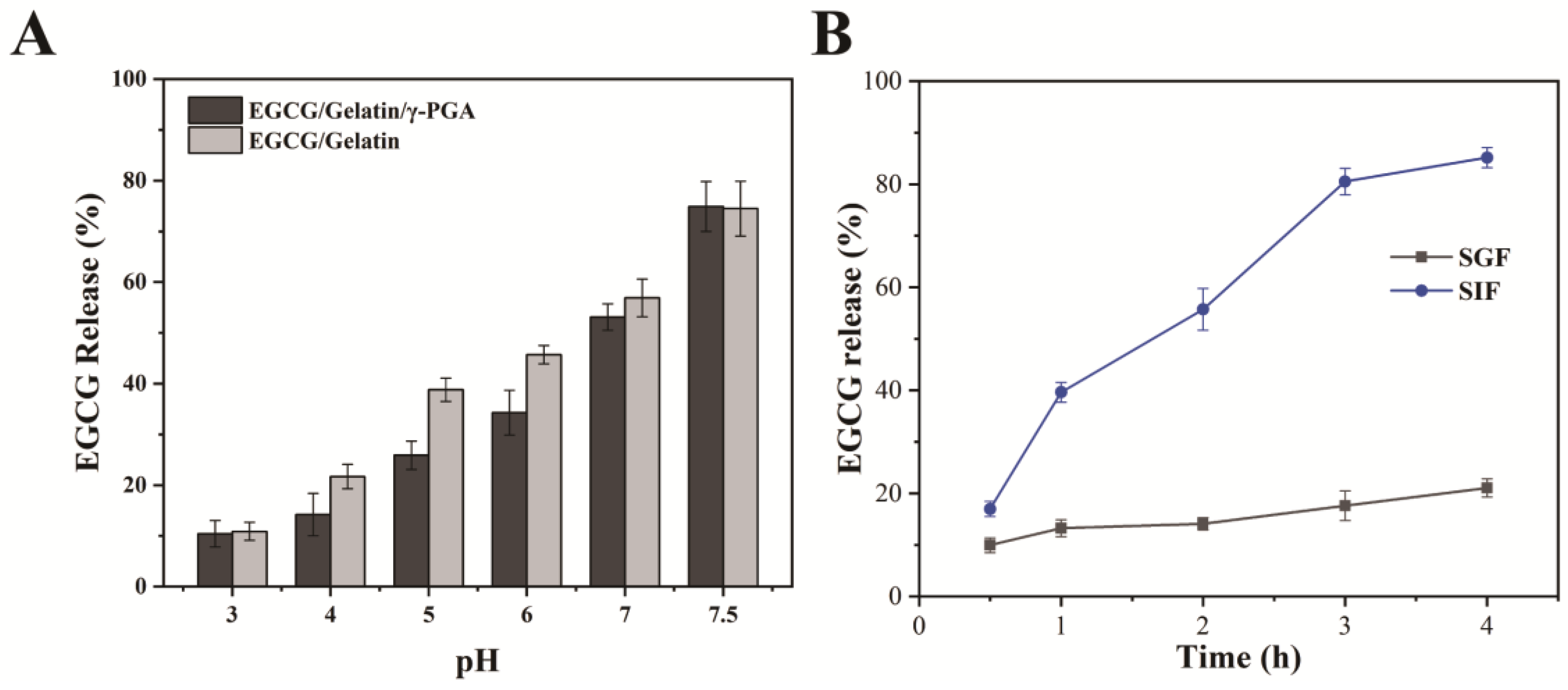
2.6. EGCG Release of Nanoparticles under Different pH Conditions
2.7. In Vitro Release of the Complex Nanoparticles
2.8. Antioxidant Measurements of Complex Nanoparticles by DPPH/ABTs
2.9. EGCG Transportation on Caco-2 Cell Monolayers
2.10. Statistical Analysis
3. Results and Discussion
3.1. Preparation of Complex Nanoparticles
3.2. Characterization of Complex Nanoparticles
3.3. Storage Stability of EGCG/gelatin/γ-PGA Nanoparticles
3.4. In Vitro Bioaccessibility of EGCG in EGCG/Gelatin/γ-PGA Nanoparticles
3.5. DPPH and ABTS Scavenging Activity of Nanoparticles
3.6. EGCG Transport on Caco-2 Monolayers
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dai, W.; Ruan, C.; Zhang, Y.; Wang, J.; Han, J.; Shao, Z.; Sun, Y.; Liang, J. Bioavailability enhancement of EGCG by structural modification and nano-delivery: A review. J. Funct. Foods 2020, 65, 103732. [Google Scholar] [CrossRef]
- Yin, Z.; Zheng, T.; Ho, C.-T.; Huang, Q.; Wu, Q.; Zhang, M. Improving the stability and bioavailability of tea polyphenols by encapsulations: A review. Food Sci. Hum. Wellness 2022, 11, 537–556. [Google Scholar] [CrossRef]
- Wen, J.-J.; Li, M.-Z.; Chen, C.-H.; Hong, T.; Yang, J.-R.; Huang, X.-J.; Geng, F.; Hu, J.-L.; Nie, S.-P. Tea polyphenol and epigallocatechin gallate ameliorate hyperlipidemia via regulating liver metabolites and remodeling gut microbiota. Food Chem. 2022, 404, 134591. [Google Scholar] [CrossRef] [PubMed]
- Rady, I.; Mohamed, H.; Rady, M.; Siddiqui, I.A.; Mukhtar, H. Cancer preventive and therapeutic effects of EGCG, the major polyphenol in green tea. Egypt. J. Basic Appl. Sci. 2018, 5, 1–23. [Google Scholar] [CrossRef]
- Cao, S.-Y.; Zhao, C.-N.; Gan, R.-Y.; Xu, X.-Y.; Wei, X.-L.; Corke, H.; Atanasov, A.G.; Li, H.-B. Effects and mechanisms of tea and its bioactive compounds for the prevention and treatment of cardiovascular diseases: An updated review. Antioxidants 2019, 8, 166. [Google Scholar] [CrossRef]
- Nikoo, M.; Regenstein, J.M.; Ahmadi Gavlighi, H. Antioxidant and antimicrobial activities of (-)-epigallocatechin-3-gallate (EGCG) and its potential to preserve the quality and safety of foods. Compr. Rev. Food Sci. Food Saf. 2018, 17, 732–753. [Google Scholar] [CrossRef]
- Barenys, M.; Gassmann, K.; Baksmeier, C.; Heinz, S.; Reverte, I.; Schmuck, M.; Temme, T.; Bendt, F.; Zschauer, T.-C.; Rockel, T.D. Epigallocatechin gallate (EGCG) inhibits adhesion and migration of neural progenitor cells in vitro. Arch. Toxicol. 2017, 91, 827–837. [Google Scholar] [CrossRef]
- Su, Y.L.; Leung, L.K.; Huang, Y.; Chen, Z.-Y. Stability of tea theaflavins and catechins. Food Chem. 2003, 83, 189–195. [Google Scholar]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef]
- Peng, Y.; Meng, Q.; Zhou, J.; Chen, B.; Xi, J.; Long, P.; Zhang, L.; Hou, R. Nanoemulsion delivery system of tea polyphenols enhanced the bioavailability of catechins in rats. Food Chem. 2018, 242, 527–532. [Google Scholar] [CrossRef]
- Tang, D.-W.; Yu, S.-H.; Ho, Y.-C.; Huang, B.-Q.; Tsai, G.-J.; Hsieh, H.-Y.; Sung, H.-W.; Mi, F.-L. Characterization of tea catechins-loaded nanoparticles prepared from chitosan and an edible polypeptide. Food Hydrocoll. 2013, 30, 33–41. [Google Scholar] [CrossRef]
- Roger, E.; Lagarce, F.; Garcion, E.; Benoit, J.-P. Biopharmaceutical parameters to consider in order to alter the fate of nanocarriers after oral delivery. Nanomedicine 2010, 5, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Li, Z.; Fan, W.; Zhong, X.; Peng, M.; Liu, Z. Nano-strategies for enhancing the bioavailability of tea polyphenols: Preparation, applications, and challenges. Foods 2022, 11, 387. [Google Scholar] [CrossRef] [PubMed]
- Rashidinejad, A.; Boostani, S.; Babazadeh, A.; Rehman, A.; Rezaei, A.; Akbari-Alavijeh, S.; Shaddel, R.; Jafari, S. Opportunities and challenges for the nanodelivery of green tea catechins in functional foods. Food Res. Int. 2021, 142, 110186. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, Y.; Zhou, X.; Huang, Z.; Zhao, L.; Liu, C. Elaboration of Cationic Soluble Soybean Polysaccharides-Epigallocatechin Gallate Nanoparticles with Sustained Antioxidant and Antimicrobial Activities. J. Agric. Food Chem. 2022, 70, 11353–11366. [Google Scholar] [CrossRef] [PubMed]
- Bhushani, J.A.; Karthik, P.; Anandharamakrishnan, C. Nanoemulsion based delivery system for improved bioaccessibility and Caco-2 cell monolayer permeability of green tea catechins. Food Hydrocoll. 2016, 56, 372–382. [Google Scholar] [CrossRef]
- Jia, Z.; Dumont, M.-J.; Orsat, V. Encapsulation of phenolic compounds present in plants using protein matrices. Food Biosci. 2016, 15, 87–104. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, X.; McClements, D.J.; Zou, L.; Liu, X.; Liu, F. Co-encapsulation of epigallocatechin gallate (EGCG) and curcumin by two proteins-based nanoparticles: Role of EGCG. J. Agric. Food Chem. 2019, 67, 13228–13236. [Google Scholar] [CrossRef]
- Evageliou, V.; Panagopoulou, E.; Mandala, I. Encapsulation of EGCG and esterified EGCG derivatives in double emulsions containing whey protein isolate, bacterial cellulose and salt. Food Chem. 2019, 281, 171–177. [Google Scholar] [CrossRef]
- Wang, L.; Chen, S.; Yu, B. Poly-γ-glutamic acid: Recent achievements, diverse applications and future perspectives. Trends Food Sci. Technol. 2022, 119, 1–12. [Google Scholar] [CrossRef]
- Wang, R.; Guo, K.; Zhang, W.; He, Y.; Yang, K.; Chen, Q.; Yang, L.; Di, Z.; Qiu, J.; Lei, P. Poly-γ-Glutamic Acid Microgel-Encapsulated Probiotics with Gastric Acid Resistance and Smart Inflammatory Factor Targeted Delivery Performance to Ameliorate Colitis. Adv. Funct. Mater. 2022, 32, 2113034. [Google Scholar] [CrossRef]
- Kashima, N.; Furuta, K.; Tanabe, I. Permeability Enhancer. U.S. Patent 20060025346, 2006. [Google Scholar]
- Tamura, M.; Hoshi, C.; Kimura, Y.; Suzuki, T.; Yamamoto-Maeda, M. Effects of γ-polyglutamic acid on the cecal microbiota and visceral fat in KK-Ay/TaJcl male mice. Food Sci. Technol. Res. 2018, 24, 151–157. [Google Scholar] [CrossRef]
- Lee, E.-H.; Son, W.-C.; Lee, S.-E.; Kim, B.-H. Anti-obesity effects of poly-γ-glutamic acid with or without isoflavones on high-fat diet induced obese mice. Biosci. Biotechnol. Biochem. 2013, 77, 130253. [Google Scholar] [CrossRef] [PubMed]
- Sarika, P.; James, N.R. Polyelectrolyte complex nanoparticles from cationised gelatin and sodium alginate for curcumin delivery. Carbohydr. Polym. 2016, 148, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Abedinia, A.; Nafchi, A.M.; Sharifi, M.; Ghalambor, P.; Oladzadabbasabadi, N.; Ariffin, F.; Huda, N. Poultry gelatin: Characteristics, developments, challenges, and future outlooks as a sustainable alternative for mammalian gelatin. Trends Food Sci. Technol. 2020, 104, 14–26. [Google Scholar] [CrossRef]
- Ranganathan, S.; Balagangadharan, K.; Selvamurugan, N. Chitosan and gelatin-based electrospun fibers for bone tissue engineering. Int. J. Biol. Macromol. 2019, 133, 354–364. [Google Scholar] [CrossRef]
- Eastoe, J.E.; Leach, A.A. Chemical constitution of gelatin. In The Science and Technology of Gelatin; Ward, A.G., Courts, A., Eds.; Academic Press Inc.: London, UK, 1977; pp. 73–108. [Google Scholar]
- Shutava, T.G.; Balkundi, S.S.; Vangala, P.; Steffan, J.J.; Bigelow, R.L.; Cardelli, J.A.; O’Neal, D.P.; Lvov, Y.M. Layer-by-layer-coated gelatin nanoparticles as a vehicle for delivery of natural polyphenols. ACS Nano 2009, 3, 1877–1885. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.P.D.; Hsieh, M.-F.; Doma, B.T., Jr.; Peruelo, D.C.; Chen, I.-H.; Lee, H.-M. Synthesis of gelatin-γ-polyglutamic acid-based hydrogel for the in vitro controlled release of epigallocatechin gallate (EGCG) from Camellia sinensis. Polymers 2013, 6, 39–58. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, S.; Chen, Y.; Chen, S. Preparation of Gamma Polyglutamic Acid (Γ-PGA)/Gelation Composite Nanoparticle and Application on Osmanthus Fragrance Slow-Release. Proc. IOP Conf. Ser. Mater. Sci. Eng. 2019, 612, 022024. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Yu, S.-H.; Tsai, G.-J.; Tang, D.-W.; Mi, F.-L.; Peng, Y.-P. Novel technology for the preparation of self-assembled catechin/gelatin nanoparticles and their characterization. J. Agric. Food Chem. 2010, 58, 6728–6734. [Google Scholar] [CrossRef]
- Neilson, A.P.; Green, R.J.; Wood, K.V.; Ferruzzi, M.G. High-throughput analysis of catechins and theaflavins by high performance liquid chromatography with diode array detection. J. Chromatogr. 2006, 1132, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, M.; Hu, C.; Liu, A.; Chen, J.; Gu, C.; Zhang, X.; You, C.; Tong, H.; Wu, M. Sargassum fusiforme fucoidan SP2 extends the lifespan of Drosophila melanogaster by upregulating the Nrf2-mediated antioxidant signaling pathway. Oxidative Med. Cell. Longev. 2019, 2019, 8918914. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Duan, L.; Guo, L.; Dou, L.-L.; Dong, X.; Zhou, P.; Li, P.; Liu, E.-H. Chemical and biological comparison of the fruit extracts of Citrus wilsonii Tanaka and Citrus medica L. Food Chem. 2015, 173, 54–60. [Google Scholar] [CrossRef]
- Hubatsch, I.; Ragnarsson, E.G.; Artursson, P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat. Protoc. 2007, 2, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Muzolf, M.; Szymusiak, H.; Gliszczyńska-Świgło, A.; Rietjens, I.M.; Tyrakowska, B.E. pH-dependent radical scavenging capacity of green tea catechins. J. Agric. Food Chem. 2008, 56, 816–823. [Google Scholar] [CrossRef]
- Luo, Z.; Guo, Y.; Liu, J.; Qiu, H.; Zhao, M.; Zou, W.; Li, S. Microbial synthesis of poly-γ-glutamic acid: Current progress, challenges, and future perspectives. Biotechnol. Biofuels 2016, 9, 134. [Google Scholar] [CrossRef]
- Hufschmid, R.; Teeman, E.; Mehdi, B.L.; Krishnan, K.M.; Browning, N.D. Observing the colloidal stability of iron oxide nanoparticles in situ. Nanoscale 2019, 11, 13098–13107. [Google Scholar] [CrossRef]
- Song, H.; Wang, Q.; He, A.; Li, S.; Guan, X.; Hu, Y.; Feng, S. Antioxidant activity, storage stability and in vitro release of epigallocatechin-3-gallate (EGCG) encapsulated in hordein nanoparticles. Food Chem. 2022, 388, 132903. [Google Scholar] [CrossRef]
- He, A.; Guan, X.; Song, H.; Li, S.; Huang, K. Encapsulation of (−)-epigallocatechin-gallate (EGCG) in hordein nanoparticles. Food Biosci. 2020, 37, 100727. [Google Scholar] [CrossRef]
- Astete, C.E.; Sabliov, C.M. Synthesis and characterization of PLGA nanoparticles. J. Biomater. Sci. Polym. Ed. 2006, 17, 247–289. [Google Scholar] [CrossRef] [PubMed]
- Amani, F.; Rezaei, A.; Damavandi, M.S.; Doost, A.S.; Jafari, S.M. Colloidal carriers of almond gum/gelatin coacervates for rosemary essential oil: Characterization and in-vitro cytotoxicity. Food Chem. 2022, 377, 131998. [Google Scholar] [CrossRef]
- You, G.; Niu, G.; Long, H.; Zhang, C.; Liu, X. Elucidation of interactions between gelatin aggregates and hsian-tsao gum in aqueous solutions. Food Chem. 2020, 319, 126532. [Google Scholar] [CrossRef]
- Dube, A.; Nicolazzo, J.A.; Larson, I. Chitosan nanoparticles enhance the intestinal absorption of the green tea catechins (+)-catechin and (−)-epigallocatechin gallate. Eur. J. Pharm. Sci. 2010, 41, 219–225. [Google Scholar] [CrossRef]
- Hu, B.; Ting, Y.; Zeng, X.; Huang, Q. Cellular uptake and cytotoxicity of chitosan–caseinophosphopeptides nanocomplexes loaded with epigallocatechin gallate. Carbohydr. Polym. 2012, 89, 362–370. [Google Scholar] [CrossRef]
- Yang, R.; Liu, Y.; Gao, Y.; Yang, Z.; Zhao, S.; Wang, Y.; Blanchard, C.; Zhou, Z. Nano-encapsulation of epigallocatechin gallate in the ferritin-chitosan double shells: Simulated digestion and absorption evaluation. Food Res. Int. 2018, 108, 1–7. [Google Scholar] [CrossRef]
- Fu, Q.; Wang, H.; Xia, M.; Deng, B.; Shen, H.; Ji, G.; Li, G.; Xie, Y. The effect of phytic acid on tight junctions in the human intestinal Caco-2 cell line and its mechanism. Eur. J. Pharm. Sci. 2015, 80, 1–8. [Google Scholar] [CrossRef]
- Rejman, J.; Oberle, V.; Zuhorn, I.S.; Hoekstra, D. Size-dependent internalization of particles via the pathways of clathrin-and caveolae-mediated endocytosis. Biochem. J. 2004, 377, 159–169. [Google Scholar] [CrossRef]
- Bae, K.-C.; Park, J.-H.; Na, A.-Y.; Kim, S.-J.; Ahn, S.; Kim, S.-P.; Oh, B.-C.; Cho, H.-C.; Kim, Y.W.; Song, D.-K. Effect of green tea extract/poly-γ-glutamic acid complex in obese type 2 diabetic mice. Diabetes Metab. J. 2013, 37, 196–206. [Google Scholar] [CrossRef]



| (a) EGCG/gelatin/γ-PGA nanoparticles | |||
|---|---|---|---|
| gelatin/ γ-PGA | Mean Particle Size (nm) | PDI | Zeta Potential (mV) |
| 1:1 | 112.4 ± 4.4 | 0.475 ± 0.026 | −24.3 ± 0.3 |
| 2:1 | 149.9 ± 5.3 | 0.341 ± 0.041 | −22.2 ± 1.0 |
| 4:1 | 155.1 ± 7.3 | 0.585 ± 0.034 | −23.9 ± 0.9 |
| 6:1 | 212.7 ± 6.5 | 0.564 ± 0.069 | −19.2 ± 0.4 |
| 8:1 | N/A b | N/A | N/A |
| (b) Gelatin/γ-PGA nanoparticles | |||
| 1:1 | 104.2 ± 2.8 | 0.272 ± 0.035 | −22.1 ± 0.6 |
| 2:1 | 116.9 ± 6.7 | 0.458 ± 0.053 | −21.4 ± 0.3 |
| 4:1 | 118.1 ± 1.1 | 0.434 ± 0.062 | −20.9 ± 0.2 |
| 6:1 | N/A | N/A | N/A |
| 8:1 | N/A | N/A | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Shen, H.; Li, Y.; Yang, K.; Lei, P.; Gu, Y.; Sun, L.; Xu, H.; Wang, R. Preparation of Type-A Gelatin/Poly-γ-Glutamic Acid Nanoparticles for Enhancing the Stability and Bioavailability of (-)-Epigallocatechin Gallate. Foods 2023, 12, 1748. https://doi.org/10.3390/foods12091748
Zhang W, Shen H, Li Y, Yang K, Lei P, Gu Y, Sun L, Xu H, Wang R. Preparation of Type-A Gelatin/Poly-γ-Glutamic Acid Nanoparticles for Enhancing the Stability and Bioavailability of (-)-Epigallocatechin Gallate. Foods. 2023; 12(9):1748. https://doi.org/10.3390/foods12091748
Chicago/Turabian StyleZhang, Weijie, Huangchen Shen, Ying Li, Kai Yang, Peng Lei, Yian Gu, Liang Sun, Hong Xu, and Rui Wang. 2023. "Preparation of Type-A Gelatin/Poly-γ-Glutamic Acid Nanoparticles for Enhancing the Stability and Bioavailability of (-)-Epigallocatechin Gallate" Foods 12, no. 9: 1748. https://doi.org/10.3390/foods12091748
APA StyleZhang, W., Shen, H., Li, Y., Yang, K., Lei, P., Gu, Y., Sun, L., Xu, H., & Wang, R. (2023). Preparation of Type-A Gelatin/Poly-γ-Glutamic Acid Nanoparticles for Enhancing the Stability and Bioavailability of (-)-Epigallocatechin Gallate. Foods, 12(9), 1748. https://doi.org/10.3390/foods12091748