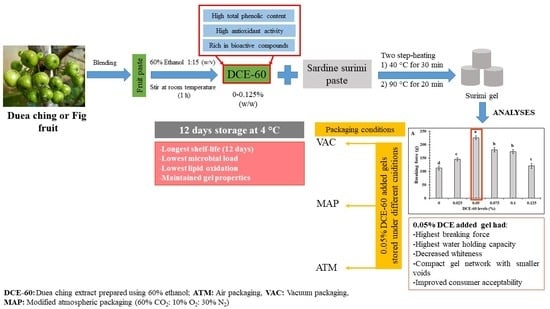
Ethanolic Extract of Duea Ching Fruit: Extraction, Characterization and Its Effect on the Properties and Storage Stability of Sardine Surimi Gel
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Effect of Extraction Media on Compositions and Antioxidant Activities of Fig (Duea Ching) Extract
2.2.1. Preparation of Fig Extract Using EtOH at Different Concentrations
2.2.2. Analyses
Total Phenolic Content (TPC)
Calcium Content
Antioxidant Activities (AOX)
LC/MS Profiling and Identification
2.3. Effect of DCE on the Properties of Sardine Surimi Gel
2.3.1. Preparation of Surimi Gel with DCE at Different Levels
2.3.2. Analysis
Breaking Force (BF) and Deformation (DF)
Expressible Moisture Content (EMC) and Whiteness
Textural Profile Analysis (TPA)
Sensory Evaluation
Microstructure
2.4. Effect of DCE on Microbial Load, Lipid Oxidation and Properties of Surimi Gel Stored under Different Packaging Conditions during Refrigerated Storage
2.4.1. Preparation of Gels Containing DCE Stored under Different Packaging Conditions
Gelling Properties
Lipid Oxidation and Microbiological Analyses
2.5. Statistical Analysis
3. Results and Discussion
3.1. Composition and Antioxidant Activities of Different Duea Ching Extracts
3.1.1. Total Phenolic Content (TPC)
3.1.2. Calcium Content
3.1.3. Antioxidant Activities (AOX)
3.1.4. Identification and Profiling of Compounds in Selected Duea Ching Extracts (DCE-60)
3.2. Effect of DCE-60 at Various Levels on the Textural and Sensory Properties of Sardine Surimi Gel
3.2.1. Breaking Force (BF) and Deformation (DF)
3.2.2. Expressible Moisture Content (EMC)
3.2.3. Whiteness
3.2.4. Textural Properties
3.2.5. Acceptability
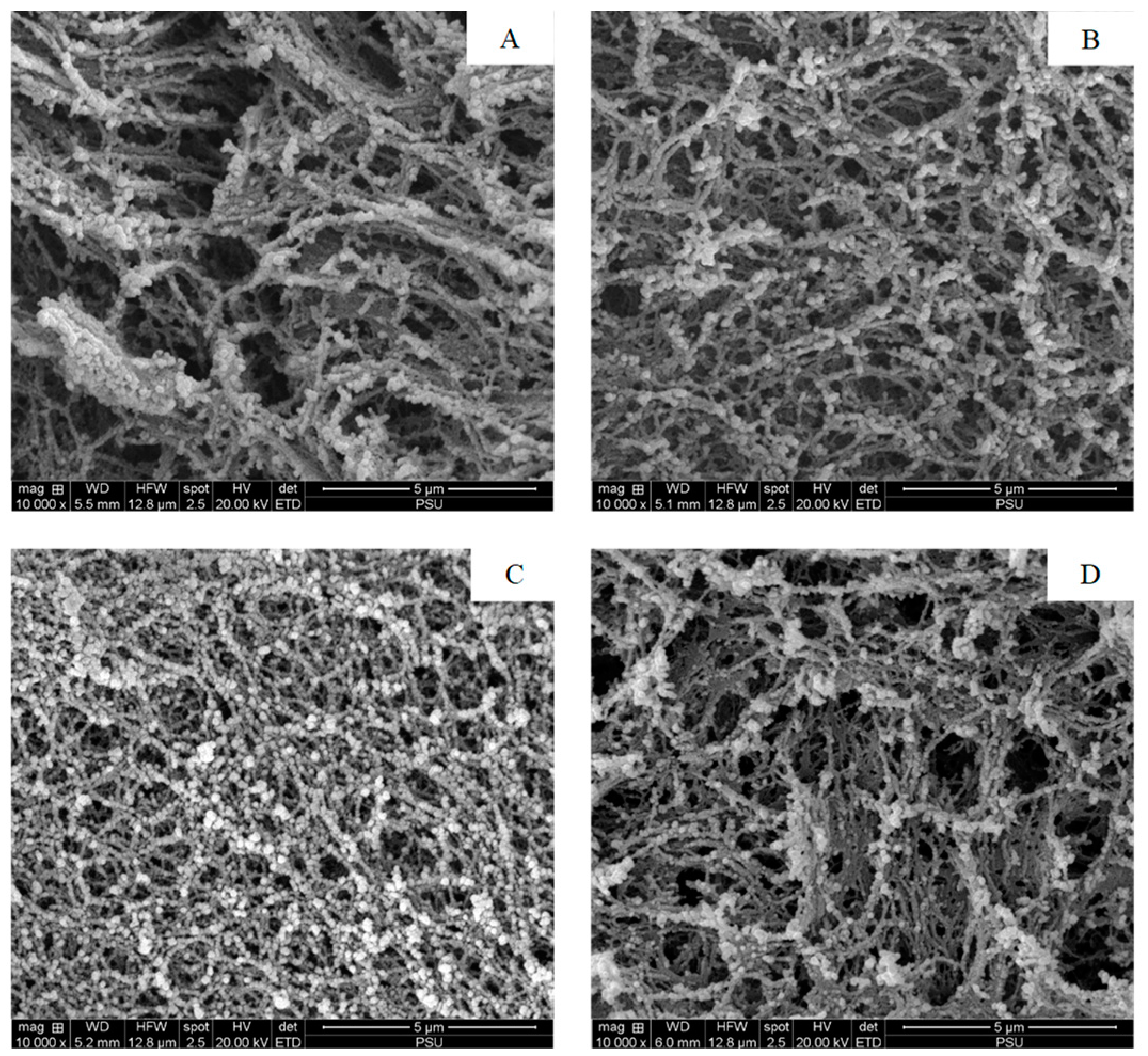
3.2.6. Microstructure
3.3. Effect of DCE-60 Addition on the Microbial Load, Lipid Oxidation and Gel Properties of Sardine Surimi Gel Packed under Varying Conditions during Refrigerated Storage
3.3.1. TVC and PBC
3.3.2. PV and TBARS
3.3.3. BF and DF
3.3.4. EMC
3.3.5. Whiteness
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pothasin, P.; Compton, S.G.; Wangpakapattanawong, P. Riparian ficus tree communities: The distribution and abundance of riparian fig trees in Northern Thailand. PLoS ONE 2014, 9, e108945. [Google Scholar] [CrossRef]
- Arunachalam, K.; Parimelazhagan, T. Evaluation of nutritional composition and antioxidant properties of underutilized Ficus talboti King fruit for nutraceuticals and food supplements. J. Food Sci. Technol. 2014, 51, 1260–1268. [Google Scholar] [CrossRef]
- Mawa, S.; Husain, K.; Jantan, I. Ficus carica L. (Moraceae): Phytochemistry, traditional uses and biological activities. Evid.-Based Complement. Altern. Med. 2013, 2013, 974256. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.H.; Benjakul, S.; Sae-leaw, T.; Balange, A.K.; Maqsood, S. Protein–polyphenol conjugates: Antioxidant property, functionalities and their applications. Trends Food Sci. Technol. 2019, 91, 507–517. [Google Scholar] [CrossRef]
- Benjakul, S.; Singh, A.; Chotphruethipong, L.; Mittal, A. Protein-polyphenol conjugates: Preparation, functional properties, bioactivities and applications in foods and nutraceuticals. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2021; Volume 98, pp. 281–320. [Google Scholar]
- Buamard, N.; Benjakul, S. Improvement of gel properties of sardine (Sardinella albella) surimi using coconut husk extracts. Food Hydrocoll. 2015, 51, 146–155. [Google Scholar] [CrossRef]
- Priyadarshini, M.B.; Balange, A.; Xavier, M.; Nayak, B.B. Effect of spray-dried cluster bean seed protein extract on the gel properties of single washed Nile tilapia surimi. J. Food Process. Preserv. 2022, 46, e17104. [Google Scholar] [CrossRef]
- Sharma, S.; Majumdar, R.K.; Mehta, N.K.; Nirmal, N.P. Effects of pineapple peel ethanolic extract on the physicochemical and textural properties of surimi prepared from silver carp (Hypophthalmichthys molitrix). Foods 2022, 11, 3223. [Google Scholar] [CrossRef]
- Buamard, N.; Benjakul, S. Cross-linking activity of ethanolic coconut husk extract toward sardine (Sardinella albella) muscle proteins. J. Food Biochem. 2017, 41, e12283. [Google Scholar] [CrossRef]
- Singh, A.; Benjakul, S. Proteolysis and its control using protease inhibitors in fish and fish products: A review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Chanarat, S.; Benjakul, S. Impact of microbial transglutaminase on gelling properties of Indian mackerel fish protein isolates. Food Chem. 2013, 136, 929–937. [Google Scholar] [CrossRef]
- Chanarat, S.; Benjakul, S.; Xiong, Y. Nonprotein nitrogenous compounds and gelling property of white cheek shark (Carcharhinus dussumieri) mince as affected by washing and microbial transglutaminase. J. Texture Stud. 2014, 45, 307–316. [Google Scholar] [CrossRef]
- Balange, A.; Benjakul, S. Enhancement of gel strength of bigeye snapper (Priacanthus tayenus) surimi using oxidised phenolic compounds. Food Chem. 2009, 113, 61–70. [Google Scholar] [CrossRef]
- Zardetto, S. Effect of modified atmosphere packaging at abuse temperature on the growth of Penicillium aurantiogriseum isolated from fresh filled pasta. Food Microbiol. 2005, 22, 367–371. [Google Scholar] [CrossRef]
- Temdee, W.; Singh, A.; Zhang, B.; Benjakul, S. Effect of vacuum packaging on shelf-life extension of cooked and peeled harpiosquillid mantis shrimp (Harpiosquilla raphidea) during refrigerated storage. Int. J. Food Sci. Technol. 2022, 57, 4451–4462. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2002. [Google Scholar]
- Tagrida, M.; Benjakul, S. Ethanolic extract of Betel (Piper betle L.) and Chaphlu (Piper sarmentosum Roxb.) dechlorophyllized using sedimentation process: Production, characteristics, and antioxidant activities. J. Food Biochem. 2020, 44, e13508. [Google Scholar] [CrossRef]
- Mittal, A.; Singh, A.; Zhang, B.; Visessanguan, W.; Benjakul, S. Chitooligosaccharide conjugates prepared using several phenolic compounds via ascorbic acid/H2O2 free radical grafting: Characteristics, antioxidant, antidiabetic, and antimicrobial activities. Foods 2022, 11, 920. [Google Scholar] [CrossRef] [PubMed]
- Wijayanti, I.; Benjakul, S.; Sookchoo, P. Effect of high pressure heating on physical and chemical characteristics of Asian sea bass (Lates calcarifer) backbone. J. Food Sci. Technol. 2021, 58, 3120–3129. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Putri, G.A.U.; Mittal, A.; Hong, H.; Yesilsu, A.F.; Benjakul, S. Protein hydrolysate from splendid squid (Loligo formosana) fins: Antioxidant, functional properties, and flavoring profile. Turk. J. Fish. Aquat. Sci. 2021, 22, TRJFA21005. [Google Scholar] [CrossRef]
- Singh, A.; Benjakul, S.; Prodpran, T. Effect of chitooligosaccharide from squid pen on gel properties of sardine surimi gel and its stability during refrigerated storage. Int. J. Food Sci. Technol. 2019, 54, 2831–2838. [Google Scholar] [CrossRef]
- Yan, X.; Murphy, B.T.; Hammond, G.B.; Vinson, J.A.; Neto, C.C. Antioxidant activities and antitumor screening of extracts from cranberry fruit (Vaccinium macrocarpon). J. Agric. Food Chem. 2002, 50, 5844–5849. [Google Scholar] [CrossRef]
- Tagrida, M.; Benjakul, S. Betel (Piper betle L.) leaf ethanolic extracts dechlorophyllized using different methods: Antioxidant and antibacterial activities, and application for shelf-life extension of Nile tilapia (Oreochromis niloticus) fillets. RSC Adv. 2021, 11, 17630–17641. [Google Scholar] [CrossRef]
- Kallel, F.; Driss, D.; Chaari, F.; Belghith, L.; Bouaziz, F.; Ghorbel, R.; Chaabouni, S.E. Garlic (Allium sativum L.) husk waste as a potential source of phenolic compounds: Influence of extracting solvents on its antimicrobial and antioxidant properties. Ind. Crops Prod. 2014, 62, 34–41. [Google Scholar] [CrossRef]
- Nour, V.; Stampar, F.; Veberic, R.; Jakopic, J. Anthocyanins profile, total phenolics and antioxidant activity of black currant ethanolic extracts as influenced by genotype and ethanol concentration. Food Chem. 2013, 141, 961–966. [Google Scholar] [CrossRef]
- Carreón-Delgado, D.F.; Hernández-Montesinos, I.Y.; Rivera-Hernández, K.N.; del Sugeyrol Villa-Ramírez, M.; Ochoa-Velasco, C.E.; Ramírez-López, C. Evaluation of pretreatments and extraction conditions on the antifungal and antioxidant effects of garlic (Allium sativum) peel extracts. Plants 2023, 12, 217. [Google Scholar] [CrossRef]
- Palmeira, L.; Pereira, C.; Dias, M.I.; Abreu, R.M.V.; Corrêa, R.C.G.; Pires, T.C.S.P.; Alves, M.J.; Barros, L.; Ferreira, I.C.F.R. Nutritional, chemical and bioactive profiles of different parts of a Portuguese common fig (Ficus carica L.) variety. Food Res. Int. 2019, 126, 108572. [Google Scholar] [CrossRef]
- Souza, J.M.A.; Leonel, S.; Leonel, M.; Garcia, E.L.; Ribeiro, L.R.; Ferreira, R.B.; Martins, R.C.; de Souza Silva, M.; Monteiro, L.N.H.; Duarte, A.S. Calcium nutrition in fig orchards enhance fruit quality at harvest and storage. Horticulturae 2023, 9, 123. [Google Scholar] [CrossRef]
- Arvaniti, O.S.; Samaras, Y.; Gatidou, G.; Thomaidis, N.S.; Stasinakis, A.S. Review on fresh and dried figs: Chemical analysis and occurrence of phytochemical compounds, antioxidant capacity and health effects. Food Res. Int. 2019, 119, 244–267. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, J.K.C. USDA National Nutrient Database for Standard Reference Dataset for What We Eat in America, Nhanes (Survey-SR); USDA Agricultural Research Service: Washington, DC, USA, 2017.
- Petcharat, T.; Benjakul, S. Effect of gellan and calcium chloride on properties of surimi gel with low and high setting phenomena. RSC Adv. 2017, 7, 52423–52434. [Google Scholar] [CrossRef]
- Singh, A.; Idowu, A.T.; Benjakul, S.; Kishimura, H.; Aluko, R.E.; Kumagai, Y. Debittering of salmon (Salmo salar) frame protein hydrolysate using 2-butanol in combination with β-cyclodextrin: Impact on some physicochemical characteristics and antioxidant activities. Food Chem. 2020, 321, 126686. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Singh, A.; Aluko, R.E.; Benjakul, S. Pacific white shrimp (Litopenaeus vannamei) shell chitosan and the conjugate with epigallocatechin gallate: Antioxidative and antimicrobial activities. J. Food Biochem. 2021, 45, e13569. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Barber, X.; Pérez-Álvarez, J.A.; Fernández-López, J. Assessment of chemical, physico-chemical, techno-functional and antioxidant properties of fig (Ficus carica L.) powder co-products. Ind. Crops Prod. 2015, 69, 472–479. [Google Scholar] [CrossRef]
- Czubinski, J.; Dwiecki, K. A review of methods used for investigation of protein–phenolic compound interactions. Int. J. Food Sci. Technol. 2017, 52, 573–585. [Google Scholar] [CrossRef]
- Rodrigues, S.; Pinto, G.A. Ultrasound extraction of phenolic compounds from coconut (Cocos nucifera) shell powder. J. Food Eng. 2007, 80, 869–872. [Google Scholar] [CrossRef]
- Liigand, P.; Kaupmees, K.; Haav, K.; Liigand, J.; Leito, I.; Girod, M.; Antoine, R.; Kruve, A. Think negative: Finding the best electrospray ionization/MS mode for your analyte. Anal. Chem. 2017, 89, 5665–5668. [Google Scholar] [CrossRef]
- Liu, X.; Wang, P.; Li, R.; Hyden, B.; An, X.; Jing, R.; Zhao, X.; Zhang, Y.; Qiao, H.; Han, Y.; et al. Cellular and metabolic characteristics of peach anther-derived callus. Sci. Hortic. 2023, 311, 111796. [Google Scholar] [CrossRef]
- Zhang, X.; Ye, B. Isolation of prunin from Bauhinia variegata and its antioxidant activity in rats Fed an atherogenic diet. Nat. Prod. Commun. 2020, 15, 1934578X20967875. [Google Scholar]
- Baliga, M.S.; Saxena, A.; Kaur, K.; Kalekhan, F.; Chacko, A.; Venkatesh, P.; Fayad, R. Chapter 50-polyphenols in the prevention of ulcerative colitis: Past, present and future. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 655–663. [Google Scholar]
- Hagenn, S.F.; Borge, G.I.A.; Bengtsson, G.B.; Bilger, W.; Berge, A.; Haffner, K.; Solhaug, K.A. Phenolic contents and other health and sensory related properties of apple fruit (Malus domestica Borkh., cv. Aroma): Effect of postharvest UV-B irradiation. Postharvest Biol. Technol. 2007, 45, 1–10. [Google Scholar] [CrossRef]
- Chua, L.S. A review on plant-based rutin extraction methods and its pharmacological activities. J. Ethnopharmacol. 2013, 150, 805–817. [Google Scholar] [CrossRef]
- Liu, S.; He, F.; Lin, N.; Chen, Y.; Liang, Z.; Liao, L.; Lv, M.; Chen, Y.; Chen, S.; Zhou, J.; et al. Pseudomonas sp. ST4 produces variety of active compounds to interfere fungal sexual mating and hyphal growth. Microb. Biotechnol. 2020, 13, 107–117. [Google Scholar] [CrossRef]
- Friesen, K.; Chang, C.; Nickerson, M. Incorporation of phenolic compounds, rutin and epicatechin, into soy protein isolate films: Mechanical, barrier and cross-linking properties. Food Chem. 2015, 172, 18–23. [Google Scholar] [CrossRef]
- Singh, A.; Benjakul, S.; Prodpran, T.; Nuthong, P. Effect of psyllium (Plantago ovata Forks) husk on characteristics, rheological and textural properties of threadfin bream surimi gel. Foods 2021, 10, 1181. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Majumdar, R.K.; Mehta, N.K. Gelling properties and microstructure of the silver carp surimi treated with pomegranate (Punica granatum L.) peel extract. J. Food Sci. Technol. 2022, 59, 4210–4220. [Google Scholar] [CrossRef] [PubMed]
- Gani, A.; Benjakul, S.; Nuthong, P. Effect of virgin coconut oil on properties of surimi gel. J. Food Sci. Technol. 2018, 55, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Hong, H.; Benjakul, S. Threadfin bream surimi gel containing squid fin protein hydrolysate: Textural properties, acceptability, and volatile profile. J. Food Sci. 2022, 87, 2337–2349. [Google Scholar] [CrossRef]
- Quan, T.H.; Benjakul, S.; Hozzein, W.N. Quality and storage stability of fish tofu as affected by duck albumen hydrolysate-epigalocatechin gallate conjugate. LWT-Food Sci. Technol. 2020, 120, 108927. [Google Scholar] [CrossRef]
- ICMSF (International Commission on Microbiological Specifications for Foods). Microorganisms in Foods. Sampling for Microbiological Analysis: Principles and Specific Applications, 2nd ed.; University of Toronto Press: Toronto, ON, USA, 1974. [Google Scholar]
- Zhao, Y.; Kong, H.; Zhang, X.; Hu, X.; Wang, M. The effect of Perilla (Perilla frutescens) leaf extracts on the quality of surimi fish balls. Food Sci. Nutr. 2019, 7, 2083–2090. [Google Scholar] [CrossRef]
- Masniyom, P.; Benjakul, S.; Visessanguan, W. Shelf-life extension of refrigerated seabass slices under modified atmosphere packaging. J. Sci. Food Agric. 2002, 82, 873–880. [Google Scholar] [CrossRef]
- Mao, L.; Wu, T. Gelling properties and lipid oxidation of kamaboko gels from grass carp (Ctenopharyngodon idellus) influenced by chitosan. J. Food Eng. 2007, 82, 128–134. [Google Scholar] [CrossRef]
- Singh, A.; Mittal, A.; Benjakul, S. Undesirable discoloration in edible fish muscle: Impact of indigenous pigments, chemical reactions, processing, and its prevention. Compr. Rev. Food Sci. Food Saf. 2022, 21, 580–603. [Google Scholar] [CrossRef]
- Benjakul, S.; Visessanguan, W.; Thongkaew, C.; Tanaka, M. Effect of frozen storage on chemical and gel-forming properties of fish commonly used for surimi production in Thailand. Food Hydrocoll. 2005, 19, 197–207. [Google Scholar] [CrossRef]
- Sae-Leaw, T.; Buamard, N.; Vate, N.K.; Benjakul, S. Effect of squid melanin-free ink and pre-emulsification on properties and stability of surimi gel fortified with seabass oil during refrigerated storage. J. Aquat. Food Prod. Technol. 2018, 27, 1–5. [Google Scholar] [CrossRef]


| Sample | Ethanol Concentration (%, v/v) | Total Phenolic Content (mg GAE/g Dry Extract) | Calcium Content (mg/kg Dry Extract) | A-RSA (µmol TE/g Dry Extract) | D-RSA (µmolTE/g Dry Extract) | FRAP (µmol TE/g Dry Extract) | MCA (µmol EE/g Dry Extract) |
|---|---|---|---|---|---|---|---|
| DCE-W | 0 | 308.51 ± 2.11 d | 1082.03 ± 4.96 c | 191.44 ± 4.17 e | 93.32 ± 2.92 e | 114.90 ± 2.18 e | 17.94 ± 0.20 c |
| DCE-20 * | 20 | 411.14 ± 2.96 c | 1089.01 ± 4.04 b | 220.65 ± 4.59 d | 101.54 ± 3.09 d | 140.61 ± 3.01 c | 18.13 ± 0.10 c |
| DCE-40 | 40 | 617.83 ± 3.07 b | 1090.23 ± 5.88 b | 369.05 ± 5.01 c | 175.74 ± 3.36 c | 251.87 ± 2.05 b | 20.32 ± 0.13 b |
| DCE-60 | 60 | 722.53 ± 2.82 a | 1099.17 ± 6.21 a | 501.48 ± 5.23 a | 212.69 ± 4.00 a | 312.47 ± 2.57 a | 21.19 ± 0.09 a |
| DCE-80 | 80 | 615.58 ± 3.53 b | 1092.12 ± 6.58 b | 423.77 ± 4.93 b | 189.36 ± 3.18 b | 121.63 ± 2.79 d | 20.35 ± 0.10 b |
| DCE-100 | 100 | 310.19 ± 1.96 d | 1087.81 ± 5.59 b | 212.01 ± 5.00 d | 102.07 ± 2.74 d | 116.00 ± 2.94 e | 18.01 ± 0.17 c |
| Compound Name | Formula | m/z | Mass (g/mol) | Abundance (×106) |
|---|---|---|---|---|
| Negative ion mode analysis | ||||
| Quinic acid | C7 H12 O6 | 191.06 | 192.06 | 1.43 |
| Procyanidin B2 | C30 H26 O12 | 577.13 | 578.14 | 0.55 |
| Hydroquinone | C6 H6 O2 | 109.03 | 110.04 | 0.21 |
| Indole-4-carboaldehyde | C9 H7 NO | 144.05 | 145.05 | 2.04 |
| Chlorogenic acid | C16 H18 O9 | 353.09 | 354.10 | 1.72 |
| (±)-Catechin | C15 H14 O6 | 289.07 | 290.08 | 0.58 |
| 2,5-Dihydroxybenzaldehyde | C7 H6 O3 | 137.03 | 138.03 | 0.36 |
| 4-Acetoxyphenol | C8 H8 O3 | 151.04 | 152.05 | 1.05 |
| Dihydroxyphenylacetic acid | C8 H8 O4 | 167.04 | 168.04 | 0.65 |
| (±)-Taxifolin | C15 H12 O7 | 303.05 | 304.06 | 0.26 |
| Rutin | C27 H30 O16 | 609.15 | 610.15 | 2.34 |
| Isovitexin | C21 H20 O10 | 431.10 | 432.11 | 0.58 |
| Quercetin 3-galactoside | C21 H20 O12 | 463.09 | 464.10 | 2.74 |
| Umbelliferone | C9 H6 O3 | 161.02 | 162.03 | 0.16 |
| Naringenin-7-O-Glucoside | C21 H22 O10 | 433.12 | 434.12 | 8.41 |
| Methyl N-(α-methylbutyryl) glycine | C9 H16 O4 | 187.10 | 188.11 | 0.23 |
| Abscisic acid (cis, trans) | C15 H20 O4 | 263.13 | 264.14 | 0.27 |
| (±)-Naringenin | C15 H12 O5 | 271.06 | 272.07 | 1.41 |
| Positive ion mode analysis | ||||
| Procyanidin B2 | C30 H26 O12 | 579.15 | 578.14 | 0.08 |
| Rutin | C27 H30 O16 | 611.16 | 610.15 | 0.23 |
| Isovitexin | C21 H20 O10 | 433.11 | 432.11 | 0.23 |
| Quercetin | C15 H10 O7 | 303.05 | 302.04 | 0.15 |
| 2,3-dinor-8-iso-PGF2a | C18 H30 O5 | 349.20 | 326.21 | 0.24 |
| DCE-60 Levels (%) | Hardness (N) | Springiness (cm) | Cohesiveness (Ratio) | Gumminess (N) | Chewiness (N·cm) |
|---|---|---|---|---|---|
| 0 | 171.79 ± 1.44 d | 0.31 ± 0.04 d | 0.36 ± 0.06 c | 60.35 ± 0.90 d | 60.93 ± 0.47 c |
| 0.025 | 202.78 ± 2.01 b | 0.34 ± 0.06 c | 0.42 ± 0.04 b | 112.11 ± 1.47 b | 94.70 ± 0.59 a |
| 0.050 | 250.09 ± 1.96 a | 0.56 ± 0.02 a | 0.50 ± 0.02 a | 141.10 ± 1.81 a | 95.46 ± 0.52 a |
| 0.075 | 198.63 ± 1.46 c | 0.43 ± 0.03 b | 0.36 ± 0.03 c | 72.72 ± 1.41 c | 72.31 ± 0.67 b |
| 0.100 | 173.65 ± 3.98 d | 0.39 ± 0.05 c | 0.33 ± 0.03 c | 60.84 ± 1.54 d | 71.59 ± 0.64 b |
| 0.125 | 164.52 ± 5.19 e | 0.32 ± 0.02 d | 0.33 ± 0.02 c | 55.13 ± 1.43 e | 61.25 ± 0.54 c |
| DCE-60 Levels (%) | Appearance | Color | Odor | Taste | Texture | Overall |
|---|---|---|---|---|---|---|
| 0 | 6.88 ± 0.44 a | 6.98 ± 0.34 a | 6.36 ± 0.36 a | 7.04 ± 0.29 a | 6.25 ± 0.30 b | 6.29 ± 0.27 bc |
| 0.025 | 6.90 ± 0.41 a | 6.82 ± 0.39 a | 6.42 ± 0.34 a | 7.01 ± 0.27 a | 6.40 ± 0.40 ab | 6.41 ± 0.22 b |
| 0.050 | 6.89 ± 0.33 a | 6.83 ± 0.44 a | 6.50 ± 0.42 a | 6.93 ± 0.31 a | 6.95 ± 0.26 a | 6.92 ± 0.34 a |
| 0.075 | 6.84 ± 0.36 a | 6.61 ± 0.32 a b | 6.36 ± 0.43 a | 6.72 ± 0.25 a | 6.83 ± 0.22 a | 6.77 ± 0.28 a |
| 0.100 | 6.55 ± 0.38 ab | 6.10 ± 0.35 b | 6.33 ± 0.43 a | 6.21 ± 0.29 b | 6.24 ± 0.34 b | 6.21 ± 0.30 bc |
| 0.125 | 6.22 ± 0.30 b | 6.01 ± 0.43 b | 6.33 ± 0.42 a | 6.07 ± 0.22 b | 6.19 ± 0.30 b | 6.08 ± 0.31 c |
| Packaging Condition | Storage Time (Days) | Total Viable Count (log CFU/g Surimi Gel) | Psychrophilic Bacteria Count (log CFU/g Surimi Gel) | Peroxide Value (mg Hydroperoxide Equivalents/ kg Surimi Gel) | TBARS (mg MDA/kg Surimi Gel) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Control | D60-0.05 | Control | D60-0.05 | Control | D60-0.05 | Control | D60-0.05 | ||
| 0 | ND | ND | ND | ND | 20.32 ± 0.09 Aex | 20.27 ± 0.07 Aex | 3.08 ± 0.03 Aex | 3.05 ± 0.02 Afx | |
| 2 | 2.34 ± 0.07 Adx | 2.28 ± 0.11 Ae # | 2.22 ± 0.03 Adx | 2.18 ± 0.02 Aex | 23.91 ± 0.05 Adx | 21.11 ± 0.09 Bex | 4.25 ± 0.03 Adx | 3.14 ± 0.04 Bex | |
| 4 | 2.89 ± 0.09 Acx | 2.53 ± 0.08 Bdx | 2.79 ± 0.06 Acx | 2.44 ± 0.02 Bdx | 40.69 ± 0.11 Abx | 29.02 ± 0.04 Bdx | 4.97 ± 0.02 Acx | 3.72 ± 0.04 Bdx | |
| ATM | 6 | 4.36 ± 0.10 Abx | 3.87 ± 0.09 Bcx | 3.96 ± 0.04 Abx | 3.71 ± 0.01 Bcx | 45.38 ± 0.08 Aax | 33.36 ± 0.09 Bcx | 8.13 ± 0.03 Abx | 4.01 ± 0.02 Bcx |
| 8 | 5.84 ± 0.07 Aax | 4.99 ± 0.10 Bbx | 5.62 ± 0.03 Aax | 4.83 ± 0.03 Bbx | 38.66 ± 0.08 Acx | 40.12 ± 0.10B Ax | 10.46 ± 0.04 Aax | 7.56 ± 0.05 Bbx | |
| 10 | >6 | 5.68 ± 0.07 * ax | >6 | 5.63 ± 0.02 * ax | − | 38.59 ± 0.09 * bx | − | 8.03 ± 0.06 * ax | |
| 12 | >6 | >6 | >6 | >6 | − | − | − | − | |
| VAC | 0 | ND | ND | ND | ND | 20.30 ± 0.07 Afx | 20.25 ± 0.06 Acx | 3.05 ± 0.02 Adx | 3.01 ± 0.04 Acx |
| 2 | 1.33 ± 0.08 * fz | ND | 1.16 ± 0.04 *fz | ND | 20.77 ± 0.03 Aez | 20.35 ± 0.04 Bcy | 3.09 ± 0.05 Adz | 3.00 ± 0.04 Acy | |
| 4 | 1.49 ± 0.07 Aez | 1.40 ± 0.07 Adz | 1.65 ± 0.09 Aez | 1.37 ± 0.08 Bez | 21.49 ± 0.08 Adz | 20.99 ± 0.02 Bbz | 3.21 ± 0.05 Acz | 3.09 ± 0.05 Bbz | |
| 6 | 1.83 ± 0.09 Adz | 1.44 ± 0.08 Adz | 2.15 ± 0.08 Adz | 1.79 ± 0.10 Bdz | 22.06 ± 0.12 Acz | 21.07 ± 0.10 Bbz | 3.25 ± 0.07 Acz | 3.11 ± 0.03 Bbz | |
| 8 | 2.60 ± 0.08 Acz | 2.38 ± 0.10 Bcz | 2.49 ± 0.10 Acz | 2.24 ± 0.09 Bcz | 22.39 ± 0.11 Acz | 21.34 ± 0.08 Baz | 3.26 ± 0.03 Acz | 3.14 ± 0.03 Bbz | |
| 10 | 3.74 ± 0.11 Aby | 3.62 ± 0.12 Abz | 3.61 ± 0.08 Abz | 3.35 ± 0.07 Bbz | 23.15 ± 0.09 Aby | 21.41 ± 0.07 Baz | 3.69 ± 0.06 Aby | 3.22 ± 0.02 Baz | |
| 12 | 5.17 ± 0.14 Aa # | 4.59 ± 0.09B Ay | 4.98 ± 0.09 Aa # | 4.37 ± 0.11B Ay | 25.67 ± 0.06 Aa # | 20.38 ± 0.07 Bcy | 4.01 ± 0.04 Aa # | 3.23 ± 0.05 Bay | |
| MAP | 0 | ND | ND | ND | ND | 20.54 ± 0.10 Afx | 20.46 ± 0.11 Af | 3.05 ± 0.06 Aex | 3.01 ± 0.04 Aex |
| 2 | 2.00 ± 0.13 * ey | ND | 1.82 ± 0.12 *ey | ND | 21.26 ± 0.15 Aey | 20.51 ± 0.08 Bfy | 3.12 ± 0.04 Aey | 3.05 ± 0.03 Bdey | |
| 4 | 2.35 ± 0.10 Ady | 2.10 ± 0.08 Bey | 2.29 ± 0.10 Ady | 1.91 ± 0.11 Bey | 24.45 ± 0.08 Ady | 21.37 ± 0.09 Bey | 3.36 ± 0.09 Ady | 3.11 ± 0.08 Bdy | |
| 6 | 2.60 ± 0.11 Acy | 2.42 ± 0.14 Bdy | 2.47 ± 0.09 Acy | 2.30 ± 0.09 Bdy | 32.22 ± 0.09 Acy | 25.61 ± 0.12 Bdy | 4.52 ± 0.12 Acy | 3.14 ± 0.06 Bdy | |
| 8 | 4.45 ± 0.13 Aby | 3.86 ± 0.13 Bcy | 3.98 ± 0.12 Aby | 3.19 ± 0.13 Bcy | 38.91 ± 0.14 Aay | 29.36 ± 0.15Bcy | 6.91 ± 0.13 Aby | 3.48 ± 0.09 Bcy | |
| 10 | 5.86 ± 0.09 Aax | 4.79 ± 0.09 Bby | 5.39 ± 0.14 Aay | 4.05 ± 0.08 Bby | 35.17 ± 0.11 Abx | 34.96 ± 0.11 Aby | 7.66 ± 0.08 Aax | 4.05 ± 0.12 Bby | |
| 12 | − | 5.80 ± 0.11 * ax | − | 5.74 ± 0.11 * ax | − | 38.04 ± 0.13 * ax | − | 5.52 ± 0.08 * ax | |
| Packaging Condition | Storage Time (Days) | Breaking Force (g) | Deformation (mm) | EMC (%) | Whiteness | ||||
|---|---|---|---|---|---|---|---|---|---|
| Control | D60-0.05 | Control | D60-0.05 | Control | D60-0.05 | Control | D60-0.05 | ||
| ATM | 0 | 108.91 ± 5.49 Bax | 220.34 ± 6.10 Aax | 3.25 ± 0.17 Bax | 5.00 ± 0.16 Aax | 10.63 ± 0.24 Adx | 6.29 ± 0.18 Bfx | 82.66 ± 0.27 Aax | 78.85 ± 0.21 Bax |
| 2 | 107.04 ± 4.97 Bax | 207.89 ± 5.77 Aby | 3.19 ± 0.11 Bay | 4.82 ± 0.15 Aay | 10.79 ± 0.19 Adx | 7.38 ± 0.20 Bex | 81.97 ± 0.18 Aax | 77.74 ± 0.19 Bax | |
| 4 | 100.12 ± 5.38 Bax | 175.36 ± 5.93 Acy | 3.10 ± 0.10 Bay | 4.26 ± 0.11 Aby | 11.38 ± 0.22 Acx | 8.74 ± 0.14 Bdx | 81.10 ± 0.21 Aby | 77.96 ± 0.20 Bbx | |
| 6 | 87.88 ± 5.08 Bbxy | 145.14 ± 5.23 Ady | 2.84 ± 0.09 Bby | 4.00 ± 0.08 Acz | 18.59 ± 0.20 Abx | 9.90 ± 0.17 Bcx | 76.34 ± 0.22 Acz | 76.66 ± 0.15 Acx | |
| 8 | 70.14 ± 4.22 Bcz | 100.66 ± 6.15 Aez | 2.25 ± 0.14 Bcy | 3.36 ± 0.17 Adz | 22.44 ± 0.25 Aaz | 12.87 ± 0.21 Bbx | 75.17 ± 0.25 Adz | 74.12 ± 0.18 Bdx | |
| 10 | − | 78.81 ± 5.36 Afz | − | 2.91 ± 0.14 * ez | − | 15.94 ± 0.20 * ax | − | 74.00 ± 0.20 * dx | |
| 12 | − | − | − | − | − | − | − | − | |
| VAC | 0 | 104.08 ± 7.33 Bax | 213.04 ± 4.75 Aax | 3.36 ± 0.12 Bax | 5.02 ± 0.19 Aax | 10.84 ± 0.20 Aex | 6.18 ± 0.16 Bex | 83.24 ± 0.17 Aax | 77.89 ± 0.26 Bax |
| 2 | 106.78 ± 8.25 Bax | 216.91 ± 4.83 Aax | 3.31 ± 0.09 Bax | 4.97 ± 0.11 Aax | 10.89 ± 0.19 Aex | 6.21 ± 0.17 Bey | 82.99 ± 0.20 Aax | 77.04 ± 0.16 Bax | |
| 4 | 106.96 ± 6.24 Bax | 201.78 ± 5.64 Aax | 3.28 ± 0.15 Bax | 4.91 ± 0.15 Aax | 11.01 ± 0.21 Aey | 6.38 ± 0.10 Bez | 81.55 ± 0.24 Aax | 76.83 ± 0.20 Bax | |
| 6 | 92.24 ± 5.04 Bby | 188.26 ± 6.69 Abx | 3.07 ± 0.13 Babx | 4.88 ± 0.14 Aax | 12.13 ± 0.15 Ady | 7.05 ± 0.14 Bdz | 81.00 ± 0.15 Abx | 76.75 ± 0.17 Bax | |
| 8 | 91.35 ± 5.30 Bbx | 173.34 ± 5.95 Acx | 3.01 ± 0.11 Babx | 4.84 ± 0.18 Aax | 12.96 ± 0.19 Acz | 7.46 ± 0.11 Bcz | 80.32 ± 0.14 Acx | 74.34 ± 0.19 Bbx | |
| 10 | 83.08 ± 4.48 Bcx | 170.81 ± 6.44 Acx | 2.97 ± 0.14 Bbx | 4.79 ± 0.13 Aabx | 14.73 ± 0.18 Abz | 7.91 ± 0.20 Bbz | 77.21 ± 0.20 Adx | 74.05 ± 0.21 Bbx | |
| 12 | 72.19 ± 5.61 Bd # | 167.54 ± 5.16 Acx | 2.55 ± 0.15 Bc # | 4.63 ± 0.17 Abx | 17.38 ± 0.22 Aa # | 9.43 ± 0.15 Bay | 76.17 ± 0.19 Ae # | 73.91 ± 0.17 Bbx | |
| MAP | 0 | 106.38 ± 6.62 Bax | 215.30 ± 5.26 Aax | 3.24 ± 0.08 Bax | 5.10 ± 0.14 Aax | 10.75 ± 0.24 Adx | 6.20 ± 0.09 Bfx | 81.29 ± 0.18 Aax | 77.90 ± 0.26 Bax |
| 2 | 104.37 ± 6.08 Bax | 210.22 ± 6.01 Aax | 3.21 ± 0.11 Bay | 5.05 ± 0.09 Aax | 10.77 ± 0.20 Adx | 6.18 ± 0.10 Bfy | 81.97 ± 0.12 Aay | 77.75 ± 0.28 Bax | |
| 4 | 103.91 ± 5.90 Bax | 203.67 ± 6.22 Aax | 3.21 ± 0.10 Bax | 5.01 ± 0.11 Aax | 10.84 ± 0.19 Ady | 6.51 ± 0.11 Bey | 80.44 ± 0.18 Aby | 77.20 ± 0.14 Bax | |
| 6 | 90.91 ± 4.17 Bby | 190.34 ± 5.38 Abx | 3.16 ± 0.06 Bax | 4.64 ± 0.12 Aby | 12.06 ± 0.13 Acy | 6.99 ± 0.13 Bdy | 77.14 ± 0.20 Acy | 74.01 ± 0.11 Bby | |
| 8 | 82.07 ± 5.36 Bcy | 164.21 ± 5.74 Acy | 3.00 ± 0.09 Bbx | 4.35 ± 0.09 Acy | 17.13 ± 0.14 Aby | 7.59 ± 0.18 Bcy | 76.05 ± 0.19 Ady | 73.85 ± 0.19 Bcy | |
| 10 | 75.15 ± 4.49 Bdy | 150.19 ± 6.13 Ady | 2.75 ± 0.08 Bcy | 4.09 ± 0.11 Ady | 20.69 ± 0.20 Aay | 8.48 ± 0.10 Bby | 73.30 ± 0.13 Aey | 73.14 ± 0.21 Acy | |
| 12 | − | 141.06 ± 5.69 * ey | − | 3.22 ± 6.10 * ey | − | 10.76 ± 0.12 * ax | − | 73.02 ± 0.18 * cx | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buamard, N.; Singh, A.; Zhang, B.; Hong, H.; Singh, P.; Benjakul, S. Ethanolic Extract of Duea Ching Fruit: Extraction, Characterization and Its Effect on the Properties and Storage Stability of Sardine Surimi Gel. Foods 2023, 12, 1635. https://doi.org/10.3390/foods12081635
Buamard N, Singh A, Zhang B, Hong H, Singh P, Benjakul S. Ethanolic Extract of Duea Ching Fruit: Extraction, Characterization and Its Effect on the Properties and Storage Stability of Sardine Surimi Gel. Foods. 2023; 12(8):1635. https://doi.org/10.3390/foods12081635
Chicago/Turabian StyleBuamard, Natchaphol, Avtar Singh, Bin Zhang, Hui Hong, Prabjeet Singh, and Soottawat Benjakul. 2023. "Ethanolic Extract of Duea Ching Fruit: Extraction, Characterization and Its Effect on the Properties and Storage Stability of Sardine Surimi Gel" Foods 12, no. 8: 1635. https://doi.org/10.3390/foods12081635
APA StyleBuamard, N., Singh, A., Zhang, B., Hong, H., Singh, P., & Benjakul, S. (2023). Ethanolic Extract of Duea Ching Fruit: Extraction, Characterization and Its Effect on the Properties and Storage Stability of Sardine Surimi Gel. Foods, 12(8), 1635. https://doi.org/10.3390/foods12081635