Pectin from Fruit- and Berry-Juice Production by-Products: Determination of Physicochemical, Antioxidant and Rheological Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterization of by-Products and Sample Preparation and Extraction
2.2. Pectin Yield, Ash Content and Moisture Content
2.3. Equivalent Weight, Methoxyl Content, Degree of Esterification
2.4. Fourier-Transform Infrared Spectroscopy (FT-IR) of Pectin Samples
2.5. Monosaccharide Composition and Galacturonic Acid Content
2.6. Content of Total Phenolic Compounds, Antiradical Scavenging Activity (DPPH Method), Quantification of Phenolic Acids by HPLC
2.7. Rheology of Pectin Gel Samples
3. Results and Discussion
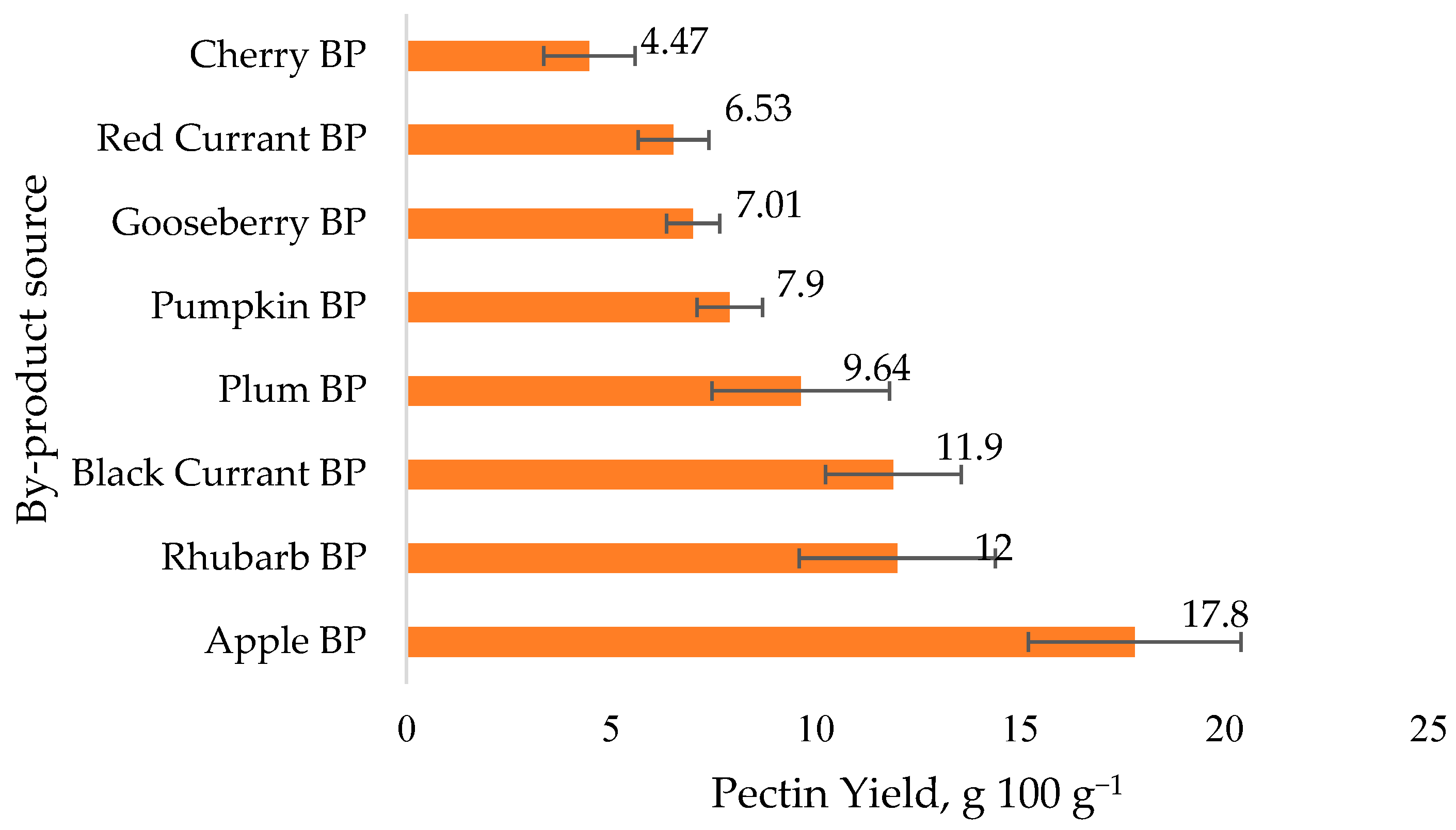
3.1. Extraction of Pectin and Pectin Yield
3.2. Ash and Moisture Content
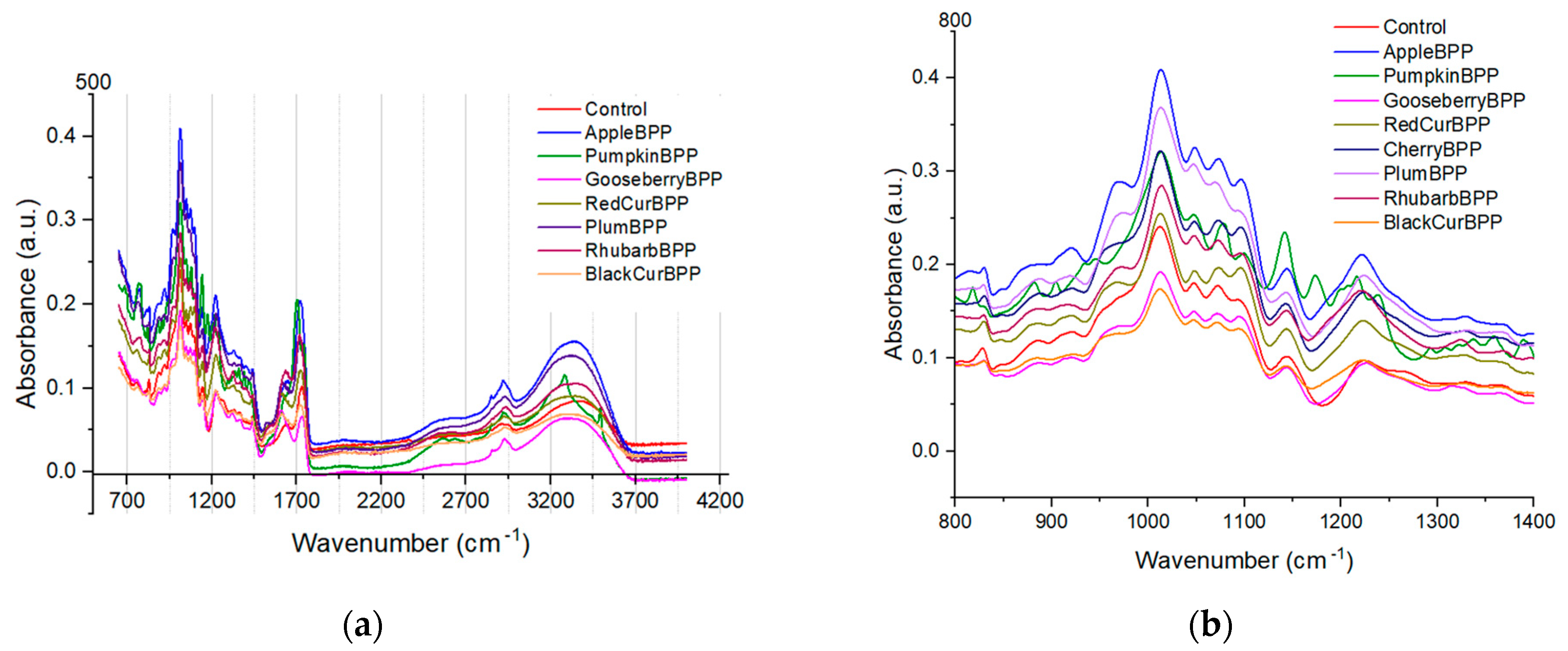
3.3. Fourier-Transform Infrared Spectroscopy (FT-IR) of Pectin Samples
3.4. Equivalent Weight, Methoxyl Content, Degree of Esterification
3.5. Monosaccharide Composition, Galacturonic Acid Content
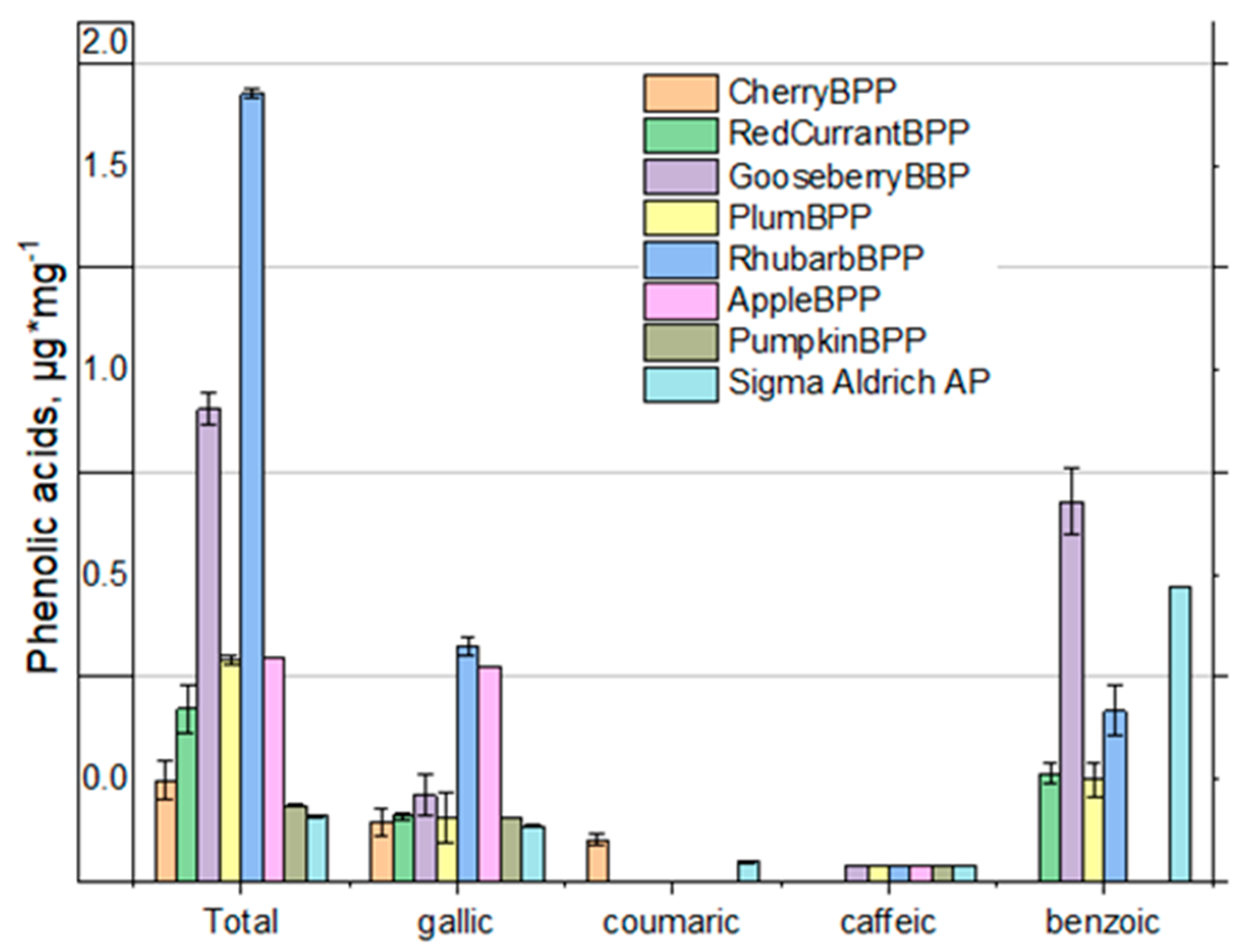
3.6. Content of Total Phenolics, Antiradical Scavenging Activity (DPPH Method)
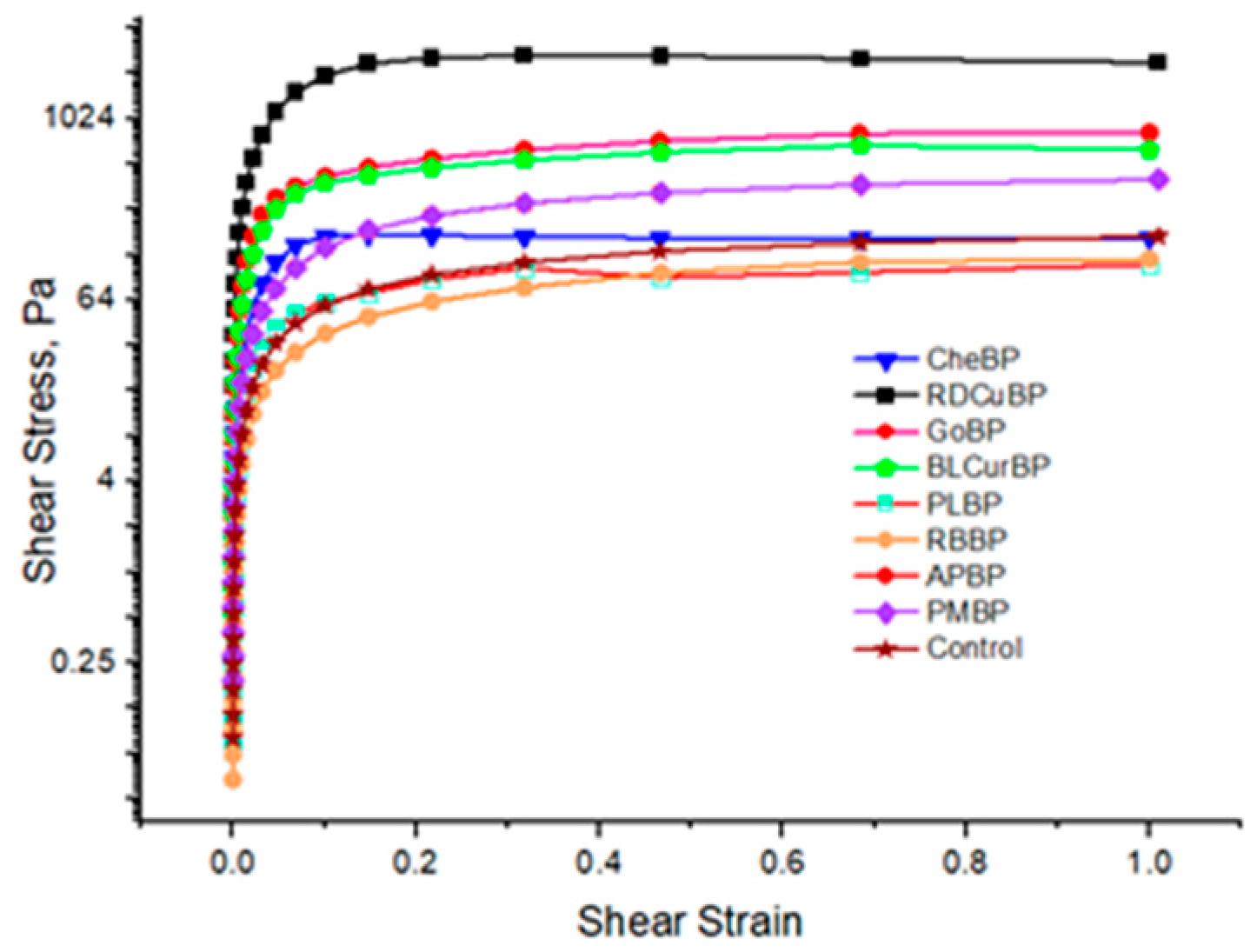
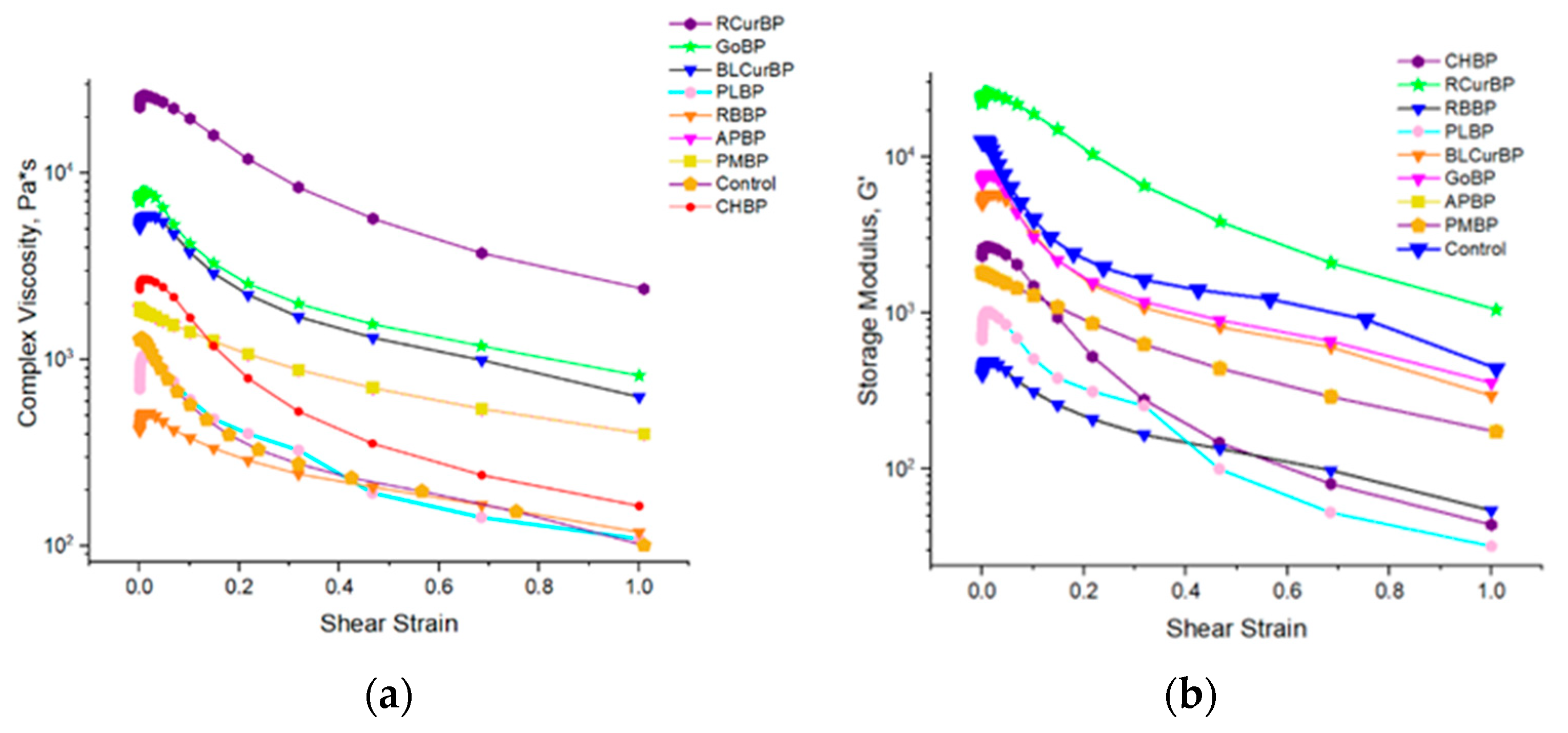
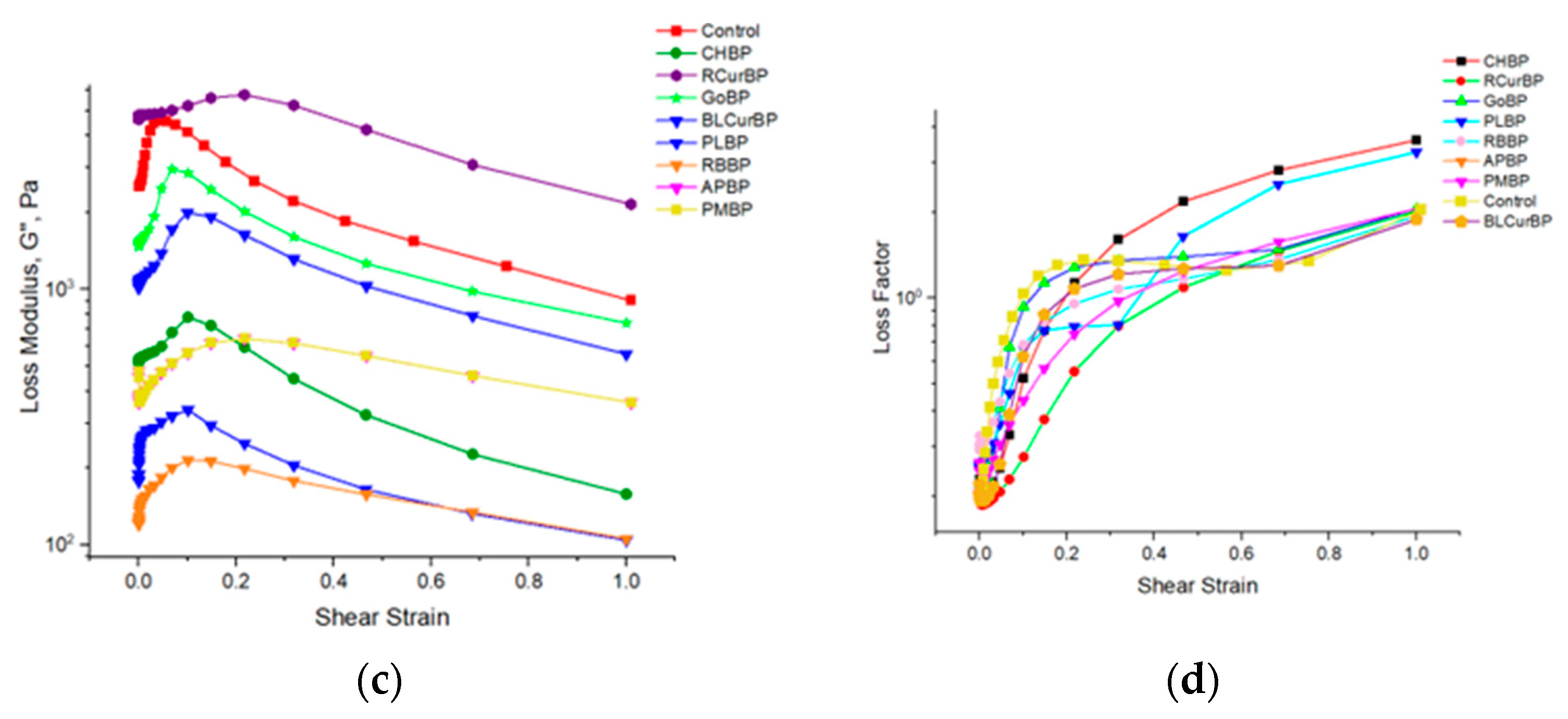
3.7. Rheology of Pectin Gel Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Maran, J.P.; Priya, B. Ultrasound-assisted extraction of pectin from sisal waste. Carbohydr. Polym. 2015, 115, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.; Morris, G.A. The anti-diabetic potential of polysaccharides extracted from members of the cucurbit family: A review. Bioact. Carbohydr. Diet. Fibre 2014, 3, 106–114. [Google Scholar] [CrossRef]
- Yang, J.-S.; Mu, T.-H.; Ma, M.-M. Extraction, structure, and emulsifying properties of pectin from potato pulp. Food Chem. 2018, 244, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Weng, P.; Liu, Y.; Wu, Z.; Wang, L.; Liu, L. Citrus pectin research advances: Derived as a biomaterial in the construction and applications of micro/nano-delivery systems. Food Hydrocoll. 2022, 133, 107910. [Google Scholar] [CrossRef]
- Dranca, F.; Vargas, M.; Oroian, M. Physicochemical properties of pectin from Malus domestica “Fălticeni” apple pomace as affected by non-conventional extraction techniques. Food Hydrocoll. 2019, 100, 105383. [Google Scholar] [CrossRef]
- Sila, D.; Van Buggenhout, S.; Duvetter, T.; Fraeye, I.; De Roeck, A.; Van Loey, A.; Hendrickx, M. Pectins in Processed Fruits and Vegetables: Part II-Structure-Function Relationships. Compr. Rev. Food Sci. Food Saf. 2009, 8, 86–104. [Google Scholar] [CrossRef]
- Bush, P.L. Pectin: Chemical Properties, Uses and Health Benefits; Nova Science Publishers, Inc.: New York, NY, USA, 2014. [Google Scholar]
- Yoo, S.-H.; Lee, B.-H.; Lee, H.; Lee, S.; Bae, I.Y.; Lee, H.G.; Fishman, M.L.; Chau, H.K.; Savary, B.J.; Hotchkiss, A.T., Jr. Structural Characteristics of Pumpkin Pectin Extracted by Microwave Heating. J. Food Sci. 2012, 77, C1169–C1173. [Google Scholar] [CrossRef]
- Alam, K.; Iqbal, M.; Hasan, A.; Al-Maskari, N. Applied Biotechnology Reports Rheological Characterization of Biological Hydrogels in Aqueous State. J. Appl. Biotechnol. Rep. 2020, 7, 172–176. [Google Scholar] [CrossRef]
- Siddiqui, A.; Chand, K. Effect of Process Parameters on Extraction of Pectin from Sweet Lime Peels. J. Inst. Eng. Ser. A 2021, 102, 469–478. [Google Scholar] [CrossRef]
- Salami, A.; Asefi, N.; Kenari, R.E.; Gharekhani, M. Extraction of pumpkin peel extract using supercritical CO2 and subcritical water technology: Enhancing oxidative stability of canola oil. J. Food Sci. Technol. 2021, 58, 1101–1109. [Google Scholar] [CrossRef]
- Chen, J.; Cheng, H.; Zhi, Z.; Zhang, H.; Linhardt, R.J.; Zhang, F.; Chen, S.; Ye, X. Extraction temperature is a decisive factor for the properties of pectin. Food Hydrocoll. 2021, 112, 106160. [Google Scholar] [CrossRef]
- Khamsucharit, P.; Laohaphatanalert, K.; Gavinlertvatana, P.; Sriroth, K.; Sangseethong, K. Characterization of pectin extracted from banana peels of different varieties. Food Sci. Biotechnol. 2018, 27, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Sandarani, M. A Review: Different Extraction Techniques of Pectin Pharmacognosy & Natural Products A Review: Different Extraction Techniques of Pectin. J. Pharmacogn. Nat. Prod. 2020, 3, 1–6. [Google Scholar] [CrossRef]
- Kratchanova, M.; Pavlova, E.; Panchev, I. The effect of microwave heating of fresh orange peels on the fruit tissue and quality of extracted pectin. Carbohydr. Polym. 2004, 56, 181–185. [Google Scholar] [CrossRef]
- Konrāde, D. Influence of Processing and Extraction Technologies on Physicochemical and Rheological Properties of Pectin Obtained from Pumpkin by-products. Agric. Food 2022, 10, 367–382. [Google Scholar]
- Dona, J.S.M. Isolation and Characterization of Pectin from Pumpkin (Cucurbita maxima) Waste and Its Food Application. Asian Food Sci. J. 2019, 13, 1–9. [Google Scholar] [CrossRef]
- Santos, E.E.; Amaro, R.C.; Bustamante, C.C.C.; Guerra, M.H.A.; Soares, L.C.; Froes, R.E.S. Extraction of pectin from agroindustrial residue with an ecofriendly solvent: Use of FTIR and chemometrics to differentiate pectins according to degree of methyl esterification. Food Hydrocoll. 2020, 107, 105921. [Google Scholar] [CrossRef]
- Vriesmann, L.C.; Teófilo, R.F.; Petkowicz, C.L.D.O. Optimization of nitric acid-mediated extraction of pectin from cacao pod husks (Theobroma cacao L.) using response surface methodology. Carbohydr. Polym. 2011, 84, 1230–1236. [Google Scholar] [CrossRef]
- Zouambia, Y.; Ettoumi, K.Y.; Krea, M.; Moulai-Mostefa, N. A new approach for pectin extraction: Electromagnetic induction heating. Arab. J. Chem. 2017, 10, 480–487. [Google Scholar] [CrossRef]
- Yağcı, S.; Göğüş, F. Response surface methodology for evaluation of physical and functional properties of extruded snack foods developed from food-by-products. J. Food Eng. 2008, 86, 122–132. [Google Scholar] [CrossRef]
- Wandee, Y.; Uttapap, D.; Mischnick, P.; Rungsardthong, V. Production of pectic-oligosaccharides from pomelo peel pectin by oxidative degradation with hydrogen peroxide. Food Chem. 2021, 348, 129078. [Google Scholar] [CrossRef]
- Koubala, B.; Mbome, L.; Kansci, G.; Mbiapo, F.T.; Crepeau, M.-J.; Thibault, J.-F.; Ralet, M.-C. Physicochemical properties of pectins from ambarella peels (Spondias cytherea) obtained using different extraction conditions. Food Chem. 2008, 106, 1202–1207. [Google Scholar] [CrossRef]
- Dao, T.A.T.; Webb, H.K.; Malherbe, F. Optimization of pectin extraction from fruit peels by response surface method: Conventional versus microwave-assisted heating. Food Hydrocoll. 2021, 113, 106475. [Google Scholar] [CrossRef]
- Cinkmanis, I.; Muizniece-Brasava, S.; Viluma, I.; Vucane, S.; Aboltins, A.; Keke, A. Extraction of pectin from apple pomace. In Proceedings of the 19th International Scientific Conference Engineering for Rural Development Proceedings, Jelgava, Łotwa, 20–22 May 2020; pp. 1934–1939. [Google Scholar] [CrossRef]
- Dhiman, A.K.; Attri, S. Extraction of Pectin from Ripe Pumpkin (Cucurbita moschata Duch ex. Poir) Extraction of Pectin from Ripe Pumpkin (Cucurbita moschata Duch ex. Poir) Using Eco-Friendly Technique. Indian J. Ecol. 2017, 44, 685–689. [Google Scholar]
- Gawkowska, D.; Cybulska, J.; Zdunek, A. Structure-Related Gelling of Pectins and Linking with Other Natural Compounds: A Review. Polymers 2018, 10, 762. [Google Scholar] [CrossRef] [PubMed]
- Gamonpilas, C.; Buathongjan, C.; Kirdsawasd, T.; Rattanaprasert, M.; Klomtun, M.; Phonsatta, N.; Methacanon, P. Pomelo pectin and fiber: Some perspectives and applications in food industry. Food Hydrocoll. 2021, 120, 106981. [Google Scholar] [CrossRef]
- Wathoni, N.; Shan, C.Y.; Shan, W.Y.; Rostinawati, T.; Indradi, R.B.; Pratiwi, R.; Muchtaridi, M. Characterization and antioxidant activity of pectin from Indonesian mangosteen (Garcinia mangostana L.) rind Heliyon. Heliyon 2019, 5, e02299. [Google Scholar] [CrossRef]
- Riekstina-Dolge, R.; Kruma, Z.; Karklina, D.; Dimins, F. Physical-Chemical Parameters of Latvian Apple Juices and Their Suitability for Cider Production. World Acad. Sci. Eng. Technol. Int. J. Nutr. Food Eng. 2014, 8, 263–267. [Google Scholar]
- Kaczmarczyk, M.M.; Miller, M.J.; Freund, G.G. The health benefits of dietary fiber: Beyond the usual suspects of type 2 diabetes mellitus, cardiovascular disease and colon cancer. Metabolism 2012, 61, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, S.; Monis, S.A.; Roy, N.; Sruthi, D.; Sangamithra, A.; John, S.G. Effect of antioxidants and dietary fiber from apple and strawberries on value addition into mutton patties. Ann. Univ. Dunarea Jos Galati Fascicle VI Food Technol. 2017, 41, 95–105. [Google Scholar]
- Helkar, P.B.; Sahoo, A. Review: Food Industry By-Products used as a Functional Food Ingredients. Int. J. Waste Resour. 2016, 6, 248. [Google Scholar] [CrossRef]
- Soengas, P.; Sotelo, T.; Velasco, P.; Elena, M. Antioxidant Properties of Brassica Vegetables. Funct. Plant Sci. Biotechnol. 2011, 5, 43–55. [Google Scholar]
- Ajila, C.; Leelavathi, K.; Rao, U.P. Improvement of dietary fiber content and antioxidant properties in soft dough biscuits with the incorporation of mango peel powder. J. Cereal Sci. 2008, 48, 319–326. [Google Scholar] [CrossRef]
- Moslemi, M. Reviewing the recent advances in application of pectin for technical and health promotion purposes: From laboratory to market. Carbohydr. Polym. 2020, 254, 117324. [Google Scholar] [CrossRef] [PubMed]
- Thilakarathna, S.H.; Rupasinghe, H.V.; Needs, P.W. Apple peel bioactive rich extracts effectively inhibit in vitro human LDL cholesterol oxidation. Food Chem. 2013, 138, 463–470. [Google Scholar] [CrossRef]
- Toivonen, R.K.; Emani, R.; Munukka, E.; Rintala, A.; Laiho, A.; Pietilä, S.; Pursiheimo, J.-P.; Soidinsalo, P.; Linhala, M.; Eerola, E.; et al. Fermentable fibres condition colon microbiota and promote diabetogenesis in NOD mice. Diabetologia 2014, 57, 2183–2192. [Google Scholar] [CrossRef] [PubMed]
- Munarin, F.; Tanzi, M.C.; Petrini, P. Advances in biomedical applications of pectin gels. Int. J. Biol. Macromol. 2012, 51, 681–689. [Google Scholar] [CrossRef]
- Kowalska, H.; Czajkowska, K.; Cichowska, J.; Lenart, A. What’s new in biopotential of fruit and vegetable by-products applied in the food processing industry. Trends Food Sci. Technol. 2017, 67, 150–159. [Google Scholar] [CrossRef]
- Stenmarck, A.; Jensen, C.; Quested, T.; Moates, G.; Buksti, M.; Cseh, B.; Juul, S.; Parry, A.; Politano, A.; Redlingshofer, B.; et al. Estimates of European Food Waste Levels; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2016; Available online: https://edepot.wur.nl/378674 (accessed on 16 October 2022).
- Kandemir, K.; Piskin, E.; Xiao, J.; Tomas, M.; Capanoglu, E. Fruit Juice Industry Wastes as a Source of Bioactives. J. Agric. Food Chem. 2022, 70, 6805–6832. [Google Scholar] [CrossRef]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary fibre and fibre-rich by-products of food processing: Characterisation, technological functionality and commercial applications: A review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Lucera, A.; Costa, C.; Marinelli, V.; Saccotelli, M.A.; Del Nobile, M.A.; Conte, A. Fruit and Vegetable By-Products to Fortify Spreadable Cheese. Antioxidants 2018, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Rabetafika, H.N.; Bchir, B.; Blecker, C.; Richel, A. Fractionation of apple by-products as source of new ingredients: Current situation and perspectives. Trends Food Sci. Technol. 2014, 40, 99–114. [Google Scholar] [CrossRef]
- Figuerola, F.; Hurtado, M.L.; Estévez, A.M.; Chiffelle, I.; Asenjo, F. Fibre concentrates from apple pomace and citrus peel as potential fibre sources for food enrichment. Food Chem. 2005, 91, 395–401. [Google Scholar] [CrossRef]
- Xu, Y.; Fan, M.; Ran, J.; Zhang, T.; Sun, H.; Dong, M.; Zhang, Z.; Zheng, H. Variation in phenolic compounds and antioxidant activity in apple seeds of seven cultivars. Saudi J. Biol. Sci. 2016, 23, 379–388. [Google Scholar] [CrossRef]
- Hughes, D.A. Dietary antioxidants and human immune function. Nutr. Bull. 2000, 25, 35–41. [Google Scholar] [CrossRef]
- O’Shea, N.; Arendt, E.K.; Gallagher, E. Dietary fibre and phytochemical characteristics of fruit and vegetable by-products and their recent applications as novel ingredients in food products. Innov. Food Sci. Emerg. Technol. 2012, 16, 1–10. [Google Scholar] [CrossRef]
- Konrade, D.; Klava, D.; Sabovics, M.; Kince, T.; Kruma, Z. Plant by-products as source of natural antioxidants for production of crispbreads. Food Sci. 2016, 6, 3001. [Google Scholar]
- Plazzotta, S.; Manzocco, L.; Nicoli, M.C. Fruit and vegetable waste management and the challenge of fresh-cut salad. Trends Food Sci. Technol. 2017, 63, 51–59. [Google Scholar] [CrossRef]
- Turon, X.; Venus, J.; Arshadi, M.; Koutinas, M.; Lin, C.S.K.; Koutinas, A. Food Waste and Byproduct Valorization through Bio-processing: Opportunities and Challenges. Bioresources 2018, 9, 5774–5777. [Google Scholar] [CrossRef]
- Sudha, M.; Baskaran, V.; Leelavathi, K. Apple pomace as a source of dietary fiber and polyphenols and its effect on the rheological characteristics and cake making. Food Chem. 2007, 104, 686–692. [Google Scholar] [CrossRef]
- Kalisz, S.; Oszmiański, J.; Kolniak-Ostek, J.; Grobelna, A.; Kieliszek, M.; Cendrowski, A. Effect of a variety of polyphenols compounds and antioxidant properties of rhubarb (Rheum rhabarbarum). LWT-Food Sci. Technol. 2019, 118, 108775. [Google Scholar] [CrossRef]
- Mosleh, G.; Zaeri, M.; Hemmati, S.; Mohagheghzadeh, A. A comprehensive review on rhubarb astringent/laxative actions and the role of aquaporins as hub genes. Phytochem. Rev. 2022. [Google Scholar] [CrossRef]
- E.U. Fruit and Vegetable Market Observatory. EU Fruit and Vegetables Market Observatory the Apple Market in the EU: Volume 1: Production, Areas and Yields Volume 1: Production, Superficies et Rendements; E.U. Fruit and Vegetable Market Observatory: Brussels, Belgium, 2022. [Google Scholar]
- Pashova, S. Chemical composition of plum fruits. J. Mt. Agric. Balk. 2019, 9, 239–249. [Google Scholar]
- Kosmala, M.; Milala, J.; Kołodziejczyk, K.; Markowski, J.; Zbrzeźniak, M.; Renard, C.M. Dietary fiber and cell wall polysaccharides from plum (Prunus domestica L.) fruit, juice and pomace: Comparison of composition and functional properties for three plum varieties. Food Res. Int. 2013, 54, 1787–1794. [Google Scholar] [CrossRef]
- Yan, H.; Kerr, W.L. Total phenolics content, anthocyanins, and dietary fiber content of apple pomace powders produced by vacuum-belt drying. J. Sci. Food Agric. 2013, 93, 1499–1504. [Google Scholar] [CrossRef]
- Perussello, C.A.; Zhang, Z.; Marzocchella, A.; Tiwari, B.K. Valorization of Apple Pomace by Extraction of Valuable Compounds. Compr. Rev. Food Sci. Food Saf. 2017, 16, 776–796. [Google Scholar] [CrossRef]
- Virk, B.S.; Sogi, D.S. Extraction and Characterization of Pectin from Apple (Malus Pumila. Cv Amri) Peel Waste. Int. J. Food Prop. 2007, 2912, 693–703. [Google Scholar] [CrossRef]
- Waldbauer, K.; McKinnon, R.; Kopp, B. Apple Pomace as Potential Source of Natural Active Compounds. Planta Med. 2017, 83, 994–1010. [Google Scholar] [CrossRef]
- Vaqué, L.G. Food Loss and Waste in the European Union: A New Challenge for the Food Law. Eur. Food Feed. Law Rev. 2015, 10, 20–34. [Google Scholar]
- Gupta, P.; Ray, J.; Aggarwal, B.K. Food Processing Residue Analysis and its Functional Components as Related to Human Health: Recent Developments. Austin J. Nutr. Food Sci. 2015, 3, 1068. [Google Scholar]
- Sun, D.; Chen, X.; Zhu, C. Physicochemical properties and antioxidant activity of pectin from hawthorn wine pomace: A comparison of different extraction methods. Int. J. Biol. Macromol. 2020, 158, 1239–1247. [Google Scholar] [CrossRef]
- Thiex, N.; Novotny, L.; Crawford, A. Determination of Ash in Animal Feed: AOAC Official Method 942.05 Revisited. J. AOAC Int. 2012, 95, 1392–1397. [Google Scholar] [CrossRef] [PubMed]
- Guiñé, R.P.F.; Pinho, S.; Barroca, M. IChemE Study of the convective drying of pumpkin. Food Bioprod. Process. 2010, 89, 422–428. [Google Scholar] [CrossRef]
- Ruthes, A.C.; Martínez-Abad, A.; Tan, H.; Bulone, V.; Vilaplana, F. Sequential fractionation of feruloylated hemicelluloses and oligosaccharides from wheat bran using subcritical water and xylanolytic enzymes. Green Chem. 2017, 19, 1919–1931. [Google Scholar] [CrossRef]
- Rudjito, R.C. Valorisation of Cereal by-Products: A Biorefinery Approach. Doctoral Dissertation, KTH Royal Institute of Technology, Stockholm, Sweden, 2022. Available online: https://www.diva-portal.org/smash/record.jsf?pid=diva2%3A1635248&dswid=597 (accessed on 26 January 2023).
- Marinova, G.; Batchvarov, V. Evaluation of the methods for determination of the free radical scavenging activity by DPPH. Bulg. J. Agric. Sci. 2011, 17, 11–24. [Google Scholar]
- Priecina, L.; Karlina, D. Total polyphenols, Flavonoid Content and Antiradical Activity of Celery, Dill, Parsley, Onion and Garlic Dried in Conventive and Microwave-Vacuum Dryers. Int. Proc. Chem. Biol. Environ. Eng. (IPCBEE) 2013, 53, 107–112. [Google Scholar]
- Kruma, Z.; Tomsone, L.; Galoburda, R.; Straumite, E.; Kronberga, A.; Åssveen, M. Total phenols and antioxidant capacity of hull-less barley and hull-less oats. Agron. Res. 2016, 14, 1361–1371. [Google Scholar]
- Koerner, P.J.; Lee, J.; Rimmer, C.A.; Schaneberg, B.T. Determination of Total Phenolic Content Using the Folin-C Assay: Single-Laboratory Validation, First Action 2017.13. J. AOAC Int. 2019, 102, 320–321. [Google Scholar] [CrossRef]
- Lee, K.J.; Oh, Y.C.; Cho, W.K.; Ma, J.Y. Antioxidant and Anti-Inflammatory Activity Determination of One Hundred Kinds of Pure Chemical Compounds Using Offline and Online Screening HPLC Assay. Evid. Based Complement. Altern. Med. 2015, 2015, 165457. [Google Scholar] [CrossRef]
- Inbaraj, B.S.; Lu, H.; Kao, T.; Chen, B. Simultaneous determination of phenolic acids and flavonoids in Lycium barbarum Linnaeus by HPLC–DAD–ESI-MS. J. Pharm. Biomed. Anal. 2010, 51, 549–556. [Google Scholar] [CrossRef]
- Ptichkina, N.; Markina, O.; Rumyantseva, G. Pectin extraction from pumpkin with the aid of microbial enzymes. Food Hydrocoll. 2008, 22, 192–195. [Google Scholar] [CrossRef]
- Dabbaghi, M.; Namjoshi, S.; Panchal, B.; Grice, J.E.; Prakash, S.; Roberts, M.S.; Mohammed, Y. Viscoelastic and Deformation Characteristics of Structurally Different Commercial Topical Systems. Pharmaceutics 2021, 13, 1351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, S.; Zhang, B.; Drago, S.R.; Zhang, J. Relationships between the gelatinization of starches and the textural properties of extruded texturized soybean protein-starch systems. J. Food Eng. 2016, 174, 29–36. [Google Scholar] [CrossRef]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP); Bampidis, V.; Azimonti, G.; Bastos, M.D.L.; Christensen, H.; Dusemund, B.; Durjava, M.F.; Kouba, M.; López-Alonso, M.; Puente, S.L.; et al. Safety and efficacy of a feed additive consisting of locust bean gum for all animal species (Dupont Nutrition and Health). EFSA J. 2022, 20, e07435. [Google Scholar] [CrossRef] [PubMed]
- Dotto, J.M.; Chacha, J.S. The potential of pumpkin seeds as a functional food ingredient: A review. Sci. Afr. 2020, 10, e00575. [Google Scholar] [CrossRef]
- Pińkowska, H.; Krzywonos, M.; Wolak, P.; Złocińska, A. Pectin and Neutral Monosaccharides Production during the Simultaneous Hydrothermal Extraction of Waste Biomass from Refining of Sugar—Optimization with the Use of Doehlert Design. Molecules 2019, 24, 472. [Google Scholar] [CrossRef]
- Szymanska-Chargot, M.; Zdunek, A. Use of FT-IR Spectra and PCA to the Bulk Characterization of Cell Wall Use of FT-IR Spectra and PCA to the Bulk Characterization of Cell Wall Residues of Fruits and Vegetables along a Fraction Process. Food Biophys. 2013, 8, 29–42. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). FAO Statistical Pocketbook; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018; Available online: http://faostat.fao.org (accessed on 10 October 2022).
- Cui, J.; Zhao, C.; Feng, L.; Han, Y.; Du, H.; Xiao, H.; Zheng, J. Pectins from fruits: Relationships between extraction methods, structural characteristics, and functional properties. Trends Food Sci. Technol. 2021, 110, 39–54. [Google Scholar] [CrossRef]
- Rodsamran, P.; Sothornvit, R. Microwave heating extraction of pectin from lime peel: Characterization and properties compared with the conventional heating method. Food Chem. 2019, 278, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Yazdanpanah, S.; Manochehr, Y. Extraction of pectin from skin and cap of pumpkin by microwave. J. Food Res. 2021, 31, 177–190. [Google Scholar] [CrossRef]
- O’Shea, N.; Ktenioudaki, A.; Smyth, T.P.; McLoughlin, P.; Doran, L.; Auty, M.A.E.; Arendt, E.; Gallagher, E. Physicochemical assessment of two fruit by-products as functional ingredients: Apple and orange pomace. J. Food Eng. 2015, 153 (Suppl. C), 89–95. [Google Scholar] [CrossRef]
- Leyva-Corral, J.; Quintero-Ramos, A.; Camacho-Dávila, A.; Zazueta-Morales, J.D.J.; Aguilar-Palazuelos, E.; Ruiz-Gutiérrez, M.G.; Meléndez-Pizarro, C.O.; Ruiz-Anchondo, T.D.J. Polyphenolic compound stability and antioxidant capacity of apple pomace in an extruded cereal. LWT-Food Sci. Technol. 2016, 65, 228–236. [Google Scholar] [CrossRef]
- Luceri, C.; Giannini, L.; Lodovici, M.; Antonucci, E.; Abbate, R.; Masini, E.; Dolara, P. p-Coumaric acid, a common dietary phenol, inhibits platelet activity in vitro and in vivo. Br. J. Nutr. 2007, 97, 458–463. [Google Scholar] [CrossRef]
- Liu, J.; Bi, J.; McClements, D.J.; Liu, X.; Yi, J.; Lyu, J.; Zhou, M.; Verkerk, R.; Dekker, M.; Wu, X.; et al. Impacts of thermal and non-thermal processing on structure and functionality of pectin in fruit- and vegetable- based products: A review. Carbohydr. Polym. 2020, 250, 116890. [Google Scholar] [CrossRef]
- Crandall, P.G.; Wicker, L. Pectin Internal Gel Strength: Theory, Measurement, and Methodology. In Chemistry and Function of Pectins; American Chemical Society: Washington, DC, USA, 1986; Volume 310, pp. 8–88. [Google Scholar]
- Mierczyńska, J.; Cybulska, J.; Zdunek, A. Rheological and chemical properties of pectin enriched fractions from different sources extracted with citric acid. Carbohydr. Polym. 2017, 156, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yuan, K.; Descallar, F.B.A.; Li, A.; Yang, X.; Yang, H. Gelation behaviors of some special plant-sourced pectins: A review inspired by examples from traditional gel foods in China. Trends Food Sci. Technol. 2022, 126, 26–40. [Google Scholar] [CrossRef]
- Chandel, V.; Biswas, D.; Roy, S.; Vaidya, D.; Verma, A.; Gupta, A. Current Advancements in Pectin: Extraction, Properties and Multifunctional Applications. Foods 2022, 11, 2683. [Google Scholar] [CrossRef]
| By-Product Source | Moisture,% | Ash, g 100 g−1 (DW) | Total Titratable Acidity (TA) |
|---|---|---|---|
| Cherry BP | 63.2 ± 1.4 | 0.82 ± 0.2 | 4.25 ± 0.8 |
| Red Currant BP | 57.1 ± 0.9 | 1.24 ± 0.2 | 4.18 ± 0.2 |
| Gooseberry BP | 58.0 ± 1.3 | 0.92 ± 0.4 | 2.95 ± 0.1 |
| Black Currant BP | 55.4 ± 1.2 | 0.98 ± 0.3 | 4.22 ± 0.2 |
| Plum BP | 60.9 ± 0.8 | 0.76 ± 0.1 | 3.95 ± 0.3 |
| Rhubarb BP | 62.7 ± 2.4 | 0.62 ± 0.2 | 3.22 ± 0.1 |
| Apple BP | 64.9 ± 1.8 | 0.98 ± 0.1 | 3.65 ± 0.2 |
| Source | Moisture, % | Ash, g 100 g−1 |
|---|---|---|
| Cherry BPP | 6.0 ± 0.1 | 1.94 ± 0.05 |
| Red Currant BPP | 6.4 ± 0.1 | 1.42 ± 0.06 |
| Gooseberry BPP | 7.4 ± 0.2 | 2.24 ± 0.08 |
| Black Currant BPP | 6.2 ± 0.1 | 2.68 ± 0.02 |
| Plum BPP | 5.2 ± 0.1 | 2.11 ± 0.24 |
| Rhubarb BPP | 7.2± 0.2 | 2.14 ± 0.08 |
| Apple BPP | 6.5 ± 0.1 | 2.88 ± 0.14 |
| Pumpkin BPP | 7.2 ± 0.1 | 2.65 ± 0.18 |
| Source | DE, % | EW, g mol−1 | ME, % |
|---|---|---|---|
| Cherry BPP | 64.06 ± 3.83 b | 1390.0 ± 69.0 a | 4.27 ± 1.04 a |
| Red currant BPP | 57.12 ± 4.07 a | 716.9 ± 80.95 b | 6.35 ± 1.84 c |
| Gooseberry BPP | 57.14 ± 7.18 a | 895.8 ± 316.8 c | 5.53 ± 0.77 b |
| Black currant BPP | 54.69 ±2.67 a | 465.0 ± 72.04 d | 8.13 ± 0.45 e |
| Plum BPP | 45.16 ± 3.74 c | 531.6 ± 95.8 e,d | 5.15 ± 2.10 b |
| Rhubarb BPP | 54.32 ± 0.44 a | 417.2 ± 34.0 d | 5.52 ± 2.85 b |
| Apple BPP | 56.03 ± 3.03 a | 581.0 ± 46.1 e | 6.86 ± 0.77 c |
| Pumpkin BPP | 51.11 ± 0.69 a,c | 426.0 ± 8.7 d | 7.61 ± 0.30 d,e |
| Control | 56.69 ± 0.39 a | 708.5 ± 24.5 b | 5.73 ± 0.11 b,c |
| Source of Pectin | TPC, µg GAE g−1 DW | Antiradical Scavenging Activity, g 100 g −1 |
|---|---|---|
| Control | 2.803± 0.002 b,c | 0.56 ± 0.04 e |
| Apple BPP | 2.398 ± 0.047 b | 2.27 ± 0.82 d |
| Rhubarb BPP | 2.173 ± 0.024 b | 8.79 ± 0.44 c,b |
| Plums BPP | 3.102 ± 0.047 c,b | 11.86 ± 1.12 b |
| Red Currant BPP | 2.516 ± 0.048 b | 7.57 ± 0.88 c |
| Gooseberry BPP | 2.657 ± 0.118 b | 15.97 ± 1.23 b |
| Pumpkin BPP | 2.076 ± 0.048 b | 3.03 ± 1.01 d |
| Black Currant BPP | 4.668 ± 0.405 a | 37.29 ± 2.03 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konrade, D.; Gaidukovs, S.; Vilaplana, F.; Sivan, P. Pectin from Fruit- and Berry-Juice Production by-Products: Determination of Physicochemical, Antioxidant and Rheological Properties. Foods 2023, 12, 1615. https://doi.org/10.3390/foods12081615
Konrade D, Gaidukovs S, Vilaplana F, Sivan P. Pectin from Fruit- and Berry-Juice Production by-Products: Determination of Physicochemical, Antioxidant and Rheological Properties. Foods. 2023; 12(8):1615. https://doi.org/10.3390/foods12081615
Chicago/Turabian StyleKonrade, Daiga, Sergejs Gaidukovs, Francisco Vilaplana, and Pramod Sivan. 2023. "Pectin from Fruit- and Berry-Juice Production by-Products: Determination of Physicochemical, Antioxidant and Rheological Properties" Foods 12, no. 8: 1615. https://doi.org/10.3390/foods12081615
APA StyleKonrade, D., Gaidukovs, S., Vilaplana, F., & Sivan, P. (2023). Pectin from Fruit- and Berry-Juice Production by-Products: Determination of Physicochemical, Antioxidant and Rheological Properties. Foods, 12(8), 1615. https://doi.org/10.3390/foods12081615







