Development and Characterization of an Edible Zein/Shellac Composite Film Loaded with Curcumin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Films
2.3. Preparation of Curcumin-Loaded Films
2.4. Physical Properties of the Films
2.4.1. Film Thickness
2.4.2. WVP
2.4.3. WS (Water Solubility)
2.4.4. Mechanical Properties
2.4.5. Optical Properties
2.4.6. WCA
2.5. Characterization of the Films
2.5.1. SEM
2.5.2. FT-IR
2.5.3. XRD
2.5.4. DSC
2.5.5. TGA
2.6. Curcumin Release Tests
2.7. pH Response Tests
2.8. Antioxidant Properties
2.8.1. DPPH Radical Scavenging Activity
2.8.2. ABTS Radical Scavenging Activity
2.9. Antibacterial Properties
2.10. Statistical Analyses
3. Results and Discussion
3.1. Physical Properties of ZS–Cur Films
3.1.1. WVP, WS, TS, and EB Analyses
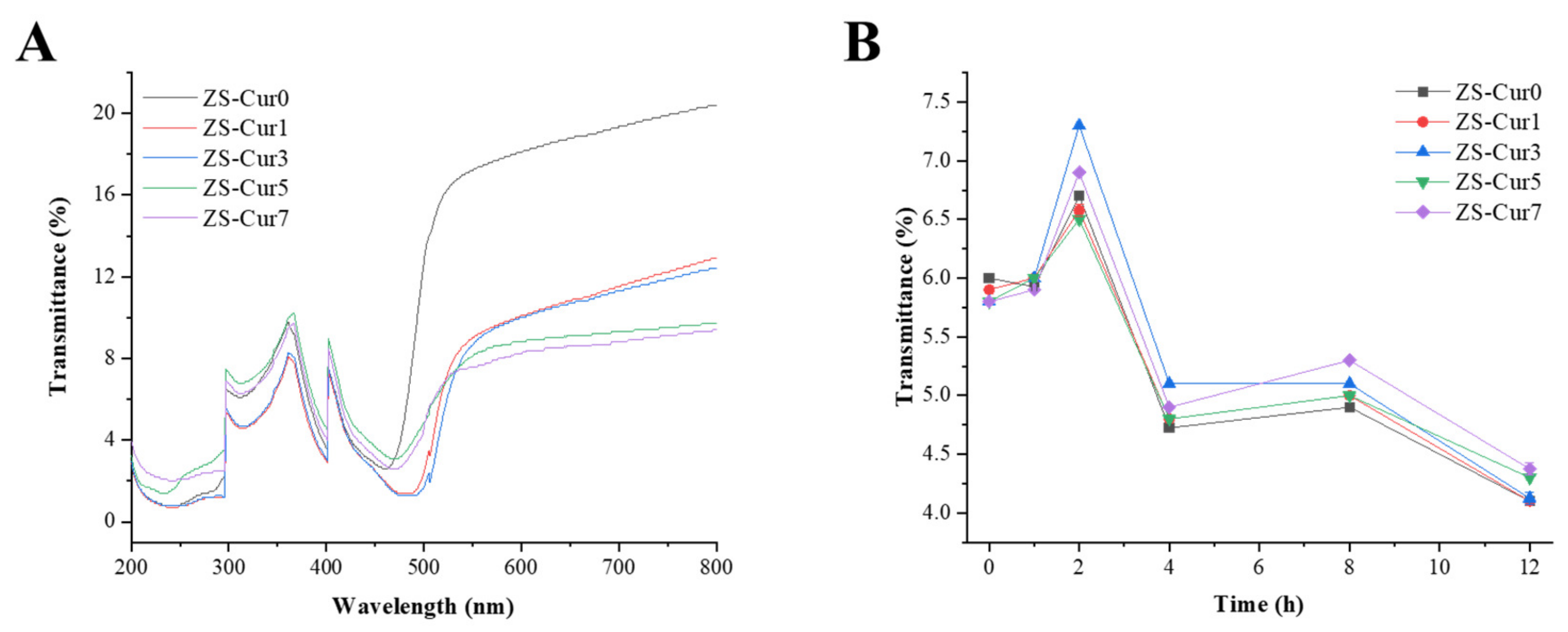
3.1.2. Optical Properties
3.1.3. WCA
3.2. Characterization of ZS–Cur Films
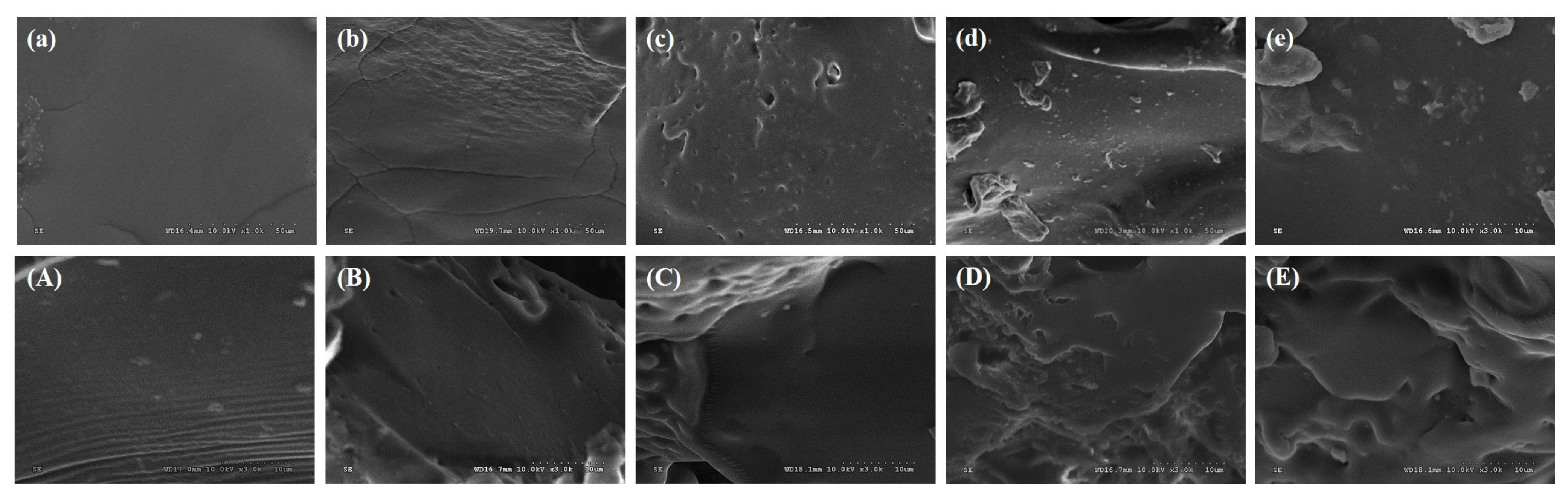
3.2.1. SEM
3.2.2. FT-IR Spectroscopy
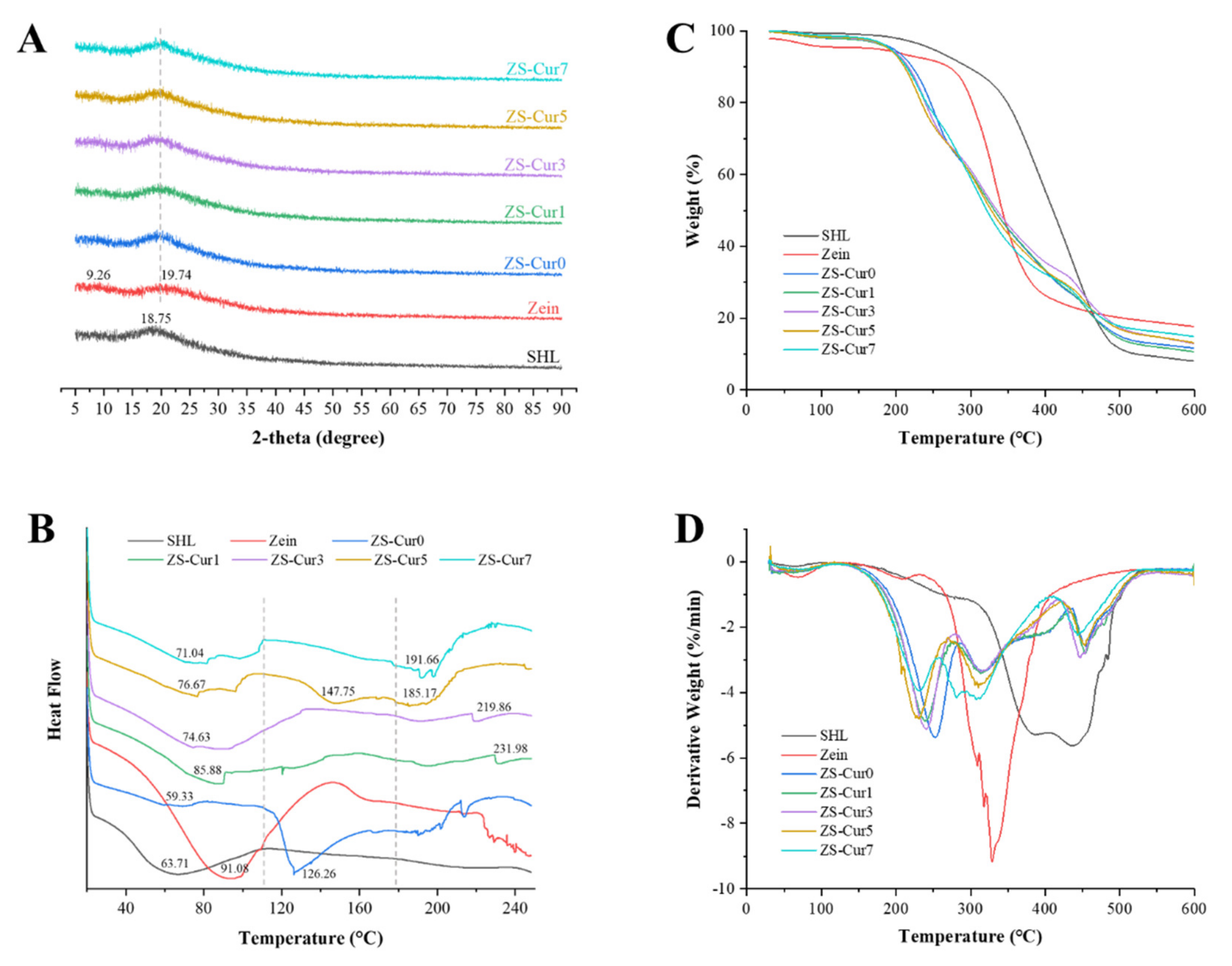
3.2.3. XRD
3.2.4. Thermal Performance Analyses
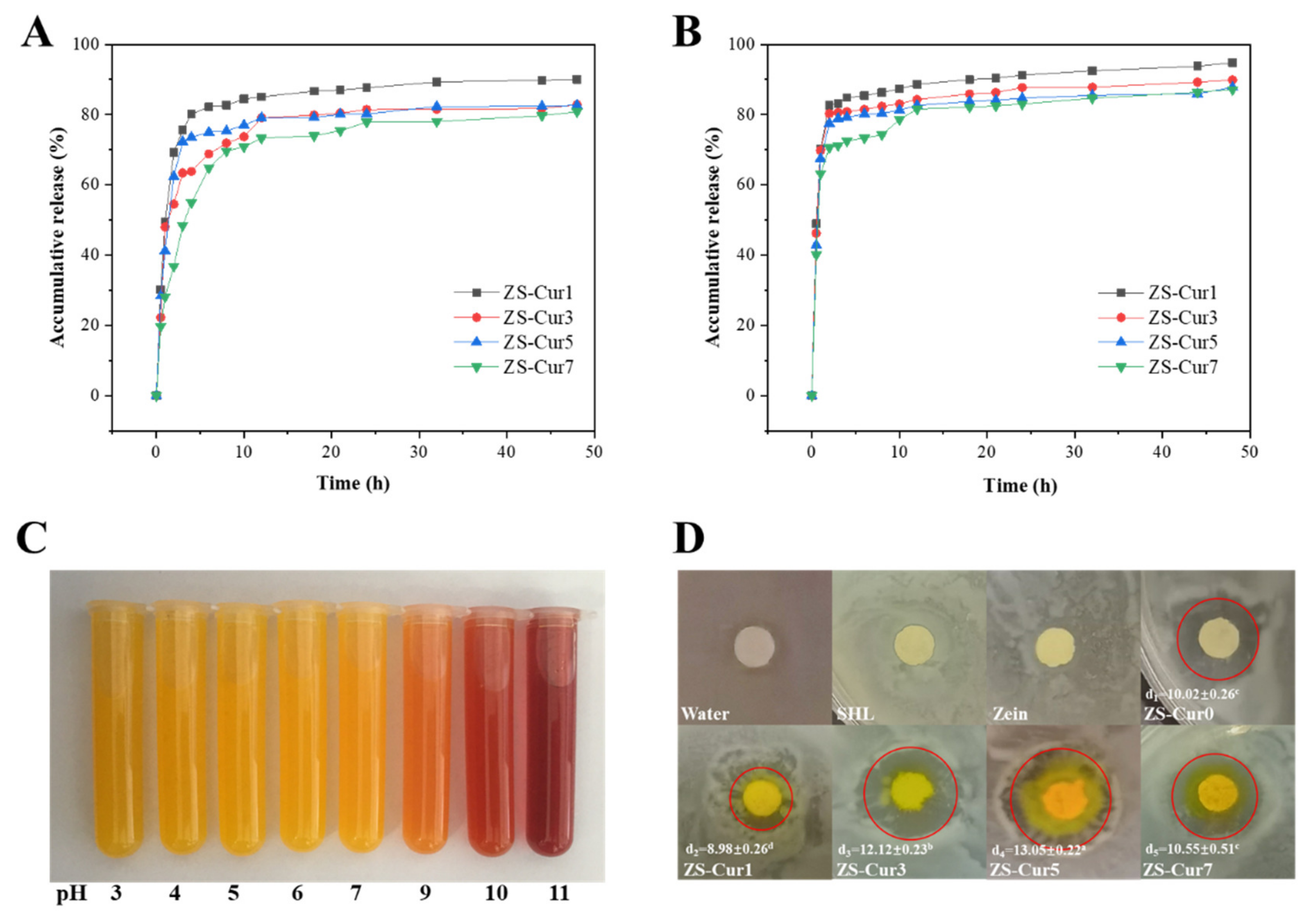
3.3. Curcumin Release Properties
3.4. pH Response of ZS–Cur Films
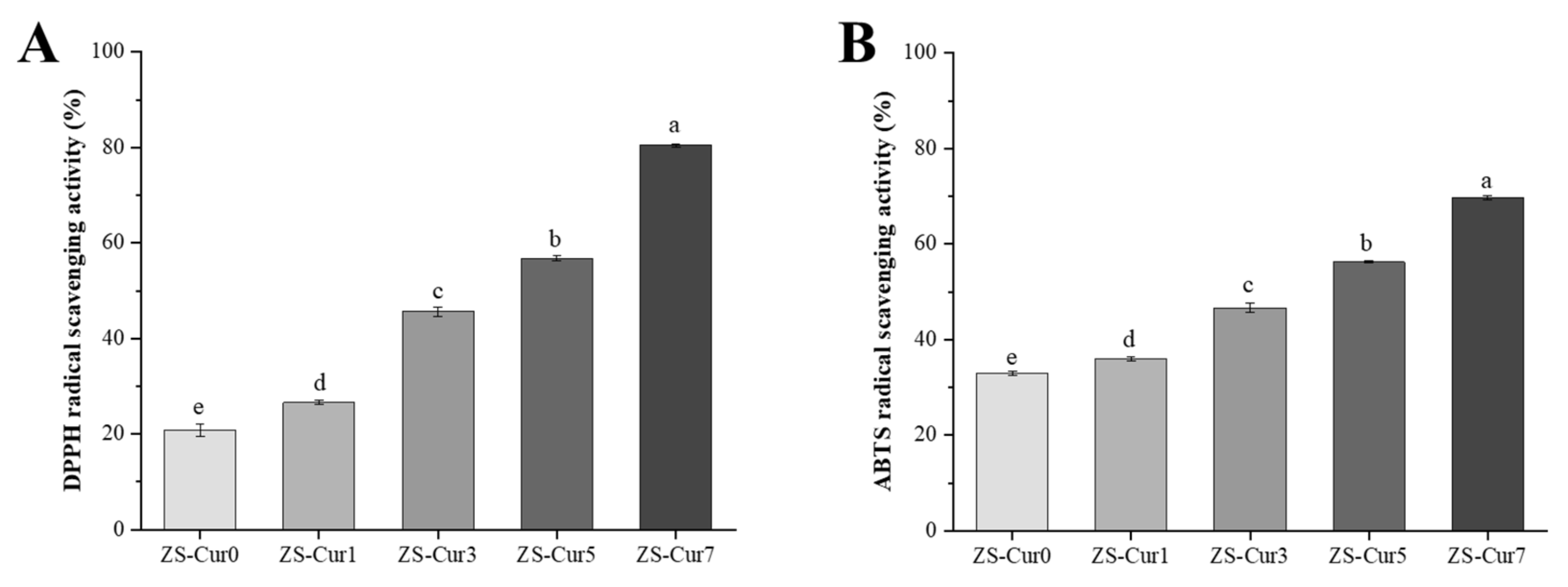
3.5. Antioxidant Properties of ZS–Cur Films
3.6. Antibacterial Properties of ZS–Cur Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chang-Bravo, L.; Lopez-Cordoba, A.; Martino, M. Biopolymeric matrices made of carrageenan and corn starch for the antioxidant extracts delivery of Cuban red propolis and yerba mate. React. Funct. Polym. 2014, 85, 11–19. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.W. Preparation of nanocellulose from micro-crystalline cellulose: The effect on the performance and properties of agar-based composite films. Carbohydr. Polym. 2016, 135, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Liu, Y.; Kang, S.; Cui, M.; Xu, H. Development of pH-responsive antioxidant soy protein isolate films incorporated with cellulose nanocrystals and curcumin nanocapsules to monitor shrimp freshness. Food Hydrocoll. 2021, 120, 106893. [Google Scholar] [CrossRef]
- Shukla, R.; Cheryan, M. Zein: The industrial protein from corn. Ind. Crops Prod. 2001, 13, 171–192. [Google Scholar] [CrossRef]
- Shi, K.; Yu, H.; Rao, S.L.; Lee, T.-C. Improved Mechanical Property and Water Resistance of Zein Films by Plasticization with Tributyl Citrate. J. Agric. Food Chem. 2012, 60, 5988–5993. [Google Scholar] [CrossRef]
- Lawton, J.W. Zein: A history of processing and use. Cereal Chem. 2002, 79, 1–18. [Google Scholar] [CrossRef]
- Xu, H.; Chai, Y.W.; Zhang, G.Y. Synergistic Effect of Oleic Acid and Glycerol on Zein Film Plasticization. J. Agric. Food Chem. 2012, 60, 10075–10081. [Google Scholar] [CrossRef]
- Solano, A.C.V.; de Gante, C.R. Development of biodegradable films based on blue corn flour with potential applications in food packaging. Effects of plasticizers on mechanical, thermal, and microstructural properties of flour films. J. Cereal Sci. 2014, 60, 60–66. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, L.L.; Che, X.X.; Zhang, H.; Shi, N.Q.; Li, C.L.; Chen, Y.; Kong, W. Zein-based films and their usage for controlled delivery: Origin, classes and current landscape. J. Control. Release 2015, 206, 206–219. [Google Scholar] [CrossRef]
- Turasan, H.; Barber, E.A.; Malm, M.; Kokini, J.L. Mechanical and spectroscopic characterization of crosslinked zein films cast from solutions of acetic acid leading to a new mechanism for the crosslinking of oleic acid plasticized zein films. Food Res. Int. 2018, 108, 357–367. [Google Scholar] [CrossRef]
- Masamba, K.; Li, Y.; Hategekimana, J.; Zehadi, M.; Ma, J.G.; Zhong, F. Evaluation of mechanical and water barrier properties of transglutaminase cross-linked zein films incorporated with oleic acid. Int. J. Food Sci. Technol. 2016, 51, 1159–1167. [Google Scholar] [CrossRef]
- Tamburini, D.; Dyer, J.; Bonaduce, I. The characterisation of shellac resin by flow injection and liquid chromatography coupled with electrospray ionisation and mass spectrometry. Sci. Rep. 2017, 7, 14784. [Google Scholar] [CrossRef]
- Patel, A.; Heussen, P.; Hazekamp, J.; Velikov, K.P. Stabilisation and controlled release of silibinin from pH responsive shellac colloidal particles. Soft Matter 2011, 7, 8549–8555. [Google Scholar] [CrossRef]
- Hagenmaier, R.D.; Baker, R.A. Reduction in gas exchange of citrus fruit by wax coatings. J. Agric. Food Chem. 1993, 41, 283–287. [Google Scholar] [CrossRef]
- McGuire, R.G.; Hagenmaier, R.D. Shellac Coatings for Grapefruits that Favor Biological Control ofPenicillium digitatumbyCandida oleophila. Biol. Control. 1996, 7, 100–106. [Google Scholar] [CrossRef]
- Valencia-Chamorro, S.A.; Perez-Gago, M.B.; del Rio, M.A.; Palou, L. Effect of antifungal hydroxypropyl methylcellulose (HPMC)-lipid edible composite coatings on postharvest decay development and quality attributes of cold-stored ‘Valencia’ oranges. Postharvest Biol. Technol. 2009, 54, 72–79. [Google Scholar] [CrossRef]
- Soradech, S.; Nunthanid, J.; Limmatvapirat, S.; Luangtana-anan, M. Utilization of shellac and gelatin composite film for coating to extend the shelf life of banana. Food Control 2017, 73, 1310–1317. [Google Scholar] [CrossRef]
- Limmatvapirat, S.; Limmatvapirat, C.; Luangtana-Anan, M.; Nunthanid, J.; Oguchi, T.; Tozuka, Y.; Yamamoto, K.; Puttipipatkhachorn, S. Modification of physicochemical and mechanical properties of shellac by partial hydrolysis. Int. J. Pharm. 2004, 278, 41–49. [Google Scholar] [CrossRef]
- Pearnchob, N.; Dashevsky, A.; Bodmeier, R. Improvement in the disintegration of shellac-coated soft gelatin capsules in simulated intestinal fluid. J. Control. Release Off. J. Control. Release Soc. 2004, 94, 313–321. [Google Scholar] [CrossRef]
- Wang, J.; Chen, L.; He, Y. Preparation of environmental friendly coatings based on natural shellac modified by diamine and its applications for copper protection. Prog. Org. Coat. 2008, 62, 307–312. [Google Scholar] [CrossRef]
- The, D.P.; Debeaufort, F.; Luu, D.; Voilley, A. Moisture barrier, wetting and mechanical properties of shellac/agar or shellac/cassava starch bilayer bio-membrane for food applications. J. Membr. Sci. 2008, 325, 277–283. [Google Scholar]
- Bai, J.; Alleyne, V.; Hagenmaier, R.D.; Mattheis, J.P.; Baldwin, E.A. Formulation of zein coatings for apples (Malus domestica Borkh). Postharvest Biol. Technol. 2003, 28, 259–268. [Google Scholar] [CrossRef]
- Na, Z.; Weng, W.; Polytechnic, Z.T. The Research Progress of Natural Edible Curcumin. Shandong Chem. Ind. 2017, 46, 72–73. [Google Scholar] [CrossRef]
- Tianmi, L.I.; Sijia, Q.U.; Han, J. Preparation of Chitosan/Curcumin/γ-Polyglutamic Acid Edible Composite Film and Its Preservative Effect on Bacon and Sausage. Food Sci. 2019, 47, 308–314. [Google Scholar]
- Liu, J.R.; Wang, H.L.; Wang, P.F.; Guo, M.; Jiang, S.W.; Li, X.J.; Jiang, S.T. Films based on kappa-carrageenan incorporated with curcumin for freshness monitoring. Food Hydrocoll. 2018, 83, 134–142. [Google Scholar] [CrossRef]
- Wang, C.Z.; Jing, H. Anti-inflammatory and anti-oxidant effects of curcumin:An update. J. Med. Postgrad. 2012, 25, 658–660. [Google Scholar]
- Liang, G.; Yang, S.L.; Jiang, L.J.; Zhao, Y.; Shao, L.L.; Xiao, J.; Ye, F.Q.; Li, Y.R.; Li, X.K. Synthesis and anti-bacterial properties of mono-carbonyl analogues of curcumin. Chem. Pharm. Bull. 2008, 56, 162–167. [Google Scholar] [CrossRef]
- Bhargava, N.; Sharanagat, V.S.; Mor, R.S.; Kumar, K. Active and intelligent biodegradable packaging films using food and food waste-derived bioactive compounds: A review. Trends Food Sci. Technol. 2020, 105, 385–401. [Google Scholar] [CrossRef]
- Xiao, J.Q.; Gu, C.Q.; Zhu, D.X.; Huang, Y.K.; Luo, Y.S.; Zhou, Q.Q. Development and characterization of an edible chitosan/zein-cinnamaldehyde nano-cellulose composite film and its effects on mango quality during storage. Lwt-Food Sci. Technol. 2021, 140, 110809. [Google Scholar] [CrossRef]
- Zhang, L.M.; Liu, Z.L.; Han, X.B.; Sun, Y.; Wang, X.Y. Effect of ethanol content on rheology of film-forming solutions and properties of zein/chitosan film. Int. J. Biol. Macromol. 2019, 134, 807–814. [Google Scholar] [CrossRef]
- Jiang, L.W.; Jia, F.G.; Han, Y.L.; Meng, X.Y.; Xiao, Y.W.; Bai, S.G. Development and characterization of zein edible films incorporated with catechin/?-cyclodextrin inclusion complex nanoparticles. Carbohydr. Polym. 2021, 261, 117877. [Google Scholar] [CrossRef] [PubMed]
- Khoshgozaran-Abras, S.; Azizi, M.H.; Hamidy, Z.; Bagheripoor-Fallah, N. Mechanical, physicochemical and color properties of chitosan based-films as a function of Aloe vera gel incorporation. Carbohydr. Polym. 2012, 87, 2058–2062. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, T.; Zhang, H.; Zhang, H.; Chi, Y.; Zhao, X.; Li, H.; Wen, Y. Blending with shellac to improve water resistance of soybean protein isolate film. J. Food Process Eng. 2020, 43, e13515. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, Y. Physical and antimicrobial properties of zein and methyl cellulose composite films with plasticizers of oleic acid and polyethylene glycol. Lwt 2021, 140, 110811. [Google Scholar] [CrossRef]
- Farajpour, R.; Emam Djomeh, Z.; Moeini, S.; Tavakolipour, H.; Safayan, S. Structural and physico-mechanical properties of potato starch-olive oil edible films reinforced with zein nanoparticles. Int. J. Biol. Macromol. 2020, 149, 941–950. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.; Sun, Y.; Wang, X.; Li, L. Effect of α-tocopherol antioxidant on rheological and physicochemical properties of chitosan/zein edible films. Lwt 2020, 118, 108799. [Google Scholar] [CrossRef]
- Ahammed, S.; Liu, F.; Khin, M.N.; Yokoyama, W.H.; Zhong, F. Improvement of the water resistance and ductility of gelatin film by zein. Food Hydrocoll. 2020, 105, 105804. [Google Scholar] [CrossRef]
- Kevij, H.T.; Salami, M.; Mohammadian, M.; Khodadadi, M. Fabrication and investigation of physicochemical, food simulant release, and antioxidant properties of whey protein isolate-based films activated by loading with curcumin through the pH-driven method. Food Hydrocoll. 2020, 108, 106026. [Google Scholar] [CrossRef]
- Wu, J.L.; Sun, X.Y.; Guo, X.B.; Ji, M.Y.; Wang, J.H.; Cheng, C.; Chen, L.; Wen, C.L.; Zhang, Q.Q. Physicochemical, Antioxidant, In Vitro Release, and Heat Sealing Properties of Fish Gelatin Films Incorporated with beta-Cyclodextrin/Curcumin Complexes for Apple Juice Preservation. Food Bioprocess Technol. 2018, 11, 447–461. [Google Scholar] [CrossRef]
- Ma, Q.M.; Cao, L.L.; Liang, T.G.; Li, J.; Lucia, L.A.; Wang, L.J. Active Tara Gum/PVA Blend Films with Curcumin-Loaded CTAC Brush-TEMPO-Oxidized Cellulose Nanocrystals. Acs Sustain. Chem. Eng. 2018, 6, 8926–8934. [Google Scholar] [CrossRef]
- Huang, Y.K.; Gu, C.Q.; He, S.; Zhu, D.X.; Liu, X.C.; Chen, Z.Y. Development and characterization of an edible chitosan-whey protein nano composite film for chestnut (Castanea mollissima Bl.) preservation. J. Food Sci. 2020, 85, 2114–2123. [Google Scholar] [CrossRef]
- Xiao, Y.Q.; Liu, Y.N.; Kang, S.F.; Xu, H.D. Insight into the formation mechanism of soy protein isolate films improved by cellulose nanocrystals. Food Chem. 2021, 359, 129971. [Google Scholar] [CrossRef]
- Musso, Y.S.; Salgado, P.R.; Mauri, A.N. Smart edible films based on gelatin and curcumin. Food Hydrocoll. 2017, 66, 8–15. [Google Scholar] [CrossRef]
- Pirsa, S.; Sani, I.K.; Pirouzifard, M.K.; Erfani, A. Smart film based on chitosan/Melissa officinalis essences/ pomegranate peel extract to detect cream cheeses spoilage. Food Addit. Contam. Part A-Chem. Anal. Control. Expo. Risk Assess. 2020, 37, 634–648. [Google Scholar] [CrossRef]
- Riaz, A.; Lagnika, C.; Abdin, M.; Hashim, M.M.; Ahmed, W. Preparation and Characterization of Chitosan/Gelatin-Based Active Food Packaging Films Containing Apple Peel Nanoparticles. J. Polym. Environ. 2020, 28, 411–420. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Preparation of carbohydrate-based functional composite films incorporated with curcumin. Food Hydrocoll. 2020, 98, 105302. [Google Scholar] [CrossRef]
- Xia, C.; Wang, W.B.; Wang, L.; Liu, H.S.; Xiao, J. Multilayer zein/gelatin films with tunable water barrier property and prolonged antioxidant activity. Food Packag. Shelf Life 2019, 19, 76–85. [Google Scholar] [CrossRef]
- Deng, L.L.; Kang, X.F.; Liu, Y.Y.; Feng, F.Q.; Zhang, H. Characterization of gelatin/zein films fabricated by electrospinning vs solvent casting. Food Hydrocoll. 2018, 74, 324–332. [Google Scholar] [CrossRef]
- Gillgren, T.; Barker, S.A.; Belton, P.S.; Georget, D.M.R.; Stading, M. Plasticization of Zein: A Thermomechanical, FTIR, and Dielectric Study. Biomacromolecules 2009, 10, 1135–1139. [Google Scholar] [CrossRef]
- Bisharat, L.; Berardi, A.; Perinelli, D.R.; Bonacucina, G.; Casettari, L.; Cespi, M.; AlKhatib, H.S.; Palmieri, G.F. Aggregation of zein in aqueous ethanol dispersions: Effect on cast film properties. Int. J. Biol. Macromol. 2018, 106, 360–368. [Google Scholar] [CrossRef]
- Kim, S.; Xu, J. Aggregate formation of zein and its structural inversion in aqueous ethanol. J. Cereal Sci. 2008, 47, 1–5. [Google Scholar] [CrossRef]
- Limmatvapirat, S.; Limmatvapirat, C.; Puttipipatkhachorn, S.; Nuntanid, J.; Luangtana-Anan, M. Enhanced enteric properties and stability of shellac films through composite salts formation. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. e.V 2007, 67, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Almutawah, A.; Barker, S.A.; Belton, P.S. Hydration of gluten: A dielectric, calorimetric, and fourier transform infrared study. Biomacromolecules 2007, 8, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.X.; Liu, F.G.; Yang, J.; Yang, W.; Yuan, F.; Gao, Y.X. Physical, structural, thermal and morphological characteristics of zeinquercetagetin composite colloidal nanoparticles. Ind. Crops Prod. 2015, 77, 476–483. [Google Scholar] [CrossRef]
- Tang, C.; Ozcam, A.E.; Stout, B.; Khan, S.A. Effect of pH on Protein Distribution in Electrospun PVA/BSA Composite Nanofibers. Biomacromolecules 2012, 13, 1269–1278. [Google Scholar] [CrossRef]
- Dong, S.; Guo, P.; Chen, Y.; Chen, G.Y.; Ji, H.; Ran, Y.; Li, S.H.; Chen, Y. Surface modification via atmospheric cold plasma (ACP): Improved functional properties and characterization of zein film. Ind. Crops Prod. 2018, 115, 124–133. [Google Scholar] [CrossRef]
- Morsy, R.; Hosny, M.; Reicha, F.; Elnimr, T. Developing a potential antibacterial long-term degradable electrospun gelatin -based composites mats for wound dressing applications. React. Funct. Polym. 2017, 114, 8–12. [Google Scholar] [CrossRef]
- Sun, C.X.; Dai, L.; Gao, Y.X. Interaction and formation mechanism of binary complex between zein and propylene glycol alginate. Carbohydr. Polym. 2017, 157, 1638–1649. [Google Scholar] [CrossRef]
- Luo, Y.C.; Zhang, B.C.; Whent, M.; Yu, L.L.; Wang, Q. Preparation and characterization of zein/chitosan complex for encapsulation of alpha-tocopherol, and its in vitro controlled release study. Colloids Surf. B-Biointerfaces 2011, 85, 145–152. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, D.J.; Karthick, S.N.; Hemalatha, K.V.; Raj, C.J.; Ok, S.; Choe, Y. Curcumin Dye Extracted from Curcuma longa L. Used as Sensitizers for Efficient Dye-Sensitized Solar Cells. Int. J. Electrochem. Sci. 2013, 8, 8320–8328. [Google Scholar]
- Zhou, Y.Y.; Tang, R.C. Modification of curcumin with a reactive UV absorber and its dyeing and functional properties for silk. Dye. Pigment. 2016, 134, 203–211. [Google Scholar] [CrossRef]
- Liu, Q.; Cui, H.P.; Muhoza, B.; Duhoranimana, E.; Xia, S.Q.; Hayat, K.; Hussain, S.; Tahir, M.U.; Zhang, X.M. Fabrication of low environment-sensitive nanoparticles for cinnamaldehyde encapsulation by heat-induced gelation method. Food Hydrocoll. 2020, 105, 105789. [Google Scholar] [CrossRef]
- Qiao, Y.C.; Duan, L.J. Curcumin-loaded polyvinyl butyral film with antibacterial activity. E-Polymers 2020, 20, 673–681. [Google Scholar] [CrossRef]
- Wang, L.; Mu, R.J.; Li, Y.Z.; Lin, L.Z.; Lin, Z.Y.; Pang, J. Characterization and antibacterial activity evaluation of curcumin loaded konjac glucomannan and zein nanofibril films. Lwt-Food Sci. Technol. 2019, 113, 108293. [Google Scholar] [CrossRef]
- Giteru, S.G.; Ali, M.A.; Oey, I. Solvent strength and biopolymer blending effects on physicochemical properties of zein-chitosan-polyvinyl alcohol composite films. Food Hydrocoll. 2019, 87, 270–286. [Google Scholar] [CrossRef]
- Wei, Y.; Yu, Z.P.; Lin, K.S.; Sun, C.X.; Dai, L.; Yang, S.F.; Mao, L.K.; Yuan, F.; Gao, Y.X. Fabrication and characterization of resveratrol loaded zein-propylene glycol alginate-rhamnolipid composite nanoparticles: Physicochemical stability, formation mechanism and in vitro digestion. Food Hydrocoll. 2019, 95, 336–348. [Google Scholar] [CrossRef]
- Esposito, M.; Di Pierro, P.; Regalado-Gonzales, C.; Mariniello, L.; Giosafatto, C.V.L.; Porta, R. Polyamines as new cationic plasticizers for pectin-based edible films. Carbohydr. Polym. 2016, 153, 222–228. [Google Scholar] [CrossRef]
- Liu, Y.J.; Liu, D.D.; Zhu, L.; Gan, Q.; Le, X.Y. Temperature-dependent structure stability and in vitro release of chitosan-coated curcumin liposome. Food Res. Int. 2015, 74, 97–105. [Google Scholar] [CrossRef]
- Gao, S.; Guo, J.; Nishinari, K. Thermoreversible konjac glucomannan gel crosslinked by borax. Carbohydr. Polym. 2008, 72, 315–325. [Google Scholar] [CrossRef]
- Du, Y.; Wang, L.; Mu, R.; Wang, Y.; Li, Y.; Wu, D.; Wu, C.; Pang, J. Fabrication of novel Konjac glucomannan/shellac film with advanced functions for food packaging. Int. J. Biol. Macromol. 2019, 131, 36–42. [Google Scholar] [CrossRef]
- Kaur, M.; Santhiya, D. UV-shielding antimicrobial zein films blended with essential oils for active food packaging. J. Appl. Polym. Sci. 2020, 138, 49832. [Google Scholar] [CrossRef]
- Sun, C.X.; Xu, C.Q.; Mao, L.K.; Wang, D.; Yang, J.; Gao, Y.X. Preparation, characterization and stability of curcumin-loaded zein-shellac composite colloidal particles. Food Chem. 2017, 228, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xu, C.Q.; Mao, L.K.; Liu, F.G.; Sun, C.X.; Dai, L.; Gao, Y.X. Fabrication and characterization of binary composite nanoparticles between zein and shellac by anti-solvent co-precipitation. Food Bioprod. Process. 2018, 107, 88–96. [Google Scholar] [CrossRef]
- Corradini, E.; Mattoso, L.; Guedes, C.; Rosa, D. Mechanical, thermal and morphological properties of poly (ε-caprolactone)/zein blends. Polym. Adv. Technol. 2004, 15, 340–345. [Google Scholar] [CrossRef]
- Sessa, D.J.; Selling, G.W.; Biswas, A. Reaction of Zein with Methylenediphenyl Diisocyanate in the Melt State: Thermal, Mechanical, and Physical Properties. Ind. Eng. Chem. Res. 2012, 51, 9199–9203. [Google Scholar] [CrossRef]
- Gomez-Estaca, J.; Lopez-de-Dicastillo, C.; Hernandez-Munoz, P.; Catala, R.; Gavara, R. Advances in antioxidant active food packaging. Trends Food Sci. Technol. 2014, 35, 42–51. [Google Scholar] [CrossRef]
- Xin, S.; Xiao, L.; Dong, X.; Li, X.; Wang, Y.; Hu, X.; Sameen, D.E.; Qin, W.; Zhu, B. Preparation of chitosan/curcumin nanoparticles based zein and potato starch composite films for Schizothorax prenati fillet preservation. Int. J. Biol. Macromol. 2020, 164, 211–221. [Google Scholar] [CrossRef]
- Ma, Q.Y.; Du, L.; Wang, L.J. Tara gum/polyvinyl alcohol-based colorimetric NH3 indicator films incorporating curcumin for intelligent packaging. Sens. Actuators B-Chem. 2017, 244, 759–766. [Google Scholar] [CrossRef]
- Ak, T.; Gulcin, I. Antioxidant and radical scavenging properties of curcumin. Chem. -Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef]
- Wang, Y.J.; Pan, M.H.; Cheng, A.L.; Lin, L.I.; Ho, Y.S.; Hsieh, C.Y.; Lin, J.K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef]
- Chen, H.Z.; Zhang, M.; Bhandari, B.; Yang, C.H. Novel pH-sensitive films containing curcumin and anthocyanins to monitor fish freshness. Food Hydrocoll. 2020, 100, 105438. [Google Scholar] [CrossRef]
- Priyadarsini, K.I.; Maity, D.K.; Naik, G.H.; Kumar, M.S.; Unnikrishnan, M.K.; Satav, J.G.; Mohan, H. Role of phenolic O-H and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin. Free. Radic. Biol. Med. 2003, 35, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Dudonne, S.; Vitrac, X.; Coutiere, P.; Woillez, M.; Merillon, J.M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-O.; Lee, K.W.; Lee, H.J.; Lee, C.Y. Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J. Agric. Food Chem. 2002, 50, 3713–3717. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Xu, F.F.; Yuan, L.M.; Hu, H.X.; Yao, X.Y.; Liu, J. Comparison of the physical and functional properties of starch/polyvinyl alcohol films containing anthocyanins and/or betacyanins. Int. J. Biol. Macromol. 2020, 163, 898–909. [Google Scholar] [CrossRef]
- Liu, D.; Dang, S.; Zhang, L.; Munsop, K.; Li, X.X. Corn starch/polyvinyl alcohol based films incorporated with curcumin-loaded Pickering emulsion for application in intelligent packaging. Int. J. Biol. Macromol. 2021, 188, 974–982. [Google Scholar] [CrossRef]
- Andreuccetti, C.; Carvalho, R.A.; Grosso, C.R.F. Effect of hydrophobic plasticizers on functional properties of gelatin-based films. Food Res. Int. 2009, 42, 1113–1121. [Google Scholar] [CrossRef]
- Budi Santosa, F.X.; Padua, G.W. Tensile properties and water absorption of zein sheets plasticized with oleic and linoleic acids. J. Agric. Food Chem. 1999, 47, 2070–2074. [Google Scholar] [CrossRef]
- Gennadios, A. Protein-Based Films and Coatings; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Otoni, C.G.; Avena-Bustillos, R.J.; Azeredo, H.M.; Lorevice, M.V.; Moura, M.R.; Mattoso, L.H.; McHugh, T.H. Recent Advances on Edible Films Based on Fruits and Vegetables—A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1151–1169. [Google Scholar] [CrossRef]
- Pena-Serna, C.; Lopes, J.F. Influence of ethanol and glycerol concentration over functional and structural properties of zein-oleic acid films. Mater. Chem. Phys. 2013, 142, 580–585. [Google Scholar] [CrossRef]
- Ren, X.; Hou, T.; Liang, Q.; Zhang, X.; Hu, D.; Xu, B.; Chen, X.; Chalamaiah, M.; Ma, H. Effects of frequency ultrasound on the properties of zein-chitosan complex coacervation for resveratrol encapsulation. Food Chem. 2019, 279, 223–230. [Google Scholar] [CrossRef]
- Saberi, B.; Chockchaisawasdee, S.; Golding, J.B.; Scarlett, C.J.; Stathopoulos, C.E. Development of biocomposite films incorporated with different amounts of shellac, emulsifier, and surfactant. Food Hydrocoll. 2017, 72, 174–184. [Google Scholar] [CrossRef]
- Soradech, S.; Limatvapirat, S.; Luangtana-Anan, M. Stability enhancement of shellac by formation of composite film: Effect of gelatin and plasticizers. J. Food Eng. 2013, 116, 572–580. [Google Scholar] [CrossRef]
- Wang, Q.; Padua, G.W. Properties of zein films coated with drying oils. J. Agric. Food Chem. 2005, 53, 3444–3448. [Google Scholar] [CrossRef]
- Yuan, Y.; He, N.; Xue, Q.; Guo, Q.; Dong, L.; Haruna, M.H.; Zhang, X.; Li, B.; Li, L. Shellac: A promising natural polymer in the food industry. Trends Food Sci. Technol. 2021, 109, 139–153. [Google Scholar] [CrossRef]

| Sample | WVP (g∙mm∙m−2∙h−1∙kPa−1) | WS (%) | TS (MPa) | EB (%) |
|---|---|---|---|---|
| ZS–Cur0 | 0.405 ± 0.015 a | 13.266 ± 0.855 a | 0.725 ± 0.021 b | 0.642 ± 0.013 a |
| ZS–Cur1 | 0.396 ± 0.007 ab | 13.079 ± 0.543 a | 0.787 ± 0.025 a | 0.557 ± 0.022 b |
| ZS–Cur3 | 0.380 ± 0.013 b | 12.906 ± 0.802 a | 0.809 ± 0.017 a | 0.475 ± 0.013 c |
| ZS–Cur5 | 0.388 ± 0.011 ab | 12.586 ± 0.651 a | 0.717 ± 0.036 b | 0.424 ± 0.016 d |
| ZS–Cur7 | 0.391 ± 0.016 ab | 12.307 ± 0.523 a | 0.688 ± 0.018 b | 0.386 ± 0.008 e |
| Sample | Color Parameters | Opacity | |||||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | △E | WI | YI | ||
| ZS–Cur0 | 85.60 ± 0.24 a | 1.27 ± 0.27 e | 40.54 ± 0.87 e | 39.68 ± 0.85 e | 56.96 ± 0.86 a | 67.66 ± 1.56 e | 6.20 ± 0.06 e |
| ZS–Cur1 | 83.83 ± 0.63 b | 4.82 ± 0.65 d | 45.42 ± 1.80 d | 45.23 ± 1.63 d | 51.54 ± 1.56 b | 77.39 ± 2.66 d | 7.12 ± 0.16 d |
| ZS–Cur3 | 78.32 ± 0.62 c | 12.86 ± 0.54 c | 50.11 ± 0.64 c | 52.98 ± 0.35 c | 43.90 ± 0.35 c | 91.40 ± 0.81 c | 7.81 ± 0.02 c |
| ZS–Cur5 | 71.66 ± 0.68 d | 23.35 ± 0.14 b | 55.77 ± 0.59 b | 63.86 ± 0.26 b | 33.22 ± 0.23 d | 111.19 ± 0.29 b | 8.95 ± 0.13 b |
| ZS–Cur7 | 69.77 ± 0.85 e | 24.82 ± 0.48 a | 61.78 ± 0.90 a | 70.20 ± 1.17 a | 26.88 ± 1.18 e | 126.54 ± 3.14 a | 10.02 ± 0.14 a |
| Sample | WCA (º, t = 0 s) | Images | WCA (º, t = 60 s) | Images | ∆WCA (º) |
|---|---|---|---|---|---|
| ZS–Cur0 | 33.92 ± 1.55 c |  | 19.19 ± 0.93 d |  | 14.73 |
| ZS–Cur1 | 35.46 ± 1.33 c |  | 19.83 ± 1.32 d |  | 15.63 |
| ZS–Cur3 | 39.33 ± 1.14 b |  | 23.23 ± 0.89 c |  | 16.10 |
| ZS–Cur5 | 40.4 ± 1.23 ab |  | 25.47 ± 1.78 b |  | 14.93 |
| ZS–Cur7 | 42.1 ± 1.47 a |  | 36.43 ± 1.01 a |  | 5.67 |
| Peak 1 (°C) | Weight Loss (%) | Peak 2 (°C) | Weight Loss (%) | Peak 3 (°C) | Weight Loss (%) | Residue (%) | |
|---|---|---|---|---|---|---|---|
| Shellac | 438.89 | 65.54 | 8.03 | ||||
| ZS–Cur0 | 250.93 | 22.48 | 318.90 | 45.79 | 455.34 | 77.37 | 11.67 |
| ZS–Cur1 | 238.63 | 19.71 | 318.81 | 45.78 | 454.24 | 77.10 | 10.68 |
| ZS–Cur3 | 239.57 | 19.94 | 313.90 | 43.18 | 447.36 | 72.26 | 12.95 |
| ZS–Cur5 | 226.07 | 16.24 | 310.26 | 43.60 | 453.88 | 75.55 | 13.16 |
| ZS–Cur7 | 228.68 | 15.34 | 312.37 | 46.20 | 442.84 | 73.80 | 14.92 |
| Zein | 328.82 | 38.37 | 17.66 |
| Sample | pH-Response | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 3 | 4 | 5 | 6 | 7 | 9 | 10 | 11 | ||
| ZS–Cur1 | Color |  |  |  |  |  |  |  |  |
| L* | 85.72 ± 1.11 a | 84.19 ± 0.57 b | 83.29 ± 0.87 b | 81.44 ± 0.44 c | 81.62 ± 0.75 c | 76.78 ± 0.52 d | 66.23 ± 1.65 e | 61.12 ± 0.50 f | |
| a* | 4.62 ± 1.01 f | 5.55 ± 0.23 def | 6.09 ± 1.94 de | 6.87 ± 0.16 d | 5.10 ± 0.47 ef | 12.02 ± 0.48 c | 14.60 ± 0.94 b | 29.65 ± 0.52 a | |
| b* | 81.18 ± 1.32 a | 79.08 ± 0.90 b | 81.04 ± 0.41 a | 80.07 ± 0.73 ab | 77.73 ± 0.83 c | 66.04 ± 0.92 d | 59.38 ± 0.39 e | 55.35 ± 0.53 f | |
| △E | 76.79 ± 1.18 a | 74.99 ± 0.90 b | 77.12 ± 0.59 a | 76.53 ± 0.68 a | 74.05 ± 0.80 b | 64.89 ± 0.89 d | 63.42 ± 0.97 e | 67.92 ± 0.65 c | |
| ZS–Cur3 | Color |  |  |  |  |  |  |  |  |
| L* | 80.07 ± 0.49 a | 79.38 ± 0.29 a | 77.02 ± 0.29 b | 76.32 ± 0.14 b | 74.78 ± 0.21 c | 71.44 ± 0.80 d | 60.88 ± 0.38 e | 55.05 ± 1.06 f | |
| a* | 10.21 ± 0.50 h | 11.56 ± 0.32 g | 13.19 ± 0.51 f | 15.36 ± 0.55 e | 17.14 ± 0.54 d | 20.56 ± 0.85 c | 22.41 ± 0.63 b | 28.58 ± 0.21 a | |
| b* | 76.34 ± 0.41 a | 75.31 ± 0.12 b | 74.22 ± 0.80 c | 70.78 ± 0.18 d | 66.84 ± 0.27 e | 60.77 ± 0.24 f | 58.13 ± 0.53 g | 50.76 ± 0.80 h | |
| △E | 73.59 ± 0.38 a | 72.96 ± 0.03 a | 72.76 ± 0.79 a | 70.16 ± 0.16 b | 67.41 ± 0.15 c | 64.16 ± 0.68 d | 67.29 ± 0.70 c | 67.55 ± 0.76 c | |
| ZS–Cur5 | Color |  |  |  |  |  |  |  |  |
| L* | 78.53 ± 0.28 a | 76.89 ± 0.53 b | 75.51 ± 0.22 c | 73.74 ± 0.53 d | 72.88 ± 0.25 e | 60.92 ± 0.12 f | 59.00 ± 0.66 g | 49.31 ± 1.20 h | |
| a* | 13.28 ± 0.72 f | 13.98 ± 0.30 ef | 14.55 ± 0.15 e | 15.36 ± 0.23 d | 15.89 ± 0.25 d | 24.53 ± 0.48 c | 27.98 ± 0.81 b | 31.87 ± 0.68 a | |
| b* | 70.06 ± 0.31 a | 68.68 ± 0.53 b | 67.31 ± 0.22 c | 65.05 ± 0.16 d | 61.93 ± 0.56 e | 59.53 ± 0.29 f | 56.39 ± 0.81 g | 46.82 ± 1.24 h | |
| △E | 68.46 ± 0.29 bc | 67.74 ± 0.37 cd | 66.98 ± 0.25 d | 65.65 ± 0.22 e | 63.25 ± 0.47 f | 69.13 ± 0.34 b | 69.07 ± 0.98 b | 70.15 ± 1.20 a | |
| ZS–Cur7 | Color |  |  |  |  |  |  |  |  |
| L* | 75.53 ± 0.21 a | 74.08 ± 0.18 b | 72.56 ± 0.55 c | 72.15 ± 0.10 c | 71.63 ± 0.25 c | 57.46 ± 0.60 d | 52.28 ± 0.98 e | 44.27 ± 1.47 f | |
| a* | 16.75 ± 0.15 f | 17.70 ± 0.35 e | 18.10 ± 0.14 e | 18.89 ± 0.04 d | 19.19 ± 0.09 d | 27.5 ± 0.91 c | 35.14 ± 0.87 b | 38.87 ± 0.76 a | |
| b* | 69.07 ± 0.83 a | 66.47 ± 0.26 b | 65.34 ± 0.55 b | 63.78 ± 0.78 c | 62.52 ± 0.44 d | 53.29 ± 1.36 e | 43.66 ± 1.28 f | 34.14 ± 0.34 g | |
| △E | 69.13 ± 0.71 b | 67.44 ± 0.25 c | 67.03 ± 0.45 cd | 66.00 ± 0.71 de | 65.17 ± 0.42 e | 67.45 ± 1.00 c | 68.02 ± 1.39 bc | 70.85 ± 1.36 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, T.; Chen, W.; Zhong, Q.; Chen, W.; Xu, Y.; Wu, J.; Chen, H. Development and Characterization of an Edible Zein/Shellac Composite Film Loaded with Curcumin. Foods 2023, 12, 1577. https://doi.org/10.3390/foods12081577
Han T, Chen W, Zhong Q, Chen W, Xu Y, Wu J, Chen H. Development and Characterization of an Edible Zein/Shellac Composite Film Loaded with Curcumin. Foods. 2023; 12(8):1577. https://doi.org/10.3390/foods12081577
Chicago/Turabian StyleHan, Tao, Wenxue Chen, Qiuping Zhong, Weijun Chen, Yaping Xu, Jiawu Wu, and Haiming Chen. 2023. "Development and Characterization of an Edible Zein/Shellac Composite Film Loaded with Curcumin" Foods 12, no. 8: 1577. https://doi.org/10.3390/foods12081577
APA StyleHan, T., Chen, W., Zhong, Q., Chen, W., Xu, Y., Wu, J., & Chen, H. (2023). Development and Characterization of an Edible Zein/Shellac Composite Film Loaded with Curcumin. Foods, 12(8), 1577. https://doi.org/10.3390/foods12081577





