From Waste to Taste: Application of Fermented Spent Rootlet Ingredients in a Bread System
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Analysis of Dough Properties
2.2.1. Water Content
2.2.2. Preparation of Dough
2.2.3. Dough Rheology
2.2.4. Bread Fermentation Quality
2.2.5. Dough Development and Starch Pasting Properties
2.2.6. Gluten Network Development
2.3. Bread Production Process
2.4. Bread Analysis
2.4.1. Bake Loss
2.4.2. Specific Volume
2.4.3. Breadcrumb Structure
2.4.4. Breadcrumb Texture
2.5. Extraction and Quantification of Antifungal Compounds from BR Ingredients
2.6. Shelf-Life Evaluation
2.7. Release of Reducing Sugars
2.8. Sensory Evaluation
2.9. Statistical Analysis
3. Results
3.1. Compositional Analysis
3.2. Dough Analysis
3.2.1. Water Absorption
3.2.2. Gluten Network Development
3.2.3. Dough Development and Starch Pasting Properties
3.2.4. Bread Fermentation Capacity
3.2.5. Dough Rheology
3.3. Baked Bread Analysis
3.3.1. Baking Loss
3.3.2. Specific Volume
3.3.3. Crumb Structure-Cell Diameter
3.3.4. Breadcrumb Texture
Hardness
Resilience
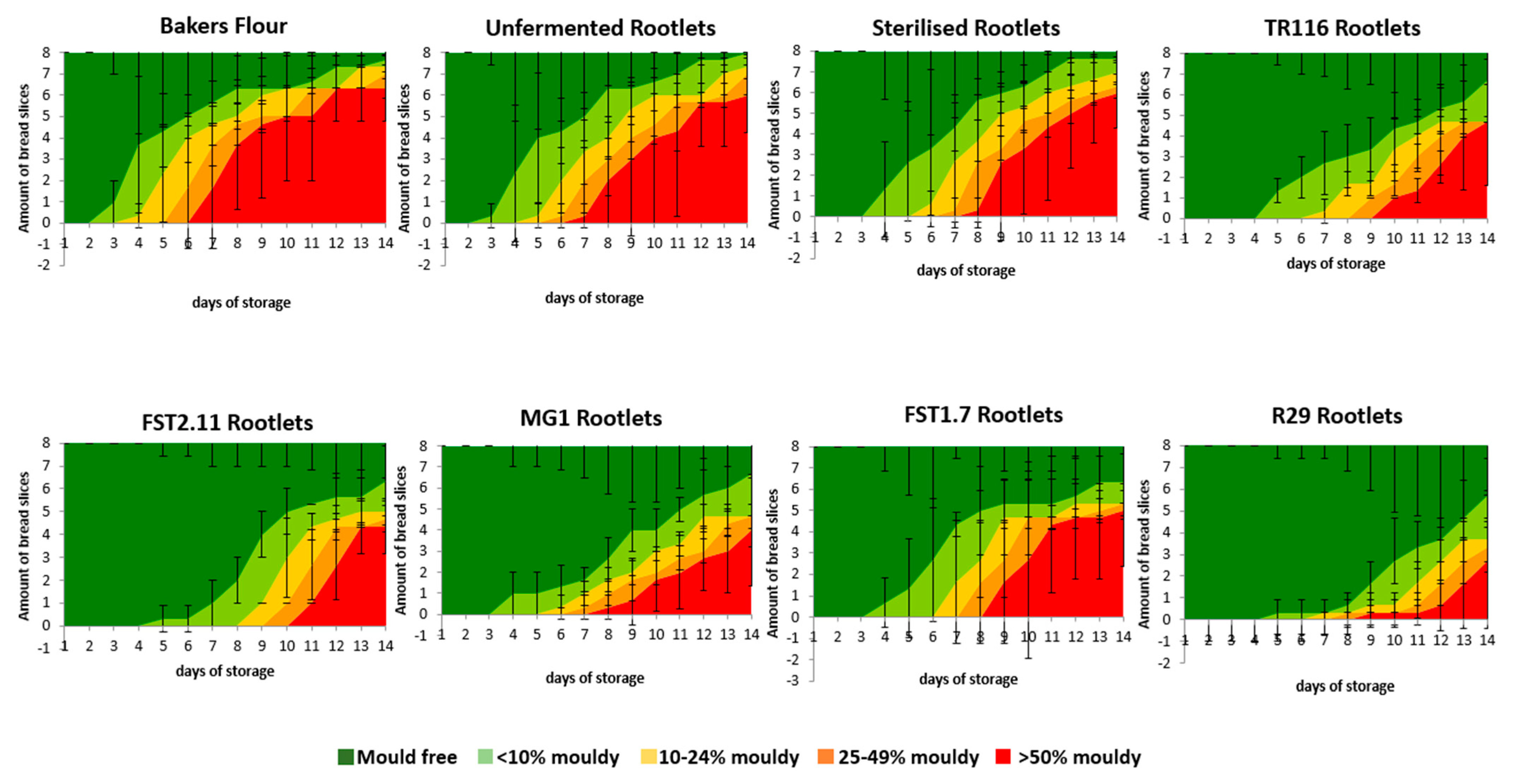
3.3.5. Microbial Shelf-Life Properties
Antifungal Compounds in BR Ingredients
Bread Fungal Shelf-Life
3.3.6. In Vitro Starch Hydrolysis
3.3.7. Sensory Analysis of the BR Bread
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Briggs, D.E. Malts and Malting, 1st ed.; Blackie Academic & Professional: London, UK, 1998. [Google Scholar]
- Kunze, W. Technology Brewing and Malting, 3rd ed.; VLB: Berlin, Germany, 2004. [Google Scholar]
- Waters, D.M.; Kingston, W.; Jacob, F.; Titze, J.; Arendt, E.K.; Zannini, E. Wheat bread biofortification with rootlets, a malting by-product. J. Sci. Food Agric. 2013, 93, 2372–2383. [Google Scholar] [CrossRef] [PubMed]
- Neylon, E.; Arendt, E.K.; Lynch, K.M.; Zannini, E.; Bazzoli, P.; Monin, T.; Sahin, A.W. Rootlets, a Malting by-Product with Great Potential. Fermentation 2020, 6, 117. [Google Scholar] [CrossRef]
- Salama, A.A.; El-Sahn, M.A.; Mesallam, A.S.; Shehata, A.M.E. Evaluation of the quality of bread, biscuit and butcher’s sausage supplemented with rootlets of malt sprouts. Nahrung 1997, 41, 228–231. [Google Scholar] [CrossRef]
- Chiş, M.S.; Pop, A.; Păucean, A.; Socaci, S.A.; Alexa, E.; Man, S.M.; Bota, M.; Muste, S. Fatty acids, volatile and sensory profile of multigrain biscuits enriched with spent malt rootlets. Molecules 2020, 25, 442. [Google Scholar] [CrossRef]
- Aprodu, I.; Simion, A.B.; Banu, I. Valorisation of the Brewers’ Spent Grain through Sourdough Bread Making. Int. J. Food Eng. 2017, 13, 1–9. [Google Scholar] [CrossRef]
- Ktenioudaki, A.; Alvarez-Jubete, L.; Smyth, T.J.; Kilcawley, K.; Rai, D.K.; Gallagher, E. Application of bioprocessing techniques (sourdough fermentation and technological aids) for brewer’s spent grain breads. Food Res. Int. 2015, 73, 107–116. [Google Scholar] [CrossRef]
- Neylon, E.; Arendt, E.K.; Zannini, E.; Sahin, A.W. Fermentation as a tool to revitalise brewers’ spent grain and elevate techno-functional properties and nutritional value in high fibre bread. Foods 2021, 10, 1639. [Google Scholar] [CrossRef] [PubMed]
- Neylon, E.; Arendt, E.K.; Zannini, E.; Sahin, A.W. Fundamental study of the application of brewers spent grain and fermented brewers spent grain on the quality of pasta. Food Struct. 2021, 30, 100225. [Google Scholar] [CrossRef]
- Schettino, R.; Verni, M.; Acin-albiac, M.; Vincentini, O.; Krona, A.; Knaapila, A.; Di Cagno, R.; Gobbetti, M.; Rizzello, C.G.; Coda, R. Bioprocessed brewers’ spent grain improves nutritional and antioxidant properties of pasta. Antioxidants 2021, 10, 742. [Google Scholar] [CrossRef] [PubMed]
- Verni, M.; Pontonio, E.; Krona, A.; Jacob, S.; Pinto, D.; Rinaldi, F.; Verardo, V.; Díaz-de-Cerio, E.; Coda, R.; Rizzello, C.G. Bioprocessing of Brewers’ Spent Grain Enhances Its Antioxidant Activity: Characterization of Phenolic Compounds and Bioactive Peptides. Front. Microbiol. 2020, 11, 1831. [Google Scholar] [CrossRef]
- Waters, D.M.; Jacob, F.; Titze, J.; Arendt, E.K.; Zannini, E. Fibre, protein and mineral fortification of wheat bread through milled and fermented brewer’s spent grain enrichment. Eur. Food Res. Technol. 2012, 235, 767–778. [Google Scholar] [CrossRef]
- Cantatore, V.; Filannino, P.; Gambacorta, G.; De Pasquale, I.; Pan, S.; Gobbetti, M.; Di Cagno, R. Lactic Acid Fermentation to Re-cycle Apple By-Products for Wheat Bread Fortification. Front. Microbiol. 2019, 10, 2574. [Google Scholar] [CrossRef] [PubMed]
- Immonen, M.; Maina, N.H.; Wang, Y.; Coda, R.; Katina, K. Waste bread recycling as a baking ingredient by tailored lactic acid fermentation. Int. J. Food Microbiol. 2020, 327, 108652. [Google Scholar] [CrossRef]
- Pontonio, E.; Lorusso, A.; Gobbetti, M.; Rizzello, C.G. Use of fermented milling by-products as functional ingredient to develop a low-glycaemic index bread. J. Cereal Sci. 2017, 77, 235–242. [Google Scholar] [CrossRef]
- Schettino, R.; Pontonio, E.; Rizzello, C.G. Use of fermented hemp, chickpea and milling by-products to improve the nutritional value of semolina pasta. Foods 2019, 8, 604. [Google Scholar] [CrossRef] [PubMed]
- Pontonio, E.; Dingeo, C.; Gobbetti, M.; Rizzello, C.G. Maize milling by-products: From food wastes to functional ingredients through lactic acid bacteria fermentation. Front. Microbiol. 2019, 10, 561. [Google Scholar] [CrossRef] [PubMed]
- Neylon, E.; Nyhan, L.; Zannini, E.; Monin, T.; Münch, S.; Sahin, A.W.; Arendt, E.K. Food Ingredients for the Future: In-Depth Analysis of the Effects of Lactic Acid Bacteria Fermentation on Spent Barley Rootlets. Fermentation 2023, 9, 78. [Google Scholar] [CrossRef]
- AOAC 992.23-1992; Crude Protein in Cereal Grains and Oilseeds. Generic Combustion Method. AOAC International, Official Methods: Rockville, MD, USA, 1998.
- Baldini, M.; Fabietti, F.; Giammarioli, S.; Onori, R.; Orefice, L.; Stacchini, A. Metodi di Analisi Utilizzati per il Controllo Chimico Degli Alimenti; Rapporti ISTISAN 1996/34; 1996. Available online: https://www.iss.it/documents/20126/45616/Rapp_ISTISAN_96_34_def.pdf/e3149ce3-508d-28f5-d4d2-f30c8479213c?t=1581103178196 (accessed on 20 December 2022).
- AOAC 923.03-1923; Ash of Flour. Direct Method. AOAC International, Official Methods: Rockville, MD, USA, 2005.
- ISO 712:2009; Cereals and Cereal Products—Determination of Moisture Content 2009. Available online: https://www.iso.org/obp/ui/#iso:std:iso:712:ed-4:v1:en (accessed on 20 December 2022).
- AOAC 986.25-1988(2002); Proximate Analysis of Milk-Based Infant. AOAC International, Official Methods: Rockville, MD, USA, 2002.
- AOAC 2017.16; Total Dietary Fiber in Foods. AOAC International, Official Methods: Rockville, MD, USA, 2017.
- Hoehnel, A.; Bez, J.; Sahin, A.W.; Coffey, A.; Arendt, E.K.; Zannini, E. Leuconostoc citreum TR116 as a Microbial Cell Factory to Functionalise High-Protein Faba Bean Ingredients for Bakery Applications. Foods 2020, 9, 1706. [Google Scholar] [CrossRef]
- Rice, T.; Sahin, A.W.; Heitmann, M.; Lynch, K.M.; Jacob, F.; Arendt, E.K.; Coffey, A. Application of mannitol producing Leuconostoc citreum TR116 to reduce sugar content of barley, oat and wheat malt-based worts. Food Microbiol. 2020, 90, 103464. [Google Scholar] [CrossRef]
- Sahin, A.W.; Rice, T.; Coffey, A. Genomic analysis of Leuconostoc citreum TR116 with metabolic reconstruction and the effects of fructose on gene expression for mannitol production. Int. J. Food Microbiol. 2021, 354, 109327. [Google Scholar] [CrossRef]
- Sahin, A.W.; Rice, T.; Zannini, E.; Axel, C.; Coffey, A.; Lynch, K.M.; Arendt, E.K. Leuconostoc citreum TR116: In-situ production of mannitol in sourdough and its application to reduce sugar in burger buns. Int. J. Food Microbiol. 2019, 302, 80–89. [Google Scholar] [CrossRef]
- Boeck, T.; Ispiryan, L.; Hoehnel, A.; Sahin, A.W.; Coffey, A.; Zannini, E.; Arendt, E.K. Lentil-Based Yogurt Alternatives Fermented with Multifunctional Strains of Lactic Acid Bacteria—Techno-Functional, Microbiological, and Sensory Characteristics. Foods 2022, 11, 2013. [Google Scholar] [CrossRef] [PubMed]
- Sahin, A.W.; Rice, T.; Zannini, E.; Lynch, K.M.; Coffey, A.; Arendt, E.K. The incorporation of sourdough in sugar-reduced biscuits: A promising strategy to improve techno-functional and sensory properties. Eur. Food Res. Technol. 2019, 245, 1841–1854. [Google Scholar] [CrossRef]
- Ryan, L.A.M.; Zannini, E.; Dal Bello, F.; Pawlowska, A.; Koehler, P.; Arendt, E.K. Lactobacillus amylovorus DSM 19280 as a novel food-grade antifungal agent for bakery products. Int. J. Food Microbiol. 2011, 146, 276–283. [Google Scholar] [CrossRef]
- Peyer, L.C.; Zarnkow, M.; Jacob, F.; De Schutter, D.P.; Arendt, E.K. Sour brewing: Impact of Lactobacillus amylovorus FST2.11 on technological and quality attributes of acid beers. J. Am. Soc. Brew. Chem. 2017, 75, 207–216. [Google Scholar] [CrossRef]
- Peyer, L.C.; Bellut, K.; Lynch, K.M.; Zarnkow, M.; Jacob, F.; De Schutter, D.P.; Arendt, E.K. Impact of buffering capacity on the acidification of wort by brewing-relevant lactic acid bacteria. J. Inst. Brew. 2017, 123, 497–505. [Google Scholar] [CrossRef]
- Lynch, K.M.; Lucid, A.; Arendt, E.K.; Sleator, R.D.; Lucey, B.; Coffey, A. Genomics of Weissella cibaria with an examination of its metabolic traits. Microbiology 2015, 161, 914–930. [Google Scholar] [CrossRef]
- Galle, S.; Schwab, C.; Arendt, E.; Gänzle, M. Exopolysaccharide-forming Weissella strains as starter cultures for sorghum and wheat sourdoughs. J. Agric. Food Chem. 2010, 58, 5834–5841. [Google Scholar] [CrossRef]
- Belz, M.C.E.; Axel, C.; Arendt, E.K.; Lynch, K.M.; Brosnan, B.; Sheehan, E.M.; Coffey, A.; Zannini, E. Improvement of taste and shelf life of yeasted low-salt bread containing functional sourdoughs using Lactobacillus amylovorus DSM 19280 and Weissella cibaria MG1. Int. J. Food Microbiol. 2019, 302, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Wolter, A.; Hager, A.S.; Zannini, E.; Galle, S.; Gänzle, M.G.; Waters, D.M.; Arendt, E.K. Evaluation of exopolysaccharide producing Weissella cibaria MG1 strain for the production of sourdough from various flours. Food Microbiol. 2014, 37, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Zannini, E.; Mauch, A.; Galle, S.; Gänzle, M.; Coffey, A.; Arendt, E.K.; Taylor, J.P.; Waters, D.M. Barley malt wort fermentation by exopolysaccharide-forming Weissella cibaria MG1 for the production of a novel beverage. J. Appl. Microbiol. 2013, 115, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Zannini, E.; Jeske, S.; Lynch, K.; Arendt, E.K. Development of novel quinoa-based yoghurt fermented with dextran producer Weissella cibaria MG1. Int. J. Food Microbiol. 2018, 268, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Axel, C.; Brosnan, B.; Zannini, E.; Peyer, L.C.; Furey, A.; Coffey, A.; Arendt, E.K. Antifungal activities of three different Lactobacillus species and their production of antifungal carboxylic acids in wheat sourdough. Appl. Microbiol. Biotechnol. 2016, 100, 1701–1711. [Google Scholar] [CrossRef]
- Schmidt, M.; Lynch, K.M.; Zannini, E.; Arendt, E.K. Fundamental study on the improvement of the antifungal activity of Lactobacillus reuteri R29 through increased production of phenyllactic acid and reuterin. Food Control 2018, 88, 139–148. [Google Scholar] [CrossRef]
- Peyer, L.C.; Zannini, E.; Jacob, F.; Arendt, E.K. Growth study, metabolite development, and organoleptic profile of a malt-based substrate fermented by lactic acid bacteria. J. Am. Soc. Brew. Chem. 2015, 73, 303–313. [Google Scholar] [CrossRef]
- Dal Bello, F.; Clarke, C.I.; Ryan, L.A.M.; Ulmer, H.; Schober, T.J.; Ström, K.; Sjögren, J.; van Sinderen, D.; Schnürer, J.; Arendt, E.K. Improvement of the quality and shelf life of wheat bread by fermentation with the antifungal strain Lactobacillus plantarum FST 1.7. J. Cereal Sci. 2007, 45, 309–318. [Google Scholar] [CrossRef]
- Peyer, L.C.; Axel, C.; Lynch, K.M.; Zannini, E.; Jacob, F.; Arendt, E.K. Inhibition of Fusarium culmorum by carboxylic acids released from lactic acid bacteria in a barley malt substrate. Food Control 2016, 69, 227–236. [Google Scholar] [CrossRef]
- Wolter, A.; Hager, A.S.; Zannini, E.; Arendt, E.K. Influence of sourdough on in vitro starch digestibility and predicted glycemic indices of gluten-free breads. Food Funct. 2014, 5, 564–572. [Google Scholar] [CrossRef]
- Delcour, J.A.; Hoseney, R.C. Yeast-leavened products. In Principles of Cereal Science and Technology; AACC International: St. Paul, MN, USA, 2010; pp. 177–206. [Google Scholar]
- Hager, A.S.; Ryan, L.A.M.; Schwab, C.; Gänzle, M.G.; O’Doherty, J.V.; Arendt, E.K. Influence of the soluble fibres inulin and oat β-glucan on quality of dough and bread. Eur. Food Res. Technol. 2011, 232, 405–413. [Google Scholar] [CrossRef]
- Rosell, C.M.; Santos, E.; Collar, C. Physical characterization of fiber-enriched bread doughs by dual mixing and temperature constraint using the Mixolab®. Eur. Food Res. Technol. 2010, 231, 535–544. [Google Scholar] [CrossRef]
- Rosell, C.M.; Collar, C.; Haros, M. Assessment of hydrocolloid effects on the thermo-mechanical properties of wheat using the Mixolab. Food Hydrocoll. 2007, 21, 452–462. [Google Scholar] [CrossRef]
- Brosnan, B.; Coffey, A.; Arendt, E.K.; Furey, A. The QuEChERS approach in a novel application for the identification of antifungal compounds produced by lactic acid bacteria cultures. Talanta 2014, 129, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Sahin, A.W.; Axel, C.; Arendt, E.K. Understanding the function of sugar in burger buns: A fundamental study. Eur. Food Res. Technol. 2017, 243, 1905–1915. [Google Scholar] [CrossRef]
- Brennan, C.S.; Tudorica, C.M. Evaluation of potential mechanisms by which dietary fibre additions reduce the predicted glycaemic index of fresh pastas. Int. J. Food Sci. Technol. 2008, 43, 2151–2162. [Google Scholar] [CrossRef]
- Ktenioudaki, A.; O’Shea, N.; Gallagher, E. Rheological properties of wheat dough supplemented with functional by-products of food processing: Brewer’s spent grain and apple pomace. J. Food Eng. 2013, 116, 362–368. [Google Scholar] [CrossRef]
- Gómez, M.; Martinez, M.M. Fruit and vegetable by-products as novel ingredients to improve the nutritional quality of baked goods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2119–2135. [Google Scholar] [CrossRef] [PubMed]
- Ortolan, F.; Steel, C.J. Protein Characteristics that Affect the Quality of Vital Wheat Gluten to be Used in Baking: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 369–381. [Google Scholar] [CrossRef]
- Noort, M.W.J.; van Haaster, D.; Hemery, Y.; Schols, H.A.; Hamer, R.J. The effect of particle size of wheat bran fractions on bread quality—Evidence for fibre-protein interactions. J. Cereal Sci. 2010, 52, 59–64. [Google Scholar] [CrossRef]
- Nawrocka, A.; Szymańska-Chargot, M.; Miś, A.; Wilczewska, A.Z.; Markiewicz, K.H. Aggregation of gluten proteins in model dough after fibre polysaccharide addition. Food Chem. 2017, 231, 51–60. [Google Scholar] [CrossRef]
- Delcour, J.A.; Hoseney, C.R. Proteins of cereals. In Principles of Cereal Science and Technology; AACC International: St. Paul, MN, USA, 2010; p. 61. [Google Scholar]
- Di Cagno, R.; Rizzello, C.G.; De Angelis, M.; Cassone, A.; Giuliani, G.; Benedusi, A.; Limitone, A.; Surico, R.F.; Gobbetti, M. Use of selected sourdough strains of Lactobacillus for removing gluten and enhancing the nutritional properties of gluten-free bread. J. Food Prot. 2008, 71, 1491–1495. [Google Scholar] [CrossRef]
- Doǧan, I.S. Dynamic rheological properties of dough as affected by amylases from various sources. Nahrung-Food 2002, 46, 399–403. [Google Scholar] [CrossRef]
- Stewart, G.G.; Russell, I.; Anstruther, A. Handbook of Brewing, 3rd ed.; Taylor & Francis Group: Sarasota, FL, USA, 2017; ISBN 9781498751919. [Google Scholar]
- Rizvi, S.M.H.; Beattie, A.D.; Rossnagel, B.; Scoles, G. Thermostability of barley malt proteases in Western Canadian two-row malting barley. Cereal Chem. 2011, 88, 609–613. [Google Scholar] [CrossRef]
- Caballero, P.A.; Gómez, M.; Rosell, C.M. Improvement of dough rheology, bread quality and bread shelf-life by enzymes combination. J. Food Eng. 2007, 81, 42–53. [Google Scholar] [CrossRef]
- Indrani, D.; Prabhasankar, P.; Rajiv, J.; Venkateswara Rao, G. Scanning Electron Microscopy, Rheological Characteristics, and Bread-Baking Performance of Wheat-Flour Dough as Affected by Enzymes. J. Food Sci. 2003, 68, 2804–2809. [Google Scholar] [CrossRef]
- Bouachra, S.; Begemann, J.; Aarab, L.; Hüsken, A. Prediction of bread wheat baking quality using an optimized GlutoPeak®-Test method. J. Cereal Sci. 2017, 76, 8–16. [Google Scholar] [CrossRef]
- Lynch, K.M.; Coffey, A.; Arendt, E.K. Exopolysaccharide producing lactic acid bacteria: Their techno-functional role and potential application in gluten-free bread products. Food Res. Int. 2018, 110, 52–61. [Google Scholar] [CrossRef]
- Galle, S.; Schwab, C.; Bello, F.D.; Coffey, A.; Gänzle, M.; Arendt, E. Comparison of the impact of dextran and reuteran on the quality of wheat sourdough bread. J. Cereal Sci. 2012, 56, 531–537. [Google Scholar] [CrossRef]
- Galle, S.; Arendt, E.K. Exopolysaccharides from Sourdough Lactic Acid Bacteria. Crit. Rev. Food Sci. Nutr. 2014, 54, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Rosell, C.; Rojas, J.A.; Benedito de Barber, C. Influence of hydrocolloids on dough rheology and bread quality. Food Hydrocoll. 2001, 15, 75–81. [Google Scholar] [CrossRef]
- Kaditzky, S.; Seitter, M.; Hertel, C.; Vogel, R.F. Performance of Lactobacillus sanfranciscensis TMW 1.392 and its levansucrase deletion mutant in wheat dough and comparison of their impact on bread quality. Eur. Food Res. Technol. 2008, 227, 433–442. [Google Scholar] [CrossRef]
- Kim, H.J.; Yoo, S.H. Effects of combined α-amylase and endo-xylanase treatments on the properties of fresh and frozen doughs and final breads. Polymers 2020, 12, 1349. [Google Scholar] [CrossRef] [PubMed]
- Gómez, M.; Jiménez, S.; Ruiz, E.; Oliete, B. Effect of extruded wheat bran on dough rheology and bread quality. LWT 2011, 44, 2231–2237. [Google Scholar] [CrossRef]
- Sudha, M.L.; Vetrimani, R.; Leelavathi, K. Influence of fibre from different cereals on the rheological characteristics of wheat flour dough and on biscuit quality. Food Chem. 2007, 100, 1365–1370. [Google Scholar] [CrossRef]
- Collar, C.; Santos, E.; Rosell, C.M. Significance of dietary fiber on the viscometric pattern of pasted and gelled flour-fiber blends. Cereal Chem. 2006, 83, 370–376. [Google Scholar] [CrossRef]
- Thiele, C.; Gänzle, M.G.; Vogel, R.F. Contribution of sourdough Lactobacilli, yeast, and cereal enzymes to the generation of amino acids in dough relevant for bread flavor. Cereal Chem. 2002, 79, 45–51. [Google Scholar] [CrossRef]
- Codinǎ, G.G.; Mironeasa, S.; Mironeasa, C. Variability and relationship among Mixolab and Falling Number evaluation based on influence of fungal α-amylase addition. J. Sci. Food Agric. 2012, 92, 2162–2170. [Google Scholar] [CrossRef] [PubMed]
- Grewal, N.; Faubion, J.; Feng, G.; Kaufman, R.C.; Wilson, J.D.; Shi, Y.C. Structure of waxy maize starch hydrolyzed by maltogenic α-amylase in relation to its retrogradation. J. Agric. Food Chem. 2015, 63, 4196–4201. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Li, X.; Bai, Y.; Jin, Z.; Svensson, B. Maltogenic α-amylase hydrolysis of wheat starch granules: Mechanism and relation to starch retrogradation. Food Hydrocoll. 2022, 124, 107256. [Google Scholar] [CrossRef]
- Axel, C.; Zannini, E.; Arendt, E.K. Mold spoilage of bread and its biopreservation: A review of current strategies for bread shelf life extension. Crit. Rev. Food Sci. Nutr. 2017, 57, 3528–3542. [Google Scholar] [CrossRef]
- Axel, C.; Röcker, B.; Brosnan, B.; Zannini, E.; Furey, A.; Coffey, A.; Arendt, E.K. Application of Lactobacillus amylovorus DSM19280 in gluten-free sourdough bread to improve the microbial shelf life. Food Microbiol. 2015, 47, 36–44. [Google Scholar] [CrossRef]
- Ryan, L.A.M.; Dal Bello, F.; Arendt, E.K. The use of sourdough fermented by antifungal LAB to reduce the amount of calcium propionate in bread. Int. J. Food Microbiol. 2008, 125, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Melini, V.; Melini, F. Strategies to extend bread and GF bread shelf-life: From Sourdough to antimicrobial active packaging and nanotechnology. Fermentation 2018, 4, 9. [Google Scholar] [CrossRef]
- Kendall, C.W.C.; Esfahani, A.; Jenkins, D.J.A. The link between dietary fibre and human health. Food Hydrocoll. 2010, 24, 42–48. [Google Scholar] [CrossRef]
- Östman, E.M.; Nilsson, M.; Liljeberg Elmståhl, H.G.M.; Molin, G.; Björck, I.M.E. On the effect of lactic acid on blood glucose and insulin responses to cereal products: Mechanistic studies in healthy subjects and in vitro. J. Cereal Sci. 2002, 36, 339–346. [Google Scholar] [CrossRef]
- Lotong, V.; Chambers IV, E.; Chambers, D.H. Determination of the sensory attributes of wheat sourdough bread. J. Sens. Stud. 2000, 15, 309–326. [Google Scholar] [CrossRef]


| Species | Leuconostoc citreum | Lactobacillus amylovorus | Weissella cibaria | Limosilactobacillus reuteri | Lactiplantibacillus plantarum |
|---|---|---|---|---|---|
| Strain | TR116 | FST 2.11 | MG1 | R29 | FST 1.7 |
| Metabolism | Heterofermentative | Homofermentative | Heterofermentative | Heterofermentative | Heterofermentative |
| Fermentation substrate | Fructose | Sucrose | Sucrose | Sucrose | Sucrose |
| Source | Yellow pea sourdough | Brewing environment | Sourdough | Human intestine | Malted barley |
| Special traits | Mannitol producer and antifungal producer | Antimicrobial producer and high acid producer | Dextran exopolysaccharide producer | Mannitol producer and antifungal producer | Antifungal producer and high acid producer |
| Reference | [26,27,28,29,30,31] | [32,33,34] | [35,36,37,38,39,40] | [41,42,43] | [34,43,44,45,46] |
| Analyte | BF | BR-UnF |
|---|---|---|
| Protein | 12.96 ± 0.79 | 35.80 ± 1.50 |
| Fat | 1.20 ± 0.08 | 1.77 ± 0.11 |
| Ash | 0.55 ± 0.05 | 5.98 ± 0.30 |
| Moisture | 12.95 ± 0.30 | 12.74 ± 0.30 |
| Total Carbohydrate | 65.31 ± 1.53 | 0 < 7.02 < 15.72 |
| Total fibre | 7.03 ± 1.27 | 36.64 ± 8.51 |
| Soluble fibre | 2.63 ± 0.63 | 1.24 ± 0.30 |
| High molecular weight of dietary fibre | 4.41 ± 1.1 | 35.40 ± 8.50 |
| Ingredient | BF | BR-UnF | BR-Ster | BR-TR116 | BR-MG1 | BR-FST2.11 | BR-R29 | BR-FST1.7 |
|---|---|---|---|---|---|---|---|---|
| BF | 100 | 95 | 95 | 95 | 95 | 95 | 95 | 95 |
| BR ingredient | - | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Salt | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 |
| Sugar | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Sunflower oil | 3.2 | 3.2 | 3.2 | 3.2 | 3.2 | 3.2 | 3.2 | 3.2 |
| Dry Yeast | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Water (FWA%) | 64.3 a | 68.2 c,d | 68.9 d | 66.90 b | 67.90 c | 67.50 b,c | 66.70 b | 67.30 b,c |
| BF | BR-UnF | BR-Ster | BR-TR116 | BR-MG1 | BR-FST2.11 | BR-R29 | BR-FST1.7 | |
|---|---|---|---|---|---|---|---|---|
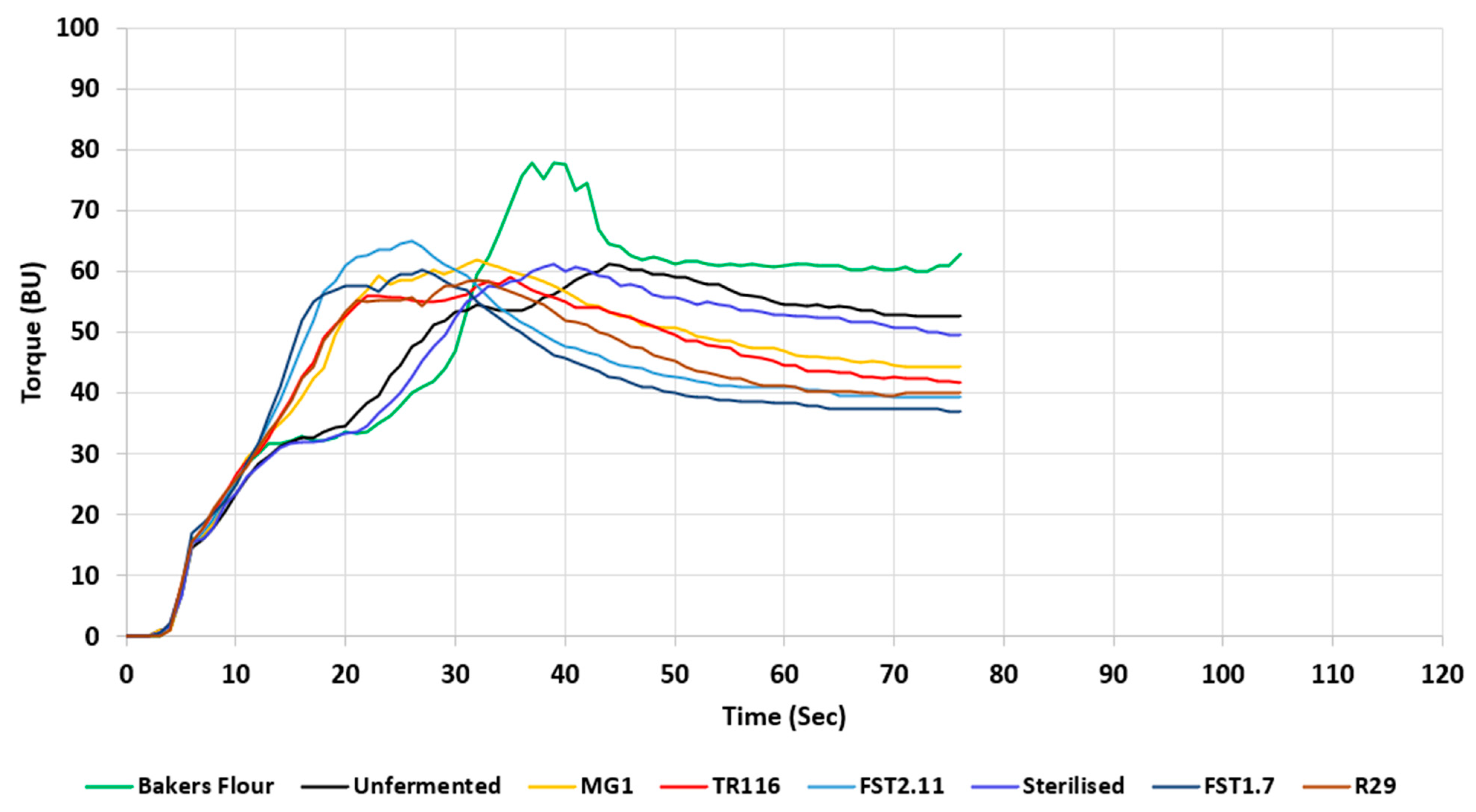
| Torque Max (BU) | 78.33 ± 1.54 c | 64.00 ± 1.00 a,b | 61.67 ± 2.53 a,b | 60.33 ± 4.04 a,b | 62.333 ± 1.53 a,b | 65.33 ± 1.16 b | 58.67 ± 1.54 a | 60.00 ± 1.00 a |
| Peak Max Time (s) | 39.67 ± 1.53 c | 44.00 ± 1.00 c | 40.00 ± 2.00 c | 33.67 ± 3.22 b | 32.67 ± 2.08 b | 25.67 ± 1.16 a | 32.33 ± 0.58 b | 26.67 ± 1.16 a |
| DDT (min) | 1.32 ± 0.27 a | 7.04 ± 0.23 d | 7.49 ± 0.32 d | 5.61 ± 0.07 c | 4.95 ± 1.00 a,b,c,d | 4.21 ± 0.03 b | 3.66 ± 0.15 b | 3.87 ± 0.10 b |
| C2 (Nm) | 0.43 ± 0.01 g | 0.33 ± 0.00 b,c | 0.38 ± 0.00 f | 0.35 ± 0.01 d,e | 0.36 ± 0.00 e | 0.31 ± 0.01 a,b | 0.34 ± 0.00 c,d | 0.30 ± 0.01 a |
| C3 (Nm) | 1.63 ± 0.01 d | 1.24 ± 0.02 a | 1.52 ± 0.01 b | 1.56 ± 0.02 b,c | 1.53 ± 0.00 b | 1.63 ± 0.00 c,d | 1.60 ± 0.01 c,d | 1.62 ± 0.01 c,d |
| C4 (Nm) | 1.50 ± 0.01 e,f | 0.71 ± 0.03 a | 1.38 ± 0.01 b | 1.44 ± 0.01 c,d | 1.41 ± 0.01 b,c | 1.51 ± 0.01 f | 1.47 ± 0.01 d,e | 1.50 ± 0.01 e,f |
| C5 (Nm) | 2.55 ± 0.03 d | 1.12 ± 0.03 a | 2.23 ± 0.03 b | 2.34 ± 0.02 c | 2.28 ± 0.01 b,c | 2.65 ± 0.03 e | 2.59 ± 0.05 d,e | 2.61 ± 0.02 d,e |
| Hm (mm) | 50.93 ± 2.48 c | 46.40 ± 1.31 b,c | 40.43 ± 0.57 a,b | 43.17 ± 3.26 b | 44.87 ± 1.52 b,c | 37.00 ± 0.30 a | 36.57 ± 2.53 a | 34.97 ± 3.10a |
| Vol. of CO2 (mL) | 2045 ± 73a,b,c | 2451 ± 30d | 2399 ± 100d | 2029 ± 69a,b | 2547 ± 68d | 2318 ± 131c,d | 1919 ± 59a | 2302 ± 173 b,c,d |
| CO2 retention coefficient (%) | 97.20 ± 3.33 a | 99.63 ± 0.12 a | 99.70 ± 0.00 a | 99.57 ± 0.15 a | 99.73 ± 0.12 a | 99.67 ± 0.06 a | 99.60 ± 0.17 a | 99.73 ± 0.06 a |
| Damping factor | 0.390 ± 0.012 d | 0.383 ± 0.009 d | 0.357 ± 0.010 c | 0.346 ± 0.012 a,b,c | 0.352 ± 0.011 b,c | 0.328 ± 0.006 a | 0.344 ± 0.011 a,b,c | 0.333 ± 0.014 a,b |
| BF | BR-UnF | BR-Ster | BR-TR116 | BR-MG1 | BR-FST2.11 | BR-R29 | BR-FST1.7 | |
|---|---|---|---|---|---|---|---|---|
| Predicted fibre content (g/100 g) | 4.74 | 5.57 | 5.55 | 5.66 | 5.63 | 5.61 | 5.61 | 5.60 |
| Digestible starch content (g/100 g) | 40.61 ± 1.46 c | 38.68 ± 0.80 a | 35.64 ± 0.56 a,b | 36.80 ± 1.14 a,b | 37.17 ± 0.66b | 36.11 ± 1.02 a,b | 36.32 ± 0.76 a,b | 36.85 ± 0.73 a,b |
| Bake loss (%) | 14.24 ± 0.91 a,b,c,d,e | 13.54 ± 0.58 b,c,e | 13.56 ± 0.53 b,c | 14.33 ± 0.47 d,e | 14.19 ± 0.38 a,d,e | 13.85 ± 0.31 a,b,c,d,e | 13.36 ± 0.58 a,b,c | 13.54 ± 0.93 a,b,c,d,e |
| Specific volume (mL/g) | 3.74 ± 0.20 d,e | 3.55 ± 0.14 c,d | 3.09 ± 0.18 b | 3.68 ± 0.22 c,d,e | 3.80 ± 0.13 e | 2.80 ± 0.15 a | 3.35 ± 0.29 b,c | 2.63 ± 0.19 a |
| Cell diameter (mm) | 2.93 ± 0.15 e | 2.16 ± 0.15 d | 1.94 ± 0.18 b,c | 1.95 ± 0.17 b,c | 2.05 ± 0.16 c,d | 1.83 ± 0.22 b | 1.83 ± 0.15 b | 1.63 ± 0.16 a |
| Breadcrumb hardness (N) | 2.33 ± 0.32 c | 1.77 ± 0.26 a,b | 3.14 ± 0.42 d | 1.96 ± 0.30 b | 1.61 ± 0.22 a | 3.59 ± 0.53 d | 2.39 ± 0.35 c | 4.11 ± 0.55 d |
| Breadcrumb resilience (N) | 0.56 ± 0.01 c,d | 0.53 ± 0.02 b | 0.55 ± 0.01 b,c | 0.55 ± 0.02 b,c | 0.56 ± 0.01 d | 0.52 ± 0.02 a | 0.54 ± 0.01 b | 0.53 ± 0.01 a |
| Antifungal Compound | BR-UnF | BR-Ster | BR-TR116 | BR-MG1 | BR-FST2.11 | BR-R29 | BR-FST1.7 |
|---|---|---|---|---|---|---|---|
| Hydroxyphenyllactic acid | n.d. | n.d. | 0.637 ± 0.091 a | n.d. | 2.483 ± 0.247 b | 2.239 ± 0.087 b | 9.136 ± 0.104 c |
| 4-Hydroxybenzoic acid | 0.928 ± 0.006 d | 1.215 ± 0.109 c,d | 1.177 ± 0.036 c | 0.881 ± 0.101 a,c,d | 0.864 ± 0.078 a,b,d | 0.653 ± 0.034 a | 0.861 ± 0.004 b,c |
| Vanillic acid | 1.316 ± 0.018 b | 1.551 ± 0.171 b | 1.270 ± 0.038 b | 1.367 ± 0.150 b | 1.144 ± 0.157 a,b | 1.199 ± 0.057 b | 0.553 ± 0.006 a |
| Phenyllactic acid | n.d. | n.d. | 5.254 ± 0.179 b | 0.562 ± 0.078 a | 5.223 ± 0.740 b | 15.645 ± 0.589 c | 13.387 ± 0.074 c |
| Hydroferulic acid | n.d. | n.d. | n.d. | 0.924 ± 0.050 a | 0.973 ± 0.132 a | n.d. | 0.815 ± 0.019 a |
| Coumaric acid | n.d. | 0.537 ± 0.090 a | 0.842 ± 0.057 b | n.d. | n.d. | n.d. | n.d. |
| Ferulic acid | 1.008 ± 0.095 a | 1.656 ± 0.197 b | 2.220 ± 0.079 c | n.d. | n.d. | 4.068 ± 0.164 d | n.d. |
| BF | BR-UnF | BR-Ster | BR-TR116 | BR-MG1 | BR-FST2.11 | BR-R29 | BR-FST1.7 | |
|---|---|---|---|---|---|---|---|---|
| Odour | ||||||||
| Intensity | 5.78 ± 2.21 a | 6.39 ± 1.85 a | 6.67 ± 2.28 a | 6.33 ± 2.11 a | 6.78 ± 1.83 a | 6.67 ± 2.11 a | 6.22 ± 1.86 a | 6.22 ± 2.07 a |
| Citrus | 1.67 ± 1.46 a | 2.06 ± 1.26 a | 1.50 ± 1.86 a | 1.72 ± 1.36 a | 1.83 ± 0.99 a | 2.56 ± 1.65 a | 2.17 ± 1.34 a | 2.78 ± 1.80 a |
| Vegetable | 1.28 ± 1.27 a | 2.28 ± 1.49 a | 2.44 ± 1.50 a | 1.83 ± 0.99 a | 1.78 ± 1.44 a | 2.44 ± 1.38 a | 2.06 ± 1.70 a | 1.67 ± 1.14 a |
| Cereals/grains | 6.06 ± 2.36 a | 7.11 ± 1.32 a | 7.28 ± 1.36 a | 7.00 ± 1.41 a | 6.83 ± 1.34 a | 6.06 ± 1.30 a | 6.06 ± 1.43 a | 6.56 ± 1.54 a |
| Taste | ||||||||
| Sour | 0.78 ± 1.26 a | 1.61 ± 1.42 a,b | 0.72 ± 0.83 a | 2.17 ± 1.86 b | 2.22 ± 1.66 b | 4.22 ± 2.34 c | 2.89 ± 2.14 b,c | 4.33 ± 2.83 c |
| Flavour | ||||||||
| Intensity | 4.39 ± 1.61 a | 5.72 ± 1.64 a | 5.67 ± 2.11 a | 5.44 ± 1.85 a | 5.11 ± 2.05 a | 6.22 ± 1.77 a | 5.94 ± 1.95 a | 6.17 ± 1.95 a |
| Muddy/earthy | 0.89 ± 1.02 a | 2.22 ± 2.37 a | 2.89 ± 2.37 a | 2.50 ± 2.38 a | 1.67 ± 1.81 a | 2.17 ± 2.33 a | 1.83 ± 2.20 a | 1.44 ± 1.25 a |
| Fruity | 0.44 ± 0.51 a | 1.56 ± 1.76 b,c | 0.61 ± 0.70 a,b | 1.39 ± 1.38 b,c | 1.56 ± 1.50 b,c | 3.56 ± 2.45 d | 2.39 ± 1.42 c,d | 2.89 ± 2.49 c,d |
| Vegetable | 1.06 ± 0.94 a | 2.39 ± 1.46 a,b | 3.06 ± 1.55 b | 2.61 ± 1.65 b | 2.39 ± 1.58 a,b | 2.50 ± 1.47 a,b | 2.50 ± 1.86 a,b | 1.89 ± 1.41 a,b |
| Aftertaste | 1.28 ± 1.74 a | 2.72 ± 2.44 a | 2.94 ± 2.21 a | 2.22 ± 1.96 a | 2.11 ± 1.64 a | 3.39 ± 2.66 a | 3.06 ± 2.41 a | 3.17 ± 2.36 a |
| Texture | ||||||||
| Hardness | 4.00 ± 2.28 a | 3.44 ± 2.28 a | 3.83 ± 1.95 a | 3.44 ± 1.62 a | 3.50 ± 1.76 a | 3.89 ± 1.45 a | 3.33 ± 1.71 a | 3.61 ± 1.65 a |
| Chewiness | 4.50 ± 2.23 a | 4.67 ± 2.11 a | 4.50 ± 2.01 a | 4.78 ± 1.77 a | 5.22 ± 2.07 a | 4.94 ± 1.63 a | 4.56 ± 1.98 a | 4.94 ± 1.83 a |
| Overall acceptability | 8.17 ± 1.72 a | 7.56 ± 1.82 a | 7.33 ± 2.11 a | 7.67 ± 1.64 a | 8.11 ± 1.41 a | 7.28 ± 1.41 a | 7.50 ± 1.98 a | 7.44 ± 1.46 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neylon, E.; Nyhan, L.; Zannini, E.; Sahin, A.W.; Arendt, E.K. From Waste to Taste: Application of Fermented Spent Rootlet Ingredients in a Bread System. Foods 2023, 12, 1549. https://doi.org/10.3390/foods12071549
Neylon E, Nyhan L, Zannini E, Sahin AW, Arendt EK. From Waste to Taste: Application of Fermented Spent Rootlet Ingredients in a Bread System. Foods. 2023; 12(7):1549. https://doi.org/10.3390/foods12071549
Chicago/Turabian StyleNeylon, Emma, Laura Nyhan, Emanuele Zannini, Aylin W. Sahin, and Elke K. Arendt. 2023. "From Waste to Taste: Application of Fermented Spent Rootlet Ingredients in a Bread System" Foods 12, no. 7: 1549. https://doi.org/10.3390/foods12071549
APA StyleNeylon, E., Nyhan, L., Zannini, E., Sahin, A. W., & Arendt, E. K. (2023). From Waste to Taste: Application of Fermented Spent Rootlet Ingredients in a Bread System. Foods, 12(7), 1549. https://doi.org/10.3390/foods12071549







